Abstract
Colorectal cancer (CRC) is one of the most common cancers in the world. When treating patients, therapeutic agents have side effects; hence, the use of natural compounds found in medicinal plants including pomegranate. Ultrasound assisted extraction (UAE) is a new technique evolving to the detriment of traditional methods such as maceration. In this study, we investigated the antioxidant and anticancer effect of pomegranate peel extracts obtained by maceration and UAE at three different ultrasonic power levels (P1 = 10 W; P2 = 50 W; P3 = 100 W) on HCT-116 colorectal cancer cells. Phytochemical screening highlighted the presence of primary and secondary metabolites in pomegranate peels. In addition, the ethanolic extract obtained by UAE at 50 W was shown to be the most concentrated in phenolic and flavonoid compounds and have the most powerful antioxidant activity, which reached a maximum activity of 92% as determined by DPPH test. Similarly, the MTT cell viability test showed that the extract obtained by UAE at 50 W had the most potent inhibitory effect compared to the other extracts. In conclusion, the UAE at 50 W was shown to be the most suitable and efficient extraction technique to obtain bioactive compounds from pomegranate peel extracts that can be used in the treatment of CRC.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related deaths worldwide, where it accounts for nearly 1 million deaths per year, in 2020 [1].
Researchers predict that the burden of CRC will increase from 1.9 million cases in 2020 to 3.2 new million cases and 1.6 million deaths a year globally, constituting an increase of 63% and 73%, respectively [2,3]. Early stage screening procedures do have the potential, however, to significantly reduce the incidence and mortality of CRC and improve survival rates [3]. The World Health Organization (WHO), the American Cancer Society, and the Agency for Health Care Policy and Research (AHCPR) have recommended an annual fecal occult blood test (FOBT) and sigmoidoscopy every 5 years and colonoscopy every 10 years in adults aged 50 and over in an effort to decrease CRC incidence and mortality [4,5].
Significant progress has been made in the treatment of CRC with the approval of new synthesized therapeutic agents that improve the condition of patients diagnosed with resectable or metastatic tumors [6]. However, these treatments are linked to many serious side effects, such as diarrhea, myelosuppression, alopecia, severe neutropenia, reversible hypertension, and proteinuria, among others [6,7]. Therapeutic approaches using plant sources have been increasingly popular in recent years, as safer alternatives with lessened side effects and long-term positive results, compared with conventional anticancer drugs. Moreover, a number of widely used and successful anticancer therapeutics originate from natural sources, such as camptothecin, taxol, paclitaxel, and vinblastine, thus highlighting the importance of plant extracts in the identification and development of drug leads [8,9].
Punica granatum L., also known as pomegranate, is an edible fruit that is consumed fresh or processed into juice, and it is native to several Asian countries; it has been cultivated and naturalized over the whole Mediterranean region, including Afghanistan, India, China, Japan, Russia, and the United States [10,11]. The peel takes up about 50% of the total weight of the fruit, and it generates a large amount of waste [12]. The chemical composition of pomegranate shows high concentrations of primary and secondary metabolites, which are particularly observed in the peel. As such, pomegranate peels are essentially rich in polyphenols, such as flavonoids (catechin, gallate, quercetin, rutin, kaempferol, luteolin, naringenin), anthocyanins (cyanidin, delphinidin, pelargonidin), ellagitannins (punicalin, punicalagin, casuarinin, corilagin, granatin), hydroxybenzoic, and hydroxycinnamic acids (gallic acid, ellagic acid, caffeic acid, chlorogenic acid); they are also rich in dietary fibers (galactose, cellulose), in addition to other components, such as minerals, glycosides, and alkaloids [13,14]. These metabolites have the ability to trap reactive oxygen species (ROS) and prevent carcinogenesis in vivo and in vitro [15]. Pomegranate has been widely used in traditional medicine and fold remedies for the treatment of chronic diseases, such as type 2 diabetes, atherosclerosis, cardiovascular and inflammatory diseases, and several types of cancer [16,17,18,19]. In addition, pomegranate peel has been shown to be effective in treating ulcers, diarrhea, dysentery, intestinal parasites, and gum disease [20].
Conventional extraction methods, including hydro-distillation, maceration, percolation, and high-pressure water extraction techniques, have been widely applied in natural product extractions from plant tissues [21]. However, these methods have several disadvantages, including prolonged extraction time, the use of large volumes of solvents or toxic solvents, non-optimal protocols, and, especially, the loss of quality of thermally sensitive bioactive molecules [22,23].
Concurrently, there has been an increasing interest in the optimization of techniques for the extraction and isolation of bioactive compounds from natural plant sources. For example, new extraction methods such as ultrasound assisted extraction (UAE), allows lower energy consumption while maintaining the quality of the final extract [23]. This process is affected by several adjustable variables, which include the solvent, the frequency, sonication power, temperature, and time [24]. Indeed, these parameters vary depending on the sample and on the target molecule, and they require precise control for optimal extraction in order to obtain the best possible quality of the extract, combined with the knowledge that the chemical composition differs within the same species according to the cultivar, growing region, climate, maturity, cultural practice, and storage, which all affect biological properties [25].
The main objective of this study is to find new approaches to treat colorectal cancer from natural compounds extracted from pomegranate peel and to prove their antioxidant and anticancer activities, specifically on HCT-116 colorectal cancer cells. In addition, this study aims to highlight the importance of the UAE technique compared to the traditional maceration extraction method and to optimize ultrasound parameters to maximize the extraction and quality of bioactive molecules in pomegranate peel from the local ecotype grown in the Middle East area, Lebanon.
2. Materials and Methods
2.1. Powder Preparation
Pomegranate fruits were obtained from the supermarket. They were peeled, and the seeds were removed. Peels were then washed with distilled water and dried in the shade at room temperature. The obtained dried peels were milled and stored in a desiccator until further use.
2.2. Reagents and Chemicals
All chemicals used were of analytical grade. Absolute ethanol and methanol were purchased from BDH England. Aluminum chloride was purchased from Merck Germany. Gallic acid, Folin–Ciocalteau reagent, Rutin, and DPPH were purchased from Sigma Aldrich, St. Louis, MO, USA. Phosphate buffer solution (PBS) was purchased from Gibco, UK. The HCT-116 cell line was obtained from ATCC.
2.3. Extraction by Maceration
A total of 70% ethanol was added to pomegranate peel powder at a ratio of 10:1 (v/w), macerated for 35 min at room temperature with gentle shaking, and then filtered under vacuum. The filtrate was roto-evaporated at 40 °C and freeze-dried to obtain the extracted powder, which was stored at −20 °C until further use.
2.4. Ultrasound Extraction
Ultrasound extraction was completed using the “Branson sonifier 250” sonicator.
An amount of 70% ethanol was added to pomegranate peel powder at a ratio of 10:1 (v/w) and sonicated at ultrasonic powers of 10 W, 50 W, or 100 W for 35 min on ice and then filtered under vacuum. The filtrate was roto-evaporated and freeze dried to obtain the extracted powder, which was stored at −20 °C until further use.
2.5. Calculation of Yield
The yield was calculated using the following equation:
where m2 = mass of the flask with dry extract, m1 = mass of the empty flask, and m0 = initial mass of the sample.
2.6. Qualitative Study—Phytochemical Screening
The chemical compositions of pomegranate extracts were determined using the qualitative tests shown in Table 1.

Table 1.
Qualitative detection of primary and secondary metabolites of pomegranate peel extracts.
2.7. Quantitative Study
2.7.1. Total Phenolic Compounds (TPC)
- −
- Preparation of the standard gallic acid curve:
The total phenolic content was determined using the Folin–Ciocalteu method [26]. A standard curve was first performed using gallic acid as the molecule of reference. Different concentrations of gallic acid solutions (3.125; 6.25; 12.5; 25; 50; 100, and 200 μg/mL) were prepared by serial dilution from a stock solution of 400 μg/mL. The powder form of gallic acid was dissolved in distilled water. A total of 100 μL of each gallic acid concentration was mixed with 500 μL of the Folin reagent (10%). After 5 min of incubation, 2 mL of sodium carbonate 7% Na2CO3 were added, thoroughly mixed by vortex, and then incubated for 30 min in the dark at room temperature. The blank was composed of 100 μL of distilled water, 500 μL 10% Folin (10%), and 2 mL of Na2CO3. The experiment was carried out in triplicate. The absorbance of the solutions was then read on the Hitachi U-2900 UV-Vis spectrophotometer at 765 nm.
- −
- Preparation of extracts:
A concentration of 1 mg/mL of each extract was prepared. An amount of 500 μL of the Folin reagent (10%) was added to 100 μL of each extract and then incubated for 5 min at room temperature. Then, 2 mL of Na2CO3 (7%) were added, mixed, and incubated for 30 min in the dark at room temperature. Each extract was made in triplicate. The blank was composed of 100 μL of solvent (70% ethanol), 500 μL 10% Folin, and 2 mL of Na2CO3. The absorbance of all samples was then read on the Hitachi U-2900 UV-Vis spectrophotometer at 765 nm.
The phenolic content of each extract was calculated using the following equation:
where GAE = gallic acid equivalent, V = extract volume, D = dilution factor, and m = extract weight.
2.7.2. Total Flavonoid Compounds (TFC)
- −
- Preparation of the standard rutin calibration curve:
The calibration curve was prepared using rutin as a standard as described in Quettier-Deleu et al. (2000) [27]. Different concentrations of rutin solutions (3.125; 6.25; 12.5; 25; 50 and 100 μg/mL) were prepared by serial dilution from a stock solution of 400 μg/mL. Rutin in powder form was dissolved in methanol. An amount of 1 mL of 2% methanolic aluminum chloride was added to 1 mL of each concentration. The solutions were mixed by vortexing and incubated for 30 min in the dark at room temperature. The experiment was carried out in triplicate. The blank was composed of 1 mL of methanol mixed with 1 mL of 2% methanolic aluminum chloride. The absorbance was then read on the Hitachi U-2900 UV-Vis spectrophotometer at 415 nm.
- −
- Preparation of extracts:
A concentration of 1 mg/mL of each extract was prepared. An amount of 1 mL of each extract was mixed with 1 mL of 2% methanolic aluminum chloride. All samples were incubated for 30 min in the dark at room temperature. Each extract was prepared in triplicate. The blank was composed of 1 mL 70% ethanol and 1 mL of 2% methanolic aluminum chloride. The absorbance of all extracts was then read on the Hitachi U-2900 UV-Vis spectrophotometer at 415 nm.
The flavonoid content of each extract was calculated using the following equation:
where RE = rutin equivalent, V = extract volume, D = dilution factor, and m = extract weight.
2.8. Evaluation of Anti-Oxidant Activity by DPPH Test and Anticancer Activities
To measure the antioxidant activity of the extracts, the 2,2-diphenyl-1-picryl-hydrazyl (DPPH) test was applied as described in Farhan et al. [28]. A concentration of 0.15 nM DPPH was prepared by dissolving 3 mg of DPPH powder in 50 mL of absolute ethanol. Increasing concentrations (5; 25; 50; 100; 200; 300; 400; 500, and 600 μg/mL) of each extract were prepared by dilution from a stock solution of 1 mg/mL. For each extract, 1 mL of each concentration was mixed with 1 mL of DPPH (0.15 nM). A control was prepared by mixing 1 mL of ethanol (70%) with 1 mL of DPPH. The blank was composed only of 70% ethanol. All solutions were mixed and then incubated for 30 min in the dark at room temperature. The absorbances of all samples were read on the Hitachi U-2900 UV-Vis spectrophotometer at 517 nm. The rate of scavenging DPPH radicals by extracts was expressed as a percentage of inhibition according to the following equation:
where Ac = absorbance of the control, and Ae = absorbance of the extract.
The IC50 of each extract was determined (IC50 is the concentration required to scavenge 50% of DPPH).
2.9. Evaluation of Antiproliferative Activity by Cell Culture
2.9.1. Trypan Blue Cell Count
RPMI culture medium was removed from the plate containing the HCT-116 adherent cells and rinsed with 3 mL of PBS. Then, 1 mL of trypsin was added and incubated at 37 °C for 2 to 3 min, which was then inhibited by adding 2 mL of the culture medium. The cells were centrifuged for 5 min at 1200 rpm at 20 °C, the supernatant was discarded, and the pellet was re-suspended in 6 mL of RPMI. Afterwards, 10 μL of the pellet suspension were mixed with 10 μL of trypan blue, and cells were counted using a Neubauer hemocytometer slide under the microscope.
2.9.2. MTT Cell Viability Test
HCT-116 cells were seeded in 96-well plates at a density of 1 × 104 cells per well and incubated at 37 °C for 24 h in a 5% CO2 incubator. The culture medium was then discarded, and the cells were treated with increasing concentrations of each extract for a total period of 72 h. Cell viability was determined by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), which was added to each well and incubated for 3 h. Then, isopropanol was added to dissolve the purple formazan crystals formed, and the plate was agitated for 15 min. Finally, the absorbance was read by Elisa at a wavelength of 570 nm, and the rate of cell proliferation was determined according to the following equation:
where Ae = absorbance of experimental wells, and Ac = absorbance of control wells.
2.10. Statistical Analysis
Three independent experiments were conducted to determine the mean + SD.
3. Results
3.1. Extraction Yield
Both maceration and ultrasonic extraction techniques were applied to pomegranate peel samples using 70% ethanol as a solvent for a duration of 35 min. In addition, three ultrasonic powers (10 W; 50 W; and 100 W) were applied to optimize ultrasound extraction conditions. The extraction yields of the different extracts were obtained as described in Equation (1).
Figure 1 shows that the yields of the different extracts varied depending on the extraction method used. In addition, different efficiency rates were obtained with the UAE technique depending on the ultrasonic powers applied. The results obtained showed that the maceration extraction had the lowest yield (R = 6.61%), while UAE was more efficient. Particularly, the highest efficiency was obtained at an ultrasonic power of 50 W (R = 61.67%), while the other powers applied (10 W and 100 W) gave lower efficiencies (53.89% and 60.08%, respectively).
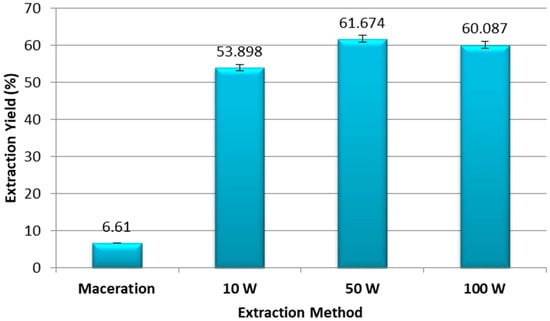
Figure 1.
Percentage of extraction yield from ethanolic extracts of pomegranate peel obtained by maceration and ultrasound assisted extraction at 10 W, 50 W, and 100 W. Experiments were repeated three times (n = 3). Bars display mean ± S.D.
3.2. Phytochemical Screening
Phytochemical screening of the pomegranate peel ethanolic extracts obtained by maceration and UAE was based on a set of qualitative tests based on the chemical reactions between the extracts and the reagents listed in Table 1. These reactions highlight the presence or absence of primary and secondary metabolites based on a change in color detectable to the naked eye. Here, we showed that the ethanolic extracts of pomegranate peel obtained by maceration and UAE were rich in primary and secondary metabolites. The ethanolic extracts obtained by maceration and UAE techniques (10 W; 50 W; and 100 W) all contained phenols, terpenoids, flavonoids, reducing sugars, proteins and amino acids, alkaloid, sterols, and steroids. Flavanones were only observed in the ethanolic extracts obtained using UAE. In addition, none of the extracts contained resin, quinones, anthraquinones, phlabotannins, or saponins.
3.3. Quantitative Tests
Total Flavonoid Compounds (TFC)
The total level of phenols in the pomegranate peel extracts were measured by the Folin–Ciocalteu colorimetric method, which is based on a mixture of tungstate and molybdate. The phenolic compounds present in the solution reduce the heteropolyphosphotungstate–molybdate complexes, thus resulting in the formation of a blue chromogen that is measured by spectrophotometry. The results obtained were based on the gallic acid calibration curve, which was the phenolic molecule of reference used. The gallic acid equivalence (GAE) of each extract was determined by the following equation according to the calibration curve:
y = 0.0039x + 0.0082; R2 = 0.9981
The total phenolic compounds (TPC) present in the extracts were calculated using Equation (2) and expressed in mg GAE/g of ethanolic extract of pomegranate peel (Figure 2).
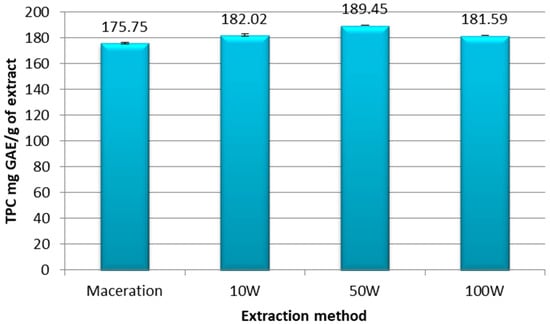
Figure 2.
Total phenolic compounds expressed in mg GAE/g extract of ethanolic extracts of pomegranate peel obtained by maceration or ultrasound at a power of 10 W; 50 W; and 100 W. Experiments were repeated three times (n = 3). Bars display mean ± S.D.
Our results indicate that the levels of phenolic compounds present in the extracts depended on the extraction method used and the ultrasonic power applied. The UAE technique at the three different ultrasonic powers yielded samples richer in phenolic compounds compared to the maceration extract, which was the least concentrated in phenol with a TPC = 175.75 mg GAE/g of extract. More specifically, UAE at 10 W contained 182.02 mg GAE/g, which increased to 189.45 mg GAE/g of extract with the 50 W power. The TPC level then dropped to 181.59 mg GAE/g of extract when the power was increased to 100 W, as is shown in Figure 2, which may have been due to the degradation of phenolic compounds exposed to higher ultrasonic power.
Flavonoids are secondary metabolites belonging to the polyphenol family and are an important class of natural products and biologically active compounds. The aluminum chloride colorimetric method was used to estimate flavonoid levels in the pomegranate peel extracts. Indeed, aluminum chloride forms stable complexes with the ketone or hydroxyl groups of flavones and flavonols, thus giving a yellow coloration to the solution. The results obtained were based on the rutin calibration curve, which was the flavonoid reference molecule used. The rutin equivalence (RE) of each extract was determined by the following equation according to the calibration curve:
The total flavonoid compounds (TPC) in the extracts were obtained by applying the Equation (3) and were expressed in mg RE/g of ethanolic extract of pomegranate peel, as is shown in Figure 3.
y = 0.0149x − 0.0052; R2 = 0.9997
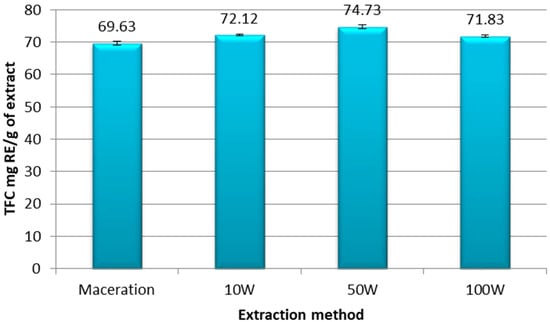
Figure 3.
Total flavonoid compounds expressed in mg RE/g of extract of ethanolic extracts of pomegranate peel obtained by maceration and UAE at 10 W, 50 W, and 100 W. Experiments were repeated three times (n = 3). Bars display mean ± S.D.
Our results showed that the maceration extraction yielded the lowest flavonoid level (TFC = 69.63 mg RE/g extract) compared to the UAE extracts at the three different powers (Figure 3). More specifically, the richest extract in flavonoids was obtained with the UAE technique at 50 W, with 74.73 mg RE/g of extract. A higher power of 100 W caused a relapse in the level of TPC in the sample (71.83 mg RE/g of extract), as is shown in Figure 3.
3.4. DPPH Antioxidant Activity
The antioxidant activity of the extracts was determined using the DPPH free radical scavenging assay, which consists of measuring the ability of the extracts to trap this radical. The inhibition percentage was calculated as described in Equation (4). Our results proved that all pomegranate peel extracts obtained by maceration and UAE exerted strong antioxidant activity in a concentration-dependent manner, thus indicating that ethanolic extracts of pomegranate peel are rich in antioxidant molecules capable of scavenging free radicals (Figure 4). The extracts obtained by maceration and UAE at 10 W and 100 W ultrasonic powers showed similar maximum antioxidant potentials, with 90.8%, 89.9%, and 90% maximum scavenging activity, respectively. On the other hand, the extract obtained by UAE at 50 W exhibited the strongest free radical scavenging activity with 92.8% at 600 μg/mL.
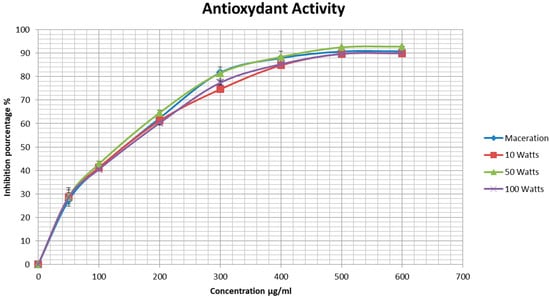
Figure 4.
Antioxidant activity of ethanolic extracts of pomegranate peels measured by the DPPH radical scavenging test. Experiments were repeated three times (n = 3). Bars display mean ± S.D.
In addition, the IC50 of each sample was determined and is presented in Table 2. The extract obtained from the UAE at 50 W had the lowest value (189.76 μg/mL) and, therefore, the strongest antioxidant activity. This was followed by the maceration extract with an IC50 value of 197.55 μg/mL. The extracts obtained by UAE at 10 W and 100 W had similar IC50 values (205.69 μg/mL and 204.98 μg/mL, respectively).

Table 2.
IC50 values of ethanolic extracts of pomegranate peels.
These results show that the extraction with an UAE at the ultrasonic power of 50 W was the richest in bioactive molecules that confer strong antioxidant activity.
3.5. MTT Antiproliferative Activity
We examined the anti-proliferative activity of the pomgranate peel ethanolic extracts against HCT-116 colorectal cancer cells using the MTT assay. The cells were assessed at 48 h and 72 h of treatment with various concentrations of the extracts. Cell viablity was determined according to Equation (5). The results showed that ethanolic extracts of pomegranate peel obtained by maceration and UAE decreased viability in a concentration- and time-dependent manner (Figure 5). For example, at 48 h of treatment, the cell viabilty of HCT-116 cells using the maceration extract at 5 μg/mL and 200 μg/mL was 80.9% and 56.16% that of the control cells, respectively. Additionally, at 72 h of treatment, it was 76.30% and 46.69% at 5 μg/mL and 200 μg/mL of extract, respectively.
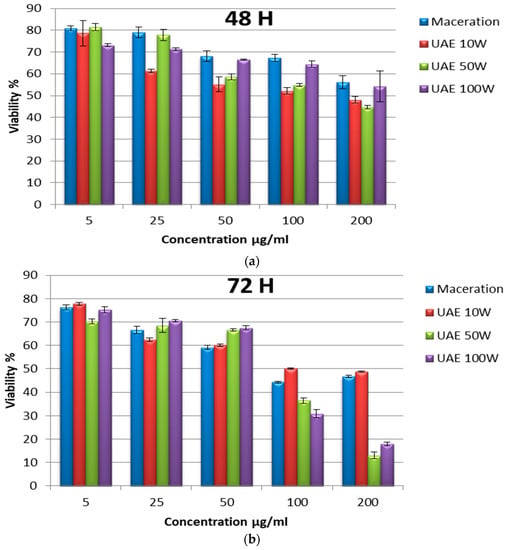
Figure 5.
Proliferation rate of HCT-116 colorectal cells treated with different concentrations of ethanolic extracts of pomegranate peels by maceration and ultrasound (10 W; 50 W; and 100 W) at 48 h (a) and 72 h (b). Experiments were repeated three times (n = 3). Bars display mean ± S.D.
Ultrasound assisted extraction at the power of 10 W exerted a slightly higher antiproliferative effect compared to the maceration extract at 48 h of treatment, with a cell viablity equal to 78.63% and 48.06% that of the control cells at 5 μg/mL and 200 μg/mL of extract, respectively. However, no significant difference was observed at 72 h of treatment.
The extract obtained by UAE at 100 W had a more effective anti-proliferative effect compared to the maceration and UAE at 10 W for extracts, particularly for those observed at 72 h of treatment. Finally, the extract obtained by UAE at 50 W exhibited the most powerful anti-proliferative effect on the HCT-116 cells. For example, cell viability using 5 μg/mL and at 200 μg/mL of extract was 81.50% and 44.72% that of control cells, respectively, at 48 h of treatment.
Because the extract obtained by UAE at 50 W exerted the highest inhibitory effect on HCT-116 colorectal cancer cell proliferation, the morphology of these cells treated with different concentrations of the extract were observed, as is depicted in Figure 6. The untreated cells were confluent. They were adherent to the wall with an epithelial morphology and with cell extensions clearly visible, while the treated cells began to lose their adhesion to the wall and showed narrowing of the extensions as the extract concentration increased. In addition, rounded and floating cells were observed in the media, which indicated cell death after 48 h and 72 h of treatment. We noticed that after 72 h of treatment, most of the cells shrank and became rounded. They detached from the wall, thus losing their adhesion capacity, especially at high concentrations (Figure 6b).

Figure 6.
Morphology of HCT-116 colorectal cancer cells treated with different concentrations of pomegranate peel ethanol extracts obtained by UAE at 50 W after 48 h (a) and 72 h (b) of treatment.
These results demonstrate that ethanolic extracts of pomegranate peels are able to inhibit the proliferation of colorectal cancer cells in a dose- and time-dependent manner; hence, they demonstrated their importance as natural anticancer agents. In addition, the extraction carried out by UAE, particularly at the ultrasonic power of 50 W, was shown to be more efficient than the maceration technique.
4. Discussion
Medicinal plants are known for their various pharmacological effects, and have been used for the prevention and treatment of human diseases since ancient times. This is attributed to the presence of primary and secondary plant metabolites, such as phenolic compounds, alkaloids, flavonoids, and tannins, etc. [29]. Of particular interest, pomegranate has been shown to have several therapeutic properties and plays a role in the prevention and treatment of various diseases, including cardiovascular diseases, diabetes, erectile dysfunction, Alzheimer’s disease, obesity, reproductive disorders, and arthritis [13].
The industrial processing of pomegranate fruits results in huge amounts of by-products, such as the pomegranate peels, which are regarded as waste in the food industry. Contextually, the utilization of these by-products for medicinal and dietary applications has been shooting up in recent decades due to their health benefits [30]. Interestingly, beside the pomegranate fruit itself, this non-edible part of pomegranate has been shown to be rich in bioactive molecules that are responsible for several biological activities, such as antimicrobial, antioxidant, anti-inflammatory, anticancer activities, among others. However, these bioactive compounds are very sensitive to environmental factors, such as heat and light, in addition to often having low solubility in water, all of which contribute to the lack of long-term stability. The challenge proposed in the pharmaceutical field is to carry out the extraction of these compounds in their active form while maintaining their chemical configuration and, consequently, their biological properties.
The phytochemical screening carried out in this study proved that ethanol (70%) is a good solvent for the extraction of bioactive molecules present in the pomegranate peel. According to the results of the statistical analysis obtained by Živković et al. [31], the most dominant factor influencing the extraction of total polyphenols was ethanol concentration. In addition, the study realized by More and Arya [21] confirmed that an ethanol–water mixture was the most appropriate solvent for the extraction of phenolic compounds from pomegranate peels. In addition, results obtained by García et al. [32] support the effectiveness of a solvent having an intermediate percentage of ethanol for the extraction of TPC from pomegranate peels using pressurized liquid extraction, but the combination of pressurized water and ethanol didn’t enhance the recovery of punicalagin. These results prove that the presence of metabolites depends on the choice of solvent used and the extraction method applied, all while taking into consideration the sample and bioactive molecules of interest [33].
New “green” extraction techniques, such as UAE, are being developed with the aim of reducing energy consumption during the extraction process, lowering operation temperatures, and using alternatives to petroleum-based solvents, in addition to keeping the process cost-effective while maintaining high safety and quality of the final extract [21,34]. In this study, the importance of ultrasound extraction and its effectiveness was highlighted compared to the conventional maceration method; in addition, the conditions under which ultrasonic extraction must take place were optimized.
The chemical tests realized in our study highlighted the presence of phenols, flavonoids, terpenoids, sterols and steroids, alkaloids, reducing sugars, and proteins in ethanolic extracts of pomegranate peels. Moreover, ultrasonic extraction gave a much higher yield compared to the maceration extract in less time. Results showed that maceration-based extraction had an efficiency of 6.61%, while, in the case of UAE, the yield exceeded 50% for the same extraction time (Figure 1). In addition, higher ultrasonic powers increased the yield obtained. If fact, ultrasound-assisted extraction has been successfully employed for the extraction of target compounds such as polyphenols, carotenoids, aromas, and polysaccharides from various plant matrices (both whole plant and by-products) [35]. Indeed, ultrasonic waves cause the formation and collapse of bubbles (cavitation phenomenon), thus producing strong mechanical effects in the medium that disrupt the plant tissue and break the cell walls, which thereby improve solvent penetration and the release of bioactive compounds. When a liquid is subjected to high pressure, van der Waals’ intermolecular forces weaken and are no longer able to maintain cohesion, which causes the formation of gas-filled microbubbles [36]. Sound waves make microbubbles unstable, causing them to collapse violently and create extreme localized conditions in the cavity. This cavity collapses and generates shock waves that produce mechanical effects, such as cell wall disruption, particle size reduction, intensive mixing, and hot spots. The cavities created induce the structural modification of plant tissues, which facilitates the penetration of the solvent into the cells, where the primary and secondary metabolites can dissolve [37]. Several studies demonstrated the efficacy of UAE compared to traditional methods [38,39]. Further studies also highlighted the importance of combining ultrasound with another extraction method, such as pressurized liquid extraction, as a clean and environmentally friendly alternative for extracting phenolic compounds from pomegranate peels [40]. Compared to other advanced extraction techniques, UAE is more economic, eco-friendly, and convenient [41]. It is a simple and fast technique that consumes little energy, time, and materials, thus producing pure products at higher yields [42]. Moreover, UAE is easy to handle, safe, and reproducible, due to the fact that it can be operated under conditions of atmospheric pressure and at an ambient temperature, which are suitable for heat-sensitive compounds [43].
Similarly, quantitative tests performed to determine the level of TPC and TFC in pomegranate peel extracts proved that ultrasonic extraction yielded extracts that were more concentrated in secondary metabolites compared to maceration extraction. In addition, increasing the ultrasonic power applied to the sample from 10 to 50 W resulted in an even more concentrated extract in phenolic and flavonoid compounds. High-intensity ultrasound can dissolve or disperse primary and secondary metabolites in the solvent to preserve the entire and natural composition of the parent plant, which leads to extracts having higher concentrations of primary and secondary metabolites [21]. However, an increase to 100 W caused a relapse in the rate of phenolic and flavonoid compounds (Figure 2 and Figure 3). This means that the most suitable power to perform UAE on pomegranate peel is 50 W.
In fact, some studies have shown that the variation in ultrasonic power can lead to a certain selectivity for target molecules [44], and a high intensity could promote the degradation of certain extracted compounds [45]. Here, it is suggested that a high power of 100 W causes the degradation and destruction of polyphenol compounds and flavonoids; hence, the fall in TPC and TFC values that were obtained. Indeed, very high ultrasonic powers, high temperature, and prolonged periods of ultrasonication in an aqueous solvent can lead to the formation of free radicals (ROS) [46]. These radicals are responsible for the degradation and destruction of phenolic compounds, since they act as reducing agents and become oxidized by these radicals.
One of the common causes that is responsible for initiating cancer is oxidative stress that takes place in cells. Free radicals that accumulate in the body at a high concentration cause oxidative stress that generates DNA damage and consequently leads to cancer. Antioxidants present in the body or in food prevent this cellular oxidation by neutralizing these free radicals and decreasing their accumulation, which consequently decreases the risk cancer initiation [47]. Secondary metabolites found in some plants, including phenolic acids, polyphenols, and flavonoids, trap free radicals, such as peroxide, hydroperoxide, or lipid peroxyl, thus inhibiting the oxidative mechanism that leads to degenerative diseases [48]. Particularly, polyphenols, such as flavonoids, are biologically active compounds that exhibit many health-promoting properties, which mainly include antioxidant, anti-inflammatory, antibacterial, and anticancer activities [49].
In our study, ethanolic extracts of pomegranate peels were shown to have a strong antioxidant activity that reached 90% at a concentration of 600 μg/mL in vitro. Moreover, the extract from UAE at 50 W was the richest sample in phenolic compounds, and it exhibited strongest antioxidant power (IC50 = 189.76 μg/mL, compared to the other two applied powers (10 W and 100 W) and the maceration extract, which had higher IC50 values (Figure 4; Table 2). These results prove that phenolic compounds and flavonoids are able to scavenge free radicals, and their abundance in an extract contributes to its antioxidant activity. García et al. identified a total of eight phenolic acids and over six flavonoids in pomegranate peels that were associated with antioxidant properties, antimicrobial activity, and cytotoxicity [32].
In addition, the results of the MTT viability test showed a positive correlation with the rates of TPC and TFC obtained. The inhibition of the proliferation of the HCT-116 colorectal cancer cell line was observed after treatment with the extracts obtained by both extraction methods, which highlights the anticancer activity of the pomegranate peel. This inhibition was dose-dependent, since higher concentrations decreased the viability rate, and it was also time-dependent, with the lowest cell viability rates observed at 72 h of treatment. The most powerful anti-proliferative effect was obtained with the UAE extract at 50 W, which was also the richest in phenolic and flavonoid compounds. The treatment of HCT-116 colorectal cells with 200 μg/mL caused a 44% decrease in cell viability after 48 h that dropped to 12% after 72 h (Figure 5). This confirms that high levels of secondary metabolites in the extract obtained by UAE at 50 W gave the extract an important anti-proliferative property against colorectal cancer.
Various epidemiological studies showed that the ingestion of a phytochemical-rich diet is associated with a decreased risk of developing colon cancer [50]. Several studies prove the potential of polyphenols in cancer treatment via several regulatory mechanisms: polyphenols promote apoptosis and cellular senescence, regulate autophagy, and inhibit the proliferation and migration of cancer cells. They are less cytotoxic to normal cells than to cancer cells. In addition, they can induce cellular stress through interaction with chemo-therapeutic agents and the reduction or reversal of multidrug resistance [49]. Polyphenols can also alter epigenetic modifications that play a crucial role in cancer prevention and treatment, such as DNA methylation and histone changes, as well as regulate the expression of non-coding miRNAs that modulate gene expression [51]. Additionally, polyphenols can alter the activity of human topoisomerase II, which is essential for the survival of actively growing tissues. This enzyme is responsible for maintaining genomic integrity and is required to cut both DNA strands, as well as to ensure proper DNA replication and chromosome segregation; hence, polyphenols are described as a potent topoisomerase II poisons [52]. Kusmardi et al. showed that the ethanolic extract of pomegranate induced apoptosis by increasing caspase-3 expression [53]. Another study conducted by Ahmed et al. demonstrated the antitumor activity of pomegranate peel extracts in CRC through the down-regulation of the Wnt/β-Catenin pathway, whose target genes play an ultimate role in tissue homeostasis, as well as in the initiation and progression of CRC through the regulation of various cellular processes, including proliferation, stem cell fate, survival, differentiation, migration, and angiogenesis [54]. It is possible that the ethanolic extracts of pomegranate peels exert their anticancer effects through similar mechanisms, which further cements their potential to develop anti-CRC cancer therapeutics.
5. Conclusions
In conclusion, the pomegranate peel, which is considered as a food-waste, is rich in bioactive molecules that are effective for the treatment of colorectal cancer. This study highlights the need to keep developing new extraction strategies, such as ultrasonic extraction, and to optimize the parameters in order to obtain high-quality extracts.
UAE is a highly efficient, safe, and emerging technique that reduces extraction time and energy consumption. UAE is indeed more efficient than the traditional maceration method, since it makes it possible to obtain higher yields in less time. UAE also provides the advantage of controlling the extraction conditions and adapting them according to the sample and the desired target molecules to be isolated.
We have shown that it is preferable to apply moderate ultrasonic powers to the sample during the extraction process in order to prevent the denaturation of bioactive molecules and to maintain the effectiveness of the extract. In the case of Lebanese pomegranate peel extract, the ultrasonic power of 50 W was the most suitable to extract active metabolites, since it had the highest concentration of phenolic and flavonoid compounds, and it provided the extract with the most powerful antioxidant and anticancer activities compared to the other extracts obtained by maceration or ultrasound at 10 W and 100 W. In addition, all pomegranate peel extracts had the ability to inhibit the proliferation of HCT-116 colorectal cancer cells after treatment for 48 h and 72 h, with the extract obtained by UAE at 50 W exerting the most toxic effect on HCT cell proliferation. The current study revealed that a Lebanese variety of pomegranate is an abundant source of bioactive compounds with promising antioxidant and anticancer properties in vitro; hence, we showed the importance of its utility in the pharmaceutical industry as potential source for natural therapeutic agents against colorectal cancer. However, this still warrants further investigation and needs to be evaluated in human clinical trials. In addition, pomegranate peel extract forms potential ingredients for the food industry. It offers important low-cost food supplements that must be included in the population diet.
Author Contributions
Conceptualization, C.H. and A.H.; investigation, K.H., A.D. and A.B.; writing—review and editing, C.H.; visualization, K.H., M.S. and E.B.; project administration, A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Anis Daou and Adnan Badran.
Acknowledgments
The authors wish to thank the Lebanese University for its financial support towards this research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colorectal Cancer Awareness Month 2022—IARC. Available online: https://www.iarc.who.int/featured-news/colorectal-cancer-awareness-month-2022/ (accessed on 5 March 2023).
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Cardoso, R.; Zhu, A.; Guo, F.; Heisser, T.; Hoffmeister, M.; Brenner, H. Incidence and Mortality of Proximal and Distal Colorectal Cancer in Germany. Dtsch. Ärztebl. Int. 2021, 118, 281–287. [Google Scholar] [CrossRef]
- Brown, J.J.; Asumeng, C.K.; Greenwald, D.; Weissman, M.; Zauber, A.; Striplin, J.; Weng, O.; List, J.M.; Farley, S.M.; Winawer, S.J. Decreased colorectal cancer incidence and mortality in a diverse urban population with increased colonoscopy screening. BMC Public Health 2021, 21, 1280. [Google Scholar] [CrossRef]
- Heiken, J.P. Colon Cancer Screening. Cancer Imaging 2001, 2, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Mayer, R.J. Systemic Treatment of Colorectal Cancer. Gastroenterology 2008, 134, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.-I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Mohan, L. Plant-Based Drugs as an Adjuvant to Cancer Chemotherapy. In Alternative Medicine—Update; Akram, M., Ed.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83962-332-5. [Google Scholar]
- Bassiri-Jahromi, S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 2018, 12, 345. [Google Scholar] [CrossRef]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-?B pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2004, 113, 423–433. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J. Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.; Dimienescu, O.; Bălan, A.; Dima, L.; Toma, S.; Bîgiu, N.; Blidaru, A. Pharmacological and Therapeutic Properties of Punica granatum Phytochemicals: Possible Roles in Breast Cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Ma, J.; Gao, W.; Zhang, L.; Li, J.; Li, J.; Zang, J. Pomegranate Peel as a Source of Bioactive Compounds: A Mini Review on Their Physiological Functions. Front. Nutr. 2022, 9, 887113. [Google Scholar] [CrossRef]
- Gómez-García, F.J.; López, A.L.; Guerrero-Sánchez, Y.; Siles, M.S.; Díaz, F.M.; Alonso, F.C. Chemopreventive effect of pomegranate and cocoa extracts on ultraviolet radiation-induced photocarcinogenesis in SKH-1 mice. PLoS ONE 2020, 15, e0232009. [Google Scholar] [CrossRef]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer Activity of Punica granatum (Pomegranate): A Review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Longo, L.; Vasapollo, G.; Bellis, L.; Miceli, A. Biochemical, antioxidant and anti-inflammatory properties of pomegranate fruits growing in Southern Italy (Salento, Apulia). Acta Aliment. 2012, 41, 190–199. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Sakr, S.H.; De Feo, V.; Camele, I. Study of Bio-Pharmaceutical and Antimicrobial Properties of Pomegranate (Punica granatum L.) Leathery Exocarp Extract. Plants 2021, 10, 153. [Google Scholar] [CrossRef]
- Suručić, R.; Tubić, B.; Stojiljković, M.P.; Djuric, D.M.; Travar, M.; Grabež, M.; Šavikin, K.; Škrbić, R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol. Cell. Biochem. 2020, 476, 1179–1193. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing Factors Affecting the Phytochemical and Nutritional Properties of Pomegranate (Punica granatum L.) Peel Waste: A Review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- More, P.R.; Arya, S.S. Intensification of bio-actives extraction from pomegranate peel using pulsed ultrasound: Effect of factors, correlation, optimization and antioxidant bioactivities. Ultrason. Sonochem. 2020, 72, 105423. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2018, 101, 342–350. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Muneeswari, S.; Naganyashree, S.; Shivamathi, C.S. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate Fruit as a Rich Source of Biologically Active Compounds. BioMed Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef] [PubMed]
- Farhan, H.; Rammal, H.; Hijazi, A.; Hamad, H.; Badran, B. Phytochemical Screening and Extraction of Polyphenol from Stems and Leaves of a Lebanese Euphorbia Macrolada Schyzoceras Boiss. Ann. Biol. Res. 2012, 3, 149–156. [Google Scholar]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Farhan, H.; Rammal, H.; Hijazi, A.; Hamad, H.; Daher, A.; Reda, M.; Badran, B. In Vitro Antioxidant Activity of Ethanolic and Aqueous Extracts from Crude Malva Parviflora L. Grown in Lebanon. Asian J. Pharm. Clin. Res. 2012, 5, 234–238. [Google Scholar]
- Kaushik, B.; Sharma, J.; Yadav, K.; Kumar, P.; Shourie, A. Phytochemical Properties and Pharmacological Role of Plants: Secondary Metabolites. Biosci. Biotechnol. Res. Asia 2021, 18, 23–35. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Petrotos, K.; Giavasis, I.; Gerasopoulos, K.; Mitsagga, C.; Papaioannou, C.; Gkoutsidis, P. Optimization of the Vacuum Microwave Assisted Extraction of the Natural Polyphenols and Flavonoids from the Raw Solid Waste of the Pomegranate Juice Producing Industry at Industrial Scale. Molecules 2021, 26, 1033. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2020, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2005, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Dent, M.; Dragovic-Uzelac, V.; Garofulic, I.E.; Bosiljkov, T.; Jezek, D.; Brncic, M. Comparison of Conventional and Ultrasound-assisted Extraction Techniques on Mass Fraction of Phenolic Compounds from Sage (Salvia officinalis L.). Chem. Biochem. Eng. Q. 2015, 29, 475–484. [Google Scholar] [CrossRef]
- Momchev, P.; Ciganović, P.; Jug, M.; Marguí, E.; Jablan, J.; Končić, M.Z. Comparison of Maceration and Ultrasonication for Green Extraction of Phenolic Acids from Echinacea purpurea Aerial Parts. Molecules 2020, 25, 5142. [Google Scholar] [CrossRef]
- Sumere, B.R.; de Souza, M.C.; dos Santos, M.P.; Bezerra, R.M.N.; da Cunha, D.T.; Martinez, J.; Rostagno, M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L.; Huang, Q.; Wang, J.; Lin, Q.; Liu, M.; Lee, W.Y.; Song, H. Ultrasonic-Assisted Extraction of Raspberry Seed Oil and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. PLoS ONE 2016, 11, e0153457. [Google Scholar] [CrossRef]
- Syahir, A.; Sulaiman, S.; Mel, M.; Othman, M.; Sulaiman, S.Z. An Overview: Analysis of ultrasonic-assisted extraction’s parameters and its process. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 12165. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Miljevic, B.; Hedayat, F.; Stevanovic, S.; Fairfull-Smith, K.; Bottle, S.; Ristovski, Z.D. To Sonicate or Not to Sonicate PM Filters: Reactive Oxygen Species Generation Upon Ultrasonic Irradiation. Aerosol Sci. Technol. 2014, 48, 1276–1284. [Google Scholar] [CrossRef]
- Muangnoi, C.; Phumsuay, R.; Jongjitphisut, N.; Waikasikorn, P.; Sangsawat, M.; Rashatasakhon, P.; Paraoan, L.; Rojsitthisak, P. Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 4722. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cheregi, M.C.; Danet, A.F. Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches. Molecules 2009, 14, 480–493. [Google Scholar] [CrossRef]
- Kluska, M.; Woźniak, K. Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. Int. J. Mol. Sci. 2021, 22, 6602. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K. Role of pomegranate and citrus fruit juices in colon cancer prevention. World J. Gastroenterol. 2014, 20, 4618–4625. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef]
- Bandele, O.J.; Clawson, S.J.; Osheroff, N. Dietary Polyphenols as Topoisomerase II Poisons: B Ring and C Ring Substituents Determine the Mechanism of Enzyme-Mediated DNA Cleavage Enhancement. Chem. Res. Toxicol. 2008, 21, 1253–1260. [Google Scholar] [CrossRef]
- Kusmardi, K.; Baihaqi, L.A.; Estuningtyas, A.; Sahar, N.; Sunaryo, H.; Tedjo, A. Ethanol Extract of Pomegranate (Punica granatum) Peel in Increasing the Expression of Caspase-3 in DSS-Induced Mice. Int. J. Inflamm. 2021, 2021, 410. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.H.; El-Abhar, H.S.; Hassanin, E.A.K.; Abdelkader, N.F.; Shalaby, M.B. Punica granatum suppresses colon cancer through downregulation of Wnt/β-Catenin in rat model. Rev. Bras. Farm. 2017, 27, 627–635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).