Abstract
The increasing antimicrobial resistance (AMR) of pathogens is a significant threat to human and animal health, but it is also an environmental challenge for water resources. The present study aimed to quantify heterotrophic bacteria resistant to five groups of antibiotics (ABs) in a selected Yantra River stretch (including its tributary, the Belitsa River); to assess AMR prevalence among Enterobacteriaceae; and to assess the impact of urban effluents or rural runoff on AMR prevalence along the river course at eight sampling points. Culture-dependent methods were used in a population-based study of total AMR and for AB susceptibility testing of Enterobacteriaceae isolates. The data reveal significant differences in AMR dissemination and a lower (up to 10%) proportion of different types of antibiotic-resistant bacteria (ARB) in the Yantra River water compared to the Belitsa River (up to 20%). The incidence of resistant Enterobacteriaceae isolates was in the range of 1% to gentamicin to 36% to ampicillin, including multidrug resistance of 19%, and different AMR patterns of isolates from each river. The prevalence of AMR among aquatic bacteria highlights the need for adequate waste water treatment and for management, monitoring and control of treatment processes to limit anthropogenic pressure through discharge of untreated or incompletely treated waste water and to ensure the ecological well-being of receiving waters.
1. Introduction
Antibiotics are important and widely used pharmaceuticals for treatment of infectious diseases in human and veterinary medicine. In treatment, antibiotic substances are not completely eliminated, and their residuals or metabolites that might retain their antibiotic activity on leaving an organism enter sewerage or receiving water resources [1,2,3,4]. There are data that show that the excretion of beta-lactams, quinolones, tetracyclines, phenicols and trimethoprim exceeds half of the administered dose, and around 19% of a ciprofloxacin dose is excreted as active metabolites [4]. Contamination of surface water with ABs was detected in concentrations varying from nanograms to micrograms per liter depending on usage intensity, chemical stability, waste water (WW) treatment effectiveness for AB removal, etc. [5,6,7]. The most often detected ABs in water environments belong to the groups of penicillins, tetracyclines, macrolides and fluoroquinolones [6,8]. In surface waters of EU countries, AB content varied from 0.0006 to 0.548 μg/L: only clarithromycin and sulfamethoxazole were found in concentrations above 0.1 μg/L; 16 ABs had a mean concentration of ≤0.01 μg/L; and 17 had concentrations in the range of 0.01–0.06 μg/L [4]. Contamination risk for water resources was the reason that clarithromycin, azithromycin and erythromycin were included in the Watch List of Potentially Dangerous Compounds of the European Frame Water Directive 2000/60/EC in 2015 and ciprofloxacin and amoxicillin were added in 2018 [4,9].
The overuse of ABs, often misused or ineffective, as well as their application for prophylaxis or growth stimulation in animal breeding, has led to the acquisition and dissemination of AMR among target pathogens, and increasing AMR is considered as one of the main threats to human and animal health. Despite linking the problem primarily to clinical pathogens, there is increasing evidence for interrelation between increasing AMR and anthropogenic impact on water resources [1,10,11,12]. AMR hotspots have been found in water environments that are subjected to anthropogenic pressure [6,13,14]. Water resources are considered an important reservoir of ABs, ARB and antibiotic-resistant genes (ARGs), which facilitates the exchange of ARGs between pathogenic and non-pathogenic bacteria and helps AMR expansion in the environment [12,14].
The dissemination of ABs, ARB and ARGs in inland surface water is due to its role as the receiver of WW with various levels of treatment and runoff from agricultural land, farms or septic pits [15,16,17,18]. Conventional WW treatment is insufficiently effective to remove ABs, ARB and ARGs, and as a result, these pollutants enter water resources. In this way, the so-called urban water cycle (which includes water supply, sewerage and WW treatment) facilitates the spread of ABs, ARB and ARGs and participates in the potential transmission routes of AMR from water to humans and vice versa [12,14]. It is well known that discharged effluents and non-point pollution sources take part in the transportation of bacteria in water resources and assist dissemination of ARB and ARGs [1,7,19,20,21]. Numerous comparative studies on the microbiome upstream and downstream from WW discharge points have showed a significant increase in ARB populations and changes in AMR patterns. For example, urban WW discharge into the Arga River has increased tetracycline and beta-lactam resistance of Enterobacteriaceae and tetracycline and co-trimoxazole resistance of Aeromonas spp. [1]. A similar effect of WW discharge has been reported for Enterobacteriaceae isolates from the Dambovita River [22]. An AMR survey of the Danube River found increased E. coli resistance along the river [23,24]. Published data on the evidenced resistance to “last instance” ABs raise a concern: phenotypes for the production of extended-spectrum beta-lactamases (ESBLs) and carbapenemases have been detected among E. coli, Enterobacter spp. and Klebsiella spp. isolated from fresh water [24,25,26].
Most investigations into AMR in water environments have been focused on bacteria of fecal origin, because of their indicator value for fecal pollution and pathogens-associated health risk, and for transmission routes from humans to water and vice versa [18,21,27]. Due to the low density of E. coli and coliform population in freshwater habitats compared to heterotrophic plate count (HPC) bacteria, considerable scientific interest has also been addressed to investigating the AMR of autochthonous aquatic bacteria (such as Aeromonas, Acinetobacter, Pseudomonas, etc.), which, because of their abundance, play important an role in AMR dissemination [16,28].
Different culture-based approaches are used to study the prevalence of AMR in aquatic communities: quantification of cultivable resistant bacteria in the presence of a selective AB agent or direct assessment of total AMR to multiple antibiotics using the disk diffusion method. The first allows quantification of the bacteria resistant to a specific AB, as well as subsequent isolation, identification and testing of AB sensitivity of the bacterial isolates, including multiple AB resistance (MAR) [17,28,29,30,31,32]. Using this approach of dosing an AB to a culture medium, Ash et al. (2002) quantified and isolated ampicillin-resistant bacteria from freshwater samples of 16 U.S. rivers and measured their resistance to beta-lactam and non-beta-lactam ABs [28]. Kim et al. (2015) examined taxonomic diversity and AMR of penicillin- or tetracycline-resistant isolates from four major freshwater bodies of Korea and determined Aeromonas and Pseudomonas to be major penicillin-resistant genera [29]. Ayandiran et al. (2014) evaluated the population and AMR pattern of heterotrophic bacteria in water and sediments of a heavy-metal-polluted Nigerian river [32].
The second culture-based approach of a direct AB susceptibility assay of a water sample microbiome makes it possible to assess the occurrence of resistance to a particular AB in at least one strain present in a water sample and, in that way, MAR manifestation in an aquatic community [16,33]. Using this direct AMR assay, Moore et al. (2010) determined the diversity of total AMR of waterborne bacteria in 11 Irish rivers and established a mean MAR to 3.7 major AB classes, with diminishing antibacterial effectiveness of aminoglycosides > fluoroquinolones > glycopeptides > macrolides > tetracyclines > beta-lactams [33].
Despite the importance of water resources as a receiver, reservoir and source of ABs, ARB and ARGs, no data on AMR dissemination in Bulgarian surface water are available. Although there are no regulations concerning the admissible values of AB, ARB and ARG content in natural water, the potential health and ecological risks for acquisition and dissemination of AMR through water give rise to a need for information of the AMR prevalence of water resources in Bulgaria. Because of this reason, the present study address a culture-based assessment of resistance to widely used ABs among the aquatic microbiome of a selected Bulgarian river, in a river stretch subjected to heavy anthropogenic impact. The object of the study was a stretch of the Yantra River, flowing through the urban territory of Veliko Tarnovo and its surroundings, including its tributary, the Belitsa River. It is known that here the river body is contaminated with untreated effluents or through diffuse pollution from small settlements without a sewage system or from agricultural activities, as well as with some of the untreated WW from the town of Veliko Tarnovo that is discharged because of insufficient capacity of the municipal waste water treatment plant (WWTP). In the WWTP, waste water is subjected to biological treatment with activated sludge, and it is disinfected only in the event of an epidemic threat.
The aims of our study are: (a) to quantify heterotrophic bacteria resistant to five AB groups in the selected river stretch; (b) to assess the prevalence of AMR among Enterobacteriaceae isolates; and (c) to evaluate the impact of urban effluents or rural runoff on the AMR of aquatic bacterial communities along the river course.
The obtained results will provide an opportunity to fill gaps in knowledge about the AMR of Bulgarian river water, even for a small river section; to assess the effect of untreated or conventionally treated waste water discharges and to clarify the importance of surface runoff from less urbanized areas, including unsewered settlements, on the spread of AMR among riverine bacteria; and to assess whether the analyzed culture-depended parameters (population proportion of resistant heterotrophic bacteria and incidence of resistant Enterobacteriaceae) can indicate changes in the river water quality.
2. Materials and Methods
2.1. Description of the Studied River Section
The Yantra River is a right tributary of the Danube River flowing in at 536 km. The study was carried out in a river section beginning in the village of Shemshevo and covering its passage through the urban territory of Veliko Tarnovo and ending at the village of Samovodene (Figure 1). The examined section also includes the confluence of the Belitsa River, a right tributary of the Yantra River, studied on its lower course starting outside the town of Debelets and following its passage through the town until its confluence with the Yantra River, as well as the inflow of the Dryanovska River into the Belitsa River on the outskirts of the city.
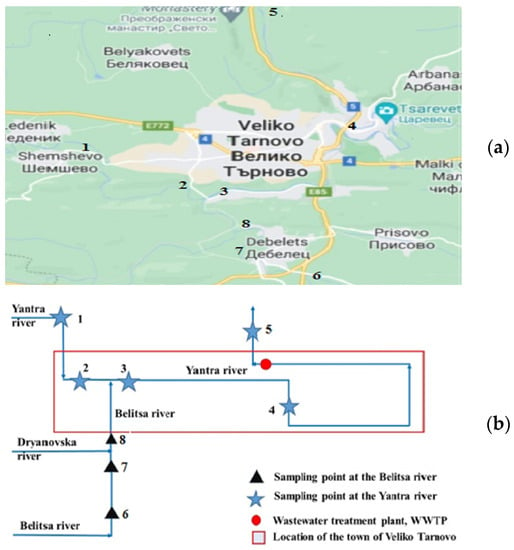
Figure 1.
Map (a) and scheme (b) of the studied Yantra River stretch and its tributary in the urban area of the town of Veliko Tarnovo, including the river water sampling points.
In the selected section, the river passes through agricultural territories, small settlements without sewage networks (as in the villages of Shemshevo and Samovodene) or those with sewage but without WW treatment (as in the town of Debelets, with 3900 inhabitants) and the medium-sized town of Veliko Tarnovo (about 74,000 inhabitants), which treats some effluents in the municipal WWTP. The ecological status of the Yantra River stretch is affected directly by the discharged untreated domestic or mixed WW and indirectly by surface runoff from agricultural land (including agriculture, livestock and poultry) and seepage from septic tanks into groundwater. Environmental release of contaminated water can also occur during combined sewer overflow events.
2.2. River Water Sampling Points
Eight water sampling points (SPs) from the Yantra River and the Belitsa River (Table 1) were selected to assess the current state of the water body and the influence of river tributaries and point sources of pollution, such as untreated WW discharge. SP 1 was chosen to reflect the state of the river in a rural area about 2 km before its passage through the town of Veliko Tarnovo. SPs 2, 3 and 4 allow tracing the state of the Yantra River as it passes through the urban territory of Veliko Tarnovo and the potential influence of diffuse and point sources of fecal pollution, because some of the domestic and industrial WW of the town is discharged untreated into the Yantra River. In the same way, the sewage of the town of Debelets discharges into the Belitsa River. Two SPs were chosen to provide an opportunity to evaluate the influence of discharged untreated waste water: SP 2 on the Yantra River and SP 7 on the Belitsa River. Selection of SPs 2, 3 and 8 allows comparison of the water quality of both rivers and assessment of the effect of the Belitsa River confluence on the Yantra River.

Table 1.
River water sampling points (SPs).
Water samples were collected during all seasons of 2019 and two times in 2020. A volume of 250 mL water was taken in duplicate from a given SP following EN ISO 19458:2006 [34].
2.3. Enumeration of Total Cultivable Heterotrophic Bacteria and Antibiotic-Resistant Bacteria
The number of culturable heterotrophic bacteria in river water was analyzed by surface spreading of a certain volume of the sample and its decimal dilutions on R2A agar (HiMedia Laboratories Pvt. Ltd., Mumbai, India) that was AB-free or supplemented with a given AB. Incubation was for 7 days at a temperature of 25 °C. Each water sample was analyzed for enumeration of total HPC bacteria (on an AB-free medium) and bacteria resistance to each individual AB (on an AB-dosed medium). Based on the obtained HPC data pairs (each measured as CFU/mL), the percentage ratio of bacteria resistant to the individual ABs was calculated.
The HPC bacteria resistant to five AB substances from different classes were enumerated: beta-lactams (ampicillin, AMP—32 mg L−1); tetracyclines (tetracycline hydrochloride, TE—8 mg L−1); phenicols (chloramphenicol, C—16 mg L−1); quinolones (ciprofloxacin hydrochloride monohydrate, CIP—4 mg L−1); and antifolates (sulphamethoxazole, Sul—256 mg L−1) (HiMedia Laboratories Pvt. Ltd., Mumbai, India).
2.4. Enumeration and Isolation of E. coli and Coliforms
The samples of river water were analyzed for enumeration of E. coli and coliform bacteria in accordance with EN ISO 9803-1:2014 [35]. Analyses were carried out by the method of membrane filtration and consecutive incubation of the membrane filters on the selective culture media lactose TTC agar with tergitol® (Merck KGaA, Darmstadt, Germany) at 35 °C for 24 h. Typical colonies of lactose-fermenting bacteria were isolated—at least 10, or all of the colonies on the membrane filter in the case that there were fewer than 10. After the colonies had been sub-cultured on soybean casein digest agar (HiMedia Laboratories Pvt. Ltd., Mumbai, India) and cultivated, they were tested for oxidase and indole production; all the oxidase-negative colonies that produced indole were counted as presumptive E. coli, while the ones that did not produce indole were counted as coliform bacteria. The pure bacteria cultures were stored at −20 °C.
2.5. Biochemical Identification of Enterobacteriaceae Isolates
Isolates of fecal indicator bacteria were identified biochemically by the ENTEROtest 24N MICROLATEST® test (Erba Lachema s.r.o., Brno, Czech Republic). The resulting ID score indicates the extent to which the taxon can be distinguished from other taxa. The strain can be distinguished perfectly when ID ≥ 99%, or very well at ID ≥ 95%, and cannot be sufficiently distinguished without additional tests at ID < 90%. The strains were considered to be identifiable at ID ≥ 90%.
2.6. Determination of Antibiotic-Resistance Pattern of Bacterial Isolates
Antimicrobial susceptibility of isolates from the Enterobacteriaceae family was evaluated by the disk diffusion method. Thirteen ABs from six classes were tested: seven beta-lactams (AMP 10 μg—ampicillin; AMC 20/10 μg—amoxicillin/clavulanic acid; CX 30 μg—cefoxitin; CTX 30 μg—cefotaxime; CTR 30 μg—ceftriaxone; CPM 30 μg—cefepime; and IPM 10 μg—imipenem); two aminoglycosides (S 10 μg—streptomycin and GEN 10 μg—gentamicin); quinolones (CIP 5 μg—ciprofloxacin); antifolates (COT 1.25/23.75 μg—trimethoprim/sulfamethoxazole and co-trimoxazole); tetracycline, TE 30 μg; and chloramphenicol, C 30 μg.
The tested strain was inoculated on the surface of Mueller Hinton agar (HiMedia, Mumbai, India) as a calibrated suspension (0.5 MacFarland). Disks with the tested ABs (HiMedia, Mumbai, India) were placed on the surface of the inoculated agar. After 18 h of incubation at 35 °C, the inhibition zone diameter around each AB disk was measured (in mm). The strains were classified as S—sensitive, R—resistant or I—susceptible with increased exposure [36,37]. According to EUCAST, AMR to at least one AB from at least three different classes was defined as MAR.
2.7. Statistical Analysis of the Data
One-way analysis of variance (ANOVA) was performed to assess the significance of differences between the sampling locations on the percentage of bacteria resistant to individual ABs as the dependent variable and sampling location as the factor. p < 0.05 was considered statistically significant. The Pearson correlation coefficient was calculated to find out whether there was a linear association between bacteriological parameters of water (HPC, E. coli and coliforms) and dissemination of resistance to different ABs.
3. Results
3.1. Microbiological Characteristics of River Water
According to the EU Water Framework Directive 2000/60/EC, the ecological status of the Yantra River and the Belitsa River in terms of physico-chemical and biological quality elements and specific pollutants has been assessed as ‘moderate’ (due to the increased values of nitrite and total nitrogen, orthophosphates and total phosphorus) and ‘good’ for priority substances (such as pesticides, polycyclic aromatic hydrocarbons, etc.) [38]; however, no microbiological parameters were included in the water environmental assessment. Our results are not consistent with a ‘moderate’ ecological quality of river water, because of the high numbers of fecal indicators and HPC bacteria determined at all river water SPs (Table 2).

Table 2.
Microbiological characteristics of the Yantra River and the Belitsa River water.
According to the only regulation of microbiological water quality, Directive 2006/7/EC concerning bathing water quality [39], the water in seven out of eight SPs of the Yantra River and the Belitsa River failed the requirements of 1000 CFU/100 mL for the number of E. coli, and the water quality had to be classified as ‘poor’. If the water quality is interpreted according to the five-category microbiologically based classification of Kirschner et al. (2009) [40], the E. coli pollution of the Yantra River water is: moderate (>102 to 103 CFU/100 mL) only at SP 1; critical (>103 to 104 CFU/100 mL) at SPs 4, 5 and 6; strong (>104 to 105 CFU/100 mL) predominantly at SPs 2, 3 and 8; and excessive (>105 CFU/100 mL) at SP 7.
The present data reveal significant fluctuations in the number of HPC and fecal indicators between the individual SPs over the river course, due to the different contamination rates of water in the particular river sections by effluent discharge or water runoff from unsewered settlements, agricultural land and pastures, and farms. The fecal bacteria burden was the lowest at SP 1 of the Yantra River and increased by up to 2 logs at SPs 2 and 3. The same trend of E. coli or coliform increase was found for the Belitsa River, with the highest values in the samples from SP 7.
The lowest number of HPC bacteria was detected in the water sampled from SPs 6 and 8 of the Belitsa River, while the lowest number of HPC bacteria from the Yantra River were in the samples from SPs 1 and 4. However, the water at SP 1 did not differ significantly (p < 0.05) in HPCs from SPs 2, 3 and 4 in the urban territory, in contrast to significant differences in the content of fecal bacteria. Where the Yantra River passes through the territory of the town of Veliko Tarnovo, the population of HPC bacteria increased at SPs 2 and 3 and then decreased at SP 4. A similar water quality change was found during the passage of the Belitsa River through the territory of the town of Debelets.
If the HPC data are interpreted according to the classification system used by Kavka & Poetsh (2002) [41], the number of HPC bacteria as an indicator for organic pollution of surface water demonstrates a critical organic pollution level (>104 to 105 CFU/mL) at five SPs (respectively, SPs 1, 4, 5, 6 and 8) or strong organic pollution level (>105 to 7.5 × 105 CFU/mL) at SPs 2, 3 and 7.
Although the water at SPs with the highest number of HPCs (SPs 2, 3 and 7) also contained the highest number of E. coli or coliforms, the relationship between both variables is weak. Weak positive correlations between HPC and E. coli numbers (r = 0.47) or HPC and total coliforms (r = 0.53) were found. The lack of significant correlations is probably due to the different fecal/organic loads from the discharged effluents or the diffuse agricultural or urban runoff along the river course. For example, the groundwater from two captured fountains in the ‘old’ city (the area located close to SP 4) had an E. coli content of 1.7 ± 0.3 × 103 CFU/100 mL and 1.4 ± 0.7 × 103 CFU/100 mL, indicating that the surface runoff is a possible diffuse source of pollution of the river body, although negligible changes were found in the E. coli content of the river water. Underground water containing a low number of E. coli in a capture fountain located close to SP 3 (2 ± 1 CFU/100 mL) showed a minor effect of the surface runoff on the river body, unlike the impact of sewage discharge on the Belitsa River inflow.
3.2. Prevalence of Antibiotic-Resistant Bacteria in the River Water
AMR data of heterotrophic bacteria in the Yantra River and Belitsa River water are presented in Table 3, according to sampling point. In all SPs, the population proportion of bacteria resistant to tetracycline was the lowest, followed by sulfamethoxazole (in five out of eight SPs), while the prevalence of bacteria resistant to the other tested ABs varied.

Table 3.
Percentage of heterotrophic bacteria resistant to individual antibiotics in the river water of the selected sampling points.
AMR data on the aquatic microbiome of the Yantra River and its tributary, the Belitsa River, show significant quantitative differences between the populations of bacteria resistant to the examined ABs. In general, the Belitsa River water is distinguished by a higher proportion of bacteria resistant to ampicillin, ciprofloxacin and chloramphenicol, but a lower proportion of sulfamethoxazole-resistant bacteria compared to the Yantra River. In the Yantra River water, the population proportion of the different types of ARB was lower than 10% (with a few exceptions). The share of ciprofloxacin-, chloramphenicol- and ampicillin-resistant bacteria at the SPs along the Belitsa River had permanently high values (up to 20%), while at the SPs along the Yantra River, significant population dynamics of these ARB types were observed. In the waters of the Belitsa River, after the confluence of the Dryanovska River, the highest level of ARB was recorded at SP 8 for the entire studied section of the Yantra River.
The differing abundance of bacteria resistant to the examined ABs in each river section seems to reflect the different urbanization levels and anthropogenic impact on the surrounding watershed areas. Upstream from the urban territory of Veliko Tarnovo (at SP 1), the lowest levels of HPC bacteria resistant to individual ABs were established. Compared to SP 1, the abundance of bacteria resistant to all tested ABs significantly increased (p < 0.05) at SPs 2 and 3 when the river crossed through the town. The increased populations of different types of ARB at SP 2 confirm the impact of untreated effluents coming from the west industrial urban zone, while changes in the ARB populations at SP 3 manifest the confluence of the Belitsa River. A decrease in the populations of various ARB types was found at SP 4, which indicates the absence of other significant pollution sources where the Yantra River meanders through the territory of the ‘old’ town of V. Tarnovo, although surface runoff could have an influence due to the higher AMR level of HPC bacteria in underground water than the river water at SP 4. The AMR level of HPC bacteria in the underground water of a captured fountain in the ‘old’ town was 31.8 ± 21.2% to ampicillin; 5.8 ± 3.0% to tetracycline; and 13.5 ± 3.3% to ciprofloxacin. Expectations of a higher proportion of ARB at SP 5 than at SP 4, resulting from WW discharge from the municipal WWTP downstream from SP 4, were met—a slight, but statistically significant increase (p < 0.05) in the ARB populations was detected at SP 5, except for ampicillin-resistant bacteria.
Apart from the population abundance of bacteria resistant to the selected ABs, the river water at the individual SPs also differs in the type of predominant ARB. At SPs 1, 4 and 5 on the Yantra River, chloramphenicol-resistant bacteria were predominant, while at SP 2, ampicillin- and ciprofloxacin-resistant bacteria were predominant. At SP 3, the populations of bacteria resistant to individual ABs were similar in abundance, except for tetracycline-resistant bacteria. At all SPs on the Belitsa River, resistance to ciprofloxacin was the highest. These differences in AMR prevalence between the SPs located on each river could be related to the differently composed effluents discharged from the urban areas and runoff from agricultural land, in addition to seepage from septic tanks in the rural areas. Fluctuations in the number of bacteria resistant to the individual ABs in the water from a single sampling location could be related to seasonal dynamics of the total number of HPCs in such a river type with non-constant annual water flow, as well as to the changing characteristics of pollution sources related to the morbidity of the population or farmed animals.
The two examined river sections differ in the population ratio of total HPC bacteria and those resistant to particular ABs. For the Yantra River microbiome, the results show a significant correlation between the bacterial resistance to a specific AB and the total number of HPC bacteria: r = 0.89 for bacteria resistant to AMP; r = 0.94 for those resistant to C; r = 0.95 for CIP-resistant ones; and r = 0.84 for those resistant to Sul. For the Belitsa River, a weak correlation was found between the different types of ARB and total number of HPC bacteria (r = −0.52), with the exception of CIP (r = −0.87). Data for the Yantra River water show that an increase in the number of HPC bacteria leads to an increase in the population proportion of those resistant to the various ABs, while a weak inverse dependence was established for the SPs of the Belitsa River.
Summarized AMR data for both river stretches reveal:
- -
- the lowest rate of different ARB in the upstream river section outside the urban territory of Veliko Tarnovo (SP 1), especially of tetracycline- and sulfamethoxazole-resistant bacteria;
- -
- raised dissemination of different types of ARB in the Yantra River in the urban territory of Veliko Tarnovo in comparison with the less urbanized area near SP 1, with the lowest value for tetracycline-resistant bacteria and a similar abundance of the other types of ARB;
- -
- a broader spread of ARB in the Belitsa River stretch compared to the Yantra River, so the confluence of the Belitsa River together with effluents discharged contribute to the increasing AMR to all ABs, except sulfamethoxazole, in the main water body.
While in the Yantra River stretch, located in a more highly urbanized territory of the town of Veliko Tarnovo, the increase in the AMR of heterotrophic bacteria seems to be more strongly influenced by the discharge of untreated WW, in the less urbanized section of the Belitsa River, in which, apart from sewage discharge, there is significant diffuse pollution from agricultural activities, the spread of AMR seems to be more strongly influenced by the features of the surrounding territories than by the urbanization level. Data showing a higher proportion of bacteria resistant to ampicillin (48.2 ± 20.8%) or tetracycline (7.4 ± 2.4%) in the waste water influent near to SP 7 support the observed increase in the AMR of HPC bacteria in the river water from SP 7 as a result of their discharge, while the close values for bacteria resistant to ciprofloxacin (19.2 ± 2.8%), sulfamethoxazole (9.8 ± 2.2%) or chloramphenicol (6.3 ± 2.4%) suggest a greater contribution of diffuse pollution from adjacent territories than effluents (unpublished data).
3.3. AMR Phenotype of Enterobacteriaceae Isolated from River Water
In total, 138 Enterobacteriaceae strains were isolated and identified to species or genus level and their AMR phenotype was assessed (Figure 2; Table 4). AMR data are presented as a percentage ratio of the number of isolates resistant to each individual AB to the total tested strain number.
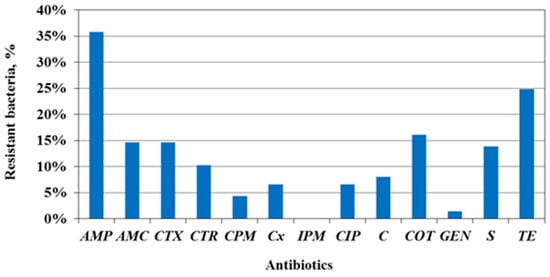
Figure 2.
AMR phenotype of Enterobacteriaceae isolates from water from the Yantra River and its tributary, the Belitsa River; tested antibiotics: AMP—ampicillin; AMC—amoxicillin/clavulanic acid; CTX—cefotaxime; CTR—ceftriaxone; CPM—cefepime CX—cefoxitin; IPM—imipenem; CIP—ciprofloxacin; C—chloramphenicol; COT—co-trimoxazole; GEN—gentamicin; S—streptomycin; TE—tetracycline.

Table 4.
AMR phenotype of the Enterobacteriaceae isolates from the Yantra River.
The most common resistance among Enterobacteriaceae isolates was to the ‘old’ ABs—ampicillin (36%), tetracycline (25%) and co-trimoxazole (16%). The lowest resistance was determined to be to gentamicin (1%) and cefepime (4%), a fourth-generation cephalosporin. All isolates were susceptible to imipenem. If the AMR data are interpreted according to the criteria for clinical isolates of ECDC, a high prevalence level (from 25 to <50%) is detected against AMP and TE; a medium level (10 to <25%) is detected against ABs from three classes; a low level (5 to <10%) is also detected to three AB classes; and a very low level (1 to <5%) is detected to CPM and GEN.
In this study, 114 of the isolates were identified as E. coli and 48% of those were resistant to at least one AB (Table 4).
The greatest resistant fractions were determined to be to AMP (27%), TE (23%) and S (17%), and the lowest to GEN (2%), as well as a void of resistance to IPM. The E. coli phenotypes demonstrated a high AMR prevalence solely against AMP; a medium level to AMC, C, S and TE; and a low or very low level to the other tested ABs.
Unlike E. coli, 87% of the tested coliforms (n, 24) showed resistance to at least one AB. Most of them were resistant to beta-lactams, including third-generation cephalosporins, such as cefotaxime (46% of isolates) and ceftriaxone (42%), and fourth-generation cefepime (8%). Half of the coliforms expressed a resistant phenotype to COT, and 33% were resistant to TE, but all were susceptible to C, GEN and S and had low resistance to CIP. The intrinsic resistance to AMP, AMC and CX contributes to a higher AMR of coliforms.
The MAR of the Enterobacteriaceae isolates was 19%, as 16% of E. coli strains (n, 18) and 33% (n, 8) of coliforms were multidrug-resistant. However, only among E. coli strains was MAR to five or six AB classes detected (respectively, 4% and 2%) (Figure 3).
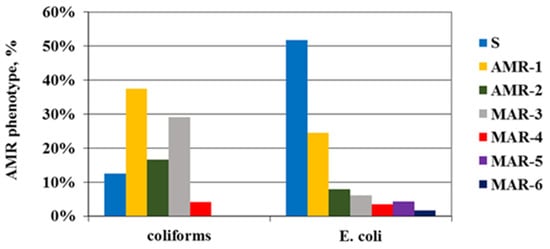
Figure 3.
AMR and MAR phenotype of the resistant Enterobacteriaceae isolates depending on the number of AB classes with manifested resistance. S—sensitive; AMR1,2—resistance to one or two classes of antibiotics; MAR3÷6—multiple antibiotic resistance.
Phenotypes of all multidrug-resistant strains are presented in Table 5. The MAR phenotype of E. coli isolates predominantly included resistance to AMP (in 89% of all MAR strains), TE and S (in 78%) and AMC and COT (in 56%). Four multiple-resistant E. coli isolates from the Belitsa River (SPs 6 and 8) were potential producers of extended-spectrum beta-lactamases (ESBLs).

Table 5.
AMR phenotype of the multidrug-resistant Enterobacteriaceae isolates.
Among the tested coliforms, only Klebsiella strains manifested an MAR phenotype, while Enterobacter and Citrobacter isolates were resistant to one AB class. Multidrug-resistant Klebsiella strains were isolated from SPs 2 and 3 of the Yantra River, unlike those of E. coli, isolated mainly from the Belitsa River. Furthermore, all Klebsiella isolates were ESBL producers exhibiting resistance to cephalosporins of the third generation as well as TE and COT. Coliforms demonstrated resistance to cephalosporins more often than E. coli.
In order to distinguish the contribution of multidrug-resistant E. coli/coliform isolates for the total AMR phenotype expressed to a particular AB, an MAR/AMR ratio was calculated for E. coli or coliform isolates resistant to each individual AB (Table 6).

Table 6.
Contribution of the MAR phenotypes for the total AMR to the individual antibiotics.
For E. coli isolates, the established MAR/AMR ratio for each tested AB had values greater than 50% for TE and beta-lactams (except for CTX) and even higher values for S, C, CIP and COT. This supposes that the incidence of multidrug-resistant E. coli has a decisive contribution to the total exhibited AMR in the Yantra River water. Although multidrug-resistant coliform isolates expressed resistance to only four AB classes, their incidence was responsible for most of the total resistance to those ABs among the coliforms tested, and their MAR/AMR ratios for the individual ABs ranged from 60 to 100% (except for AMP). The obtained data reveal the significant contribution of the MAR strains to the expressed AMR phenotype of Enterobacteriaceae isolated from the Yantra River water.
3.4. AMR Prevalence Depending on the Sampling Point
Based on the AMR data, a comparison between the Enterobacteriaceae strains isolated from the Yantra River and its tributary, the Belitsa River, was made (Figure 4a), as well as between the isolates from two particular SPs, each one representative of the water body on which it is located (Figure 4b).
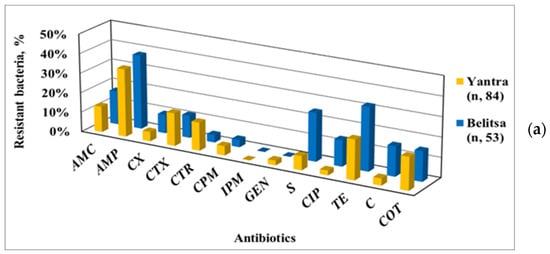
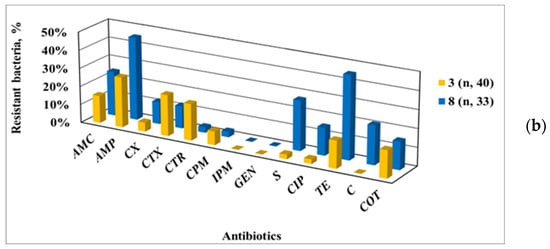
Figure 4.
Antibiotic-resistant phenotypes of Enterobacteriaceae strains isolated from the Yantra River and its tributary, the Belitsa River (a), and, in particular, from their representative sampling points (b) SP 3, localized on the Yantra River, and SP 8 on the Belitsa River.
Among the isolates from the Yantra River, a greater resistance to cephalosporins and GEN was found, as opposed to the higher resistance to S, CIP, TE and C of those from the Belitsa River. This trend was clearly demonstrated by comparing the AMR data of SP 3 (on the Yantra River) with SP 8 (on the Belitsa River), despite the tributary inflow being taken into account for the total AMR of the Yantra River at SP 3. So, the presented data show the contribution of the Belitsa River tributary to AMR spreading among Enterobacteriaceae in the Yantra River water.
Significant spatial variations in the AMR level of Enterobacteriaceae isolates were observed along the river where it passes through the urban territory of Veliko Tarnovo: upstream (SP 1); inside (SPs 2, 3 and 4); and downstream from the urban area (SP 5). The comparative data (Figure 5) demonstrate these AMR differences: at SP 1, resistance only to AMC, CX, S and TE was found; at SP 3, there was an increased AMR to cephalosporins, CIP and COT, under the combined effect of the tributary and sewerage effluents; at SP 4, there were various changes in AMR levels, shown by an increased resistance to TE, C, COT, GEN and C but decreased resistance to cephalosporins.
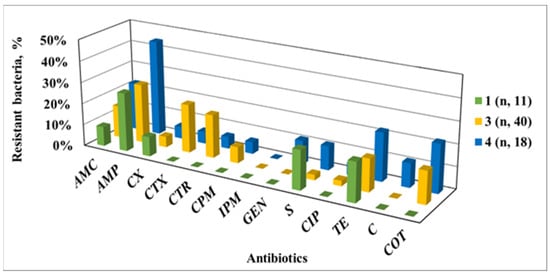
Figure 5.
AMR phenotypes of Enterobacteriaceae strains isolated from the Yantra River passing through the territory of the town of Veliko Tarnovo; SP 1—outside of the urban territory; SP 3—in the south-west urban territory, after the inflow of the tributary of the Belitsa River and a waste water collector; SP 4—after the meanders of the river as it passes through the city.
Similar changes in the level of AMP were found for the Belitsa River (Figure 6). At SP 7, after passing through the urban area of Debelets, the proportion of bacteria resistant to cephalosporins, CIP, COT, S and C increased compared to SP 6. The inflow of the Dryanovska River upstream of SP 8 led to an even greater increase in the fraction of bacteria resistant to TE, C and aminopenicillins.
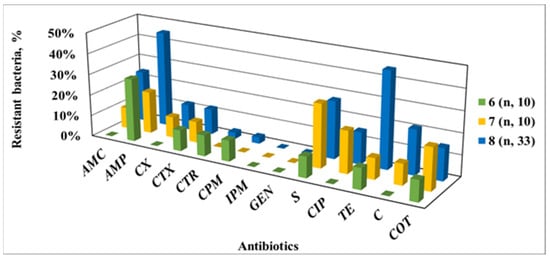
Figure 6.
AMR phenotypes of Enterobacteriaceae isolates from the Belitsa River depending on the water sampling point; SP 6—upstream of the urban territory of the town of Debelets; SP 7—in the urban territory after the inflow of sewage water; SP 8—downstream from the town, after the confluence of the Drynovska River.
The obtained data show changes experienced in the share of Enterobacteriaceae resistant to the individual ABs along the river stretch, comprising the river passing through settlements discharging sewage water, due to insufficient capacity of the WWTP (V.Tarnovo) or the absence of facilities for sewage treatment (Debelets). They reveal the impacts of the urbanized territories on the spread of Enterobacteriaceae resistant to various ABs in the studied river stretch through point and diffuse sources of organic and fecal water pollution. The direct discharge of untreated waste water had a strong influence on the spreading of resistant Enterobacteriaceae in the river water, as well as the combined effect of surface runoff from unsewered settlement and agricultural activities, but there are insufficient data to prioritize the urbanization impact or diffuse fecal contamination from the rural area for AMR prevalence.
4. Discussion
4.1. Prevalence of AMR among Riverine Heterotrophic Bacteria
Our investigations of the AMR of riverine bacteria target ABs widely used for many years in human and veterinary medicine, such as ampicillin, tetracycline and sulfamethoxazole. Ampicillin is categorized as a high-priority, ‘critically important antimicrobial’ (CIA) by the World Health Organization (WHO), and sulfamethoxazole and tetracycline are recognized as ‘highly important antimicrobials’. Fluoroquinolones and third-generation cephalosporins are categorized as the highest-priority CIAs in human medicine [42]. AB residuals, an emergent pollutant of water resources, can play the role of a driving force for the selection of ARB and acquisition of AMR.
The present study assessed AMR prevalence in the water of the selected river sections known to be under pressure from treated WW (in a WWTP without a tertiary stage), untreated sewage or diffuse pollution from small settlements without sewage systems, or runoff from agricultural activities and livestock husbandry, by quantifying the HPC bacteria resistant to various ABs. Although our study did not analyze the content of antibiotic residues or their metabolites in both river water and discharged waste water, the obtained results reveal the contribution of water pollution sources to the spread of AMR among riverine bacteria. The data established significant quantitative differences between the Yantra River and its tributary, the Belitsa River, in bacteria resistant to the studied AB groups related to the different levels of urbanization (comprising population size, construction of a sewage system, construction of a WWTP and treatment efficiency, and economic activities) in the respective catchment areas. The established spatial variations in the AMR of the heterotrophic microbiome in the individual SPs of the examined river stretches demonstrate the influence of the specific point and/or non-point sources of organic and fecal pollution. In the water of the Yantra River, after it passes through rural areas with small settlements without a sewerage system (SPs 1, 5 and 6), a lower AMR level of the aquatic community was found compared to the more highly urbanized areas subjected to strong anthropogenic impact (SPs 2, 3 and 7).
The quantitative relationships between the total number of HPC bacteria and the incidence of the different types of ARB depended on the river stretch. For the Yantra River microbiome, a significant correlation between the resistance to the individual ABs and the total number of HPC bacteria was found, as opposed to a weak correlation for the Belitsa River. A lack of correlation for the Belitsa River indicates that the increased proportion of certain types of ARB in a given SP that suffered from effluent discharge was not solely a result of direct water pollution. It can be assumed that the immediate surroundings of a specific SP in the rural areas with a low degree of urbanization may have a stronger influence on AMR spreading compared to direct WW pollution points in territories with high levels of urbanization.
Differences in the predominant types of ARB depending on the sampling point also clearly suggest the impact of the adjacent territory and water pollution by the human activities performed there. This influence is best demonstrated by the population proportion of ciprofloxacin-resistant bacteria, which had the greatest abundance in the Belitsa River during its passage through a rural area with diffuse fecal pollution (SP 6) and pollution from domestic and industrial effluents (SPs 7 and 8). In the Yantra River water at SPs 2 and 3, which is subjected to similar pollution, an increased population share of bacteria resistant to ciprofloxacin was also registered.
Ciprofloxacin and sulfamethoxazole are synthetic ABs that are absent in the environment, and their residuals in surface water can exert selective pressure on aquatic bacteria. Despite the fact that in this study, specific sources of ciprofloxacin contamination of river water have not been clarified, the increased proportion of bacteria resistant to this AB suggests its presence and impact on aquatic communities. It is also interesting to note that the share of sulfometaxazole-resistant bacteria in the Yantra River (SP 1) and Belitsa River (SP 6) sections located in less urbanized rural areas was lower compared to SPs located in the urban area of Veliko Tarnovo. Also, sulfamethoxazole-resistant bacteria in the Belitsa River water were less abundant than in the Yantra River water. Because of that, our study could continue to identify potential sources of contamination with these ABs, such as poultry farms and livestock farms or food processing activities in the rural areas, as well as tracking waste water pollution due to the use of ABs by the local population, including the discharge of hospital waste water into the municipal sewerage.
In the Yantra River water, the population proportion of tetracycline-resistant bacteria was the lowest, while the highest were those of bacteria resistant to ampicillin and chloramphenicol. Our data on the low abundance of tetracycline-resistant bacteria are consistent with the findings of Harnitz (2013) for a Polish river, in which the water was found to contain tetracyclines [17]. The low level of tetracycline resistance is in line with the observations of Kim et al. (2012) in four Korean freshwater bodies, in which the average number of tetracycline-resistant bacteria was up to three orders of magnitude lower than that of penicillin-resistant bacteria [29].
Assessing the prevalence of penicillin or ampicillin resistance in water environments, numerous studies have found a high population proportion of beta-lactam-resistant bacteria [16,28,29]. In the Yantra River water, the proportion of ampicillin-resistant bacteria was lower than 10% (with one exception), but in its tributary, the Belitsa River, values up to 21.8% were registered. A broad range of ampicillin resistance has been detected in a survey of sixteen US rivers, with considerable differences in the number of ampicillin-resistant bacteria registered in the river bodies: in half of the sampling points, the resistant fraction was in the range of 10 to 30%, but there were those in which it was lower than 10% or higher than 50% [28]. A high level of beta-lactam resistance, including to third-generation cephalosporin, has been observed among opportunistic pathogens isolated from heavy-metal-polluted rivers, co-selected with multiple resistance to copper, lead and cadmium [30].
The obtained population data revealed different ascending orders of bacteria resistant to the individual ABs for the studied river sections: for the Yantra River, the pattern was TE < CIP < Sul/C/AMP, while for the Belitsa River, it was TE < Sul < C/AMP < CIP. This AMR pattern agrees with the ascending degree of bacterial resistance to quinolones, phenicols and beta-lactams established by Kim et al. (2015) for freshwater communities [29], but some differences are found with the AMR ordering by Moore et al. (2010) [33].
Despite few data being available on the AMR of the freshwater microbiome and different culture-based methods used, the researchers support the understanding that the total AMR of native aquatic bacteria can be considered as an indicator of changes in the water environment [16,17]. Our quantitative data on the different types of ARB clearly indicate changes in the river water status related to ongoing organic and fecal pollution in the specific catchment area or the different urbanization levels and human impact. Although no quantitative relationships were inferred between the AMR prevalence in the river water and the urbanization level of the adjacent territory, as was achieved by Sanderson et al. (2018) and Honda et al. (2016) [19,43], our data well indicate the negative anthropogenic impact on the microbiological status and AMR prevalence in the Yantra River and Belitsa River.
The specific features of the water pollution sources, and the specific physico-chemical, hydraulic and biological characteristics of the river body, contribute to significant spatial–temporal variations of the populations of the different types of ARB detected in the studied river water. The seasonal variation in population of heterotrophic bacteria under the conditions of a moderate continental climate, uneven annual water runoff of the studied river (maximum in March–July and minimum in the period of August–October), as well as the changing characteristics of pollution sources related to the morbidity of the population or farmed animals, are the basis for fluctuations in the populations of different ARB types, despite the fact that many other abiotic and biotic factors also could have an influence.
In our study we collected water samples from five sites on the main stream and three sites on the tributary, but did not analyze the water pollution in detail, including the amount of ABs. The detection of antibiotic residuals or their metabolites in surface water is a difficult task, due to a cumulative effect in sediments, benthic biofilms and aquatic organisms or hydraulic events such as flooding and sediment movement, but it is important to establish the critical points of potential pollution by tracing the pathway of key antibiotics in the water body. Because some components of the river body such as benthic biofilms and sediments can facilitate the acquisition of AMR due to close cell-to-cell contact and possibilities for horizontal gene transfer, our study could continue by analyzing river water samples and also sediments and benthic biofilms to determine the content of ABs, and the abundance of ARB and ARGs, and clarifying the relationship between pollutant levels and the prevalence of AMR among aquatic bacteria.
Knowledge of the occurrence, concentration and fate of ARB, ARGs and related antibiotics in surface water will contribute to a better understanding and management of environmental hazards caused by these emergent pollutants. Although there are few data, mainly reported for waste water [6,7,11], it would be important for the relationships between AB content and the corresponding ARB and ARGs in river water bodies to be clarified.
4.2. Prevalence of AMR among Enterobacteriaceae Isolates from the Yantra River Stretch
It was apparent from the investigations of the aquatic community that AMR was widespread among environmental bacteria. To assess the specific contribution of humans and animals to the spreading of ARB in a water environment, special attention was addressed to evaluating the antibiotic susceptibility of Enterobacteriaceae isolates.
Fecal pollution of water resources poses significant risks to human and animal health since numerous pathogens are often associated with feces. E. coli and fecal coliforms are human and animal commensals that enter water resources through untreated effluents, treated waste water or surface runoff from rural areas and agricultural land. Some species are pathogenic, and as a result of common habitats with other pathogens or ARB in living organisms or in the WW treatment process, they can acquire resistance to various ABs. The increased content of Enterobacteriaceae in fecal polluted river water suggests the possibility of a raised AMR incidence in their population, as well as in the entire aquatic community due to the opportunity for horizontal gene transfer. Concentrations of E. coli and total coliforms in the Yantra River water were generally high, indicating that the studied river stretch experienced high levels of microbial pollution as a result of the river passing through settlements discharging untreated or incompletely treated sewage water, as well as through rural areas with diffuse fecal contamination. In the slightly polluted water from SP 1, the E. coli content was two orders of magnitude lower than in the water from SPs 2 and 3, and the difference between SP 6 and SP 7 was in the same range (Table 2). However, weak positive correlations between HPC and E. coli numbers (r = 0.47) or HPC and total coliforms (r = 0.53) in the water were found. The E. coli content of the river water at SPs 1, 4, 5 and 6 comprised below 0.1% of the HPC population, but had higher values at SPs 2, 3 and 8 (0.2–0.6%) with a maximum at SP 7 (2%).
The obtained AMR data underline changes in the incidence of Enterobacteriaceae resistant to the tested ABs along the river stretch. Study of the AB susceptibility of Enterobactericeae isolates found considerable incidence of AMR, including MAR; spatial variations in AMR in the examined river stretch; a higher AMR rate in isolates from the Belitsa River than the Yantra River; and ranking of AMR incidence in the following order: quinolones/amphenicols/aminoglycosides < beta-lactams < antifolates < tetracyclines.
Not surprisingly, the study found the highest resistance of Enterobacteriaceae to ampicillin (36%), despite the fact that a beta-lactam resistance of 12.5% was determined on the basis of the seven tested beta-lactam ABs. A similar level of ampicillin resistance of E. coli isolates has been reported for the largest EU river, the Danube River—33.3% [23], and 32% for a Korean river [44], but there is evidence of a wider ampicillin resistance range—from 24.3% found for a Spanish river [1], 43.6% [31], 55.17% [22] to the highest value of 86.6% in a Chinese river [45].
Despite the different rates of ESBL resistance reported for a number of European and Asian rivers [19,26,45], a small fraction of Enterobacteriaceae isolates from the studied river stretch turned out to be resistant to third- and fourth-generation cephalosporins. The observed low Enterobacteriaceae resistance to cefepime and susceptibility to imipenem is of particular importance due to their status as “last resort” ABs. However, some of the isolated MAR strains were potential ESBL producers—four E. coli strains and eight Klebsiella spp. The AMR level to cefoxitin as an indicator of plasmid AmpC-mediated resistance was low as well.
Our data on the Yantra River water reveal that the Enterobacteriaceae resistance was generally high to the older and more commonly used ABs. The incidence of tetracycline-resistant Enterobacteriaceae was high, despite the broad range of tetracycline resistances reported for other rivers—from low [1,23] to high abundance values [15,19,44,45]. The high rate of streptomycin resistance found in the Yantra River was in compliance with other findings [19,45], while resistance to gentamicin, the other tested aminoglycoside, was very rare.
A comparison of the resistance phenotypes of E. coli isolates from the Yantra River water with WHO/ECDC data on clinical isolates or isolates from swine feces and lagoons in animal breeding found a much lower level of AMR among riverine bacteria [46]. According to the WHO/ECDC report Antimicrobial resistance surveillance in Europe 2020, the percentage of invasive E. coli isolates with resistant phenotype in Bulgaria had a population-weighted mean value of 54.6% for AMP, 14.9% to third-generation cephalosporin, 23.8% to fluoroquinolones, 10.2% to aminoglycosides and 0.2% to carbapenems [46]. A study on the AMR profile of E. coli isolates from swine feces and lagoons in Bulgaria to ABs used in animal husbandry found high AMR prevalence (between 50% and 75%) to amoxicillin, ampicillin, tetracycline and chloramphenicol, and between 25% and 50% to co-trimoxazole, doxycycline and nalidixic acid [47].
The MAR level of Enterobacteriaceae isolated from the Yantra River was 19%. The multidrug-resistant E. coli isolates expressed resistance to up to five or six classes of ABs, predominantly to AMP, TE, S and COT. Coliforms were multi-resistant to up to four AB classes, but the MAR incidence contributed to the majority of the total AMR to these ABs. The calculated MAR/AMR ratios of the tested E. coli or coliforms reveal the significant contribution of the MAR strains to the manifested AMR phenotype of Enterobacteriaceae isolates. Multidrug-resistant Klebsiella strains were isolated from the fecal polluted water of SPs 2 and 3 of the Yantra River, while those of E. coli were mainly isolated from SP 8 of the Belitsa River. The MAR incidence among the tested Enterobacteriaceae isolates was higher than the values reported for the Arga River (5.5%) or the Danube River (9.7% of the isolated E. coli and 2.2% of Klebsiella spp.) [1,23,24], but lower compared with the MAR value of 48.6% for isolates from the Mur River [48] or 77.9% for those from the Ave River [15].
Our AMR data established changes in the incidence of Enterobacteriaceae resistant to the individual ABs along the Yantra River, as a result of the river’s passage through urbanized areas with direct sewage discharge or rural areas with predominantly diffuse fecal pollution. In the rural areas (SPs 1, 5 and 6), despite the agricultural activities and sewage absence, lower AMR levels were detected among the tested Enterobacteriaceae strains, as well as sensitivity to GEN, CIP and C and a rare incidence of MAR. At SPs 3, 7 and 8, an increase in the different resistant phenotypes was found under the impact of inflowing sewage water or polluted river tributaries. The Belitsa River inflow contributed to the increased incidence of resistant Enterobacteriaceae at SP 3 of the Yantra River, but it is important to note that their incidence remained lower compared to SP 8.
No apparent relationship was found between the level of fecal pollution of the river water and the AMR pattern of the Enterobacteriaceae strains. For the Yantra River, the correlation between AMR pattern and the number of Enterobacteriaceae was significant only for CTX (r = 0.85), CTR (r = 0.86) and COT (r = 0.86). For the Belitsa River, negative correlations were found for CTR and CPM (r = −0.92) and positive ones for CIP (r = 0.90), COT (r = 0.98) and S (r = 0.84). The discharged effluents probably contributed to a significant increase in the proportion of antibiotic-resistant Enterobacteriaceae in the river water, but the lack of direct correlation dependence for the entire river section and separately for the Yantra and Belitsa River stretches is probably related to a high incidence of E. coli strains susceptible to ABs in the waste water.
The data on the spatial AMR variations of Enterobacteriaceae isolates depending on SP are consistent with the already discussed data on the prevalence of AMR among HPC bacteria. In the SPs where untreated waste water was directly discharged, an AMR increase in both parameters—HPC bacteria and Enterobacteriaceae—was demonstrated. Therefore, the quantitative data, such as the percentage of the different types of resistant HPC bacteria or resistant Enterobacteriaceae strains, indicate well the AMR changes in the river microbiome suffered under the anthropogenic pressure of urban effluents or the complex water pollution from point and diffuse fecal sources. Determining the contribution of specific sources of pollution to AMR dissemination along the river course will enable the prioritization of measures needed for the limitation of AMR spreading among the river microbiome.
5. Conclusions
To the best of our knowledge, our study is the first to assess the prevalence of AMR among heterotrophic bacteria and Enterobacteriaceae in Bulgarian river water. Thus, AMR studies of Bulgarian surface water have begun, which also provides an opportunity to enrich the published data on this issue.
Our study determined the AMR level of riverine heterotrophic bacteria and fecal indicators of the Enterobacteriaceae family in a selected section of the Yantra River basin and confirmed the influence of existing (and still neutralized) sources of organic and fecal pollution on increased AMR. Quantitative data on the percentage of HPC bacteria and Enterobacteriaceae resistant to selected ABs describe well AMR changes of the river microbiome under anthropogenic pressure. Owing to the obtained AMR data, the impact of particular pollution sources was spatially differentiated along the river course. Established differences between the Yantra River and its tributary, the Belitsa River, in terms of the population proportion of HPC bacteria resistant to the studied ABs as well as the AMR incidence and AMR pattern of Enterobacteriaceae confirm the influence of pollution sources with specific characteristics. The total AMR of aquatic bacteria and AMR prevalence among Enterobacteriaceae can be considered as indicators of water quality changes in a particular river catchment area being caused by some kind of organic and fecal pollution or different levels of urbanization and various human activities. Both parameters indicate the negative anthropogenic impact on the microbiological status and AMR prevalence in the Yantra River and the Belitsa River, but although we cannot track the contribution of individual pollution sources within mixed (point and diffuse) fecal water pollution, monitoring of these indicators is particularly important in the context of the water cycle and sustainable management of water resources.
Our data on the prevalence of AMR among autochthonous aquatic bacteria and allochthonous Enterobacteriaceae highlight the need for adequate waste water treatment and for management, monitoring and control of treatment processes to limit anthropogenic pressures through discharge of untreated or incompletely treated waste water and to ensure the ecological well-being of receiving water bodies. Thus, prioritizing the problems caused by various sources of river water pollution and solving them will help to limit AMR dissemination and reduce the ecological hazard to water resources and the potential health risk for the population of adjacent territories.
Author Contributions
Conceptualization, Z.T.; methodology, Z.T. and H.N.; investigation, Z.T.; data curation, Z.T.; writing—original draft preparation, Z.T.; writing—review and editing, H.N.; project administration, Z.T. and H.N. All authors have read and agreed to the published version of the manuscript.
Funding
(1) Grant BG05M2OP001-1.002-0019: “Clean technologies for sustainable environment—waters, wastes, energy for circle economy” (Clean & Circle) for the building and developing of a Centre of Competence, part of Operational Programme “Science and Education for Smart Growth”, which was co-financed by the European Union through European Structural and Investment Funds. (2) Grant No. KП-06-H31/20: “Antibiotic resistant bacteria and antibiotic resistance genes in Bulgarian natural waters and waters under anthropogenic impact” financed by the National Science Fund, Bulgaria.
Data Availability Statement
Data are available on authors’ request.
Acknowledgments
The technical assistance of Vanja Slaveva is greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AB | Antibiotic |
| AMC | Amoxicillin/clavulanic acid |
| AMP | Ampicillin |
| AMR | Antimicrobial resistance |
| ANOVA | One-way analysis of variance |
| ARB | Antibiotic-resistant bacteria |
| ARG | Antibiotic resistance gene |
| C | Chloramphenicol |
| CIA | Critically important antimicrobial |
| CIP | Ciprofloxacin |
| CFU | Colony form unit |
| CLSI | Clinical and Laboratory Standards Institute |
| COT | Co-trimoxazole (trimethoprim/sulfamethoxazole) |
| CTR | Ceftriaxone |
| CTX | Cefotaxime |
| CPM | Cefepime |
| CX | Cefoxitin |
| ECDC | European Centre for Disease Prevention and Control |
| EU | European Union |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| GEN | Gentamycin |
| HPC | Heterotrophic plate count |
| IPM | Imipenem |
| MAR | Multiple antibiotic resistance |
| P | Probability |
| r | Pearson correlation coefficient |
| S | Streptomycin |
| SPs | Sampling points |
| Sul | Sulfamethoxazole |
| TE | Tetracycline |
| WHO | World Health Organization |
| WW | Waste water |
| WWTP | Waste water treatment plant |
References
- Goñi-Urriza, M.; Capdepuy, M.; Arpin, C.; Raymond, N.; Caumette, P.; Quentin, C. Impact of an urban effluent on antibiotic resistance of riverine Enterobacteriaceae and Aeromonas spp. Appl. Environ. Microbiol. 2000, 66, 125–132. [Google Scholar] [CrossRef]
- Singh, A.K.; Kaur, R.; Verma, S.; Singh, S. Antimicrobials and antibiotic resistance genes in water bodies: Pollution, risk, and control. Front. Environ. Sci. 2022, 10, 830861. [Google Scholar] [CrossRef]
- Kulik, K.; Lenart-Boro, K.; Wyrzykowska, K. Impact of antibiotic pollution on the bacterial population within surface water with special focus on mountain rivers. Water 2023, 15, 975. [Google Scholar] [CrossRef]
- Sanseverino, I.; Cuenca, A.N.; Loos, R.; Marinov, D.; Lettieri, T. State of the Art on the Contribution of Water to Antimicrobial Resistance; EUR 29592 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-98478-5. [Google Scholar] [CrossRef]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018, 618, 409–418. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Сhemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef]
- Daverio, E.; Ghiani, M.; Bernasconi, C. Antibiotics and Antibiotic-Resistant Bacteria in Aquatic Environment: A Review. 2004. EC, JRC, p.77. EN 21201. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC28124 (accessed on 1 August 2023).
- Commission of the European Communities. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; Office for Official Publications of the European Communities: Luxembourg, 2000; Volume 43, p. 327. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2000:327:FULL (accessed on 1 August 2023).
- Liguori, K.; Keenum, I.; Davis, B.C.; Calarco, J.; Milligan, E.; Harwood, V.J.; Pruden, A. Antimicrobial resistance monitoring of water environments: A framework for standardized methods and quality control. Environ. Sci. Technol. 2022, 56, 9149–9160. [Google Scholar] [CrossRef]
- Fletcher, S. Understanding the contribution of environmental factors in the spread of antimicrobial resistance. Environ. Health Prev. Med. 2015, 20, 243–252. [Google Scholar] [CrossRef]
- Manaia, C.M.; Macedo, G.; Fatta-Kassinos, D.; Nunes, O.C. Antibiotic resistance in urban aquatic environments: Can it be controlled? Appl. Microbiol. Biotechnol. 2016, 100, 1543–1557. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Kassinos, D.F.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Bacterial diversity and antibiotic resistance in water habitats: Searching the links with the human microbiome. FEMS Microbiol. Rev. 2014, 38, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Vasconcelos, A.; Mendes, Â.; Martins, F.; Lopes, E.; Machado, A.; Bordalo, A.A.; Vaz-Pires, P.; Vieira, N.; da Costa, P.M.; Bessa, L.J. River water analysis using a multiparametric approach: Portuguese river as a case study. J. Water Health 2018, 16, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Moore, P.J.A.; Millar, B.C.; Goldsmith, C.E.; Loughrey, A.; Rooney, P.J.; Rao, J.R. The presence of antibiotic resistant bacteria along the River Lagan. Agricult. Water Manag. 2010, 98, 217–221. [Google Scholar] [CrossRef]
- Harnisz, M. Total resistance of native bacteria as an indicator of changes in the water environment. Environ. Pollut. 2013, 174, 85–92. [Google Scholar] [CrossRef]
- Ferreira da Silva, M.; Vaz-Moreira, I.; Gonzalez-Pajuelo, M.; Nunes, O.C.; Manaia, C.M. Antimicrobial resistance patterns in Enterobacteriaceae isolated from an urban wastewater treatment plant. FEMS Microbiol. Ecol. 2007, 60, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Watanabe, T.; Sawaittayotin, V.; Masago, Y.; Chulasak, R.; Tanong, K.; Chaminda, G.T.; Wongsila, K.; Sienglum, C.; Sunthonwatthanaphong, V.; et al. Impacts of urbanization on the prevalence of antibiotic resistant Escherichia coli in the Chaophraya River and its tributaries. Water Sci. Technol. 2016, 73, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Jofre, J.; Balcazar, J.L. Prevalence of antibiotic resistance genes and bacterial community composition in a river influenced by a wastewater treatment plant. PLoS ONE 2013, 8, e78906. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestępski, S. The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Sci. Total Environ. 2017, 15, 577, 367–375. [Google Scholar] [CrossRef]
- Marinescu, F.; Marutescu, L.; Savin, I.; Lazar, V. Antibiotic resistance markers among Gram-negative isolates from wastewater and receiving rivers in South Romania. Rom. Biotechnol. Lett. 2015, 20, 10055–10069. [Google Scholar]
- Zarfel, G.; Folli, B.; Lipp, M.; Pfeifer, B.; Baumert, B.; Farnleitner, A.; Kirschner, A.; Kittinger, C. Spread of non-wild type antibiotic resistant phenotypes in the river Danube. In Joint Danube Survey. A Comprehensive Analysis of Danube Water Quality; Liška, I., Wagner, F., Sengl, M., Deutsch, K., Slobodník, J., Eds.; ICPDR-International Commission for the Protection of the Danube River: Vienna, Austria, 2015; pp. 169–172. [Google Scholar]
- Kittinger, C.; Lipp, M.; Folli, B.; Kirschner, A.; Baumert, R.; Galler, H. Enterobacteriaceae isolated from the river Danube: Antibiotic resistances, with a focus on the presence of ESBL and carbapenemases. PLoS ONE 2016, 11, e0165820. [Google Scholar] [CrossRef]
- Zurfluh, K.; Hachler, H.; Nuesch-Inderbinen, M.; Stephan, R. Characteristics of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013, 79, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Kürekci, C.; Aydin, M.; Yipel, M.; Katouli, M.; Gündogdu, A. Characterization of extended spectrum β-lactamase (ESBL)-producing Escherichia coli in Asi (Orontes) River in Turkey. J. Water Health 2017, 15, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Koczura, R.; Mokracka, J.; Jabłońska, L.; Gozdecka, E.; Kubek, M.; Kaznowski, A. Antimicrobial resistance of integron- harboring Escherichia coli isolates from clinical samples, wastewater treatment plant and river water. Sci. Total Environ. 2012, 414, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ash, R.J.; Mauck, B.; Morgan, M. Antibiotic resistance of Gram-negative bacteria in rivers, United States. Emerg. Inf. Dis. 2002, 8, 240–247. [Google Scholar] [CrossRef]
- Kim, T.; Joung, W.Y.; Han, J.H.; Jung, W.; Kim, S.B. Antibiotic resistance among aquatic bacteria in natural freshwater environments of Korea. J. Water Health 2015, 13, 1085–1097. [Google Scholar] [CrossRef]
- Djouadi, L.N.; Selama, O.; Abderrahmani, A.; Bouanane-Darenfed, A.; Abdellaziz, L.; Amziane, M.; Fardeau, M.L.; Nateche, F. Multiresistant opportunistic pathogenic bacteria and detection of nontuberculous mycobacteria. J. Water Health 2017, 15, 566–578. [Google Scholar] [CrossRef]
- Biyela, P.T.; Lin, J.; Bezuidenhout, C.C. The role of aquatic ecosystems as reservoirs of antibiotic resistant bacteria and antibiotic resistance genes. Water Sci. Technol. 2004, 1, 45–50. [Google Scholar] [CrossRef]
- Ayandiran, T.A.; Ayandele, A.A.; Dahunsi, S.O.; Ajala, O.O. Microbiological assessment and prevalence of antibiotic resistance in polluted Oluwa iver, Nigeria. Egypt. J. Aquatic Res. 2014, 40, 291–299. [Google Scholar] [CrossRef]
- Moore, J.E.; Rao, J.R.; Moore, P.J.A.; Millar, B.C.; Goldsmith, C.E.; Loughrey, A.; Rooney, P.J. Determination of total antibiotic resistance in waterborne bacteria in rivers and streams in Northern Ireland: Can antibiotic-resistant bacteria be an indicator of ecological change? Aquat. Ecol. 2010, 44, 349–358. [Google Scholar] [CrossRef]
- EN ISO 19458:2006; Water Quality. Sampling for Microbiological Analysis. ISO: Geneva, Switzerland, 2006.
- EN ISO 9308-1:2014; Water Quality. Enumeration of Escherichia coli and Coliforms. Part 1: Membrane Filtration Method. ISO: Geneva, Switzerland, 2014.
- CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. 2013. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Expert_Rules/2021/Intrinsic_Resistance_and_Unusual_Phenotypes_Tables_v3.3_20211018.pdf (accessed on 1 August 2023).
- Ministry of Environment and Water (MOEW). Regional Environment and Water Inspectorate—Veliko Tarnovo. Annual Report on the State of the Environment for 2020. Available online: https://www.riosvt.org/wp-content/uploads/2021/05/RIOSV-VT-reg.doklad_2020-1-1.pdf (accessed on 1 August 2023). (In Bulgarian).
- Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:064:0037:0051:EN:PDF (accessed on 1 August 2023).
- Kirschner, A.; Jakwerth, S.; Kolarevic, S.; Sommer, R.; Blaschke, A.P.; Kavka, G.; Reischner, G.; Farnleitner, A. Bacterial faecal indicators. In Joint Danube Survey 3. A Comprehensive Analysis of Danube Water Quality; Liška, I., Wagner, F., Sengl, M., Deutsch, K., Slobodník, J., Eds.; ICPDR-International Commission for the Protection of the Danube River: Vienna, Austria, 2015; pp. 155–161. [Google Scholar]
- Kavka, G.; Poetsch, E. Microbiology. In Joint Danube Survey. Technical Report of the International Commission for the Protection of the Danube River; Literathy, P., Koller-Kreimel, V., Liška, I., Eds.; ICPDR-International Commission for the Protection of the Danube River: Vienna, Austria, 2002; pp. 138–150. [Google Scholar]
- WHO. Critically Important Antimicrobials for Human Medicine, 6th ed.; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Sanderson, C.E.; Fox, J.T.; Dougherty, E.R.; Cameron, A.D.S.; Alexander, K.A. The changing face of water: A dynamic reflection of antibiotic resistance across landscapes. Front. Microbiol. 2018, 9, 1894. [Google Scholar] [CrossRef]
- Yoo, H.N.; Ki, S.J.; Kang, J.H.; Ham, Y.S.; Cha, S.M.; Lee, S.W.; Lee, S.Y.; Kim, J.H. Occurrence of antibiotic resistant E. coli in surface sater: A study in a typical urban watershed, Korea. Water Pract. Technol. 2010, 5, wpt2010056. [Google Scholar] [CrossRef]
- Han, N.; Sheng, D.; Xu, H. Role of Escherichia coli strain subgroups, integrons, and integron-associated gene cassettes in dissemination of antimicrobial resistance in aquatic environments of Jinan, China. Water Sci. Technol. 2012, 66, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- WHO/ECDC. World Health Organization Regional Office for Europe/European Centre for Disease Prevention and Control. In Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf (accessed on 1 August 2023).
- Dimitrova, L.; Kaleva, M.; Zaharieva, M.M.; Stoykova, C.; Tsvetkova, I.; Angelovska, M.; Ilieva, Y.; Kussovski, V.; Naydenska, S.; Najdenski, H. Prevalence of Antibiotic-Resistant Escherichia coli Isolated from Swine Faeces and Lagoons in Bulgaria. Antibiotics 2021, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Zarfel, G.; Lipp, M.; Gürtl, E.; Folli, B.; Baumert, R.; Kittinger, C. Troubled water under the bridge: Screening of river Mur water reveals dominance of CTX-M harboring Escherichia coli and for the first time an environmental VIM-1 producer in Austria. Sci. Total Environ. 2017, 593–594, 399–405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).