Abstract
The current alternative of using aromatic-based wax soak solvents has been found to be hazardous and imposes a high cost on field expenditures. These solvents are widely used in the oil and gas industry to soften up wax before the pigging process. However, their impacts on the environment are quite concerning. Plus, they also impose hazardous exposure and are found to be damaging to both plastic and rubber hoses. To replace the current alternative with other hydrocarbon-based wax soak solvents, ionic liquids were found to have the capability to increase the solvent power capabilities and the efficiency of wax soak solvent in dissolving heavy crude oil. To optimise the application of hydrocarbon solvents and ionic liquid in wax dissolution, the affinity of ionic liquids in three solvents was studied. The solvents were condensate, ethyl acetate, and xylene. It was found that from four types of ionic liquids tested, only BMIMCL and HMIMBr were miscible in all three of the solvents used. Dissolution of hard deposited paraffin wax in condensate, ethyl acetate and xylene was conducted using the spectral analysis method, and it was found that the dissolution of paraffin wax was greatest in ethyl acetate. However, enchantment on wax dissolution was also observed for gas condensate and xylene. The wax soaking time was also optimised, in which the dissolution of wax was found to reach a saturation level when the wax had been soaked for over 90 min in the solvents, especially for gas condensate. Further study on the effect of ionic liquid introduced alongside condensate, ethyl acetate and xylene, aiming to enhance the solvent power, was also conducted using the spectral analysis method. The introduction of ionic liquids to all hydrocarbon-based wax soak solvents used in this project was proven to significantly increase the UV-VIS absorbance of the extracted solvents after paraffin wax had been soaked for 30 min.
1. Introduction
Between 1992 to 2002, there were over fifty-one severe cases of production pipeline blockage due to plugging by deposited wax in the Gulf of Mexico, in which remediation activities of the entire pipeline cost over several millions of USD per day and required at least 40 days of operation shutdown. The deposition of wax in a pipeline is usually caused by heavy components of crude oil from heavy saturate forming hard paraffin and asphaltene waxes, which are catalysed when the temperature of the pipeline falls below the Wax Appearance Temperature (WAT) or simply due to ageing factors [1]. As the oil and gas industry moves towards the production of heavy crude oil, the deposition of wax is inevitable; hence, several remission and remediation methods have been introduced to avoid such a big risk in terms of pipeline blockage [2].
The deposition of wax in a pipeline is usually caused by heavy components of crude oil from heavy saturate forming hard paraffin and asphaltene waxes [1]. Mechanically removing deposited wax in the pipeline by pigging remains the most applicable remediation method, as pigging activities impose lower costs compared to other deposited wax remission methods, such as thermal management and chemical wax inhibition [3]. To avoid total pipeline blockage by deposited wax, the pipeline will need to be pigged once over several weeks where, despite being a cheaper alternative, pigging activities still come at a high cost and present high risk of the pipeline being plugged by the pig during the pigging activities [4]. Hence, total capital and operational expenditures from pigging activities will need to be further reduced by improving pigging efficiency. Pigging activities are common practice in oil and gas industries throughout the world.
The hardness of the deposited wax could impose significant effects on the pig efficiency, where hard wax imposes a high risk of damage and failure of the pig brushes, scrappers and seals, leading to pipeline blockage by the pig during pigging [4,5]. Studies have been conducted on multiple types of pigs and it has been found that the pig efficiency was reduced to about 50% when it was used to scrape off hard wax [5]. As the hardness of the wax increases, the pigging efficiency will significantly decrease. Wax plugs have the capability to withstand high differential pressure without moving, and when the breaking force, which is the wax shear strength, is greater than the baseline force, the differential pressure will push the pig driving force further, hence causing severe damages to parts of the pig, especially to brushes and caps [6]. Reducing the hardness of the deposited wax would significantly improve pigging efficiency as damage to the pig could be reduced.
Industry current practice is to soak a high volume of aromatics-based solvents on the hard-deposited wax inside the pipeline before pigging activities commence, to reduce the hardness of the deposited wax and to ease the pigging processes. Aromatic-based solvents such as toluene and xylene have been found to have high efficiency in the dissolution of heavy crude oil and wax [6,7,8,9,10]. Despite having the highest solvent power among other hydrocarbon-based solvents, studies have suggested looking for cheaper and environmentally safer alternatives, as solvents derived from toluene and xylene impose hazardous exposure and have been found to be damaging to both plastic and rubber hoses [8,11,12]. Therefore, due to high environmental risk and high costs, industries have been looking for a safer and much cheaper alternative to wax solvents derived from toluene and xylene [2]. The abundant presence of gas condensate as a byproduct of the production of crude oil and gas could offer the industry a readily available hydrocarbon-based wax solvent at a much cheaper cost and which is environmentally safer than solvents derived from toluene and xylene.
Despite being less efficient than aromatics, solvents such as naphtha, condensate, mineral oil and lighter hydrocarbon have been found to have the capability to dissolve and also reduce the viscosity of heavy crude oil [3,10,11,13]. To increase the efficiency of these hydrocarbon solvents, studies have been conducted and proved that the addition of ionic liquids alongside a hydrocarbon-based solvent was found to significantly improve and enhance the performance of hydrocarbon-based solvents in the dissolution of heavy crude oil and tank bottom sludge [2,14,15,16]. The wide application of ionic liquids is due to its unique characteristics of high thermal and chemical stability, low toxicity compared to aromatic-based products and its special flexibility in tuning its cation and anion components to suit target tasks. Hence, to optimize the application of hydrocarbon solvents and ionic liquids, this study aimed to evaluate condensate, ethyl-acetate and xylene performance with and without ionic liquid as wax soak solvents after determining the affinity and the performance of condensate, ethyl-acetate and xylene as wax soak solvents in the presence of ionic liquid, which were 1-Butyl-3-Methylimidazolium Chloride (BMIMCL), 1-Butyl-3-Methylimidazolium Tetrafluoroborate (BMIMBF4), 1-Methyl-3-Octylimidazolium Chloride (OMIMCL) and 1-Hexyl-3-Methylimidazolium Bromide (HMIMBr) at different wax soaking times, different mass ratios of IL:wax and different combination pairs of solvent mixture.
2. Materials and Methods
2.1. Materials
Both crude oil wax and crude oil condensate were sourced from PETRONAS, Kerteh, Malaysia while the ethyl acetate, xylene, toluene, 1-Butyl-3-Methylimidazolium Chloride (BMIMCL), 1-Butyl-3-Methylimidazolium Tetrafluoroborate (BMIMBF4), 1-Methyl-3-Octylimidazolium Chloride (OMIMCL) 1-Hexyl-3-Methylimidazolium Bromide (HMIMBr) used in this study were sourced from Sigma Aldrich, St. Louis, MO, USA.
2.2. Wax Characterisation by GC/MS
A quantity of 0.20 g of crude oil wax was weighed, and 20 mL of toluene was then transferred into the beaker containing the wax. The beaker containing a mixture of toluene and wax was stirred and put in an ultrasonic bath for 5 min for the wax to fully dissolve. The mixture solution of toluene and wax was then analysed by the Centre of Analytical Laboratory (CAL) UTP with a Gas Chromatograph Mass Spectrometer with a Direct Insertion Probe and Pyrolysis Cell (GCMS-DIPPC) Agilent 7890A, Agilent Technologies, USA.
2.3. Condensate Characterisation by GC/MS
Condensate sourced from PETRONAS, Kerteh, Malaysia was analysed by the Centre of Analytical Laboratory (CAL) UTP with a Gas Chromatograph Mass Spectrometer with a Direct Insertion Probe and Pyrolysis Cell (GCMS-DIPPC) Agilent 7890A, Agilent Technologies, USA.
2.4. Synthesis of Hydrocarbon Solvents with Ionic Liquids
A weight of 0.10 g of ionic liquid was added to 20 mL of solvent. The mixture was then slowly shaken and was then put in an ultrasonic bath to promote ionic liquid dissolution for 30 min. After 24 h, the mixture miscibility was recorded.
2.5. Analysis of Wax Dissolution in Hydrocarbon Solvents
Around 0.20 g of wax was moulded with the tip of a 3 mL syringe. This moulded technique was used to make sure that the shape of the wax was constant throughout this study. A volume of 20 mL of solvent was then transferred into the vial containing the wax that was previously moulded. The mixture containing wax was then left for soaking for 30 min. After 30 min, the solution inside the vial was then fully and carefully extracted using a pipette. The used soaked solvent was then analysed using UV-VIS. The remaining undissolved wax in the vial was left to dry for 72 h at room temperature under a fume-hood. After 72 h, the mass of the vial containing the remaining wax was weighed and recorded. The experiment was then repeated with soaking times of 1.5 h and 2.5 h in the same experimental conditions.
2.6. Analysis of Wax Dissolution in Hydrocarbon Solvents and Ionic Liquids
An amount of 20 mg of ionic liquid was added to a vial containing 20 mL of solvent. The vial was then capped and slowly shaken, and then soaked in an ultrasonic bath for 30 min to promote ionic liquid dissolution in the solvent. This step was crucial to ensure the dissolution of ionic liquids inside the solvents used. The mixture of ionic liquid and solvent was left for 24 h. After 24 h, about 0.20 g of wax moulded with the tip of a 3 mL syringe was transferred into the vial. The mixture containing wax was then left for 30 min for soaking. After 30 min, the solution inside the vial was fully extracted and analysed with UV-VIS. The remaining wax in the vial was left to dry for 72 h. After 72 h, the mass of the vial containing the remaining wax was weighed and recorded. The experiment was repeated with 60 mg and 100 mg of ionic liquid and with different soaking times of 90 min and 150 min.
3. Results and Discussions
3.1. Characterisation of Crude Oil Wax
The characterisation analysis of the wax using GC/MS was compared with the database from Agilent Technologies and the total percentage of area under peak was then calculated for every carbon number present in the wax. Figure 1 shows the percentage of the number of carbons present in the crude oil wax coursed from PETRONAS, Kerteh, Malaysia. The deposited wax from PETRONAS, Kerteh, Malaysia was found to be composed of almost 78% of C20 to C36 straight-chain hydrocarbons compared to the database from Agilent Technologies.
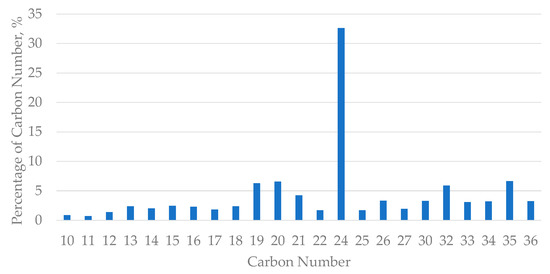
Figure 1.
Percentage of the number of carbons present in crude oil wax sourced from PETRONAS, Kerteh, Malaysia.
This result was compared with the previous literature findings and it was found that the wax used in this project was a paraffinic wax. Hence, condensate as a wax soak solvent could be used in the dissolution of wax used in this project, as condensate was found to have the capability to dissolve paraffin wax [8,12]. Plus, in addition to ionic liquids, the dissolution of the paraffin wax could be improved.
3.2. Characterisation of Crude Oil Condensate
The characterisation analysis of the condensate using GC/MS was compared with the database from Agilent Technologies and the total percentage of area under peak was then calculated for every carbon number present in the condensate. As can be seen from Figure 2 below, the total percentage of C8, C9, C10 and C11 compositions present in the condensate was about 78%, in which the gas condensate was found to have a high composition of C9 hydrocarbons.
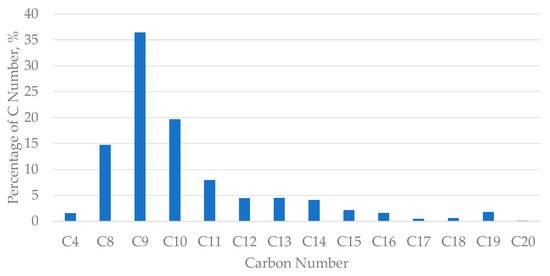
Figure 2.
Percentage of the number of carbons present in condensate sourced from PETRONAS, Kerteh, Malaysia.
The previous literature findings found that the general composition of liquid crude oil condensate is usually composed of more than 70% of C7 and heavier, with little presence of C4 and heavier C5. Hence, the results derived from this project using this condensate sourced from PETRONAS, Kerteh, Malaysia was projected to have similar results with previous findings in the literature [17,18,19], as the characteristics of the gas condensate samples were quite similar.
3.3. Miscibility of Ionic Liquid in Condensate, Ethyl Acetate and Xylene
The miscibility test of the ionic liquid in hydrocarbon was tested at the highest mass ratio of ionic liquid to wax used in this project. The ionic liquids used in this study were BMIMBF4, BMIMCL, HMIMBr and OMIMCL. These ionic liquids were then mixed with the solvents (condensate, ethyl acetate and xylene) and placed in an ultrasonic bath for 30 min and left for 24 h before the visible miscibility test results were taken. The results of the experiment are summarised below.
Based on Table 1 above, it was found that BMIMBF4 and OMIMCL formed another layer at the bottom of the flask. However, for BMIMCL and HMIMBr, there was no layer or layer of IL droplets seen settling at the bottom of the flask, which can be seen in Figure 3 below. This means that BMIMCL and HMIMBr were fully dissolved inside the condensate, ethyl acetate and xylene.

Table 1.
Miscibility of Ionic Liquid in hydrocarbon solvents.
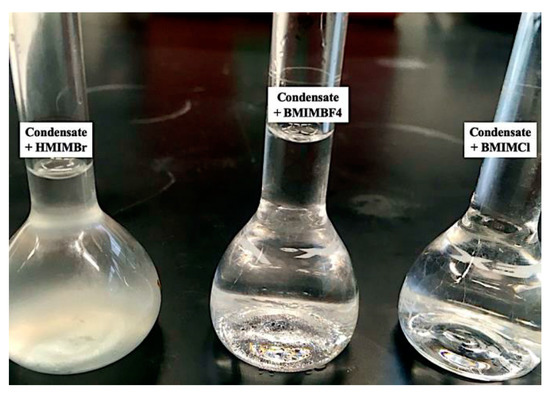
Figure 3.
Example of miscibility of ILs in condensate.
The miscibility of ionic liquids heavily depends on the length of the aliphatic chain of its cation and anion. The longer the aliphatic chain, the more aliphatic the ionic liquid, and the more immiscible it becomes [20]. Furthermore, the anion of ionic liquids had the most influencing feature in determining the miscibility of ionic liquids compared to the cation of ionic liquids [21]. Thus, for BMIMBF4, even though it has a shorter aliphatic chain than HMIMBr, it was found to be immiscible in all three solvents. This was due to its anion head of BF4-, which conflicted with its hydrophilic nature. This anion head of BF4 was resistant to being dissolved and miscible in hydrocarbon solvent.
From the miscibility tests of all four of ionic liquids tested in condensate, ethyl acetate and xylene, only BMIMCL and HMIMBr were tested and carried forward into the next phase of this project. This is because only these two ionic liquids were found to be miscible in all three of the solvents.
3.4. Dissolution of Paraffin Wax in Hydrocarbon
The concentration of wax in the extracted solvent could be estimated by using Beer-Lambert’s law, but, in this experiment, when 20 mg of wax was fully dissolved in 20 mL of all solvents, it appeared to have impurities which were expected to be either inorganic or asphaltene remaining from the wax; hence, the exact concentration of the paraffin wax present could not be easily determined using Beer-Lambert’s law. The results of this experiment were then fully analysed by using the UV-VIS absorbance of the extracted solvent; the higher the value of the UV-VIS absorbance, the higher the amount of wax that had dissolved in the solvent. However, if the extracted solvents were too dark in colour, some dilution factors were needed. This was mostly true for the cases with 90 min and 150 min of exposure time.
Figure 4 below shows the UV-VIS absorbance of the extracted solvents for all three types of solvents at three different soaking times: 30 min, 90 min and 150 min. The graph below shows that, in all three types of solvents tested, the wax dissolution reached its saturation level after the wax had been soaked for over 90 min, especially in the case of gas condensate. The UV-VIS absorbance showed a large increment in value when the wax had been soaked for 30 min and for 90 min, but when the wax was soaked for 150 min, the UV-VIS absorbance of the extracted solvent was almost similar to that when the wax had been soaked for 90 min. This result was especially true for condensate, where it reached a plateau at a 90 min soaking time in terms of dissolution enhancement.
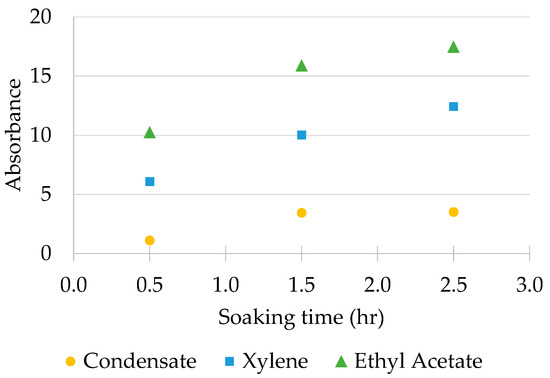
Figure 4.
UV-VIS absorbance of soak solvent against wax soaking time.
Based on the results obtained from this experiment, as shown in Figure 4 above, it was concluded that the optimum soaking time of the wax for gas condensate could be set to 90 min, as when the wax was soaked for over 90 min, little to no increment of wax dissolution could be seen.
At wax soaking time of 90 min, the UV-VIS absorbance of the extracted solvents was further analysed to study the performance of condensate, ethyl acetate and xylene, as was the soak solvent. As shown in Figure 5 below, the UV-VIS absorbance of the extracted solvent was the highest in ethyl acetate, followed by absorbance in xylene and condensate.
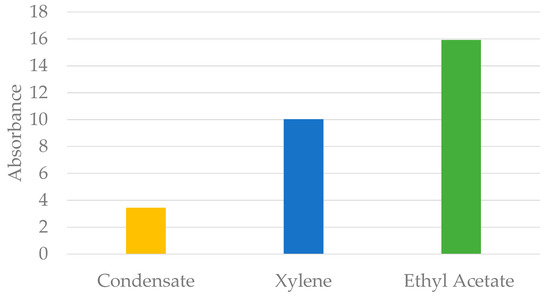
Figure 5.
UV-VIS absorbance of extracted soak solvent at 1.5 h of soaking time.
According to Beer-Lambert’s law, the absorbance is directly proportional to the concentration of particles in a particular solvent. Hence, it was concluded that the concentration of wax dissolved in the solvent was highest when using ethyl acetate as the wax soak solvent, followed by xylene and condensate. This result is in accordance with previous findings from the literature, in which xylene was found to be more efficient as the wax soak solvent compared to condensate. Thus, it was concluded that the performances of soak solvents could be summarised as follows: Ethyl Acetate > Xylene > Condensate.
3.5. Dissolution of Paraffin Wax in Hydrocarbon Solvents in the Presence of Ionic Liquid
Ionic liquid was introduced to the hydrocarbon solvent, forming a mixture of hydrocarbon solvent and ionic liquid as the wax soak solvent Wax was then soaked inside the prepared solvents. It was found that, at a 90-min soaking time, when ionic liquid was introduced into the three solvents used, the UV-VIS absorbance of all the samples showed the same trend. Hence, it was concluded that, at 90 min, the dissolution of wax in gas condensate had reached its saturation level. Thus, in this experiment, to further study and analyse the effect of adding ionic liquid to wax soak solvent, the wax in this experiment was soaked for 30 min instead.
The weight of 20 mg, 60 mg and 100 mg of respective BMIMCL and HMIMBr were added to each of the three solvents, forming a combination pair consisting of one type of solvent with one type of ionic liquid. In Figure 6 below, the UV-VIS absorbance of the extracted solvent of each combination pair is shown at different mass ratios of ionic liquid to wax. For all six combination pairs, it was found that increasing the mass ratio of ionic liquid to wax did not affect the UV-VIS absorbance; hence, it was concluded that the concentration of wax dissolved in the mixture of wax soak solvent would not be affected by increasing the amount of ionic liquid present in the wax soak solvent.
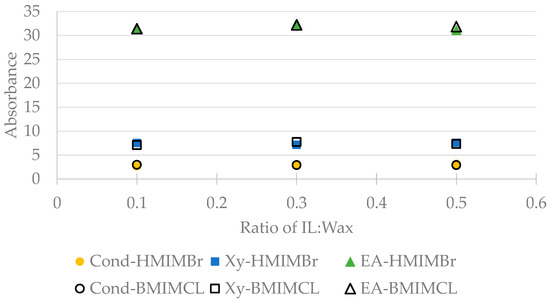
Figure 6.
UV-VIS absorbance of soak solvent in the presence of ionic liquid at 0.5 h of soaking time.
Although increasing the mass ratio of ionic liquid to wax did not affect the dissolution of wax, adding ionic liquid to the hydrocarbon solvents was found to significantly improve the dissolution of wax. The UV-VIS absorbance analysis of the previous experiment without the presence of ionic liquid was compared with UV-VIS absorbance analysis in the presence of ionic liquid, as shown in Figure 7 below. The results of UV-VIS absorbance of the extracted solvent, in the presence of ionic liquid for 30 min of wax soaking time, had significantly increased compared to UV-VIS absorbance without the presence of ionic liquid.
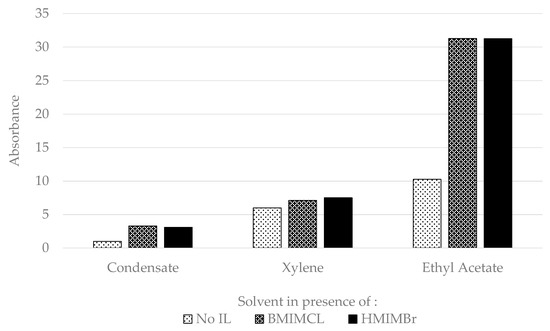
Figure 7.
UV-VIS absorbance of extracted solvent with and without the presence of ionic liquid at 0.1 of IL:Wax mass ratio.
The ionic liquid enabled the hard crude oil wax to dissolve more easily in the solvent due to its cation and anion charges pulling apart the hard-wax properties according to its respective charges. The mechanisms worked in such a way that the cation and the anion of the ionic liquid were simultaneously attracted to the wax and the solvent, thus increasing the dissolution of the wax in the solvent.
As can be seen in Figure 8 below, the percentage of improvement of UV-VIS absorbance in the presence of ionic liquid was found to be significant in ethyl acetate and condensate compared to without the presence of ionic liquid in the hydrocarbon solvent. Adding BMIMCL and HMIMBr in ethyl acetate and condensate significantly improved the dissolution of wax by over 200%, whereby adding BMIMCL and HMIMBr in xylene only improved the wax dissolution by 15% to 23%, respectively. Hence, in future studies, it is not recommended to add ionic liquid if the wax soak solvent used is xylene, as it will only impose a higher cost in addition to the high cost of xylene itself.
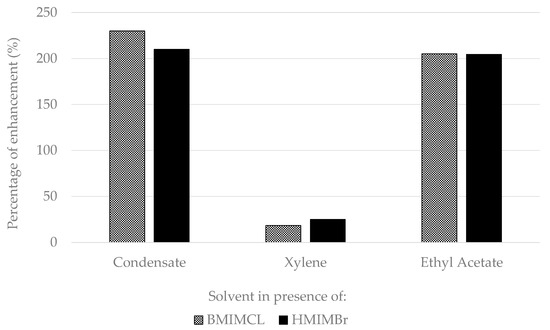
Figure 8.
Percentage of enhancement of extracted solvent UV-VIS absorbance in the presence of ionic liquid.
In addition, introducing BMIMCL in ethyl acetate and condensate as the wax soak solvent improved the wax dissolution slightly more than adding HMIMBr to ethyl acetate and condensate, and this might be due to the shorter aliphatic chain of BMIMCL compared to HMIMBr. This result differed from that obtained from xylene, in which adding HMIMBr to xylene seemed to improve the absorbance slightly more than by adding BMIMCL to xylene as the wax soak solvent.
Further studies need to be conducted at the molecular level to analyse the interactions between the aliphatic cation chain and the anion head of the ionic liquid with the hydrocarbon solvent to gain a better understanding of the mechanisms of wax dissolution in hydrocarbon solvent in the presence of ionic liquid.
In conclusion, introducing ionic liquids to hydrocarbon solvents was found to increase the solvent power and efficiency, as was soak solvent. It was also found that adding more ionic liquid to the mass ratio of IL:wax did not affect the percentage of improvement of the absorbance, and hence, the dissolution of wax.
4. Conclusions
The results obtained from this project had proven that there exists a more efficient wax soak solvent, which is ethyl acetate, while xylene came second before condensate as most efficient wax soak solvents. Introducing ionic liquids to each of the hydrocarbon solvents proved to increase the solvent power and the efficiency of the solvent in dissolving paraffin wax. The addition of a high volume of ionic liquid to the same amount of solvents was found to not be effective in enhancing the dissolution of wax in the solvents.
For gas condensate, the wax soaking time and the mass ratio of IL to wax were also investigated. Soaking the wax for more than 90 min proved that the dissolution of wax in all of the solvents had reached their saturation levels.
This project was conducted at the macroscopic level, which was intended to analyse the trend in hydrocarbon solvents as wax soak solvents with and without the presence of ionic liquid. In conclusion, the results obtained from this project are in accordance with the findings from previous experiments in the literature, in which, in the presence of ionic liquid, the dissolution of paraffin wax significantly improves compared to in hydrocarbon solvents without the presence of ionic liquids.
The findings from this project could potentially replace aromatic-based wax soak solvents with a mixture of condensate and ionic liquid. Although a high volume of condensate might be needed, the overall cost of softening up the deposited wax can be reduced by using a readily available wax soak solvent.
This project achieved all of its objectives, including the optimisation of wax soak solvents by optimising the wax soaking time, mass ratio of ionic liquid and optimising the combination pairs of wax soak solvents. This project also successfully evaluated the performance of hydrocarbon solvents with and without ionic liquid as wax soak solvents.
Author Contributions
Methodology, M.R.S.Z.A. and M.H.N.; writing—original draft preparation, M.R.S.Z.A. and M.H.N.; writing—review and editing, M.H.N., M.M. and M.G.; Supervision, M.H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Yayasan Universiti Teknologi PETRONAS (Y-UTP), Universiti Teknologi PETRONAS; Grant Number 015LC0-266.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Acknowledgments
Sincerest appreciation to both Centre for Research in Ionic Liquid (CORIL) and Universiti Teknologi PETRONAS (UTP) for equipping the best facilities to complete this project. Thank you also to Malaysia Petroleum Management and PETRONAS for their cooperation in providing the samples for this project. Lastly, thank you to YUTP for funding this project and also to RIC in UTP for their assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Speight, J.G. The Chemistry and Technology of Petroleum, 4th ed.; CRC Press Taylor & Francis Group: Laramie, WY, USA, 2006. [Google Scholar]
- Sakthivel, S.; Velusamy, S.; Gardas, R.L.; Sangwai, J.S. Eco-Efficient Method for the Dissolution Enhancement of Heavy Crude Oil Using Ionic Liquids. In Proceedings of the Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 11–14 October 2015; p. 7. [Google Scholar]
- Hart, A. A review of technologies for transporting heavy crude oil and bitumen via pipelines. J. Pet. Explor. Prod. Technol. 2014, 4, 327–336. [Google Scholar] [CrossRef]
- Mendes, P.R.S.; Braga, A.M.B.; Azevedo, L.F.A.; Correa, K.S. Resistive Force of Wax Deposits During Pigging Operations. J. Energy Resour. Technol. 1999, 121, 167–171. [Google Scholar] [CrossRef]
- Wang, Q.; Sarica, C.; Chen, T.X. An Experimental Study on Mechanics of Wax Removal in Pipeline. J. Energy Resour. Technol. 2005, 127, 302–309. [Google Scholar] [CrossRef]
- Kang, P.-S.; Lee, D.-G.; Lim, J.-S. Status of Wax Mitigation Technologies in Offshore Oil Production. In Proceedings of the Twenty-fourth International Ocean and Polar Engineering Conference, Busan, Republic of Korea, 15–20 June 2014; p. 8. [Google Scholar]
- Li, W.; Huang, Q.; Wang, W.; Ren, Y.; Dong, X.; Zhao, Q.; Hou, L. Study on Wax Removal during Pipeline-Pigging Operations. SPE Prod. Oper. 2019, 34, 216–231. [Google Scholar] [CrossRef]
- Sakthivel, S.; Velusamy, S.; Gardas, R.L.; Sangwai, J.S. Experimental Investigation on the Effect of Aliphatic Ionic Liquids on the Solubility of Heavy Crude Oil Using UV–Visible, Fourier Transform-Infrared, and 13C-NMR Spectroscopy. Energy Fuels 2014, 28, 6151–6162. [Google Scholar] [CrossRef]
- Zhu, T.; Walker, J.A.; Liang, J. Evaluation of Wax Deposition and Its Control during Production of Alaska North Slope Oils; United States Department of Energy National Energy Technology Laboratory: Fairbanks, AK, USA, 2008.
- Turbakov, M.S.; Riabokon, E.P. Improving of Cleaning Efficiency of Oil Pipeline from Paraffin. In Proceedings of the SPE Russian Oil and Gas Exploration & Production Technical Conference and Exhibition, Moscow, Russia, 14–16 October 2014; p. 9. [Google Scholar]
- Southgate, J. Wax Removal Using Pipeline Pigs. Ph.D. Thesis, Durham University, Durham, UK, 2004. [Google Scholar]
- Straub, T.J. An Investigation into Practical Removal of Downhole Paraffin by Thermal Methods and Chemical Solvents. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 13–14 March 1989; p. 8. [Google Scholar]
- Khaibullina, K. Technology to Remove Asphaltene, Resin and Paraffin Deposits in Wells Using Organic Solvents. In Proceedings of the Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 26–28 September 2016. [Google Scholar]
- Sakthivel, S.; Velusamy, S.; Gardas, R.L.; Sangwai, J.S. Nature friendly Application of Ionic Liquids for Dissolution Enhancement of Heavy Crude Oil. In Proceedings of the Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015. [Google Scholar]
- Turosung, S.N.; Ghosh, B. Application of Ionic Liquids in the Upstream oil Industry—A Review. Int. J. Petrochem. Res. 2017, 1, 50–60. [Google Scholar] [CrossRef]
- Jos-Alberto, M.-H.; Jorge, A. Current Knowledge and Potential Applications of Ionic Liquids in the Petroleum Industry. In Ionic Liquids: Applications and Perspectives; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011; p. 22. [Google Scholar]
- Roussennac, B. Gas Condensate Well Test Analysis. Master’s Thesis, Stanford University, Stanford, CA, USA, 2001. [Google Scholar]
- Speight, J.G. Gas Condensate. In Natural Gas; Elsevier: Amsterdam, The Netherlands, 2019; pp. 325–358. [Google Scholar]
- Thornton, O.F. Gas-Condensate Reservoirs—A Review; API-46-150; API: New York, NY, USA, 1946; pp. 150–159. [Google Scholar]
- Marciniak, A. The Solubility Parameters of Ionic Liquids. Int. J. Mol. Sci. 2010, 11, 1973–1990. [Google Scholar] [CrossRef] [PubMed]
- Klähn, M.; Stüber, C.; Seduraman, A.; Wu, P. What Determines the Miscibility of Ionic Liquids with Water? Identification of the Underlying Factors to Enable a Straightforward Prediction. J. Phys. Chem. B 2010, 114, 2856–2868. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).