Abstract
The use of alumina-containing nepheline raw materials as an alternative source of alumina is relevant in the context of the limited bauxite reserves in Kazakhstan. Nepheline processing can result in products such as alumina, sodium and potassium salts, silicate products and rare metals. In terms of economic value, alumina is the most important. This article considers an advanced technology for nepheline processing for the extraction of alumina that is first purified from potassium. The application of calcium sulphate and calcium oxide as additives to the nepheline raw materials is studied. The optimal conditions for two-stage leaching with calcium additives in the form of calcium sulphate and calcium hydroxide are determined.
1. Introduction
The reserves of Bayer bauxite are limited in Kazakhstan, which affects the industrial production of alumina, but it is possible to use non-bauxite raw materials, such as the nepheline syenites of the Kubasadyr deposit, for the same purpose. The generally accepted processing method for nepheline consists of sintering the ore with limestone. The main disadvantages of the sintering process are high energy consumption, high capital costs and significant atmospheric emissions. Due to technological advancements, it is possible to use a hydrochemical alkaline-sulphate method for the processing of nepheline syenites. The technology includes preliminary chemical enrichment and leaching with a new active calcium additive. The global aluminium industry is growing rapidly, and aluminium is a vital structural material [1].
The traditional source of aluminium is bauxite. However, quality reserves of bauxite are limited, and alternative sources of aluminium are being sought. These include aluminosilicate minerals such as kaolins or nepheline [2,3,4,5,6].
Nepheline is an important aluminium non-bauxite source [7,8]. The transition to alkaline aluminosilicate rocks is significant because they generate other valuable products at the same time as alumina without any waste. The structure and chemical composition of nepheline varies in different deposits [9]. The value of nepheline depends on the content of K2O, Na2O and Al2O3 and on the presence of rare elements [10,11,12,13]. The excessive amount of silica found in nepheline is a negative aspect of this rock as it is of no value. However, it can be isolated as individual valuable chemicals.
Nephelines are processed only in Russia, Canada, Norway, Turkey, and Brazil [14].
Currently, there are only two nepheline processing facilities in the world, the Achinsk Alumina Refinery and Pikalevsky Alumina Refinery, which are located in Russia. In these plants, the lime sintering process is used to produce soda, potash, and Portland cement in addition to alumina.
Kazakhstan has discovered significant amounts of non-bauxite aluminium raw materials. Alkaline nepheline is present in high-alumina minerals found in ultrabasic and granitoid rocks. There are three primary types of rocks that contain nepheline: nepheline rocks of alkaline or ultrabasic massifs, nepheline–lycite rocks of alkaline–basic massifs and nepheline syenites of alkaline granitoid massifs
Several dozens of nepheline rock massifs have been found in different regions of the Republic of Kazakhstan [15,16]. The processing of nepheline in Kazakhstan requires the development of an effective technology that takes into account the peculiarities of the mineral composition of the raw materials.
With the feasibility of processing substandard alumina-containing raw materials, ways to expand the raw material base of Kazakhstan’s alumina industry must be found, with a focus on the broader use of low-quality but more common natural aluminium raw materials, namely, nepheline ores.
Processing nepheline, a material that is highly siliceous, involves the use of a substantial amount of waste sludge. It is difficult to create a waste-free production in this case. In this regard, it is more advantageous to process rocks of this type with preliminary chemical enrichment, which enables the partial desilicification of ore. When decomposing nepheline using the hydroalkali method, this method is a viable option.
The sintering method is an industrial method for the processing of alkaline aluminosilicate feedstock into alumina [17]. Alumina is the most important economic element, but cement production from belite sludge may not be profitable in some situations. Therefore, a high content of alumina in the raw material charge is of primary importance. The preparation of such a nepheline–limestone–soda charge consists of thorough grinding and mixing of all components in compliance with the necessary stoichiometric proportions. The sintering process is carried out in tubular rotary furnaces, in which the charge is heated to a temperature of 1270 °C. The main reactions during sintering are the formation of sodium aluminate and bicalcium silicate. During the subsequent leaching, bicalcium silicate is converted into belite sludge, from which cement can be produced. It has significant disadvantages, such as high energy consumption, high capital costs, process complexity, high cost of sintering furnaces, the loss of expensive rare earth elements and high atmospheric emissions [13,17,18]. It is estimated that the aluminium industry produces 0.45–0.5 Gt of carbon dioxide (CO2) equivalent emissions per year and is responsible for 1% of anthropogenic GHG emissions and 2.5% of CO2 emissions [17].
Acidic methods of processing aluminosilicate raw materials are known. There are known works on the hydrochloric acid leaching of nepheline at temperatures of 90–150 °C, aluminium extraction from solution by crystallisation of AlCl3∙H2O followed by calcination with HCl release [1].
The dissolution of nepheline syenite ore with oxalic acid reduces the Fe2O3 content and produces a high value-added concentrate for many industrial purposes. Considering the results of the dissolution tests carried out, a nepheline syenite concentrate was obtained with 0.15% Fe2O3 content when 0.4 mol/dm3 oxalic acid and sulfuric acid were used for 2 h leaching [5].
Chemical leaching of nepheline syenite with dilute sulphuric acid after magnetic separation could recover only ~40% of the potassium values [18].
The use of acid methods in industry is limited by several significant drawbacks: the necessity of expensive acid-proof apparatus, difficulties in separating and washing silica residue, difficulties in regeneration of acid solutions and the problem of utilising sludge, which mainly consists of silicic acid gels. In addition, the processing of alkaline aluminosilicates (nepheline) will lose the alkali contained in these rocks, as well as the acids used for the decomposition of silicic acid.
The hydrochemical processing method consists of autoclave leaching at high temperatures and highly concentrated solutions of NaOH in the presence of calcium compounds. Calcium compounds introduced into the aluminosilicate leaching process have a decisive influence on alumina recovery. Many studies have been devoted to the effect of calcium in aluminosilicate systems [19,20,21].
Although nepheline decomposes in solutions without calcium addition, alumina is not extracted from the solution. Since the process of aluminium leaching from aluminosilicates is based on the formation of sodium-calcium hydrosilicate (SCHS) Na2O × 2CaO × SiO2∙H2O, its composition is determined by the dosage of calcium oxide. This compound is an unstable compound in alkaline solutions [22]. After desilicification of the solution, alkali is regenerated from the unstable SCHS compound by hydro-rolysis to yield a precipitate of alkali-free calcium silicate. Sodium and potassium alkalies, which are found in nepheline ore, are also present in the resulting beneficiation solution. The separation of potassium and sodium alkali during desiliconisation results in the formation of SCHS since potassium alkali does not create compounds like SCHS. The presence of potassium alkali should be considered in the hydrochemical processing of nepheline, unlike bauxite.
The physicochemical properties of NaOH and KOH solutions differ significantly. The electrolyte activity coefficient in NaOH solutions is about an order of magnitude smaller than in potassium solutions, and consequently, the chemical activity of sodium solutions is lower [23].
Experiments proved that during the alkaline treatment of nepheline, first, excess silica enters the solution. If the silica content in the rock is greater than that needed for the formation of alkaline hydroaluminosilicate, then the alumina from the rock does not enter the solution at all, and in the alkaline solution, only silica remains in the form of sodium and potassium metasilicate. If there is not enough alumina to completely bind all the silica in the solution, then it will only move into the solution. Nepheline cannot be processed without the use of calcium aluminate additives [24]. In alumina production, calcium oxide plays an important role in binding SiO2 from aluminosilicate raw materials (bauxite, nepheline, kaolinite, etc.) to form aluminium-free compounds.
At present, calcium oxide is obtained from limestone (calcium carbonate), which requires additional capital investment, energy costs, etc. To produce 1 ton of alumina, it is necessary to roast about 3 tons of limestone. Replacing lime with natural limestone will eliminate the limestone roasting operation and, therefore, improve technical and economic performance. Calcium sulphate is of particular interest as a calcium-containing reagent, significant amounts of which are in the form of the toxic technogenic products of phosphogypsum. The replacement of calcium sulphate with a manmade compound will improve the technological and economic feasibility of nepheline processing and will allow for the use of both of its main components: calcium for binding silicon in the form of calcium silicate and sulphate for obtaining the scarce fertiliser potassium sulphate from the potassium contained in nepheline.
Phosphogypsum is a multitonne waste product from phosphate fertiliser production, consisting mainly of gypsum CaSO4 at 94–95%, undecomposed apatite Ca5(PO4)3 at 1.77% and monocalcium phosphate Ca(H2PO4)2 at 0.18% [25].
According to expert estimates, the accumulated phosphogypsum reserves in the dumps of industries amount to approximately 140 million tonnes, with an annual increase of 14 million tonnes [26].
There are 14 million tonnes of phosphogypsum in the dumps of Kazphosphate LLP, and the company gives it away at a price of 1 tenge per tonne [27].
The purpose of this article is to develop an effective technology for processing nepheline syenites to study the influence of the forms of calcium additive in the leaching process. The possibility of partial or complete replacement of CaO by CaSO4 at complex technology of nepheline processing is considered. The use of CaSO4 will reduce the energy costs associated with limestone firing.
A hydrometallurgical alkali-sulphate technology for processing nepheline ore was developed, including preliminary chemical activation and leaching in HMAS with the use of CaSO4 as a calcium additive in the first stage, which provides the amount of constituents necessary for the formation of potassium sulphate. As a result, we extract potassium and purify the aluminate solution from potassium. Potassium has a negative effect on aluminium recovery. In the second stage, leaching should be carried out in recycled aluminous-alkali solution with CaO added in the amount necessary for the complete binding of silica in CaO∙SiO2. The addition of a calcium additive in the second stage to a ratio of CaO:SiO2 = 1.6 is optimal, as above such a ratio, SiO2 does enter the solution, the presence of which negatively affects the regeneration of the solution and the quality of the obtained aluminium hydroxide. The recovery of Al2O3 remained at approximately 85%.
2. Materials and Methods
X-ray fluorescence analysis to determine chemical composition was performed on a Venus 200 wave dispersion spectrometer (Panalyical B.V., Almelo, The Netherlands). The sample, ground to a particle size of 0.074 mm, \was applied to a boric acid substrate under a force of 5 tonnes. The sample was evenly distributed throughout the cuvette, compressed and placed in the apparatus. Chemical analysis was performed using an Optima 2000 DV (Perkin Elmer, Waltham, MA, USA) inductively coupled plasma optical emission spectrometer (Optima, Perkin Elmer). X-ray experimental data were obtained using a BRUKERD8 ADVANCE (Karlsruhe, Germany) device using copper radiation at an accelerating voltage of 36 kW and a current of 25 mA. The sample, ground to a particle size of 0.056 mm, was placed in a cuvette pretreated with alcohol. The sample was evenly distributed throughout the cuvette, compressed and placed in the apparatus. International Centre for Diffraction Data ICDD PDF-2 2023 database was used to confirm the accuracy of phase identification. Electron probe microanalysis (EPMA) was performed on a JEOL JXA-8230 (Tokyo, Japan) and scanning electron microscopy was used to clarify the elemental composition of the particles in different areas of the cake after leaching as a function of duration.
An average representative sample of nepheline syenites from the Kubasadyr deposit was used in this work, which was obtained by sampling and quarting. Leaching in alkaline solution at temperatures of 200–300 °C was carried out in an autoclave with an overpressure of up to 100 bar, which allowed the temperature to increase to 300 °C with the help of an electric heater with a power of 5 kW. The accuracy of temperature maintenance in the autoclave was ±2 °C. The design of the autoclave enabled sampling with quenching throughout the experiment. The design of the autoclave also allows the introduction of the solid phase at a given temperature and stirring by a falling stirrer with a frequency of 0.1–1 Hz.
3. Results and Discussion
To develop an effective technology for the direct extraction of alumina from nepheline syenites, the influence of the form of calcium added during leaching was investigated. CaO and CaSO4 were used as the calcium additives.
Nepheline syenites of the Kubasadyr deposit, Republic of Kazakhstan, were used as a raw material. Chemical composition in wt% was as follows: Al2O3 21.2; SiO2 55.86; Fe2O3 3.18; CaO 3.64; Na2O 5.09; K2O 5.52; others 5.14.
The use of CaSO4 as a calcium additive instead of CaO is justified by the fact that it will reduce the energy costs associated with limestone firing.
Leaching was carried out in a high-modulus aluminate solution (HMAS) with caustic modulus αk = 30.0 (the caustic modulus of alkaline solutions (αk) was determined from the ratio αk = Na2O/Al2O3 × 1.645) at a temperature of 280 °C, L:S ratio of 5 and duration of 90 min (Table 1), and calcium was added to achieve a CaO:SiO2 ratio of 1.0 [24].

Table 1.
Leach cake of nepheline concentrate with different forms of calcium added.
The chemical composition of HMAS, g/dm3 was Na2O 235.0; Al2O3 13.7. Ga 0.28; αk = 30.0.
Leaching resulted in a medium-modulus aluminate solution of composition g/dm3: Na2O 161.0; Al2O3 38.87; SiO2 0.1; αk = 10.5.

Table 2.
X-ray phase composition of leach cakes.
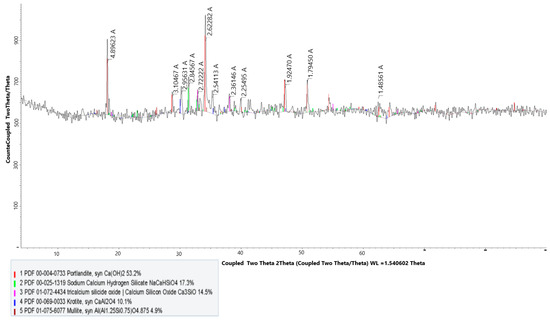
Figure 1.
X-ray diffractogram of leach cake with CaO.
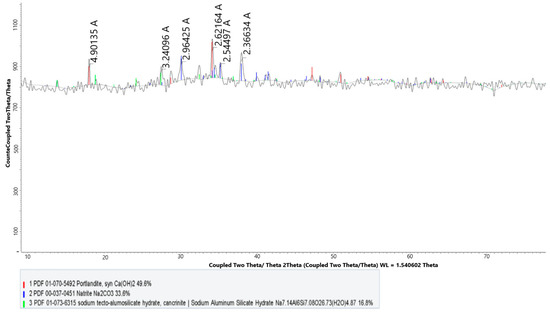
Figure 2.
X-ray diffractogram of leach cake with CaSO4.
Analysis of the results showed that it is possible to use CaSO4 as a calcium additive in the leaching of nepheline ore instead of CaO.
The mechanism of ore leaching using CaO or CaSO4 as a calcium additive can be represented by Reactions (1)–(3),
where R is Na or K.
2ROH + SiO2 → R2SiO3 + H2O
R2SiO3 + CaO + H2O → 2ROH + CaSiO3
R2SiO3 + CaSO4 + 2H2O → R2SO4 + CaSiO3 · 2H2O,
Thus, the developed hydrometallurgical alkaline-sulphate technology for nepheline ore processing, including preliminary chemical activation and leaching in HMAS using CaSO4 as a calcium additive, allows for Al2O3 extraction in solution at a recovery rate of 94–95%. As a result of leaching, a medium-modulus aluminate solution with αk = 10.5 was obtained.
However, the replacement of CaO with CaSO4 leads to the formation of sodium and/or potassium sulphates in solution and, consequently, to the consumption of caustic alkali (Reactions (1)–(3)), so it is logical to use CaSO4 only in the amount necessary for the formation of potassium sulphate; this can be realised by dividing the leaching process into two stages. The first stage includes leaching in an alkaline solution with the addition of a part of the calcium in the form of CaSO4, which is necessary for the binding of potassium during leaching into a sulphate form. In the second stage, leaching is carried out in a recycled aluminous-alkaline solution with the addition of CaO in the amount necessary for the complete binding of silica in the form of CaO∙SiO2.
From the alkaline solution in the first leaching stage, using the difference in solubility, potassium sulphate can be selectively extracted by crystallisation.
The technological scheme for processing nepheline ore is shown in Figure 3. In the two-stage leaching process, Stage I includes leaching with the recycled alkaline solution with CaSO4 added in the amount necessary for the formation of potassium sulphate, and Stage II of leaching contains the recycled HMAS with the rest of the calcium introduced in the form of CaO to obtain CaO:SiO2 = 1.
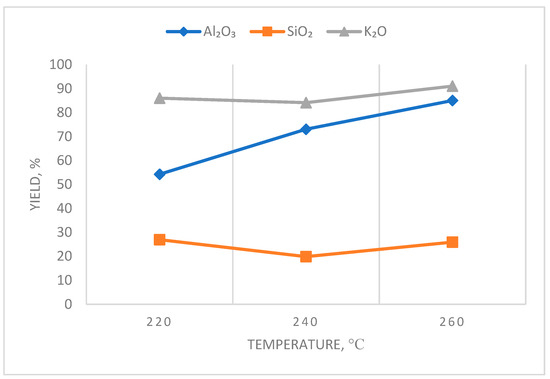
Figure 3.
Effect of temperature in Stage II of leaching on elemental recovery.
Stage I of leaching was carried out in a solution with a Na2O content of 240 g/L, temperature of 280 °C and duration of 120 min.
Thus, samples of nepheline purified from potassium were prepared in the optimal solution with a Na2O concentration of 240 g/dm3, 280 °C and duration of 1200 min with 70% CaO added at the rate of CaO:SiO2 = 1. Under these conditions, the maximum recovery of K2O in solution was 92.13%, that of Al2O3 was 6.57 and that of SiO2 was 22.84.
The chemical composition of the spent cake from leaching stage I was Al2O3 11.86%; SiO2 21.9%; Fe2O3 1.4%; CaO 17.7%; Na2O 11.73%; K2O 0.89% and MgO 0.52%.
The optimal ratio of CaO:SiO2 = 1.6 was chosen because, at this ratio, SiO2 did not enter the solution, the presence of which negatively affects the regeneration of the solution and the quality of the obtained aluminium hydroxide.
The aim of leaching Stage II was to release aluminium into solution and to bind the remaining silicon to calcium to produce calcium silicate. For this purpose, the effects of the temperature, duration and CaO:SiO2 ratio on the recovery of Al2O3 and SiO2 were investigated.
The study of the influence of CaO:SiO2 on the degree of Al2O3 extraction at Stage II of leaching was carried out with the addition of Ca(OH)2, and it was quenched at a temperature of 130 °C to obtain the molar ratio CaO:SiO2 = 1:2. The influence of temperature in Stage II of the leaching process on the degree of recovery of elements is presented in Table 3 and Figure 3.

Table 3.
Effect of the Stage II leaching temperature on the element recovery rate.
According to the results, as the temperature of leaching in Stage II increases, the recovery rate of Al2O3 in solution increases and reaches 91.37% at 280 °C.
The influence of the amount of calcium added in the stoichiometric ratio of CaO:SiO2 in Stage II of leaching on the degree of elemental recovery was investigated (Table 4, Figure 4).

Table 4.
Effect of the amount of calcium added in the stoichiometric CaO:SiO2 ratio in Stage II of leaching on the recovery of elements.
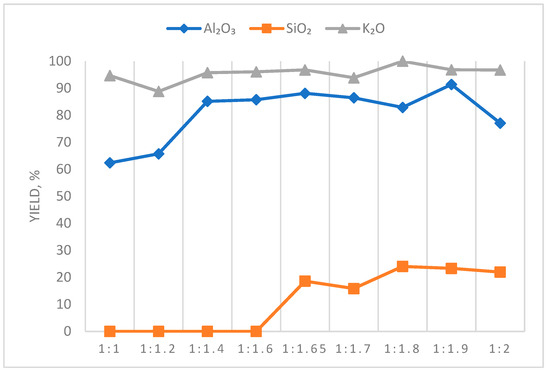
Figure 4.
Effect of the amount of calcium added on the recovery of major elements.
According to the results obtained, when calcium oxide is added at a molar ratio of CaO:SiO2 greater than 1.6, SiO2 starts to be released into solution, and the recovery of Al2O3 remains at approximately 85%.
Thus, the technological parameters for leaching, including two-stage leaching with the use of HMAS and calcium sulphate, were determined (Figure 5).
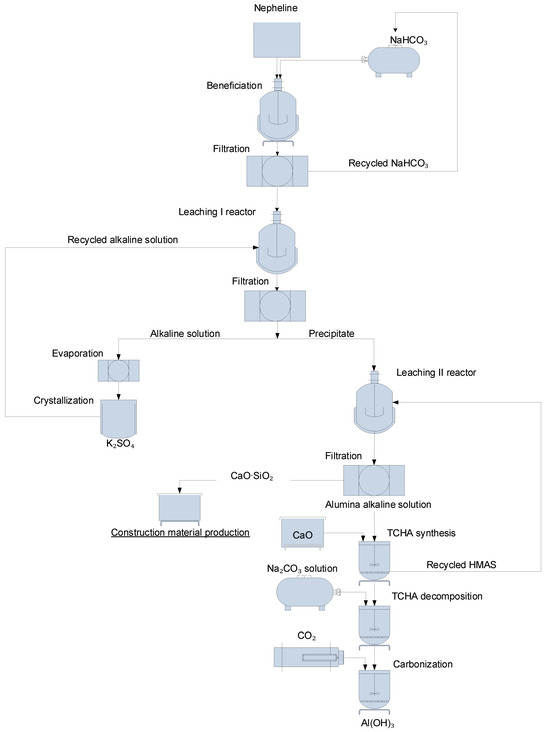
Figure 5.
Complex technological scheme for processing nepheline syenites using fractional calcium additive.
4. Conclusions
A hydrometallurgical alkali-sulphate technology for processing nepheline ore was developed, including preliminary chemical activation and leaching in HMAS with the use of CaSO4 as a calcium additive in the first stage, which provides the amount of constituents necessary for the formation of potassium sulphate. In the second stage, leaching should be carried out in recycled aluminous-alkali solution with CaO added in the amount necessary for the complete binding of silica in CaO∙SiO2. The addition of a calcium additive in the second stage to a ratio of CaO:SiO2 = 1.6 is optimal, as above such a ratio, SiO2 does enter the solution, the presence of which negatively affects the regeneration of the solution and the quality of the obtained aluminium hydroxide. The recovery of Al2O3 remained at approximately 85%.
Author Contributions
N.A.: project administration, writing—original draft preparation, writing—review and editing; R.A.: conceptualisation, methodology, writing—review and editing; S.G.: methodology, writing—original draft preparation; A.K.: formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No, AP14869579).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brough, D.; Jouhara, H. The aluminium industry: A review on state-of-the-art technologies, environmental impacts and possibilities for waste heat recovery. Int. J. Thermofluids 2020, 1–2, 100007. [Google Scholar] [CrossRef]
- Kassa, A.; Shibeshi, N.; Tizazu, B. Characterisation and Optimisation of Calcination Process Parameters for Extraction of Aluminum from Ethiopian Kaolinite. Int. J. Chem. Eng. 2022, 2022, 5072635. [Google Scholar] [CrossRef]
- Kyriakogona, K.; Giannopoulou, I.; Panias, D. Extraction of Aluminium from Kaolin: A Comparative Study of Hydrometallurgical Processes. In Proceedings of the 3rd World Congress on Mechanical, Chemical, and Material Engineering, Rome, Italy, 8–10 June 2017. [Google Scholar]
- Padilla, I.; Lupez-Delgado, A.; Romero, M. Kinetic study of the transformation of sodalite to nepheline. Am. Ceram. Soc. 2022, 105, 4336–4347. [Google Scholar] [CrossRef]
- Bagani, M.; Balomenos, E.; Panias, D. Nepheline syenite as an alternative source for aluminium production. Minerals 2021, 11, 734. [Google Scholar] [CrossRef]
- Bagani, M.; Efthymios, B.; Dimitros, P. Exploitation of kaolin as an alternative source in alumina production. Mater. Proc. 2021, 5, 24. [Google Scholar] [CrossRef]
- Aghazadeh, V.; Shayanfar, S.; Hassanpour, P. Aluminum hydroside crystallization from aluminate solution using carbon dioxide gas: Effect of pH and seeing. Miner. Process. Extr. Metall. 2019, 130, 1–7. [Google Scholar]
- Burat, F.; Kangal, O.; Onal, G. An alternative mineral in the glass and ceramic industry: Nepheline syenite. Miner. Eng. 2006, 19, 370–371. [Google Scholar] [CrossRef]
- Antao, S.; Nicholls, J. Crystal chemistry of three volcanic K-rich nepheline samples from Oldoinyo Lengai, Tanzania and Mount Nyiragongo, Eastern Congo, Africa. Front. Earth Sci. 2018, 6, 155. [Google Scholar] [CrossRef]
- Jena, S.K.; Dhawan, N.; Rao, D.S.; Misra, P.K.; Mishra, B.K. Studies on extraction of potassium values from nepheline syenite. Int. J. Miner. Process. 2014, 133, 13–22. [Google Scholar] [CrossRef]
- Unkear, A.T.; Budakoglu, M.; Doner, Z. The Evolution of the REE-Bearing Özvatan Nepheline Syenite-Carbonatite Complex. Central Turkey: Mineralogical. Geochemical. and Stable Isotopic Approaches. Minerals 2023, 13, 667. [Google Scholar] [CrossRef]
- Haseli, P.; Majewski, P.; Christo, F.; Keane, P.; Jafarian, M.; Bruno, F. A review paper on the extraction of potassium from non-soluble resources with the use of acid and alkaline solution and molten salts. Minerals Engineering 2023, 204, 108365. [Google Scholar] [CrossRef]
- Seitenov, R.A.; Lipin, V.A.; Akhmedov, S.N.; Medvedev, V.V. Comparative economic efficiency of processing high-potassium aluminosilicate raw materials into Alumina and related products. In Light Metals 2024; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2024; pp. 82–89. [Google Scholar]
- Abouzeid, A.-Z.; Negm, A.-T. Characterization and beneficiation of Egyptian Nepheline Syenite Ore. Int. J. Mineral. 2014, 2014, 128246. [Google Scholar] [CrossRef]
- Akhmadiyeva, N.K.; Abdulvaliyev, R.A.; Akcil, A.; Manapova, A.I. Pre-activation of nepheline before the enrichment. Complex Use Miner. Resour. 2023, 327, 82–89. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Kuldeyev, E.; Abdulvaliyev, R.; Pozmogov, V.; Beisembekova, K.; Gladyshev, S.; Tastanov, E. Prospects of aluminumindustry development in Kazakhstan. News Natl. Acad. Sci. Repub. Kazakhstan 2017, 3, 151–160. [Google Scholar]
- Mohammadloo, K.; Barakan, S.; Shayanfar, S.; Azhazadeh, V. Kinetics Studies of Sintered Nepheline Syenite Alkaline Leaching under Atmospheric Pressure. Trans. Indian Inst. Met. 2021, 74, 2105–2445. [Google Scholar] [CrossRef]
- Kangal, M.O.; Bulut, G.; Yesilyurt, Z.; Basturkcu, H.; Burat, F. Characterization and production of Turkish nepheline syenites for industrial applications. Physicochem. Probl. Miner. Process. 2018, 5, 605–616. [Google Scholar]
- Jiang, C.; Li, K.; Zhang, J.; Qin, Q.; Liu, Z.; Liang, W.; Sun, M.; Wang, Z. The effect of CaO(MgO) on the structure and properties of aluminosilicate system by molecular dynamics simulation. J. Mol. Liq. 2018, 286, 762–769. [Google Scholar] [CrossRef]
- Wu, T.; He, S.; Liang, Y.; Wang, Q. Molecular dynamics simulation of the structure and properties for the CaO–SiO2 and CaO–Al2O3 systems. J. Non-Cryst. Solids 2015, 411, 145–151. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Aparicio-Rebollo, E.; Fernandez-Jimenez, A.; Palomo, A. Effect of calcium on the alkaline activation of aluminosilicate glass. Ceram. Int. 2016, 42, 7697–7707. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Zu, D.; Zhang, C.; Yu, H.; Tu, G. Formation characteristics of sodium calcium silicate compounds based on solid-state reaction. Ceram. Int. 2022, 48, 25958–25967. [Google Scholar] [CrossRef]
- Abdulvaliyev, R.; Akhmadiyeva, N.; Gladyshev, S.; Samenova, N.; Kolesnikova, O.; Mankesheva, O. Behavior of calcium compounds under hydrothermal conditions during alkaline leaching of aluminosilicates with synthesis of fillers for composites. J. Compos. Sci. 2023, 7, 508. [Google Scholar] [CrossRef]
- Akhmadiyeva, N.; Gladyshev, S.; Abdulvaliyev, R.; Sukurov, B.; Amanzholova, L. Selective extraction of potassium from raw nepheline materials. Heliyon 2024, 10, e29461. [Google Scholar] [CrossRef] [PubMed]
- Beisembekova, L.K.; Smagulova, D.A.; Mahmut, G.; Omarov, A.T.; Tanasheva, M.A. Technology of complex processing of phosphogypsum and polyhalite into chemical ameliorant. Bull. KazNU. Chem. Ser. 2011, 4, 42–45. [Google Scholar] [CrossRef]
- Miller, O. Phosphogypsum: Both a Problem and a Panacea. 2021. Available online: https://kazakh-zerno.net/186600-fosfogips-i-problema-i-panaceja (accessed on 1 April 2024).
- Zhantasov, K.T.; Ziyat, A.J.; Lavrov, B.A.; Zhantasov, M.K. Mineralogical and chemical composition of phosphogypsum—A waste product of extraction phosphoric acid production. Sci. Herit. 2021, 78, 24–29. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).