3.1. Changes in Corrosion Morphology
Figure 1 illustrates the markedly different corrosion behaviors of the three graphite anodes in fluoride molten salt, which are attributable to their distinct structures, grain sizes, preparation processes, and origins (virgin or recycled). The overall surface of the original graphite anode is relatively flat and intact, with clear corner contours and a complete structure. After corrosion analysis, a spectrum of corrosion morphologies was observed, ranging from slight surface roughening to complete structural disintegration.
The #1 anode, with its original relatively flat and intact surface and sharp edges, was partially retained, indicating a certain degree of resistance to the corrosive melt. However, higher magnification images revealed the onset of nonuniform structural degradation at the microscale. This manifests initially as granular exfoliation, where fine particles detach from the bulk, leading to measurable increases in surface looseness and porosity. Furthermore, the formation of interstitial pores and corrosive debris wedged within the initially compact grain boundaries signifies the penetration of the molten salt along the grain boundaries, selectively attacking the weaker interfaces and initiating a granular disintegration process. In stark contrast, the #2 anode developed a radically distinct surface morphology, being entirely encapsulated by a continuous, monolithic layer that EDS analysis identified as resolidified fluoride salt. This layer formed through intense wetting and subsequent cooling, where the thermal contraction coefficient mismatch between the salt and the graphite substrate generated significant internal stresses. These stresses are relieved through a network of numerous thermal-stress-induced microcracks that traverse the salt layer. The presence of this brittle, adherent salt layer has a complex dual effect on the morphological evolution of the underlying anode. It could act as a physical barrier, temporarily shielding the graphite surface from direct contact with the molten salt. Conversely, the pervasive and interconnected cracking network likely serves as direct capillaries for the continued infiltration of the corrosive melt, posing a significant risk of localized subsurface corrosion and eventual spallation of the layer under operational thermal cycling, which would repeatedly open and close these microcracks. The most severe and pervasive corrosion was observed on the #3 anode, which exhibited a morphology characterized by extensive material removal superimposed with aggressive localized pitting. Macroscopically, the surface transitioned from relatively flat to the naked eye to heavily roughened, becoming extensively covered with a layer of corrosion products that altered its visual appearance and texture. At the microstructural level, SEM revealed a severely compromised surface topography characterized by a high density of irregularly shaped pits of varying depths, suggesting an uneven corrosion rate across the surface. Concurrently, the prominent exposure of the underlying graphite aggregates, which protrude from the surface, points towards a selective corrosion process where the matrix material surrounding these aggregates has been preferentially etched away. This complex morphology indicates that the corrosion mechanism was not confined to preferential attack at the grain boundaries but also involved direct and pervasive degradation of the graphite grains themselves, leading to a general surface recession and a pronounced increase in surface area and structural fragility.
The surface and cross-sectional morphologies of the graphite anodes before and after corrosion were characterized by SEM.
Figure 2 presents comparative microstructural features of the three anodes, including pre corrosion surface views at 50×, postcorrosion surface views at 200× and 5000×, and cross-sectional views at 100× after exposure.
Graphite anode #1 exhibited characteristics typical of intergranular corrosion. Low-magnification (200×) observation revealed extensive granular spalling and microcracks on the surface, suggesting a uniform corrosion pattern. Higher-magnification (5000×) SEM images further revealed a loose, honeycomb-like surface structure scattered with granular debris. This morphology results from the destruction of the intergranular bonding forces in graphite, leading to grain detachment. Cross-sectional analysis indicated that the corrosion had progressed inwards, forming a distinct corrosion layer with a diffuse boundary with the underlying matrix, and the thickness of the corrosion layer was approximately 200–300 µm. This suggests that the molten salt penetrated along the grain boundaries, not only attacking the surface but also advancing into the interior of the material, thereby compromising the overall structural integrity. This corrosion mode is expected to significantly degrade the mechanical strength and electrical conductivity of the anode.
A notable feature of graphite anode #2 is the presence of a continuous and dense adherent layer covering its surface. At low magnification (200×), a network of cracks is visible on this layer, which is attributable to shrinkage stresses generated by the thermal expansion mismatch between the adherent layer and the graphite substrate during cooling. High-magnification (5000×) imaging confirmed that the layer was tightly bonded to the substrate but was internally cracked. While this adherent layer may partially act as a barrier against direct molten salt erosion, the crack network provides pathways for salt infiltration, potentially inducing localized corrosion. The cross-sectional view clearly reveals a well-defined interface with a certain layer thickness and strong bonding, indicating significant high-temperature interactions between the anode material and the molten salt.
Graphite anode #3 displays the most severe corrosion damage. At low magnification (200×), the surface appeared highly rough and structurally compromised and was covered with corrosion products and macroscopic defects. High-magnification (5000×) images show numerous irregular pits and fragmented, uplifted graphite layers, which is consistent with uniform corrosion superimposed with localized pitting. This indicates that corrosion attacked not only the grain boundaries but also the graphite grains directly and aggressively. Cross-sectional observations revealed a coarse corrosion layer containing abundant pores and internal defects. These defects facilitate the rapid penetration of molten salt deeper into the material, severely impairing the structural integrity of the cross-section. This morphology reflects the poorest tolerance of the #3 anode to fluoride molten salt corrosion.
Table 2 summarizes the morphology and thickness of the corrosion layer of various graphite anodes. The characteristics of the corrosion layers reveal distinct degradation levels among the anodes. Anode #2 demonstrated superior corrosion resistance, evidenced by having the thinnest (100–130 µm) and most continuous layer, which effectively shielded the substrate despite minor internal cracking. In contrast, the loose, honeycomb-like structure of the anode #1 thicker layer (200–300 µm) suggests poorer cohesion and easier pathways for corrosive agents. Anode #3 experienced the most severe degradation, indicated by the thickest and most variable layer (180–400 µm) with a rough, defective morphology, highlighting a structurally compromised state.
3.2. Changes in Corrosion Elements
To examine the surface elemental composition of the graphite anode in the fluoride molten salt, EDS analysis of the #2 graphite anode was performed, and the results are presented in
Figure 3. The SEM image in
Figure 3a shows the local morphology of the graphite anode surface, which has a relatively rough structure with visible pores, providing a basis for interpreting the subsequent elemental distribution. As shown in the carbon distribution map in
Figure 3b, carbon is the predominant element, accounting for approximately 97.7% of the material, and is uniformly distributed across the surface, which is consistent with the inherent carbon-based nature of graphite. With respect to other elements, the chlorine distribution map in
Figure 3c reveals a sparse presence of chlorine, indicating a low surface concentration prior to corrosion of approximately 0.9%. The EDS spectrum in
Figure 3d,e quantitatively summarizes the relative content of each element: carbon constitutes the vast majority, whereas other elements—such as Si, Cl, K, and Ca—are present in extremely low quantities.
In the context of graphite corrosion in fluoride molten salt, graphite is primarily composed of carbon, and the corrosion process generally involves interactions between the carbon and molten salt components. The low concentrations of elements such as chlorine—which may originate from chloride impurities in the molten salt or the environment—along with trace amounts of calcium and silicon, likely derived from impurities in the graphite or external sources, indicate that the graphite anode surface is relatively “clean” before corrosion, dominated by carbon with only minor impurities. This initial elemental profile serves as a reference for tracking changes in surface composition during the subsequent corrosion process in fluoride molten salt. Both the interaction of the original trace impurities with the molten salt and the direct reaction between the carbon and the molten salt collectively govern the corrosion behavior and evolution of the surface elemental composition.
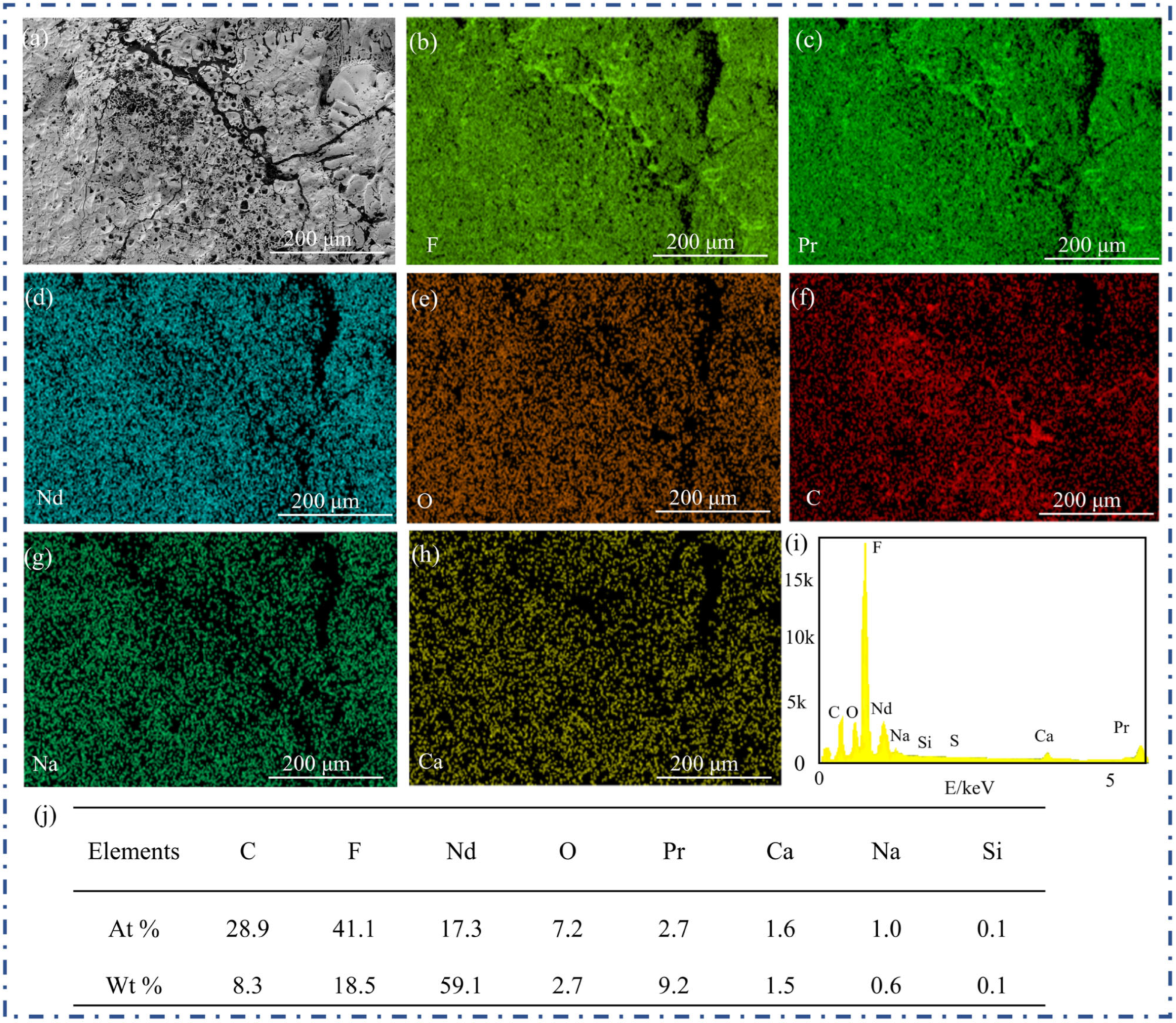
To elucidate the surface elemental characteristics and corrosion behavior of the graphite anode after exposure to fluoride molten salt, EDS analysis of #2 was conducted, and the results are presented in
Figure 4. The SEM image in
Figure 4a displays the local topography of the graphite anode surface after corrosion. A distinct reticular white shell structure, or aggregated white deposits, is observed covering the surface, with white substances filling cracks that extend into the graphite matrix. These features indicate substantial morphological changes induced by corrosion.
In terms of the elemental distribution, the F element map in
Figure 4b shows a widespread and relatively uniform distribution of fluorine across the surface. Given the corrosive nature of the fluoride molten salt environment, the abundant presence of F suggests strong interactions between the molten salt and the graphite anode. The distributions of Pr in
Figure 4c and Nd in
Figure 4d are also relatively uniform, which is consistent with the formation of deposition products enriched in these rare earth elements—supporting the macroscopic observation of white substances. The O distribution in
Figure 4e shows a moderate presence, which may originate from residual oxygen-containing impurities or oxidation during the corrosion process. Although the C element in
Figure 4f remains detectable, its distribution is less uniform than that in the pre correction state, reflecting the consumption or structural alteration of carbon due to corrosion. The Na distributions in
Figure 4g and Ca distributions in
Figure 4h are present only in trace amounts, likely originating from impurities in the molten salt or the graphite itself.
The EDS spectrum in
Figure 4i,j quantitatively summarizes the relative contents of each element. F, at approximately 18.5%, constituted a significant proportion, and Nd (approximately 59.1%) and Pr (approximately 9.2%) were also present in considerable amounts, whereas C, O, Na, Si, S, and Ca were detected at relatively low concentrations. Upon completion of the experiment, the graphite anode surface was covered with a white layer of solidified salt. EDS analysis confirmed the predominant presence of F, Nd, and Pr in this layer. The corrosion process not only altered the surface morphology but also created the composition. The originally carbon-dominated surface is supplemented with elements derived from the molten salt. The presence of O may be attributed to trace oxygen involvement in the reaction system, while the trace levels of Na and Ca further confirm the role of impurities in influencing the corrosion process.
In summary, combined morphological and elemental analyses demonstrate that the graphite anode undergoes a complex corrosion reaction in fluoride molten salt, resulting in the formation of new deposition products and notable changes in the surface elemental distribution.
3.3. Phase Changes
To comprehensively investigate the corrosion behavior and phase evolution of graphite anodes in a fluoride molten salt system at 1050 °C, XRD analysis was performed, and the results are presented in
Figure 5.
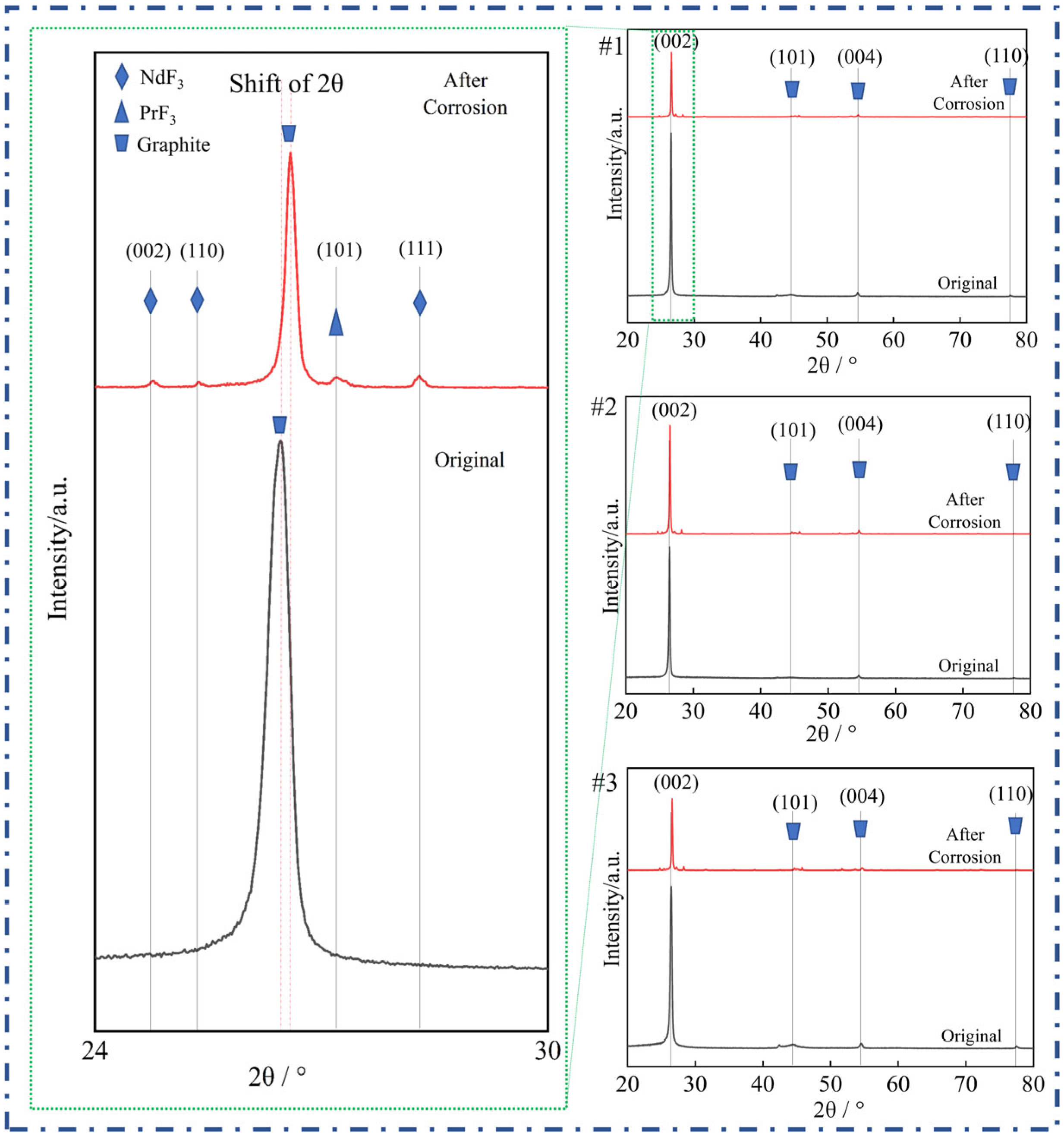
For the original graphite anode, within the characteristic diffraction angle (2θ) range, the diffraction peak of graphite—particularly the (002) peak corresponding to its basal plane—shows high intensity and a sharp profile (as seen in the black reference curves in all subfigures of
Figure 5). This indicates a typical and well-ordered crystalline structure. In graphite, carbon atoms are arranged in an ordered hexagonal layered structure, and the strong, sharp (002) peak reflects the high crystallinity of the graphite material, which is a fundamental characteristic of its structure.
After the graphite anode is subjected to molten salt corrosion at 1050 °C, significant phase changes occur, as clearly observed in the XRD patterns. The most obvious change is that the characteristic diffraction peaks of graphite shifted and weakened significantly. In the detailed view on the left (covering the 24–30° 2θ range) and the subfigures (#1, #2, #3) on the right, the intensity of the graphite peaks in the corroded samples (red curves) is substantially lower than that of the original graphite (black curves). In some regions, the graphite peaks nearly disappear.
The peak height of the graphite (002) crystal plane directly reflects the integrity of the long-range ordered structure of graphite. The data for the crystal plane position, crystal plane spacing, peak height, and grain size corresponding to the (002) plane are shown in
Table 3.
After corrosion, all anodes exhibited a positive shift in 2θ, corresponding to a contraction in d
002. The extent of this shift, however, was strongly dependent on the initial microstructure of the graphite. Anode #1 showed the smallest shift (Δ2θ = +0.03°). This can be attributed to its high initial crystallinity and structural density, as evidenced by its sharp and intense original (002) peak. This robust structure provided superior resistance to the corrosive attack, minimizing lattice distortion. Anode #2 exhibited a moderate shift (Δ2θ = +0.06°). The most critical factor for #2 appears to be the protective, continuously solidified salt layer formed on its surface. This layer acted as an effective barrier that significantly impeded the penetration of molten salt, thereby limiting the interlayer contraction. Anode #3 demonstrated the most pronounced changes (Δ2θ = +0.13°). Its high porosity (20.03%,
Table 1) and pre-existing microcracks from regeneration facilitated extensive molten salt infiltration. Severe corrosion led to significant disruption of the graphitic lattice.
The significant attenuation of the graphite (002) diffraction peak after corrosion is a direct consequence of the degradation of long-range crystallographic order, which diminishes the volume of material capable of coherent X-ray scattering. This phenomenon is driven by several interconnected mechanisms: First, the corrosive attack by F− ions preferentially erode defective regions, such as grain boundaries and edge planes, thereby reducing the number of coherent scattering domains. This is evidenced by the substantial decrease in peak height, particularly in Sample #3 (66.9% reduction), where the disintegration of larger crystalline domains into smaller, misoriented fragments disrupts constructive interference. Second, the corrosion induces lattice distortion. This distortion, stemming from point defects and layer misalignment, reduces the periodicity of the atomic planes and introduces phase shifts in the scattered X-rays, leading to partial destructive interference and a net decrease in peak intensity. The extent of degradation is further governed by the initial microstructure. Samples with high inherent defect densities (e.g., the porous, regenerated Sample #3) or abundant grain boundaries (e.g., the fine-grained Sample #1) offer numerous pathways for corrosive infiltration, resulting in more severe destruction of ordered domains. In contrast, the formation of a protective salt layer on Sample #2 effectively mitigated these processes, accounting for its minimal peak intensity loss (16.4%) and lattice parameter change. Collectively, the attenuation of the (002) peak intensity unequivocally reflects a corrosion-induced decrease in crystallinity, governed by the decrease in the scattering domain size and number, coupled with an increase in lattice disorder.
This observation directly reflects the disruption of the layered crystal structure of graphite under the combined effects of high temperature (1050 °C) and reactive ions in the fluoride molten salt. The ordered arrangement of carbon atoms in the hexagonal lattice is disturbed, defects are introduced, and parts of the ordered structure are destroyed. From a structural perspective, this degradation explains the deterioration in the physical properties of graphite anodes during corrosion, such as reduced mechanical strength and electrical conductivity, due to the loss of structural integrity.
In addition to the attenuation of the graphite peaks, new diffraction features emerge in the corroded samples. Specifically, characteristic peaks corresponding to PrF3 and NdF3 are observed, indicating the formation of these rare earth fluorides. For NdF3, peaks corresponding to the (002), (110), and (111) planes are identified, whereas for PrF3, the (101) plane is detected (as labeled in the left-hand detailed view and the right-hand subfigures).
The weakening of the graphite diffraction peaks, together with the emergence of PrF
3 and NdF
3 peaks, provides direct evidence of the corrosion of the graphite anodes in the fluoride molten salt. The attenuation of the graphite signals reflects the degradation of the bulk graphite structure, which is consistent with the corrosion behavior of carbon materials reported in molten fluoride salts, including disruption of the lattice order and increased defect density [
5,
30]. In contrast, the appearance of PrF
3 and NdF
3 peaks indicates the formation of corrosion products resulting from interactions between the graphite anode and the molten salt medium.
In summary, the XRD results in
Figure 5 clearly demonstrate the corrosion behavior of the graphite anodes in the fluoride molten salt at 1050 °C. The originally well-ordered graphite structure underwent significant degradation, while new fluoride phases formed as corrosion products. This phase evolution not only alters the chemical composition of the graphite anode surface but also affects its physical and chemical properties, providing important insight into the service life and performance degradation mechanism of graphite anodes in high-temperature fluoride molten salt environments.
3.4. Raman Analysis
Raman spectroscopy is a non-destructive and highly sensitive technique for structural characterization, offering unique advantages in the microstructural analysis of graphite materials. It is particularly sensitive to changes in the crystalline order, defect types, and layer stacking [
31]. Long-term contact between graphite and fluoride molten salts (used as the anode) leads to salt penetration, chemical reactions, and thermal stress at elevated temperatures. The combined effects of these factors significantly alter the graphite structure, ultimately affecting its electrochemical performance and service life.
The first-order Raman spectrum of graphite is dominated by three characteristic peaks whose assignments are based on group theory and the double resonance mechanism. The G peak (~1582 cm
−1) corresponds to the E
2g phonon mode and arises from the in-plane stretching vibration of sp
2-hybridized carbon atoms. This peak serves as a hallmark of the graphitic structure, with its full half-height width (FWHM) being a sensitive indicator of in-plane crystallinity and its position being influenced by stress and doping. The D peak (~1350 cm
−1) is a defect-induced peak activated by the intervalley double resonance process. It is associated with defects that break the in-plane symmetry, such as edges, grain boundaries, and vacancies. The intensity ratio of the D to G band (I
D/I
G) is commonly used to quantify the defect density. The D’ band (~1620 cm
−1) is a weak peak arising from the intravalley double resonance process, which is also activated by defects, particularly in-plane defects such as point defects. In the second-order Raman spectrum, the G’ band (~2700 cm
−1), also known as the 2D band, is an overtone of the D band. This peak is highly sensitive to the electronic structure and thus the stacking order of the graphitic layers. For example, single-layer graphene has a single, sharp 2D peak, whereas the Bernal-stacked bilayer and few-layer graphite have a characteristic multipeak structure due to interlayer coupling. The 2D band line shape is, therefore, a key fingerprint for identifying the number of layers and the stacking configuration [
1,
32,
33,
34].
Evolution of in-plane Crystallinity and Defects (G, D, and D’ Bands). The Raman spectra of the three types of graphite anodes before and after corrosion are shown in
Figure 6.
The key Raman spectral data of the three types of graphite anodes before and after corrosion are summarized in
Table 4, including the positions of the D peak, 2D peak, G peak, D’ peak, intensity, and I
D/I
G transformation.
The first-order Raman spectrum provides critical insights into the in-plane structural integrity of graphite. Before corrosion, the G band of the graphite anode was located at 1581.8–1582.2 cm
−1 with a narrow FWHM of 18–20 cm
−1. This is consistent with the characteristics of highly crystalline graphite reported in the literature, indicating a well-ordered in-plane lattice structure with minimal disturbance to the sp
2 carbon stretching vibrations [
35]. After corrosion, the FWHM of the G band substantially widened from 18–20 to 24–26 cm
−1, as shown in
Table 4. The FWHM is generally linked to the amorphization of the sp
2 carbon network. The FWHM broadening from the original narrow range directly indicates the amorphization of the sp
2 carbon network, likely induced by the interactions between F and the carbon lattice at elevated temperatures [
33]. This interaction, along with the propagation of microcracks due to thermal stress, disrupts the periodicity of the in-plane lattice, leading to peak broadening and a consequent decrease in crystallinity [
36].
The defect evolution was further quantified by the D and G bands. Prior to corrosion, the D band was weak, and the intensity variation of peak D is shown in
Figure 6b. The ratio changes in the I
D/I
G are shown in
Figure 6c, and the original graphite anodes with I
D/I
G ratios in the range of 0.10–0.17 have a low initial defect density. After corrosion, the I
D/I
G ratio clearly increased to 0.11–0.24. The relationship between the I
D/I
G ratio and the in-plane crystallite size (La) was first established by Tuinstra and Koenig, who demonstrated that the I
D/I
G ratio is inversely proportional to La [
33,
34,
37]. Importantly, this inverse relationship is primarily valid for the low-defect regime. The observed increase in I
D/I
G after corrosion unequivocally indicates a reduction in La and a greater density of defects. This grain refinement is attributed to the penetration of fluoride molten salt through open pores, which not only creates fresh edge defects on the pore walls but also promotes the fragmentation of large graphite grains into smaller domains, thereby increasing the D band intensity via the intervalley double resonance process.
The appearance of the D’ band at 1619–1621 cm
−1 after corrosion offers direct evidence for the generation of in-plane point defects, as shown in
Figure 6a. This band, activated by the intravalley double resonance mechanism, confirms that the high-temperature exposure to F
− ions introduces atomic-scale disorder [
38]. We propose that F
− ions temporarily adsorb onto dangling bonds at the graphite surface, disrupting the periodic arrangement of carbon atoms and creating vacancy-like defects, even in the absence of stable fluorocarbon formation, as detected by XRD.
Disruption of the interlayer stacking order (2D Band). The second-order 2D band serves as a sensitive probe for interlayer stacking. The initial graphite exhibited a well-resolved doublet at 2690 cm
−1 and 2727 cm
−1, characteristic of Bernal (AB) stacking in three-dimensional ordered graphite. After corrosion, this characteristic doublet merged into a single, broad peak. As quantified in
Table 4, the 2D band position shifted substantially (e.g., by 29 cm
−1 for sample #3), and its FWHM increased significantly from approximately 42 ± 2 cm
−1 to over 63 ± 2 cm
−1 for all samples, with #3 exhibiting the most severe broadening, to 66 cm
−1. This transformation aligns perfectly with the 2D band profile of turbotrain graphite, which features random layer rotations and translations. This finding confirms that the molten salt penetrates the graphite interlayers, screening the van der Waals forces and disrupting the ordered AB stacking. Concurrently, the refinement of in-plane crystallites reduces the interlayer correlation length, collectively manifesting as the collapse of the bimodal 2D structure into a unimodal one.
Based on the Raman spectral evolution summarized in
Table 4 and the comprehensive multi characterization results, the corrosion mechanism of graphite anodes in fluoride molten salt can be delineated into three sequential stages, with distinct quantitative differences observed across the three graphite types (#1, #2, and #3) that correlate strongly with their initial microstructural properties.
In the molten salt penetration stage, fluoride salts infiltrate the graphite bulk through open pores, preferentially attacking pore walls and generating edge defects, which is reflected by the initial increase in the D band intensity. As shown in
Table 4, the original D band intensity of #3 graphite is notably greater than those of #1 and #2, which is consistent with its highest initial porosity, facilitating more extensive salt infiltration. After corrosion, the D band intensity increases across all samples, with #3 exhibiting the most significant increase (from 562 to 719 a.u., +27.9%), followed by #2 (from 179 to 426 a.u., +137.9%) and #1 (from 234 to 300 a.u., +28.2%). This variation indicates that while salt penetration initiates defect formation in all samples, the extent is modulated by the initial pore connectivity and salt-accessible surface area.
The defect generation and grain refinement stage involves high-temperature chemical interactions between F− ions and basal plane carbon, introducing in-plane point defects (evidenced by the appearance of the D’ band in corroded samples) and thermal-stress-induced microcrack propagation, leading to pronounced grain refinement. The ID/IG ratio of all graphite anodes has increased, corresponding to a decrease in grain size. Concurrently, the G band FWHM broadens from 19 ± 1 to 24 ± 1 cm−1 (#1), 18 ± 1 to 25 ± 1 cm−1 (#2), and 20 ± 1 to 26 ± 1 cm−1 (#3), directly reflecting the loss of in-plane crystallinity. Notably, #2 graphite, which has a protective cooled fluoride salt layer on its surface, exhibits a moderate increase in ID/IG and G band broadening, indicating that the salt layer mitigates but does not entirely suppress defect generation.
In the stacking disorder stage, molten salt molecules intercalate into the graphite galleries, weakening the interlayer van der Waals interactions and disrupting the AB stacking order. This is unambiguously reflected by the fusion of the 2D band doublet into a single peak. The 2D peak position shifts from 2711 to 2722 cm−1 (#1), 2701 to 2702 cm−1 (#2), and 2690 to 2719 cm−1 (#3), accompanied by a significant increase in the FWHM (from ~40 to 55, 50, and 65 cm−1 for #1, #2, and #3, respectively). The largest shift and broadening in the #3 graphite confirmed that regenerated graphite underwent the most severe interlayer disorder, likely due to its high initial defect density and extensive salt intercalation.
Collectively, these quantitative Raman, XRD, and SEM results validate the tri-phasic corrosion sequence while highlighting the critical role of the initial graphite microstructure (porosity, grain size, and defect density) and surface salt layer formation (in #2) in dictating the extent of each corrosion stage, providing a mechanistic framework for optimizing graphite anode durability in fluoride molten salt systems.
3.5. Infrared Spectroscopy Analysis
Infrared spectroscopy serves as a powerful analytical technique for probing molecular vibrations and identifying functional groups, rendering it highly valuable for characterizing the structural properties of carbon materials. Owing to its sensitivity to chemical bonding and surface chemistry, this method is particularly effective for investigating redox behavior, surface functionalization, doping, and other modification processes in graphite [
39]. When graphite anodes are exposed to fluoride molten salt environments, their chemical structure and surface functional groups undergo significant alterations as a result of high-temperature conditions, salt penetration, and associated electrochemical reactions. These molecular-level changes can be effectively monitored via infrared spectroscopy. For example, in the case of graphite oxide, a broad absorption band at approximately 3435 cm
−1 is common, which is attributed to O–H stretching vibrations, suggesting the presence of adsorbed water or surface hydroxyl groups. A characteristic peak near 1632 cm
−1 corresponds to C=O stretching vibrations from carboxyl groups, confirming the introduction of oxygen-containing functionalities. The absorption at approximately 1590 cm
−1 is associated with the C=C stretching vibration of sp
2-hybridized carbon within the graphitic lattice (E
2 symmetry mode), reflecting the degree of graphitic crystallinity. Moreover, the peak at approximately 1351 cm
−1 is generally assigned to structural defects in graphite and may also include contributions from bending vibrations of surface hydrogen-containing moieties such as CH
2 and CH
3 [
40].
By comparing the infrared spectra of various graphite anodes before and after corrosion in fluoride molten salts, it is possible to trace their structural evolution, including the formation and cleavage of chemical bonds. This analysis offers essential molecular-level insights into the performance degradation and durability of graphite anodes under aggressive molten salt conditions. As shown in
Figure 7, noticeable changes in the infrared spectra of different graphite anodes after corrosion further illustrate the structural alterations discussed above.
Figure 7a,b clearly shows a consistent attenuation of the infrared absorption peaks at 1351, 1384, 1590, 1632, and 3435 cm
−1 across all three types of graphite anodes after corrosion in the fluoride molten salt environment. These spectral changes provide direct evidence of microstructural and chemical transformations resulting from the combined effects of high temperature, molten salt infiltration, and fluoride-ion attack. A detailed interpretation of these spectral changes, organized by wavenumber region, is presented below.
In the high-wavenumber region, the pronounced decrease in the broad absorption band near 3435 cm−1, which is associated with O–H stretching vibrations, indicates a substantial loss of surface hydroxyl groups and adsorbed water. This decrease is attributed to two main processes: the thermal desorption of water molecules at elevated temperatures and the chemical reaction between F− and hydroxyl groups, which likely results in the production of volatile hydrogen fluoride. The variation in the extent of this decrease among the three graphite samples can be correlated with differences in their pore structure and surface-active-site density. Graphite with higher porosity and more active sites experiences greater interfacial contact with the molten salt, leading to more extensive removal of hydroxyl species.
In the medium-wavenumber region, the spectral changes offer key insights into the degradation of the graphitic framework. The decrease in the peak at 1632 cm−1, assigned to C=O stretching vibrations in carbonyl or carboxyl groups, reflects a net decrease in the number of oxygen-containing functional groups. This can be explained by two factors: first, pre-existing oxygenated functional groups are likely etched away through reactions with F−, forming volatile fluorine-containing compounds; second, the oxygen-free molten salt environment inhibits the formation of new oxygen-containing groups during corrosion. More importantly, the decrease in intensity at 1590 cm−1, corresponding to the in-plane C=C stretching vibration of the sp2-carbon lattice (G band), signals a decrease in graphitic crystallinity. The combined action of thermal stress and chemical attack by fluoride ions disrupts the long-range order of the graphitic layers, induces lattice distortion, and partially damages the conjugated π-system, thereby weakening the C=C stretching vibration.
In the low-wavenumber region, the spectral changes reveal complex behavior related to defect evolution and removal of hydrocarbon species. The absorption near 1351 cm−1, known as the D band, is associated with structural defects in sp2 carbon materials. The decrease in its intensity suggests a reorganization of the defect structure, possibly due to the disruption of local C–C bonds by infiltrating F− ions at high temperatures. Additionally, the peak at 1384 cm−1, which is commonly linked to C–H bending vibrations in aliphatic hydrocarbons (e.g., –CH2 and –CH3), also has a reduced intensity, indicating the desorption of hydrocarbon impurities under high-temperature conditions. Concurrently, F− may undergo substitution reactions with edge C–H groups, forming C–F bonds and further reducing the content of hydrogen-containing groups, contributing to the overall decline in absorbance in this region.
A comprehensive comparative analysis of the FTIR spectra acquired before and after corrosion revealed a multifaceted degradation mechanism for graphite anodes in fluoride-based molten salt environments. This mechanism encompasses the depletion of surface functional groups coupled with a fundamental deterioration of the graphitic crystalline order. Variations in corrosion severity across the three graphite variants underscore the critical role of initial physicochemical properties—including porous architecture and surface-active site density—in governing their electrochemical durability within such corrosive operational environments.