Abstract
The effects of transglutaminase (TGase), reductant, and thermal treatment on the cross-linking of white proteins in soft-shell turtle eggs were investigated. Egg white proteins were denatured by reductant (0.83% 2-mercaptoethanol, 2-ME) pretreatment and thermal pretreatment (95 °C and 5 min), and the denatured proteins were then catalyzed by TGase (1.0 unit/mL). SDS–PAGE showed that without any pretreatments, three major egg white proteins (210 kDa, 115 kDa, and 76 kDa proteins) were inferior substrates for TGase. Only portions of the 210 kDa protein (7.9%), 115 kDa protein (11.4%), and 76 kDa protein (42.9%) were polymerized by TGase into high-molecular-weight (MW) protein polymers (>180 kDa) after incubation for 3 h at 40 °C. However, the combined use of TGase with 0.83% 2-ME and thermal pretreatment led to a significant increase (p < 0.05) in the rate of white protein polymerization after 3 h: 210 kDa protein (90.8%), 115 kDa protein (69.5%), and 76 kDa protein (72.2%). Particle size analysis indicated that these cross-linked high-MW protein polymers were 2000–10,000 nm in size. Based on the experimental results, egg white proteins denatured by 2-ME and heat pretreatment are more prone to TGase-induced cross-linking.
1. Introduction
The soft-shell turtle (Pelodiscus sinensis) is the most commonly eaten species, as it provides a healthy food source and its eggs can be utilized [1]. In East Asia, since the 1980s, the farming of soft-shell turtles has advanced rapidly. In addition, soft-shell turtles and their eggs are becoming abundant [2]. In recent years, there has been tremendous public enthusiasm in Asian countries for soft-shell turtle eggs as a portion of a healthy diet. Moreover, some researchers have reported that soft-shell turtle eggs could prevent hypertension and lower serum cholesterol [3]. Previous research has shown that soft-shell turtle eggs have a shiny yellowish shell and that the average diameter of these spherical eggs is approximately 2 cm. The average weight of soft-shell turtle eggs is 5.78 g, and the weight ratio is 16.4% eggshell, 33.5% egg white, and 50.0% egg yolk [4]. The egg white proteins show a gel-forming ability, Alavi et al. [5] reported that TGase treatment significantly enhanced the cross-linking of heat-denatured egg white proteins, resulting in a significant increase in the gel strength of egg white protein gels.
In foods, TGase is typically used to convert the solubility, thermal stability, and hydration of proteins. TGase catalyzes the exchange between acyl donors (such as the γ-carboxamide group of peptide-bound glutamine residues) and acyl acceptors (including the ε-amino group of lysine residues in some proteins and various primary amines) in the acyl transfer reaction [6]. In lysine and glutamine residues, many food proteins (soybean glycinin, casein, or myosin) have been determined to be good candidates for invertase due to advancements. However, globular proteins, such as egg white proteins and whey proteins, are inferior substrates for TGase. These globular proteins cannot be used for TGase enzymatic catalysis [7]. However, TGase treatment improves the susceptibility of proteins because the structure is unfolded by unstable globular proteins. Therefore, lysine and glutamine residues receive more exposure during TGase-catalyzed reactions. Various strategies have been used to increase the cross-linking efficiency of TGase, such as using reductants or thermal denaturation [8].
Hsiao et al. [9] reported that thermal treatment applied to native proteins results in the dissociation and denaturation of the proteins, a process by which the proteins unfold and form protein filaments due to the formation of disulfide bonds and hydrophobic interactions. The denaturation of hen egg protein (ovalbumin) by a reducing agent or prethermal treatment (95 °C and 5 min) improved the degree of TGase cross-linking with ovalbumin [10]. The utilization of a reducing agent reduced disulfide bonds, opened the globular structure of α-lactalbumin, and reacted with TGase [11]. Furthermore, the reductant enhanced the level of enzymatic cross-linking between TGase and β-lactoglobulin [12]. With a reductant, the disulfide bonds of proteins are broken to create sulfhydryl groups. Disulfide bonds are reduced, resulting in modifications to the quaternary and tertiary structures of proteins. In protein studies, 2-ME is a common reductant that utilizes disulfide bond reductants [13]. The reducing agents cause the cleavage of disulfide bonds, resulting in the formation of sulfhydryl groups. Among these reducing agents, 2-ME (C2H6OS) is commonly used in protein studies as a disulfide bond reductant [14]. Additionally, 2-ME acts as an antioxidant that is capable of scavenging radicals while also being able to break disulfide bonds. However, few papers have researched the influences of the enzyme (TGase), reductants, and thermal treatment on egg white protein cross-linking.
In our previous study, we examined the enhancements in enzymatic cross-linking of hen egg white protein (ovalbumin) through TGase when subjected to heat and 2-ME pretreatment [10]. We have previously shown that hen egg protein (ovalbumin) can polymerize to large polymers when exposed to 0.83% 2-ME and thermal pretreatment, followed by TGase (1.0 units/mL) for 3 h. Due to the enhanced cross-linking of heat-denatured egg white proteins by TGase treatment, there was a notable increase in the gel strength of the egg white protein gels. However, there have been no studies examining the impact of a reducing agent, TGase, and heat treatment on the cross-linking of egg white proteins from soft-shell turtle eggs. Therefore, the influences of TGase, thermal treatment, and 2-ME on the cross-linking of soft-shell turtle egg white were investigated. The aim was to improve the efficiency of cross-linking egg white proteins, including the 210, 115, and 76 kDa proteins, through the utilization of TGase, thermal treatment, and 2-ME.
2. Materials and Methods
2.1. Preparation of Egg White
A total of 500 fresh, unfertilized eggs produced by 3- to 4-year-old soft-shell turtles were obtained from a turtle farm located in Pingtung City, Taiwan. These 500 turtle eggs (2725 g) were rinsed using water to remove dirt. After the washed turtle eggs had been broken, a dropper was utilized to acquire egg whites for analysis. All collected turtle egg whites were placed in an ice bath. Then, the mixture was stirred gently to prevent foam and create a homogeneous mixture. The egg white (890 g) was filtered through a sifting screen to remove impurities. After three days of freeze-drying, the dried egg white samples (36.5 g), which contained 54% of protein, were stored in a freezer (−20 °C).
2.2. Egg White Sample Preparation with/without Reductants, Thermal Treatment, and TGase
The freeze-dried egg white (400 mg) was dissolved in 100 mL of distilled water, and the concentration of the egg white solution was 4 mg/mL, including 2.16 mg protein/mL. The experiments were carried out under one of the following conditions: (1) egg white without treatment, (2) egg white with thermal treatment (95 °C and 5 min), (3) egg white with 2-ME (0.83%, v/v) and thermal treatment (95 °C and 5 min), and (4) egg white with TGase treatment at 1.0 units/mL. The samples were incubated before analysis by SDS–PAGE at 40 °C for 1 h.
2.3. Effects of the Incubation Time on Cross-Linking of Egg White Proteins with/without Reductants, Thermal Treatment, and TGase
In this research, the influence of a combination of reductants, thermal treatment, and TGase on the cross-linking of egg white protein (4 mg/mL) was examined. The experiments were carried out under one of the following conditions: (1) TGase (1.0 units/mL)-treated egg white, (2) egg white thermal pretreatment (95 °C and 5 min) and TGase (1.0 units/mL) treatment, and (3) egg whites pretreated with 2-ME (0.83%, v/v), thermal treatment (95 °C and 5 min), and TGase treatment at 1.0 units/mL. Samples were analyzed using SDS–PAGE after incubation at 40 °C for 0, 1, 2, and 3 h.
2.4. SDS-Polyacrylamide Gel Electrophoresis (SDS–PAGE)
Egg white samples were analyzed by SDS–PAGE following a modified method described by Chen et al. [15], and these samples were analyzed by SDS–PAGE. The 0.25 mL sample was mixed with 0.75 mL of the sample buffer solution (70 mM Tris-HCl, pH 6.8). The sample buffer solution included bromophenol blue, SDS, 2-ME, and glycerol (0.02, 2, 5, and 10%). Separately, each sample (10.0 µL) and protein markers (6.5 µL) were added to the individual wells of the gel. After gel electrophoresis, SDS–PAGE gels were soaked for 30 min in a fixative solution including methanol and acetic acid (10 and 7%). Then, the cells were stained overnight with Coomassie brilliant blue (R-250) staining solution. After removing excess dye, a flatbed scanner (Canon LiDE 120, Canon Inc., Tokyo, Japan) was used to digitize SDS–PAGE gels. Subsequently, gel images obtained from SDS–PAGE were subjected to analysis using Gel-Pro Analyzer software.
2.5. Analysis of Surface Hydrophobicity (H0)
The influences of reductants and thermal treatment on the change in surface hydrophobicity (H0) of egg white proteins (4 mg/mL) were investigated. Experiments were performed under the following conditions: egg whites treated with 2-ME (0.83%, v/v) and heat (95 °C and 5 min). The surface hydrophobicity of egg white protein samples was determined by the method described by Hsiao et al. [9] with modifications. The egg white protein samples were solubilized in 50 mL of 0.01 M phosphate buffer at pH 8.0. Then, from 0.05 to 1 mg/mL, the samples were serially diluted to different protein concentrations using the same buffer. H0 was analyzed, and eight-aniline-one-naphthalene sulfonic acid (a fluorescent probe) was added to each sample. After 15 min, the fluorescence intensity of each sample was measured with a microplate reader (Infinite® M200 Pro, Tecan, Männedorf, Switzerland). The excitation wavelength was set to 390 nm, and another emission wavelength was 470 nm. The primary slope of the plot of the fluorescence intensity to the protein concentrations using the H0 index was expressed.
2.6. Determination of Reactive Sulfhydryl (SH) Groups
In this research, the influences of reductants and thermal treatment on changes in the reactive sulfhydryl (SH) groups of egg white protein (4 mg/mL) were investigated. Egg whites were treated with 2-ME (0.83%, v/v) and heat (95 °C and 5 min). 2-ME and thermal pretreatment with/without samples were analyzed by reactive sulfhydryl (SH) groups. Stefanović et al. [16] determined the SH groups of egg white protein samples. In proteins, at 412 nm and expressed in μmol/g protein, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was spectrophotometrically analyzed by reactive SH groups. The protein samples were diluted to achieve a protein concentration of 1 mg/mL for each sample. Egg white protein solutions were treated with reductant (2-ME), and an additional procedure for reducing agent removal was followed. To precipitate the protein, a mixed and diluted reductant-treated egg white protein sample (0.5 mL, 1 mg/mL) was mixed with a trichloroacetic acid solution (1 mL, 12%, w/v) for 1 h. The supernatant was discarded after centrifugation at 5000× g for 10 min, and the precipitated protein was redissolved in a standard buffer (0.5 mL). Ultimately, a total of 0.02 mL of reagent (Ellman, Hicksville, NY, USA), consisting of DTNB at a concentration of 4 mg/mL mixed with a standard buffer solution, was added to 1 mL of the diluted egg white sample and incubated for 15 min. A standard buffer was used to replace the protein solution, which exhibited absorbance at 412 nm with the sample in connection with a reagent blank.
2.7. Particle Size Analysis
After 2-ME, thermal pretreatment, and TGase treatment, the particle size distribution of egg white protein (4 mg/mL) was analyzed. The egg white samples pretreated with 2-ME (0.83%, v/v), thermal-pretreated (95 °C and 5 min), and TGase-treated (1.0 units/mL) were incubated at 40 °C for 0 and 3 h. The samples were diluted with 0.05 M phosphate-buffered saline (pH 6.8). A particle size analyzer (NanoBrook 90Plus PALS, Brookhaven Instruments Co., Holtsville, NY, USA) was used to analyze the particle sizes of the samples through dynamic light scattering measurements. At 25 °C, the dynamic light scattering technique was used to express the size distribution curve of the nanoparticles as the frequency (%) versus diameter (nm) at a scattering angle of 90°.
2.8. Analytical Approach by Transmission Electron Microscopy (TEM)
TEM analyses were used to image egg white proteins (4 mg/mL) with thermal and 2-ME pretreatment followed by TGase treatment at 40 °C for 0 and 3 h. The egg white samples pretreated with 2-ME (0.83%, v/v), thermal-pretreated (95 °C and 5 min), and TGase-treated (1.0 units/mL) were incubated. For the TEM analysis, samples were dropped onto carbon-film-coated, 300 mesh, Cu grids with diluted water to air dry. On film, at a magnification of 5000×, using a JEM-1400 microscope (JEOL Ltd., Tokyo, Japan), images were captured with the accelerating voltage set to 100 kV.
2.9. Statistical Analysis
For the analysis of the experimental data, the Statistical Analysis System (SAS, version 9.4, SAS Institute Inc., Cary, NC, USA) was used. Data are presented as the mean ± standard deviation. The results were calculated using one-way ANOVA and Duncan’s multiple range test. There was statistical significance among the sample groups exposed to different treatments. Three assays were performed for all treatments with significance levels set at p < 0.05.
3. Results and Discussion
3.1. Influences of Thermal Treatment and TGase on Egg White Proteins
The effects of TGase (1.0 units/mL) treatment, 2-ME (0.83%) pretreatment, and thermal pretreatment (95 °C for 5 min) on egg white proteins (4 mg/mL) were investigated. Experiments were conducted under one of four conditions: (1) egg white without treatment, (2) egg white with thermal pretreatment, (3) egg white with 2-ME pretreatment and thermal pretreatment, and (4) egg white with TGase treatment. In this study, egg white samples with 2-ME pretreatment and/or thermal pretreatment were incubated at 95 °C for 5 min. After incubation, TGase was added to the samples, which were incubated for a further 1 h at 40 °C. The samples were subsequently subjected to analysis using SDS–PAGE. As shown in Figure 1, the SDS–PAGE analysis revealed that three major proteins, 210 kDa protein, 115 kDa protein, and 76 kDa protein, were observed in egg white (Figure 1, L1). We noticed that several protein bands from heated egg white were decreased on SDS–PAGE, and the intensities of protein bands corresponding to 210 kDa protein, 115 kDa protein, and 76 kDa protein in egg white with thermal treatment (95 °C for 5 min) decreased to 47.9 ± 2.0, 64.2 ± 3.4, and 34.5 ± 2.6%, respectively. However, an increase in high-MW polymers on the top of the gel was observed (red arrow, Figure 1, L2). The results revealed that the thermal aggregation of proteins was most likely caused by thermal treatment at 95 °C for 5 min [17,18]. These results indicate that portions of the 210 kDa protein, 115 kDa protein, and 76 kDa protein formed protein aggregates after thermal treatment. In the food system, Matsudomi et al. [19] indicated that the thermal-induced gelation of egg whites is among the great features of egg whites. Furthermore, the influences of 2-ME (0.83%, v/v) and thermal treatment (95 °C for 5 min) on 4 mg/mL egg white were studied (Figure 1, L3). The result was compared to egg white without treatment (Figure 1, L1). No significant changes in the 210 kDa protein, 115 kDa protein, or 76 kDa protein were observed.
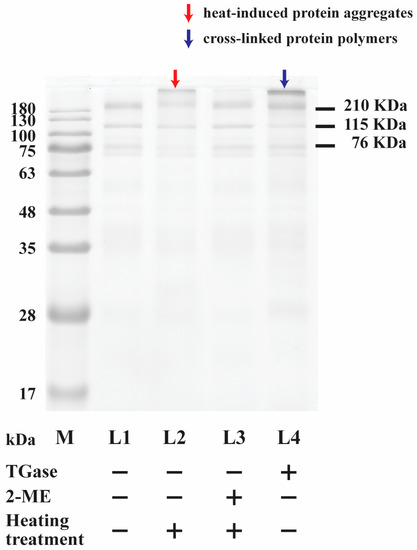
Figure 1.
SDS–PAGE image showing egg white treated with/without TGase, 2-ME, and thermal treatment (95 °C, 5 min) for 1 h at 40 °C. (L1) Egg white without treatment, (L2) egg white with thermal treatment, (L3) egg white with 2-ME (v/v) and thermal treatment, and (L4) egg white with TGase treatment. M = protein marker.
The cross-linking of egg white incubated at 40 °C with the influence of TGase (1.0 units/mL) for 1 h was studied. Compared to the proteins without TGase incubation at 40 °C for 1 h (Figure 1, L1), a small fraction of proteins was observed to polymerize into higher-molecular-weight proteins (>180 kDa) by TGase (blue arrows, Figure 1, L4). Thus, the intensities of higher-MW proteins were increased by TGase, inducing the cross-linking of egg white proteins. This finding indicates that egg white protein polymerization occurred in the presence of TGase. Simultaneously, only some of the egg white proteins were catalyzed by TGase. On the other hand, a portion of egg white proteins are inferior substrates for TGase. Some researchers have found that a portion of proteins from hen eggs can act as substrates of TGase. The secondary protein structure changed, as the contents of α-helix and β-turn forms increased with TGase treatment, whereas the β-sheet structure decreased [20]. In egg white protein, ovomucoid, some studies have demonstrated that a glutamine residue is found, and this acts as an effective acyl donor for TGase. Despite the glutamine residue being exposed, the protein on the surface of the ovomucoid structure plays the role of a substrate, but the protein flexibility and surface hydrophobicity only improve after heating treatment for TGase is performed [21]. According to a study by Jaros et al. [22], egg white proteins are characterized as globular proteins, making them less favorable substrates for TGase. Nonetheless, when egg white proteins are destabilized, their structure unfolds. This leads to the enhanced accessibility of glutamine and lysine residues by TGase, promoting the efficiency of the catalytic reaction. As a result, there is potential to enhance the susceptibility of globular proteins to TGase treatment. Djoullah et al. [8] reported that several strategies can be employed to enhance the cross-linking efficiency by TGase. These strategies include the use of reducing agents and thermal denaturation techniques.
3.2. Influences of Reductants and Thermal Treatment on Egg White Proteins
Foroumadi and Saeedi [23] demonstrated that proteins subjected to 2-ME and thermal pretreatment (95 °C and 5 min) were denatured by breaking disulfide bonds. Furthermore, an increased H0 value suggested the exposure of hydrophobic components within the protein’s globular structure, which occurred because of denaturation [24]. Therefore, we analyzed the changes in SH groups and the surface hydrophobicity (H0) of egg white samples with/without 2-ME and thermal pretreatment. As shown in Table 1, the SH groups and H0 of egg white samples without 2-ME and thermal pretreatment were 15.3 ± 3.5 (μmol/g) and 14,782.1 ± 817.9, respectively. However, the SH groups and H0 of egg white samples with 2-ME and thermal pretreatment were increased to 30.8 ± 1.8 μmol/g and 40,756.9 ± 922.7, respectively. These findings align with the observations made by Chen et al. [10]. They reported that when ovalbumin was pretreated with heat and 2-ME, followed by TGase treatment for 3 h, there was a significant increase in the SH groups in ovalbumin, from 89.3 ± 1.2 SH groups (μmol/g) to 119.5 ± 3.7 SH groups (μmol/g). Based on the experimental results, the SH group concentration and H0 of egg white samples with 2-ME and thermal pretreatment were significantly higher (p < 0.05) than those of samples without 2-ME and thermal pretreatment. Some researchers have investigated whether the H0 of thermal protein samples was obviously greater than that of unheated protein samples, and changes in H0 reflected variations in the physical and chemical properties of the protein structure by Hsiao et al. [9] and Feng et al. [25]. These results demonstrate that the structure of the egg white protein was changed and exposed to more hydrophobic groups. Moreover, after treatment with 2-ME and thermal pretreatment, egg white samples were significantly enhanced in terms of the SH group. These results indicate that 2-ME cleaved disulfide bonds on egg white proteins. Karimi et al. [26] noted that 2-ME plays an important role in stabilizing protein structures with disulfide bonds. There was a close association with the breakdown and inactivation of the protein structure and function.

Table 1.
The reactive sulfhydryl (SH) groups and surface hydrophobicity (H0) of egg white samples with/without 2-ME (0.83%) and thermal treatment (95 °C, 5 min).
3.3. SDS–PAGE Analysis of TGase on Cross-Linked Egg White Proteins with Reductants and Thermal Pretreatment
The effects of the incubation time on the cross-linking of egg white proteins with/without 2-ME pretreatment, thermal pretreatment, and TGase treatment were investigated. Egg white samples with 2-ME pretreatment and/or thermal pretreatment were incubated at 95 °C for 5 min. After incubation, TGase was added into samples, incubated at 40 °C for 0, 1, 2, or 3 h, and then analyzed by SDS–PAGE (Figure 2). As shown in Figure 2A, there were no significant changes in the egg white proteins with TGase in the 0 h incubation period, indicating that cross-linking of egg white proteins did not immediately occur. However, cross-linked high-MW polymers (>180 kDa) on the top of the gel were observed following treatment with TGase for 1 h. SDS–PAGE analysis showed that the intensities of protein bands corresponding to 210 kDa protein, 115 kDa protein, and 76 kDa protein and incubated with TGase at 40 °C for 3 h decreased to 92.1 ± 3.0, 88.6 ± 4.0, and 57.1 ± 5.3%, respectively (Figure 3). We noticed that only a portion of the egg white proteins were polymerized into high-MW protein aggregates after 3 h of incubation, which indicated that egg white proteins in their native state are not good substrates for TGase. The SDS–PAGE analysis showed that egg white proteins were subjected to heat pretreatment followed by incubation with TGase for 0 h, and heat-induced high-MW polymers (>180 kDa) were observed, indicating that the aggregation of egg white proteins occurred (Figure 2B). The polymerization of a portion of egg white proteins by TGase into higher-MW proteins after 3 h of incubation was observed; these higher-MW proteins contained egg white proteins and heat-induced and TGase-induced high-MW protein aggregates. Gharbi and Labbafi [17] reported that the intensity of some protein bands is partly caused by TGase and heating factors, which induce the cross-linking of high-molecular-weight protein polymers. Based on our results, it was found that egg white proteins are not highly suitable substrates for TGase. Nonetheless, by subjecting the proteins to heat treatment, cross-linking through TGase can still be achieved.
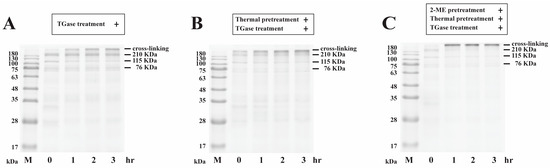
Figure 2.
SDS–PAGE image displaying the profiles of egg white samples (4 mg/mL) that underwent different treatments: with or without 0.83% 2-ME, thermal pretreatment (95 °C, 5 min), and TGase treatment (1.0 unit/mL) for 0, 1, 2, and 3 h at 40 °C. (A) Egg white with TGase treatment, (B) egg white with thermal pretreatment and TGase treatment, (C) egg white with 2-ME, thermal pretreatment, and TGase treatment. M = protein marker.
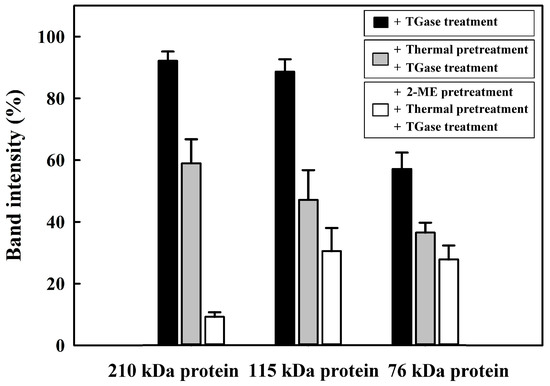
Figure 3.
Densitogram corresponding to SDS–PAGE analysis of egg white (4 mg/mL) treated with/without 2-ME, thermal pretreatment, and TGase treatment for 3 h at 40 °C.
To enhance the accessibility of egg white proteins to TGase even further, in this research, the effect of TGase on the cross-linking of egg white proteins with 2-ME and thermal pretreatment was evaluated. The egg white proteins were pretreated with 2-ME and thermal pretreatment, followed by incubation with TGase for 3 h at 40 °C, and cross-linked high-MW polymers were observed (Figure 2C). The intensities of the protein bands on SDS–PAGE corresponding to the 210 kDa protein, 115 kDa protein, and 76 kDa protein were decreased to 9.2 ± 1.5, 30.5 ± 7.5, and 27.8 ± 4.5%, respectively (Figure 3). Compared to thermal pretreatment or thermal pretreatment and TGase treatment, the combined use of 2-ME, thermal pretreatment, and TGase treatment caused the egg white proteins to aggregate more quickly. For the above experimental results, TGase induced egg white proteins, including 210 kDa protein, 115 kDa protein, and 76 kDa protein to undergo polymerization at the following rates after 3 h: TGase treatment < thermal pretreatment and TGase treatment < 2-ME, thermal pretreatment, and TGase treatment. Our findings demonstrate that the denaturation of egg white proteins, specifically the 210, 115, and 76 kDa proteins, through 2-ME and thermal pretreatment, significantly enhanced their enzymatic cross-linking degree by TGase. In this research, the results were consistent with those of Jaros et al. [22], who pointed out that, in the natural state, several spherical proteins are not good substrates for TGase. The experimental results demonstrated that TGase was cross-linked after thermal treatment and/or the addition of a reductant.
3.4. Particle Size and Microstructure Analysis of Egg White Proteins with 2-ME, Thermal Pretreatment, and TGase
After incubation at 40 °C for 0 and 3 h, the particle size distributions of egg white proteins after 2-ME, thermal pretreatment, and TGase treatment were examined (Figure 4). For the average particle size of ovalbumin, there was a 30% yield for particles sized from 0 to 200 nm in 0 h. For the average particle size polymerization on egg white proteins, there was a 67% yield of particles sized between 200 and 2000 nm. However, thermal-induced aggregation of high-MW egg white proteins resulted in a particle yield of 3% in the 2000–10,000 nm range (Figure 4A). For the average particle size of egg white protein, there was a 21% yield of 0–200 nm particles after 3 h. For the average particle size of aggregated egg white proteins, there was a 47% yield of particles sized between 200 and 2000 nm. In contrast, for thermal-induced and TGase-induced large protein aggregates, there was a 32% yield of 2000–10,000 nm particles (Figure 4B). After 3 h of incubation, these results demonstrated that TGase-polymerized egg white protein, polymerized egg white protein, and thermally induced egg white protein form high-molecular-weight aggregates sized between 2000 and 10,000 nm. Chen et al. [10] first denatured ovalbumin by pretreatment with 2-ME and heat and then incubated the product with TGase at 40 °C. Following a 3 h incubation period, TGase induced the polymerization of a fraction of aggregated ovalbumin and denatured ovalbumin to form cross-linked ovalbumin polymers. According to the findings of Giosafatto et al. [27], denatured proteins exhibit greater flexibility compared to their native forms, rendering them more accessible to TGase-induced cross-linking. Bashash et al. [28] illustrated that egg white protein is a naturally occurring polymer that is used to make protein hydrogels, and a driving force is initially needed to open the native protein structure, which allows aggregation. The action of 2-ME results in the breaking of disulfide bonds and the formation of sulfhydryl groups in egg white protein. In egg white proteins, sulfhydryl and disulfide bonds play key roles in thermally induced gelation. Among them, the SH group participates in disulfide-bond-stabilized protein aggregation [29]. Lysine and glutamine residues are sensitized to TGase responses, and preheating the egg white proteins causes the egg white proteins to irreversibly unfold, as reported by Alavi et al. [5].
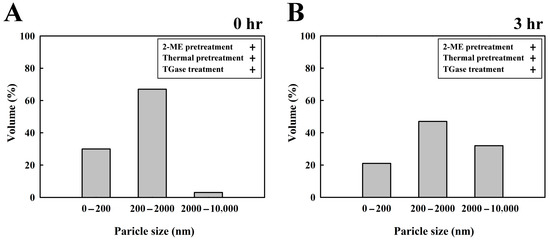
Figure 4.
Particle size distribution of egg white (4 mg/mL) with 2-ME, thermal pretreatment, and TGase treatment for 0 and 3 h at 40 °C. (A) Egg white treated for 0 h, (B) egg white treated for 3 h. The experiments on the samples were performed in triplicate.
After 2-ME treatment, thermal pretreatment, and TGase treatment, the microstructure of egg white proteins incubated at 40 °C for 0 and 3 h was analyzed (Figure 5). In the TEM images, aggregation is shown by green arrows, and thermal induction is shown by red arrows. Egg white protein aggregates were observed at 0 h (Figure 5A). The cross-linked network structure shown by the blue arrow of the TGase egg white protein polymer was formed by egg white protein. Aggregated egg white proteins are indicated by green arrows, and thermally induced egg white proteins are indicated by red arrows after 3 h of incubation (Figure 5B). Through 2-ME and thermal pretreatment, egg white proteins were denatured, which increased the degree of enzymatic cross-linking of egg white proteins with TGase. Therefore, in this research, the results show that 2-ME split egg white protein disulfide bonds into sulfhydryl groups. In native proteins, thermal pretreatment exposed the original hydrophobic groups, forming thermally induced protein aggregation through hydrophobic interactions. Then, in lysine and glutamine residues, TGase catalyzed the transfer of acyl groups, leading to the constitution of covalently cross-linked ovalbumin and enhanced protein aggregation.
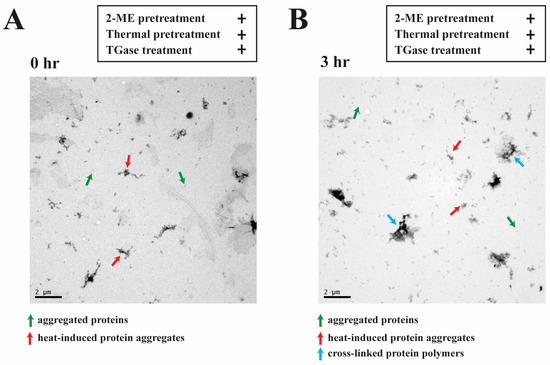
Figure 5.
Transmission electron microscopy of egg white (4 mg/mL) with 2-ME, thermal pretreatment, and TGase treatment for 0 and 3 h at 40 °C. (A) Egg white treated for 0 h, (B) egg white treated for 3 h.
3.5. Reaction Scheme for TGase on Cross-Linked Egg White Proteins with 2-ME and Thermal Pretreatment
Asgharzadeh et al. [30] reported that food proteins may be polymers resulting from cross-linking induced by TGase from lower-molecular-weight proteins. The 210 kDa protein, 115 kDa protein, and 76 kDa protein are the major proteins in egg white. In this research, pretreating these proteins with 2-ME and thermal pretreatment followed by TGase treatment was investigated. After 3 h of incubation, according to the TEM, particle size, and SDS–PAGE results, TGase-induced egg white protein aggregation was observed. Thus, in this research, the reaction scheme of 2-ME pretreatment and TGase thermal pretreatment on the egg white protein cross-linking process was proposed (Figure 6). Egg white proteins with 2-ME pretreatment, thermal pretreatment, and TGase treatment were incubated at 40 °C for 0 h (Figure 6, left). The mean particle sizes of egg white proteins, aggregated high-MW proteins, and thermally induced high-MW proteins were 0–200, 200–2, and 2000–10,000 nm, respectively. Following 3 h of incubation, a fraction of the egg white proteins aggregated, and thermally induced high-MW proteins were polymerized to cross-linked high-MW protein polymers (>180 kDa, 2000–10,000 nm) induced by TGase (Figure 6, right). The average particle sizes of egg white protein, aggregated, heat-induced HMW protein, and TGase-induced HMW protein were 0–200, 200–2000, 2000–10,000, and 2000–10,000 nm, respectively. Thus, in this research, the cross-linking of egg white proteins, including 210 kDa protein, 115 kDa protein, and 76 kDa protein, was achieved through catalysis by 2-ME and thermal pretreatment with TGase.
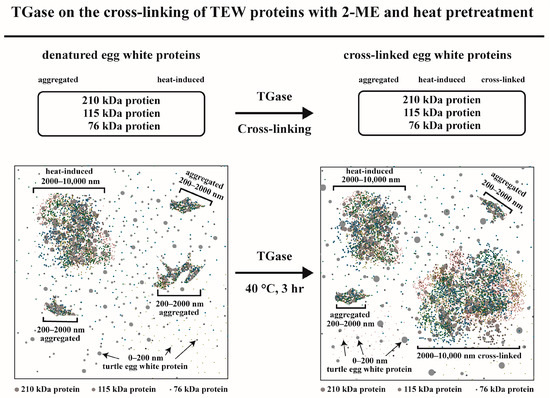
Figure 6.
Reaction scheme for TGase showing the cross-linking of egg white proteins with 2-ME and thermal pretreatment for 0 and 3 h at 40 °C.
4. Conclusions
We examined the influence of utilizing TGase with a reducing agent and thermal pretreatment on the cross-linking of turtle egg white proteins. Our results reveal that egg white proteins exhibit low reactivity as substrates for TGase. The denaturation of turtle egg white proteins, including the 210, 115, and 76 kDa proteins, through 2-ME and thermal pretreatment, resulted in a substantial enhancement in the level of enzymatic cross-linking by TGase. Following 3 h of incubation, polymerization and cross-linking of the denatured egg white proteins were observed. Thus, the cross-linking of egg white proteins was significantly improved using TGase in combination with 2-ME and heat pretreatment. Our results indicate that turtle egg white proteins can, in fact, serve as good substrates for TGase after thermal and 2-ME pretreatment. The level of enzymatic cross-linking in egg white proteins was elevated by utilizing TGase in combination with 2-ME and thermal pretreatment. However, the use of 2-ME would likely not pass food safety standards. Therefore, safer reducing agents, such as NADPH-sulfite reductase and L-cysteine, can be considered as replacements for 2-ME.
Author Contributions
Conceptualization, J.-F.H.; data curation, C.-H.J. and C.-J.C.; formal analysis, M.-C.K.; funding acquisition, J.-F.H.; methodology, M.-I.K.; supervision, J.-F.H.; validation, Y.L.; writing—original draft, C.-C.C. and J.-F.H.; writing—review and editing, J.-F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology in Taiwan through grant support (MOST 109-2221-E-030-003-MY2 and MOST 111-2221-E-030-006-MY3).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Ministry of Science and Technology in Taiwan for their grant support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, C.T.; Hsu, S.L.; Chang, C.M.J. Co-solvent-modified supercritical carbon dioxide extractions of cholesterol and free amino acids from soft-shell turtle fish egg. Sep. Purif. Technol. 2008, 60, 215–222. [Google Scholar] [CrossRef]
- Nong, N.T.P.; Chen, Y.K.; Shih, W.L.; Hsu, J.L. Characterization of novel dipeptidyl peptidase-IV inhibitory peptides from soft-shelled turtle yolk hydrolysate using orthogonal bioassay-guided fractionations coupled with in vitro and in silico study. Pharmaceuticals 2020, 13, 308. [Google Scholar] [CrossRef]
- Rawendra, R.D.; Chang, C.I.; Chen, H.H.; Huang, T.C.; Hsu, J.L. A novel angiotensin converting enzyme inhibitory peptide derived from proteolytic digest of Chinese soft-shelled turtle egg white proteins. J. Proteom. 2013, 94, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Kuo, M.I. Physicochemical and functional properties of Chinese soft-shell turtle (Pelodiscus sinensis) egg. Food Res. Int. 2016, 85, 36–43. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Chen, L. Acid-induced gelation of thermal co-aggregates from egg white and hempseed protein: Impact of microbial transglutaminase on mechanical and microstructural properties of gels. Food Hydrocoll. 2020, 107, 105960. [Google Scholar] [CrossRef]
- Ma, X.; Lozano-Ojalvo, D.; Chen, H.; Lopez-Fandiño, R.; Molina, E. Effect of high pressure-assisted crosslinking of ovalbumin and egg white by transglutaminase on their potential allergenicity. Innov. Food Sci. Emerg. Technol. 2015, 29, 143–150. [Google Scholar] [CrossRef]
- Miwa, N. Innovation in the food industry using microbial transglutaminase: Keys to success and future prospects. Anal. Biochem. 2020, 597, 113638. [Google Scholar] [CrossRef]
- Djoullah, A.; Husson, F.; Saurel, R. Gelation behaviors of denaturated pea albumin and globulin fractions during transglutaminase treatment. Food Hydrocoll. 2018, 77, 636–645. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Lu, C.P.; Kuo, M.I.; Hsieh, J.F. Coacervation of β-conglycinin, glycinin and isoflavones induced by propylene glycol alginate in heated soymilk. Food Chem. 2016, 200, 55–61. [Google Scholar] [CrossRef]
- Chen, C.C.; Kao, M.C.; Chen, C.J.; Jao, C.H.; Hsieh, J.F. Improvement of enzymatic cross-linking of ovalbumin and ovotransferrin induced by transglutaminase with heat and reducing agent pretreatment. Food Chem. 2023, 409, 135281. [Google Scholar] [CrossRef]
- Gaspar, A.L.C.; de Góes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Eissa, A.S.; Khan, S.A. Modulation of hydrophobic interactions in denatured whey proteins by transglutaminase enzyme. Food Hydrocoll. 2006, 20, 543–547. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A.; Kundu, S.; Saha, S.; Sarkar, H.S.; Sahoo, P. Development of a new fluorescent probe for cysteine detection in processed food samples. Anal. Bioanal. Chem. 2019, 411, 6203–6212. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Winter, D. Systematic evaluation of protein reduction and alkylation reveals massive unspecific side effects by iodine-containing reagents. Mol. Cell. Proteom. 2017, 16, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, L.Y.; Li, W.T.; Chang, K.L.; Tseng, H.W.; Chen, B.Y.; Chen, C.J.; Hsieh, J.F. Ultrasound-assisted transglutaminase catalysis of the cross-linking and microstructure of αs-casein, β-casein and κ-casein. Processes 2021, 9, 1630. [Google Scholar] [CrossRef]
- Stefanović, A.B.; Jovanović, J.R.; Dojčinović, M.B.; Lević, S.M.; Nedović, V.A.; Bugarski, M.B.; Knežević-Jugović, Z.D. Effect of the controlled high-intensity ultrasound on improving functionality and structural changes of egg white proteins. Food Bioprocess Technol. 2017, 10, 1224–1239. [Google Scholar] [CrossRef]
- Gharbi, N.; Labbafi, M. Effect of processing on aggregation mechanism of egg white proteins. Food Chem. 2018, 252, 126–133. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Acevedo, N.C. Effects of pre-heating soybean protein isolate and transglutaminase treatments on the properties of egg-soybean protein isolate composite gels. Food Chem. 2020, 318, 126421. [Google Scholar] [CrossRef] [PubMed]
- Matsudomi, N.; Kanda, Y.; Yoshika, Y.; Moriwaki, H. Ability of αs-casein to suppress the heat aggregation of ovotransferrin. J. Agric. Food Chem. 2004, 52, 4882–4886. [Google Scholar] [CrossRef]
- Marcet, I.; Sáez, S.; Rendueles, M.; Díaz, M. Edible films from residual delipidated egg yolk proteins. J. Food Sci. Technol. 2017, 54, 3969–3978. [Google Scholar] [CrossRef]
- Porta, R.; Giosafatto, C.V.L.; di Pierro, P.; Sorrentino, A.; Mariniello, L. Transglutaminase-mediated modification of ovomucoid: Effects on its trypsin inhibitory activity and antigenic properties. Amino Acids 2013, 44, 285–292. [Google Scholar] [CrossRef]
- Jaros, D.; Partschefeld, C.; Henle, T.; Rohm, H. Transglutaminase in dairy products: Chemistry, physics, applications. J. Texture Stud. 2006, 37, 113–155. [Google Scholar] [CrossRef]
- Foroumadi, A.; Saeedi, M. 2-Mercaptoethanol. In Encyclopedia of Toxicology; Wexler, P., Ed.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 201–202. [Google Scholar]
- Wang, Z.; Li, Y.; Jiang, L.; Qi, B.; Zhou, L. Relationship between secondary structure and surface hydrophobicity of soybean protein isolate subjected to heat treatment. J. Chem. 2014, 2014, 475389. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef]
- Karimi, M.; Ignasiak, M.T.; Chan, B.; Croft, A.K.; Radom, L.; Schiesser, C.H.; Pattison, D.I.; Davies, M.J. Reactivity of disulfide bonds is markedly affected by structure and environment: Implications for protein modification and stability. Sci. Rep. 2016, 6, 38572. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Rigby, N.M.; Wellner, N.; Ridout, M.; Husband, F.; Mackie, A.R. Microbial transglutaminase-mediated modification of ovalbumin. Food Hydrocoll. 2012, 26, 261–267. [Google Scholar] [CrossRef]
- Bashash, M.; Varidi, M.; Varshosaz, J. Ultrasound-triggered transglutaminase-catalyzed egg white-bovine gelatin composite hydrogel: Physicochemical and rheological studies. Innov. Food Sci. Emerg. Technol. 2022, 76, 102936. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. J. Agric. Food Chem. 2005, 53, 5726–5733. [Google Scholar]
- Asgharzadeh, S.; Shareghi, B.; Farhadian, S.; Tirgir, F. Effect of free L-cysteine on the structure and function of α-chymotrypsin. J. Mol. Liq. 2019, 280, 79–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).