Abstract
Betulin is a birch bark-derived lupane-type pentacyclic triterpene with a wide spectrum of biological activities. Given their enhanced antiproliferative potential and enhanced pharmacological profile, betulin derivatives are continuously investigated in scientific studies. The objective of the current study was to in vitro assess the antiproliferative properties of novel synthesized 1,2,4-triazole derivatives of diacetyl betulin. The compounds were investigated using three cancer cell lines: A375 (melanoma), MCF-7 (breast cancer), HT-29 (colorectal cancer), and HaCaT (human keratinocytes). Bet-TZ1 had the lowest recorded IC50 values (ranging from 22.41 to 46.92 μM after 48 h of exposure) than its precursor and other tested compounds in every scenario, with the highest cytotoxicity against the A375 cell line. Bet-TZ3 demonstrated comparable cytotoxicity to the previously mentioned compound, with an IC50 of 34.34 μM against A375. Both compounds caused apoptosis in tested cells, by inducing specific nuclear morphological changes and by increasing the expression of caspase 9, indicating significant cytotoxicity, which was consistent with the literature and viability evaluation. Bet-TZ1 and Bet-TZ3 inhibit cancer cell migration, with the former having a stronger effect than the latter. The HET−CAM test indicated that all compounds have no irritative potential, suggesting that they can be used locally.
1. Introduction
The structural diversity and the high number of phytocompounds found in nature have always exceeded the representatives found in the synthetic libraries developed by chemists. As a consequence, several phytocompounds with therapeutic effects, such as morphine, codeine, and artemisinin, among many others, have been successfully used in therapy [1]. In particular, approximately 50% of all approved anticancer drugs since the 1940s originated from natural compounds; they act through a wide range of mechanisms involving a variety of molecular targets and signaling pathways [2]. Simultaneously, organic synthesis has produced an array of small molecules with cytotoxic properties that are currently used as systemic chemotherapy; unfortunately, this approach comes with severe side effects due to non-selective cytotoxicity, multidrug resistance, and high risk of cancer relapse [3]. Finding new therapeutic alternatives is therefore critical in cancer management in order to improve the therapeutic outcome and the patient’s life quality as well; a promising solution to such challenges is the continuous investigation of phytocompounds which may come with increased efficacy and milder side effects [4,5]. Despite many phytocompounds displaying clear therapeutic potential, their application is currently limited by their innate physico-chemical properties and toxicity [6].
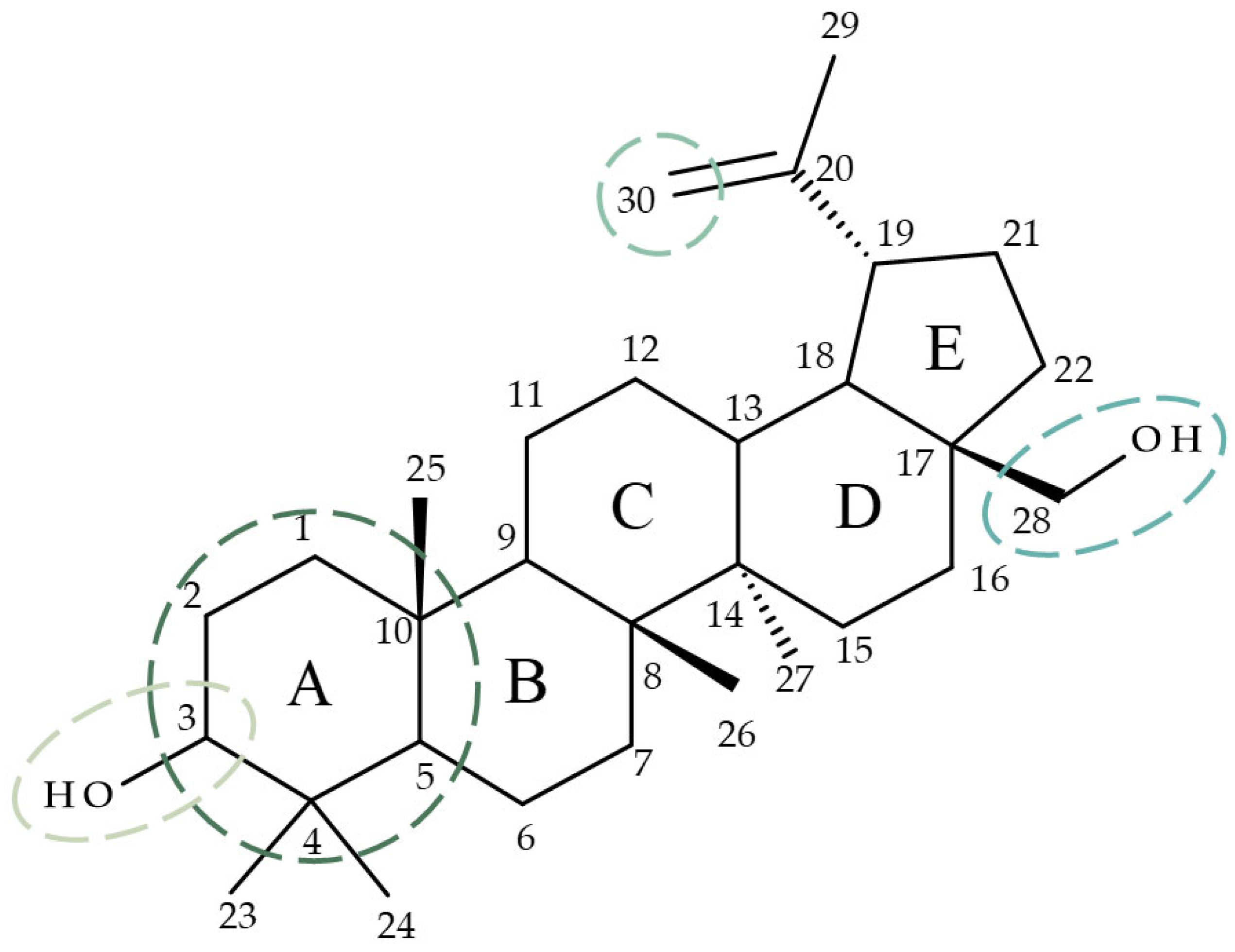
Betulin (Bet; lup-20(29)-ene-3b,28-diol) is a lupane-derived pentacyclic triterpene isolated predominantly from the birch bark [7]. Bet has numerous documented biological activities including anticancer, anti-inflammatory, and anti-HIV [8]; these effects were attributed to the modulation of several signaling pathways such as NF-kB (Nuclear factor kappa-light-chain-enhancer of activated B cells), Nrf-2 (Nuclear factor erythroid 2-related factor 2), and COX-2 (cyclooxygenase-2) [9]. The anticancer effect of Bet, in particular, has been explained through cell viability and angiogenesis inhibition, cell migration suppression, and cell cycle arrest in the G0/G1 phase [10]. As the structure of Bet consists of four six-membered rings, an additional five-membered ring, one isopropenyl group at C19, and two hydroxyl groups at C3 and C28 [11], the compound exhibits high lipophilicity which greatly reduces its bioavailability [12]. A frequently employed strategy in drug development combines the potential of phytocompounds with the advantages of chemical derivatization with the resulting semisynthetic compounds usually displaying enhanced selectivity and biological activity as well as improved pharmacokinetic properties [13]. For betulin, its complex structure allows for various modifications in different positions, such as C3 [14], C28 [15], C30 [16], and ring A [17] (Figure 1) that may result in semisynthetic derivatives with optimized pharmacological profiles.
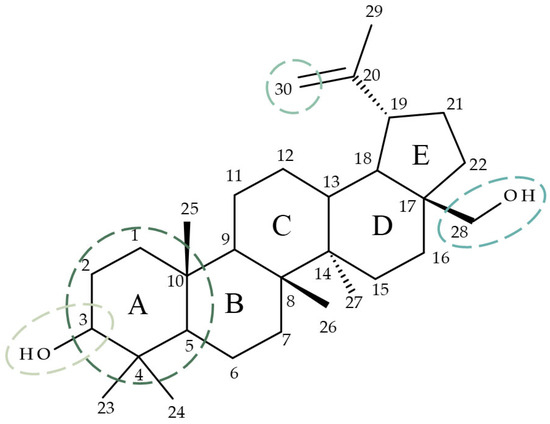
Figure 1.
Target positions frequently employed for betulin derivatization.
Currently, heterocyclic scaffolds are included in the structure of more than 85% of the approved drugs, with the majority being represented by nitrogen-containing heterocycles, such as triazoles [18]. Triazoles are five-membered heterocycles that can be divided given the position of the two nitrogen atoms into two isomers, 1,2,3-triazoles and 1,2,4-triazoles [19]. They have been extensively used in drug design as they increase the stability of molecules, and can act as linkers [20] and bioisosteres of amide bonds [21]. Additionally, both isomers are highly water soluble and, together with their derivatives, display a wide spectrum of biological properties including anticancer, antioxidative, and anti-inflammatory [22].
As an example, this approach was previously used to develop novel Bet-1,2,3-triazole derivatives with improved cytotoxicity against human ductal carcinoma (T47D), human adenocarcinoma (MCF-7), and glioblastoma (SNB-19) cell lines [23]. Considering the intrinsic limitations of betulin as a therapeutic agent, such as low bioavailability, as well as the advantages provided by triazoles in terms of pharmacological effects, the synthesis of Bet-triazoles derivatives should lead to a significant improvement in the pharmacologic potential of the unmodified compound.
The current study expands on the synthesis of diacetylbetulin derivatives containing a 5-substituted-1,2,4-TZ at C30 (Bet-TZ1-4) followed by their cytotoxicity assessment in several cancer cells such as melanoma (A375), breast cancer (MCF-7), and colorectal cancer (HT-29) as well as in human keratinocytes (HaCaT). A preliminary toxicity assessment was conducted through HET−CAM assay regarding their irritant potential.
2. Results
2.1. Chemistry
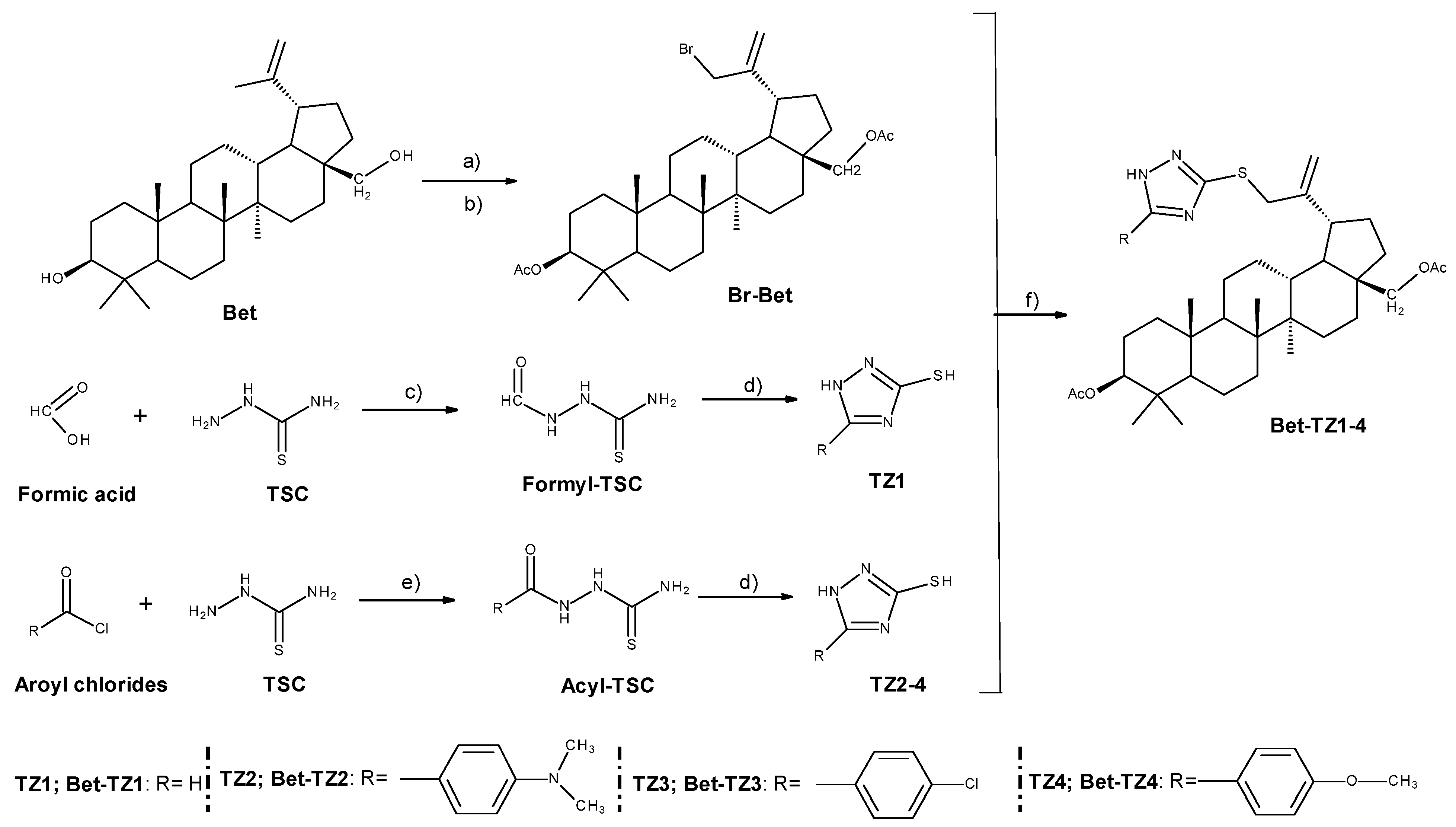
Figure 2 depicts the synthetic route as well as the reaction conditions required to synthesize betulin-triazole derivatives (Bet-TZ1-4). By using slightly modified methods previously reported [24], high yields (above 50%) of triazole derivatives (TZ1-4) and brominated diacetyl-betulin (Br-Bet) were obtained. The subsequent alkylation of the SH group on the triazole ring (TZ1-4) with Br-Bet in dimethylformamide (DMF)/K2CO3 produced moderate yields (32–41%) of betulin-triazole derivatives (Bet-TZ1-4). Despite the purity of the precursors, TLC examination revealed that the reaction produced additional lipophilic side products that were easily separated by column chromatography using CHCl3:ethyl acetate 1:1 as eluent for Bet-TZ2-4 or CHCl3:ethyl acetate 2:1 for Bet-TZ1, due to the significant Rf value differences. During TLC (thin layer chromatography) analysis, there was also an additional spot of low intensity above the reference spot for each compound. This spot migrated with the main one, regardless of the eluents used. As a result, after separation by column chromatography, only fractions with a faint trace were retained, resulting in yield values below 50%. Following NMR (nuclear magnetic resonance) tests, it was discovered that the respective spot corresponds to a tautomer. As a result, if it was previously known that this tautomerism occurs, all the discarded fractions containing the tautomer would have been kept, producing compounds with higher yields. All synthesized compounds’ structures were confirmed using 1H, 13C NMR, and FTIR spectroscopy. All spectral results are available in the Supplementary Materials section of the manuscript.
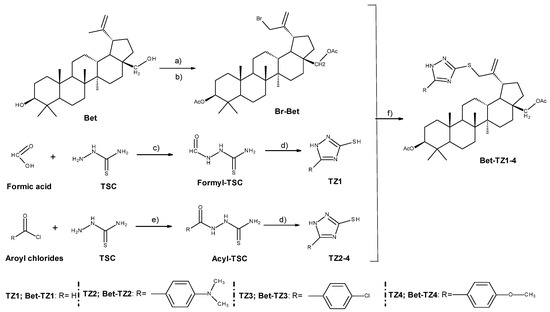
Figure 2.
Synthesis of betulin-triazole derivatives (Bet-TZ1−4); Bet (betulin); Br-Bet (3,28-O-diacetyl-30-bromo-betulin); TSC (thiosemicarbazide); TZ1 (1H-1,2,4-triazole-3-thiol); TZ2 (5-[4-(dimethylamino) phenyl]-1H-1,2,4-triazole-3-thiol); TZ3 (5-(4-chlorophenyl)-1H-1,2,4-triazole-3-thiol); TZ4 (5-(4-methoxyphenyl)-1H-1,2,4-triazole-3-thiol); Bet-TZ1 3,28-O-diacetyl-30-(1H-1,2,4-triazole-3-yl-sulfanyl)-betulin); Bet-TZ2 (3,28-O-diacetyl-30-{5-[4-(dimethylamino) phenyl]-1H-1,2,4-triazole-3-yl-sulfanyl}-betulin); Bet-TZ3 (3,28-O-diacetyl-30-[5-(4-chlorophenyl)-1H-1,2,4-triazole-3-yl-sulfanyl]-betulin); Bet-TZ4 (3,28-O-diacetyl-30-[5-(4-methoxyphenyl)-1H-1,2,4-triazole-3-yl-sulfanyl]-betulin). Reaction conditions: (a) acetic anhydride, pyridine, DMAP, r.t., 12 h; (b) NBS, CCl4, r.t., 48 h; (c) reflux, 30 min; (d) H2O, NaOH, reflux, 1 h; (e) pyridine/DMF, 1 h, 50 °C; (f) DMF, K2CO3, r.t., 72 h.
The deacetylation of Bet was successful and its 1H and 13C NMR spectra confirm the structure according to the existing literature [25]. Moreover, the bromination of diacetyl-Bet (Br-Bet) led to a mixture of allylic and vinylic bromine derivatives, as previously reported in the literature [26]. The 1H NMR spectra of Bet-TZ1-4 show the peaks for the betulin scaffold in the 5.1–0.6 ppm region. The peaks for C(30)H2, C(29)H2, and H3 from the betulin backbone resonate at about 3.8 ppm, 4.9–5.0 ppm, and 4.3–4.4 ppm, respectively. Their integral ratio is 2:2:1, suggesting that only the allylic bromine derivative reacted with the triazole derivatives.
When analyzing the aromatic region corresponding to the 1H NMR spectra for Bet-TZ1-4, where the protons of the triazole derivatives resonate, we observed that the peaks were doubled, but if integrated, the integral values were in appropriate ratios compared to the protons from Bet. We attributed this behavior to the existence of a slow exchange tautomeric equilibrium that can occur in 3,5-disubstituted 1,2,4-triazoles [27,28]. Out of the three tautomeric forms possible, two tautomers with long enough stability to be individually detected by NMR spectroscopy were observed (Table 1).

Table 1.
Percentage of triazole tautomers, as calculated from the 1H NMR integral values for the H36 peaks (Bet-TZ1) or the NH peaks (Bet-TZ2-4).
In order to prove beyond any doubt that the doubling of the triazole derivative peaks is due to the existence of a tautomeric equilibrium, to each solution of Bet-TZ1-4 in DMSO-d6, for which the initial experiments were recorded, we added a drop of trifluoroacetic acid (TFA) and then recorded all the NMR spectra again. Adding TFA speeded up the tautomeric equilibrium leading to a fast exchange system and the NMR spectra show only one set of peaks for the triazole derivatives in appropriate integral ratios compared to the protons from Bet.
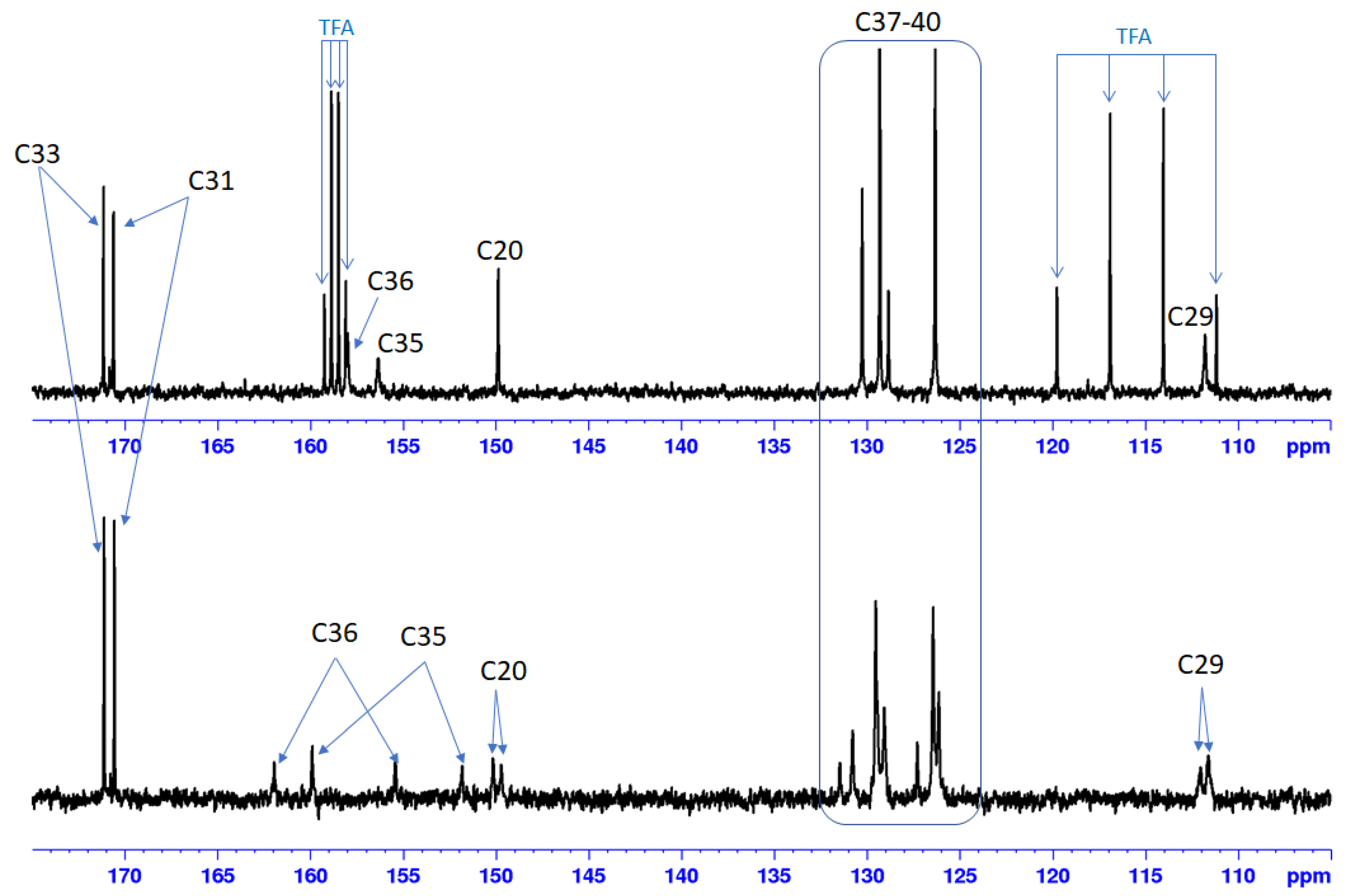
The 13C NMR spectra of Bet-TZ1-4 show broad peaks for C19 (44–45 ppm), C20 (149–150 ppm), C29 (110–112 ppm), and C30 (35–36 ppm) of the Bet residue. The H, C-HMBC spectra of Bet-TZ2-4 show long range correlation peaks, over three bonds, between the triazole’s C35 and H30 from betulin (Figures S10, S16, S22 and S28), proving that the reactions took place. The complete assignment of the peaks is given in the experimental section and all the 1D and 2D NMR spectra are available in the Supporting Information. In the case of Bet-TZ2, where the two tautomeric forms are almost in a 1:1 ratio, we can observe peaks (broad) for C36 and C35 from each isomer (Figure 3) and doubled peaks for the carbons of the p-chlorophenyl residue for C20 and C29. After adding TFA, we can observe that the peaks are no longer doubled and are noticeably narrower.
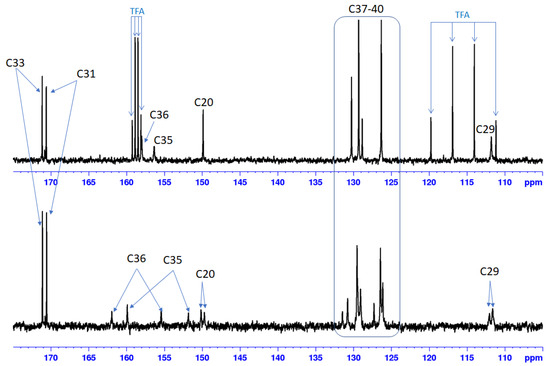
Figure 3.
13C NMR spectra (175–105 ppm region) of Bet-TZ3 in DMSO-d6 (bottom) and in DMSO-d6 with one drop of TFA (top) indicating the loss of signals corresponding to the tautomer form after acid addition.
2.2. Evaluation of Diacetylbetulin Derivatives Cytotoxic Effect
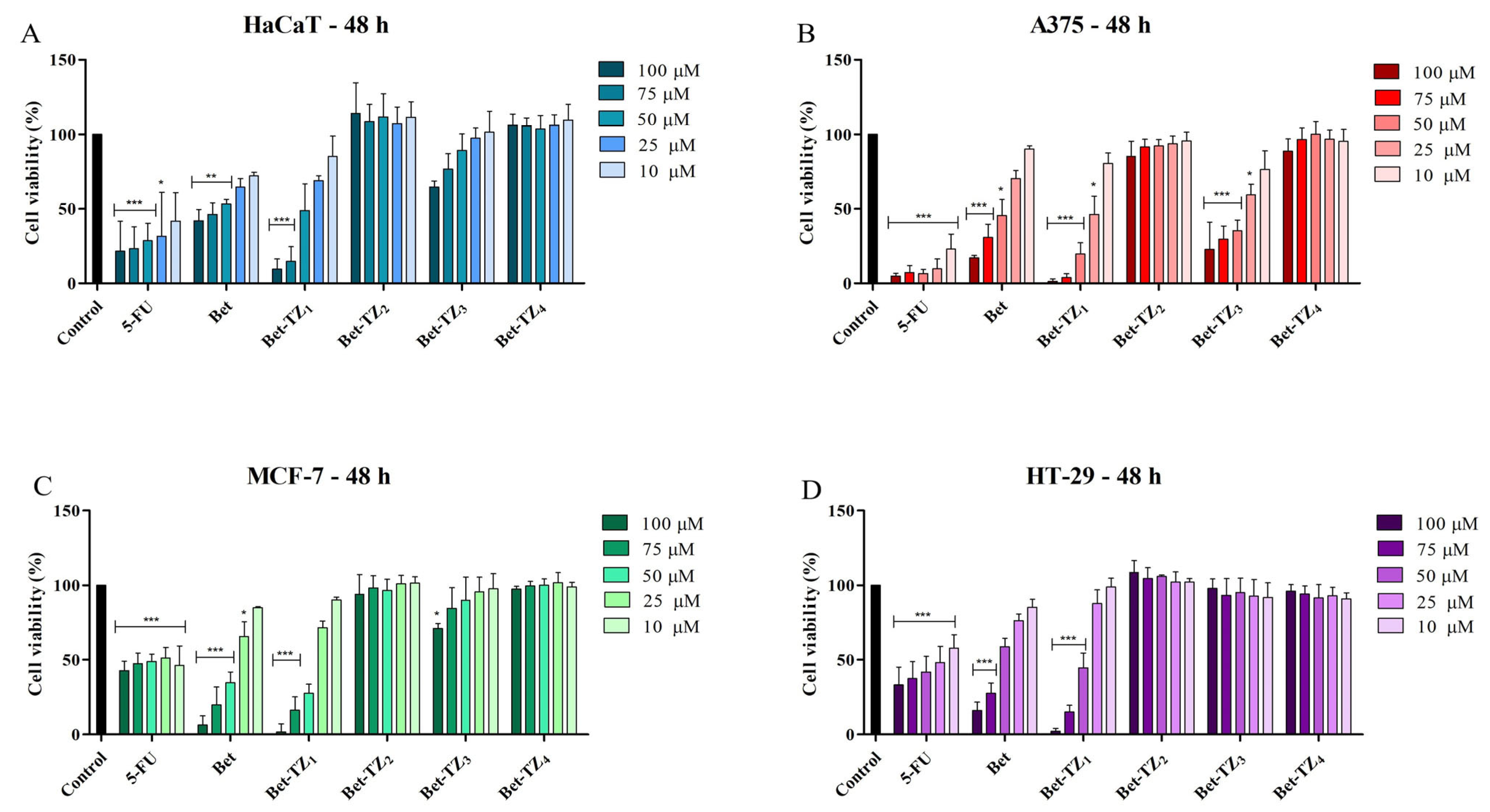
The viability of HaCaT, A375, MCF-7, and HT-29 cells, after a 48-h treatment period with the novel synthesized compounds (10, 25, 50, 75, and 100 μM), was assessed using the Alamar blue assay. Incubation of HaCaT cells with the tested compounds revealed significant inhibition of cell viability at 48 h after Bet-TZ1 was used in the highest concentrations (100 and 75 μM). This inhibitory effect at 48 h was stronger compared to the effect of 5-FU (positive control) and to the parent compound (Bet) at the corresponding concentrations, as follows: 9.44 ± 6.90% (Bet-TZ1 100 μM) and 14.76 ± 9.84% (Bet-TZ1 75 μM) vs. 21.57 ± 14.62% (5-FU 100 μM), 27.25 ± 14.90% (5-FU 75 μM), (42.00 ± 7.79% Bet 100 μM) (46.26 ± 7.64% Bet 75 μM) (Figure 4A). A 48-h incubation of A375 cells with 100, 75, and 50 μM Bet-TZ1 and Bet-TZ3 promoted a significant reduction of cell viability in a concentration-dependent manner vs. Bet, as follows: 1.22 ± 1.09%, 3.83 ± 2.68%, 19.78 ± 7.36% (Bet-TZ1), 22.78 ± 18.44%, 29.65 ± 8.98%, 35.58 ± 6.92% (Bet-TZ3) vs. 17.04 ± 1.71%, 30.83 ± 9.01%, 45.64 ± 10.63% (Bet) (Figure 4B). Bet-TZ1 also produced a cytotoxic effect on MCF-7 cells at 100, 75, and 50 μM (1.73 ± 5.48%, 16.47 ± 8.93%, 27.85 ± 5.74%) compared to Bet alone (42.78 ± 6.47%, 47.54 ± 7.00%, 48.92 ± 5.05%) (Figure 4C). The other tested compounds did not influence the cell viability of HaCaT, A375, MCF-7, and HT-29 in a statistically significant manner.
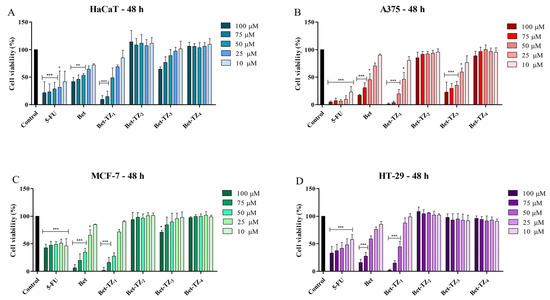
Figure 4.
Cell viability after 48-h treatment with 5-FU, Bet, Bet-TZ1-4 (100, 75, 50, 25, and 10 μM) of HaCaT (A), A375 (B), MCF-7 (C), and HT-29 (D) cells. The results represent viability percentages compared to the control group, considered 100% (* p < 0.05, ** p < 0.01, and *** p < 0.001). The results represent the mean values ± SD of three separate experiments executed in triplicate.
Table 2 presents the calculated IC50 values (μM) after 48-h treatment with 5-FU, Bet, Bet-TZ1, and Bet-TZ3 on HaCaT, A375, MCF-7, and HT-29 cell lines.

Table 2.
The calculated IC50 values (μM) of 5-FU, Bet, Bet-TZ1, and Bet-TZ3 on HaCaT, A375, MCF-7, and HT-29 cell lines; the compounds deemed ineffective have IC50 values above 100 μM.
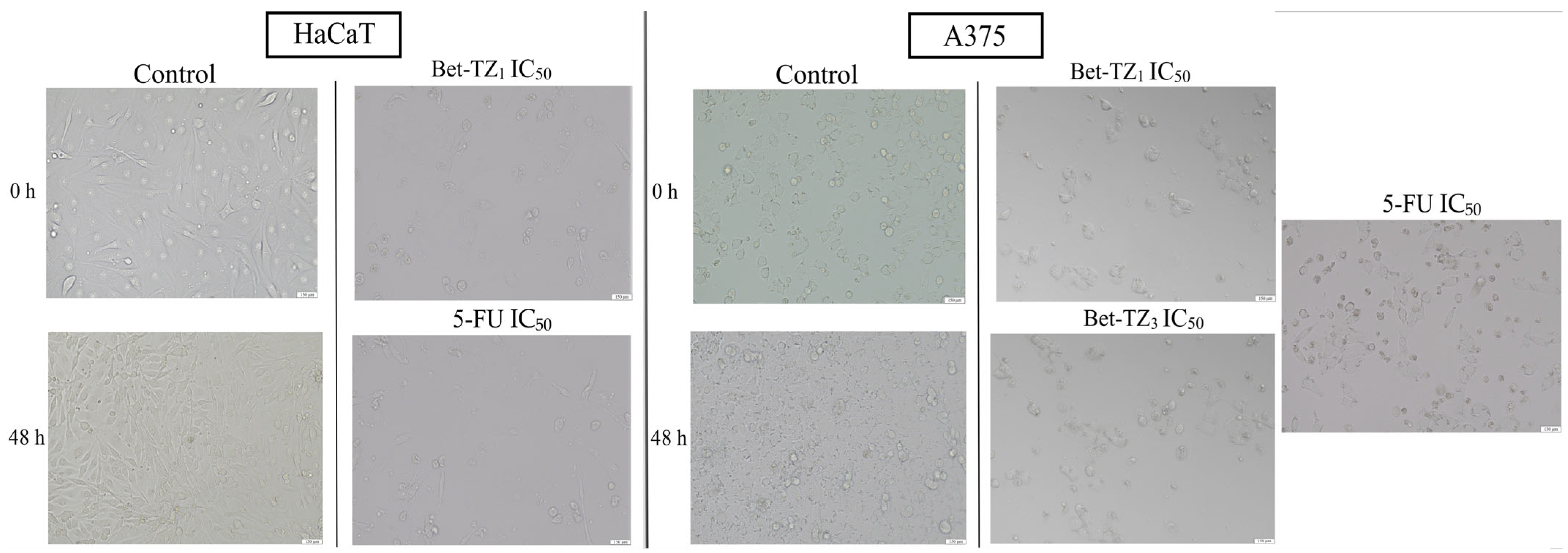
2.3. Diacetylbetulin Derivatives Effect on Cell Morphology
In HaCaT cells, no significant morphological changes were recorded in terms of confluence and aspect between the control group and the Bet, Bet-TZ2, Bet-TZ3, and Bet-TZ4-treated cells. However, Bet-TZ1 treatment decreased the number of cells, rendering them rounder and detached, in a similar manner to the 5-FU positive control (Figure 5). In A375 cells, treatment with both Bet-TZ1 and Bet-TZ3, respectively, at their IC50 induced morphological changes (rounder and detached cells) and a decreased number of cells, in agreement with the results of cell viability testing (Figure 5).
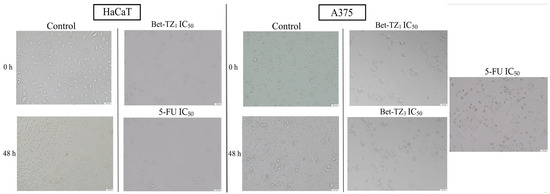
Figure 5.
The evaluation of morphological changes of HaCaT and A375 cells after a 48-h treatment with Bet-TZ1, Bet-TZ3, and 5-FU (IC50); the scale bar was 150 μm.
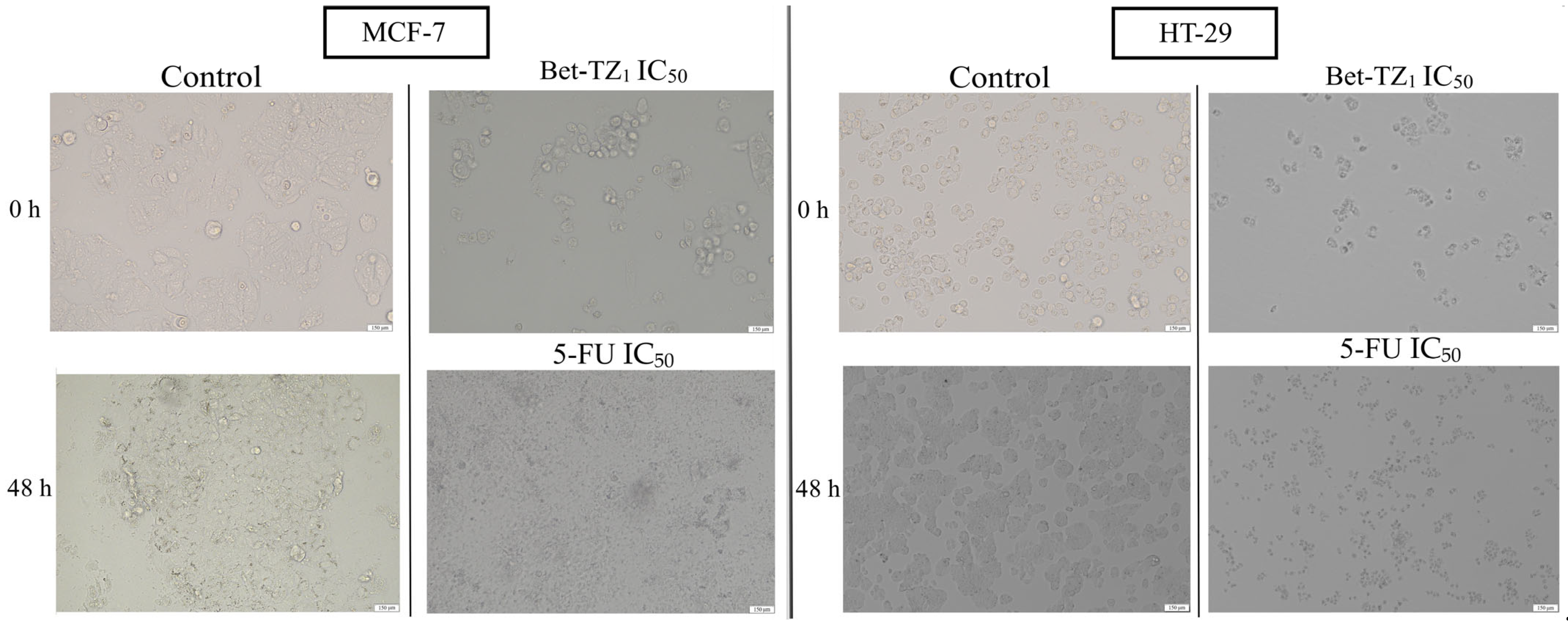
Treatment with Bet-TZ1 (IC50) induced similar changes in MCF-7 and HT-29 cells, in terms of number and cell morphology, changes comparable to those caused by 5-FU used as a positive control (Figure 6).
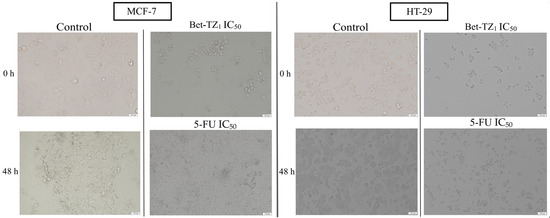
Figure 6.
The evaluation of morphological changes of MCF-7 and HT-29 cells after 48-h treatment with Bet-TZ1 and 5-FU (IC50); the scale bar was 150 μm.
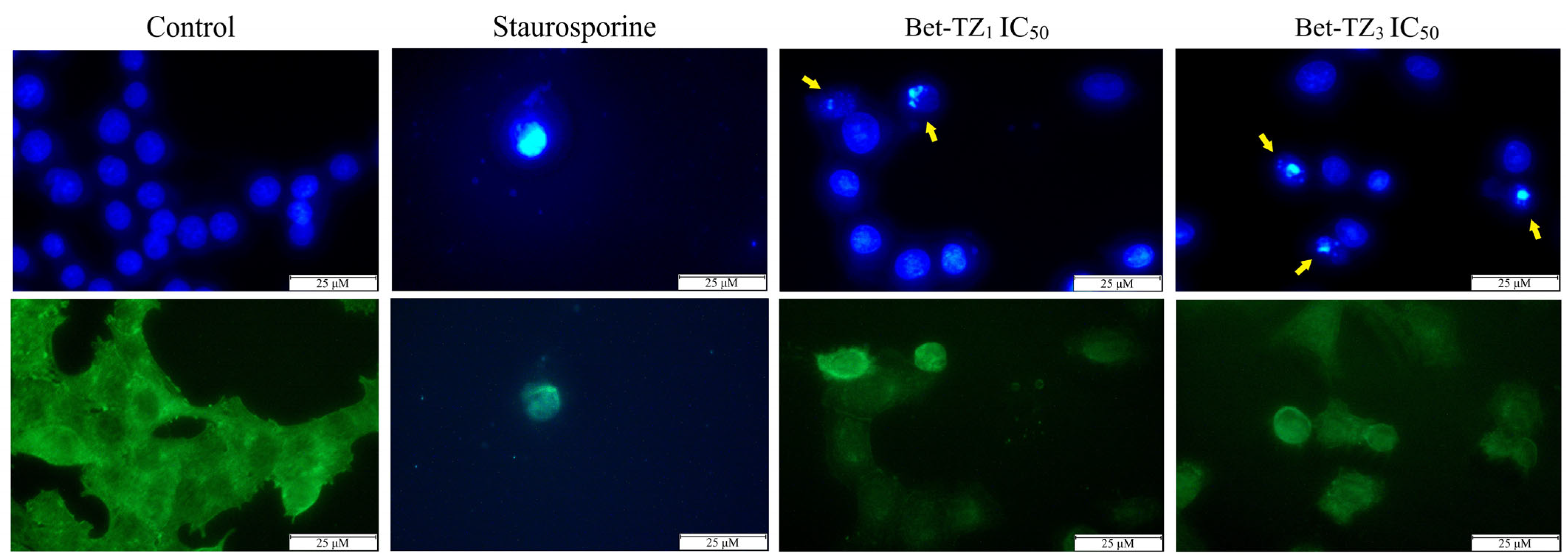
The cells’ cytoskeleton and nuclei undergo characteristic morphological changes during apoptosis. To determine whether the cytotoxic effects recorded in HaCaT, A375, MCF-7, and HT-29 cells after a 48-h treatment with Bet-TZ1 (IC50) and Bet-TZ3 (IC50—A375 and 100 μM—MCF-7 and HT-29), respectively, occurred as a result of apoptotic cell death induction, cells’ nuclei were stained with Hoechst solution, while the cytoskeleton was labeled with beta-tubulin antibody and Alexa fluor 488. Treatment with both Bet-TZ1 and Bet-TZ3, respectively, induced morphological changes in A375 cells that are consistent with apoptosis. Specific observed hallmarks included small and bright nuclei indicative of nuclear condensation, nuclear fragmentation, and small, round-shaped cells whose membrane start to disorganize, thus leading to the formation of apoptotic bodies (Figure 7). In contrast, the necrotic cell death induced by staurosporine used as a positive control was mainly accompanied by morphological changes of the cell shape that occur due to cell membrane disruption (Figure 7).
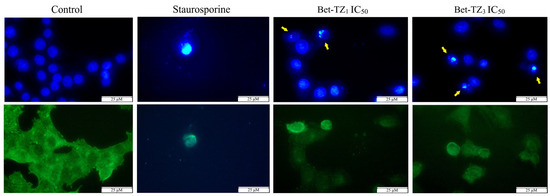
Figure 7.
The impact of 48-h treatment with Bet-TZ1 and Bet-TZ3 (IC50) on A375 nuclei; blue—Hoechst staining was used for the nuclei while beta-tubulin—green staining—was used to highlight the cytoskeleton. Staurosporine (5 μM) was used as a control (positive) for necrotic cell death. The scale bar was 50 μm. Yellow arrows indicate specific apoptotic-related morphological changes.
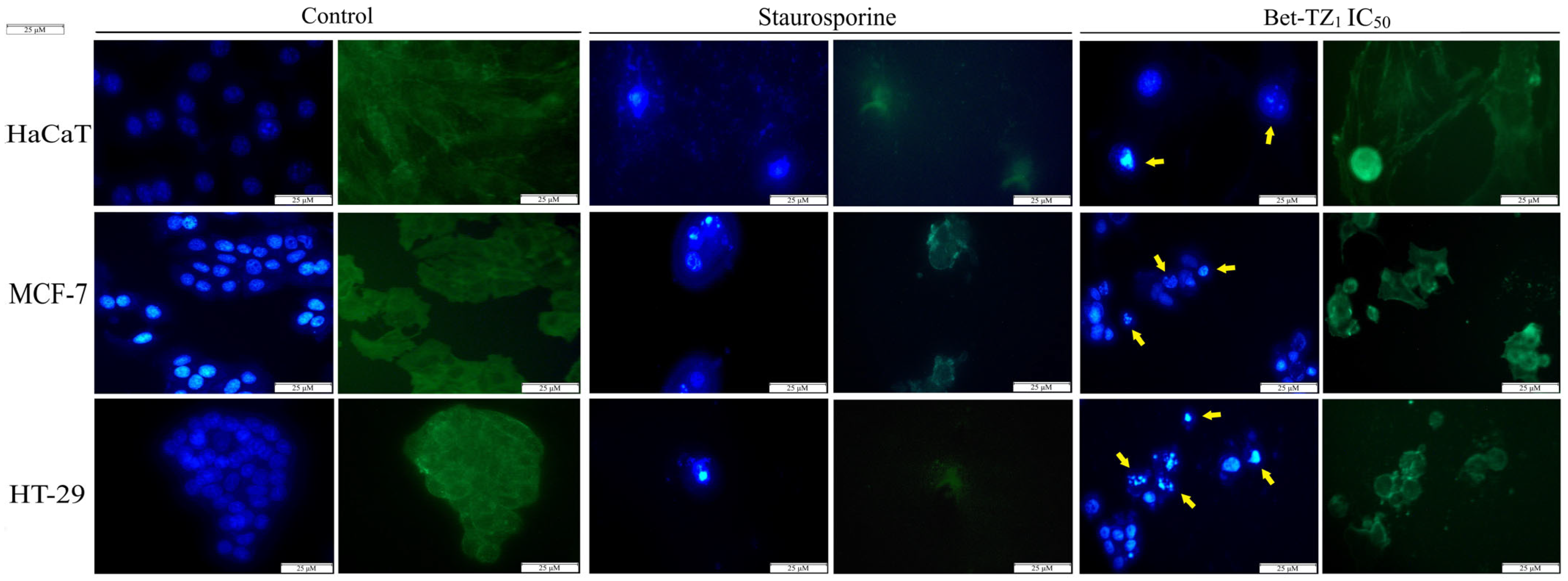
Similar morphological changes, consistent with apoptosis, occurred in HaCaT, MCF-7, and HT-29 cell lines, after treatment with Bet-TZ1 (IC50) (Figure 8).
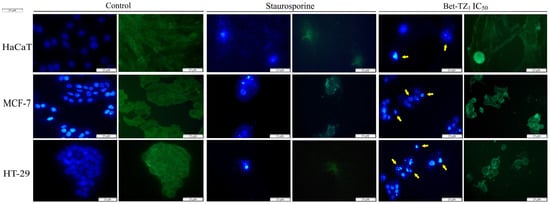
Figure 8.
The impact of a 48-h treatment with Bet-TZ1 (IC50) on HaCaT, MCF-7, and HT-29 nuclei; blue—Hoechst staining was used for the nuclei while beta-tubulin—green staining—was used to highlight the cytoskeleton. The effect of staurosporine (5 μM) was recorded as a control (positive) for necrotic cell death. The scale bar was 50 μm. Yellow arrows indicate specific apoptotic-related morphological changes.
2.4. Real-Time PCR Quantification of Apoptotic Markers
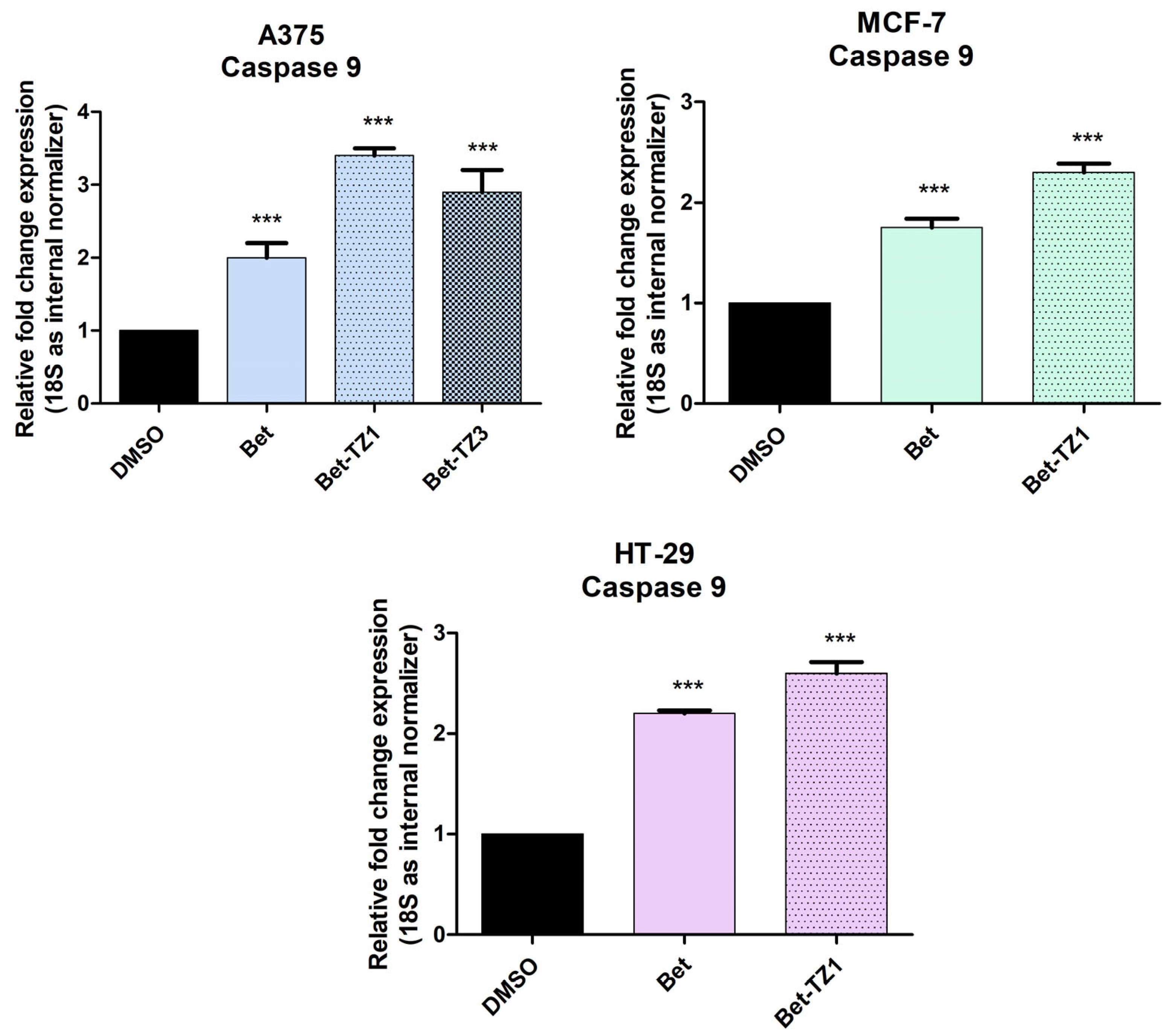
To further establish the proapoptotic effect of Bet-TZ1 and Bet-TZ3, RT-PCR (reverse transcription polymerase chain reaction) was employed to quantify caspase 9 expression in all three Bet-TZ1-treated cell lines, and the A375 cell line treated with Bet-TZ3, at a sub-cytotoxic concentration (10 μM). Betulin increased caspase 9 expression in all treated cells, according to the results (Figure 9). Bet-TZ1 and Bet-TZ3 both promote caspase 9 expression in the A375 cell line, outperforming their parent compound, with Bet-TZ1 being the most active. This scenario also occurs in the MCF-7 and HT-29 cell lines, where Bet-TZ1 increases caspase 9 expression compared to both control and Bet.
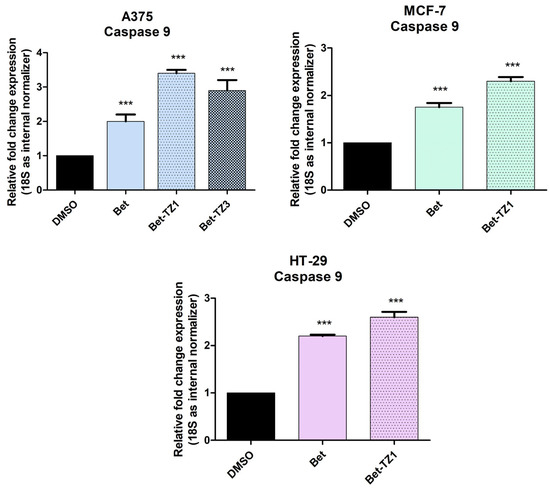
Figure 9.
The recorded fold change expression in mRNA of caspase 9 in cells treated with a 10 μM concentration of Bet, Bet-TZ1 (A375, MCF-7, HT-29), and Bet-TZ3 showing an increase in caspase 9 expression as a result of compound stimulation of cancer cells. The results were normalized to 18 S and DMSO was used as control. Data represent the mean values ± SD of three separate experiments. One-way ANOVA with Dunnett’s post hoc test was applied to determine the statistical differences compared to control (*** p < 0.001).
2.5. Scratch Assay
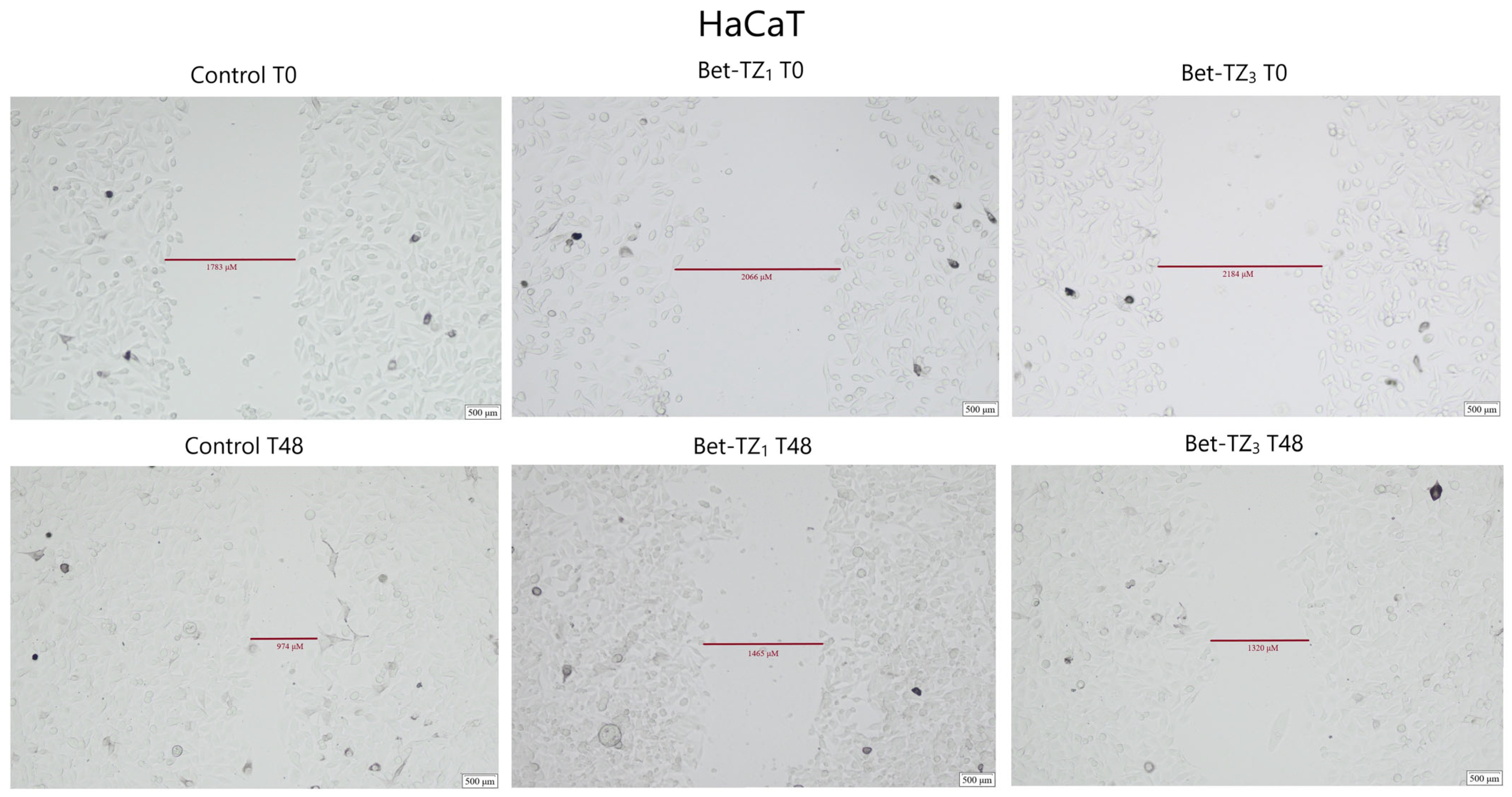
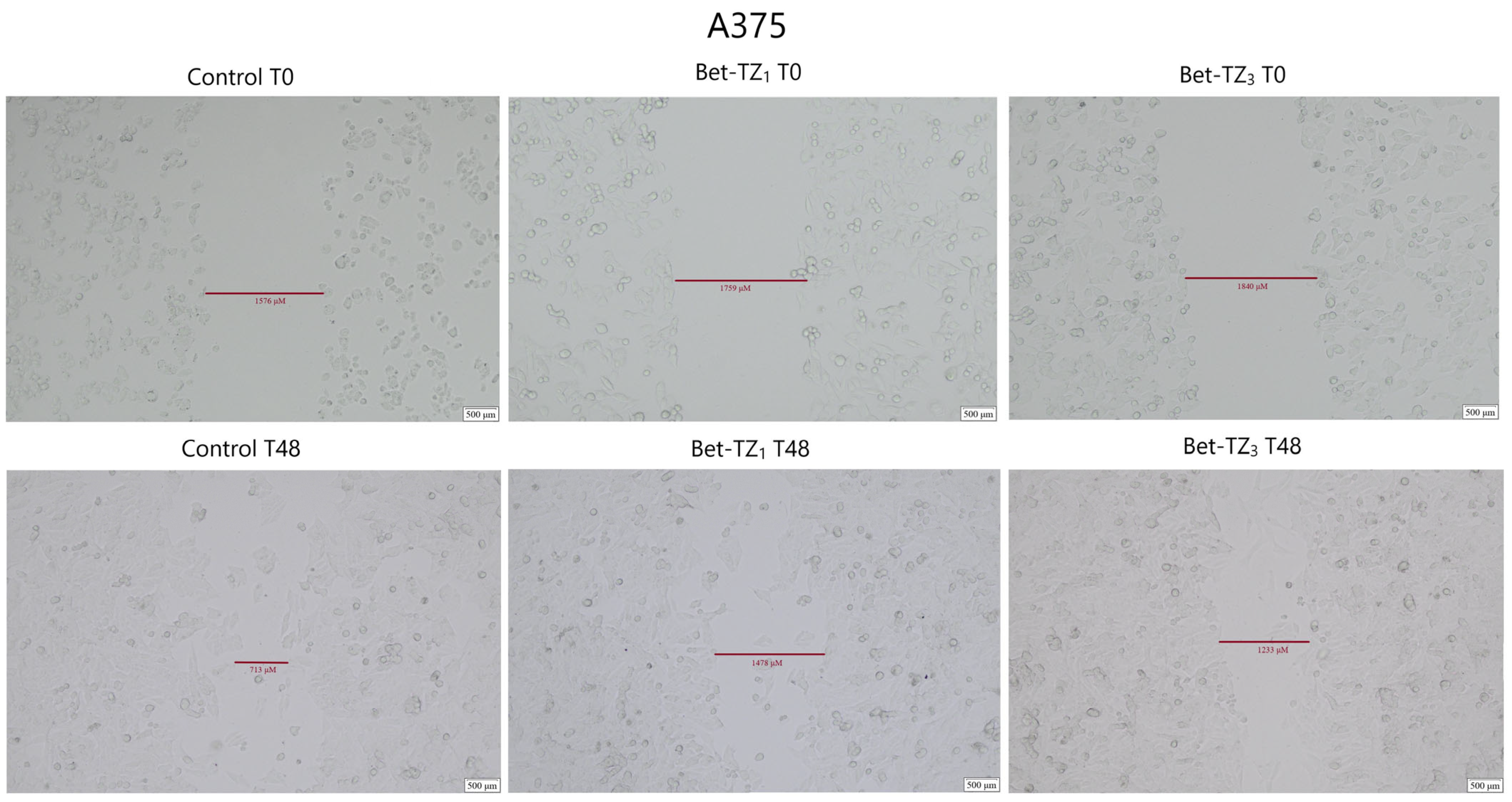
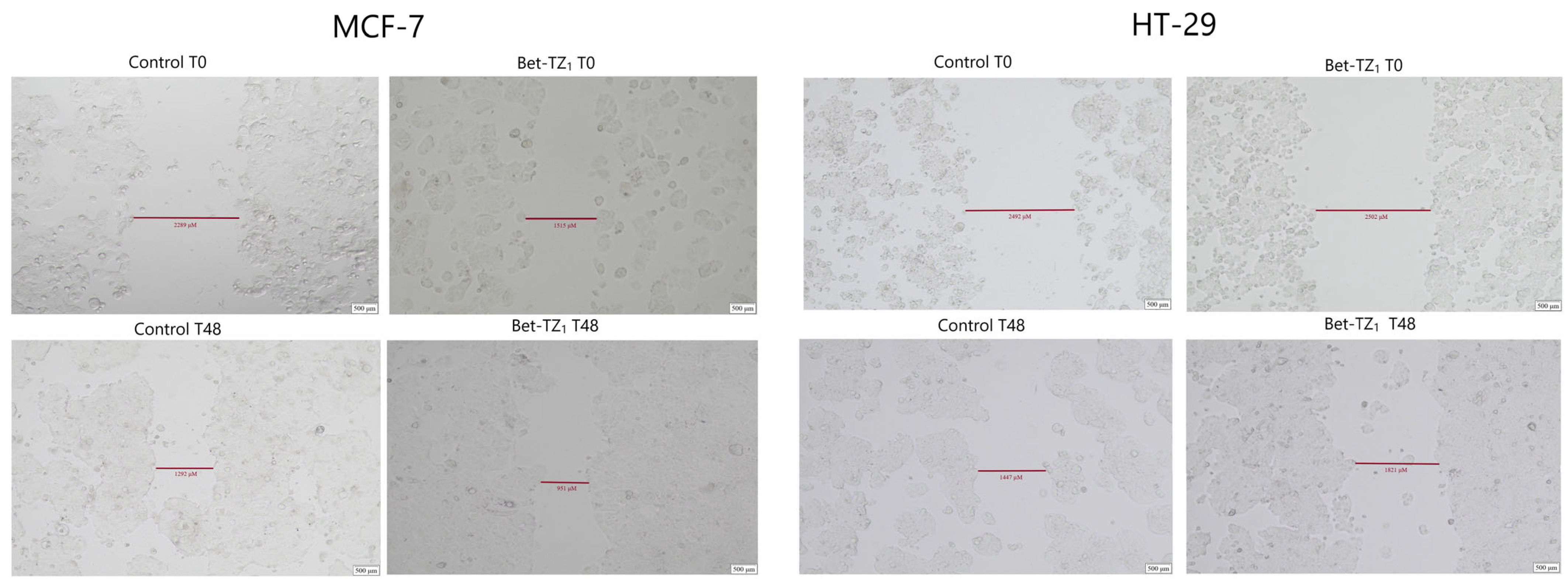
The antimigratory potential on HaCaT, A375, HT-29, and MCF-7 was assessed after 48-h treatment with Bet-TZ1 at 10 μΜ (Figure 10, Figure 11, Figure 12 and Figure 13) and on HaCaT and A375 after 48-h treatment with Bet-TZ3 at 10 μΜ (Figure 10 and Figure 11).
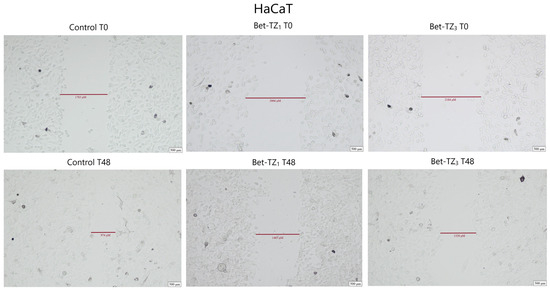
Figure 10.
The effects of Bet-TZ1 (10 μΜ) and Bet-TZ3 (10 μΜ) on immortalized human keratinocytes’ HaCaT migration capacity. Cell migration was measured at 0 h and 48 h after stimulation, indicating that the tested compounds reduced the scratch closure time when compared to untreated cells used (control).
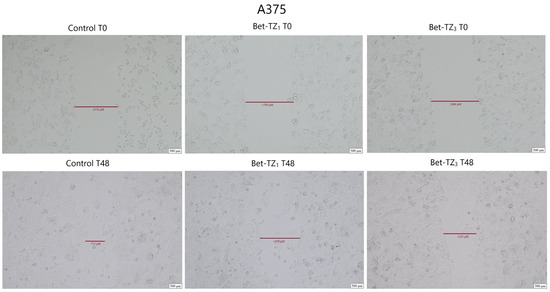
Figure 11.
The effects of Bet-TZ1 (10 μΜ) and Bet-TZ3 (10 μΜ) on malignant melanoma cells’ A375 migration capacity. Cell migration was measured at 0 h and 48 h after stimulation, indicating that the tested compounds reduced the scratch closure time when compared to untreated cells (control).
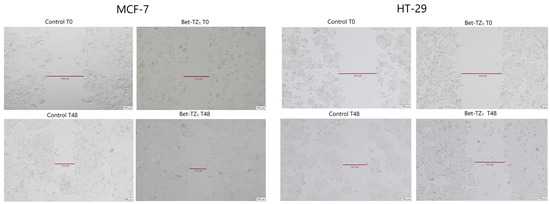
Figure 12.
The effects of Bet-TZ1 (10 μΜ) on breast cancer cells’ MCF-7 and HT-29 migration capacity. Cell migration was measured at 0 h and 48 h after stimulation, indicating that the tested compounds reduced the scratch closure time when compared to untreated cells (controls).
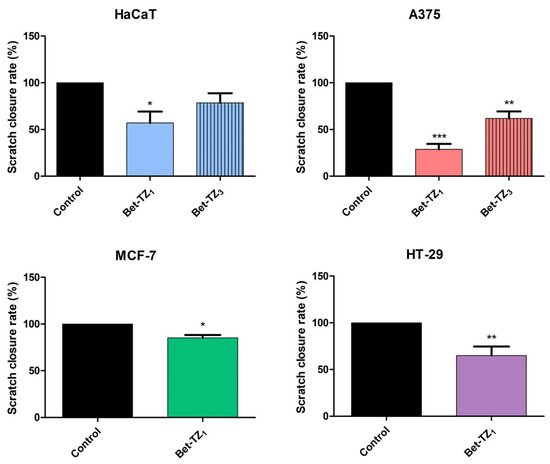
Figure 13.
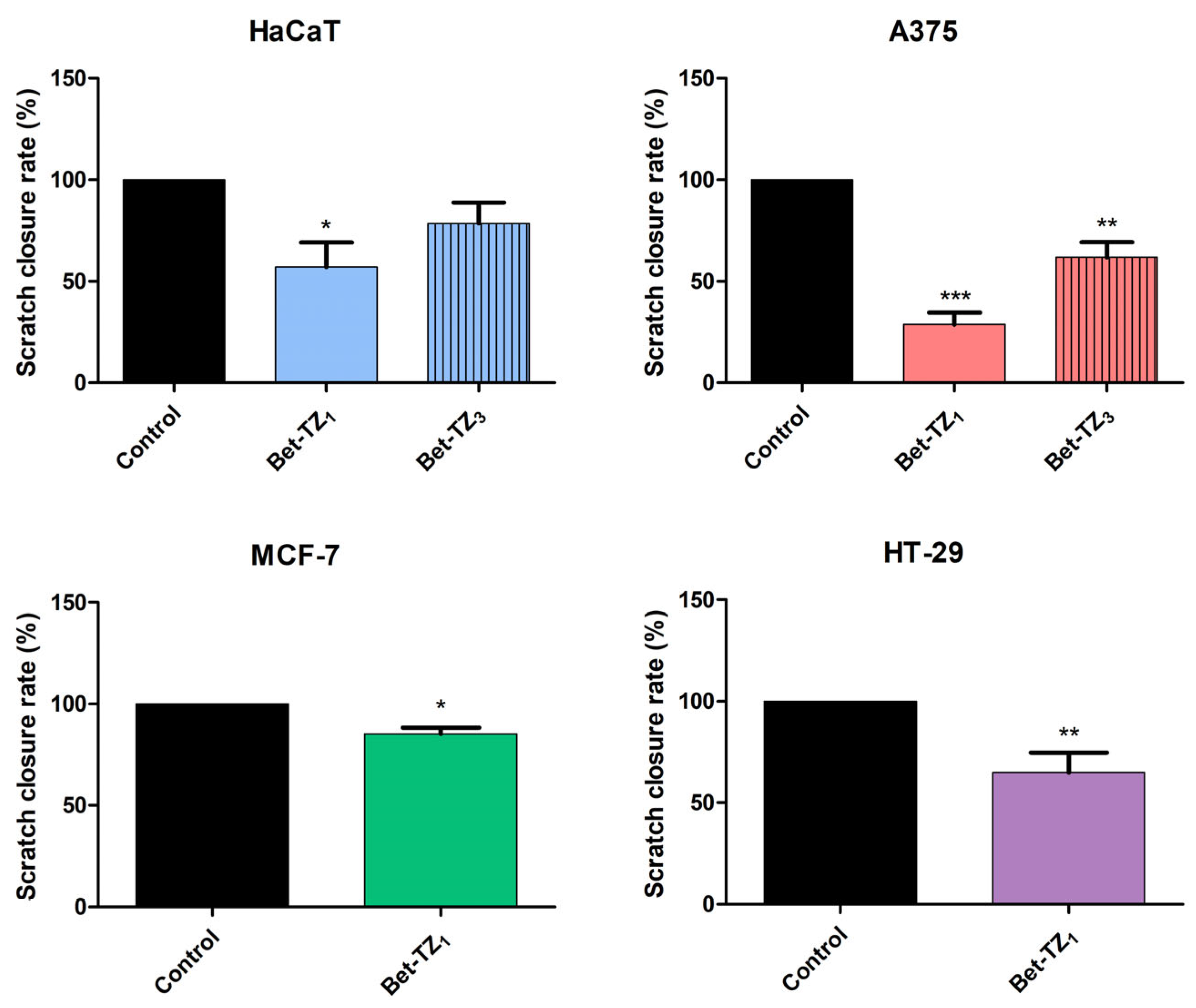
Scratch migration assay of Bet-TZ1 (10 μΜ) on HaCaT, A375, MCF-7, and HT-29 cells and of Bet-TZ3 (10 μΜ) on HaCaT and A375 cells. The presented values signify the remanent gap size 48 h post treatment and were calculated as a percentage of the initial gap size used as control (100%). Results are recorded as the mean values ± SD by one-way ANOVA test, followed by a Dunnett’s post hoc test (for HaCaT and A375 cells) and with unpaired t-test (for MCF-7 and HT-29 cells). (*** p < 0.001, ** p < 0.01, and * p < 0.05).
Both Bet-TZ1 and Bet-TZ3 exhibited lower scratch closure rates for HaCaT and A375 cells (57.03% and 78.54%—HaCaT; 28.74% and 61.83%—A375) vs. control (100%), while Bet-TZ1 expressed lower scratch closure rates compared to Bet-TZ3 on both HaCaT and A375 cells (Figure 12). Bet-TZ1 decreased the scratch closure area also on MCF-7 and HT-29 cells, as follows: 85.13% and 64.91% vs. control (100%) (Figure 12).
2.6. HET−CAM Test
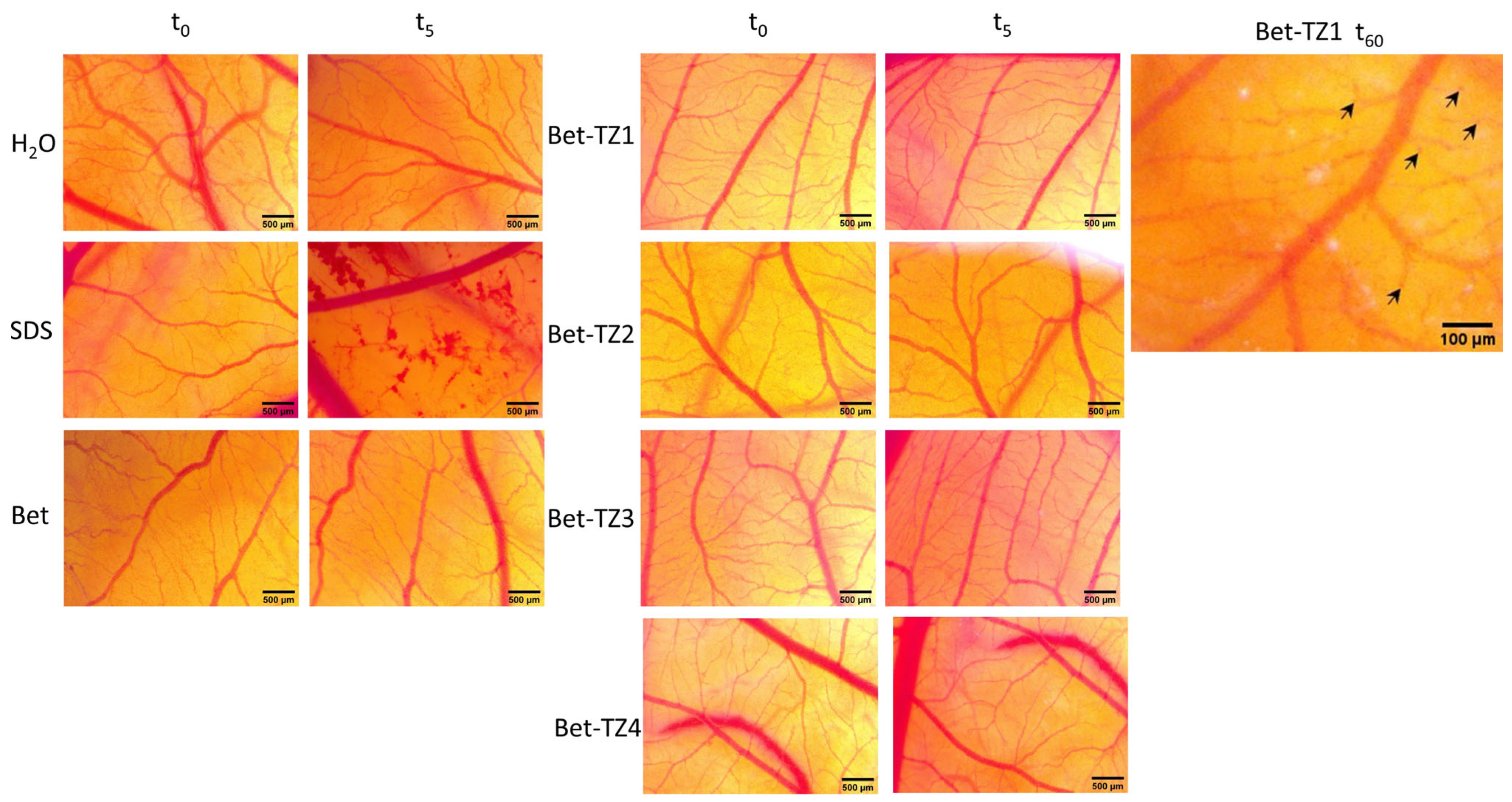
The HET−CAM test was used to assess the irritative potential of Bet and Bet-TZ1-4. By monitoring the effects induced 24 h after the application of 300 µL of each sample one can notice that Bet and Bet-TZ2-4 did not produce any interference with the circulation process; by contrast, Bet-TZ1 triggered some localized spotted hemorrhages, without influencing the embryo’s viability (Figure 14).
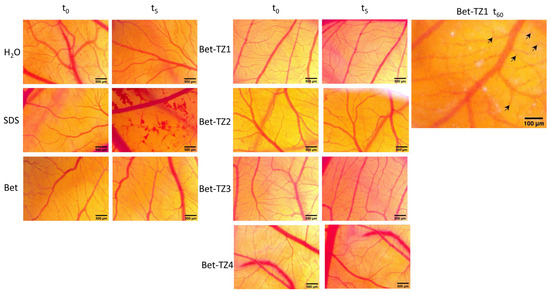
Figure 14.
The HET−CAM method-based irritation test. Stereomicroscope images of the chorioallantoic membrane were captured before (T0), 300 s (T5) post treatment with 300 μL Bet and Bet-TZ1-4, respectively (tested at 100 μM), and after 24 h for Bet-TZ1 indicating localized spotted hemorrhages (indicated by black arrows), without influencing the embryo’s viability; distilled water and sodium dodecylsulfate (SDS) 0.5%, were used as negative and positive control, respectively. Scale bars were set at 500 μm.
The irritation potential of each compound was investigated by using the Luepke scale [29] which assigns scores ranging from 0 to 21 based on the degree of severity of the reaction of the chorioallantoic membrane. Non-irritant compounds are classified within the range of 0 to 0.9; slight irritation is indicated by scores between 1 and 4.9; moderate irritation is depicted by scores ranging from 5 to 8.9 and strongly irritant compounds are assigned scores between 9 and 21. According to the findings in Table 3, the compounds under investigation did not exhibit any signs of irritation, indicating that they are appropriate for both mucosal and cutaneous applications.

Table 3.
The irritation factor for Bet and Bet-TZ1-4.
3. Discussion
Bet is the main triterpene isolated from the outer bark of Betula species, which possesses tremendous therapeutic potential [30]. Chemical modulation of key positions in betulin’s molecule allowed the synthesis of various derivatives with improved pharmacological profile. The 1,2,4-triazole and its derivatives are common heterocycles found in the structure of various drugs due to their innate physicochemical properties such as dipole character, rigidity, and the capacity to form hydrogen bonds, which provide them with a suitable pharmacological profile [31,32]. The use of 1,2,4-triazoles as linkers [33] or pharmacophores [34,35,36] in the structure of various triterpenes has been previously reported in the literature; however, to the best of our knowledge, this study represents the first report regarding the introduction of 5-substituted-1,2,4-triazole-3-thiols to the C30 position of Bet. An interesting property of azoles is the annular tautomerism where the proton linked to a heteroatom can migrate to other heteroatoms within the heterocycle [37]; thus, the 1,2,4-triazoles can be found in three tautomeric forms, namely, 1H-, 2H-, and 4H-1,2,4-triazoles. Although, in general, the 1H tautomer was considered the only stable tautomer in solution, some derivatives, such as 3(5)-chloro-1,2,4-triazole and 3(5)-bromo-1,2,4-triazole, were identified as 4H tautomers in solution, contradicting the previous knowledge on the topic [38]. Currently, the effect of different tautomers on the biological effect is not clearly understood. The impact of one sole tautomer on the therapeutic properties of that compound is always dependent on the duration of the tautomeric equilibrium in relation to a given biological process. On one hand, the rapid interconversion of tautomers in relation to a certain biological process can lead to both forms being consumed. On the other hand, slow interconversion in a similar setting could end up in one tautomer being favored as the only active species [39]. Therefore they might induce a different impact on the biological effect, as some tautomers can be rapidly interchanged into the most favorable form to interact with the target while slow interconversion of others could result in the existence of a single active tautomer [40]. We established that all Bet-TZ1-4 compounds exist in DMSO solutions in two stable forms in various percentages, as detected through 1H NMR spectroscopy, with one major component (59% to 81%) that we hypothesize is responsible for the anticancer effect. However, because we have not identified a specific protein target for our compounds, we cannot determine which tautomer is responsible for the biological effect. To visualize the exact tautomer form in the binding site, a highly precise crystallographic analysis of the target–ligand complex would be required.
The Bet-TZ1-4 cytotoxicity was assessed against HaCaT (human keratinocytes), A375 (melanoma), MCF-7 (breast cancer), and HT-29 (colorectal cancer) cell lines using the Alamar blue assay. The results in HaCaT cells showed that Bet-TZ2-4 semisynthetic derivatives significantly inhibit cell viability even at the highest tested concentration (100 µM); however, when used in high concentrations (75 and 100 µM), Bet-TZ1 significantly inhibited cell viability, compared to 5-FU used as a reference. These results indicate that Bet-TZ2-4 does not cytotoxically affect non-malignant cells and can be administered in elevated doses without significant side effects; in contrast, Bet-TZ1 exhibits clear cytotoxic effects against non-malignant keratinocytes. The selectivity index (SI) for Bet-TZ1, calculated as the IC50 HaCaT/IC50 cancer cell line, was less than 2 in all three cases (SIA375 1.90, SIMCF-7 1.27, and SIHT−29 0.91), indicating non-selective cytotoxic activity. However, the use of only the selectivity index to assess the anticancer potential of a drug candidate has been shown to be a poor and insufficient predictor [41].
Shy et al. reported the synthesis of similar betulin derivatives only by using 1,2,3-triazole as a C30-substituent while maintaining the free C3-hydroxyl group; experimental results showed that all triazole derivatives exerted superior cytotoxic effects compared to the parent compound but the presence of large lipophilic aromatic side chains favored their bioactivity [42].
The cytotoxicity of Bet-TZ1-4 against the melanoma cell line was similar or slightly enhanced compared to Bet. In particular, the effect of higher doses of Bet-TZ1 (75 and 100 µM) against A375 was superior to Bet and even 5-FU; a dose-dependent cytotoxic effect was also recorded for Bet-TZ3 after 48 h of exposure. In breast and colorectal cancer cells, the lowest dose of Bet-TZ1 and Bet-TZ3 (10 µM) and all samples of Bet-TZ2 and Bet-TZ4 showed inferior cytotoxicity compared with 5-FU and Bet alone; doses above 25 µM of Bet-TZ1 and Bet-TZ3 exhibited significantly stronger cytotoxic activity compared to Bet and 5-FU. In A375 cells, the IC50 values of Bet-TZ1 and Bet-TZ3 were 22.41 μΜ and 34.34 μΜ, compared to Bet alone (46.19 μΜ) and to 5-FU (1.06 μΜ). Intriguingly, despite a higher IC50 value on A375 cells, when compared to Bet-TZ1, Bet-TZ3 can be viewed as a more effective agent due to the lack of cytotoxic effect on non-malignant HaCaT cells that reveals a selective cytotoxic effect against melanoma cells. In MCF-7 and HT-29 cells, Bet-TZ3 was not active (IC50 > 100 μΜ) whereas Bet-TZ1 had an IC50 of 33.52 μΜ and 46.92 μΜ, outperforming the parent-compound Bet (37.29 μΜ and 55.67 μΜ). Comparatively, the most active compounds were Bet-TZ1, in particular in higher concentrations, against all tested cell lines, and Bet-TZ3 when used in A375 cells. While the unsubstituted triazole derivative was cytotoxic to both malignant and non-malignant cells, a substituted phenyl attached to the 5th position of the triazole ring reduced toxicity to non-malignant cells. Bet-TZ3, a p-chloro-phenyl triazole derivative of Bet, was more selective, being cytotoxic primarily to the A375 melanoma cell line. This case was previously encountered in a series of substituted 1,2,3-triazole derivatives of betulinic acid (linked to the triterpene in the same 28th position), with the p-fluoro-phenyl derivative being the most active compound [42]. It appears that p-halogeno-triazole groups linked at the allylic position of a lupane-type triterpene are beneficial for inducing in vitro cancer cell cytotoxicity. However, this hypothesis requires a larger compound series to be synthesized and tested in order to be validated. Considering these results, only these two compounds in concentrations equivalent to their IC50 values were further evaluated in terms of potential effect on cell morphology.
The induction of apoptosis is a well known cellular mechanism for Bet and other triterpenes [43], as well as their semisynthetic derivatives [44]. In the current study, a morphology assessment also showed that the newly synthesized betulin derivatives triggered apoptotic processes, identified as nuclei condensation and fragmentation which led to the formation of characteristic apoptotic bodies [45]. Apoptosis was also identified as the underlying mechanism for similar betulinic acid-triazole derivatives [34]; the authors established that large triazole substituents in the C30 position favorably influence the compound’s cytotoxicity becoming an important element of the pharmacophore with the C3 substituent affecting its bioavailability. For betulinic acid derivatives, having two hydrophilic groups in C28 and C3 positions seemed to hinder cell membrane penetration, with acetylation of at least one such group being able to overcome this challenge. In this case, both hydroxylic groups in the C20 and C3 positions bear the lipophilic acetate moiety that apparently facilitates cell entrance. Our previous experiments on betulinic acid derivatives bearing 1,2,4-triazole scaffolds in the C30 position, together with acetylated C3-hydroxyl, supported such conclusions both in terms of the mechanism of action and the structure–activity relationships [46,47].
Betulin was also previously shown to trigger apoptosis in cancer cells via the intrinsic signaling pathway. However, unlike betulinic acid, Bet does not primarily affect the pro/anti-apoptotic protein (BAX, Bcl-2) normal ratio [48,49], but rather induces an upregulated/increased caspase activity [50,51]. Caspases 3 and 9 are key players in the onset of the intrinsic apoptotic pathway [52], and as stated above, are correlated with Bet pro-apoptotic activity. However, given the fact that MCF-7 is a caspase 3-deficient cell line [53], we proceeded to quantify the expression of caspase 9 in cell lines treated with Bet-TZ1 and Bet-TZ3, where a significant cytotoxic activity was recorded. As expected, Bet did increase the expression of caspase 9, but so did both Bet derivatives in each tested setting. There are currently no triazole-betulin derivatives tested for anti-apoptotic activity similar to the ones described in this study. However, previous studies reported that the synthesis of other types of betulin derivatives with various functional groups in positions 3, 28, or 30 has resulted in pro-apoptotic agents with increased caspase activity [54,55,56]. According to these findings, the increased expression of various caspases and the associated pro-apoptotic features appear to be more related to the triterpenic structural core than to the various functional groups present in their structure. As a result, future research focusing on additional Bet derivatizations may shed light on the big picture of betulin derivatives-induced apoptosis in cancer cells.
It has been shown that the migration of cancer cells is an important marker for tumor cell invasion and metastasis while inhibiting cell migration represents a strong and desirable quality for a potent anticancer agent [57]. The antimigratory effect of the leading compounds, Bet-TZ1 and Bet-TZ3, was assessed in non-cancer and cancer cell lines; results revealed that although both compounds inhibit the migration of tested cells, the effect of Bet-TZ1, containing the unsubstituted 1,2,4-triazole, was superior to Bet-TZ3. The ability of the triterpene scaffold to prevent cancer cell migration was previously revealed for numerous betulin and betulinic acid derivatives displaying various chemical modulations in several cancer cell lines where the cytotoxic activity was clearly distinct from the anti-invasive capacity [58,59].
The HET–CAM assay represents a one of a kind model in biomedical testing since the chorioallantoic membrane provides a rich vascular network that enables a wide variety of non-invasive biological studies [60]. It is considered a better and more valid alternative to more invasive tests used to assess the local irritative potential of different substances, providing information regarding vascular events such as coagulation, hyperemia, and hemorrhage [61]. In our study, Bet, as well as its Bet-TZ1-4 derivatives did not induce irritative phenomena thus predicting their safe use on skin and mucosae. These findings are in agreement with other studies supporting the non-irritative and wound-healing effect of triterpenes [62]; one such example is a randomized phase 3 trial developed by Frew et al. [63] in which a gel containing Bet accelerated the healing of superficial partial thickness burns.
These findings collectively show that Bet can be an ideal starting point for developing potent cytotoxic compounds for cancer cells. This is an important step because, unlike its acid counterpart (BA), Bet is more readily obtained and considerably less expensive. Future research could focus on expanding the synthesized series in order to assess precise structure–activity relationships and possibly pinpoint specific targets for the active compounds.
4. Materials and Methods
4.1. Chemistry
The reagents utilized for the chemical synthesis were commercially acquired from Merk (Darmstadt, Germany) and were subsequently used without any supplementary purification.
4.1.1. Instruments
The 1D (1H and 13C) and 2D NMR (H,H-COSY, H,C-HSQC, and H,C-HMBC) experiments were performed utilizing a Bruker Avance NEO Spectrometer 400 MHz (Bruker, Karlsruhe, Germany) that was equipped with a QNP direct detection probe (5 mm) and z-gradients. The spectra were recorded under standard conditions in either hexadeuterodimethyl sulfoxide (DMSO-d6) or deuterochloroform (CDCl3) and were referenced to the residual peak of the solvent (1H: 2.51 ppm for DMSO-d6 or 7.26 ppm for CDCl3; 13C: 39.5 ppm for DMSO-d6 or 77.0 for CDCl3). For Bet-TZ1-4 derivatives, we first recorded the 1D and 2D NMR spectra in DMSO-d6. Then, in each NMR tube containing the solutions of Bet-TZ1-4, a drop of trifluoroacetic acid (TFA) was added; the solutions were vortexed for 5 min at 500 rpm and then the 1D and 2D NMR experiments were recorded again. All the NMR experiments were recorded using standard parameter sets, as provided by Bruker. A Biobase melting point instrument (Biobase Group, Jinan, China) was utilized to record the melting points. Thin-layer chromatography was performed using 60 F254 silica gel-coated plates (Merck KGaA, Darmstadt, Germany). Fourier-transform infrared spectroscopy (FTIR) experiments were conducted with KBr pellets using a Shimadzu IR Affinity-1S apparatus (400–4000 cm−1 range and a 4 cm−1 resolution). Methanolic solutions were utilized to record LC/MS spectra in the negative ion mode, using an Agilent (Santa Clara, CA, USA) 6120 Quadrupole LC/MS system that was outfitted with an ESI ionization source, UV detector, and a SB−C18 Zorbax Rapid Resolution column. The samples were analyzed under the following conditions: 25 °C, 0.4 mL/min, and l = 250 nm. The mobile phase was composed of a 1 mM isocratic mixture comprising 15% ammonium formate and 85% methanol.
4.1.2. Synthesis Procedure for Br-Bet
The process of Bet acetylation was carried out using a modified version of a previously described method [64,65]. This involved the reaction of Bet (1 equivalent) with acetic anhydride (4 equivalents) in pyridine and dimethylaminopyridine (DMAP) (0.1 equivalent) at room temperature for a duration of 12 h. The reaction mixture underwent dilution with water and was subjected to triple extraction with CHCl3. The organic phase was dehydrated using anhydrous MgSO4, followed by solvent elimination through rotary evaporation. The freshly obtained 3-O, 28-O-diacetyl-betulin was subsequently used without undergoing supplementary purification. Subsequently, a solution of 2.5 g of acetylated Bet, approximately equivalent to 5 mmol, was prepared in 50 mL of CCl4. Following this, 1.78 g of recently recrystallized NBS, equivalent to 10 mmol, was introduced into the solution. The reaction continued at room temperature for a duration of 48 h. Subsequently, the solution was subjected to filtration, followed by solvent evaporation. The resulting product was chromatographed over silica, utilizing a mixture of CHCl3 and ethyl acetate in a volume ratio of 40:1.
3,28-O-diacetyl-betulin, white powder, m.p. 216–218 ℃, yield 82%; 1H NMR (CDCl3, 400.13 MHz, δ, ppm): 4.68 (s, 1H, H29a), 4.58 (s, 1H, H29b), 4.46 (dd, J = 6.0 Hz, J = 10 Hz, 1H, H3), 4.24 (d, J = 10.9 Hz, 1H, H28a), 3.84 (d, J = 11.0 Hz, H28b), 2.44 (m, 1H, H19), 2.07 (s, 3H, H34), 2.04 (s, 3H, H32), 1.98.1.90 (m, 1H, H15a), 1.84 (d, J = 13.0 Hz, 1H, H21a), 1.76 (dd, J = 12.4 Hz, J = 8.4 Hz, 1H, H7a), 1.68–1.59 (m, 10H, H1a, H2a, H12a, H13, H18, H22, H30), 1.50–1.49 (m, 1H, H6a), 1.41–1.39 (m, 5H, H9, H11a, H15b, H16), 1.30–1.18 (m, 3H, H6b, H11b, H21b), 1.11–1.02 (m, 6H, H2b, H7b, H12b, H27), 0.96–0.93 (m, 4H, H1b, H26), 0.84–0.83 (m, H23–25), 0.78 (d, J = 9.0 Hz, 1H, H5). 13C NMR (CDCl3, 100.6 MHz, δ, ppm): 171.6 (C33), 171.0 (C31), 150.1 (C20), 109.9 (C29), 80.9 (C3), 62.8 (C28), 55.4 (C5), 50.3 (C9), 48.8 (C18), 47.7 (C19), 46.3 (C17), 42.7 (C14), 44.9 (C8), 38.4 (C1), 37.8 (C4), 37.5 (C13), 37.1 (C10), 34.5 (C7), 34.1 (C16), 29.7 (C21), 29.6 (C15), 27.9 (C23), 27.0 (C12), 25.1 (C2), 23.7 (C22), 21.3 (C32), 21.0 (C34), 20.8 (C11), 19.1 (C30), 18.2 (C6), 16.5 (C24), 16.1 (C27), 16.0 (C25), 14.7 (C26).
3,28-O-diacetyl-30-bromo-betulin (Br-Bet), white powder, m.p. 187–190 ℃, yield 65%; 1H NMR (CDCl3, 400.13 MHz, δ, ppm): 5.13 (s, 1H, H29a), 5.02 (s, 1H, H29b), 4.45 (m, 1H, H3), 4.25 (m, 1H, H28a), 3.97 (s, 2H, H30), 3.84 (m, H28b), 2.44 (m, 1H, H19), 2.07 (s, 3H, H34), 2.03 (s, 3H, H32), 1.84–0.76 (m, betulinic protons). 13C NMR (CDCl3, 100.6 MHz, δ, ppm)171.5 (C33), 171.0 (C31), 150.8 (C20), 113.3 (C29), 80.9 (C3), 62.5–14.6 (betulinic carbons). ESI-MS Rt = 4.74 min, m/z = 604 [M-H+]−.
4.1.3. Synthesis Procedure for TZ1
The procedure to synthesize 1,2,4-triazole-3-thiol (TZ1) was established based on the available methods in the literature [66,67] and was previously reported by our group along with the corresponding spectral data [46]. First, 0.5 moles of formic acid (90%, 20 mL) was stirred with 0.1 moles thiosemicarbazide for 30 min, when 1-formyl-3-thiosemicarbazide began to crystalize. Cold water was added and the emulsion was filtered and kept in an ice bath for the crystallization of 1H-1,2,4-triazole-3-thiol to occur. The crystals were filtered, dried, and utilized immediately without additional purification. For the following stage, 30 mmoles of NaOH, 20 mL H2O, and 28.1 mmoles of 1-formyl-3-thiosemicarbazide were added into a 50 mL round-bottom flask and were refluxed for 1 h. After completion, the reaction was cooled and the final product was precipitated (concentrated HCl) and filtered.
4.1.4. Synthesis Procedure for TZ2-4
The procedure for synthesizing 5-substituted-1,2,4-triazole-3-thiol was carried out in accordance with established methodologies as previously reported [24]. A solution containing 20 mmol of thiosemicarbazide was prepared by dissolving it in 50 mL of DMF while being stirred magnetically. Then, 22 mmol of pyridine and 20 mmol of aroyl chloride were added to the solution. The process of magnetic stirring was sustained at room temperature for a duration of 30 min, following which the temperature was elevated to 50 °C and sustained for an approximate duration of 1 h. The endpoint of the reaction was verified by means of TLC. The aroyl-thiosemicarbazides that were obtained were subjected to precipitation using aqueous hydrochloric acid, followed by filtration and subsequent drying. Furthermore, the 5-substituted-1H-1,2,4-triazole-3-thiols (TZ2-4) were synthesized through the cyclization of 10 mmol of aroyl-thiosemicarbazide in ethanolic NaOH at reflux. The reaction was monitored using TLC until completion. The 5-substituted-1H-1,2,4-triazole-3-thiols were obtained through precipitation with HCl 4% and the further filtration of the resulting precipitate. Spectral data for TZ2 and TZ4 were previously reported [47]. Spectral data for TZ3 are listed below.
5-(4-chlorophenyl)-1H-1,2,4-triazole-3-thiol (TZ3); white powder, m.p. 296−298 °C (uncorrected), yield 65%; 1H NMR (400.13 MHz, DMSO-d6, d, ppm): 13.93 (s, 1H), 13.75 (s, 1H), 7.93 (d, J = 8.6 Hz, 2H), 7.61 (d, J = 8.6 Hz, 2H). 13C NMR (100.6 MHz, DMSO-d6, d, ppm): 167.1, 149.3, 135.2, 129.2, 127.4, 124.3. ESI−MS Rt = 0.5 min, m/z = 210 [M-H+]−.
4.1.5. Synthesis Procedure for Bet-TZ1-4
A quantity of 0.2 mmoles of 3,28-O-diacetyl-30-bromo-betulin (BetBr) and 0.3 mmoles of anhydrous K2CO3 were added in 5 mL of DMF and were stirred for 10 min at 25 °C. In the next stage, 0.2 mmoles of triazole derivative (TZ1-4) was added and the mixture was stirred for an additional 72 h at room temperature. In the following stage, the mixture was diluted with 50 mL H2O and then extracted with CHCl3 (4 × 15 mL). Anhydrous MgSO4 was used to dry the organic phase and after solvent removal, the obtained product was chromatographed using a 2:1 ratio of CHCl3 to ethyl acetate.
3,28-O-diacetyl-30-(1H-1,2,4-triazole-3-yl-sulfanyl)-betulin (Bet-TZ1), white powder, m.p. 113–119 °C (uncorrected), yield 38%; 1H NMR (DMSO-d6 + TFA, 400.13 MHz, δ, ppm): 8.49 (, s, 1H, H36), 4.95 (s, 1H, H29a), 4.91 (s, 1H, H29b), 4.36 (dd, J = 4.6 Hz, J = 10 Hz, 1H, H3), 4.23 (d, J = 10.8 Hz, 1H, H28a), 3.80 (s, 2H, H30) 3.72 (d, J = 10.9 Hz, H28b), 2.47 (m, 1H, H19),2.03 (m, 1H, H2a) 2.02 (s, 3H, H34), 1.99 (s, 3H, H32), 1.74–0.94 (betulinic protons), 0.80–0.78 (m, 10H, H5, H23–25). 13C NMR (DMSO-d6+ TFA, 400.13 MHz, δ, ppm): 170.9 (C33), 170.3 (C31), 156.4 (C35), 149.7 (C20), 146.3 (C36), 111.6 (C29), 80.1 (C3), 61.4 (C28), 54.7 (C5), 49.6 (C9), 49.2 (C18), 46.1 (C17), 44.6 (C19), 42.3 (C14), 40.5 (C8), 37.9 (C1), 37.5 (C4), 37.1 (C13), 36.7 (C10), 36.5 (C30), 33.9 (C7), 33.7 (C16), 30.9 (C21), 29.3 (C15), 27.6 (C23), 26.7 (C12), 26.2 (C2), 23.5 (C22), 21.1 (C32), 20.8 (C34), 20.6 (C11), 17.8 (C6), 16.5 (C24), 15.9 (C27), 15.7 (C25), 14.6 (C26). FTIR [KBr] (cm−1) relevant peaks: 3117 (N-H stretch); 2945, 2872 (C-H stretch); 1735, 1244, 1030 (ester C=O, C-C-O, O-C-C stretch); ESI-MS Rt = 2.85 min, m/z = 625 [M-H+]−.
3,28-O-diacetyl-30-{5-[4-(dimethylamino)phenyl]-1H-1,2,4-triazole-3-yl-sulfanyl}-betulin (Bet-TZ2); white powder, m.p. 113–119 °C (uncorrected), yield 41%; 1H NMR (DMSO-d6 + TFA, 400.13 MHz, δ, ppm): 7.85 (d, J = 8.8 HZ, 2H, H38), 7.00 (d, J = 8.8 Hz, 2H, H39), 5.00 (s, 1H, H29a) 4.90 (s, 1H, H29b), 4.31 (dd, J = 4.8 Hz, J = 11.3 Hz, 1H, H3), 4.22 (d, J = 10.9 Hz, 1H, H28a), 3.90 (d, J = 14.4 Hz, 1H, H30a, AB spin system), 3.81 (d, J = 14.4 Hz, 1H, H30b, AB spin system), 3.73 (d, J = 10.9 Hz, H28b), 3.02 (s, 6H, H41), 2.47 (m, 1H, H19), 2.06 (m, 1H, H2a) 2.00 (s, 3H, H34), 1.97 (s, 3H, H32), 1.70–0.90 (betulinic protons), 0.75–0.72 (m, 10H, H5, H23–25). 13C NMR (DMSO-d6+ TFA, 400.13 MHz, δ, ppm): 171.0 (C33), 170.5 (C31), 160.6 (C40), 156.3 (C35), 155.8 (C35), 150.4 (C40) 149.6 (C20), 127.7 (C38), 116.1 (C37), 113.7 (C39), 111.5 (C29), 80.2 (C3), 61.7 (C28), 54.9 (C5), 49.7 (C9), 49.1 (C18), 46.2 (C17), 44.3 (C19), 42.4 (C14), 41.0 (C41), 40.6 (C8), 37.8 (C1), 37.5 (C4), 37.2 (C13), 36.7 (C10, C30), 34.0 (C7), 33.7 (C16), 31.0 (C21), 29.4 (C15), 27.8 (C23), 26.8 (C12), 26.4 (C2), 23.6 (C22), 21.1 (C32), 20.9 (C34), 20.7 (C11), 17.9 (C6), 16.6 (C24), 16.0 (C27), 15.8 (C25), 14.7 (C26). FTIR [KBr] (cm−1) relevant peaks: 3232 (N-H stretch); 2945, 2873 (C-H stretch); 1735, 1244, 1029 (ester C=O, C-C-O, O-C-C stretch); ESI-MS Rt = 2.25 min, m/z = 744 [M-H+]−.
3,28-O-diacetyl-30-[5-(4-chlorophenyl)-1H-1,2,4-triazole-3-yl-sulfanyl]-betulin (Bet-TZ3); white powder, m.p. 128–135 °C (uncorrected), yield 32%; 1H NMR (DMSO-d6 + TFA, 400.13 MHz, δ, ppm): 7.97–7.95 (m, 2H, H38), 7.48 (d, J = 6.6 Hz, 2H, H39), 5.03 (s, 1H, H29a) 4.91 (s, 1H, H29b), 4.36 (dd, J = 4.5 Hz, J = 11.3 Hz, 1H, H3), 4.22 (d, J = 10.8 Hz, 1H, H28a), 3.93 (d, J = 14.5 Hz, 1H, H30a, AB spin system), 3.80 (d, J = 14.5 Hz, 1H, H30b, AB spin system) 3.73 (d, J = 10.9 Hz, H28b), 2.57 (m, 1H, H19), 2.06 (m, 1H, H2a) 2.00 (s, 3H, H34), 1.98 (s, 3H, H32), 1.72–0.91 (betulinic protons), 0.76–0.68 (m, 10H, H5, H23–25). 13C NMR (DMSO-d6+ TFA, 400.13 MHz, δ, ppm): 170.9 (C33), 170.3 (C31), 157.7 (C36), 156.0 (C35), 149.6 (C20), 130.0 (C40), 129.0 (C39), 128.5 (C37), 126.0 (C38), 114.5 (C29), 80.6 (C3), 61.6 (C28), 54.7 (C5), 49.6 (C9), 49.0 (C18), 46.1 (C17), 44.5 (C19), 42.3 (C14), 40.5 (C8), 37.9 (C1), 37.4 (C4), 37.1 (C13), 36.6 (C10, C30), 33.9 (C7), 33.7 (C16), 30.7 (C21), 29.4 (C15), 27.7 (C23), 26.7 (C12), 26.1 (C2), 23.4 (C22), 21.0 (C32), 20.8 (C34), 20.6 (C11), 17.8 (C6), 16.4 (C24), 15.9 (C27), 15.7 (C25), 14.6 (C26). FTIR [KBr] (cm−1) relevant peaks: 3230 (N-H stretch); 2945, 2872 (C-H stretch); 1735, 1246, 1029 (ester C=O, C-C-O, O-C-C stretch); ESI-MS Rt = 3.05 min, m/z = 735 [M-H+]−.
3,28-O-diacetyl-30-[5-(4-methoxyphenyl)-1H-1,2,4-triazole-3-yl-sulfanyl]-betulin (Bet-TZ4); white powder, m.p. 132–138 °C (uncorrected), yield 35%; 1H NMR (DMSO-d6 + TFA, 400.13 MHz, δ, ppm): 7.88 (d, J = 8.8 HZ, 2H, H38), 7.04 (d, J = 8.8 Hz, 2H, H39), 5.00 (s, 1H, H29a) 4.90 (s, 1H, H29b), 4.36 (dd, J = 4.8 Hz, J = 11.1 Hz, 1H, H3), 4.22 (d, J = 10.9 Hz, 1H, H28a), 3.89 (d, J = 14.5 Hz, 1H, H30a, AB spin system), 3.81–3.79 (m, 4H, H30b, H41) 3.72 (d, J = 11.0 Hz, H28b), 2.47 (m, 1H, H19), 2.06 (m, 1H, H2a) 2.00 (s, 3H, H34), 1.97 (s, 3H, H32), 1.71–0.90 (betulinic protons), 0.76–0.69 (m, 10H, H5, H23–25). 13C NMR (DMSO-d6+ TFA, 400.13 MHz, δ, ppm): 170.7 (C33), 170.1 (C31), 160.6 (C40), 156.9 (C36), 156.4 (C35), 149.6 (C20), 127.5 (C38), 120.6 (C37), 114.3 (C39), 111.2 (C29), 79.9 (C3), 61.5 (C28), 55.3 (C41), 54.6 (C5), 49.5 (C9), 48.9 (C18), 46.0 (C17), 44.3 (C19), 42.2 (C14), 40.4 (C8), 37.6 (C1), 37.3 (C4), 37.0 (C13), 36.5 (C10), 36.4 C30), 33.8 (C7), 33.6 (C16), 30.7 (C21), 29.2 (C15), 27.6 (C23), 26.6 (C12), 26.0 (C2), 23.3 (C22), 20.9 (C32), 20.7 (C34), 20.5 (C11), 17.7 (C6), 16.4 (C24), 15.7 (C27), 15.6 (C25), 14.5 (C26). FTIR [KBr] (cm−1) relevant peaks: 3230 (N-H stretch); 2945, 2872 (C-H stretch); 1735, 1247, 1030 (ester C=O, C-C-O, O-C-C stretch); ESI-MS Rt = 2.14 min, m/z = 731 [M-H+]−.
4.2. Biological Assessment
4.2.1. Cell Culture
The selected cell lines for the study, namely, HaCat (immortalized human keratinocytes) were acquired from CLS Cell Lines Service GmbH (Eppelheim, Germany), whereas A375 (human malignant melanoma cells), HT-29 (human colorectal adenocarcinoma), and MCF7 (human breast adenocarcinoma) were purchased from American Type Culture Collection (ATCC, Lomianki, Poland). The aforementioned cells were obtained as frozen items and were subsequently stored in liquid nitrogen. Dulbecco’s Modified Eagle Medium (DMEM) High Glucose added with 1% Penicillin/Streptomycin mixture (100 IU/mL) and with 10% fetal bovine serum (FBS) was used to culture HaCaT and A375 cells, while HT-29 cells were cultured using McCoy’s 5A Medium, supplemented with the same 10% FBS and 1% antibiotic mixture. The MCF7 cells were propagated in Eagle’s Minimum Essential Medium (EMEM), supplemented with 10% FBS, 1% antibiotic mixture, and 0.01 mg/mL human recombinant insulin. All cells were maintained in a humified incubator with 5% CO2 at 37 °C. After reaching 80–90% confluence, cells were stimulated with the tested compounds (10, 25, 50, 75, and 100 μΜ) for 24 h and 48 h, respectively. The cell number was determined with Trypan Blue using a cell counting device (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
4.2.2. Cell Viability Assessment
The Alamar blue colorimetric determination was used to assess the cell viability of HaCaT, A375, MCF7, and HT-29 cells, after stimulation with increasing concentrations (10, 25, 50, 75, and 100 μΜ) of four diacetylbetulin derivatives, betulin, and 5-fluorouracil as a positive control, at the same concentrations for 48 h. The cells (1 × 104) were seeded into 96-well plates and incubated (37 °C and 5% CO2) until an 80–85% confluence was reached. The used medium was discarded using an aspiration station and swapped with fresh medium specific for each cell line, containing the compounds. The tested concentrations (10, 25, 50, 75, and 100 μM) were prepared from 20 mM compound stock solutions so that the final concentration of DMSO did not exceed 0.5%. After 48 h, 0.01% Alamar blue was used to counterstain all cells, after which the cells incubated for an additional 3 h. The absorbance measurements were carried out at 2 wavelengths (570 nm, and 600 nm) using a xMark™ Microplate Spectrophotometer, Bio-Rad (Hercules, CA, USA). The experiments were performed in triplicate.
4.2.3. Immunofluorescence Assay—Morphological Assessment of Apoptotic Cells
The assessment of nuclear localization and any signs of apoptosis (shrinkage, fragmentation) and cytoplasmatic alterations were determined using Hoechst staining, while the cytoplasmatic localization was assessed using beta-tubulin staining. HaCaT, A375, MCF-7, and HT-29 cells were seeded onto 12-well plates at 2 × 105 cells/well initial density. After reaching 80–90% confluence, the cells were stimulated with Bet-TZ1 using its 48-h-treatment IC50 values obtained for each cell line and with Bet-TZ3 at its 48-h-treatment IC50 value for the A375 cell line. Separately, some wells were stimulated with 5-fluorouracil using the concentration corresponding to its IC50 values for each cell line at 48 h. After 48 h, the old medium was removed and the cells were fixed with methanol for 15 min, permeabilized with Triton X 0.01% in phosphate buffer saline (PBS) for an additional 15 min, and finally blocked with Bovine serum albumin 3% (BSA) for 30 min at room temperature. Afterward, the cells were stained with beta-tubulin monoclonal antibody at a dilution of 1:2000 in BSA 3% for 1 h (room temperature) and subsequently incubated with Alexa Fluor 488 goat-anti mouse secondary antibody at a 1:5000 dilution in BSA 3% for 30 min in the dark. Finally, the Hoechst 33258 solution was added for 5 min. The nuclear and cytoplasmatic alterations were observed and recorded using the integrated DP74 digital camera of the inverted microscope, Olympus IX73 (Olympus, Tokyo, Japan).
4.2.4. Real-Time PCR Quantification of Apoptotic Markers
The total RNA was extracted using the peqGold RNAPureTM Package (Peqlab Biotechnology GmbH, Erlangen, Germany) following the manufacturer’s instructions, and the total concentration of RNA was measured using a DS-11 spectrophotometer (DeNovix, Wilmington, DE, USA). Reverse transcription was achieved using the Maxima® First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The Tadvanced Biometra Product line (Analytik Jena AG, Göttingen, Germany) was used for sample incubation using the following thermal cycle: 10 min at 25 °C, 15 min at 50 °C, and 5 min at 85 °C. The Quant Studio 5 real-time PCR system (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used for quantitative real-time PCR determinations. The experiment was conducted using 20 µL aliquots containing Power SYBR-Green PCR Master Mix (Thermo Fisher Scientific, Inc., Waltham, MA, USA), pure water, the sense and antisense primer, and the sample cDNA. The primer pairs used for this method included 18 S (Thermo Fisher Scientific, Inc., Waltham, MA, USA), used as housekeeping gene (sense: 5′GTAACCCGTTGAACCCCATT 3′; antisense: 5′CCATCCAATCGGTAGTAGCG3′), and Caspase-9 (sense: 5′ATGGACGAAGCGGATCGGCGGCTCC3′; antisense: 5′GCACCACTGGGGGTAAGGTTTTCTAG3′) (Eurogentec, Seraing, Belgium). Normalized, results were calculated using the comparative threshold cycle method (2−ΔΔCt).
4.2.5. Scratch Assay
The regressive effect on the invasion capacity of Bet-TZ1 (on A375, MCF7, and HT-29 cancer cells) and Bet-TZ3 (on A375 cells) and their wound healing potential on HaCaT cells was determined using the scratch test. The cells were seeded onto 12-well plates at an initial density of 2 × 105 cells/well. After reaching 80–85% confluence, the old medium was removed and each well was washed with warm PBS, then treated with 5 μg/mL mitomycin C for 2 h at 37 °C. Mitomycin C is an antibiotic that inhibits DNA synthesis and cell proliferation used to determine the true anti-migratory effect of a substance. After mitomycin C treatment, the cells were again washed with PBS, scratched onto the diameter of the well with a sterile pipette tip, and then stimulated with 10 μM Bet-TZ1 and Bet-TZ3. To establish the scratch closure rate (%), the wells were photographed at 0, 24, and 48 h using the Olympus IX73 inverted microscope (Olympus, Tokyo, Japan). The Sense Dimension software (version 1.8) was utilized for analyzing cell migration for each cell line.
4.2.6. Statistical Analysis
The statistical analysis was achieved by employing a t-test and one-way ANOVA followed by Dunnett’s post hoc test using GraphPad Prism version 6.0.0 (GraphPad Software, San Diego, CA, USA). The IC50 values were calculated using the same software, according to the correlation between the log[concentration] and cell viability. The statistically significant threshold (p < 0.05) between groups was * p < 0.05, ** p < 0.01, and *** p < 0.001.
4.3. HET−CAM Assay
We used the HET−CAM in vivo protocol to evaluate the safety profile of a particular substance against a living tissue. The standard protocol involved the usage of a developing chorioallantoic membrane within an embryonated chicken (Gallus domesticus) egg. This method complied with the Interagency Coordinating Committee on the Validation of Alternative Methods recommendations [68], which were customized to suit the specific circumstances of the study. Based on an adapted approach to the established methodology [69], the eggs were subjected to incubation conditions of 37 °C and 50% relative humidity. On the third day of incubation, 5–6 mL of albumen was extracted, subsequently leading to the creation of an opening at the top of the eggs. In the context of the developing chorioallantoic membrane of the chick embryo, a volume of 300 μL of SLS (positive control), Bet, and Bet-TZ1-4 were administered at a concentration of 100 µM. The alterations in CAM were observed through the use of stereomicroscopy, specifically, the Discovery 8 Stereomicroscope by Zeiss (Jena, Germany). The images were captured using the Zeiss Axio CAM 105 color camera, both before and 5 min after the application of the tested substances. During the five-minute duration, the impact on three specific parameters was observed (hemorrhage, lysis, and vascular plexus coagulability). Each determination was performed in triplicate. The obtained results were quantified as irritation factor (IF) values, which were determined using the provided formula. These values were then compared to a negative (distilled water) and a positive control (SLS 0.5%) with an IF of 16.29. The Luepke scale was used to interpret the IF values, where a range of 0–0.9 indicates non-irritation, 1–4.9 indicates weak irritation, 5–8.9 indicates moderate irritation, and 9–21 indicates strong irritation [70].
where IF = irritation factor; H = hemorrhage; L = vascular lysis; C = coagulation; Sec H = start of hemorrhage reactions (s); Sec L = onset of vessel lysis on CAM (s); Sec C = onset of (s).
5. Conclusions
This study presented the synthesis, cytotoxicity assessment, and influence on angiogenesis of a series of diacetylbetulin derivatives containing 5-Substituted-1,2,4-triazoles at C30 (Bet-TZ1-4). While the synthesis protocol led to obtaining good yields of target compounds, the NMR analysis revealed that, in the DMSO solution, they exist in two tautomeric forms that could have an influence on the anticancer effect, the hypothesis that remains to be explored in further studies. The cytotoxicity assessment of Bet-TZ1-4 against A375 (melanoma), MCF-7 (breast cancer), HT-29 (colorectal cancer), and HaCaT (human keratinocytes) revealed Bet-TZ1 as the lead candidate of the series against all tested lines. However, the cytotoxicity of Bet-TZ1 manifested also against the non-malignant HaCaT cell line, indicating a reduced selectivity of the derivative. Promising results were also obtained for Bet-TZ3, which exhibited a selective cytotoxic effect against melanoma cells, and along Bet-TZ1, which showed promising anti-migratory properties. A related cytotoxic correlated pro-apoptotic effect was observed for both compounds confirmed by morphological nuclear assessment and PCR results that showed an increase in the expression of caspase 9. The HET−CAM test revealed that Bet-TZ1-4 does not have an irritative potential, supporting their safety application in local treatments. Although our study obtained modest results in terms of cytotoxicity, further investigation of betulin-triazole derivatives still remains a pathway that should be explored, focusing on the synthesis of more selective derivatives against cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr12010024/s1, Figure S1. The three possible tautomeric forms of 3,5-disubstituted 1,2,4-triazoles; Figure S2. 1H NMR spectrum of 3-O,28-O-diacetyl-betulin (400 MHz, CDCl3); Figure S3. 13C NMR spectrum of 3-O,28-O-diacetyl-betulin (100 MHz, CDCl3); Figure S4. 1H NMR spectrum of 3-O, 28-O-diacetyl-30-bromo-betulin (400 MHz, CDCl3); Figure S5. 13C NMR spectrum of 3-O, 28-O-diacetyl-30-bromo-betulin (100 MHz, CDCl3); Figure S6. 1H NMR spectrum of Bet-TZ1 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA (up); Figure S7. 13C NMR spectrum of Bet-TZ1 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA; Figure S8. H, H-COSY NMR spectrum of Bet-TZ1 in DMSO-d6 with one drop of TFA; Figure S9. H, C-HSQC NMR spectrum of Bet-TZ1 in DMSO-d6 with one drop of TFA; Figure S10. H,C-HMBC NMR spectrum of Bet-TZ1 in DMSO-d6 with one drop of TFA; Figure S11. 1H NMR spectrum of Bet-TZ2 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA (up); Figure S12. FTIR spectrum of Bet-TZ2; Figure S13. 13C NMR spectrum of Bet-TZ2 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA (up); Figure S14. H,H-COSY NMR spectrum of Bet-TZ2 in DMSO-d6; Figure S15. H,C-HSQC NMR spectrum of Bet-TZ2 in DMSO-d6 with one drop of TFA; Figure S16. H,C-HMBC NMR spectrum of Bet-TZ2 in DMSO-d6 with one drop of TFA; Figure S17. FTIR spectrum of Bet-TZ2; Figure S18. 1H NMR spectrum of Bet-TZ3 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA (up); Figure S19. 13C NMR spectrum of Bet-TZ3 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA (up); Figure S20. H,H-COSY NMR spectrum of Bet-TZ3 in DMSO-d6; Figure S21. H,C-HSQC NMR spectrum of Bet-TZ3 in DMSO-d6 with one drop of TFA; Figure S22. H,C-HMBC NMR spectrum of Bet-TZ3 in DMSO-d6 with one drop of TFA; Figure S23. FTIR spectrum of Bet-TZ3 in DMSO-d6; Figure S24. 1H NMR spectrum of Bet-TZ4 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA (up); Figure S25. 13C NMR spectrum of Bet-TZ4 in DMSO-d6 (bottom) and DMSO-d6 with one drop of TFA (up); Figure S26. H,H-COSY NMR spectrum of Bet-TZ4 in DMSO-d6; Figure S27. H,C-HSQC NMR spectrum of Bet-TZ4 in DMSO-d6 with one drop of TFA; Figure S28. H,C-HMBC NMR spectrum of Bet-TZ4 in DMSO-d6 with one drop of TFA; Figure S29. FTIR spectrum of Bet-TZ4.
Author Contributions
Conceptualization, A.P., M.M. and C.Ș.; methodology, A.P., M.M., A.M. (Alexandra Mioc), M.B.-P., R.R., R.N.-G., A.M. (Andreea Milan), G.M., C.T., C.O., Ș.A., S.R., I.Ș. and C.Ș.; validation, A.P., A.M. (Alexandra Mioc) and M.M.; investigation, A.P., A.M. (Alexandra Mioc) and M.M.; writing—original draft preparation, A.P., M.M., A.M. (Alexandra Mioc), M.B.-P., A.M. (Andreea Milan), G.M., S.R., C.O., C.T. and I.Ș.; writing—review and editing A.P., A.M. (Alexandra Mioc), M.M. and C.Ș.; visualization, A.P., R.R. and R.N.-G.; supervision, M.M. and C.Ș.; project administration, M.M. and C.Ș.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Medicine and Pharmacy “Victor Babes” Timisoara, grant number 26679/09.11.2022 (M.M.).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maitra, U.; Stephen, C.; Ciesla, L.M. Drug discovery from natural products—Old problems and novel solutions for the treatment of neurodegenerative diseases. J. Pharm. Biomed. Anal. 2022, 210, 114553. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Islam, S.U.; Alghamdi, A.A.A.; Kamran, M.; Ahsan, H.; Lee, Y.S. Phytochemicals as Chemo-Preventive Agents and Signaling Molecule Modulators: Current Role in Cancer Therapeutics and Inflammation. Int. J. Mol. Sci. 2022, 23, 15765. [Google Scholar] [CrossRef] [PubMed]
- Andor, B.; Tischer, A.; Berceanu-Vaduva, D.; Lazureanu, V.; Cheveresan, A.; Poenaru, M. Antimicrobial activity and cytotoxic effect on gingival cells of silver nanoparticles obtained by biosynthesis. Rev. Chim. 2019, 70, 781–783. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Demets, O.V.; Takibayeva, A.T.; Kassenov, R.Z.; Aliyeva, M.R. Methods of Betulin Extraction from Birch Bark. Molecules 2022, 27, 3621. [Google Scholar] [CrossRef]
- Özdemir, Z.; Rybková, M.; Vlk, M.; Šaman, D.; Rárová, L.; Wimmer, Z. Synthesis and Pharmacological Effects of Diosgenin–Betulinic Acid Conjugates. Molecules 2020, 25, 3546. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sak, K.; Gupta, D.S.; Kaur, G.; Aggarwal, D.; Chaturvedi Parashar, N.; Choudhary, R.; Yerer, M.B.; Kaur, J.; Kumar, M.; et al. Anti-Inflammatory and Anticancer Properties of Birch Bark-Derived Betulin: Recent Developments. Plants 2021, 10, 2663. [Google Scholar] [CrossRef]
- John, R.; Dalal, B.; Shankarkumar, A.; Devarajan, P.V. Innovative Betulin Nanosuspension exhibits enhanced anticancer activity in a Triple Negative Breast Cancer Cell line and Zebrafish angiogenesis model. Int. J. Pharm. 2021, 600, 120511. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Boryczka, S. Chromatographic and Computational Screening of Lipophilicity and Pharmacokinetics of Newly Synthesized Betulin-1,4-quinone Hybrids. Processes 2021, 9, 376. [Google Scholar] [CrossRef]
- Grymel, M.; Zawojak, M.; Adamek, J. Triphenylphosphonium Analogues of Betulin and Betulinic Acid with Biological Activity: A Comprehensive Review. J. Nat. Prod. 2019, 82, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Das, D. Chemical derivatization of natural products: Semisynthesis and pharmacological aspects—A decade update. Tetrahedron 2021, 78, 131801. [Google Scholar] [CrossRef]
- Kuczynska, K.; Cmoch, P.; Rárová, L.; Oklešťková, J.; Korda, A.; Pakulski, Z.; Strnad, M. Influence of intramolecular hydrogen bonds on regioselectivity of glycosylation. Synthesis of lupane-type saponins bearing the OSW-1 saponin disaccharide unit and its isomers. Carbohydr. Res. 2016, 423, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Lugiņina, J.; Linden, M.; Bazulis, M.; Kumpiņš, V.; Mishnev, A.; Popov, S.A.; Golubeva, T.S.; Waldvogel, S.R.; Shults, E.E.; Turks, M. Electrosynthesis of Stable Betulin-Derived Nitrile Oxides and their Application in Synthesis of Cytostatic Lupane-Type Triterpenoid-Isoxazole Conjugates. Eur. J. Org. Chem. 2021, 2021, 2557–2577. [Google Scholar] [CrossRef]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Markelova, N.Y.; Penkov, N.V.; Shakurova, E.R.; Belosludtsev, K.N.; Parfenova, L.V. New quaternized pyridinium derivatives of betulin: Synthesis and evaluation of membranotropic properties on liposomes, pro- and eukaryotic cells, and isolated mitochondria. Chem. Biol. Interact. 2021, 349, 109678. [Google Scholar] [CrossRef]
- Grishko, V.V.; Tolmacheva, I.A.; Nebogatikov, V.O.; Galaiko, N.V.; Nazarov, A.V.; Dmitriev, M.V.; Ivshina, I.B. Preparation of novel ring-A fused azole derivatives of betulin and evaluation of their cytotoxicity. Eur. J. Med. Chem. 2017, 125, 629–639. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Strzelecka, M.; Świątek, P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef]
- Malik, M.S.; Ahmed, S.A.; Althagafi, I.I.; Ansari, M.A.; Kamal, A. Application of triazoles as bioisosteres and linkers in the development of microtubule targeting agents. RSC Med. Chem. 2020, 11, 327–348. [Google Scholar] [CrossRef]
- Lengerli, D.; Ibis, K.; Nural, Y.; Banoglu, E. The 1,2,3-triazole ‘all-in-one’ ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert Opin. Drug Discov. 2022, 17, 1209–1236. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef] [PubMed]
- Bębenek, E.; Jastrzębska, M.; Kadela-Tomanek, M.; Chrobak, E.; Orzechowska, B.; Zwolińska, K.; Latocha, M.; Mertas, A.; Czuba, Z.; Boryczka, S. Novel Triazole Hybrids of Betulin: Synthesis and Biological Activity Profile. Molecules 2017, 22, 1876. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Soica, C.; Bercean, V.; Avram, S.; Balan-Porcarasu, M.; Coricovac, D.; Ghiulai, R.; Muntean, D.; Andrica, F.; Dehelean, C.; et al. Design, synthesis and pharmaco-toxicological assessment of 5-mercapto-1,2,4-triazole derivatives with antibacterial and antiproliferative activity. Int. J. Oncol. 2017, 50, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.M.C.; Silva, G.N.; da Rocha Pitta, I.; Melo Rêgo, M.J.B.; Gnoato, S.C.B.; da Rocha Pitta, M.G. Novel betulin derivatives inhibit IFN-γ and modulates COX-2 expression. Nat. Prod. Res. 2020, 34, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Bodrikov, I.V.; Kurskii, Y.A.; Chiyanov, A.A.; Subbotin, A.Y. Electrophilic Substitution of Hydrogen in Betulin and Diacetylbetulin. Russ. J. Org. Chem. 2018, 54, 131–138. [Google Scholar] [CrossRef]
- Phalgune, U.D.; Vanka, K.; Rajamohanan, P.R. GIAO/DFT studies on 1,2,4-triazole-5-thiones and their propargyl derivatives. Magn. Reson. Chem. 2013, 51, 767–774. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Chavan, S.R.; Kavitha, M.; Maybhate, S.P.; Deshpande, S.R.; Likhite, A.P.; Rajamohanan, P.R. Structural elucidation of propargylated products of 3-substituted-1,2,4-triazole-5-thiols by NMR techniques. Magn. Reson. Chem. 2008, 46, 1168–1174. [Google Scholar] [CrossRef]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Szoka, Ł.; Isidorov, V.; Nazaruk, J.; Stocki, M.; Siergiejczyk, L. Cytotoxicity of Triterpene Seco-Acids from Betula pubescens Buds. Molecules 2019, 24, 4060. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brahmbhatt, J.G.; Pandya, P.A.; Daraji, D.G.; Patel, H.D.; Rawal, R.M.; Baran, S.K. Design, synthesis and biological evaluation of novel 5-(4-chlorophenyl)-4-phenyl-4H-1,2,4-triazole-3-thiols as an anticancer agent. J. Mol. Struct. 2021, 1231, 130000. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Marciniec, K.; Chrobak, E.; Bębenek, E.; Boryczka, S. Lipophilicity, Pharmacokinetic Properties, and Molecular Docking Study on SARS-CoV-2 Target for Betulin Triazole Derivatives with Attached 1,4-Quinone. Pharmaceutics 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Sidova, V.; Zoufaly, P.; Pokorny, J.; Dzubak, P.; Hajduch, M.; Popa, I.; Urban, M. Cytotoxic conjugates of betulinic acid and substituted triazoles prepared by Huisgen Cycloaddition from 30-azidoderivatives. PLoS ONE 2017, 12, e0171621. [Google Scholar] [CrossRef] [PubMed]
- Dangroo, N.A.; Singh, J.; Rath, S.K.; Gupta, N.; Qayum, A.; Singh, S.; Sangwan, P.L. A convergent synthesis of novel alkyne–azide cycloaddition congeners of betulinic acid as potent cytotoxic agent. Steroids 2017, 123, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, K.; Bończak, B.; Rárová, L.; Kvasnicová, M.; Strnad, M.; Pakulski, Z.; Cmoch, P.; Fiałkowski, M. Synthesis and cytotoxic activity of 1,2,3-triazoles derived from 2,3-seco-dihydrobetulin via a click chemistry approach. J. Mol. Struct. 2022, 1250, 131751. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Liebman, J.F. The annular tautomerism of imidazoles and pyrazoles: The possible existence of nonaromatic forms. Struct. Chem. 2006, 17, 439–444. [Google Scholar] [CrossRef]
- Claramunt, R.M.; López, C.; Angeles García, M.; Dolores Otero, M.; Rosario Torres, M.; Pinilla, E.; Alarcón, S.H.; Alkorta, I.; Elguero, J. The structure of halogeno-1,2,4-triazoles in the solid state and in solution. New J. Chem. 2001, 25, 1061–1068. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Hall, C.D.; El-Gendy, B.E.-D.M.; Draghici, B. Tautomerism in drug discovery. J. Comput. Aided. Mol. Des. 2010, 24, 475–484. [Google Scholar] [CrossRef]
- Larina, L.I. Tautomerism and Structure of Azoles. Adv. Heterocycl. Chem. 2018, 124, 233–321. [Google Scholar]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [PubMed]
- Shi, W.; Tang, N.; Yan, W.-D. Synthesis and cytotoxicity of triterpenoids derived from betulin and betulinic acid via click chemistry. J. Asian Nat. Prod. Res. 2015, 17, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, W.; Zhang, B.; Li, C.; Zhang, X.; Wang, Q.; Wang, Y.; Zhou, Q.; Li, X.; Shen, X.L. Central role of TRAP1 in the ameliorative effect of oleanolic acid on the mitochondrial-mediated and endoplasmic reticulum stress-excitated apoptosis induced by ochratoxin A. Toxicology 2021, 450, 152681. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Dutta, D.; Mukherjee, S.; Das, S.; Maiti, N.C.; Das, P.; Chowdhury, C. Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29). Eur. J. Med. Chem. 2015, 102, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective assessment of changes in nuclear morphology and cell distribution following induction of apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Nistor, G.; Mioc, M.; Mioc, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Prodea, A.; Milan, A.; Ghiulai, R.; Semenescu, A.; Dehelean, C.; et al. The C30-Modulation of Betulinic Acid Using 1,2,4-Triazole: A Promising Strategy for Increasing Its Antimelanoma Cytotoxic Potential. Molecules 2022, 27, 7807. [Google Scholar] [CrossRef] [PubMed]
- Nistor, G.; Mioc, A.; Mioc, M.; Balan-Porcarasu, M.; Ghiulai, R.; Racoviceanu, R.; Avram, Ș.; Prodea, A.; Semenescu, A.; Milan, A.; et al. Novel Semisynthetic Betulinic Acid−Triazole Hybrids with In Vitro Antiproliferative Potential. Processes 2022, 11, 101. [Google Scholar] [CrossRef]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Juszczak, M.; Grabarska, A.; Sifringer, M.; Kaczor, J.; Kandefer-Szerszeń, M. Betulin Elicits Anti-Cancer Effects in Tumour Primary Cultures and Cell Lines In Vitro. Basic Clin. Pharmacol. Toxicol. 2009, 105, 425–432. [Google Scholar] [CrossRef]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and Dose Dependent Melanoma Cytotoxic and Immune Stimulatory Activity of Betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef]
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Abid Ali, S.; Al-Harrasi, A. Apoptotic and antimetastatic activities of betulin isolated from Quercus incana against non-small cell lung cancer cells. Cancer Manag. Res. 2019, 11, 1667–1683. [Google Scholar] [CrossRef]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.-G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the Cytochrome c–initiated Caspase Cascade: Hierarchical Activation of Caspases-2, -3, -6, -7, -8, and -10 in a Caspase-9–dependent Manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Tian, T. MCF-7 cells lack the expression of Caspase-3. Int. J. Biol. Macromol. 2023, 231, 123310. [Google Scholar] [CrossRef] [PubMed]
- Orchel, A.; Chodurek, E.; Jaworska-Kik, M.; Paduszyński, P.; Kaps, A.; Chrobak, E.; Bębenek, E.; Boryczka, S.; Borkowska, P.; Kasperczyk, J. Anticancer Activity of the Acetylenic Derivative of Betulin Phosphate Involves Induction of Necrotic-Like Death in Breast Cancer Cells In Vitro. Molecules 2021, 26, 615. [Google Scholar] [CrossRef] [PubMed]
- Pęcak, P.; Świtalska, M.; Chrobak, E.; Boryczka, G.; Bębenek, E. Betulin Acid Ester Derivatives Inhibit Cancer Cell Growth by Inducing Apoptosis through Caspase Cascade Activation: A Comprehensive In Vitro and In Silico Study. Int. J. Mol. Sci. 2022, 24, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Xiao, M.; Lin, H.; Luo, J.; Wang, T. Novel betulin derivative induces anti-proliferative activity by G2/M phase cell cycle arrest and apoptosis in Huh7 cells. Oncol. Lett. 2018, 15, 2097–2104. [Google Scholar] [CrossRef] [PubMed]
- Entschladen, F.; Drell, T.L.; Lang, K.; Joseph, J.; Zaenker, K.S. Tumour-cell migration, invasion, and metastasis: Navigation by neurotransmitters. Lancet Oncol. 2004, 5, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Härmä, V.; Haavikko, R.; Virtanen, J.; Ahonen, I.; Schukov, H.-P.; Alakurtti, S.; Purev, E.; Rischer, H.; Yli-Kauhaluoma, J.; Moreira, V.M.; et al. Optimization of Invasion-Specific Effects of Betulin Derivatives on Prostate Cancer Cells through Lead Development. PLoS ONE 2015, 10, e0126111. [Google Scholar] [CrossRef]
- Bache, M.; Bernhardt, S.; Passin, S.; Wichmann, H.; Hein, A.; Zschornak, M.P.; Kappler, M.; Taubert, H.; Paschke, R.; Vordermark, D. Betulinic Acid Derivatives NVX-207 and B10 for Treatment of Glioblastoma—An in Vitro Study of Cytotoxicity and Radiosensitization. Int. J. Mol. Sci. 2014, 15, 19777–19790. [Google Scholar] [CrossRef]
- Winter, G.; Koch, A.B.F.; Löffler, J.; Jelezko, F.; Lindén, M.; Li, H.; Abaei, A.; Zuo, Z.; Beer, A.J.; Rasche, V. In vivo PET/MRI Imaging of the Chorioallantoic Membrane. Front. Phys. 2020, 8, 151. [Google Scholar] [CrossRef]
- de Araujo Lowndes Viera, L.M.; Silva, R.S.; da Silva, C.C.; Presgrave, O.A.F.; Boas, M.H.S.V. Comparison of the different protocols of the Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) by evaluating the eye irritation potential of surfactants. Toxicol. Vitr. 2022, 78, 105255. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, I.A.; Shelepen’kina, L.N.; Vikharev, Y.B.; Anikina, L.V.; Grishko, V.V.; Tolstikov, A.G. Synthesis and biological activity of S-containing betulin derivatives. Chem. Nat. Compd. 2005, 41, 701–705. [Google Scholar] [CrossRef]
- Uzenkova, N.V.; Petrenko, N.I.; Shakirov, M.M.; Shul’ts, E.E.; Tolstikov, G.A. Synthesis of 30-amino derivatives of lupane triterpenoids. Chem. Nat. Compd. 2005, 41, 692–700. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Wei, X.; Wu, X.; Chen, G.; Cao, G.; Shen, X.; Zhang, X.; Tang, Q.; Liang, G.; et al. Synthesis and biological evaluation of novel thiazolidinone derivatives as potential anti-inflammatory agents. Eur. J. Med. Chem. 2013, 64, 292–301. [Google Scholar] [CrossRef]
- Ainsworth, C. 1,2,4-TRIAZOLE. Org. Synth. 1960, 40, 99. [Google Scholar] [CrossRef]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM-Recommended Test Method Protocol: Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method; National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2010.
- Maghiari, A.L.; Coricovac, D.; Pinzaru, I.A.; Macașoi, I.G.; Marcovici, I.; Simu, S.; Navolan, D.; Dehelean, C. High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells. Nutrients 2020, 12, 3600. [Google Scholar] [CrossRef]
- Guercio, B.J.; Zhang, S.; Niedzwiecki, D.; Li, Y.; Babic, A.; Morales-Oyarvide, V.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance). PLoS ONE 2018, 13, e0199244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).