A Comparative Study of Homogeneous and Heterogeneous Catalyzed Glycerol Acetylation with Acetic Acid: Activity, Selectivity, and Stability
Abstract
1. Introduction
| Catalyst | HAc/Gly a | Temp (°C) | Time (h) | X (%) | S (%) b | Recycles | Ref |
|---|---|---|---|---|---|---|---|
| A15 | 9/1 | 110 | 4.5 | 97.1 | 92.2 | Not Performed | [25] |
| H-USY | 78.4 | 26.2 | Not Performed | ||||
| H-ZSM-5 | 85.6 | 33.4 | Not Performed | ||||
| A15 | 6/1 | 105 | 4.0 | 98.6 | 25.0 * | Not Performed | [18] |
| H-Y | 6/1 | 105 | 4.0 | 80.9 | 3.2 * | Not Performed | |
| H-ZSM-5 | 6/1 | 105 | 4.0 | 75.7 | 2.5 * | Not Performed | |
| Sulf-SBA-15 | 6/1 | 120 | 4.5 | 100 | 70.0 | Not Performed | [40] |
| H-ZSM-5 AT | 6/1 | 120 | 91.0 | 52.0 | Not Performed | ||
| PMo3_Na-USY | 15/1 | 120 | 3 | 68.0 | 61.0 | 5 | [23] |
| PW2_AC | 15/1 | 120 | 3 | 86.0 | 74.0 | 4 | [24] |
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.3. Catalytic Tests
3. Results
3.1. Structure Characterization
3.2. Acidity Characterization
3.2.1. Temperature-Programmed Desorption (TPD) of Pyridine and Collidine
3.2.2. Pyridine FTIR Studies
OH Region
Pyridine Adsorbed Region
3.3. Reactions Catalyzed by Acetic Acid (Autocatalytic)
3.4. Reactions Catalyzed by Solid Acids
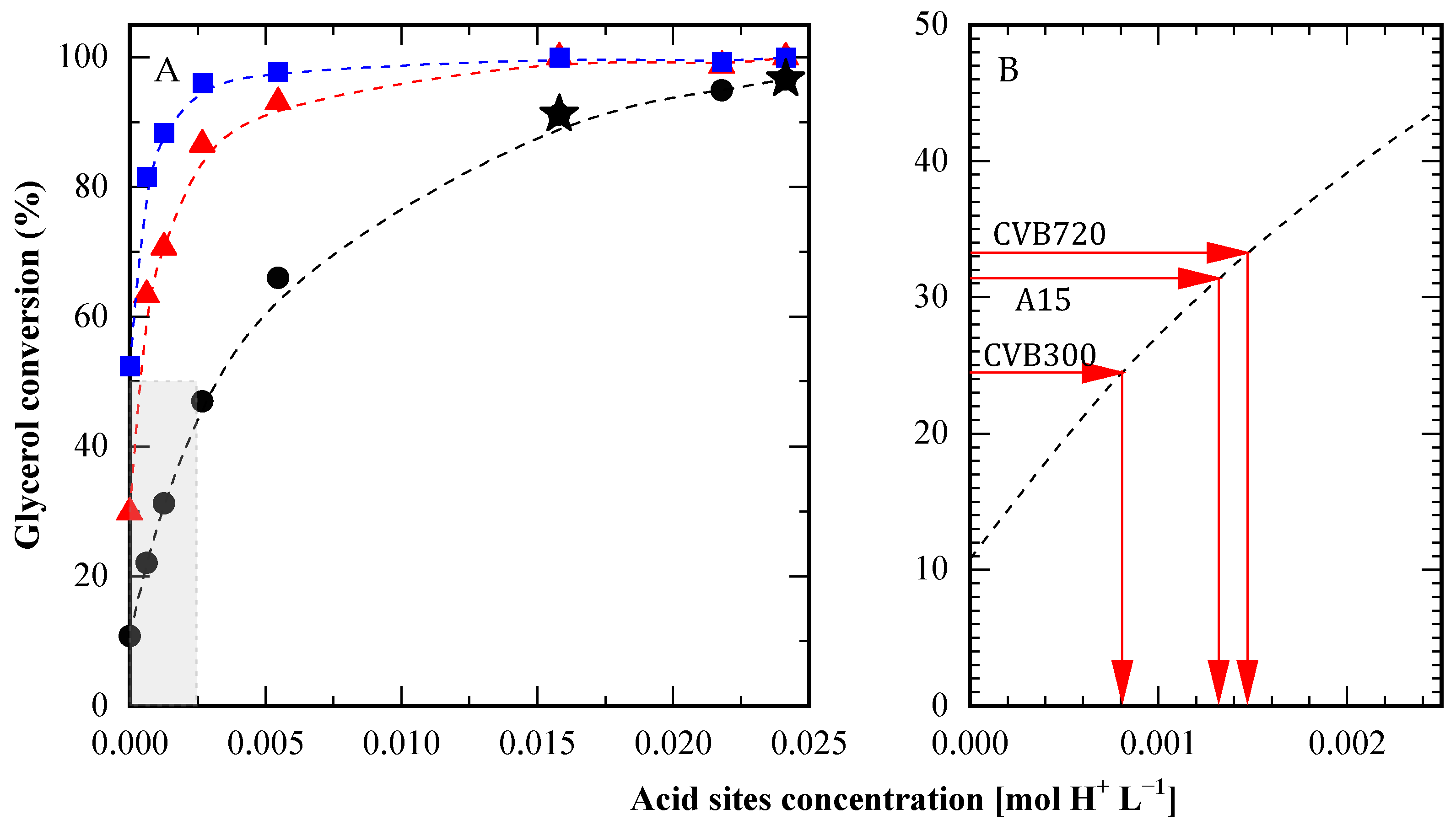
3.5. Comparison Between Heterogeneous and Homogeneous Catalysts
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reagents Molar Ratio HAc/Gly | X (%) (5 h) | X (%) (24 h) |
|---|---|---|
| 3/1 | 84.6 | 91.2 |
| 6/1 | 91.0 | 95.5 |
| 12/1 | 98.7 | 100.0 |
| Reagents Molar Ratio HAc/Gly | X: 10 % | X: 80 % | Final | ||
|---|---|---|---|---|---|
| Time (min) * | SDAG + STAG (%) | Time (min) * | STAG + SDAG (%) | SDAG + STAG (%) | |
| 3/1 | 13 | 4.0 [0] | 240 | 39.3 [3.45] | 61.3 [10.8] |
| 6/1 | 10 | 6.0 [0] | 240 | 39.6 [5.10] | 78.8 [24.1] |
| 12/1 | 4 | 4.0 [0] | 120 | 45.6 [4.76] | 92.3 [45.9] |
| TOF (h−1) (t = 30 min) | X (%) (t = 30 min) | X (%) (t = 24 h) | STAG (%) (t = 24 h) | SDAG + STAG (%) (t = 24 h) | |
|---|---|---|---|---|---|
| A15 | 120.5 | 52.00 | 100 | 29.42 | 82.50 |
| CBV720 | 113.3 | 48.70 | 98.50 | 27.71 | 80.90 |
| PTSA | 231.5 | 98.30 | 100 | 31.62 | 84.90 |



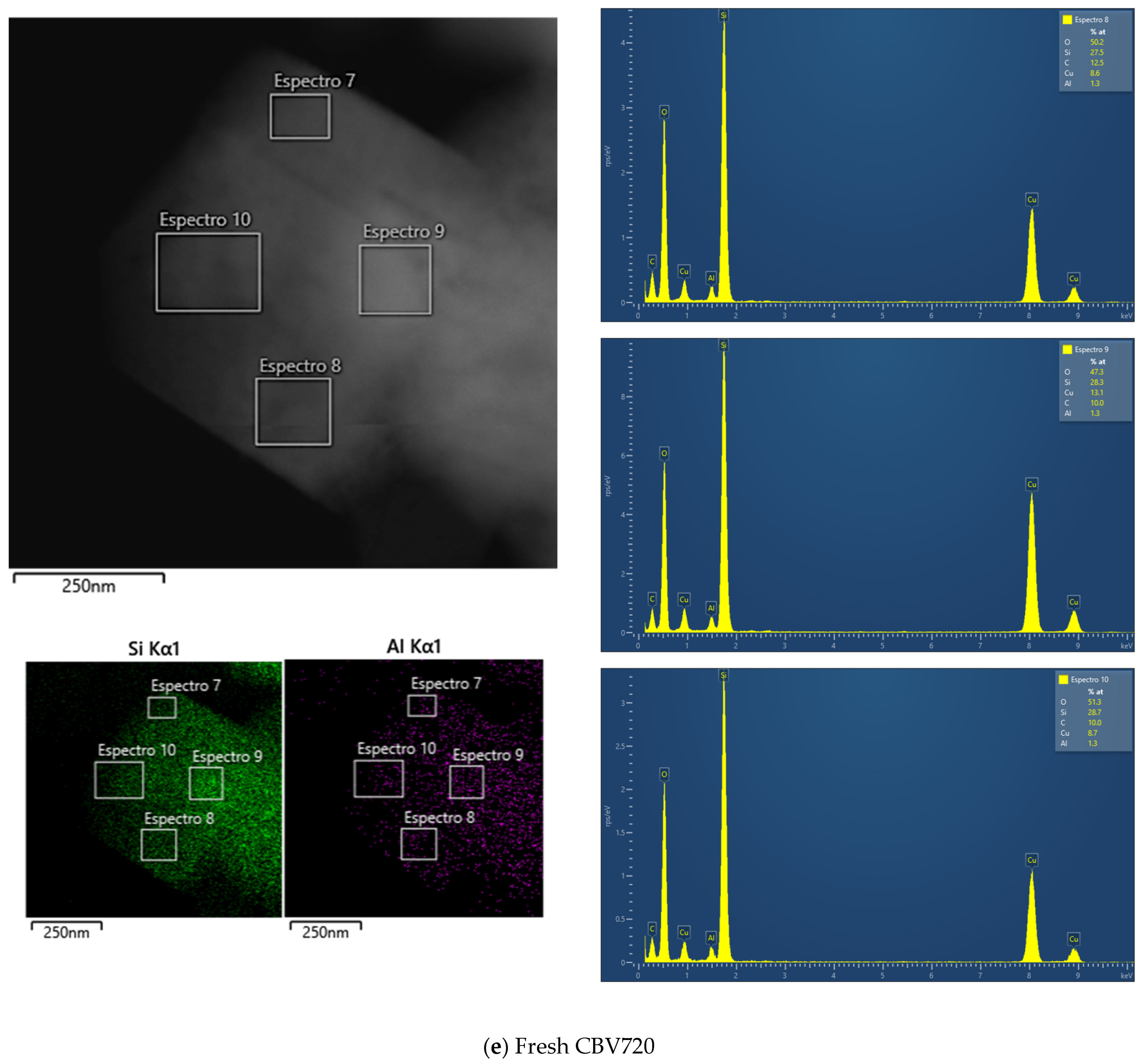
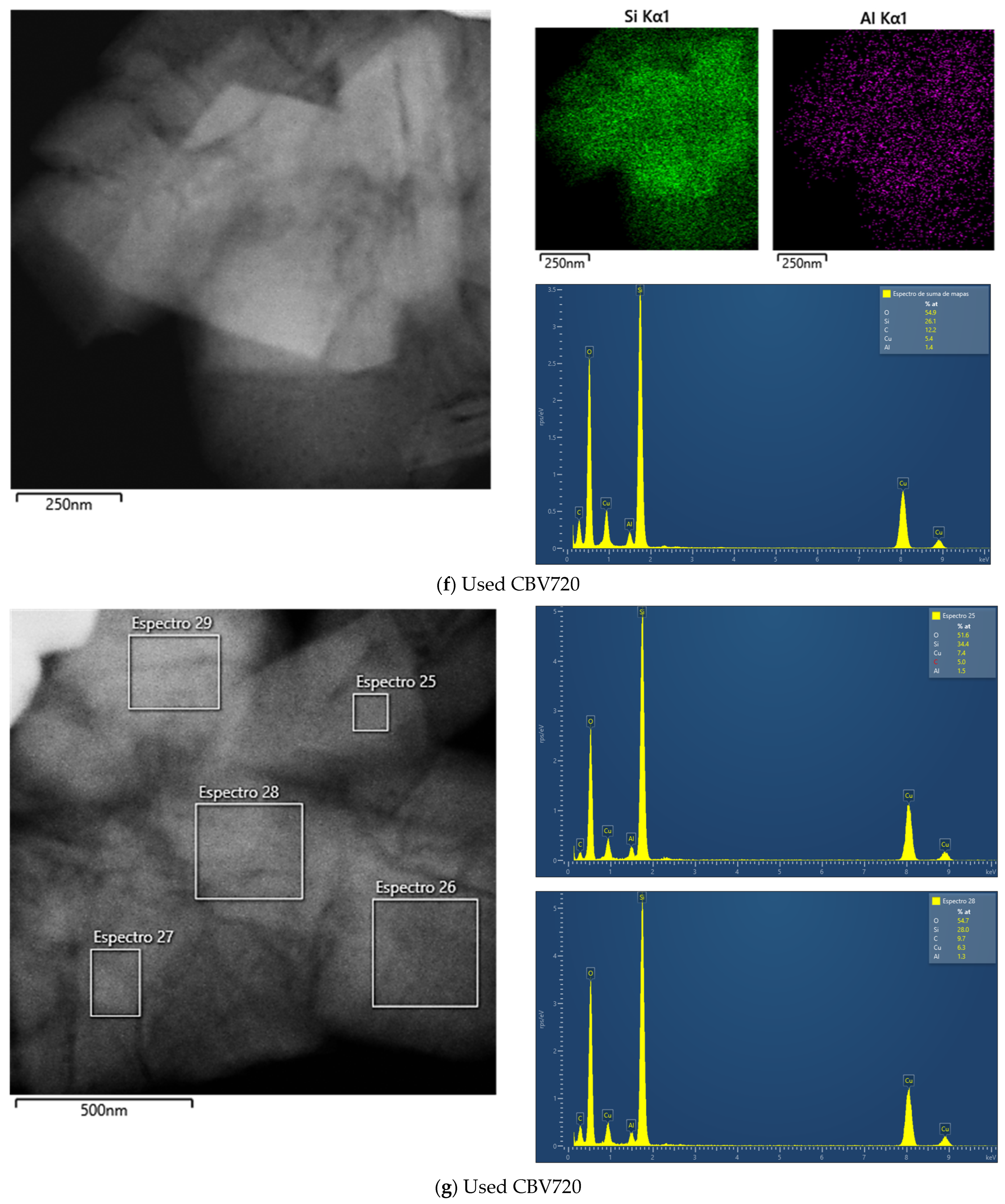
Appendix B
Appendix B.1. Textural Properties—Procedure Details
Appendix B.2. Pyridine-TPD and FTIR Procedure Details
Appendix B.2.1. Temperature-Programmed Desorption (TPD) Measurements
Appendix B.2.2. FTIR Measurements
References
- Khan, H.M.; Ali, C.; Iqbal, T.; Yasin, S.; Sulaiman, M.; Mahmood, H.; Raashid, M.; Pasha, M.; Mu, B. Current scenario and potential of biodiesel production from waste cooking oil in Pakistan: An overview. Chin. J. Chem. Eng. 2019, 27, 2238–2250. [Google Scholar] [CrossRef]
- Bozbas, K. Biodiesel as an alternative motor fuel: Production and policies in the European Union. Renew. Sust. Energ. Rev. 2008, 12, 542–552. [Google Scholar] [CrossRef]
- Zhou, H.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Hussein, R.; Ong, M.Y. Sustainability of biodiesel production in Malaysia by production of bio-oil from crude glycerol using microwave pyrolysis: A review. Green Chem. Lett. Rev. 2018, 11, 135–157. [Google Scholar] [CrossRef]
- Bansod, Y.; Ghasemzadeh, K.; D’Agostino, C. Techno-economic assessment of biodiesel-derived crude glycerol purification processes. RSC Sustain. 2025, 3, 2605–2618. [Google Scholar] [CrossRef]
- Kenkel, P.; Holcomb, R. Feasibility of On-Farm or Small Scale Oilseed Processing and Biodiesel Production in Integration of Agricultural and Energy Systems. In Proceedings of the Farm Foundation Conference, Oak Brook, IL, USA, 12–13 February 2008. [Google Scholar]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sust. Energ. Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.; Dabdawb, W.; Mansouri, N. Biodiesel -Derived Raw Glycerol to Value-Added Products: Catalytic Conversion Approach. In Handbook of Composites from Renewable Materials, 1st ed.; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2017; Volume 3, pp. 309–365. [Google Scholar] [CrossRef]
- Garcia, E.; Laca, M.; Perez, E.; Garrido, A.; Peinado, J. New class of acetal derived from glycerin as a biodiesel fuel component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- EN 14214; Automotive Fuels - Fatty acid Methyl Esters (FAME) for Diesel Engines - Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2019.
- ASTM D6751; Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2001.
- Mukhopadhyay, P.; Chakraborty, R.; Singh, S. Triacetin additive in biodiesel to reduce air pollution: A review. Environ. Chem. Lett. 2022, 20, 1193–1224. [Google Scholar] [CrossRef]
- Konwar, L.J.; Mäki-Arvela, P.; Begum, P.; Kumar, N.; Thakur, A.J.; Mikkola, J.-P.; Deka, R.C.; Deka, D. Shape selectivity and acidity effects in glycerol acetylation with acetic anhydride: Selective synthesis of triacetin over Y-zeolite and sulfonated mesoporous carbons. J. Catal. 2015, 329, 237–247. [Google Scholar] [CrossRef]
- Okoye, P.; Abdullah, A.; Hameed, B. A review on recent developments and progress in the kinetics and deactivation of catalytic acetylation of glycerol—A byproduct of biodiesel. Renew. Sust. Energ. Rev. 2017, 74, 387–401. [Google Scholar] [CrossRef]
- Mufrodi, Z.; Rochmadi, S.; Budiman, A. Chemical Kinetics for Synthesis of Triacetin from Biodiesel Byproduct. Int. J. Chem. 2012, 4, 101. [Google Scholar] [CrossRef]
- Khramov, M. Process for Production and Purification of Triacetin. US Patent 5777157, 7 July 1998. [Google Scholar]
- Melero, J.; van Grieken, R.; Morales, G.; Paniagua, M. Acidic Mesoporous Silica for the Acetylation of Glycerol: Synthesis of Bioadditives to Petrol Fuel. Energy Fuels 2007, 21, 1782–1791. [Google Scholar] [CrossRef]
- Liao, X.; Zhu, Y.; Wang, S.-G.; Li, Y. Producing triacetylglycerol with glycerol by two steps: Esterification and acetylation. Fuel Process. Technol. 2009, 90, 988–993. [Google Scholar] [CrossRef]
- De Canck, E.; Dosuna-Rodríguez, I.; Gaigneaux, E.M.; Van Der Voort, P. Periodic Mesoporous Organosilica Functionalized with Sulfonic Acid Groups as Acid Catalyst for Glycerol Acetylation. Materials 2013, 6, 3556–3570. [Google Scholar] [CrossRef]
- Ghoreishi, K.; Asim, N.; Yarmo, M.; Samsudin, M. Mesoporous phosphated and sulphated silica as solid acid catalysts for glycerol acetylation. Chem. Pap. 2014, 68, 1194–1204. [Google Scholar] [CrossRef]
- Chamack, M.; Mahjoub, A.; Akbari, A. Zirconium-modified mesoporous silica as an efficient catalyst for the production of fuel additives from glycerol. Catal. Commun. 2018, 110, 1–4. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Acetylation of glycerol to biofuel additives over sulfated activated carbon catalyst. Bioresour. Technol. 2011, 102, 9229–9235. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Esterification of glycerol with acetic acid over dodecamolybdophosphoric acid encaged in USY zeolite. Catal. Commun. 2009, 10, 481–484. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Acetylation of glycerol over heteropolyacids supported on activated carbon. Catal. Commun. 2011, 12, 573–576. [Google Scholar] [CrossRef]
- Malaika, A.; Kozłowski, M. Glycerol conversion towards valuable fuel blending compounds with the assistance of SO3H-functionalized carbon xerogels and spheres. Fuel Process. Technol. 2019, 184, 19–26. [Google Scholar] [CrossRef]
- Kong, P.S.; Aroua, M.; Daud, W.; Lee, H.; Cognet, P.; Pérès, Y. Catalytic role of solid acid catalysts in glycerol acetylation for the production of bio-additives: A review. RSC Adv. 2016, 6, 68885–68905. [Google Scholar] [CrossRef]
- Zhou, L.; Al-Zaini, E.; Adesina, A. Catalytic characteristics and parameters optimization of the glycerol acetylation over solid acid catalysts. Fuel 2013, 103, 617–625. [Google Scholar] [CrossRef]
- Bedogni, G.; Acevedo, M.; Aguzín, F.; Okulik, N.; Padró, C. Synthesis of bioadditives of fuels from biodiesel-derived glycerol by esterification with acetic acid on solid catalysts. Environ. Technol. 2018, 39, 1955–1966. [Google Scholar] [CrossRef]
- Almas, Q.; Sievers, C.; Jones, C. Role of the mesopore generation method in structure, activity and stability of MFI catalysts in glycerol acetylation. Appl. Catal. A-Gen. 2019, 571, 107–117. [Google Scholar] [CrossRef]
- Dalla Costa, B.; Decolatti, H.; Legnoverde, S.; Querini, C. Influence of acidic properties of different solid acid catalysts for glycerol acetylation. Catal. Today 2017, 289, 222–230. [Google Scholar] [CrossRef]
- Goncalves, V.L.C.; Pinto, B.P.; Silva, J.C.; Mota, C.J.A. Acetylation of glycerol catalyzed by different solid acids. Catal. Today 2008, 133–135, 673–677. [Google Scholar] [CrossRef]
- Gomes, G.C.; Zalazar, M.F.; Arroyo, P.A. New Insights into the Effect of the Zeolites Framework Topology on the Esterification Reactions: A Comparative Study from Experiments and Theoretical Calculations. Top. Catal. 2022, 65, 871–886. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Y.; Wen, G.; Xu, L.; Ding, Q.; Guan, Y.; Yang, Z.; Sun, Y.; Gao, X.; Zhang, J.; et al. Cs exchanged 12-tungstophosphoric acid supported on high-silica mesoporous Y zeolites for synthesis of ethyl lactate via catalytic esterification. Biomass Bioenerg. 2022, 165, 106552. [Google Scholar] [CrossRef]
- Chaowamalee, S.; Yan, N.; Ngamcharussrivichai, C. Propylsulfonic Acid-Functionalized Mesostructured Natural Rubber/Silica Nanocomposites as Promising Hydrophobic Solid Catalysts for Alkyl Levulinate Synthesis. Nanomaterials 2022, 12, 604. [Google Scholar] [CrossRef]
- Gautam, P.; Barman, S.; Ali, A. Catalytic Synthesis of Energy-rich Fuel Additive Levulinate Esters from Levulinic Acid using Modified Ultra-stable Zeolite Y. Chem. Eur. 2022, 7, e202203044. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, S.; Raja, D.; Binti Khusni, N.; Liub, J.; Zhang, J.; Abdulridha, S.; Xiang, H.; Jianga, S.; Guan, Y.; et al. On the effect of mesoporosity of FAU Y zeolites in the liquid-phase catalysis. Micropor. Mesopor. Mat. 2019, 278, 297–306. [Google Scholar] [CrossRef]
- Dal Pozzo, D.M.; Azevedo dos Santos, J.A.; Seabra Junior, E.; Ferreira Santos, R.; Feiden, A.; Melegari de Souza, S.N.; Burgardta, I. Free fatty acids esterification catalyzed by acid Faujasite type zeolite. RSC Adv. 2019, 9, 4900–4907. [Google Scholar] [CrossRef]
- Prinsen, P.; Luque, R.; González-Arellano, C. Zeolite catalyzed palmitic acid esterification. Micropor. Mesopor. Mat. 2018, 262, 133–139. [Google Scholar] [CrossRef]
- Freitas, E.F.; Araújo, A.A.L.; Paiva, M.F.; Dias, S.C.L.; Dias, J.A. Comparative acidity of BEA and Y zeolite composites with 12-tungstophosphoric and 12-tungstosilicic acids. Mol. Catal. 2018, 458, 152–160. [Google Scholar] [CrossRef]
- Baroi, C.; Mahto, S.; Niu, C.; Dalai, A.K. Biofuel production from green seed canola oil using zeolites. App. Catal. A-Gen. 2014, 469, 18–32. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, R.; Ye, B.; Hou, Z. Acetalization of glycerol over sulfated UiO-66 under mild condition. J. Ind. Eng. Chem. 2022, 110, 357–366. [Google Scholar] [CrossRef]
- Tonutti, L.G.; Decolatti, H.P.; Querini, C.A.; Dalla Costa, B.O. Hierarchical H-ZSM-5 zeolite and sulfonic SBA-15: The properties of acidic H and behavior in acetylation and alkylation reactions. Micropor. Mesopor. Mat. 2020, 305, 110284. [Google Scholar] [CrossRef]
- Tonutti, L.G.; Vergara, L.; Querini, C.A.; Dalla Costa, B.O. On the Location and Accessibility of Active Acid Sites in MFI Zeolites Modified by Alkaline Treatment. Processes 2024, 12, 2567. [Google Scholar] [CrossRef]
- Tonutti, L.G.; Dalla Costa, B.O.; Decolatti, H.P.; Mendow, G.; Querini, C.A. Determination of kinetic constants for glycerol acetylation by particle swarm optimization algorithm. Chem. Eng. J. 2021, 424, 130408. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rac, V.; Rakic, V.; Miladinovic, Z.; Stosic, D.; Auroux, A. Influence of the desilication process on the acidity of HZSM-5 zeolite. Thermochim. Acta 2013, 567, 73–78. [Google Scholar] [CrossRef]
- Sachse, A.; Grau-Atienza, A.; Jardim, E.O.; Linares, N.; Thommes, M.; García-Martínez, J. Development of Intracrystalline Mesoporosity in Zeolites through Surfactant-Templating. Cryst. Growth Des. 2017, 17, 4289–4305. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Gies, H.; Marler, B. Database of Zeolite Structures. Available online: https://www.iza-structure.org/databases/ (accessed on 22 September 2025).
- Anderson, M.W.; Klinowski, J. Zeolites treated with silicon tetrachloride vapour. IV. Acidity. Zeolites 1986, 6, 455–466. [Google Scholar] [CrossRef]
- Montanari, T.; Finocchio, E.; Busca, G. Infrared Spectroscopy of Heterogeneous Catalysts: Acidity and Accessibility of Acid Sites of Faujasite-Type Solid Acids. J. Phys. Chem. C 2011, 115, 937–943. [Google Scholar] [CrossRef]
- Daniell, W.; Topsøe, N.-Y.; Knözinger, H. An FTIR Study of the Surface Acidity of USY Zeolites: Comparison of CO, CD3CN, and C5H5N Probe Molecules. Langmuir 2001, 17, 6233–6239. [Google Scholar] [CrossRef]
- Nesterenko, N.S.; Thibault-Starzyk, F.; Montouilliout, V.; Yushchenko, V.V.; Fernandez, C.; Gilson, J.-P.; Fajula, F.; Ivanova, I.I. The use of the consecutive adsorption of pyridine bases and carbon monoxide in the IR spectroscopic study of the accessibility of acid sites in microporous/mesoporous materials. Kinet. Catal. 2006, 47, 40–48. [Google Scholar] [CrossRef]
- Castellà-Ventura, M.; Akacem, Y.; Kassab, E. Vibrational Analysis of Pyridine Adsorption on the Brønsted Acid Sites of Zeolites Based on Density Functional Cluster Calculations. J. Phys. Chem C. 2008, 112, 19045–19054. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Kassab, E.; Castellà-Ventura, M. Theoretical Study of Pyridine and 4,4‘-Bipyridine Adsorption on the Lewis Acid Sites of Alumina Surfaces Based on Ab Initio and Density Functional Cluster Calculations. J. Phys. Chem. B 2005, 109, 13716–13728. [Google Scholar] [CrossRef]
- Yasuda, H.; Sato, T.; Yoshimura, Y. Influence of the acidity of USY zeolite on the sulfur tolerance of Pd–Pt catalysts for aromatic hydrogenation. Catal. Today 1999, 50, 63–71. [Google Scholar] [CrossRef]
- Arribas, M.A.; Martinez, A. The influence of zeolite acidity for the coupled hydrogenation and ring opening of 1-methylnaphthalene on Pt/USY catalysts. Appl. Catal. A-Gen. 2002, 230, 203–217. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Zhao, H.; Hou, Z. Esterification of glycerol with acetic acid over SO3H-functionalized phenolic resin. Fuel 2019, 255, 115842. [Google Scholar] [CrossRef]
- Reddy, P.S.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Selective acetylation of glycerol over CeO2–M and SO42−/CeO2–M (M = ZrO2 and Al2O3) catalysts for synthesis of bioadditives. J. Ind. Eng. Chem. 2012, 18, 648–654. [Google Scholar] [CrossRef]
- Kale, S.; Umbarkar, S.B.; Dongare, M.K.; Eckelt, R.; Armbruster, U.; Martin, A. Selective formation of triacetin by glycerol acetylation using acidic ion-exchange resins as catalyst and toluene as an entrainer. Appl. Catal. A-Gen. 2015, 490, 10–16. [Google Scholar] [CrossRef]
- Vieira Grossi, C.; de Oliveira Jardim, E.; de Araújo, M.H.; Montero Lago, R.; da Silva, M. Sulfonated polystyrene: A catalyst with acid and superabsorbent properties for the esterification of fatty acids. Fuel 2010, 89, 257–259. [Google Scholar] [CrossRef]
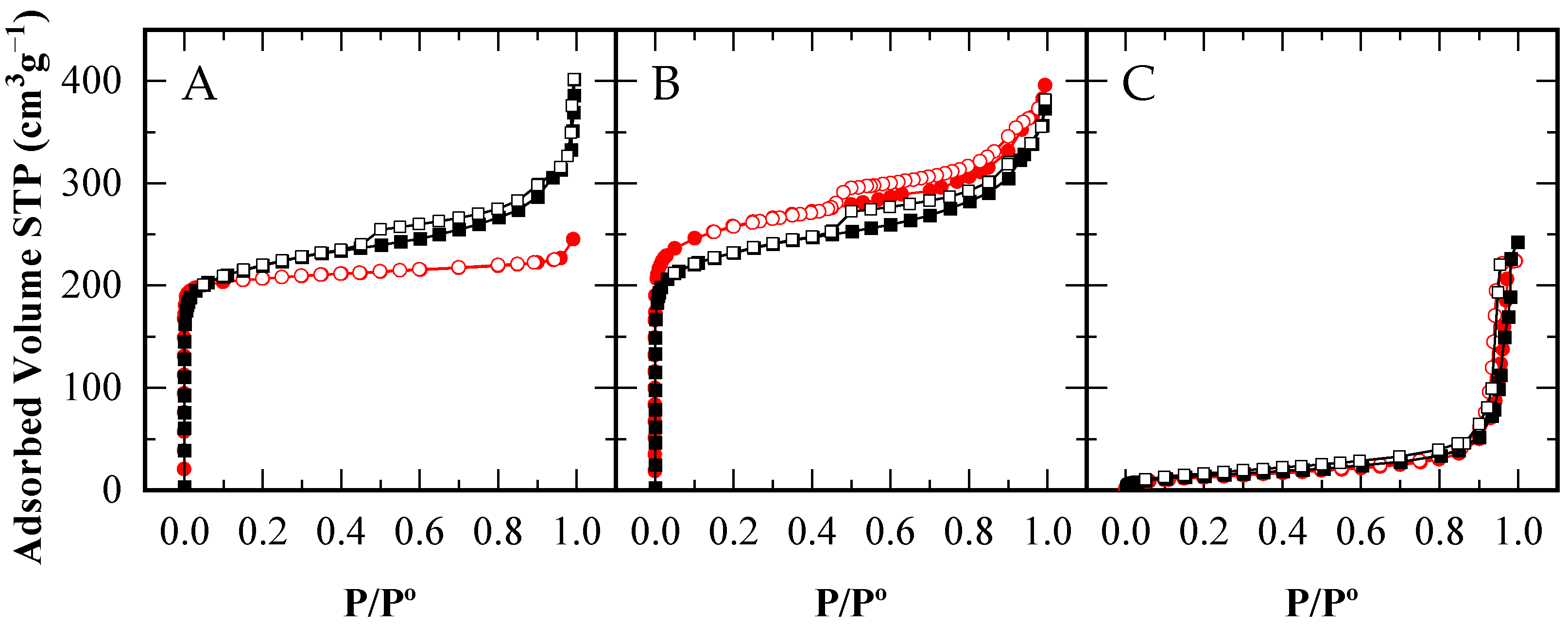
 ) and after their use in reaction (■□). Adsorption branches in filled symbols (
) and after their use in reaction (■□). Adsorption branches in filled symbols ( ) and desorption branches in open symbols (
) and desorption branches in open symbols ( ).
).
 ) and after their use in reaction (■□). Adsorption branches in filled symbols (
) and after their use in reaction (■□). Adsorption branches in filled symbols ( ) and desorption branches in open symbols (
) and desorption branches in open symbols ( ).
).





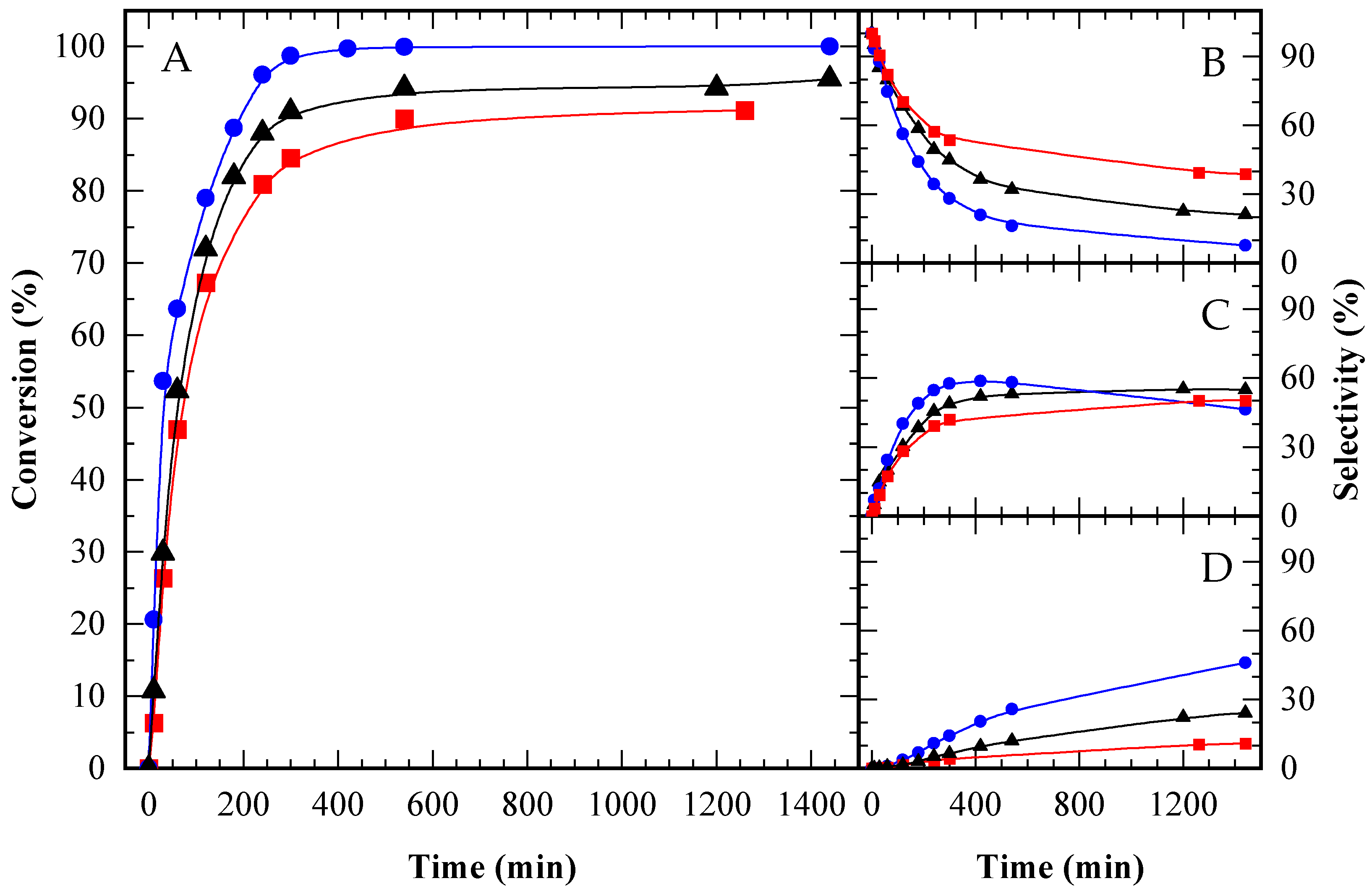
 ), CBV100 (★), and the autocatalytic run (▲), under an equal acid load of 9.5 x 10−2 mmol H+ (g glycerol)−1. HAc/Gly molar ratio: 6/1.
), CBV100 (★), and the autocatalytic run (▲), under an equal acid load of 9.5 x 10−2 mmol H+ (g glycerol)−1. HAc/Gly molar ratio: 6/1.
 ), CBV100 (★), and the autocatalytic run (▲), under an equal acid load of 9.5 x 10−2 mmol H+ (g glycerol)−1. HAc/Gly molar ratio: 6/1.
), CBV100 (★), and the autocatalytic run (▲), under an equal acid load of 9.5 x 10−2 mmol H+ (g glycerol)−1. HAc/Gly molar ratio: 6/1.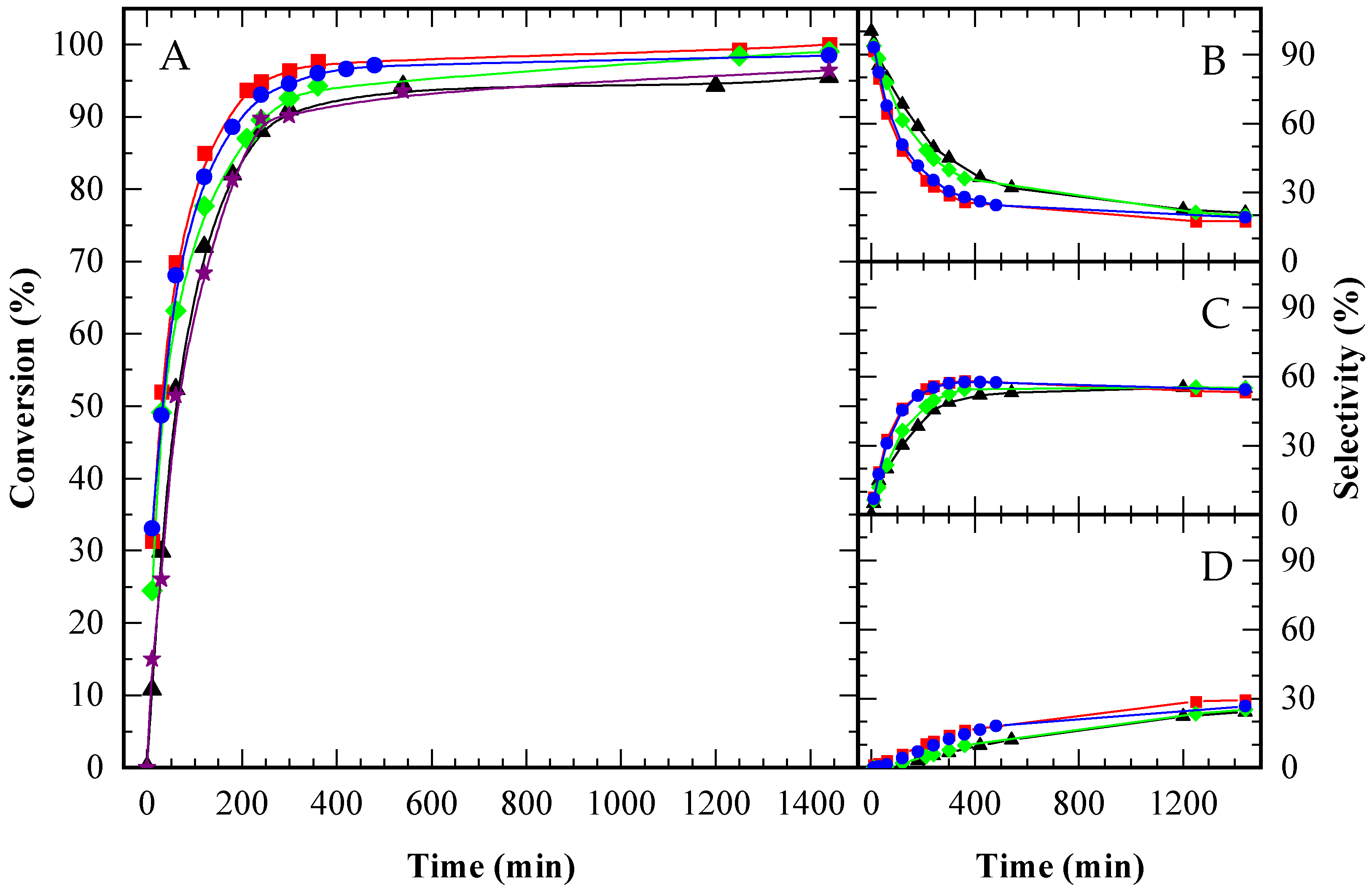
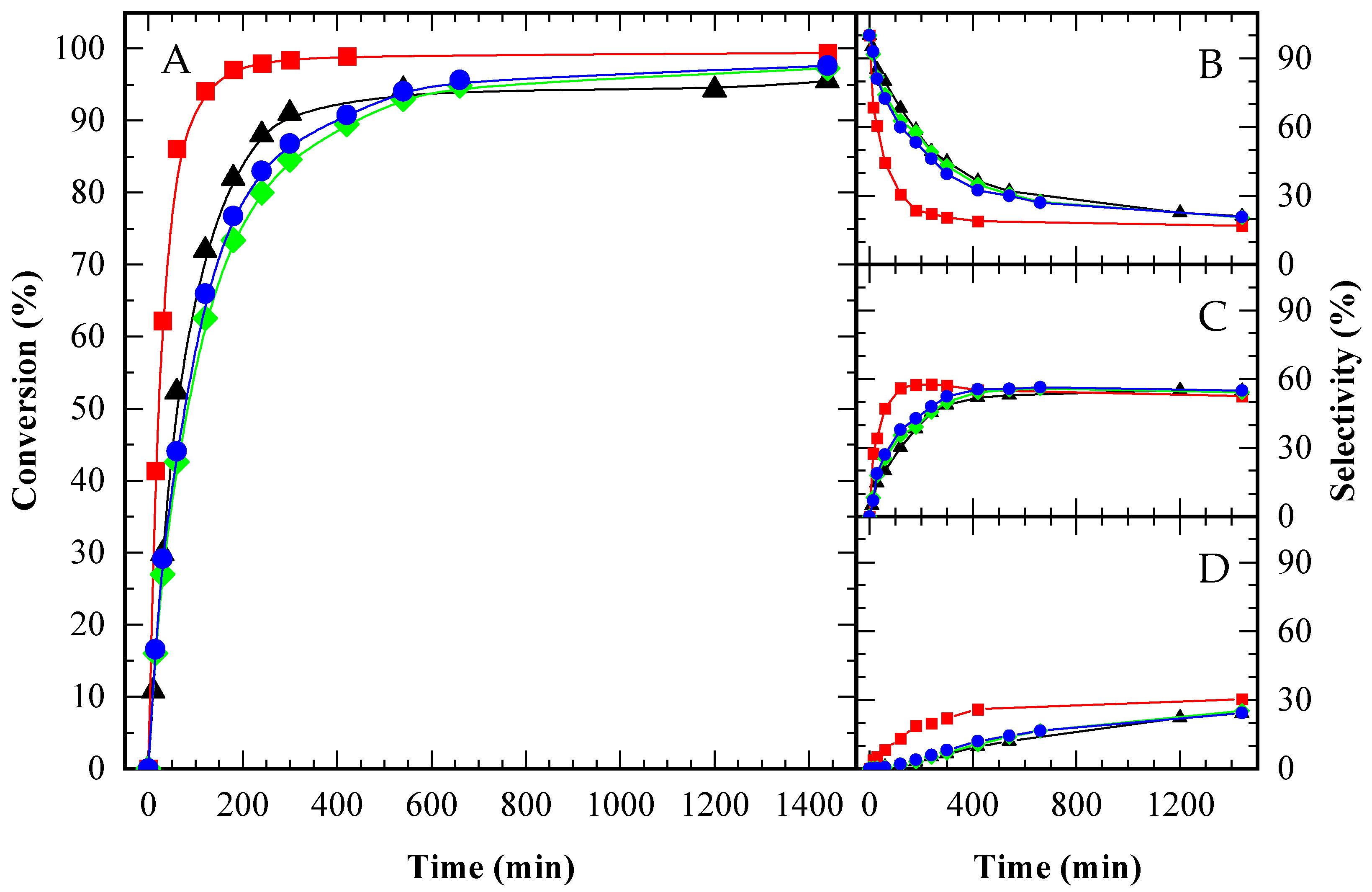
 ) and TAG (
) and TAG ( ) for A15 (A), CBV720 (B) and CBV300 (C). First test (solid symbols, continuous lines) and reuse test (empty symbols, dashed lines). HAc/Gly molar ratio: 6/1, 9.5 × 10−2 mmol H+ (g glycerol)−1.
) for A15 (A), CBV720 (B) and CBV300 (C). First test (solid symbols, continuous lines) and reuse test (empty symbols, dashed lines). HAc/Gly molar ratio: 6/1, 9.5 × 10−2 mmol H+ (g glycerol)−1.
 ) and TAG (
) and TAG ( ) for A15 (A), CBV720 (B) and CBV300 (C). First test (solid symbols, continuous lines) and reuse test (empty symbols, dashed lines). HAc/Gly molar ratio: 6/1, 9.5 × 10−2 mmol H+ (g glycerol)−1.
) for A15 (A), CBV720 (B) and CBV300 (C). First test (solid symbols, continuous lines) and reuse test (empty symbols, dashed lines). HAc/Gly molar ratio: 6/1, 9.5 × 10−2 mmol H+ (g glycerol)−1.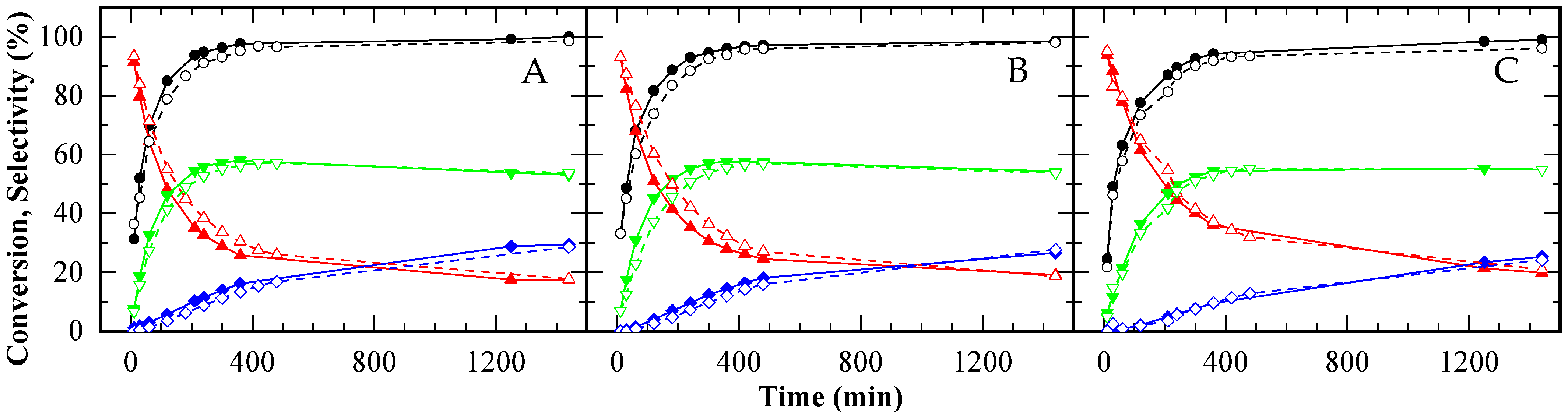

| Sample | Fresh Catalyst | After Reaction | ||||||
|---|---|---|---|---|---|---|---|---|
| Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Dp (nm) | Total Acid Sites (mmol g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Dp (nm) | Total Acid Sites (mmol g−1) | |
| A15 | - | - | 30 | 4.7 * | - | - | 30 | n.d |
| CBV300 | 0.32 | 0.06 | 1.17 | 0.94 ** | 0.34 | 0.09 | 1.17 | 0.62 |
| CBV720 | 0.37 | 0.24 | 1.22 | 0.36 ** | 0.32 | 0.28 | 1.22 | 0.32 |
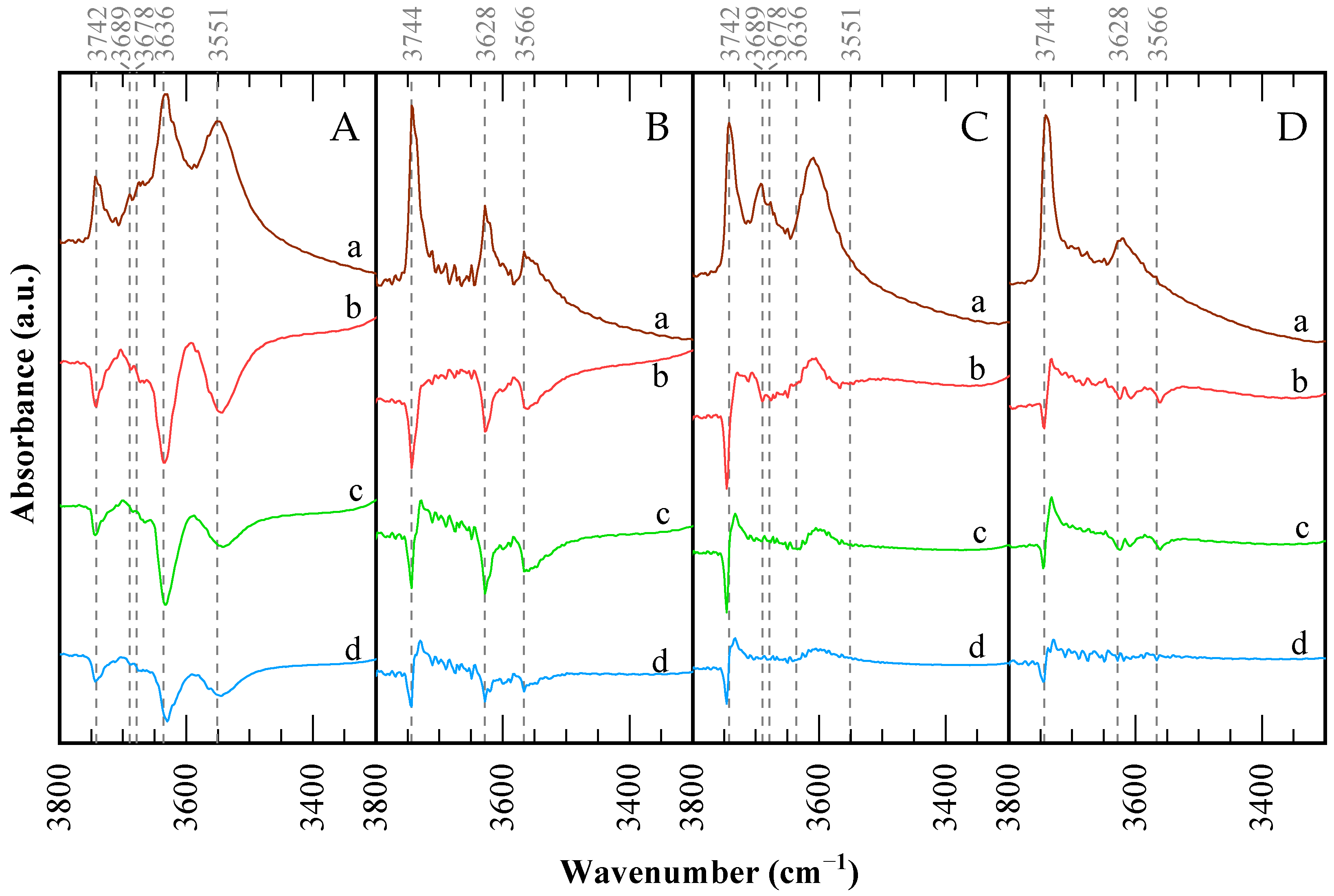
| Pyridine (mmol g−1) | Collidine (mmol g−1) | Col/Py (%) | ||
|---|---|---|---|---|
| CBV300 | Calcined | 0.938 | 0.580 | 61.8 |
| Used | 0.619 | 0.566 | 91.4 | |
| Used/Calcined (%) | 66.0 | 97.6 | - | |
| CBV720 | Calcined | 0.361 | 0.250 | 69.3 |
| Used | 0.324 | 0.259 | 79.9 | |
| Used/Calcined (%) | 89.8 | ~100 | - |
| Strength | Br/Lw | Br (μmol g−1) | Lw (μmol g−1) | |
|---|---|---|---|---|
| CBV300 | Weak | 2.192 | 344 | 157 |
| Medium | 7.391 | 396 | 54 | |
| Strong | 0.955 | 166 | 174 | |
| CBV720 | Weak | 3.539 | 43 | 12 |
| Medium | 5.764 | 78 | 14 | |
| Strong | 3.751 | 142 | 38 | |
| used CBV300 | Weak | 1.243 | 337 | 271 |
| Medium | 1.294 | 85 | 66 | |
| Strong | 0.953 | 91 | 95 | |
| used CBV720 | Weak | 1.068 | 126 | 118 |
| Medium | 1.330 | 93 | 70 | |
| Strong | 0.450 | 26 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonutti, L.G.; Vergara, L.; Linares Pérez, N.; de Oliveira Jardim, E.; Dalla Costa, B.O.; Decolatti, H.P. A Comparative Study of Homogeneous and Heterogeneous Catalyzed Glycerol Acetylation with Acetic Acid: Activity, Selectivity, and Stability. Processes 2025, 13, 3231. https://doi.org/10.3390/pr13103231
Tonutti LG, Vergara L, Linares Pérez N, de Oliveira Jardim E, Dalla Costa BO, Decolatti HP. A Comparative Study of Homogeneous and Heterogeneous Catalyzed Glycerol Acetylation with Acetic Acid: Activity, Selectivity, and Stability. Processes. 2025; 13(10):3231. https://doi.org/10.3390/pr13103231
Chicago/Turabian StyleTonutti, Lucas G., Lourdes Vergara, Noemí Linares Pérez, Erika de Oliveira Jardim, Bruno O. Dalla Costa, and Hernán P. Decolatti. 2025. "A Comparative Study of Homogeneous and Heterogeneous Catalyzed Glycerol Acetylation with Acetic Acid: Activity, Selectivity, and Stability" Processes 13, no. 10: 3231. https://doi.org/10.3390/pr13103231
APA StyleTonutti, L. G., Vergara, L., Linares Pérez, N., de Oliveira Jardim, E., Dalla Costa, B. O., & Decolatti, H. P. (2025). A Comparative Study of Homogeneous and Heterogeneous Catalyzed Glycerol Acetylation with Acetic Acid: Activity, Selectivity, and Stability. Processes, 13(10), 3231. https://doi.org/10.3390/pr13103231






