Abstract
Chitin, an abundant biopolymer with potential applications in agriculture, medicine, and bioremediation, is conventionally extracted using chemical methods that have environmental disadvantages. This study investigates the extraction of chitin from Litopenaeus vannamei shrimp waste by one-step fermentation using the bacterial strains Pseudomonas aeruginosa QF50 and Serratia sp. QCS23. A total of 4 kg of shrimp waste was treated by fermentation with culture media enriched with different concentrations of glucose (1, 5, and 10%) for 7 days at 25 °C, followed by purification and characterization processes using infrared spectroscopy and X-ray diffraction. The results demonstrated an increase in the yield of crude chitin proportional to the glucose concentration, reaching a maximum of 76.81 ± 7.64% for Pseudomonas aeruginosa QF50 and 71.30 ± 1.16% for Serratia sp. QCS23. Both strains showed high efficiencies in deproteinization (80–87%) and demineralization, with significant improvements especially shown at high glucose concentrations. Structural characterization confirmed the presence of the spectral characteristics of α-chitin, with crystallinity indices of 81% and 71% for chitins obtained with Pseudomonas aeruginosa QF50 and Serratia sp. QCS23, respectively. This study concludes that single-step fermentation with Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 is an effective and sustainable method for the extraction of high-quality chitin from shrimp exoskeleton waste, offering a promising alternative to traditional chemical methods.
1. Introduction
Chitin is the second most abundant biopolymer in the biosphere, surpassed only by cellulose, and is found in a variety of organisms including insects, mollusks, fungi, protozoa, and crustaceans [1]. Structurally, chitin is a β (1→4)-linked linear homopolymer of N-acetylglucosamine, known for its remarkable properties such as thermal stability, biocompatibility, biodegradability, and a nanofibrous surface [2]. These characteristics suggest a wide range of economically significant applications in fields such as agriculture, medicine, the food industry, textiles, cosmetics, and bioremediation [3]. Among various sources, crustacean exoskeleton wastes are particularly rich in chitin, accounting for 15–20% of their weight [4]. However, the current methods of chitin extraction are not fully aligned with the principles of green chemistry and sustainability, posing a challenge for its broader application.
Traditionally, chitin is extracted from crustacean exoskeletons using chemical methods that involve deproteinization (DP) with NaOH, followed by demineralization (DM) with acids such as HCl, HNO3, H2SO4, CH3COOH, or HCOOH [5]. These harsh chemicals are environmentally damaging, and strong acids can lead to polymer hydrolysis; moreover, high concentrations of NaOH at elevated temperatures can cause the unwanted deacetylation and depolymerization of chitin [6,7]. As a result, biological treatments have gained preference for their ability to preserve the chitin structure, including its degree of deacetylation and molecular weight, while also reducing energy consumption and chemical byproducts, thus offering a sustainable and economical alternative.
Biological treatments utilize enzymes and/or microorganisms to extract chitin through the production of organic acids and proteases during fermentation or enzymatic activity [8]. Various microorganisms have been employed for this purpose: Chakravarty et al. [9] used Bacillus megaterium NH21, Serratia marcescens DB11, and Lactobacillus plantarum for the successive fermentation of lobster exoskeletons; Gamal et al. [10] optimized chitin extraction from prawn (Penaeus merguiensis) exoskeleton waste supplemented with sucrose (5%) using a strain of Bacillus subtilis, achieving DP and DM values of 98% and 83%, respectively; Liu et al. [11] reported the extraction of chitin with superior physicochemical and structural properties from shrimp (Litopenaeus vannamei) exoskeletons using Lactobacillus rhamnoides and Bacillus amyloliquefaciens BA01; and Sedaghat et al. [12] obtained chitin from prawns (Penaeus merguiensis) using Pseudomonas aeruginosa at 30 °C and 100 rpm over 4–6 days.
In Peru, the cultivation of Litopenaeus vannamei constitutes one of the primary aquacultural activities, accounting for 72.6% of aquaculture exports [13]. To date, commercial efforts have focused solely on the meat, with no established processes for utilizing exoskeleton waste to produce byproducts like chitin [14]. Although chitin has been chemically extracted from Litopenaeus vannamei on a laboratory scale [15,16], there are no reports of its extraction from the exoskeleton of Litopenaeus vannamei through fermentative treatments.
The bacterial strain Pseudomonas aeruginosa QF50, recently identified and isolated from petroleum-contaminated soil in La Libertad, northern Peru, has demonstrated a remarkable capacity to adapt to toxic environments and to degrade petroleum-derived compounds, as documented by Cruz et al. [17]. This study aims to explore the potential of Pseudomonas aeruginosa QF50 in fermentative treatments for chitin extraction. Additionally, Serratia species are known for their ability to produce enzymes such as protease, chitinase, and chitosanase, which have been exploited for the extraction of bioactive compounds through the enzymatic degradation of proteins in biological waste from shrimp shells [18].
The primary objective of this study is to investigate the application of Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 in fermentative treatments for chitin extraction from shrimp exoskeleton waste (Litopenaeus vannamei). This method offers a more sustainable and efficient alternative to conventional chemical treatments. To assess the quality and properties of the extracted chitin, characterization techniques such as Fourier Transform Infrared Spectroscopy (FTIR) and X-ray diffraction (XRD) were utilized.
2. Materials and Methods
2.1. Shrimp Exoskeleton Preparation
A total of 4 kg of shrimp exoskeleton waste (Litopenaeus vannamei) was sourced from the Chifa Hing-Da restaurant. The collected shrimp exoskeleton waste was thoroughly washed six times with potable water to remove any adhering contaminants, and any remaining meat was carefully discarded. The waste was then disinfected using a 0.5% sodium hypochlorite solution (NaClO) for 5 min. To eliminate any residual chlorine, the exoskeletons were rinsed three times with distilled water. The cleaned exoskeletons were subsequently dried at 60 °C for 6 h, ground, and sieved to obtain a particle size range of 25–30 mm. The prepared exoskeleton was stored in airtight containers at ambient temperature until needed for further processing [19].
2.2. Preparation of Bacterial Inoculum
Pseudomonas aeruginosa QF50, previously isolated and characterized from soil contaminated with crude oil in La Libertad, Peru, was utilized following the procedures described by Cruz et al. [17]. Serratia sp. was isolated from a wastewater treatment plant (PTAR), and its microscopic characteristics were determined through Gram staining. Biochemical assays for bacterial identification were performed using the MicroScan colorimetric and enzymatic system (WalkAway 96, Beckman Coulter, Brea, Ca, USA) [20].
For inoculum preparation, a pure culture of each bacterium was enriched in a 250 mL Erlenmeyer flask containing 100 mL of Brain Heart Infusion (BHI) culture medium. The BHI medium was composed of the following ingredients per liter: 8.0 g of Brain Heart Infusion solids, 5.0 g of peptic digest of animal tissue, 16.0 g of pancreatic digest of casein, 5.0 g NaCl, 2.0 g of glucose, and 2.5 g of Na2HPO4. The pH of the medium was adjusted to 7.0 using either 0.5 M NaOH or 0.5 M HCl as needed. The culture was incubated at 30 °C for 24 h [21].
2.3. Fermentation of the Shrimp Exoskeleton
A base culture medium (BCM) was prepared with the following composition: 0.5 g/L magnesium sulfate heptahydrate (MgSO4.7H2O) and 1.0 g/L dipotassium phosphate (K2HPO4). The pH was adjusted to 7.0 ± 0.2 using either 0.5 N NaOH or 0.5 M HCl as required. Shrimp exoskeleton waste was added at a concentration of 50 g/L, and glucose (Merck, West Point, PA, USA) was incorporated at varying concentrations (1%, 5%, and 10%) to evaluate the efficiency of deproteinization and demineralization. The medium was sterilized at 121 °C for 15 min. Subsequently, 10% (v/v) of the prepared cultures of Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 was inoculated into the sterile medium. The fermentation was conducted at room temperature (approximately 25 °C) with agitation at 130 rpm for a duration of 7 days. All experimental assays were performed in triplicate [18,22].
2.4. Extraction and Purification of Chitin
Following the fermentative treatment, the samples were filtered using Whatman N° 1 filter paper and rinsed three times with distilled water. The filtered biomass was then dried at 40 °C for 24 h. For decolorization, the dried sample was treated with a 1% NaClO solution using a 1:30 solid-to-solution ratio for 30 min at room temperature (25 °C). The sample was then filtered, washed with distilled water, and subjected to two additional 10 min treatments with 1% NaClO. Extensive washing was performed to eliminate any remaining chlorine residues. The samples were finally dried at 40 °C for 24 h to a constant weight [23].
Total protein content was quantified using the Biuret method as described by Janairo et al. [24]. Deproteinization efficiency was calculated using Equation (1):
The mineral content was quantified from the ashes by the weighing method [11]. The sample was heated in a muffle furnace at 400 °C for 3 h. After cooling to room temperature, the ash was weighed using a precision balance (BIOBASE, BP10003, Jinan, Lǔ, China), which offers a readability of 0.001 g and an accuracy of ±0.002 g. The percentage of demineralization was calculated using Equation (2):
2.5. Characterization of Obtained Chitin
2.5.1. Measurement of Chitin Color
The color of the raw chitin was measured using a spectrophotometer equipped with a color measurement system (Konica Minolta CM-5, Tokyo, Japan). The system recorded the L*, a*, and b* values of the sample. The L* value represents luminosity on a scale from 0 (black) to 100 (white). The a∗ value indicates the chromaticity on the green–red axis, with positive values signifying red and negative values signifying green. The b∗ value indicates the chromaticity on the blue–yellow axis, with positive values signifying yellow and negative values signifying blue [25]. The whiteness index (WI) was calculated using Equation (3):
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
To analyze the structural characteristics, Fourier Transform Infrared Spectroscopy was performed using a Thermo Scientific Nicolet iS50 (Waltham, MA, USA) with ATR (attenuated total reflection) in the region of 4000–600 cm−1 with a resolution of 4 cm−1 [26].
2.5.3. X-ray Diffraction (XRD) Analysis
X-ray diffraction (XRD) analysis was performed to assess the crystallinity of the extracted chitin. The analysis utilized a RIGAKU Miniflex 600 diffractometer (Woodlands, TX, USA) equipped with a Cu Kα radiation source (λ = 0.154). The scanning was conducted over a 2θ range of 5–60° at a scanning speed of 2°/min and a step size of 0.02°. The diffractometer was operated at 40 kV and 15 mA [27]. The relative crystallinity index (CI) was calculated using the Segal method, as shown in Equation (4):
where I110 is the intensity of the diffraction corresponding to the (110) plane at 2θ ≈ 20° and Iam is the intensity of the amorphous diffraction at 2θ ≈ 18°.
2.6. Statistic Analysis
Data were processed using analysis of variance (ANOVA) to determine statistical differences among treatments. Results are expressed as mean ± standard deviation. The analysis was conducted using Origin 2018 software, and a significance level of p < 0.05 was established to assess statistical significance.
3. Results and Discussion
Pseudomonas aeruginosa was used due to its high proteolytic capacity as described by Heywood and Lamont [28]; similarly, the biochemical characterization of Serratia sp. QCS23 revealed its ability to ferment glucose, sorbitol, inositol, and o-nitrophenyl-beta (ONPG). Conversely, the QCS23 isolate did not show positive reactions for raffinose, urease, rhamnose, H2S, maltose, arabinose, indole, and oxidase. These findings were consistent with those reported by Yehia et al. [29] and aligned with the characteristics described in this study, presumptively agreeing with the description of Serratia marcescens in Bergey’s Manual, as mentioned in the work of Zhang et al. [30].
Figure 1 illustrates the comparative fermentation of the exoskeleton by Pseudomonas aeruginosa QF50 and Serratia sp. QCS23. For Pseudomonas aeruginosa, glucose concentrations increased from 1% to 10%, correlating with an increase in the amount of crude chitin from 58.83 ± 2.30 to a maximum of 76.81 ± 7.64%. Similarly, Serratia sp. QCS23 showed an increase in crude chitin yield from 59.25 ± 8.30 to a maximum of 71.30 ± 1.16%. These results suggest a dose-dependent relationship between glucose concentration and crude chitin production, although the consistency of this relationship varies between bacterial species. The highest yields observed in this study differ from those reported by Suryawanshi et al. [31], who obtained chitin purities of 88.6% and 98.7% from Penaeus monodon and Litopenaeus stylirostrys shrimp, respectively, using a chemical extraction with 0.25 M citric acid and 1 N HCl. These results also surpass those found by Tan et al. [32], who achieved a chitin yield of 57.7% from shrimp waste using Lactobacillus acidophilus FTDC3871 at 15% (w/w) glucose and 5% (v/w) inoculum after 72 h at 37 °C. Additionally, these findings exceeded the results of Li et al. [33], who obtained a chitin yield of 16.3%, inoculated with Lactobacillus fermentum, in shrimp byproducts with a single-step fermentation.
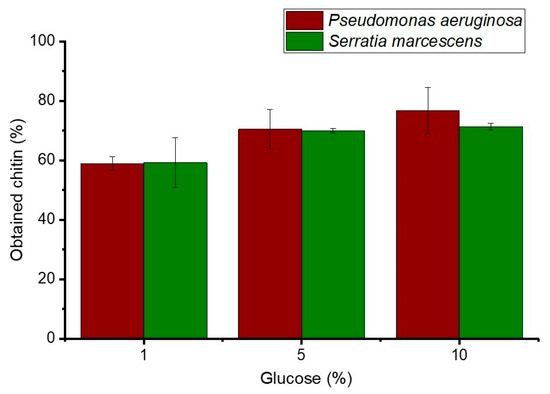
Figure 1.
Comparative analysis of chitin extraction percentages obtained through fermentation by Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 under varying glucose concentrations.
According to Table 1, both Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 demonstrated variable deproteinization efficiencies, ranging from 80 to 87% depending on the glucose concentration. The presence of statistical significance at 1% and 10% glucose for Pseudomonas aeruginosa QF50 suggests that the effectiveness of this strain is effective in deproteinization under conditions of low and high glucose concentrations. The marginal difference of 1% between the strains indicates comparable efficacy at low glucose concentrations; however, Pseudomonas aeruginosa QF50 maintains its efficiency at high concentrations. This can be attributed to the metabolic characteristics of the strain under higher glucose availability. At increased glucose concentrations, it is likely that the metabolic pathways of Pseudomonas aeruginosa QF50 shift towards the enhanced production of enzymes involved in protein breakdown [34]. For demineralization, Pseudomonas aeruginosa QF50 showed a notable increase in efficiency (24.02 ± 0.35%) at a 10% glucose concentration, suggesting enhanced effectiveness at higher glucose levels, potentially due to increased production of acids or chelating agents that aid in mineralization [35]. As glucose levels rise, they are more actively metabolized, leading to the increased production of organic acids. These acids dissolve the calcium carbonate in crustacean exoskeletons, thus exposing the internal matrix and enhancing impurity removal, including pigmentation. This process is highlighted by Zhou et al. [36], who observed a significant increase in demineralization (99%) as glucose levels reached 3%, using Lacticanttacllus pantarum LA01 to treat crab shells. Similarly, Serratia sp. QCS23 also exhibited an increase in demineralization efficiency (21.20 ± 0.78%) with increasing glucose concentrations, indicating that while it may be less efficient at lower glucose levels, its demineralization capability is significantly enhanced at higher concentrations.

Table 1.
Levels of deproteinization and demineralization of shrimp exoskeleton by Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 at different glucose concentrations.
These results exceed those reported by Ta et al. [37] who achieved an 80.9% deproteinization after two days of incubation using Yarrowia lipolytica and achieved a demineralization range of 38.2–49.4% with shrimp head waste. In contrast, Gharibzadeh et al. [38] reached a maximum value of 91.22% deproteinization using Bacillus licheniformis at 45 °C and 96.55% demineralization using Lactobacillus plantarum at 37 °C, both during a single-step process. Zare et al. [39] reported that with 10% glucose, they achieved 81% demineralization and 62% deproteinization from shrimp shell waste using co-fermentation with Lactiplantibacillus plantarum PTCC 1745 and Bacillus subtilis PTCC 1720.
Table 2 presents the most representative numerical values of the CIEL*a*b* color space results for raw chitin extracted by fermentation. For chitin extracted by Serratia marcescens, decreases at 10% glucose concentration showed a decrease in luminosity (81.46 ± 3.50), indicating the production of darker chitin, and an increase in red (3.30 ± 1.35) and yellow (17.40 ± 2.81) tones. This suggests that higher glucose concentrations may affect the composition or purity of the chitin. Conversely, chitin extracted by Pseudomonas aeruginosa QF50 showed notable consistency in luminosity (86.33 ± 0.64) across glucose concentrations, with reduced red (0.15 ± 0.21) and yellow (13.34 ± 1.63) tones, which could reflect the greater purity and stability of the chitin obtained under these conditions (Figure 2).

Table 2.
CIEL*a*b* color space results of the reflective surface of the extracted raw chitin.

Figure 2.
Images of shrimp exoskeleton samples at different stages of chitin extraction: (A) the initial exoskeleton; (B) the conditioned exoskeleton; (C) treatment with Pseudomonas aeruginosa QF50; and (D) treatment with Serratia marcescens QCS23.
In chitin production, the use of Serratia marcescens QCS23 resulted in a decreasing trend in the whiteness index, which dropped from 79.95 ± 1.11 to 74.36 ± 21.26. This decline not only indicates a reduction in whiteness but also a significant increase in result variability, which correlates with changes in glucose concentration. Conversely, the use of Pseudomonas aeruginosa QF50 demonstrated an improvement in the whiteness index, increasing from 78.35 ± 12.40 to 80.90 ± 2.53, along with a notable decrease in variability. This suggests a potential optimization of the process, yielding chitin of a whiter and more consistent quality (Figure 2). Regarding luminosity values (L*), the chitin produced surpassed the standard value of 47.9, as well as the value of 79 reported by Hahn et al. [40], who achieved this after bleaching Hermetia illucens cultivation waste with a NaOCl solution, following treatment with 0.5 M formic acid for 2 h at 40 °C and 1.25 N NaOH at 90 °C for 4 h. Additionally, the whiteness index (WI) obtained is comparable to the 83.1 reported by Ploydee and Chaiyanan [41], who used a two-step biological process with Lactobacillus pentosus L7 and Bacillus thuringiensis SA to extract chitin from shrimp. These results highlight the significant impact of microbial selection and fermentation conditions on the optical properties of chitin and its potential suitability for various applications [42].
The FT-IR spectra of the chitin extracted by Pseudomonas aeruginosa, Serratia sp., and commercial chitin are depicted in Figure 3. The spectra exhibited O-H stretching bands at 3428, 3424, and 3427 cm−1 for chitin from Pseudomonas, Serratia, and commercial sources, respectively [43]. Additionally, N-H stretching bands were observed at 3257 and 3254 cm−1 for the chitin extracted by Pseudomonas and Serratia, while commercial chitin displayed a band at 3100 cm−1 [44]. Aliphatic CH3 symmetrical stretching was identified between 2871 and 2879 cm−1 across all samples, indicating minimal variation and suggesting that the aliphatic structure is preserved across different extraction methods [45]. The Amide I band, associated with C=O secondary amide stretching, was recorded at 1655 and 1652 cm−1 for the extracted chitin, and at 1651 cm−1 for the commercial sample [46]. Bands corresponding to Amide II, which include N-H bending and C-N stretching, were found close to 1549, 1550, and 1547 cm−1 for all samples [47]. This consistency implies that the secondary structure of the amide groups is maintained regardless of the chitin source. Bands at 1418–1424 cm−1 were attributed to CH2 bending and CH3 deformation, while those at 1368–1378 cm−1 were associated with similar vibrational modes [48]. The presence of Amide III, indicated by CH2 wagging, was identified around 1308–1307 cm−1, suggesting the presence of protein components [49]. Finally, bands ranging from 1009 to 1109 cm−1 were attributed to asymmetric oxygen bridging and C-O stretching, which are characteristic of the glycosidic bonds in the chitin structure [50].
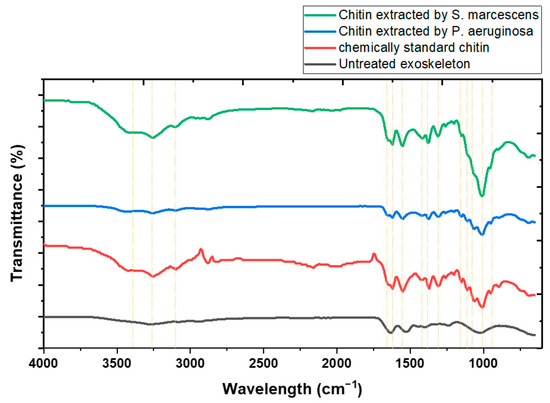
Figure 3.
IR spectrum of chitin obtained through fermentation using Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 compared to commercial standard chitin and shrimp exoskeleton.
The observed FT-IR bands around 3427–3428 cm−1 for O-H stretching and 1651–1655 cm−1 for Amide I, as well as the C-O stretching bands in the range of 1009–1109 cm−1, are characteristic of alpha chitin, as reported in the literature. Additionally, the N-H stretching bands near 3257 and 3254 cm−1 align with the spectral features of alpha chitin from the samples obtained, consistent with findings by Hisham et al. [51]. The similarity of the FT-IR bands of the chitin examined in this study to those of α-chitin extracted from shrimp (Fenneropenaeus chinensis) shell waste, as reported in another study [52], further corroborates the identification of the chitin type.
Figure 4 shows the XRD patterns of the extracted chitin, displaying two main peaks around 9–9.3 and 19.3 (2θ), accompanied by three weaker peaks at 12.2, 23.3, and 25.9–26.2 (2θ). These peaks are indicative of the ordered atomic arrangement characteristic of the crystal structure of α-chitin [53]. Additionally, a diffraction peak at approximately 29.4 (2θ) was identified, which can be attributed to the presence of calcium carbonate (CaCO3) [54]. This peak suggests residual mineral content in the extracted chitin, with a more pronounced intensity observed in samples fermented with Serratia sp. QF50. The crystallinity index (CI) of chitins extracted through single-step fermentation using S. marcescens QCS23 and Pseudomonas aeruginosa QF50 was determined to be 71% and 81%, respectively. These values are in close agreement with those reported by Xin et al. [55], who found CI values ranging from 80 to 82% in shrimp (Penaeus orientalis) chitin obtained through fermentation with MS 10017 and E. profundum; however, these findings differ from those of Gharibzdeh et al. [53], who reported a CI as high as 93.9% from green tiger shrimp waste using Bacillus licheniformis and Lactobacillus plantarum. The variation in CI percentages may be attributed to the presence of mineral traces and residual proteins that were not completely removed during the extraction process [27].
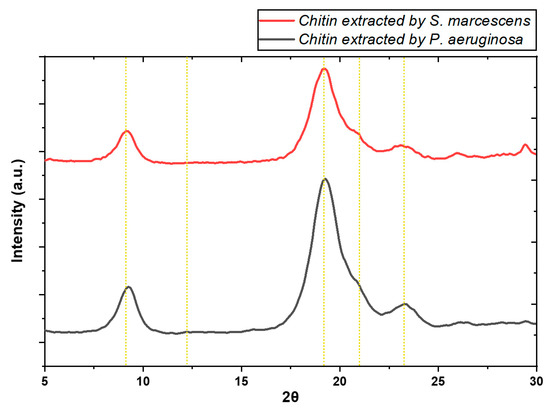
Figure 4.
XRD spectrum of chitin extracted by fermentation using Serratia sp. QCS23 and Pseudomonas aeruginosa QF50.
4. Conclusions
This study has demonstrated that one-step fermentation using Pseudomonas aeruginosa QF50 and Serratia sp. QCS23 is an effective and sustainable method for extracting chitin from shrimp exoskeletons. The use of Pseudomonas aeruginosa QF50 led to significant increases in crude chitin yield (76.81 ± 7.64%) and maintained a high quality in terms of luminosity and whiteness index (80.90 ± 2.53), even at high glucose concentrations. Serratia sp. QCS23 also showed promising results, with good yield and process efficiency. Both strains achieved high deproteinization and demineralization efficiencies, essential for the purity of the final chitin product.
This study’s findings suggest that the fermentation process can be optimized based on the bacterial strain and glucose concentration to enhance the yield and quality of chitin. The higher crystallinity index observed with Pseudomonas aeruginosa QF50 indicates a more ordered crystalline structure, which is desirable for many applications. Overall, the results indicate that bacterial fermentation is a viable and ecofriendly alternative to conventional chemical extraction methods, with the potential to contribute to the circular economy by valorizing seafood waste. These conclusions highlight the importance of continued research into microbial fermentation processes for chitin extraction and the optimization of conditions to maximize yield and quality for industrial applications.
Author Contributions
Conceptualization, C.Q.-C. and J.C.R.-S.; methodology, C.Q.-C., L.C.-C. and R.M.-R.; software, F.H.-B. and W.U.-L.; validation, L.C.-C.; formal analysis, F.H.-B. and W.U.-L.; investigation, R.M.-R., E.L.-Q. and J.G.-R.; data curation, L.C.-C.; writing—original draft preparation, C.Q.-C.; writing—review and editing, J.C.R.-S. and W.U.-L.; visualization, J.G.-R. and E.L.-Q.; supervision, C.Q.-C.; project administration, C.Q.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by the Universidad Cesar Vallejo.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the infrastructure and support of the Laboratorio de Biotecnología e Ingeniería Genética of the Facultad de Ciencias Biológicas of the Universidad Nacional de Trujillo.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pighinelli, L. Methods of Chitin Production a Short Review. Am. J. Biomed. Sci. Res. 2019, 3, 307–314. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.; De-Lima, M.A.B.; Franco, L.O.; De Campos-Takaki, G.M. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Lizárraga, A.; Escobedo-Lozano, A.; Mendez, E.; Vásquez-Olivares, A.; Martinez-Sanchez, H. Extraction, partial characterization and evaluation of in vitro digestibility of the protein associated with the exoskeleton of white shrimp (Litopenaeus vannamei). Rev. Bio Cienc. 2014, 2, 293–301. [Google Scholar]
- Gadgey, K.K.; Bahekar, A. Studies on extraction methods of chitin from crab shell and investigation of its mechanical properties. Int. J. Mech. Eng. Technol. 2017, 8, 220–231. [Google Scholar]
- Anwar, M.; Anggraeni, A.S.; Amin, M.H. Comparison of green method for chitin deacetylation. AIP Conf. Proc. 2017, 1823, 020071. [Google Scholar]
- Rojas, J.; Madrigal, J.; Ortiz, J. Effect of acid hydrolysis on tableting properties of chitin obtained from shrimp heads. Trop. J. Pharm. Res. 2015, 14, 1137–1144. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Guemiza, K.; Rouissi, T.; Sarma, S.J.; Brar, S.K. Novel biological and chemical methods of chitin extraction from crustacean waste using saline water. J. Chem. Technol. Biotechnol. 2016, 91, 2331–2339. [Google Scholar] [CrossRef]
- Chakravarty, J.; Yang, C.L.; Palmer, J.; Brigham, C.J. Chitin extraction from lobster shell waste using microbial culture-based methods. Appl. Food Biotechnol. 2018, 5, 141–154. [Google Scholar]
- Gamal, R.F.; El-Tayeb, T.S.; Raffat, E.I.; Ibrahim, H.M.; Bashandy, A.S. Optimization of chitin yield from shrimp shell waste by Bacillus subtilis and impact of gamma irradiation on production of low molecular weight chitosan. Int. J. Biol. Macromol. 2016, 91, 598–608. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, R.; Yang, H.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Chitin extraction from shrimp (Litopenaeus vannamei) shells by successive two-step fermentation with Lactobacillus rhamnoides and Bacillus amyloliquefaciens. Int. J. Biol. Macromol. 2020, 148, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, F.; Yousefzadi, M.; Toiserkani, H.; Najafipour, S. Bioconversion of shrimp waste Penaeus merguiensis using lactic acid fermentation: An alternative procedure for chemical extraction of chitin and chitosan. Int. J. Biol. Macromol. 2017, 104, 883–888. [Google Scholar] [CrossRef]
- Castro-Morán, J.J.; Ordinola-Zapata, A. La estrategia de ayuno y realimentación, una alternativa viable para optimizar el consumo de alimento balanceado en el cultivo semi-intensivo de camarón blanco Litopenaeus vannamei. Rev. Investig. Vet. Perú 2021, 32, e19546. [Google Scholar] [CrossRef]
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A review of various sources of chitin and chitosan in nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Rodrigues, C.; Lapolli, F.R.; Lobo-Recio, M.A. Physicochemical characterization of white shrimp (Litopenaeus vannamei) waste as a low-cost chitinous biomaterial. J. Polym. Environ. 2021, 29, 576–587. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean waste-derived chitosan: Antioxidant properties and future perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Quiñones, C.; Saavedra, J.B.; Urquizo, D.; Esparza, M. Biodegradation of phenol by Pseudomonas aeruginosa isolated from oil contaminated environments in Peru. Biosci. Res. 2021, 18, 1294–1300. [Google Scholar]
- Zhang, H.; Jin, Y.; Deng, Y.; Wang, D.; Zhao, Y. Production of chitin from shrimp shell powders using Serratia marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydr Res. 2012, 362, 13–20. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Williams, P.A.; Tverezovskaya, O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocoll. 2013, 31, 166–171. [Google Scholar] [CrossRef]
- Zhang, H.; Yun, S.; Song, L.; Zhang, Y.; Zhao, Y. The preparation and characterization of chitin and chitosan under large-scale submerged fermentation level using shrimp by-products as substrate. Int. J. Biol. Macromol. 2017, 96, 334–339. [Google Scholar] [CrossRef]
- Ombelet, S.; Natale, A.; Ronat, J.B.; Vandenberg, O.; Hardy, L.; Jacobs, J. Evaluation of microscan bacterial identification panels for low-resource settings. Diagnostics 2021, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.H.; Jung, W.J.; Kuk, J.H.; Oh, K.T.; Kim, Y.J.; Park, R.D. Screening of protease-producing Serratia marcescens FS-3 and its application to deproteinization of crab shell waste for chitin extraction. Carbohydr. Polym. 2008, 74, 504–508. [Google Scholar] [CrossRef]
- Poerio, A.; Petit, C.; Jehl, J.P.; Arab-Tehrany, E.; Mano, J.F.; Cleymand, F. Extraction and Physicochemical Characterization of Chitin from Cicadaorni Sloughs of the South-Eastern French Mediterranean Basin. Molecules 2020, 25, 2543. [Google Scholar] [CrossRef] [PubMed]
- Janairo, G.; Sy, M.; Yap, L.; Llanos-Lazaro, N.; Robles, J. Determination of the Sensitivity Range of Biuret Test for Undergraduate Biochemistry Experiments. J. Sci. Technol. 2011, 6, 77–83. [Google Scholar]
- Terrones, N.; Quiñones-Cerna, C.E.; Robles, M.; Cruz-Monzon, J.A.; Butrón, F.; Rodríguez, J.C. Optimization of Total Carotenoid Production by Rhodotorula mucilaginosa from Artichoke Agroindustrial Waste Using Response Surface Methodology. Environ. Res. Eng. Manag. 2023, 79, 111–121. [Google Scholar] [CrossRef]
- Demir, D.; Öfkeli, F.; Ceylan, S.; Bölgen, N. Extraction and characterization of chitin and chitosan from blue crab and synthesis of chitosan cryogel scaffolds. J. Turkish Chem. Soc. Sect. A Chem. 2016, 3, 131–144. [Google Scholar] [CrossRef]
- Huang, W.C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef] [PubMed]
- Heywood, A.; Lamont, I.L. Cell envelope proteases and peptidases of Pseudomonas aeruginosa: Multiple roles, multiple mechanisms. FEMS Microbiol. Rev. 2020, 44, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Yehia, A.; Ahmed, A.; Amira, S. Optimization of prodigiosin production by a new isolate of Serratia species. Int. J. Res. Med. Basic. Sci. 2016, 2, 30–49. [Google Scholar]
- Zhang, Y.; Shang, R.; Zhang, J.; Li, J.; Zhu, G.; Yao, M.; Sun, J.; Shen, Z. Isolation and identification of two Serratia marcescens strains from silkworm, Bombyx mori. Antonie Leeuwenhoek 2020, 113, 1313–1321. [Google Scholar] [CrossRef]
- Suryawanshi, N.; Jujjavarapu, S.E.; Ayothiraman, S. Marine shell industrial wastes–an abundant source of chitin and its derivatives: Constituents, pretreatment, fermentation, and pleiotropic applications-a revisit. Int. J. Environ. Sci. Technol. 2019, 16, 3877–3898. [Google Scholar] [CrossRef]
- Tan, J.S.; Abbasiliasi, S.; Lee, C.K.; Phapugrangkul, P. Chitin extraction from shrimp wastes by single step fermentation with Lactobacillus acidophilus FTDC3871 using response surface methodology. J. Food Process Preserv. 2020, 44, e14895. [Google Scholar] [CrossRef]
- Li, J.; Song, R.; Zou, X.; Wei, R.; Wang, J. Simultaneous preparation of chitin and flavor protein hydrolysates from the by-products of shrimp processing by one-step fermentation with Lactobacillus fermuntum. Molecules 2023, 28, 3761. [Google Scholar] [CrossRef] [PubMed]
- Solihin, J.; Waturangi, D.E.; Purwadaria, T. Induction of amylase and protease as antibiofilm agents by starch, casein, and yeast extract in Arthrobacter sp. CW01. BMC Microbiol. 2021, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Ghssein, G.; Ezzeddine, Z. A Review of Pseudomonas aeruginosa Metallophores: Pyoverdine, Pyochelin and Pseudopaline. Biology 2022, 11, 1711. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, H.; Xing, R.; Liu, S.; Li, K.; Li, R.; Yu, H.; Li, P. Chitin extraction from crab shells by two-step fermentation with Lacticanttacllus pantarum and Pseudomonas aeruginosa. Res. Sq. 2024, 1, 1–37. [Google Scholar]
- Ta, T.M.; Bui, H.H.; Trinh, T.T.; Nguyen, T.M.; Nguyen, H.N. Investigation of chitin recovery from shrimp waste by yeast fermentation. IOP Conf. Ser. Earth Environ. Sci. 2023, 1155, 012012. [Google Scholar] [CrossRef]
- Gharibzadeh, M.; Osfouri, S.; Jamekhorshid, A.; Jafari, S.A. Microbial chitin extraction and characterization from green tiger shrimp waste: A comparative study of culture mediums along with bioprocess optimization. Int. J. Biol. Macromol. 2023, 242, 125213. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Jafari, Z.; Darzi, H.H. Production of chitin and chitosan from shrimp shell wastes using co-Fermentation of Lactiplantibacillus plantarum PTCC 1745 and Bacillus subtilis PTCC 1720. Appl Food Biotechnol. 2022, 9, 311–320. [Google Scholar]
- Hahn, T.; Tafi, E.; von Seggern, N.; Falabella, P.; Salvia, R.; Thomä, J.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Stegbauer, L.; et al. Purification of chitin from pupal exuviae of the black soldier fly. Waste Biomass Valorization 2022, 13, 1993–2008. [Google Scholar] [CrossRef]
- Ploydee, E.; Chaiyanan, S. Production of high viscosity chitosan from biologically purified chitin isolated by microbial fermentation and deproteinization. Int. J. Polym. Sci. 2014, 2014, 162173. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Al Shaqsi, N.H.K.; Al Hoqani, H.A.S.; Hossain, M.A.; Al Sibani, M.A. Isolation, characterization and standardization of demineralization process for chitin polymer and minerals from the crabs waste of Portunidae segnis. Adv. Biomark. Sci. Technol. 2020, 2, 45–58. [Google Scholar] [CrossRef]
- Taokaew, S.; Zhang, X.; Chuenkaek, T.; Kobayashi, T. Chitin from fermentative extraction of crab shells using okara as a nutrient source and comparative analysis of structural differences from chemically extracted chitin. Biochem. Eng. J. 2020, 159, 107588. [Google Scholar] [CrossRef]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Kamal, M.; Adly, E.; Alharbi, S.A.; Khaled, A.S.; Rady, M.H.; Ibrahim, N.A. Exploring Simplified Methods for insect chitin extraction and application as a potential alternative bioethanol resource. Insects 2020, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Muralisankar, T.; Jayakumar, R.; Rajeevgandhi, C. A study on structural comparisons of α-chitin extracted from marine crustacean shell waste. Carbohydr. Polym. Technol. Appl. 2021, 2, 100037. [Google Scholar] [CrossRef]
- Son, Y.; Hwang, I.; Nho, C.; Kim, S.; Kim, S. Determination of carbohydrate composition in mealworm (Tenebrio molitor L.) Larvae and characterization of mealworm chitin and chitosan. Foods 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Ozel, N.; Elibol, M. Chitin and chitosan from mushroom (Agaricus bisporus) using deep eutectic solvents. Int. J. Biol. Macromol. 2024, 262, 130110. [Google Scholar] [CrossRef]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct conversion of chitin into a N-containing furan derivative. Green. Chem. 2014, 16, 2204–2212. [Google Scholar] [CrossRef]
- Hisham, F.; Maziati-Akmal, M.H.; Ahmad, F.B.; Ahmad, K. Facile extraction of chitin and chitosan from shrimp shell. Mater. Today Proc. 2021, 42, 2369–2373. [Google Scholar] [CrossRef]
- Hu, X.; Tian, Z.; Li, X.; Wang, S.; Pei, H.; Sun, H.; Zhang, Z. Green, Simple, and Effective Process for the Comprehensive Utilization of shrimp shell waste. ACS Omega 2020, 5, 19227–19235. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xie, W.; Yu, J.; Xin, R.; Shi, Z.; Song, L.; Yang, X. Extraction of chitin from shrimp shell by successive two-step fermentation of Exiguobacterium profundum and Lactobacillus acidophilus. Front. Microbiol. 2021, 12, 677126. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Halfar, J.; Adey, W.H.; Nash, M.; Paulo, C.; Dittrich, M. The role of chitin-rich skeletal organic matrix on the crystallization of calcium carbonate in the crustose coralline alga Leptophytum foecundum. Sci. Rep. 2019, 9, 11869. [Google Scholar] [CrossRef]
- Xin, R.; Xie, W.; Xu, Z.; Che, H.; Zheng, Z.; Yang, X. Efficient extraction of chitin from shrimp waste by mutagenized strain fermentation using atmospheric and room-temperature plasma. Int. J. Biol. Macromol. 2020, 155, 1561–1568. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).