1. Introduction
Recent technological advancements have enabled the fabrication of geometrically complex metal components with high precision, offering unique advantages in customized production and flexible manufacturing [
1], thereby significantly accelerating the development of the advanced manufacturing sector. Three-dimensional printing technology, known as additive manufacturing (AM) [
2], has emerged as one of the most rapidly advancing innovations in the intelligent manufacturing era. Three-dimensional objects are constructed by sequentially depositing fine material powders based on digital models. Metal powder remains critical for component-specific printing, necessitating a comprehensive evaluation of part geometry, dimensional accuracy, mechanical strength, metal compatibility, cost efficiency, production speed, and batch size [
3]. Typically, there are three powder manufacturing routes: chemical, physical, and mechanical [
4]. Milling, electrolytic, and atomization methods are amongst the most popular production methods.
Atomization method offers high quality of produced aluminium alloy particles and prospects for mass production [
5]. In this method [
6], the metal to be atomised is first melted and then exposed to a high-velocity gas jet which causes the liquid metal to break up into small droplets. These droplets subsequently solidify in flight to form the aluminium alloy powder. Studies demonstrate that fine gas-atomized metal zirconium powders exhibit small particle sizes, high sphericity, and part longevity [
7]. N. Ciftci et al. [
8] prepared iron-based glass powders using argon gas at different temperatures, revealing that hot gas atomization is helpful to produce particles with narrower size distributions and lower gas consumption. Hussain et al. [
9] found that high-temperature gas atomization promotes more melt fragmentation into finer steel droplets than low-temperature processes. Li et al. [
10] investigated the atomization of three kinds of Al alloys using melt nozzles with different inner diameters. They found that production of high-aluminium alloy powder with small average particle size occurred at the concave position of the delivery tube. The low production was at the flush position of the delivery tube with large average particle size. Microscopic morphology and solidification structure of fine powders significantly influence mechanical properties. Pinotti et. al. [
11] evaluated the influence of melt feed nozzle diameter, superheating temperature, and atomization pressure on AA2017 aluminium alloy powder. Based on the venturi phenomenon, metal aluminium powder of different sizes and shapes can be produced [
12]. Current gas atomization studies focus on the influence of powder preparation process on powder properties and yield, but few investigations on microstructural characteristics of powder have been reported.
In practical applications, the microstructure of particle will be designed according to the target performance. The particle shape will affect the fluidity, packing characteristics, and interface interaction of the particles. The internal crystal structure of powder including crystal form, phase composition, and defect affects the atomic diffusion ability inside the powders, which is the core determinant of the mechanical, electrical, magnetic, and other properties of powder materials. For example, spherical, fine-grained, and narrow-distribution powder should be used for the preparation of high-density parts; particle with rough, porous, and defect-rich microstructure suit highly active catalysts. So, understanding the relationship between microstructure and performance is important for achieving high performance of powder materials. In this work, aluminum powder was produced via vacuum induction gas atomization. Characterization and optimization between particle shape, particle size, surface morphology, crystal structure, and process parameters was made. Satellite particle, outgrowth index, and secondary dendritic arm spacing were also investigated.
2. Materials and Methods
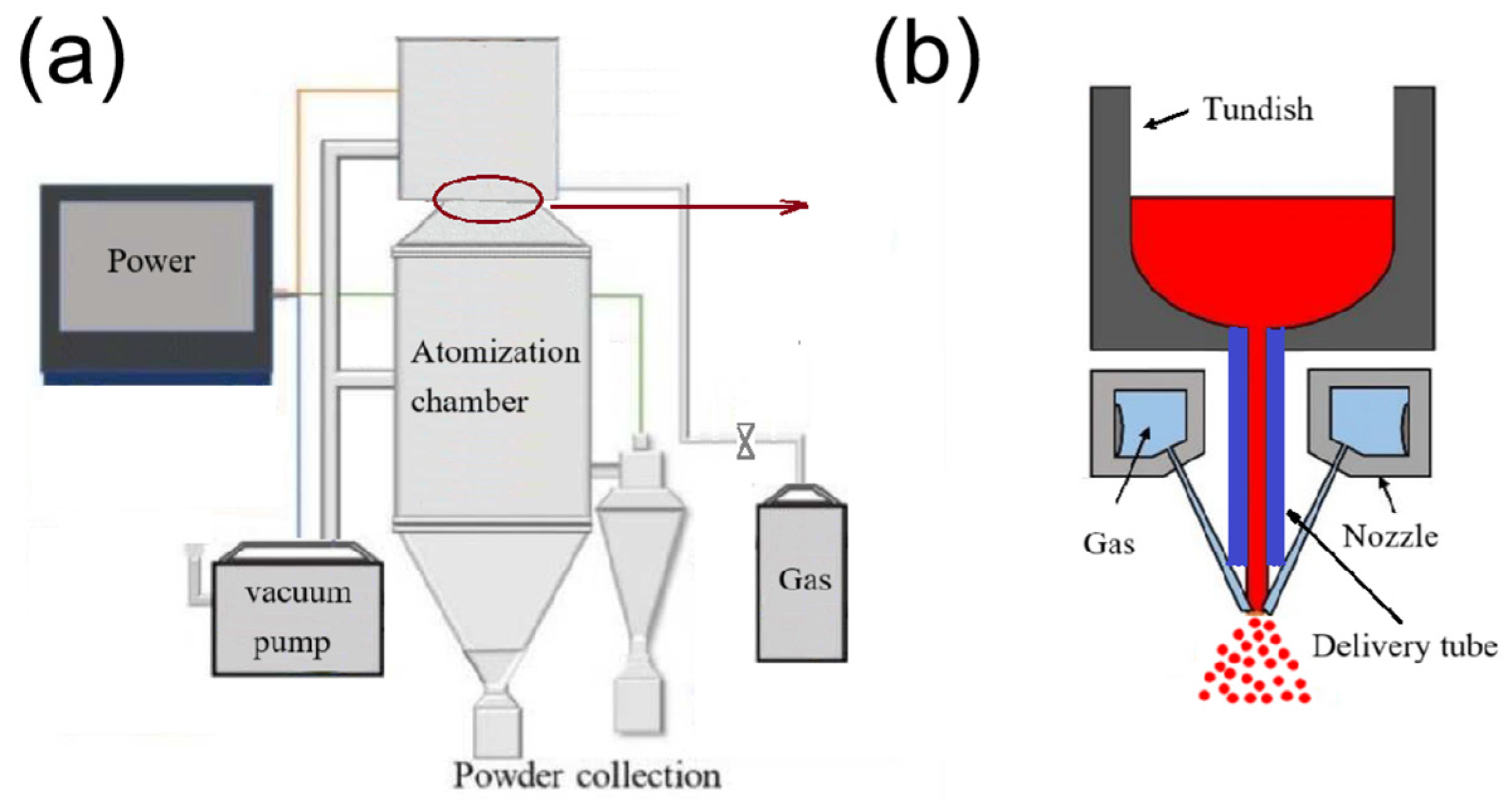
Aluminium powder was prepared using gas atomization techniques. The schematic of equipment and atomization device was shown in
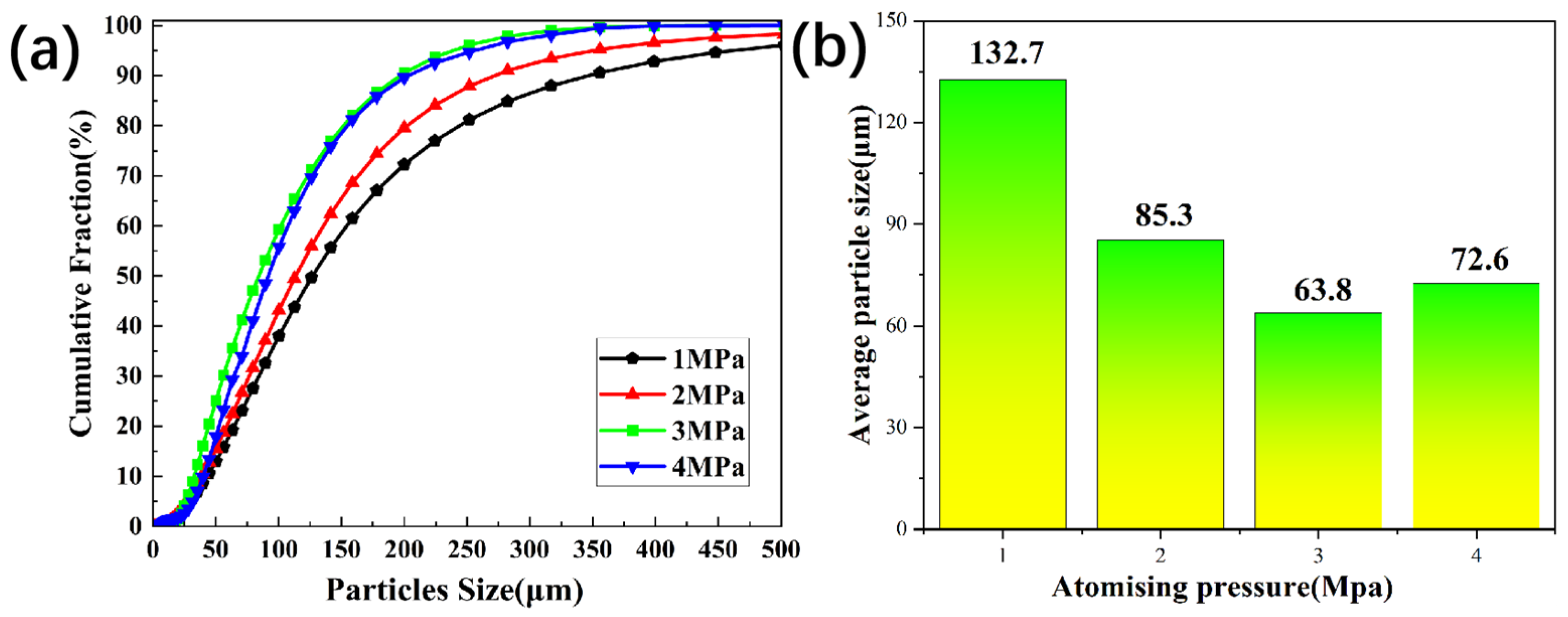
Figure 1. During the experiment, the atomization pressure was selected as 1.0, 2.0, 3.0 and 4.0 MPa, and the inner diameters of the deflector tubes were 2.5, 3.0, 3.5 and 4.0 mm, respectively. and the angle of the atomizing nozzle, the degree of superheat of the melt, and the length of the guide tube were 30°, 200 °C, and 120 mm, with all processed under vacuum. Each group of experiments was repeated three time. The nitrogen atomization system consists of an atomization chamber, a power system, a vacuum system, and a nitrogen supplier system. The atomization chamber has an observation window with a diameter of 30 cm, with a temperature thermocouple, a pressure sensor, and a heating wire. Nitrogen was introduced into the atomization device through nozzle at a certain angle to atomize liquid aluminum during the experiment. Nitrogen pressure adjustment can be acquried by controlling the air valve. The kinetic energy carried by the high-speed gas flow is used to impact the molten aluminum entering the atomization zone, atomizing the molten metal into tiny droplets. These droplets are cooled and solidified into powders in an atomization furnace. The angle of the atomizing nozzle, the degree of superheat of the melt, and the length of the delivery tube were 30°, 200 °C, and 120 mm, respectively. Variable parameters of the atomization pressure and the inner diameters of the delivery tube were shown in
Table 1. The optimal parameters were obtained according to particle size.
Powders are then classified by sieving into size ranges appropriate for various applications. Nitrogen was used to atomize the molten aluminum metal into powders.
Particle Size Distribution (PSD) was analyzed using a MASTERSIZER 2000 laser (particle size analyzer. Powder morphology and surface elemental distribution were characterized using Field Emission Scanning Electron Microscopy (FESEM; Zeiss Sigma 300) coupled with Energy Dispersive Spectroscopy (EDS). Primary dendrite arm lengths within differently sized powders were quantified using Image J(imagej 1.53k) software. Nitrogen content was measured by HORIBA EMGA-830 oxygen-nitrogen-hydrogen analyzer.
4. Discussion
4.1. Characterization of Outgrowth Index and Sphericity
Outgrowth index, defined as the ratio of the adhered satellite particles to the total surface features of particle, has been proposed to quantitatively assess satellite particles through digital filtering [
16]. The outgrowth index 0, 50%, 66%, 75%, and 80% illustrates that there are 0, 1, 2, 3, and 4 satellite sphere on the surface, respectively. Standard deviation, which characterize the inhomogeneity of the powder, were incorporated for comparison [
17]. Higher values are associated with large deviations in the motion states and solidification time of droplets, thus increasing the probability of collisions and the formation of satellite spheres. Fine particles have larger surface areas and high dispersion values, which are correlated with increasing surface energy per unit mass. The outgrowth index, standard deviation, and dispersion are described by Equations (4)–(6) [
16,
17]:
where
is the number of adherent particles, δ is the standard deviation,
is the particle size of any particle,
is represents the particle size of any particle, and
is the average particle size. Based on the analysis in
Figure 8, aluminum powder exhibits relatively high density; and the number of satellite spheres has more ratio among powders with larger particle size and more irregular shape, which reduces the powder density of aluminum powder.
Aluminum particles prepared at the pressure of 3 MPa were used to make an assessment of the surface characteristics. Based on Equations (4)–(6), Outgrowth, standard deviation and span were obtained in
Table 3.
Sphericity offers a rigorous way to describe the shape of the powder particles. The calculation formula for the sphericity is as follows [
18]:
where V is the particle volume, λ
E is the diameter of the largest internally connected sphere, and λ
i is the diameter of the internally connected disc at the i point where the sphere contains the particle profile.
Powder flowability is a very critical concept in powder metallurgy, additive manufacturing (3D printing), metal injection molding, and other industries. Metal powder fluidity refers to the difficulty and speed of metal powder flow under specific conditions. Simply put, it measures the ability of powder to “flow” like liquid. Liquid powders can quickly, uniformly, and steadily fill the mold or spread on the printing platform, while poorly liquid powders are likeas to agglomery and bridge, resulting in uneven filling and ultimately affecting product quality.
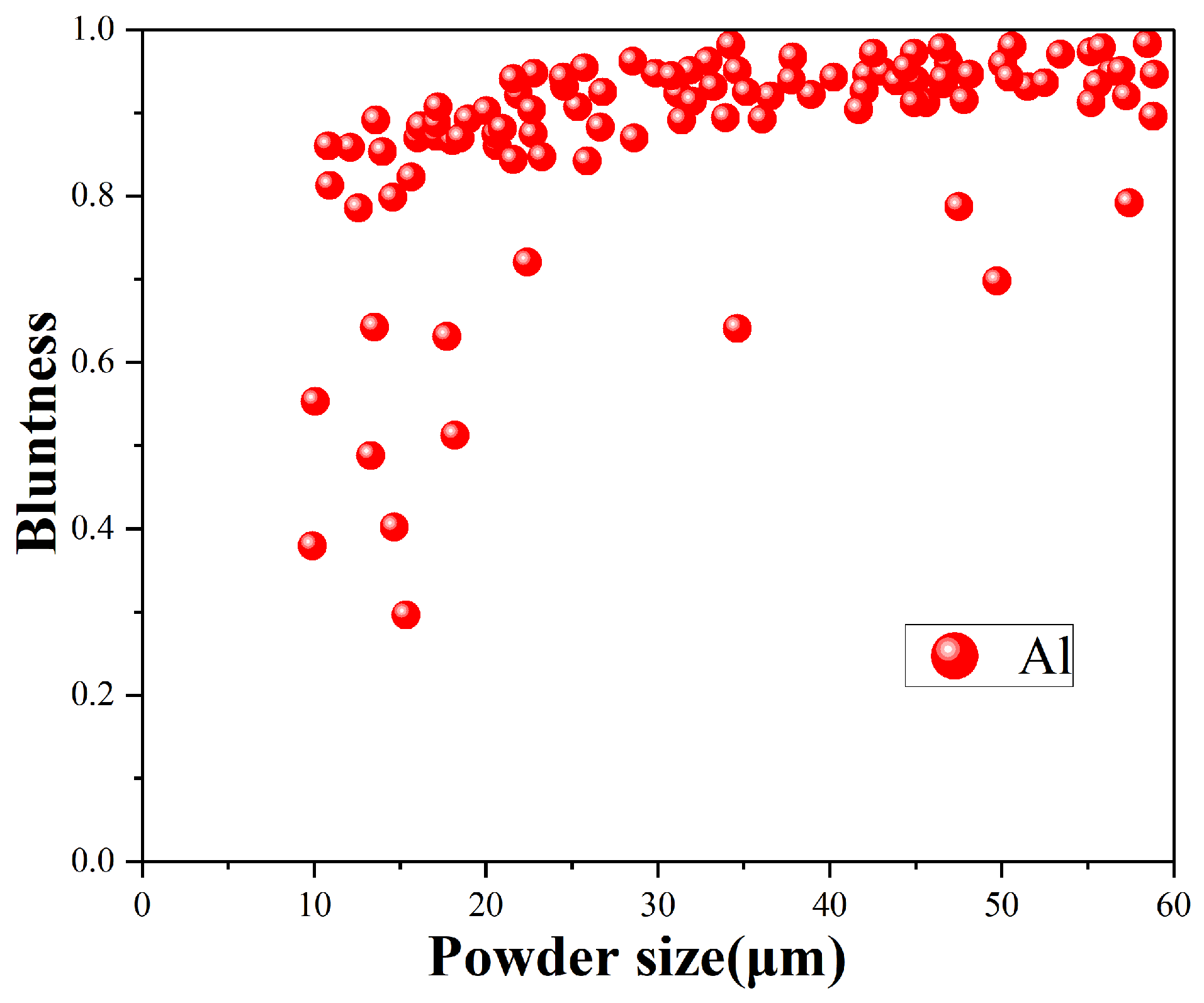
The most accurate quantification of sphericity is bluntness. the bluntness of the powders at different powder sizes are shown in
Figure 9. It can be seen that in the powders with particle size of 0–60 μm, the larger the powder size is, the tends to increase. The bluntness of the majority of powders exceeds 90%. This means that most of the aluminum powders exhibit a high sphericity.
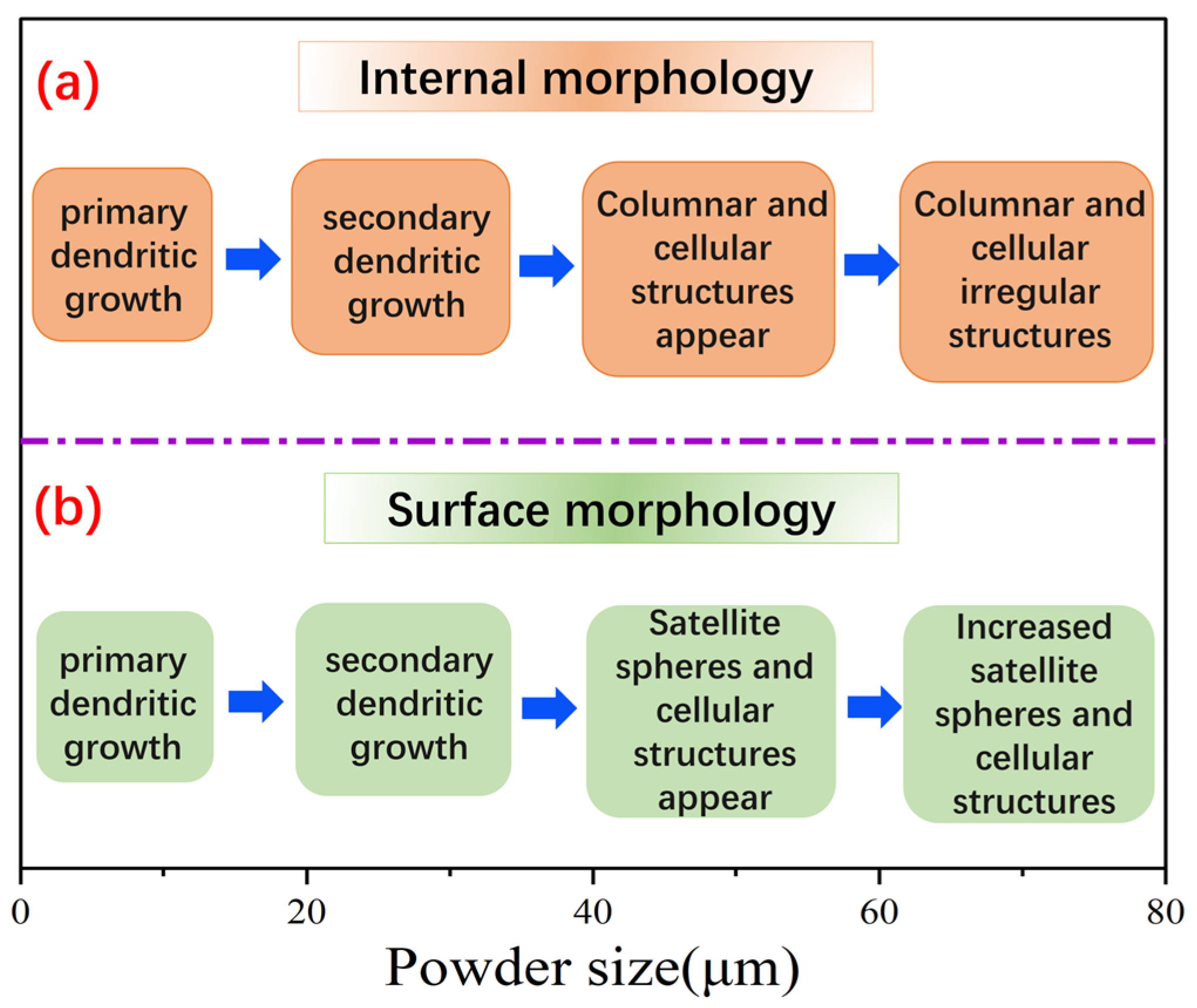
4.2. The Process of Dendrite Generation
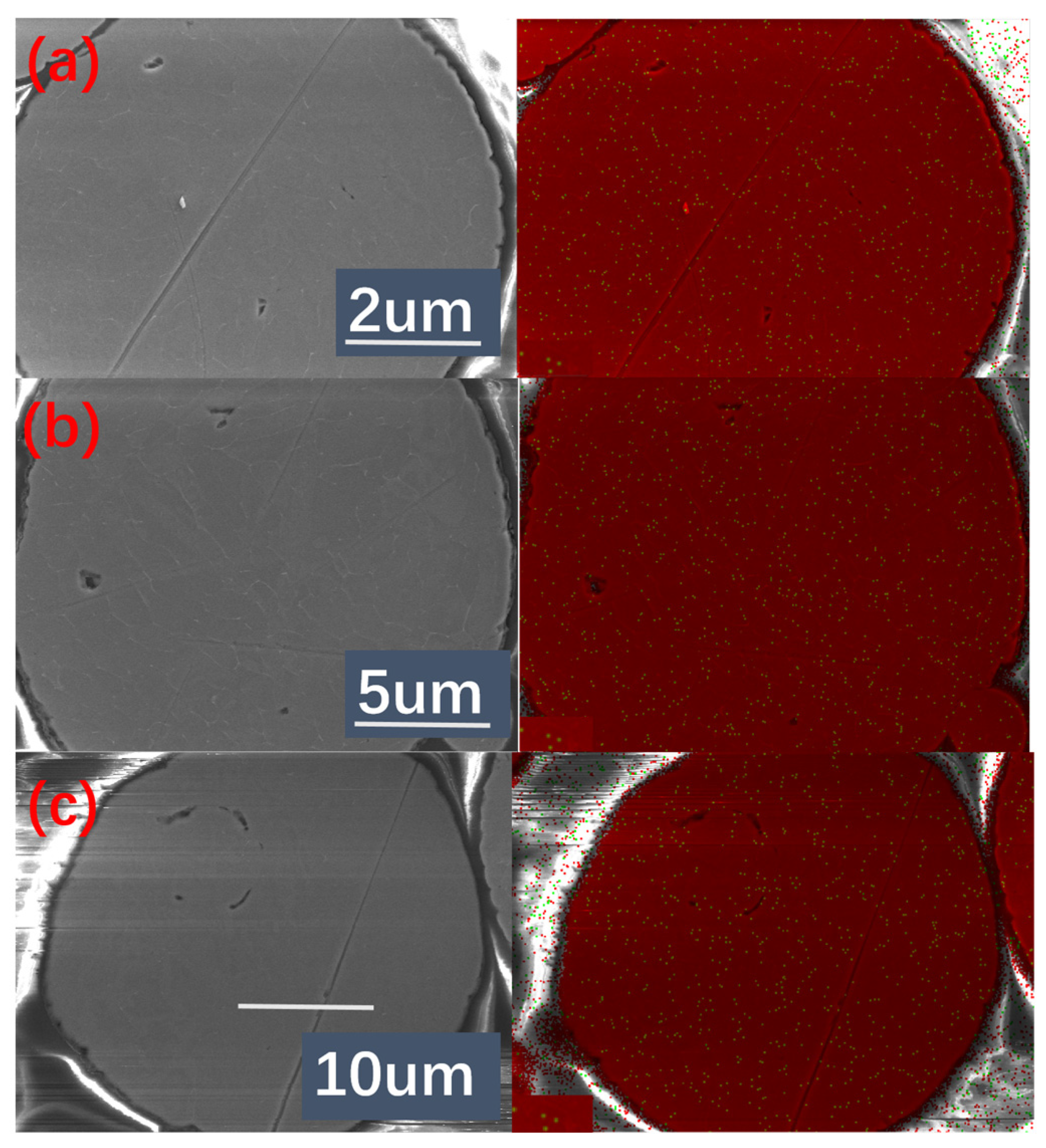
Figure 10 depicts the surface and internal dendritic structures of aluminum powders, respectively. According to the dendrite growth model (
Figure 11) [
19], small powder particles experience distinct pressure difference between their surfaces and interiors, creating different cooling rates. Surface regions develop cellular structures with primary dendrites, following by the honeycomb microstructures surrounding nucleation sites internally. With the increase of droplet size, the cooling rate decreases, and the overall solidification time increases, providing kinetic conditions for the growth of secondary dendritic structures. Eventually, the growth of primary dendritic arms on the surface and inside of the solidified particles is strengthened, and the spacing of secondary dendritic arms increases. As droplet size continues to increase, the solidification rate decreases significantly, and secondary dendrites form and grow freely inside the powder. Surface energy leads to a fast diffusion of liquid aluminum at the edges of the microstructure. Inside of the droplet with relatively low temperature change (ΔT) produces a disordered small-sized equiaxed microstructure during cooling process. Meanwhile, the morphology of the equiaxed microstructures tends to be a spherical state due to the increase of particle size, and the liquid aluminum diffusion makes the equiaxed microstructures more uniform.
The nucleation sites of primary dendrites are located at sub-boundary positions. The length of primary dendrite arms is no more than the particle size, which can be approximately regarded as the size of the solidified powder.
Figure 12 shows the variation curves of primary dendrite arm spacing with different powder sizes. When the particle size increases from 0 to 45 μm, the length of primary dendrite arms also increases from 0 to ~30 μm. As the particle size continues to grow, the length of primary dendrite arms remains stable around 30 μm. For the pure aluminum particles, influence of the trace elements in aluminum on the atomization can be ignored. No dendrite structures should be produced under conditions of the same diffusion coefficient. This abnormal phenomenon is probably related to thermal properties such as solidification enthalpy and specific heat capacity, indicating that the evolution of the microstructure is influenced by multiple factors.
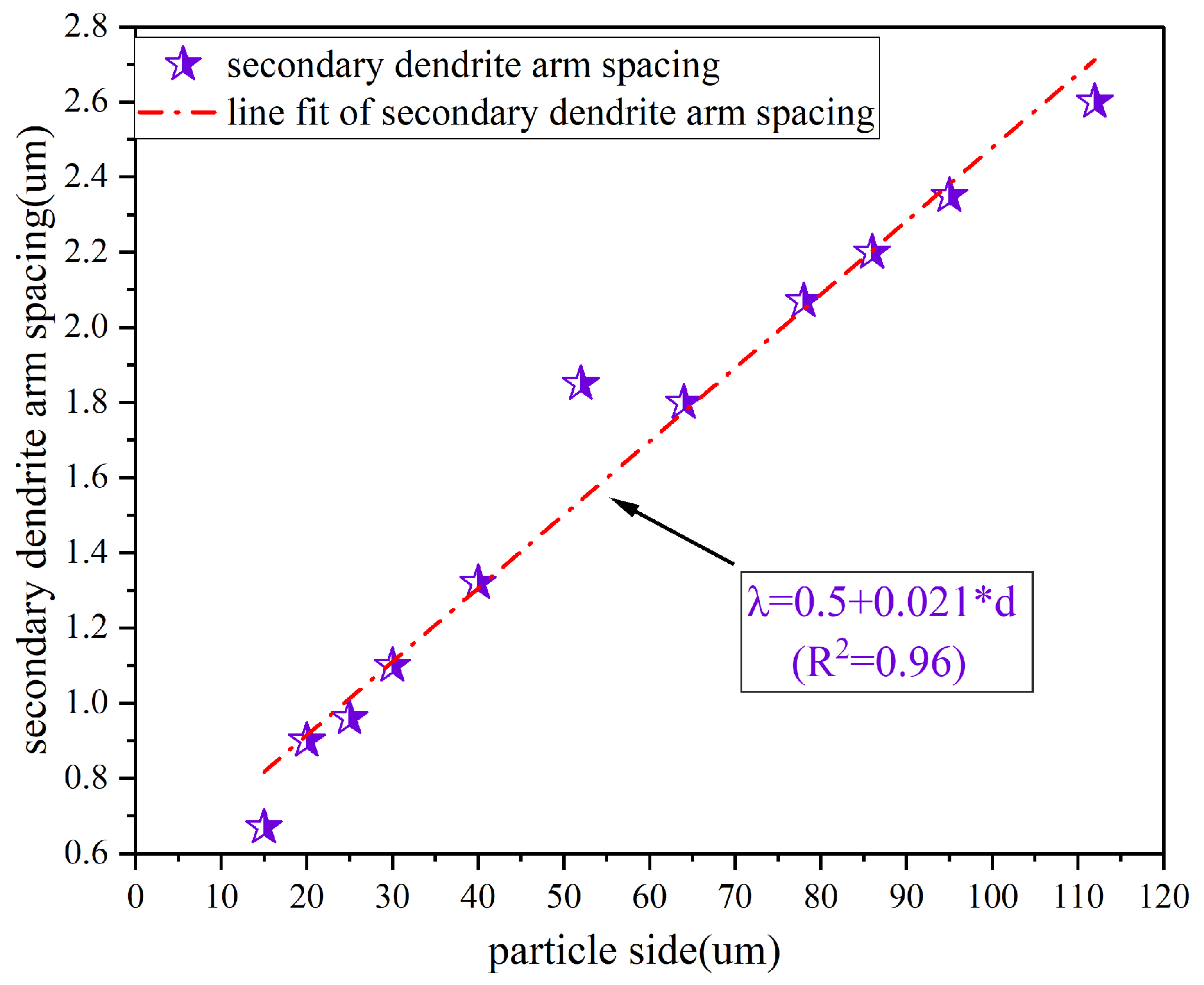
The secondary Dendrite Arm Spacing (SDAS) is an essential parameter for the assessment of the particle refinement. The SDAS values, obtained by Image J analysis, are listed in
Table 4. Relationship between the SDAS and the particle size is graphically presented in
Figure 13. There is a clear linear dependence of SDAS on the powder dimensions, exhibiting progressive expansion from 0.5 μm to 2.5 μm. The fitting relationship is determined by [
20]:
Additionally, a solidification rate using a calibration equation for the spacing of the secondary dendrite arms and the solidification rate is [
21]:
where β is a constant related to the material class. It can be found from Equation (9) that the cooling rate is negatively correlated with the particle size. The heat transfer coefficient of powders with large particle sizes is less than that of powders with small particle sizes, leading to a significant particle refinement.
Solidification of the atomized molten droplets goes through the nucleation, the growth of the primary dendrite and the secondary dendrite [
22]. When the nucleation is completed, the growth of primary dendrites begins. The latent heat released by the droplets will generate a temperature change (ΔT) perpendicular to the primary dendrites, which is a necessary prerequisite for the formation of the secondary dendrites. It can be seen from Equation (9) that the spacing and growth of secondary dendrites are determined by the solidification rate. High solidification rate makes droplet with small particle sizes semi-solid, thereby inhibiting the growth of secondary dendrites. As a result, the secondary dendrites are mostly present in powders with larger particle sizes. Combining Formulas (8) and (9), the relationship between the particle size of the powder and the solidification time can be obtained [
22].
It can be seen from the Equation (10) that the existence of the temperature change rate provides a good kinetic foundation for the growth of dendritic structures. With the increase in the particle size, the temperature change rate decreases. The solidification time governs internal dendritic structure development. For fine powders with a size range of 0–35 μm, the rapid solidification of their surfaces gives rise to the cellular or dendritic surface structures during the atomization process. However, inside the fine powder, only primary dendrites can be observed. For large powders with a size more than 35 μm, the decrease of temperature change rate makes the overall solidification time increase. Secondary dendrites have sufficient kinetic conditions for growth, so primary dendrites and secondary dendrites coexist on the surface of the powder. In addition, since the cooling rate of satellite spherical powder is higher than that of the ordinary particles, the cooling rate at the interface is accelerated, leading to the formation of cellular structures at the interface of satellite powder.
4.3. The Process of Increasing Nitrogen on the Surface of the Powder
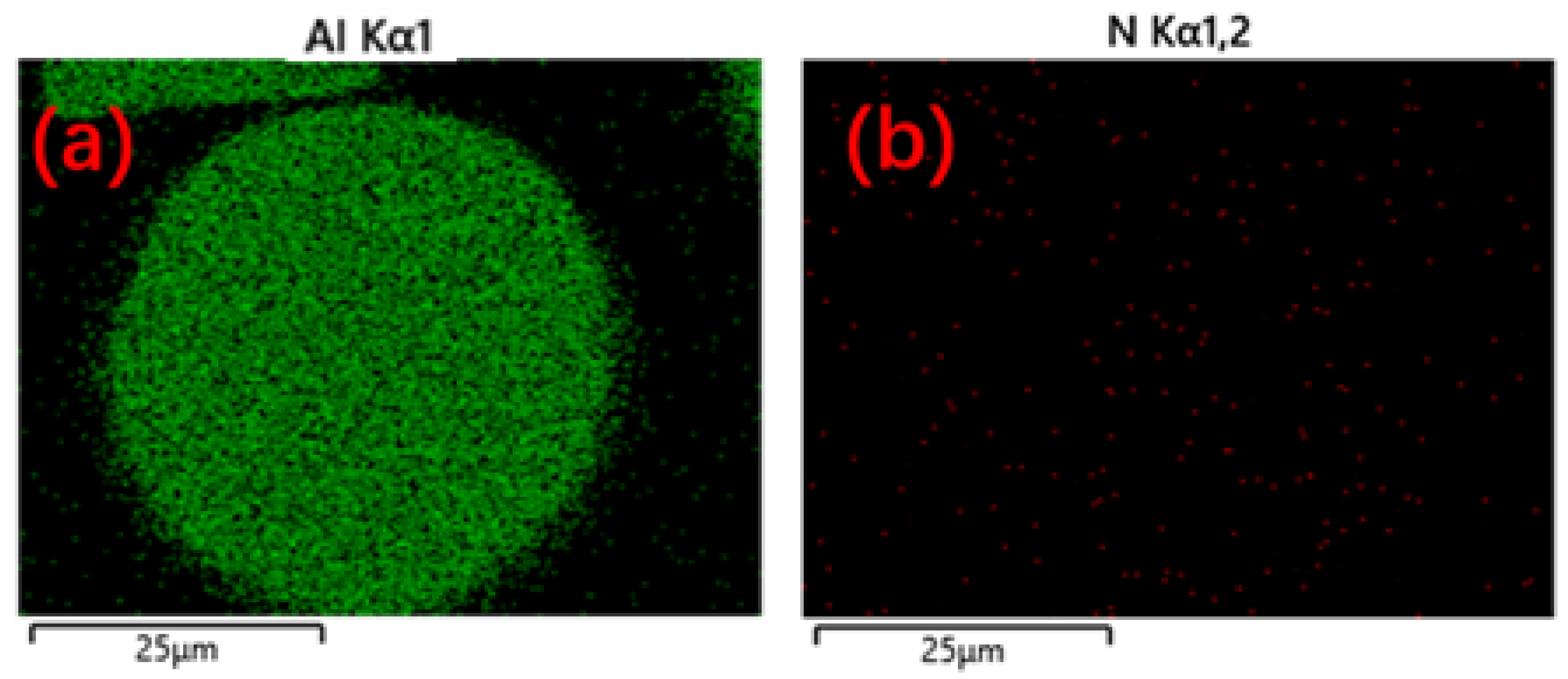
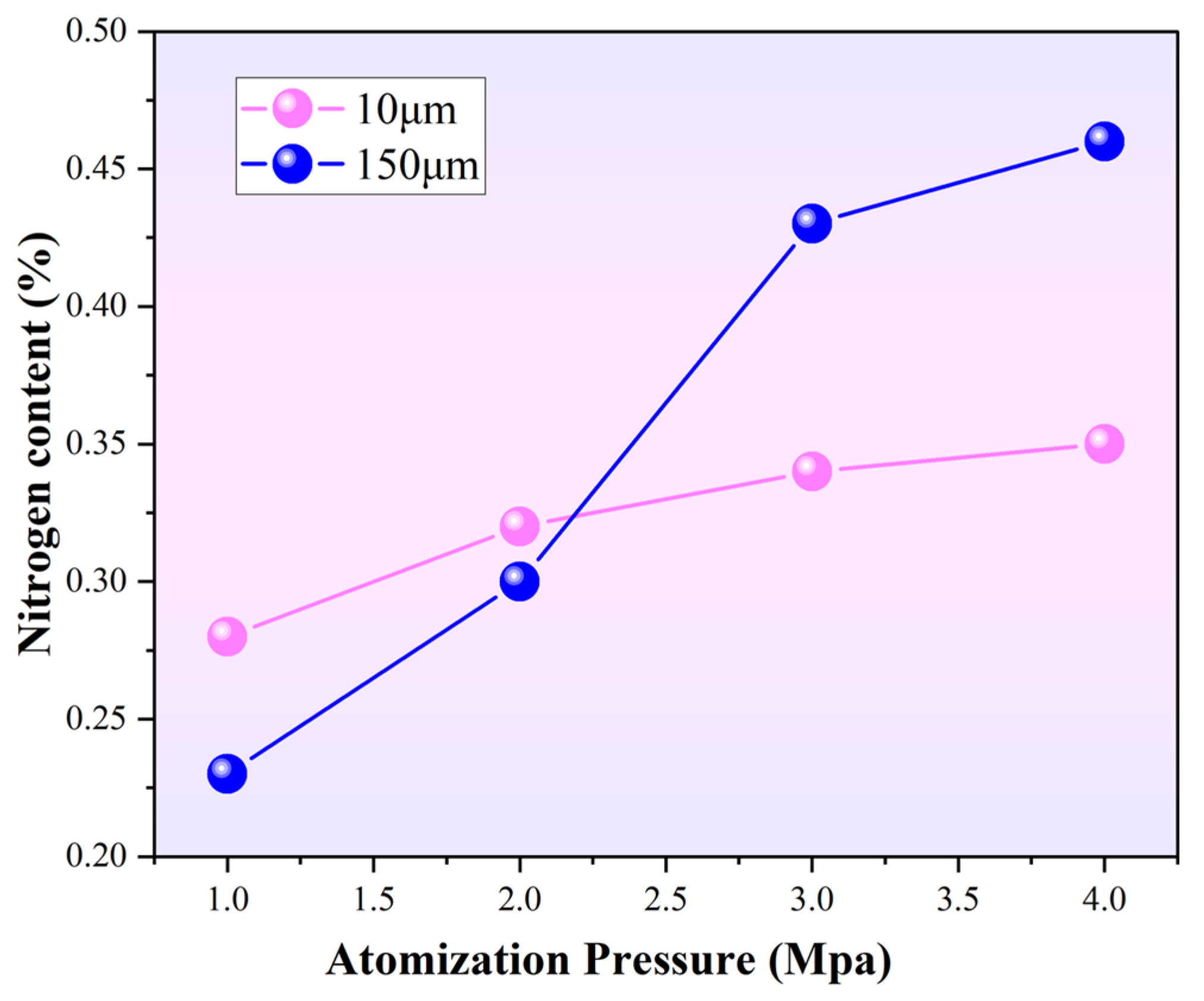
Figure 14 illustrates the variation in nitrogen content with particle size under different atomization pressures. For 10 μm powder, nitrogen content does not changes little at ~0.33 wt%. For the powders with a particle size of 150 μm, the nitrogen content increases to 0.23%, 0.30%, 0.43%, and 0.46% respectively, with the increase of atomization pressure. The powder surface can adsorb more free nitrogen during the atomization process when there is a large surface area on the particle. When the atomization pressure is low, finer powders have higher solidification rates, thus inhibiting the precipitation behavior of nitrogen. The nitrogen content decreases with the increase of powder particle size before 2.2 MPa. When the atomization pressure is more than 2.2 MPa and continually increases, the nitrogen content increases with the increase of powder particle size. Meanwhile, the enhancement of the kinetic energy of nitrogen gas may strengthen the energy exchange between the nitrogen gas and the liquid metal droplets. So, there is an increase in the nitrogen content. It can be inferred that the influence of specific surface area on nitrogen adsorption is less than that of solidification rate on the powder.
The nitrogen enhancement effect originates from nitrogen dissociation into atomic nitrogen during efficient atomization, accompanied by kinetic and surface energy conversion [
23]. The dissociation and solidification behavior of nitrogen is a complex process. The nitrogen content of particles can be changed by controlling the particle size and atomization pressure. According to Dombrowski’s wave fragmentation model [
24], the key step of the increase of nitrogen content is the melting process, not the atomization process. The main role is to accelerate the solidification of atomized droplets, thereby inhibiting the precipitation of nitrogen. In addition, high chamber pressure in the atomization furnace chamber can offer high melting pressure and solidification pressure to improve the solubility and inhibit precipitation of nitrogen, respectively. Thus, benefiting from the synergetic effect between atomization pressure and chamber pressure, the high nitrogen content powder can be finally obtained.
4.4. Formation of Satellite Powders
Satellite sphere particles are formed due to the differences in the solid-liquid morphology of the droplets and the formation of rapid collisions [
25]. As shown in
Figure 15, satellite sphere particles include three types: the first one is the point contact type shown in
Figure 15a. The point contact satellite sphere is generated at the bottom of the atomization zone. When the metal droplet is about to solidify, it rapidly collides with other powders that are about to solidify or have already solidified. The second one is the surface contact type shown in
Figure 15b; a surface contact type satellite sphere is generated in the middle of the atomized zone. During the solidification stage of the metal droplet, it undergoes rapid collisions with other powders that are about to solidify or have already solidified. The third one is the parcel type shown in
Figure 15c. The encapsulated satellite spheres are generated in the upper part of the atomization zone. When the metal droplet is in the liquid stage, it undergoes rapid collisions with the solidified powders.
A recirculation zone, in which the particles are cooled at a slow rate, is formed due to the collision of nitrogen gas in the direction of the gas atomization nozzle. Most particles have temperatures more than the solidification point. Many particles have the opportunity to collide and produce satellite spheres. The solidification rate of particle near the nozzle outlet is relatively fast due to the low temperature in the vicinity of the nozzle outlet. Droplets with different sizes have different physical sensible heat. After cooling in the recirculation zone, there are three types of droplets with different temperatures [
25]: high-temperature droplets, higher temperature droplets, and cryonic droplets. When cryonic droplets collides each other, they will produce point contact powders. As a cryonic droplet runs into a higher temperature droplet, a surface contact powder will be formed. Similarly, a parcel contact powder will grow up on the condition of collision between high temperature droplet and cryonic droplet. As shown in
Figure 9, the number of satellite spheres significantly increases with the increase of powder particle size. It can be inferred that satellite spheres on the powder surface can be reduced by controlling of atomization parameters to achieve a specific powder particle size range.