Abstract
Inspired by the vast potential of microalgae in the bioeconomy and the numerous applications and benefits associated with their cultivation, a multitude of pilot- and industrial-scale microalgae production systems have been developed in recent years. Both open and closed cultivation systems have been successfully utilized, with closed photo-bioreactors (PBRs) emerging as the most versatile option for various applications and products, enabling the implementation of advanced optimization strategies. Therefore, this short review provides a comprehensive overview of the different PBR configurations and their recent applications, primarily in large-scale but also in pilot- and laboratory-scale microalgae cultivation. A detailed discussion of the advantages, limitations, specific applications and recent advancements of each type of PBR is presented to aid researchers, engineers and industry stakeholders in selecting the most suitable PBR design for their specific goals and constraints. Moreover, this review highlights the major challenges impeding the full commercialization of microalgal products and forecasts future trends in the microalgae-based industry. The diverse potential applications of microalgae in various sectors, including biofuels, nutraceuticals, pharmaceuticals, agriculture and environmental remediation, underscore the versatility and significance of the relevant cultivation technologies. By offering valuable insights into the future commercial scale and trends of microalgal biotechnology, this work sheds light on the challenges and opportunities facing this burgeoning industry.
Keywords:
photobioreactors; PBR; microalgae; cultivation; flat-panel; tubular; bubble column; closed systems; membrane; biomass 1. Introduction
Nowadays, microalgae stand out as a promising and sustainable solution for the bioeconomy, serving as a cornerstone in promoting green growth and tackling environmental issues [1]. Their remarkable efficiency in converting sunlight and CO2 into biomass and valuable compounds positions them as a renewable and adaptable resource for diverse applications. Microalgae, spanning various classes, such as chlorophyta and cyanobacteria, exhibit notably higher photosynthetic efficiency when compared to terrestrial plants. This characteristic enables them to produce biomass up to ten times faster and more efficiently. This highlights nature’s inherent ability to offer innovative and environmentally friendly solutions, paving the way for a more sustainable future [2].
Microalgae are rich in lipids, which can be converted into liquid transportation biofuels such as biodiesel and sustainable aviation fuels (SAF). The cultivation of microalgae for biofuel production potentially offers a carbon-neutral alternative to traditional fossil fuels, provided that the productivity and effectiveness of the different production systems can be further enhanced [3]. This can be realized by improving the performance of microalgal species and/or photo-bioreactor (PBR) designs [4]. Some species of microalgae, like Arthrospira and Chlorella, are abundant in proteins, vitamins and essential fatty acids, making them valuable nutritional supplements for the food and feed industries, thereby contributing to human and animal health [5]. In addition, high-value compounds derived from microalgae, like pigments and antioxidants, find application in cosmetics and pharmaceuticals, due to their antioxidant, anti-aging, anti-inflammatory and other beneficial properties [6]. On the other hand, microalgae can be employed in applications of lower value, such as wastewater treatment to remove nutrients and pollutants. They possess the ability to assimilate contaminants, thus aiding in water purification [7]. Moreover, microalgae play a major role in carbon capture by fixing CO2 during photosynthesis. Integrating microalgae cultivation with existing industrial plants has the potential to reduce their greenhouse gas (GHG) emissions [8]. Still, there are a lot of challenges to be addressed regarding the insufficient robustness of microalgal strains to CO2-laden off-streams, the low production yields, the difficulties with biomass harvesting and processing, the limitations with product extraction and purification, the availability of stable CO2 in liquid form and the effective valorization of all biomass fractions to achieve an economically competitive biorefinery scheme. These challenges can all be directly attributed to the chosen PBR design for the cultivation of microalgae. In addition, certain microalgal species have the capability to absorb heavy metals and other pollutants from the environment, making them valuable for bioremediation efforts in contaminated areas [9].
Microalgae cultivation at a large scale involves providing optimal conditions for their growth, including light, nutrients, temperature and pH. There are various cultivation methods, each with its advantages and challenges. Initially, open systems typically consist of large outdoor ponds that expose microalgae to natural sunlight. While cost-effective, open ponds are susceptible to contamination and require careful management of the environmental conditions [10]. In contrast, closed cultivation systems involve enclosed photo-bioreactors (PBRs) that provide a controlled environment for microalgae cultivation. They offer protection from contaminants and allow precise control of the parameters, but can be more expensive to set up and operate [11]. Combining elements of both open and closed systems, hybrid approaches seek to capitalize on the strengths of each – for instance, using closed systems for initial growth and transferring to open ponds for the final cultivation stage [12]. A comparison of the advantages and disadvantages of open vs. closed systems is outlined in Table 1. The choice between them depends on the specific goals of cultivation, the scale and the economic viability of the chosen method. As technology advances, the optimization of cultivation practices and exploration of new applications are expected to contribute to the continued growth of the microalgae-based industry.

Table 1.
Advantages and challenges of microalgae cultivation systems: open vs. closed.
Beyond the system itself, microalgae cultivation involves various strategies to optimize biomass production and overall efficiency. These strategies consider factors such as the nutrient supply, light exposure, temperature control and harvesting methods [13]. Microalgae can be cultivated through different modes, including photo(auto)trophic, mixotrophic and heterotrophic cultivation, each offering unique advantages and challenges. Phototrophic cultivation relies solely on light as the energy source, making it suitable for outdoor pond systems or well-lit PBRs [14]. Mixotrophic cultivation involves supplementing light with organic carbon sources, such as sugars or waste streams, to enhance the growth rates and biomass yields [15]. Heterotrophic cultivation utilizes organic carbon sources as the primary energy and carbon substrates, making it suitable for dark fermentation or controlled bioreactor systems [16]. The choice of cultivation mode depends on factors such as the microalgal species’ characteristics, the available resources and the desired product profile, and can be coupled with the carbon supply strategy.
For example, in batch cultivation, the selected species is cultivated in a closed system without the addition of fresh nutrients during the growth phase. It is the simplest and easiest operation to implement, typically suitable for small-scale operations. However, limited biomass productivity is, in general, observed due to nutrient depletion and longer overall cultivation times [17]. It should be noticed, though, that this limitation may be overcome by high-end PBR designs, like an open thin-layer system that allows the cultivation of microalgae with an adequate cell density [18]. A continuous cultivation strategy presupposes that fresh nutrients are continuously supplied in the system, and a fraction of the culture is harvested regularly, maintaining a steady state. This approach yields higher biomass productivity and a reduced cultivation duration. However, it is a more complex operation with potential for contamination and higher energy consumption [19]. An intermediate between the above two modes, fed-batch cultivation allows nutrients to be added incrementally during the cultivation process, thereby offering extended growth periods with controlled nutrient concentrations. In general, fed-batch cultures demonstrate improved biomass productivity compared to batch cultivation, as well as better control over the nutrient levels [20].
Choosing the appropriate cultivation strategy strongly depends on the characteristics of the targeted microalgal species, the available resources and, most importantly, on the cultivation system; closed PBRs are the obvious selection when advanced processes are pursued. Additionally, the cost and availability of nutrients play a crucial role in microalgae cultivation. Fine chemicals, such as commercially sourced nutrient solutions, may offer precise control over the nutrient composition but can be expensive, especially for large-scale operations [21]. Conversely, waste streams or residues and by-products from other industries can serve as cost-effective nutrient sources, enhancing the sustainability and economic viability of microalgae cultivation [22]. The optimization of the nutrient sources and cultivation strategies is essential in enhancing biomass productivity while minimizing the costs and environmental impact.
The ever-increasing interest in PBRs for microalgae cultivation, especially at a pilot and large scale, is evidenced by the number of relevant publications over the past few years. As depicted in Figure 1a, there is a clear trend in designing, developing, applying, intensifying and optimizing numerous PBR configurations for various applications. Over the last four to five years (2020 and onwards), the majority of research articles have focused on tubular, flat-panel, bubble-column and membrane PBRs (Figure 1b). Following these trends, the present work aims to systematically present and compare the majority of the available PBR designs for microalgae cultivation. In what follows, we highlight common and innovative PBR systems developed to cultivate well-known and new species for various applications. Subsequently, we discuss the major characteristics of each PBR type and attempt to forecast the future commercial status of microalgae. The focus is placed on pilot- and industrial-scale PBRs, although laboratory-scale closed cultivation systems are also addressed to showcase the variety of PBR designs and setups.
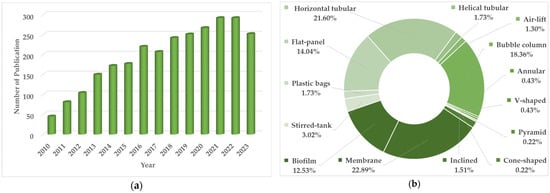
Figure 1.
(a) Number of annual publications dealing with the cultivation of microalgae in photo-bioreactors (PBRs); (b) distribution of published papers per employed PBR type and configuration. (Data from Scopus, accessed on 2 May 2024. For (a), the search was performed within ‘Article title, Abstract, Keywords’ using the following keywords: microalgae AND photobioreactors OR photo-bioreactors; 2010–2023. For (b), the search was performed within ‘Article title, Abstract, Keywords’ using the following keywords: microalgae AND photobioreactors OR photo-bioreactors AND each PBR type; 2020–2023.
2. Recent Advances in Closed Pilot- and Large-Scale PBRs for Microalgae Cultivation
Undoubtedly, the design of case-specific photo-bioreactor configurations for microalgae cultivation is a critical aspect in enhancing biomass production and optimizing the overall performance of such systems. In the following discussion, selected examples of different PBR types are explored within the context of recent publications, which are also compiled in a list in Table 2. For this short review, representative recent publications are selected to showcase the variety of PBRs, the diversity of the microalgal species and their multi-sectoral applications. Each study includes a performance indicator, particularly biomass growth and production. Moreover, the comparison of PBRs extends to Table 3, which outlines their major advantages, disadvantages and other key characteristics.

Table 2.
Most common photo-bioreactor (PBR) configurations used for the cultivation of selected species during the last few years.

Table 3.
Major advantages and disadvantages of common PBR types, as well as cost considerations (CAPEX: capital expenditures; OPEX: operational expenditures) and indicative applications for each one.
2.1. Stirred-Tank PBRs
Uyar et al. [23] used a 2.4 L typical stirred-tank PBR to cultivate Chlorella sorokiniana and compared the results regarding biomass production with other systems like air-lift and bubble column PBRs. Under the selected conditions, microalgae growth was limited to 0.064 g·L−1·day−1 in the stirred-tank PBR due to the relatively small volumetric mass transfer. Similarly, using an analogous 2 L stirred-tank PBR, Doppler et al. [24] cultivated a novel strain, i.e., Coelastrella terrestris, aiming to produce the rare keto-carotenoid adonixanthin (0.13 mg·L−1·day−1), as well as high levels of unsaturated fatty acids (85% w/w). Upon screening and optimization experiments, a promising cultivation profile was discovered for the selected species. Moreover, beyond phototrophic mode, stirred-tank PBRs can be easily employed for mixotrophic and heterotrophic cultivation as well. For instance, Occhipinti et al. [25] cultivated the polyextremophile red microalgal species Galdieria sulphuraria in such a PBR (volume 13 L), using buttermilk as a low-cost carbon source. The study found that lactose-containing substrates were suitable for the production of biomass at a rate equal to 0.55 g·L−1·day−1. With the objective of minimizing the production cost during large-scale biomass production from microalgae, Erbland et al. [26] designed and evaluated an internally illuminated cone-shaped-bottom 1.7 m3 stirred-tank PBR specifically for feed production. They cultivated Tetraselmis chuii in batch mode under optimal conditions regarding the temperature, pH, illumination and harvesting time, validating its potential as an economic source of biomass. As was demonstrated by the previous studies, stirred-tank PBRs allow for efficient gas exchange and nutrient distribution, enhancing microalgae growth, particularly for unicellular species; filamentous strains may require additional considerations to prevent shear-induced cell damage. On the other hand, light penetration is often limited due to self-shading phenomena caused by microalgal cells. Optimizing the light distribution through the use of internal light sources can improve the light availability. Additionally, they achieve effective CO2 transfer through sparging systems, which is essential in improving the photosynthetic activity.
2.2. Tubular PBRs
A 119 L horizontal tubular PBR technology, one of the most widely used configurations, was designed and evaluated while immersed in open waters by Francke et al. [27], to effectively control the temperature during outdoor operation. No major temperature peaks were recorded, and the stable cultivation and biomass production (0.23 g·L−1·day−1) of Tetradesmus obliquus was achieved, with a validated positive impact on energy and land savings. Schoeters et al. [28] cultivated the under-utilized red marine species Porphyridium purpureum using pilot-scale horizontal tubular reactors (up to 1.5 m3) protected in a greenhouse. By monitoring numerous sequential batch experiments conducted over a total of 2 years of cultivation, the authors calculated an adequate yield of CO2 to microalgal biomass bioconversion on a mass basis (13.5%), resulting in biomass productivity equal to 0.2 g·L−1·day−1. As a strategy for further optimization, the authors suggested capturing, recycling and reusing the off-gas CO2 emitted from the PBR. Pereira et al. [29] developed a system for the production of fucoxanthin (up to 0.7% w/w) using Phaeodactylum tricornutum during autumn and winter. To this end, a 15 m3 pilot-scale tubular flow-through PBR was utilized under a long-term semi-continuous cultivation regime, thereby increasing the application window of microalgae to non-favorable climatic conditions. In a similar vein, Olsen et al. [30] conducted outdoor cultivation in a 115 L horizontal tubular PBR, utilizing Scenedesmus sp. as a novel protein source. The protein content within the dried biomass was enhanced to 52.4% w/w, particularly the amount of total essential amino acids. Hashemi et al. [31] performed the production of β-carotene by Dunaliella salina in a 20 L indoor helical tubular PBR. This specific design allowed for the application of unbalanced conditions, such as salt stress, to stimulate the production of β-carotene (4.85 µg per mg of biomass) as a secondary metabolite during efficient cell growth. In a different application, Glockow et al. [32] installed an advanced 145 L helical tubular PBR in animal housing for the on-site treatment of exhaust gases, i.e., CO2 and NH3. The robust design of the PBR enabled the successful continuous cultivation of a mixed culture of Arthrospira sp. for several weeks, producing 0.3 g·L−1·day−1 biomass. In addition, Pavlou et al. [33] used a 5 L spiral helical PBR in a recirculating mode to cultivate Stichococcus sp. as a source of carbohydrates, proteins and lipids, following the biorefinery notion. Through bioprocess intensification, both biomass production and the accumulation of total biochemical products were simultaneously increased to 3.66 g·L−1 and 3.33 g·L−1, respectively. All relevant studies employed tubular configurations to maintain the microalgae in direct contact with the culture medium, ensuring, in this way, efficient nutrient uptake and gas exchange. This method secured excellent light exposure due to the cylindrical geometry and continuous flow of the culture medium, minimizing self-shading effects and ensuring uniform CO2 distribution, with minimal shear stress on cells. Consequently, this approach led to high biomass yields and productivity for the different systems.
2.3. Air-Driven PBRs
Dos Santos et al. [34] evaluated the production and composition of biomass from Chlorella minutissima in a semi-continuous 3.8 L air-lift PBR. They employed a statistical Design of Experiments (DoE) approach to optimize the operational profile during cultivation in a landfill leachate. Under these conditions, the selected microalgal species produced high levels of protein (69.6% w/w). Using a similar PBR configuration, equipped with an external sparger, Azhand et al. [35] investigated the effect of the input gas velocity on Chlorella vulgaris growth and CO2 fixation. The authors reported adequate growth and CO2 removal (94% efficiency), attributed to the optimized conditions for gas transfer within the 20 L PBR. In another study, Wolf et al. [36] applied physically simulated outdoor conditions during the cultivation of Dunaliella salina in a 1.8 L air-lift PBR. Their objective to efficiently produce β-carotene was fulfilled under batch conditions. They achieved an adequate density of the culture in the minimum time, boosting the accumulation of β-carotene to 25 mg·L−1. Mohamandia et al. [37] implemented a scale-up strategy for a 5 L bubble column PBR to investigate the effect of the mass transfer coefficient on the cultivation profile of Tisochrysis lutea, with a focus on improving the production of fucoxanthin. They found that maintaining a constant volumetric mass transfer coefficient enabled intensive fucoxanthin productivity (24.96 mg·L−1·day−1) in a growth medium enriched with starch. Pourbakhtiar et al. [38] employed a multi-purpose strategy, including CO2 removal, wastewater treatment and lipid production, in a two-stage 15 L bubble column PBR. They intensified the Chlorella vulgaris cultivation following a systematic approach to establish an optimal operational window for the accumulation of lipids up to 61.24% w/w. In a study by Saxena et al. [39], the cultivation of Spirulina (Arthrospira platensis) in a 10.1 L bubble column PBR was investigated. Their optimized strategy allowed for a simultaneous increase in biomass growth and carbohydrate accumulation (0.93 g/L and 74.44% w/w, respectively). Optimal conditions regarding limitations in nutrients like nitrogen and phosphorous were discovered. Targeting higher-value ingredients, such as bioactives, Macías-de la Rosa et al. [40] cultivated red tide-forming species Heterosigma akashiwo in an artificially illuminated 10 L bubble column PBR. They found the biomass growth and production of polyunsaturated fatty acids (PUFAs) and carotenoids to be adequate under well-controlled conditions (132.6, 2.3 and 0.16 mg·L−1·day−1, respectively). In all instances, the air-driven PBRs employed gas bubbles to circulate the culture medium, providing simultaneous mixing and aeration. Two key features were identified: an optimal bubble size and spacing can enhance the light availability throughout the culture volume, while gas–liquid mass transfer facilitates the nutrient uptake and metabolic activity of microalgal cells. Due to the lower shear stress compared to stirred-tank PBRs, they can accommodate both unicellular and filamentous microalgae.
2.4. Simple PBR Configurations
In a simpler approach, plastic bags can be used for low-value outdoor applications like digestate treatment from an anaerobic plant, as demonstrated by Barbato et al. [41]. A cost-effective 35 L PBR was installed within a biogas plant to cultivate Scenedesus dimorphus as the means to efficiently remove nitrogen and phosphorous sources from the culture medium. Furthermore, bag-type PBRs can also support higher-value applications. Indicatively, Chen at al. [42] cultivated Nannochloropsis oceanica in a deep-sea water-based medium for the autotrophic production of eicosapentaenoic acid. An outdoor-simulated experiment was conducted to explore the feasibility of the system (5 L volume), and it was found that the productivity was higher when a semi-batch strategy was used (9.9 mg·L−1·day−1). In a different approach, Carone et al. [43] designed and tested a new 17 L flat-panel configuration, based on an alveolar design, aiming for high microalgal biomass productivity and CO2 fixation rates by Monoraphidium sp. This pilot-scale system successfully demonstrated the efficient conversion of CO2 to microalgal biomass (0.05 g·L−1·day−1) with the minimum energy demands for liquid culture circulation and mixing. Additionally, a flat-panel PBR was employed for the mixotrophic cultivation of isolated Monoraphidium sp. and the treatment of synthetic dairy wastewater [44]. This mode improved the nutrient removal efficiency and biomass productivity (0.21 g·L−1·day−1), with the PBR performing better overall in terms of photosynthetic activity. Moreover, Guimarães et al. [45] investigated the effect of phosphorus limitation on selenium accumulation and uptake efficiency in Nannochloropsis oceanica. The cultivation was conducted again in a 1.8 L flat-panel PBR in batch mode under different conditions, resulting in a sufficiently high biomass concentration and productivity (0.89 g·L−1·day−1). The aforementioned designs generally provide simplicity and ease of implementation for microalgae of varying physiology and characteristics. When operated in a thin layer of culture medium, both nutrient uptake and gas exchange are facilitated. CO2 can be supplied through diffusion or bubbling systems, ensuring adequate carbon availability for microalgae growth.
2.5. Membrane PBRs
Aiming to exploit the resilience of Chlorella vulgaris in terms of nutrient (i.e., nitrogen and phosphorus) removal efficiency, Amini et al. [46] cultivated this species in a 10 L electrokinetic-assisted membrane PBR for wastewater treatment. By applying a low-voltage direct current, the authors effectively remediated common wastewater streams. Another example of applying a membrane PBR to wastewater treatment was demonstrated by Theepharaksapan et al. [47]. Their goal was to treat the nitrate-nitrogen and phosphate that remained in the effluent of a previous membrane bioreactor in a continuous mode, using Spirulina sp. The system (63 L volume) was evaluated as a promising water reuse and nutrient recovery solution. Roopashri and Makam [48] designed a new PBR prototype, namely a hollow-fiber membrane PBR, to produce microalgal biomass for biofuel production. They were able to increase both the biomass productivity (0.44 g·L−1·day−1) and lipid concentration (0.1 g/L) by applying a two-stage cultivation strategy for Tetradesmus obliquus. The use of membrane PBRs enabled the separation of microalgae from the growth medium, forming biofilms that facilitated nutrient uptake and metabolic activity. They also promoted the efficient mass transfer of gases across the membrane surface, enhancing the growth rates and biomass productivity. It is worth noting also that membrane PBRs are easily adaptable to various microalgal strains, with the potential to tailor the membrane properties to meet specific cultivation requirements.
2.6. Other PBR Designs
Beyond the most popular PBR designs discussed in the previous paragraphs, biofilm PBRs have been widely utilized to improve the light penetration during microalgae cultivation, while minimizing the land/area footprint of the cultivation system. Zeng et al. [49] developed, using 3D printing, a new light-conducting porous biofilm PBR based on a framework that not only supported the microalgal biofilm but also provided light for microalgal growth. By cultivating Chlorella sorokiniana, the authors identified a strategy to increase the biomass production by 82% compared to a flat biofilm PBR. The capacity of biofilm PBRs for bioremediation was demonstrated by Fan et al. [50] through the operation of a 7 L open continuous-flow biofilm PBR using Dunaliella salina. The goal to investigate the long-term performance of the system in terms of organic matter, phosphorus and nitrogen removal from saline wastewater was accomplished, validating the technology under operational conditions. Following similar cultivation principles, Štěrbová et al. [51] employed a 30 L short-light-path annular-column PBR to compare cultures at a pilot scale and suggested this system as a source of quality feed products. Among other microalgal strains, the findings of this study nominated Monodopsis sp. as a promising producer of fatty acids (up to 31.9% w/w) targeted for aquaculture feeding. This type of annular PBR allows also the cultivation of immobilized microalgae, as demonstrated by Hu et al. [52]. In their study, they facilitated the treatment of real food industrial wastewaters in an annular PBR by using alginate beads to immobilize a mixed consortium of Scenedesmus obliquus, Chlorella vulgaris and Chlorella sorokiniana. Ultimately, microalgal growth was doubled with enhanced photosynthesis.
In order to address the issue of microalgae oversaturation at high light intensities, Chin-On et al. [53] proposed an innovative V-shaped PBR for adequate light capture and dilution. They found that the biomass productivity of Chlorella sorokiniana was enhanced in this configuration (0.051 g·dm−2·day−1) compared to other PBR designs. Similarly, Khoobkar et al. [54] utilized a 16 L novel pyramid-shaped PBR for the cultivation of local Chlorella sp. They conducted studies with various light wavelengths (red, white and blue) and identified that red LED lighting was the optimal source, enhancing the accumulation of chlorophyll a to 2.7 g/g of biomass, particularly under high specific growth rates. In a separate study, Wang et al. [55] cultivated the underutilized microalgal species Oedocladium carolinianum in a 10 m3 inclined PBR with tailored orientation towards sunlight, facilitating the efficient production of astaxanthin (24.2 mg·L−1·day−1) under nitrogen-limited conditions. Simultaneously, they demonstrated the concomitant production of fatty acids to enhance the sustainability of the process.
In addition to the PBRs listed in Table 2, there are several more examples of intensified cultivation systems using innovative technologies. These include PBRs retrofitted with new technological components based on a rotating membrane system [56], a parallel spiral-flow column [57], a magnet-driven rotary mixing aerator [58], inclined baffles [59], an internally illuminated mirror [60], a Fibonacci-type vessel [61], a spiral-ascending CO2 dissolver [62], etc. Moreover, hybrid PBR systems have already been demonstrated, combining the benefits of closed PBRs like a bubble column coupled to a thin illumination platform [63] and a tubular design integrated with an air-lift system [64] or both closed PBRs and open raceways [65].
2.7. Comparison of PBRs
It is evident that closed PBRs offer distinct advantages for microalgae cultivation, and advancements in this technology continue to enhance their scalability, reliability and cost-effectiveness, making them increasingly attractive for both research and commercial microalgae cultivation endeavors. In addition to the discussion of the recent progress in PBR systems, a comparison of the major characteristics of each configuration is attempted in Table 3. Despite there being more common PBRs than others, e.g., stirred-tank, tubular and air-lift PBRs, each type presents unique characteristics.
Indicatively, flat-panel PBRs offer efficient light exposure and are suitable for research and small-scale applications, but they may suffer from fouling issues and limited scalability, while air-lift PBRs provide excellent mixing and are adaptable to various scales, with relatively lower maintenance and operational costs, making them suitable for commercial high-value products. Moreover, stirred-tank PBRs are versatile and widely used in industrial settings due to their scalability, efficient mixing and relatively low maintenance, making them suitable for large-scale biofuel production. Both horizontal and helical tubular PBRs allow enhanced illumination due to the small diameters of the tubes, in an easily scalable system that qualifies for several applications, like biofuels, food and feed. Overall, each PBR design varies in terms of efficiency, scalability, maintenance requirements, adaptability to different conditions, capital expenditures (CAPEX), operational expenditures (OPEX) and specific applications, highlighting the importance of selecting the most appropriate design based on the intended goals and constraints of the microalgae cultivation project.
3. Discussion of Future Commercial Scale and Trends for Microalgae
While microalgae hold great potential for various applications, including biofuel production, food supplements, cosmetics, pharmaceuticals, wastewater treatment, etc., several barriers hinder their full commercialization. These challenges extend to the configurations of the PBRs, which play the most crucial role in microalgae cultivation. First and foremost is the high cost of microalgae cultivation and production at a large scale, particularly in terms of nutrient inputs, the energy requirements for lighting and/or mixing and the infrastructure [66]. Innovations in PBR design and operation are essential in addressing these cost-related concerns. Efficient harvesting and processing methods are also important for the economic viability of microalgae-based technologies, especially considering that the current downstream methods are typically energy-intensive and costly [67]. High-end PBRs can streamline and facilitate harvesting and dewatering processes, reducing, in this way, the energy consumption and operational costs. Infrastructure requirements pose another challenge, as establishing large-scale production facilities usually demands significant investments in processing equipment and technology [68]. Novel PBR designs can optimize the space utilization and enhance the scalability, mitigating some of these infrastructure challenges.
Beyond cost considerations, achieving consistent quality and high yields at a larger scale can be challenging when attempting to advance the technology readiness level (TRL) of microalgae from lab- to commercial-scale production [69]. Regardless of the PBR configuration and production scale, all microalgae cultivation systems encounter significant performance losses, usually caused by microbial contamination, primarily by bacteria, protozoa and fungi [70]. Thus, tubular, flat-panel and other closed PBRs can be susceptible to contamination if not properly sterilized and maintained. Any breach or improper handling can introduce contaminants that thrive in the typically nutrient-rich, controlled environments of these systems. Therefore, an additional objective in designing PBRs should be the efficient prevention, monitoring and suppression of contamination, along with enhanced illumination systems to stimulate the growth of photoautotrophic microorganisms rather than heterotrophic ones, and also the design of appropriate cultivation media [71]. These challenges extend to the selection and development of high-yielding and robust microalgal strains suitable for large-scale cultivation, coupled with case-specific regulatory concerns and public perception [72], particularly concerning food applications or the use of genetically modified organisms (GMOs). Consequently, some microalgae-based products may face consumer acceptance challenges, especially in traditional markets where these products are not well known or understood. Moreover, entering markets with well-established products may be difficult due to competition with these conventional alternatives [73].
In some regions, there may be a lack of clear standards and regulatory frameworks for the commercial production and marketing of microalgae-based products. This could be mitigated in the future by systematically standardizing the research practices and operational guidelines for microalgae, greatly enhancing the reproducibility and quality of the available data on actual microalgae-based production systems [74]. The standardization of PBR designs and operational practices could also assist in addressing regulatory concerns and enhance market access for microalgae-based industries. In this respect, both tubular PBR types as well as flat-panel and stirred-tank PBRs are considered the most suitable for standardization, due to the advantages listed in Table 3. These benefits collectively enhance their reproducibility and scalability in both experimental and industrial applications. Furthermore, concerns related to environmental impacts and potential ecological risks may lead to the implementation of strict regulations. The impact of these regularity hurdles is intensified by supply chain issues, especially concerning the availability of raw materials [75]. Microalgae rely on the local availability of specific raw materials, such as nutrients and CO2; thus, challenges in securing a stable and cost-effective supply chain can arise. Additionally, transporting raw materials as feedstock, live microalgae or perishable products can be logistically unstainable [76].
In this uncertain ecosystem, creating awareness and educating potential consumers about the benefits of microalgae-related products and applications is essential for market development. Limited knowledge, along with insufficient product diversification, may negatively affect the overall market appeal of microalgae-based offerings [77]. These barriers could be addressed by securing sufficient funding for research, development and commercialization activities, mainly for small and new companies. Overall, a collaborative effort from researchers, industry stakeholders and policymakers is required to overcome the technical, economic and regulatory challenges associated with microalgae’s commercialization.
Microalgae cultivation and biomass production systems are of paramount importance in addressing the limitations discussed above. Innovation in PBRs, tailored to the specific requirements of each selected species and product, can facilitate the commercial-scale utilization of microalgae [78]. Undoubtedly, small laboratory-scale PBRs play a crucial role in research and development efforts aimed at advancing microalgae cultivation technologies at an industrial scale. These PBRs vary in design, each offering unique advantages and applications. Common types of laboratory-scale PBRs include flat-panel, column, tubular, plate, bag, etc., configurations, aiming to enhance the efficiency, scalability and automation, while reducing the costs and environmental impact [79]. New trends in lab-scale PBRs may include the integration of advanced sensors and monitoring systems for real-time data collection and process control [80]; the utilization of novel materials and coatings to improve light transmission, heat dissipation and nutrient absorption [81]; the development of modular and customizable PBR systems to accommodate diverse research needs and experimental setups [82]; the incorporation of advanced cultivation techniques such as photoperiod control and nutrient cycling to optimize the microalgae growth and productivity [83]; and the adoption of sustainable practices such as energy-efficient lighting and carbon capture in an immobilized PBR to minimize the environmental footprint [84]. However, there are several challenges when scaling up PBRs, including the different mixing mechanisms among the different scales, resulting in non-uniform distribution of nutrients and the potential formation of “dead” zones; inadequate light penetration, leading to shading effects and decreased productivity in specific areas of the larger-scale PBR; poor temperature control; an increased risk of contamination; and operational complexity. Among closed configurations, tubular PBRs are the most challenging to scale up because of their complex design and maintenance requirements. In contrast, flat-panel PBRs are easier to scale up due to their simpler construction and more manageable operational parameters.
The climate conditions, including temperature variations, can significantly impact the productivity and efficiency of PBRs for microalgae cultivation. Implementing temperature control measures is essential in optimizing microalgae productivity. This may include using cooling systems for warmer climates and heating elements for colder ones, as well as insulation or greenhouse structures to maintain stable growth temperatures, especially during winter periods [85]. In regions with distinct seasons, it is important to adjust the PBR operations and cultivation strategies accordingly. For example, elevated temperatures during the summer months can reduce productivity, necessitating advanced heat management [86]. By examining the characteristics of the PBRs outlined in Table 3, recommendations can be made for the use of specific types in warmer and colder climates: tubular and air-lift PBRs are well suited for warmer climates due to their ability to dissipate heat efficiently; in contrast, greenhouse PBRs are ideal for colder climates as they provide insulation and protection from low temperatures, while stirred-tank PBRs can be equipped with efficient heating systems.
Various industries already recognize the potential of microalgae in diverse applications. For instance, the use of cost-efficient PBRs could expand biofuel production, potentially through retrofitting existing biorefineries. Advances in large-scale microalgae cultivation methods have the potential to increase biofuel yields [87]. Moreover, the use of microalgae for bioremediation and environmental applications is expected to grow, provided that innovative solutions are developed for pollution control, carbon sequestration and ecological restoration within PBRs [88]. Similarly, microalgae-based products may find increasing acceptance in agriculture as sustainable alternatives to enhance soil health, promoting plant growth and improving crop yields [89]. The introduction of novel microalgae-derived products is expected to significantly diversify the nutraceutical and functional food sector. The vast majority of microalgal species can be potentially exploited as cell factories in modern industrial biotechnology, replacing existing proteins and useful fat sources [90]. This expansion could encompass personalized nutrition and dietary supplements, manufactured in controlled environments within automated PBRs, driving commercial viability [91]. High-end PBRs are also extensively utilized for ongoing research on new pharmaceutical products. An emphasis is placed on exploring the bioactive compounds present in microalgal biomass and their potential functionalities as therapeutic compounds [92]. Over time, the industry may experience market consolidation as larger companies enter the microalgae space. However, the establishment of a regulatory environment with clearer standards should foster a more conducive market for commercial-scale operations, thereby shaping the future of microalgae-based industries.
4. Conclusions
The future of photo-bioreactors for microalgae cultivation holds great promise in advancing various applications, particularly in the fields of biotechnology, environmental sustainability and renewable energy. Several key aspects need to be considered and addressed when discussing the next generation of industrial-scale PBR configurations. Firstly, a focus should be placed on developing modular and scalable designs, enabling the easy expansion or modification of the system. This facilitates an increased production capacity and adaptability to different environments or applications. Incorporating advanced probes/sensors and automation technologies will play a significant role in enhancing the performance of PBRs. The real-time monitoring of the environmental conditions, nutrient levels and biomass productivity can greatly improve the control and efficiency by allowing the implementation of multi-object optimization strategies. Improving the energy efficiency and optimizing the photosynthetic activity within PBRs can be achieved through advanced light management. This may involve the development of smart lighting systems that adjust to the specific needs of the selected species or the prevailing environmental conditions, particularly for outdoor use. Moreover, innovations in materials and coatings for PBR construction can improve their durability, heat dissipation and light transmission. These advancements can contribute to longer operational lifetimes and improved overall performance, while simultaneously reducing the associated capital costs.
The future is expected to witness an increased focus on genetic engineering and strain evolution to enhance the productivity of microalgae within PBRs. Tailoring strains for specific applications, such as biofuel production, novel food or pharmaceuticals, could lead to more efficient and specialized systems. Integration with other emerging technologies, such as artificial intelligence (AI) and machine learning, can further enhance the efficiency and performance of PBRs. These digital tools can analyze complex data, predict the optimal cultivation conditions and automate adjustments in real time. As all technological components linked with microalgae continue to evolve, PBRs will find diverse applications beyond biofuels and food supplements. These could include the production of high-value bioactive compounds and biopolymers, wastewater treatment and carbon capture. Accordingly, new PBR designs should place a greater emphasis on environmentally friendly practices, such as using sustainable materials, reducing energy consumption and minimizing waste production. This aligns with the growing focus on green and sustainable technologies.
Undoubtedly, exciting possibilities for industrial-scale PBRs will emerge as technological advancements continue to shape the landscape of sustainable bioprocessing and resource utilization. Collaboration between research institutions, industrial stakeholders and governments will continue to drive innovation in microalgae-based cultivation systems. These innovations have the potential to address complex challenges and eventually contribute significantly to addressing global challenges related to energy, food and environmental sustainability.
Author Contributions
Conceptualization, G.P. and A.P.; methodology, G.P.; validation, G.P. and A.P.; formal analysis, G.P. and C.K.; investigation, G.P. and A.P.; writing—original draft preparation, G.P. and A.P.; writing—review and editing, G.P. and C.K.; visualization, G.P. and A.P.; supervision, C.K.; project administration, G.P.; funding acquisition, G.P. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been developed within the framework of the “FUELGAE—Innovative sustainable on-site technologies for using microalgae to capture CO2 and produce advanced biofuels” research project, funded by the European Union’s Horizon Europe research and innovation program under grant agreement number 101122151. The views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union or CINEA. Neither the European Union nor the granting authority can be held responsible for them.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moreira, J.B.; Santos, T.D.; Duarte, J.H.; Bezerra, P.Q.M.; de Morais, M.G.; Costa, J.A.V. Role of microalgae in circular bioeconomy: From waste treatment to biofuel production. Clean Technol. Environ. Policy 2023, 25, 427–437. [Google Scholar] [CrossRef]
- Karapatsia, K.; Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. An experimental investigation of Stichococcus sp. cultivation conditions for optimal co-production of carbohydrates, proteins and lipids following a biorefinery concept. Biomass Bioenergy 2016, 89, 123–132. [Google Scholar] [CrossRef]
- Rame, R.; Purwanto, P.; Sudarno, S. Sustainable energy harnessing: Microalgae as a potential biofuel source and carbon sequestration solution. Renew. Energy Focus 2023, 47, 100498. [Google Scholar] [CrossRef]
- Bradley, T.; Rajaeifar, M.A.; Kenny, A.; Hainsworth, C.; del Pino, V.; del Valle Inclán, Y.; Povoa, I.; Mendonça, P.; Brown, L.; Smallbone, A.; et al. Life cycle assessment of microalgae-derived biodiesel. Int. J. Life Cycle Assess. 2023, 28, 590–609. [Google Scholar] [CrossRef]
- Ahmad, A.; Ashraf, S.S. Sustainable food and feed sources from microalgae: Food security and the circular bioeconomy. Algal Res. 2023, 74, 103185. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, S. Bioactive compounds in microalgae and their potential health benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Mehariya, S.; Das, P.; Thaher, M.I.; Quadir, M.A.; Khan, S.; Sayadi, S.; Hawari, A.H.; Verma, P.; Bhatia, S.K.; Karthikeyan, O.P.; et al. Microalgae: A potential bioagent for treatment of emerging contaminants from domestic wastewater. Chemosphere 2024, 351, 141245. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Chhandama, M.V.L.; Rai, P.K.; Lalawmpuii. Coupling bioremediation and biorefinery prospects of microalgae for circular economy. Bioresour. Technol. Rep. 2023, 22, 101479. [Google Scholar] [CrossRef]
- Jerney, J.; Spilling, K. Large scale cultivation of microalgae: Open and closed systems. In Biofuels from Algae. Methods in Molecular Biology; MIMB, Volume 1980; Spilling, K., Ed.; Humana: New York, NY, USA, 2018; pp. 1–8. [Google Scholar] [CrossRef]
- Al-Dailami, A.; Koji, I.; Ahmad, I.; Goto, M. Potential of photobioreactors (PBRs) in cultivation of microalgae. J. Adv. Res. Appl. Sci. Eng. Technol. 2022, 27, 32–44. [Google Scholar] [CrossRef]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Sandaka, B.P.; Kumar, J.; Melo, J.S. Biofuels from microalgae: Growing conditions, cultivation strategies, and techno-commercial challenges. In Microalgal Biomass for Bioenergy Applications; Sangeetha, J., Thangadurai, D., Eds.; Woodhead Publishing: Sawston, UK, 2024; pp. 305–340. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kim, Y.-S.; Yoon, H.-S. Effect of different cultivation modes (photoautotrophic, mixotrophic, and heterotrophic) on the growth of Chlorella sp. and biocompositions. Front. Bioeng. Biotechnol. 2021, 9, 774143. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.; Wijffels, R.H.; Dominguez, M.; Barbosa, M.J. Heterotrophic vs autotrophic production of microalgae: Bringing some light into the everlasting cost controversy. Algal Res. 2022, 64, 102698. [Google Scholar] [CrossRef]
- Guieysse, B.; Plouviez, M. Microalgae cultivation: Closing the yield gap from laboratory to field scale. Front. Bioeng. Biotechnol. 2024, 12, 1359755. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Ponnusamy, V.K.; Jeyakumar, R.B.; Sirohi, R.; Piechota, G.; Shobana, S.; Dharmaraja, J.; Lay, C.-H.; Saratale, G.D.; Shin, H.S.; et al. Microalgae cultivation strategies using cost-effective nutrient sources: Recent updates and progress towards biofuel production. Bioresour. Technol. 2022, 361, 127691. [Google Scholar] [CrossRef] [PubMed]
- Schädler, T.; Neumann-Cip, A.-C.; Wieland, K.; Glöckler, D.; Haisch, C.; Brück, T.; Weuster-Botz, D. High-density microalgae cultivation in open thin-layer cascade photobioreactors with water recycling. Appl. Sci. 2020, 10, 3883. [Google Scholar] [CrossRef]
- Tena, F.O.; Bickel, V.; Steinweg, C.; Posten, C. Continuous microalgae cultivation for wastewater treatment—Development of a process strategy during day and night. Sci. Total Environ. 2024, 912, 169082. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pawar, S.B.; Pandey, R.A.; Kanade, G.S.; Lokhande, S.K. Outdoor microalgae cultivation in airlift photobioreactor at high irradiance and temperature conditions: Effect of batch and fed-batch strategies, photoinhibition, and temperature stress. Bioprocess Biosyst. Eng. 2019, 42, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.d.; Moniz, P.; Silva, C.; Reis, A. The role of heterotrophic microalgae in waste conversion to biofuels and bioproducts. Processes 2021, 9, 1090. [Google Scholar] [CrossRef]
- Kumar, Y.; Kaur, S.; Kheto, A.; Munshi, M.; Sarkar, A.; Om Pandey, H.; Tarafdar, A.; Sindhu, R.; Sirohi, R. Cultivation of microalgae on food waste: Recent advances and way forward. Bioresour. Technol. 2022, 363, 127834. [Google Scholar] [CrossRef]
- Uyar, B.; Ali, M.D.; Uyar, G.E.O. Design parameters comparison of bubble column, airlift and stirred tank photobioreactors for microalgae production. Bioprocess Biosyst. Eng. 2024, 47, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Doppler, P.; Kriechbaum, R.; Käfer, M.; Kopp, J.; Remias, D.; Spadiut, O. Coelastrella terrestris for adonixanthin production: Physiological characterization and evaluation of secondary carotenoid productivity. Mar. Drugs 2022, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, P.S.; Del Signore, F.; Canziani, S.; Caggia, C.; Mezzanotte, V.; Ferrer-Ledo, N. Mixotrophic and heterotrophic growth of Galdieria sulphuraria using buttermilk as a carbon source. J. Appl. Phycol. 2023, 35, 2631–2643. [Google Scholar] [CrossRef]
- Erbland, P.; Caron, S.; Peterson, M.; Alyokhin, A. Design and performance of a low-cost, automated, large-scale photobioreactor for microalgae production. Aquac. Eng. 2020, 90, 102103. [Google Scholar] [CrossRef]
- Francke, L.; Löhn, S.; Weiderer, P.; Kosheleva, A.; Wieczorek, N.; Kuchta, N. A novel tubular photobioreactor immersed in open waters for passive temperature control and operated with the microalga Tetradesmus obliquus. Algal Res. 2022, 67, 102832. [Google Scholar] [CrossRef]
- Schoeters, F.; Spit, J.; Swinnen, E.; De Cuyper, A.; Vleugels, R.; Noyens, I.; Van Miert, S. Pilot-scale cultivation of the red alga Porphyridium purpureum over a two-year period in a greenhouse. J. Appl. Phycol. 2023, 35, 2095–2109. [Google Scholar] [CrossRef]
- Pereira, H.; Sá, M.; Maia, I.; Rodrigues, A.; Teles, I.; Wijffels, R.H.; Navalho, J.; Barbosa, M. Fucoxanthin production from Tisochrysis lutea and Phaeodactylum tricornutum at industrial scale. Algal Res. 2021, 56, 102322. [Google Scholar] [CrossRef]
- Olsen, M.F.L.; Pedersen, J.S.; Thomsen, S.T.; Martens, H.J.; Petersen, A.; Jensen, P.E. Outdoor cultivation of a novel isolate of the microalgae Scenedesmus sp. and the evaluation of its potential as a novel protein crop. Physiol. Plant. 2021, 173, 483–494. [Google Scholar] [CrossRef]
- Hashemi, A.; Moslemi, M.; Shariati, F.P.; Amrei, H.D. Beta-carotene production within Dunaliella salina cells under salt stress condition in an indoor hybrid helical-tubular photobioreactor. Can. J. Chem. Eng. 2020, 98, 69–74. [Google Scholar] [CrossRef]
- Glockow, T.; Velaz Martín, M.; Meisch, L.; Kapieske, D.; Meissner, K.; Correa Cassal, M.; Kaster, A.-K.; Rabe, K.S.; Niemeyer, C.M. A photobioreactor for production of algae biomass from gaseous emissions of an animal house. Appl. Microbiol. Biotechnol. 2023, 107, 7673–7684. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, A.; Penloglou, G.; Kiparissides, C. Evaluation of tolerant to CO2 excess microalgae for the production of multiple biochemicals in a 3G biorefinery. Sustainability 2023, 15, 3889. [Google Scholar] [CrossRef]
- Dos Santos, W.R.; Tagliaferro, G.V.; dos Santos, J.C.; Pereira, P.; Roma, C.; Silva, M.B.; Guimarães, D.H.P. Semi-continuous cultivation of Chlorella minutissima in landfill leachate: Effect of process variables on biomass composition. Waste Biomass Valorization 2022, 13, 1627–1638. [Google Scholar] [CrossRef]
- Azhand, N.; Sadeghizadeh, A.; Rahimi, R. Effect of superficial gas velocity on CO2 capture from air by Chlorella vulgaris microalgae in an airlift photobioreactor with external sparger. J. Environ. Chem. Eng. 2020, 8, 104022. [Google Scholar] [CrossRef]
- Wolf, L.; Cummings, T.; Müller, K.; Reppke, M.; Volkmar, M.; Weuster-Botz, D. Production of β-carotene with Dunaliella salina CCAP19/18 at physically simulated outdoor conditions. Eng. Life Sci. 2021, 21, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnia, S.; Tavakoli, O.; Faramarzi, M.A. Production of fucoxanthin from the microalga Tisochrysis lutea in the bubble column photobioreactor applying mass transfer coefficient. J. Biotechnol. 2022, 348, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pourbakhtiar, A.; Tavakoli, O.; Ahmadi, B. Design and optimization of a two-stage microalgae-assisted lipid production. Bioenergy Res. 2023, 16, 565–578. [Google Scholar] [CrossRef]
- Saxena, R.; Rodríguez-Jasso, R.M.; Chávez-Gonzalez, M.L.; Aguilar, C.N.; Quijano, G.; Ruiz, H.A. Strategy development for microalgae Spirulina platensis biomass cultivation in a bubble photobioreactor to promote high carbohydrate content. Fermentation 2022, 8, 374. [Google Scholar] [CrossRef]
- Macías-de la Rosa, A.; González-Cardoso, M.Á.; Cerón-García, M.D.C.; López-Rosales, L.; Gallardo-Rodríguez, J.J.; Seoane, S.; Sánchez-Mirón, A.; García-Camacho, F. Bioactives overproduction through operational strategies in the ichthyotoxic microalga Heterosigma akashiwo culture. Toxins 2023, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Barbato, F.; Venditti, A.; Bianco, A.; Guarcini, L.; Bottari, E.; Festa, M.R.; Cogliani, E.; Pignatelli, V. Scenedesmus dimorphus (Turpin) Kützing growth with digestate from biogas plant in outdoor bag photobioreactors. Nat. Prod. Res. 2016, 30, 185–191. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Nagarajan, D.; Cheah, W.Y. Eicosapentaenoic acid production from Nannochloropsis oceanica CY2 using deep sea water in outdoor plastic-bag type photobioreactors. Bioresour. Technol. 2018, 253, 1–7. [Google Scholar] [CrossRef]
- Carone, M.; Alpe, D.; Costantino, C.; Derossi, C.; Occhipinti, A.; Zanetti, M.; Riggio, V.A. Design and characterization of a new pressurized flat panel photobioreactor for microalgae cultivation and CO2 bio-fixation. Chemosphere 2022, 307, 135755. [Google Scholar] [CrossRef]
- Kuravi, S.D.; Mohan, S.V. Mixotrophic cultivation of Monoraphidium sp. in dairy wastewater using flat-panel photobioreactor and photosynthetic performance. Bioresour. Technol. 2022, 348, 126671. [Google Scholar] [CrossRef]
- Guimarães, B.O.; Van der Graaf, Y.; Kunert, I.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Effect of phosphorus limitation on Se uptake efficiency in the microalga Nannochloropsis oceanica. Bioresour. Technol. 2023, 367, 128239. [Google Scholar] [CrossRef]
- Amini, M.; Mohamedelhassan, E.; Liao, B. The biological performance of a novel electrokinetic-assisted membrane photobioreactor (EK-MPBR) for wastewater treatment. Membranes 2022, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Theepharaksapan, S.; Lerkmahalikit, Y.; Namyuang, C.; Ittisupornrat, S. Performance of membrane photobioreactor for integrated Spirulina strain cultivation and nutrient removal of membrane bioreactor effluent. J. Environ. Chem. Eng. 2023, 11, 110579. [Google Scholar] [CrossRef]
- Roopashri, A.N.; Makam, R. Development of operating process for continuous production of biomass by Tetradesmus obliquus (MT188616.1) in a hollow fiber membrane photobioreactor. J. Biotechnol. 2022, 359, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Chen, K.; Huang, Y.; Xia, A.; Zhu, Z.; Zhu, Z.; Liao, Q. Three-dimensional porous biofilm photobioreactor with light-conducting frameworks for high-efficiency microalgal growth. Algal Res. 2023, 69, 102942. [Google Scholar] [CrossRef]
- Fan, G.; Huang, J.; Jiang, X.; Meng, W.; Yang, R.; Guo, J.; Fang, F.; Yang, J. Microalgae biofilm photobioreactor and its combined process for long-term stable treatment of high-saline wastewater achieved high pollutant removal efficiency. J. Environ. Chem. Eng. 2023, 11, 111473. [Google Scholar] [CrossRef]
- Štěrbová, K.; Manoel, J.C.; Lakatos, G.E.; Grivalský, T.; Masojídek, J. Microalgae as an aquaculture feed produced in a short light-path annular column photobioreactor. J. Appl. Phycol. 2023, 35, 603–611. [Google Scholar] [CrossRef]
- Hu, X.; Meneses, Y.E.; Hassan, A.A.; Stratton, J.; Huo, S. Application of alginate immobilized microalgae in treating real food industrial wastewater and design of annular photobioreactor: A proof-of-concept study. Algal Res. 2021, 60, 102524. [Google Scholar] [CrossRef]
- Chin-On, R.C.; Barbosa, M.J.; Wijffels, R.H.; Janssen, M. A novel V-shaped photobioreactor design for microalgae cultivation at low latitudes: Modelling biomass productivities of Chlorella sorokiniana on Bonaire. Chem. Eng. J. 2022, 449, 137793. [Google Scholar] [CrossRef]
- Khoobkar, Z.; Shariati, F.P.; Safekordi, A.A.; Amrei, H.D. Performance assessment of a novel pyramid photobioreactor for cultivation of microalgae using external and internal light sources. Food Technol. Biotechnol. 2019, 57, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, J.; Chi, Q.; Li, Y.; Wang, H.; Gong, Y.; Liu, G.; Hu, Z.; Han, D.; Hu, Q. Critical assessment of the filamentous green microalga Oedocladium carolinianum for astaxanthin and oil production. Algal Res. 2022, 61, 102599. [Google Scholar] [CrossRef]
- Segredo-Morales, E.; González-Martín, C.; Vera, L.; González, E. Performance of a novel rotating membrane photobioreactor based on indigenous microalgae-bacteria consortia for wastewater reclamation. J. Ind. Eng. Chem. 2023, 119, 586–597. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Zhang, Q.; Ye, Q.; Cai, Q.; Wu, X. Enhancing the flow field in parallel spiral-flow column photobioreactor to improve CO2 fixation with Spirulina sp. Sci. Total Environ. 2021, 799, 149314. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, C.; Zhong, F.; Zhang, S.; Xia, A.; Huang, Y.; Liao, Q.; Zhu, X. A novel magnet-driven rotary mixing aerator for carbon dioxide fixation and microalgae cultivation: Focusing on bubble behavior and cultivation performance. J. Biotechnol. 2022, 352, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, R.; Wang, Q.; Han, Z.; Mao, Z. Novel bioreactor with inclined baffles in cost-efficiently increasing algal biomass and carbon fixation. Energy 2022, 247, 123453. [Google Scholar] [CrossRef]
- Ahangar, A.K.; Yaqoubnejad, P.; Divsalar, K.; Mousavi, S.; Taghavijeloudar, M. Design a novel internally illuminated mirror photobioreactor to improve microalgae production through homogeneous light distribution. Bioresour. Technol. 2023, 387, 129577. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.P.; Inostroza, C.; Acién, F.G. Yield and production cost of Chlorella sp. culture in a Fibonacci-type photobioreactor. Process. Biochem. 2023, 129, 209–220. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.; Xin, K.; Xu, J.; Yang, W. Developing a spiral-ascending CO2 dissolver to enhance CO2 mass transfer in a horizontal tubular photobioreactor for improved microalgal growth. ACS Sustain. Chem. Eng. 2020, 8, 18926–18935. [Google Scholar] [CrossRef]
- Deprá, M.C.; Mérida, L.G.R.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. A new hybrid photobioreactor design for microalgae culture. Chem. Eng. Res. Des. 2019, 144, 1–10. [Google Scholar] [CrossRef]
- Johnson, T.J.; Katuwal, S.; Anderson, G.A.; Gu, L.; Zhou, R.; Gibbons, W.R. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol. Prog. 2018, 34, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Cho, D.-H.; Lee, S.; Heo, J.; Tran, Q.-G.; Chang, Y.K.; Kim, H.S. Hybrid operation of photobioreactor and wastewater-fed open raceway ponds enhances the dominance of target algal species and algal biomass production. Algal Res. 2018, 29, 319–329. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae biofuels: Illuminating the path to a sustainable future amidst challenges and opportunities. Biotechnol. Biofuels 2024, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. A microalgae-based biorefinery plant for the production of valuable biochemicals: Design and economics. Com. Aid. Chem. Eng. 2016, 38, 1731–1736. [Google Scholar] [CrossRef]
- Araújo, R.; Calderón, F.V.; López, J.S.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Tasende, M.G.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Chanana, I.; Kaur, P.; Kumar, L.; Kumar, P.; Kulshreshtha, S. Advancements in microalgal biorefinery technologies and their economic analysis and positioning in energy resource market. Fermentation 2023, 9, 202. [Google Scholar] [CrossRef]
- Laezza, C.; Salbitani, G.; Carfagna, S. Fungal contamination in microalgal cultivation: Biological and biotechnological aspects of fungi-microalgae interaction. J. Fungi 2022, 8, 1099. [Google Scholar] [CrossRef]
- Habtegebriel, H.; Valdramidis, V. Descriptive statistics and meta-analysis approaches to assess the effect of microbial contamination on the cultivation of microalgal biomass and its derivatives. Algal Res. 2023, 74, 103205. [Google Scholar] [CrossRef]
- Show, P.L. Global market and economic analysis of microalgae technology: Status and perspectives. Bioresour. Technol. 2022, 357, 127329. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Selvam, S.M.; Paramasivan, B. Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour. Technol. 2022, 351, 127038. [Google Scholar] [CrossRef] [PubMed]
- Schmelling, N.M.; Bross, M. Too many big promises: What is holding back cyanobacterial research and applications? bioRxiv 2023. [Google Scholar] [CrossRef]
- Diva, S.A.; Jerusa, S.; Gavilanes, F.Z.; Pellini, T.; Rodrigues, K.C.T.T.; Silvestre, D.A.; Valencia, H.A.M.; Telles, T.S. Microalgae supply chains. In Microalgae-Based Systems: Process Integration and Process Intensification Approaches; Jacob-Lopes, E., Dias, R.R., Zepka, L.Q., Eds.; De Gruyter: Berlin, Germany, 2023; pp. 107–130. [Google Scholar] [CrossRef]
- Gómez-Ochoa, M.; Ojeda, K.; Sánchez-Tuirán, E.L.; Kafarov, V. Topology analysis of the third-generation biofuels. In 3rd Generation Biofuels; Jacob-Lopes, E., Zepka, L.Q., Severo, I.A., Maroneze, M.M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 345–366. [Google Scholar] [CrossRef]
- Vigani, M. Bioeconomy of microalgae-based fuels. In 3rd Generation Biofuels; Jacob-Lopes, E., Zepka, L.Q., Severo, I.A., Maroneze, M.M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 769–784. [Google Scholar] [CrossRef]
- Shekh, A.; Sharma, A.; Schenk, P.M.; Kumar, G.; Mudliar, S. Microalgae cultivation: Photobioreactors, CO2 utilization, and value-added products of industrial importance. J. Chem. Technol. Biotechnol. 2022, 97, 1064–1085. [Google Scholar] [CrossRef]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Vargas, J.E.O.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Porras Reyes, L.; Havlik, I.; Beutel, S. Software sensors in the monitoring of microalgae cultivations. Rev. Environ. Sci. Biotechnol. 2024, 23, 67–92. [Google Scholar] [CrossRef]
- Soriano-Jerez, Y.; Macías-de la Rosa, A.; García-Abad, L.; López-Rosales, L.; Maza-Márquez, P.; García-Camacho, F.; Bressy, C.; Cerón-García, M.C.; Molina-Grima, E. Transparent antibiofouling coating to improve the efficiency of Nannochloropsis gaditana and Chlorella sorokiniana culture photobioreactors at the pilot-plant scale. Chemosphere 2024, 347, 140669. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae cultivation in photobioreactors: Sustainable solutions for a greener future. Green Chem. Eng. 2023; pre-proof. [Google Scholar] [CrossRef]
- Tan, X.-B.; Wan, X.-P.; Yang, L.B.; Wang, X.; Meng, J.; Jiang, M.-J.; Pi, H.-J. Nutrients recycling and biomass production from Chlorella pyrenoidosa culture using anaerobic food processing wastewater in a pilot-scale tubular photobioreactor. Chemosphere 2021, 270, 129459. [Google Scholar] [CrossRef]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized microalgae-based photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency estimation, technological parameters, and prototype concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Sukačová, K.; Lošák, P.; Brummer, V.; Máša, V.; Vícha, D.; Zavřel, T. Perspective design of algae photobioreactor for greenhouses—A comparative study. Energies 2021, 14, 1338. [Google Scholar] [CrossRef]
- Shenawy, E.A.E.; Elkelawy, M.; Alm-Eldin Bastawissi, H.; Taha, M.; Panchal, H.; Sadasivuni, K.K.; Thakar, N. Effect of cultivation parameters and heat management on the algae species growth conditions and biomass production in a continuous feedstock photobioreactor. Renew. Energy 2020, 148, 807–815. [Google Scholar] [CrossRef]
- Ramírez-Mérida, L.G. Commercial facilities of microalgae-based products around the world. In Handbook of Food and Feed from Microalgae; Jacob-Lopes, E., Queiroz, M.I., Maroneze, M.M., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 33–40. [Google Scholar] [CrossRef]
- Llamas, B.; Suárez-Rodríguez, M.C.; González-López, C.V.; Mora, P.; Acién, F.G. Techno-economic analysis of microalgae related processes for CO2 bio-fixation. Algal Res. 2021, 57, 102339. [Google Scholar] [CrossRef]
- Calijuri, M.L.; Assemany, P.; Couto, E.; de Sousa Oliveira, A.P.; Lorentz, J.F.; de Assis, L.R. Recent developments and challenges: A prospectus of microalgal biomass valorization. In Valorization of Microalgal Biomass and Wastewater Treatment; Bandh, S.A., Malla, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 219–237. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, hopes, and the way forward for microalgal biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Van der Stricht, H.; Hung, Y.; Fischer, A.R.H.; Verbeke, W. Consumer segments less or more willing to adopt foods with microalgae proteins. Food Qual. Prefer. 2024, 113, 105047. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).