Abstract
The development of large-scale energy storage technologies is a key element in the transition to sustainable energy systems, where redox flow batteries (RFBs) are emerging as a promising alternative to conventional systems. The available literature reveals a notable lack of systematic studies evaluating the impact of membranes on the performance of IL-incorporating RFBs, despite this component being crucial for regulating ionic conductivity, minimizing the crossover of active species, and ensuring the operational stability of the system. This review provides a critical analysis of 81 articles published between 2015 and 2025, examining the impact of various membrane types on key parameters including conductivity, thermal and mechanical stability, energy efficiency, and power output. The findings reveal that more than 70% of the reviewed studies do not directly address the function of the membrane, underscoring the need for research focused on designing selective and robust materials for non-aqueous conditions. Finally, knowledge gaps are identified, and development prospects are proposed, along with the standardization of characterization protocols, to accelerate the practical implementation of IL-based RFBs in various scenarios.
1. Introduction
The global energy transition requires the development of electrical storage technologies capable of ensuring the massive integration of intermittent renewable sources, such as solar and wind, into generation and distribution systems [1]. In this context, redox flow batteries (RFBs) emerge as a promising alternative due to their scalability, long service life, and operational flexibility [2,3].
The operating principle of RFBs is based on the storage of energy in liquid electrolytes that circulate through electrochemical cells. Historically, the most studied systems have been those based on acid-aqueous electrolytes containing vanadium ions, whose technological maturity has enabled industrial-scale applications [4]. However, limitations in energy density, electrochemical window, and formation of hydrated metallic salts have driven the search for new electrolytes. In this context, ionic liquids (ILs) have emerged as desirable candidates due to their low volatility, high thermal stability, adjustable conductivity, and structural versatility [5,6,7].
The overall performance of an RFB depends not only on the electrolyte but also on the interaction between the electrolyte and the ion exchange membrane, which enables selective transport of species and minimizes reagent crossover. In electrochemical energy storage systems, membrane selection determines critical parameters, including coulombic efficiency, voltage efficiency, and overall system efficiency [8,9,10]. Therefore, commercial membranes such as dense polymeric Nafion, as well as modified membranes and composite materials incorporating ILs, nanomaterials, or organic covalent frameworks, have been explored to improve the selectivity and stability of RFBs [4,11,12].
Despite these advances, there are still gaps in knowledge, with more than 70% of the studies reviewed focusing on the development of new electrolytes or catalysts, not considering as a study factor the interaction of the membranes with the non-aqueous ionic solvent, nor the direct relationship of the membrane with the power values of the storage system [13,14,15]. Furthermore, methodologies for characterizing ion transport and long-term stability lack standardization, making it challenging to establish rigorous comparisons between different designs [16,17].
The present review focuses specifically on the membrane-IL interaction and its impact on electrochemical metrics.
1.1. General Principles of Redox Flow Batteries
RFBs are electrochemical systems that store energy in liquid electrolytes containing dissolved redox species, which are stored in external tanks and circulated through an electrochemical cell during charging and discharging processes [2], allowing this modular design to decouple power (determined by cell area) from capacity (defined by electrolyte volume), making them ideal technologies for large-scale stationary applications [8,18].
Among the systems studied, vanadium redox flow batteries (VRFBs) have been the most widely explored due to their use of a single chemical element in multiple oxidation states, thereby avoiding cross-contamination between compartments [19]. However, limitations associated with low energy density (25–35 Wh·L−1) and electrolyte stability have driven the search for non-aqueous alternatives [14,15].
1.2. Ionic Liquids as Electrolytes in RFBs
ILs have emerged as attractive electrolytes thanks to their unique properties: low vapor pressure, high thermal stability, wide electrochemical window, and the ability to tailor their molecular structure to adjust ionic conductivity and the solubility of redox species [5,6]. These characteristics make them promising candidates for increasing the energy density of RFBs and enabling non-aqueous configurations with higher voltages [20,21].
Recent studies have explored the integration of ILs into membraneless systems based on laminar flows or aqueous two-phase systems, demonstrating that it is possible to maintain the separation of redox species without a physical membrane [22,23]. However, most practical configurations still require selective membranes that allow the transport of support ions while minimizing the crossing of active species [13,17].
Furthermore, the use of ILs poses challenges related to relatively high viscosity, compatibility with membrane materials, and long-term stability under realistic operating conditions [12,24]. This is because ILs can cause significant chemical damage to many of the polymer membranes currently available. Furthermore, ILs require higher temperatures to reduce their viscosity, which, in turn, intensifies their chemical reactivity with the membranes and accelerates their degradation. For this reason, it is essential to develop membranes that can operate efficiently at higher temperatures, thus achieving greater stability and prolonging their useful life. These issues have driven the development of new separation materials adapted explicitly to non-aqueous and high-polarity environments.
1.3. Membranes in RFBs with ILs
The ion exchange membrane is a critical component that controls the selectivity, conductivity, and overall efficiency of an RFB. Its primary function is to facilitate the transport of charged species (H+, anions, or supporting ions) while blocking the passage of redox molecules, thereby reducing the crossover phenomenon [8,12,25].
In conventional aqueous systems, proton-exchange membranes such as Nafion are commonly used. However, these have high permeability to vanadium and are considerably expensive, which limits their applicability [16,18]. To overcome these limitations, modified or composite membranes have been investigated, such as:
- Polybenzimidazole (PBI) with ILs: improves proton selectivity and thermal stability [11].
- Modified Sulfonated Poly(Ether Ether Ketone), (SPEEK) and SPEEK/TiO2: increase durability and reduce species crossover in VRFB [26].
- Membranes based on zwitterionic polymers or composite polyelectrolytes: provide selective ion channels that reduce permeability without compromising conductivity [27].
- Membranes derived from ionic liquid polymers (PILs): allow the intrinsic conductivity of ILs to be combined with the mechanical stability of the polymer support [28].
In addition, it has been reported that surface modifications and the incorporation of nanomaterials, such as CNTs or organic covalent frameworks, can improve the relationship between selectivity and conductivity, thereby optimizing overall energy efficiency [4,12,29].
Despite these advances, fundamental challenges remain—the lack of standardization in characterization methods, the difficulty of correlating structural properties with long-term performance metrics, and the need to validate membranes under operating conditions that closely mimic industrial conditions [16,17,30]. Resolving these issues will be key to accelerating the practical implementation of ionic liquid-based RFBs in large-scale energy storage scenarios.
2. Bibliographic Search Methodology
This review was conducted using a systematic methodology for searching, selecting, and critically analyzing scientific literature to ensure the comprehensiveness of the gathered information and transparency in the synthesis of the results.
2.1. Search Strategy
The literature search was conducted between January and May 2025, mainly using international databases such as Web of Science (WoS), Scopus, ScienceDirect, PubMed, SpringerLink, and Wiley Online Library. Institutional repositories and gray literature (doctoral theses and technical reports) were also consulted to include studies not published in indexed journals but relevant to the field of redox flow battery (RFB) materials.
Combinations of keywords in English and Spanish were used, including:
- ‘redox flow battery’, ‘vanadium redox flow battery’, ‘membrane’, ‘ion exchange membrane’, ‘polymeric ionic liquid’, ‘ionic liquid electrolytes’, ‘membraneless flow battery’, ‘ion transport’, “selectivity”, ‘energy storage’.
- ‘batería de flujo redox’, ‘membranas de intercambio iónico’, ‘líquidos iónicos’, ‘electrolitos no acuosos’, ‘almacenamiento electroquímico’.
The Boolean operators AND, OR, and NOT were used to optimize the search, along with filters for date (2014–2025), document type (original articles, reviews, and theses), and subject area (energy, electrochemistry, materials science, chemical engineering).
2.2. Inclusion and Exclusion Criteria
Articles that met the following criteria were included:
Published between January 2014 and May 2025 and written in English or Spanish.
Experimental, theoretical, or review studies focused on RFBs, ionic liquids as electrolytes, or ionic membranes used in flow systems; articles reporting quantitative properties of membranes (conductivity, permeability, coulombic efficiency, energy efficiency) or analyzing relevant structural modifications.
The following were excluded:
Publications that exclusively addressed other storage technologies (lithium batteries, supercapacitors) without a direct relationship to RFB, studies without experimental data or discussion applicable to membranes or ionic liquids, and duplicate documents in different repositories.
2.3. Selection Process
The initial search yielded approximately 460 results. After removing duplicates and applying inclusion/exclusion criteria, 81 articles were selected to form the documentary basis of this review. These include publications in high-impact indexed journals (e.g., Journal of Power Sources, Electrochimica Acta, Journal of Membrane Science, Advanced Functional Materials, Energies, Journal of The Electrochemical Society), as well as doctoral theses and specialized chapters.
The DOI of the selected articles are:
- ○
- 10.1016/j.jiec.2010.07.007
- ○
- 10.1002/app.53802
- ○
- 10.1115/1.4040329
- ○
- 10.1016/j.memsci.2019.04.017
- ○
- 10.1016/j.jpowsour.2019.03.043
- ○
- 10.1002/app.51628
- ○
- 10.1002/anie.201704318
- ○
- 10.1016/j.est.2019.100840
- ○
- 10.1016/j.memsci.2021.119539
- ○
- 10.1002/cplu.201402096
- ○
- 10.1016/j.ijhydene.2016.04.060
- ○
- 10.3390/batteries5010025
- ○
- 10.1016/j.elecom.2012.03.037
- ○
- 10.1016/j.mtener.2021.100937
- ○
- 10.1016/j.electacta.2023.142619
- ○
- 10.1016/j.electacta.2018.11.123
- ○
- 10.1016/j.est.2023.107890
- ○
- 10.3390/batteries9020141
- ○
- 10.1016/j.jpowsour.2013.12.038
- ○
- 10.1002/ente.202000978
- ○
- 10.1109/PESGM.2017.8274042
- ○
- 10.1039/c3ta11459g
- ○
- 10.1002/advs.201800576
- ○
- 10.1016/j.est.2022.106270
- ○
- 10.1149/2.0471503jes
- ○
- 10.3390/batteries10010009
- ○
- 10.1016/j.compositesb.2022.109657
- ○
- 10.1039/d0ma00881h
- ○
- 10.1016/j.jpowsour.2015.03.104
- ○
- 10.1016/j.jpowsour.2017.12.049
- ○
- 10.1016/j.jpowsour.2021.230804
- ○
- 10.3390/en15134545
- ○
- 10.1149/2.0901510jes
- ○
- 10.1002/adfm.202306633
- ○
- 10.1007/s11581-023-05342-y
- ○
- 10.1002/app.43593
- ○
- 10.1149/07212.0033ecst
- ○
- 10.19799/j.cnki.2095-4239.2022.0492
- ○
- 10.1016/j.seppur.2020.117436
- ○
- 10.1016/j.molliq.2021.116533
- ○
- 10.1007/s10971-014-3299-3
- ○
- 10.1149/2.0141601jes
- ○
- 10.1149/05837.0055ecst
- ○
- 10.1016/j.jscs.2024.101931
- ○
- 10.1021/acsami.8b11581
- ○
- 10.1016/j.memsci.2019.117614
- ○
- 10.1016/j.jhazmat.2020.123047
- ○
- 10.1021/am501540g
- ○
- 10.1016/j.est.2019.100883
- ○
- 10.1021/acsami.2c10072
- ○
- 10.1166/jnn.2020.17625
- ○
- 10.1016/j.seppur.2021.119220
- ○
- 10.1016/j.jiec.2017.03.017
- ○
- 10.1149/2.1031714jes
- ○
- 10.1016/j.memsci.2019.117665
- ○
- 10.1002/celc.201700700
- ○
- 10.1021/jp507155s
- ○
- 10.1021/acsami.1c20181
- ○
- 10.3390/pr6080124
- ○
- 10.1149/2.1461809jes
- ○
- 10.1016/j.apmt.2023.101928
- ○
- 10.1021/acs.iecr.9b04592
- ○
- 10.1371/journal.pone.0187369
- ○
- 10.1128/IAI.73.7.3999-4006.2005
- ○
- 10.1016/j.ensm.2024.103296
- ○
- 10.1039/d0ma00508h
- ○
- 10.1002/ente.201500449
- ○
- 10.1039/d3ta02633g
- ○
- 10.1016/j.ijhydene.2017.02.050
- ○
- 10.1149/2.0701813jes
- ○
- 10.1016/j.jpowsour.2024.235898
- ○
- 10.1128/IAI.00654-18
- ○
- 10.1021/acsaem.3c03260
- ○
- 10.1039/9781782622727-00023
- ○
- 10.1080/15548627.2015.1100356
- ○
- 10.1149/1945-7111/ac1396
- ○
- 10.1007/978-3-030-60443-1_6
- ○
- 10.1016/j.nanoen.2018.06.044
- ○
- 10.5796/electrochemistry.17-00080
- ○
- 10.1016/j.est.2023.108584
- ○
- 10.1016/j.molliq.2024.124775
The selection process was conducted in two stages: first, a review of titles and abstracts to assess thematic relevance; second, a full reading of the texts to confirm the significance of the results and to extract key data on membranes and ionic liquids.
2.4. Organization and Analysis of Information
The selected articles were organized into a synthesis matrix that included variables such as battery type (VRFB, bromide, hybrid, membraneless), membrane type (PEM, AEM, composite, hybrid, ionic liquid-based), structural modifications, electrochemical performance metrics (conductivity, permeability, coulombic efficiency, energy efficiency, power density), experimental conditions, and main findings.
This procedure enabled the identification of research trends, technological advances, and knowledge gaps. Similarly, the information collected was critically compared to highlight both the most relevant achievements and the methodological limitations reported in the literature.
3. Results
3.1. Trends in the Literature
Analysis of the 81 selected articles reveals a clear evolution in research trends on RFBs, particularly in the role of membranes in systems incorporating ILs, over the period 2014–2017. Initial studies focused on evaluating commercial membranes, primarily Nafion and sulfonated polymer derivatives, as well as on early approaches to anion-exchange membranes and non-aqueous systems [8,31,32]. At the same time, studies emerged that explored innovative configurations, such as membraneless batteries and two-phase systems, opening the possibility of dispensing with a physical membrane due to the immiscibility of IL-based electrolytes [14,33]. From 2018 onwards, the literature has shown a shift towards the integration of hybrid and composite membranes, in which nanomaterials (TiO2, CNTs, graphene) and ionic liquids are immobilized in polymer matrices to improve selectivity and reduce redox species crossover [11,34,35].
Between 2019 and 2021, research intensified into the design of new functionalized polymers, such as IL-grafted polybenzimidazoles, polyelectrolyte-based membranes, and zwitterionic structures, which demonstrated a better relationship between ionic conductivity and selectivity compared to conventional membranes [16,36,37]. This stage also saw increased interest in multiscale modeling and ion-transport analysis, enabling the correlation of structural properties with electrochemical performance [15,38]. More recently, in the period 2022–2025, trends point to the incorporation of emerging materials, such as ionic liquid polymers (ILPs), membranes based on covalent organic frameworks (COFs), and hybrid compounds with zwitterionic properties, which offer high stability in non-aqueous environments [27,28]. Likewise, there has been growing attention to sustainability, with proposals for membranes based on biopolymers and green synthesis strategies [36,39].
The annual production of scientific articles in this area of research has increased since 2013, as depicted in Figure 1, with a slight decrease between 2019 and 2022, presumably due to the impact of the global pandemic.
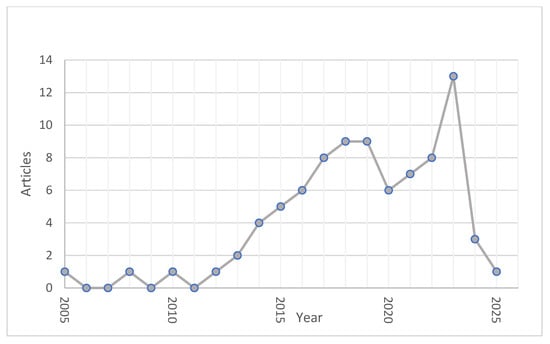
Figure 1.
Annual Scientific Production on the use of ionic liquids in RFBs.
Overall, the literature shows a progression from the use of commercial materials to the development of customized membranes, specifically designed to operate in the presence of ILs, to improve coulombic efficiency, reduce crossover losses, and increase the service life of the systems. However, despite these advances, a lack of standardization in characterization protocols and a shortage of pilot-scale studies remain, which limit the transfer of results to industrial applications. These trends reflect the need for integrated approaches that combine molecular design, the synthesis of new materials, and validation under real operating conditions to consolidate membranes’ role in the future development of RFBs based on ionic liquids.
3.2. Effect of Membranes on RFB Performance with IL
Analysis of the literature reveals that the properties of the separation membrane significantly influence the overall performance of RFBs utilizing ILs. Some initial studies have shown that commercial membranes, such as Nafion, exhibit high proton conductivity but also high permeability to redox species, leading to capacity losses and decreased coulombic efficiency [13,32]. Therefore, to overcome these limitations, modified and composite membranes have been developed that integrate nanomaterials (TiO2, CNTs) or ionic liquids immobilized in polymer matrices, thereby improving ionic selectivity and reducing the crossover phenomenon [11,26,35]. Likewise, the incorporation of organic covalent frameworks and zwitterionic structures has been shown to increase chemical and mechanical stability under non-aqueous conditions, achieving energy efficiencies greater than 80% in experimental configurations [4,27]. Recent research shows that membranes derived from PILs and grafted polybenzimidazole exhibit a better balance between conductivity and selectivity, thereby improving the power density and durability of the system [12,28]. Overall, evidence suggests that the rational design of specific membranes for ILs enables the optimization of coulombic and energy efficiency in RFBs, thereby reducing degradation from species crossover and extending their useful life. However, challenges remain in relation to standardizing methodologies and validating performance under large-scale operating conditions.
To summarize the most relevant findings from the reviewed literature, Table 1 presents a comparison of the main studies published between 2014 and 2025 on membranes applied to RFBs using ionic liquids. The information includes the membrane type, the ionic liquid class used, and the reported electrochemical performance metrics (conductivity, coulombic efficiency, energy efficiency, and power density), as well as the main findings and identified limitations. This synthesis highlights the progression from the use of commercial membranes to the design of hybrid membranes specific to non-aqueous environments, emphasizing both advances in efficiency and the challenges associated with standardization and scaling.

Table 1.
Comparison of main items regarding RFBs using non-aqueous electrolytes 2014–2025.
According to the data in Table 1, the Coulombic efficiency ranges from 70% to 94% as depicted in Figure 2. Zwitterionic membranes stand out for their highest Coulombic efficiency, suggesting high ionic selectivity and minimal self-discharge in the cell, key factors for optimizing charge retention and minimizing losses from cross-permeability.
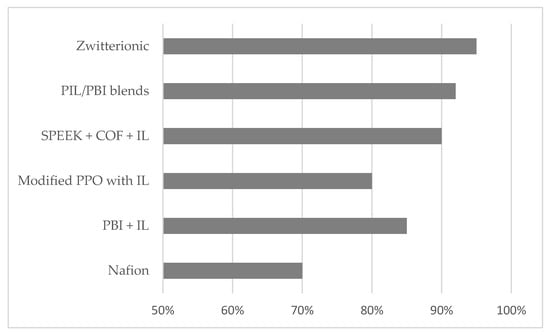
Figure 2.
Coulombic efficiency (%) related to electrolytes/membranes systems.
3.3. Types of Membranes Used
A review of the literature reveals a wide range of membranes evaluated in RFBs, with a clear evolution from commercial materials to membranes specifically designed for operation in the presence of ILs. In the early years, Studies focused on commercial proton exchange membranes, such as Nafion, widely used for their high conductivity but limited by their high permeability to vanadium and high cost [13,32]. Subsequently, anion exchange membranes (AEMs) were introduced, developed from sulfonated polymers, such as polybenzimidazole (PBI) and modified polyphenylene, which improved ionic selectivity in non-aqueous systems [11,18]. A growing trend was the use of composite or hybrid membranes incorporating nanomaterials such as TiO2, graphene, and carbon nanotubes, which enhanced mechanical stability and reduced crossover [26,35]. Recently, PILs, zwitterionic membranes, and structures based on covalent organic frameworks (COFs) have been explored, combining high selectivity and chemical stability, achieving coulombic efficiencies of over 90% in experimental configurations [4,27,28]. Overall, the trend indicates a gradual replacement of generic membranes with tailor-made materials that address the conductivity, selectivity, and stability requirements of RFBs with ILs.
3.4. Technical Information Obtained from Membranes
Based on the information collected, Table 2 and Table 3 were prepared, listing different types of membranes and their corresponding power supply, depending on the type of RFB used, along with the power provided per cm2 unit.
Table 2 is based on batteries with anion exchange membranes:

Table 2.
Classification of ionic liquid-based RFBs using an anionic membrane.
Table 2.
Classification of ionic liquid-based RFBs using an anionic membrane.
| Reference | Type of Membrane | Battery | Thickness [μm] | Power [mW/cm2] | Ion to Be Exchanged |
|---|---|---|---|---|---|
| [40] | Zeolite MFI | Ce(NO3) | 2400 | 25 | OH− |
| [40] | Alpha aluminate | Na2S4 | 2400 | 25 | OH− |
| [41] | Nafion 117 | KOH | 170 | 57 | OH− |
| [41] | Sulfonated lignin nanocomposite | KOH | 170 | 87.5 | OH− |
| [42] | Graphite | Zn-Br2 | 1000 | 560 | Br− |
| [42] | Pt@Graphite | Zn-Br2 | 1000 | 1430 | Br− |
It shows that the maximum power achieved so far in this review is 1430 mW/cm2 in a Zn/Br2 battery. The lowest value found is 25 mW/cm2.
Table 3 presents the same analysis when the membrane nature is cationic.

Table 3.
Classification of ionic liquid-based RFBs using an cationic membrane.
Table 3.
Classification of ionic liquid-based RFBs using an cationic membrane.
| Reference | Type of Membrane | Battery | Thickness [μm] | Power [mW/cm2] | Ion to Be Exchanged |
|---|---|---|---|---|---|
| [41,43] | G-C3N4 Nano-sheets | Vanadium | 32–35 | 320 | H+ |
| [41] | Polyprotic DCM-2 | Vanadium | 180 | 496 | H+ |
| [41] | Polyprotic Ha-45 | Vanadium | 200 | 456 | H+ |
| [41] | Poliprotica ICEM | Vanadium | 180 | 512 | H+ |
| [41] | Polyprotic LSCM2T | Vanadium | 180 | 510 | H+ |
| [41] | Polyprotic Qa-Radel-1.7 | Vanadium | 180 | 500 | H+ |
| [44] | Nafion 117 | Polysulphide–ferrocyanide | 180 | 100 | K+ |
| [45] | Nafion 117 | Hydrochloric acid | 180 | 98.1 | H+ |
| [46] | Nafion 117 | Zinc and bromine | 180 | 50 | H+ |
| [47] | Nafion 117 | DEP Fe | 180 | 70 | Fe3+ |
| [48] | Nafion 117 | Fe-Va | 180 | 100 | H+ |
| [49] | Nafion 212 | Vanadium | 50 | 300 | H+ |
| [49] | Nafion 212 50 wt% | Vanadium | 50 | 375 | H+ |
| [50] | Nafion/TIZRO4NTS | Cobalt/tungsten | 50 | 12 | H+ |
| [49] | SPEEK 40% | Vanadium | 50 | 250 | H+ |
| [49] | Spes | Vanadium | 50 | 200 | H+ |
| [51] | SP-p-CT320 | Zinc and bromine | 80 | 100 | H+ |
The literature contains more data on the power of vanadium batteries than on the ion exchange area of membranes. It is essential to note that the vanadium batteries considered here are only non-aqueous batteries.
Among the data collected, a power of 555 mW/cm2 was reported for a vanadium battery using the polyprotic membrane LSCM2T [41]. Although higher power values have been reported for redox flow batteries, no information is provided on the membrane used. It is important to note that membrane selection in vanadium RFBs is critical to balancing vanadium permeability, coulombic efficiency, and voltage efficiency to optimize overall battery performance [50]. In addition, information has been compiled indicating a power of 1510 mW/cm2 in a zinc battery using a commercial Nafion 117 membrane. In this case, the battery provides the thickness of the membrane used, which adds relevant details to the characterization of this system. Based on the literature, it has not been possible to establish a direct relationship between membrane type and the power a flow battery can supply. However, upon examining the provided tables, several factors, including membrane type and thickness, affect the system’s power output. An interesting finding is that, even with the same membrane, modifying the chemical elements involved in the cell reaction results in different powers. For example, consider the standardized commercial membrane Nafion 212. According to the literature reviewed, the maximum power achieved by a membrane in a flow battery is 2.78 W/cm2, specifically in a vanadium battery, as reported by Jiang et al. in their work on a ‘vanadium redox flow battery’ with high power density and long service life. Still, the membrane type is not well specified [50]. For all other combinations of batteries and membranes, the power per unit area does not approach 2.7 W/cm2.
From the analyzed data, shown in Figure 3, it can be observed that there is no direct correlation between membrane thickness and the power output of the storage system; this indicates that ionic transport across the membrane does not appear to be a controlling factor in the overall kinetics of the electrochemical processes within the system. This can be better appreciated in the following figure, which shows the membrane thickness and the power obtained. There is no clear correlation.
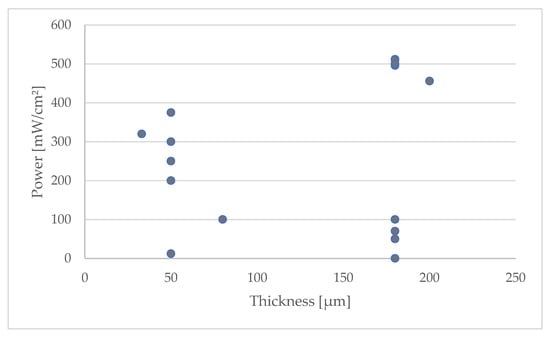
Figure 3.
Relationship between membrane thickness and system power output.
Furthermore, it has been observed that flow batteries with cation-exchange membranes have achieved higher power in an anion NARFB using the electrochemical reaction with vanadium. This contrasts with technologies based on anion-exchange membranes.
4. Challenges and Limitations
Despite significant advances in the design and optimization of membranes for IL-based RFBs, multiple challenges remain that limit their technological consolidation. One of the primary issues is redox species crossover, which reduces coulombic efficiency and leads to premature electrolyte degradation. Even in advanced membranes such as modified Nafion or SPEEK, permeability remains high in non-aqueous media [13,18]. Furthermore, there is an inherent trade-off between selectivity and ionic conductivity, as highly selective materials tend to reduce conductivity and, in turn, the achievable power density [4,11]. Another relevant challenge is long-term chemical and mechanical stability: several hybrid or polymer-based ionic liquid (PIL) membranes show promising initial performance but suffer structural degradation after hundreds of cycles, limiting their practical applicability [28,35]. In addition, most reported studies are conducted in laboratory cells under controlled conditions, making it difficult to extrapolate results to pilot- or industrial-scale systems [19,27]. Finally, there is a persistent lack of standardization in characterization protocols, which prevents rigorous comparisons between different studies [16]. Therefore, overcoming these limitations will require multidisciplinary approaches that integrate material synthesis, multiscale modeling, and experimental validation under real operating conditions.
5. Critical Discussion
Analysis of the literature reviewed between 2014 and 2025 reveals significant progress in the development of membranes for RFBs using ILs but also highlights limitations that still hinder their commercialization. One of the clearest trends is the evolution from commercial membranes, such as Nafion, towards hybrid and customized materials designed to overcome problems of permeability and compatibility with non-aqueous electrolytes [11,13,32]. While traditional membranes provided a solid foundation for the initial development of RFBs, their limitations in terms of crossover, cost, and stability drove the design of new architectures based on polybenzimidazole (PBI), polyelectrolytes, and IL-functionalized polymers [4,28].
Recent studies show that integrating ILs into the membrane matrix itself, either by grafting functional chains or by developing PILs, is a promising strategy for improving the balance between ionic conductivity and selectivity [12,28]. In this way, the use of nanomaterials such as TiO2, graphene, and CNTs has improved the mechanical and chemical stability of membranes, reducing structural degradation after multiple cycles [26,35]. However, the literature also highlights an inherent trade-off between selectivity and conductivity—more selective membranes tend to penalize achievable power density. In contrast, those with high conductivity tend to facilitate the crossing of redox species [4,11]. This dilemma represents one of the main unresolved design challenges.
The performance of ionic liquid-based redox flow batteries (RFBs) is governed by the complex interplay between the membrane’s structure, the physicochemical properties of the ionic liquid (IL) electrolyte, and their mutual compatibility.
High-viscosity ionic liquids are great for minimizing active species crossover! Although they have lower ionic mobility, their strong polarity positively influences the swelling behavior of polymeric membranes, paving the way for exciting advancements in the field. Excessive swelling in conventional polymers such as Nafion or SPEEK can enlarge ion channels, thereby increasing the permeability of redox-active species and accelerating capacity fade [13,32].
Regarding long-term interfacial stability between the membrane and the IL electrolyte, degradation mechanisms are influenced by operating temperature, mechanical stress, leaching, and fouling; many polymeric membranes are susceptible to chemical degradation when exposed to aggressive ILs at higher temperatures over extended cycles [12,24]. Differential swelling between the membrane and other cell components (e.g., electrodes, gaskets) can induce significant mechanical stress; this effect can be minimized with a well-designed cell. Otherwise, there is a risk of IL leaching into the flowing electrolyte over time [35], leading to pore fouling and a consequent increase in area-specific resistance.
All the previous factors affect the scalability of the electrochemical device.
Furthermore, the development of standardized protocols for reporting interfacial stability—such as quantifying swelling ratios in specific ILs, measuring leaching rates, and conducting long-term cycling at elevated temperatures—will be crucial to generate comparable data and help to select a proper membrane for a specific IL electrolyte to improve the long-term performance of the RFB.
The lack of standardization in characterization protocols hinders rigorous comparisons between different materials. Metrics for coulombic efficiency, energy efficiency, and permeability to vanadium or analogous species are reported under very diverse experimental conditions, making it difficult to identify universal trends [16,17]. In this regard, it is essential to adopt standardized methodologies that enable correlations between membrane structural properties and their electrochemical performance in RFBs. To move towards a consistent, reproducible database, future studies must report a minimum set of quantitative and structural parameters that characterize both the device and the materials used.
It is proposed that all studies of RFBs with ILs include the following information:
- Physicochemical properties of the membrane: type of material, active cell area (cm2), thickness (μm), porosity, surface functionalization, and type of exchange (cationic or anionic).
- Electrochemical properties: ionic conductivity (S·cm−1) and ionic selectivity at different concentration and temperature regimes.
- Cell performance data: cell voltage and individual half-reaction voltages; generated power density (mW·cm−2); coulombic efficiency, total energy efficiency and measured ohmic drop.
- Operating conditions: composition and viscosity of electrolytes, operating temperature, flow rate, and total solution volume per compartment.
- Durability and stability tests: number of charge/discharge cycles, variation in conductivity and ionic permeability over time, and post-mortem analysis of membrane structural integrity.
- In addition, it is recommended to include the energy balance associated with the cell architecture (total power generated, applied current density, and performance as a function of membrane area), together with the relationship between structure and performance, considering the effect of membrane thickness and microstructure on ion transport mechanisms.
The adoption of these criteria would allow direct comparisons between studies from different groups and detect correlations between structural variables (membrane type and electrolyte) and performance metrics (efficiency, power and stability). These measures would contribute substantially to consolidating standardization in the study of membranes for RFBs with ionic liquids and accelerate their technological validation in industrial settings.
In the field of sustainability, proposals based on biopolymers and green synthesis have emerged, offering more environmentally friendly alternatives with the potential to reduce costs [36,39]. These approaches respond to the need for technological advancement to be accompanied by sustainability and industrial scalability criteria. However, most of these proposals are still in the experimental phase and require validation at the pilot cell level. Finally, the review indicates that most of the work has been conducted at the laboratory level under controlled conditions, limiting the extrapolation of results to larger-scale systems. The lack of prolonged testing under real operating conditions is a critical gap, considering that long-term durability and stability are determinants of the commercial viability of RFBs [19,27].
6. Conclusions
Analysis of literature published between 2014 and 2025 reveals that membranes play a crucial role in determining the efficiency, stability, and service life of RFBs using ionic liquids. There has been a gradual transition from the use of commercial membranes, such as Nafion, to the development of hybrid, composite, and specialized materials designed to overcome the problems of crossover, capacity loss, and stability limitations in non-aqueous media. Strategies such as incorporating nanomaterials (TiO2, CNTs, graphene), using ionic liquid polymers, and implementing zwitterionic or organic covalent framework-based structures have improved ionic selectivity and reduced permeability, achieving coulombic efficiencies of over 90% in experimental configurations.
Anion and cation exchange membranes exhibit different performances, with the Nafion 117 membrane achieving a maximum power of 1510 mW/cm2 in a zinc battery, and the polyprotic LSCM2T membrane reaching 555 mW/cm2 in a vanadium battery.
It is essential to note that vanadium RFBs have demonstrated remarkable performance, with a reported maximum power density of 2.78 W/cm2; however, the membrane type is not well specified. In addition, flow batteries with cation-exchange membranes have achieved higher power than anion-exchange-based technologies.
However, significant challenges remain in balancing conductivity and selectivity, a central dilemma in membrane design. This is compounded by the lack of standardization in characterization protocols, which makes it difficult to compare results across studies rigorously. Furthermore, most research focuses on laboratory testing, and there is a clear need to validate these materials under pilot- and industrial-scale operating conditions.
7. Future Directions
The review highlights that, although notable advances have been made in the design of membranes for RFBs with ILs, there are still challenges that require priority attention. A key direction is the development of tailor-made membranes that balance high ionic selectivity with sufficient conductivity to achieve competitive power densities. To this end, it is recommended that the design of PILs, zwitterionic structures, and covalent organic frameworks (COFs) be further developed, as their versatility offers opportunities to modulate species transport with greater precision.
It will also be essential to standardize characterization protocols, enabling rigorous comparisons of results across laboratories and accelerating the identification of materials with greater potential. Similarly, the implementation of multiscale modeling and molecular simulations is required to correlate membrane structural properties with their electrochemical performance under real operating conditions.
On the other hand, priority should be given to validation in pilot systems to evaluate the durability, cost, and environmental sustainability of new membranes. The use of biopolymers and green synthesis methodologies also represents an opportunity to reduce the environmental footprint and promote industrial scalability. Together, these future directions provide a roadmap for designing more efficient, stable, and sustainable membranes, capable of consolidating RFBs with ILs as key solutions in the global energy transition.
Author Contributions
Conceptualization, A.S.-I.; methodology, A.S.-I.; software, A.S.-I.; validation, A.S.-I. and C.C.; formal analysis, A.S.-I. and C.C.; investigation, A.S.-I.; resources, A.S.-I. and C.C.; data curation, A.S.-I. and C.C.; writing—original draft preparation, A.S.-I.; writing—review and editing, C.C.; visualization, A.S.-I.; supervision, C.C.; project administration, A.S.-I. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
C.C. acknowledges the financial support of VINCI-PUCV through the project “INVESTIGACIÓN ASOCIATIVA INTERDISCIPLINARIA 2025” code 039.777/2025.
Data Availability Statement
This study is a review of existing scientific literature. As such, no new datasets were generated or analyzed. All data discussed or cited are available from the original publications referenced in the reference list.
Acknowledgments
A.S-I. extends his gratitude to the Doctoral Program in Smart Industry of the Engineering Faculty at Pontificia Universidad Católica de Valparaíso, Chile.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Maity, S.; Sarkar, S.; Dutta, K.; De, T.; Mukherjee, R. Renewable Energy and Green Technology. In Green Chemistry for Environmental Sustainability—Prevention-Assurance-Sustainability (P-A-S) Approach; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for Redox Flow Battery Applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Salla, M.; Zhang, F.; Zhi, Y.; Wang, Q. Membrane Fouling in Aqueous Redox Flow Batteries. J. Power Sources 2022, 527, 231180. [Google Scholar] [CrossRef]
- Meng, X.; Peng, Q.; Wen, J.; Song, K.; Peng, L.; Wu, T.; Cong, C.; Ye, H.; Zhou, Q. Sulfonated Poly(Ether Ether Ketone) Membranes for Vanadium Redox Flow Enabled by the Incorporation of Ionic Liquid-Covalent Organic Complex. J. Appl. Polym. Sci. 2023, 140, e53802. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Pérez, G.; Ortiz, A.; Ortiz, I. The Roles of Ionic Liquids as New Electrolytes in Redox Flow Batteries. Sep. Purif. Technol. 2020, 252, 117436. [Google Scholar] [CrossRef]
- Joseph, A.; Sobczak, J.; Żyła, G.; Mathew, S. Ionic Liquid and Ionanofluid-Based Redox Flow Batteries—A Mini Review. Energies 2022, 15, 4545. [Google Scholar] [CrossRef]
- Xue, K.; Wang, Y.; Lang, J.; He, T.; Dai, Z.; Zheng, Z. The Progress in Applications of Dicationic Ionic Liquids in the Energy Storage and Conversion System. Energy Storage Sci. Technol. 2023, 12, 808–821. [Google Scholar] [CrossRef]
- Chakrabarti, M.H.; Brandon, N.P.; Hajimolana, S.A.; Tariq, E.; Yufit, V.; Hashim, M.A.; Hussain, M.A.; Low, C.T.J.; Aravind, P.V. Application of Carbon Materials in Redox Flow Batteries. J. Power Sources 2014, 253, 150–166. [Google Scholar] [CrossRef]
- Wang, T.; Han, J.; Kim, K.; Muenchinger, A.; Gao, Y.; Farchi, A.; Choe, Y.-K.; Kreuer, K.-D.; Bae, C.; Kim, S. Suppressing Vanadium Crossover Using Sulfonated Aromatic Ion Exchange for High Performance Flow Batteries. Mater. Adv. 2020, 1, 2206–2218. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Pendse, A.; Gao Yuechen and Wang, K.; Bae, C.; Kim, S. High-Efficient Multifunctional Electrochemical Membrane for Lithium Redox Flow Batteries. J. Memb. Sci. 2021, 636, 119539. [Google Scholar] [CrossRef]
- Song, X.; Ding, L.; Wang, L.; He, M.; Han, X. Polybenzimidazole Membranes Embedded with Ionic Liquids for Use in High Proton Selectivity Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 295, 1034–1043. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Liang, D.; Cui, Y.; Wang, X.; Yong, Z.; Liu, F.; Wang, Z. Low Vanadium Permeability Membranes Based on Flexible Hydrophilic Side Grafted Polybenzimidazole/Polymeric Ionic Liquid for VRFBs. Batteries 2023, 9, 141. [Google Scholar] [CrossRef]
- Hudak, N.S.; Small, L.J.; Pratt, H.D.; Anderson, T.M. Through-Plane Conductivities of Membranes for Nonaqueous Redox Flow Batteries. J. Electrochem. Soc. 2015, 162, A2188–A2194. [Google Scholar] [CrossRef]
- Navalpotro, P.; Sierra, N.; Trujillo, C.; Montes, I.; Palma, J.; Marcilla, R. Exploring the Versatility of Membrane-Free Battery Concept Using Different Combinations of Immiscible Redox Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 41246–41256. [Google Scholar] [CrossRef] [PubMed]
- Nemani, V.P.; Smith, K.C. Analysis of Crossover-Induced Capacity Fade in Redox Flow Batteries with Non-Selective Separators. J. Electrochem. Soc. 2018, 165, A3144–A3155. [Google Scholar] [CrossRef]
- McGrath, M.J.; Patterson, N.; Manubay, B.C.; Hardy, S.H.; Malecha, J.J.; Shi, Z.; Yue, X.; Xing, X.; Funke, H.H.; Gin, D.L.; et al. 110th Anniversary: The Dehydration and Loss of Ionic Conductivity in Anion Exchange Membranes Due to FeCl4− Ion and the Role of Membrane Microstructure. Ind. Eng. Chem. Res. 2019, 58, 22250–22259. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Robertson, L.A.; Yu, Z.; Assary, R.S.; Cheng, L.; Zhang, L.; Sarnello, E.; Liu, X.; Li, T.; Preet Kaur, A.; et al. Crowded Electrolytes Containing Redoxmers in Different States of Charge: Solution Structure, Properties, and Fundamental Limits on Energy Density. J. Mol. Liq. 2021, 334, 116533. [Google Scholar] [CrossRef]
- Xi, J.; Li, Z.; Yu, L.; Yin, B.; Wang, L.; Liu, L.; Qiu, X.; Chen, L. Effect of Degree of Sulfonation and Casting Solvent on Sulfonated Poly(Ether Ether Ketone) Membrane for Vanadium Redox Flow Battery. J. Power Sources 2015, 285, 195–204. [Google Scholar] [CrossRef]
- Jeong, K.I.; Lim, S.H.; Hong, H.; Jeong Jae-Moon and Kim, W.V.; Kim, S.S. Enhancing Vanadium Redox Flow Batteries Performance through Local Ratio Adjustment Using Stiffness Gradient Carbon Felt. Appl. Mater. Today 2023, 35, 101928. [Google Scholar] [CrossRef]
- Das, S.; Voskian, S.; Rajczykowski, K.P.; Hatton, T.A.; Bazant, M.Z. Enabling a Stable High-Power Lithium-Bromine Flow Battery Using-Specific Ionic Liquids. J. Electrochem. Soc. 2021, 168, 070542. [Google Scholar] [CrossRef]
- Yan, Y.; Sitaula, P.; Odom, S.A.; Vaid, T.P. High Energy Density, Asymmetric, Nonaqueous Redox Flow Batteries without a Supporting Electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 49633–49640. [Google Scholar] [CrossRef]
- Navalpotro, P.; Neves, C.M.S.S.; Palma, J.; Freire, M.G.; Coutinho, J.A.P.; Marcilla, R. Pioneering Use of Ionic Liquid-Based Aqueous Biphasic Systems as Membrane-Free Batteries. Adv. Sci. 2018, 5, 1800576. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, N.; Ngo, K.; Turmine, M.; Vivier, V. Ionic Liquid Redox Flow Membraneless Battery in Microfluidic System. J. Energy Storage 2023, 57, 106270. [Google Scholar] [CrossRef]
- Xue, B.; Wu, X.; Guo, Y.; Zhang, C.; Qian, W.; Zhang, L. Review—Ionic Liquids Applications in Flow Batteries. J. Electrochem. Soc. 2022, 169, 080501. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Parrondo, J.; Ramani, V. Recent Progress in the Development of Anion Exchange Membranes for electrochemical Devices. In Electrochemistry; Banks, C., McIntosh, S., Eds.; Specialist Periodical Reports: Electrochemistry; Royal Society of Chemistry: Cambridge, UK, 2017; Volume 14, pp. 23–60. ISBN 978-1-78262-272-7/978-1-78262-114-0. [Google Scholar]
- Han, S.I.; Jon, S.H.; Kim, U.H.; Kim, G.H.; Jon, S.M. High Durable SPEEK/TiO2 Nanopaper Composite Membrane for vanadium Redox Flow Battery. J. Saudi Chem. Soc. 2024, 28, 101931. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Zhang, S.; Wang, C.; Chen, H. Zwitterionic Materials in Electrochemical Energy Storage. J. Power Sources 2025, 628, 235898. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, T.; Kraglund, M.R.; Yang, J.; Dong, J.; Tang, A.; Aili, D.; Yang, J. Anion Exchange Membranes Derived from Poly(Ionic Liquid) of Poly(Bis-Piperidinium) and Polybenzimidazole Blends. J. Mol. Liq. 2024, 402, 124775. [Google Scholar] [CrossRef]
- Wang, T.; Lee, J.; Wang, X.; Wang, K.; Bae, C.; Kim, S. Surface-Engineered Nafion/CNTs Nanocomposite Membrane with Improved Efficiency for Vanadium Redox Flow Battery. J. Appl. Polym. Sci. 2022, 139, 51628. [Google Scholar] [CrossRef]
- Jett, B.; Flynn, A.; Sigman, M.S.; Sanford, M.S. Identifying Structure-Function Relationships to Modulate Crossover in nonaqueous Redox Flow Batteries. J. Mater. Chem. A 2023, 11, 22288–22294. [Google Scholar] [CrossRef]
- Small, L.J.; Pratt, H.D., III; Fujimoto, C.H.; Anderson, T.M. Diels Alder Polyphenylene Anion Exchange Membrane for Nonaqueous Redox Batteries. J. Electrochem. Soc. 2016, 163, A5106–A5111. [Google Scholar] [CrossRef]
- Escalante-Garcia, I.L.; Wainright, J.S.; Thompson, L.T.; Savinell, R.F. Performance of a Non-Aqueous Vanadium Acetylacetonate Prototype Redox Battery: Examination of Separators and Capacity Decay. J. Electrochem. Soc. 2015, 162, A363–A372. [Google Scholar] [CrossRef]
- Navalpotro, P.; Palma, J.; Anderson, M.; Marcilla, R. A Membrane-Free Redox Flow Battery with Two Immiscible Redox. Angew. Chem. Int. Ed. 2017, 56, 12460. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Q.; Cong, C.; Meng, X.; Zhou, Q. Influence of Alkaline 2D Carbon Nitride Nanosheets as Fillers for anchoring HPW and Improving Conductivity of SPEEK Nanocomposite. Int. J. Hydrogen Energy 2017, 42, 10317–10328. [Google Scholar] [CrossRef]
- Roh, S.H.; Shin, D.S.; Kang, D.K.; Kim, S.C.; Kang, U.I.; Park, W.S.; Kurkuri, M.; Jung, H.Y. Surface Modification of Sulfonated Poly(Phenylene Oxide) Membrane for Vanadium Redox Flow Batteries. J. Nanosci. Nanotechnol. 2020, 20, 5765–5770. [Google Scholar] [CrossRef]
- Pan, J.; Tao, Y.; Zhao, L.; Yu, X.; Zhao, X.; Wu, T.; Liu, L. Green Preparation of Quaternized Vinylimidazole-Based Anion Exchange by Photopolymerization. Sep. Purif. Technol. 2021, 276, 119220. [Google Scholar] [CrossRef]
- Sengupta, S.; Lyulin, A.V.; Kritikos, G.; Karatasos, K.; Venkatnathan, A.; Pant, R.; Komarov, P.V. Multiscale Modeling Examples: New Polyelectrolyte Nanocomposite Membranes for Perspective Fuel Cells and Flow Batteries. Springer Ser. Mater. Sci. 2021, 310, 133–177. [Google Scholar] [CrossRef]
- Hinkle, K.R.; Wang, X.; Gu, X.; Jameson, C.J.; Murad, S. Computational Molecular Modeling of Transport Processes in Nanoporous. Processes 2018, 6, 124. [Google Scholar] [CrossRef]
- Alday, P.P.; Barros, S.C.; Alves, R.; Esperança, J.M.S.S.; Navarro--Segarra, M.; Sabaté, N.; Silva, M.M.; Esquivel, J.P. Biopolymer Electrolyte Membranes (BioPEMs) for Sustainable Primary Redox Batteries. Adv. Sustain. Syst. 2020, 4, 1900110. [Google Scholar] [CrossRef]
- Silambarasan, P.; Ramu, A.G.; Govarthanan, M.; Kim, W.; Moon, I.S. Cerium-Polysulfide Redox Flow Battery with Possible High Energy Density Enabled by MFI-Zeolite Membrane Working with Acid-Base Electrolytes. Chemosphere 2022, 291, 132680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Khan, H.; Upadhyay, P.; Kothandaraman, R.; Kulshrestha, V. Stable Poly(2,6-Dimethyl-1,4-Phenylene Ether) Based Cross-Linked Cationic Polyelectrolyte Membrane with Ionic Microstructure Modification for Efficient VRFB Performance. ACS Appl. Energy Mater. 2023, 6, 447–460. [Google Scholar] [CrossRef]
- Mariyappan, K.; Velmurugan, R.; Subramanian, B.; Ragupathy, P.; Ulaganathan, M. Low Loading of Pt@Graphite Felt for Enhancing Multifunctional Activity towards Achieving High Energy Efficiency of Zn–Br2 Redox Flow Battery. J. Power Sources 2021, 482, 228912. [Google Scholar] [CrossRef]
- Wu, C.; Lu, S.; Wang, H.; Chen, S.; Xiang, Y. Endowing Nonionic Membrane with Superior Ionic Selectivity by Using G-C3N4 for Redox Flow Battery. J. Power Sources 2023, 564, 232857. [Google Scholar] [CrossRef]
- Sreenath, S.; Nayanthara, P.S.; Pawar, C.M.; Ash, A.; Bhatt, B.; Verma, V.; Nagarale, R.K. An Aqueous Polysulfide Redox Flow Battery with a Semi-Fluorinated Cation Exchange Membrane. Energy Adv. 2024, 3, 203–214. [Google Scholar] [CrossRef]
- Thomya, A.; Khunatorn, Y. Analyzing Discharge Characteristics of Redox Flow Battery Using Hydrochloric Acid as a Reaactant. In Proceedings of the 2020 International Conference and Utility Exhibition on Energy, Environment and Climate Change (ICUE), Pattaya, Thailand, 20–22 October 2020; pp. 1–7. [Google Scholar]
- Thamizhselvan, R.; Naresh, R.; Sekar, R.; Ulaganathan, M.; Pol, V.G.; Ragupathy, P. Redox Flow Batteries: Pushing the Cell Voltage Limits for Sustainable Energy Storage. J. Energy Storage 2023, 61, 106622. [Google Scholar] [CrossRef]
- Xuan, T.; Cheng, X.; Wang, L. Hydrated Eutectic Electrolyte as Catholyte Enables High-Performance Redox Flow Batteries. J. Energy Storage 2024, 84, 111029. [Google Scholar] [CrossRef]
- Lu, P.; Sun, P.; Ma, Q.; Su, H.; Leung, P.; Yang, W.; Xu, Q. Rationally Designed Ternary Deep Eutectic Solvent Enabling Higher Performance for Non-Aqueous Redox Flow Batteries. Processes 2022, 10, 649. [Google Scholar] [CrossRef]
- Bai, E.; Zhu, H.; Sun, C.; Liu, G.; Xie, X.; Xu, C.; Wu, S. A Comparative Study of Nafion 212 and Sulfonated Poly(Ether Ether Ketone) Membranes with Different Degrees of Sulfonation on the Performance of Iron-Chromium Redox Flow Battery. Membranes 2023, 13, 820. [Google Scholar] [CrossRef]
- Aziz, M.A.; Shanmugam, S. High-Performance Cobalt–Tungsten All-Heteropolyacid Redox Flow Battery with a TiZrO4-Decorated Advanced Nafion Composite Membrane. ACS Appl. Energy Mater. 2021, 4, 2115–2129. [Google Scholar] [CrossRef]
- Gikunoo, E.K.; Han, D.; Vinothkannan, M.; Shanmugam, S. Synthesis and Characterization of Highly Durable Hydrocarbon-Based Composite Membrane for Zinc-Bromine Redox Flow Battery. J. Power Sources 2023, 563, 232821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).