Abstract
With the increased demand for sustainable, hypoallergenic products, plant surfactants are a promising, eco-friendly option for cleaning products due to their low toxicity or even the absence of toxicity. In the present study, surfactant-rich extracts from Chenopodium quinoa, Glycine max, and Malpighia emarginata were assessed for their stability, antioxidant capacity, toxic potential, and cleaning potential in shampoo formulations. The surfactants in the extracts were isolated and characterized by NMR, UV-Vis, and FTIR spectroscopy. The results demonstrated that the extracts remained stable within the temperature and pH ranges tested. The antioxidant properties were also determined. In the analysis of irritation potential, G. max and C. quinoa exhibited low toxicity and no toxicity, respectively. The cleaning potential analysis confirmed that the extracts could be used as primary surfactants. Seven shampoo formulations were developed, which showed potential to reduce surface tension to the range of 27.1–31.7 mN/m and interfacial tension to the range of 5.4–7.3 mN/m. The wettability, percentage of solids, density, pH, and dirt dispersion of the formulas were within standard ranges, and the sebum removal capacity of the seven formulations was similar to or even better than that of a commercial shampoo.
1. Introduction
Hair has considerable cultural, social, and economic importance and is associated with improved self-esteem and inclusion of individuals in social, cultural, or ethnic groups. Thus, it is no surprise that hair care products are among the most widely used, especially innovative formulations specific to each type of hair [1].
Shampoos consist basically of a solution composed of surfactants, which are the main components responsible for cleaning the hair and scalp, and other adequate ingredients and additives that provide benefits to the hair as well as make the appearance and consistency of the shampoo attractive [2,3]. Some of the challenges faced by the cosmetic industry regarding shampoo formulations are related to allergies, hair loss, and irritation of the skin and eyes caused by synthetic surfactants, which also affect soil and groundwater, causing harm to the environment [4].
Therefore, the cosmetics market has prioritized natural, renewable, biodegradable, and less toxic active ingredients/components that can reduce or replace synthetic compounds in formulations. According to Cosmetics Europe [5], large companies in the cosmetics industry reformulate 25 to 30% of their products every year, and approximately 10% of these reformulations depend on new active ingredients/components.
Natural surfactants constitute one of the options to meet this demand. Microorganisms and plants are producers of these natural surfactants or biosurfactants. Biosurfactants of plant origin include phospholipids, saponins, and proteins or protein hydrolysates [6]. Among these, saponins have the potential for several biotechnological applications due to their physical–chemical and biological properties [6,7,8]. Sophorolipids, rhamnolipids, and mannosylerythritol lipids are examples of microbial surfactants that have been used in cosmetics formulations [9].
With considerable biotechnological potential, plant-derived biosurfactants stand out due to their tensioactive and biological properties, as well as their greater yield in extraction processes compared with microbial biosurfactants [10]. The development of cosmetic formulations using plant-derived biosurfactants is promising and constitutes an alternative to cosmetics formulated exclusively with synthetic surfactants.
Therefore, the aim of the present study was to formulate shampoos using biosurfactant-rich extracts from three plants, namely Chenopodium quinoa (quinoa), Glycine max (soybean), and Malpighia emarginata (acerola cherry), analyzing the tensioactive properties of these extracts in formulations as well as their potential to diminish or replace the use of synthetic surfactants in shampoos.
2. Materials and Methods
2.1. Plant Material and Obtaining Extracts
Seeds from Chenopodium quinoa and Glycine max, and the dried fruit of Malpighia emarginata were used for the hydroalcoholic extraction of biosurfactants [11].
The M. emarginata fruit was macerated and dried at 50 °C for 48 h. The dried pulp was then pulverized, using a crusher, to obtain the flour. The seeds of C. quinoa and G. max were also pulverized before the extraction processes. The resulting powders were sieved in a vertical sieve to obtain fractions with a granulometry of ≤250 µm.
The ethanol concentrations and temperature values used in the extraction process were 50% and 50 °C for C. quinoa, 60% and 40 °C for G. max, and 50% and 40 °C for M. emarginata. Such conditions were determined after carrying out an experimental design as described previously by Bezerra et al. [11]. The mixtures were then agitated in an orbital shaker (24 h at 180 rpm) and were ultrasounded for 30 min (Ultrasonic bath, Unique USC-1400), centrifuged (10 min at 4500 rpm), and filtered. The extracts were concentrated to evaporate the solvent and the aqueous part was lyophilized.
2.2. Stability of Extracts
The stability of the extracts at a concentration of 10% (w/v) was evaluated at different temperatures (40, 60, 80, and 100 °C) and pH values (2, 4, 6, 8, 10, and 12). The response variables were the surface tension (ST) and the emulsification index (E24), expressed in mN/m and %, respectively [12]. Surface tension was determined using a Sigma 700 Tensiometer (KSV Instruments LTD, Helsinki, Finland) at room temperature. The emulsification index was determined following the method described by Cooper and Goldenberg [13], which consisted of a mixture of 2 mL of the emulsifying agent and 2 mL of the hydrophobic compound in test tubes, which were stirred in a vortex for 2 min and left to stand for 24 h. After that period, measurements of the height of the emulsions were carried out and the emulsification index was calculated by the ratio between the height of the emulsion layer and the total height multiplied by 100.
2.3. Radical Scavenging Activity of Extracts
The radical scavenging activity of the extracts was determined based on their capability to scavenge 2,2-diphenyl-1-picrylhydrazyl radical (DPPH∙), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid radical cation (ABTS∙+), total antioxidant capacity (TAC), hydroxyl radical (∙OH), and superoxide radical anion (O2∙−) [14,15,16]. The concentration required to eliminate 50% of each radical (IC50) was calculated from spectrophotometric measures and expressed in µg/mL.
2.4. Assessment of Eye Irritation Potential of Extracts
The hen’s egg chorioallantoic membrane test (HET-CAM) was performed following the in vitro method described by Wilson and Steck [17] for a semi-quantitative analysis of the irritant potential (IP) of the extracts at a concentration of 1% (w/v). The membranes were observed for 300 s for signs of hemorrhage, lysis, and coagulation. The times at which each process began were incorporated into Equation (1).
where H, L, and C are, respectively, the number of seconds elapsed from irritant application to first signs of hemorrhage, lysis, and coagulation, in an observation period with a 300 s maximum duration.
IP = [(301 − H) × 5] + [(303 − L) × 7] + [(301 − C) × 9]/300
The irritant potential (IP) of the extracts was defined as suggested by the guidelines listed in Table 1. All assays were repeated five times.

Table 1.
Scoring Scheme for Irritation Testing.
2.5. Cleaning Potential of Extracts
To select the best combinations of plant-derived extracts and best concentrations (4% or 10%) to develop shampoo formulations, the cleaning potential of the extracts was assessed by determining primary and secondary surfactants based on the methods described by Thompson et al. [18] and Azadbakht et al. [19]. Disodium cocoyl glutamate (DCG) was selected as the synthetic surfactant and tested in combination with the extract at different concentrations (Table 2).

Table 2.
Concentrations and combinations of plant extracts to determine the primary and secondary surfactants in formulations.
2.5.1. Artificial Sebum
The artificial sebum used to simulate fatty material was prepared according to Thompson et al. [18] with some adaptation and had the following composition: 20% olive oil, 15% coconut oil, 30% oleic acid, 15% paraffin, and 20% jojoba oil.
2.5.2. Washing Process
Hair discard from a local beauty salon was used in the simulation of the washing process. The hair was separated into locks with a standard length of 11 cm and mass of 1.5 g, which were immersed in a hexane solution containing the artificial sebum at a concentration of 10% and weighed after complete drying. Washing was performed following the bulk process described by Thompson et al. [18].
The sebum removal rate (percentage) was determined using the following equation:
where W1 is the weight of the lock with sebum, W2 is the weight of the lock after washing, and W3 is the initial weight of the lock without sebum.
Sebum removal (%) = [(W1 − W2)/(W1 − W3)] × 100
2.6. Determination of Hydrophilic/Lipophilic Balance of Extracts
The hydrophilic/lipophilic balance (HLB) of the extracts was estimated using the water solubility method described by Gadhave [20], as surfactants’ solubility in water can be used as an index of the HLB value (Table 3).

Table 3.
Water solubility and hydrophilic/lipophilic balance (HLB).
2.7. Isolation of Biosurfactants
Biosurfactants were isolated in two steps, namely pre-isolation based on the method described by Obasi et al. [21] and subsequent purification using liquid chromatography in a silica gel column 60 (70–230 mesh) with 12:3:5 (w/v) n-butanol–acetic acid–water as the mobile phase.
2.8. Characterization of Biosurfactants in Plant Extracts
The isolated biosurfactants were dissolved in deuterated chloroform and their respective NMR spectra were recorded at 25 °C using a 300 MHz spectrometer (Agilent, Santa Clara, CA, USA) operating at 300.13 MHz. Chemical shifts (δ) in relation to tetramethylsilane were expressed in ppm.
The isolated biosurfactants were also characterized by Fourier-Transform Infrared (FTIR) spectrometry (Spectrum 400, Perkin Elmer). Signals were collected from 400 to 4000 wavenumbers with a resolution of 4 cm−1.
The absorption properties of biosurfactants dispersion were analyzed by ultraviolet/visible (UV/Vis) spectrometry (Digital Spectrophotometer SP-22, Biospectro, Shanghai, China) in the wavelength range from 100 to 400 nm.
A saponin standard (Sigma Aldrich, St. Louis, MO, USA) was also used in all characterization analyses for comparison purposes.
2.9. Development of Shampoo Formulations
After determining the cleaning potential of extracts, seven combinations were chosen to develop formulations. Production was performed in partnership with a compounding pharmacy. A commercially available moisturizing shampoo was chosen for comparison with formulations that has considerable acceptability among consumers and no sulfates in its composition, which makes it closer to the proposal of the present study.
2.10. Characterization of Formulations
2.10.1. Determination of superficial and interfacial tension
A 10% (w/v) solution of the formulations was used to determine surface and interfacial tension using a Sigma 700 Tensiometer (KSV Instruments LTD, Helsinki, Finland) at room temperature. Interfacial tension was determined using n-hexadecane as the heavy phase [22].
2.10.2. Determination of pH, Wettability, Solid Content, and Density
The pH of the shampoo solutions formulated at 10% (w/v) in distilled water was measured using a pH meter at room temperature. The content of solids was determined by drying 2 g of the formulation in a previously weighed Petri dish in a laboratory oven at 105 °C for 4 h and expressed as a percentage [23].
Wettability was determined using filter paper disks that were 0.5 cm in diameter. The smooth surface of the disk was placed on the surface of 1% (w/v) formulations, and the time required for the disk to begin to submerge was considered the wettability time [19]. The density of each formulation was determined in a test tube by dividing the mass of a given shampoo sample by its volume [24].
2.10.3. Foam Formation and Dirt Dispersion Test
The foaming capacity was determined using the agitation method in a cylinder. Fifty milliliters of 10% (w/v) shampoo solutions were placed in a graduated 250 mL cylinder, which was capped and manually shaken ten times. The total volume of foam was recorded 1.5 and 10 min after agitation [23].
Two drops of shampoo were added to 10 mL of distilled water in a large test tube, followed by one drop of methylene blue. The test tube was capped and shaken manually ten times, and the amount of dye in the foam (none, light, moderate, or heavy) was determined [23].
2.10.4. Cleaning Capacity of Formulations
The cleaning capacity of the formulations was determined based on the method reported by Thompson et al. [18] and Azadbakht et al. [19], as described in Section 2.5. A 10% concentration of the formulations was used for washing the hair locks.
2.10.5. Preliminary Stability Test
The preliminary stability test was performed following the Cosmetic Product Stability Guide published by the Brazilian Health Surveillance Agency (ANVISA) [25]. After centrifugation at 3000 rpm for 30 min, the formulations were placed in transparent neutral glass flasks with lids and submitted to alternating cycles of heating and cooling for 24 h, as follows:
- Cycles of 24 h at 37 ± 2 °C and 24 h at 5 ± 2 °C for 12 days;
- Cycles of 24 h at 40 ± 2 °C and 24 h at 5 ± 2 °C for 12 days;
- Cycles of 24 h at 45 ± 2 °C and 24 h at 5 ± 2 °C for 12 days.
At the end of each cycle, the organoleptic characteristics (appearance, color, and odor) of the formulations were assessed and the pH was determined.
2.11. Statistical Analysis
The results were expressed as the mean and standard deviation of three independent determinations, calculated using Microsoft Office Excel. The data were analyzed by the analysis of variance (ANOVA) (p ≤ 0.05).
3. Results and Discussion
3.1. Stability of Extracts
Stability is a fundamental factor in cosmetic products and is governed by different guidelines, such as the Cosmetics Europe Guidelines on Stability of Cosmetic Products, which state that the manufacturer must assess pertinent criteria in each type of formulation at different temperatures to enable the deduction of the real or predicted stability of the product [26].
It is essential to ascertain the chemical and physical attributes of additives prior to their incorporation in cosmetics to determine their susceptibility to instability and degradation, which can be caused by various factors, such as exposure to light, air, and oxygen, as well as changes in pH and temperature [27]. Thus, plant extracts with potential cosmetic applications need to maintain their desired functions in the presence of environmental variations and considering the particularities of each formulation.
The effects of variations In temperature and pH on the surface tension (ST) and emulsifying index (E24) of the solutions containing the Chenopodium quinoa, Glycine max, and Malpighia emarginata extracts are described below.
For the C. quinoa extract, variations in temperature did not affect either ST or E24, which remained almost stable at 31 mN/m and 50%, respectively (Figure 1a). As for pH, ST remained stable in the pH range of 6–8, whereas more acidic and alkaline conditions (2 and 12) resulted in an increase up to 37 mN/m. Even though E24 was almost stable in the pH range of 6–8, it was 50% at the starting pH of the solution with the extract (5.6), but then reached 56% as the pH increased to 6 (Figure 1b).
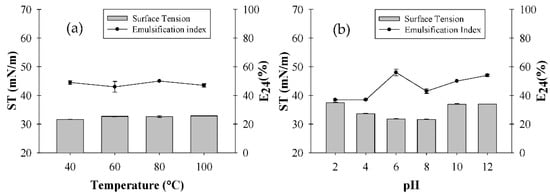
Figure 1.
Influence of temperature (a) and pH (b) on Chenopodium quinoa extract surface tension (ST) and emulsification index (E24).
Exposure to variations in temperature and pH did not affect the ST of the solution with the G. max extract, which remained at 32 mN/m. However, a slight decrease in E24 was found after heating the extract to 100 °C, decreasing from 50% at 40 °C to 43% (Figure 2a). pH variations in the range of 4–10 did not significantly change the E24 value of the emulsions compared with the initial pH (5.7), which remained at around 52% (Figure 2b). Hajimohammadi et al. [28] reported similar results on the influence of the pH and emulsification of plant extract from Glycyrrhiza glabra. The emulsification index was unaltered in the pH range of 5.5 to 13; however, the emulsification index decreased from 100% to 57% at pH 2.
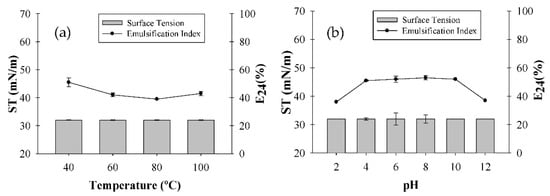
Figure 2.
Influence of temperature (a) and pH (b) on Glycine max extract surface tension (ST) and emulsification index (E24).
For the solution with the M. emarginata extract, both ST and E24 remained almost stable under all temperatures and pH values tested (Figure 3a,b).
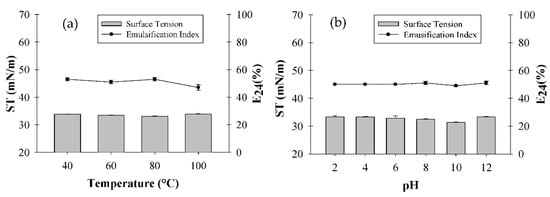
Figure 3.
Influence of temperature (a) and pH (b) on Malpighia emarginata extract’s surface tension (ST) and emulsification index (E24).
Although each plant extract has its own particularities in terms of stability, in general, biosurfactants show good stability under different temperatures and pH values [29]. The stability results of G. max and M. emarginata extracts studied here confirm this tendency, as their surface activity did not undergo significant changes, while in the case of C. quinoa, changes occurred in a pH range that is generally not used in cosmetic formulations, demonstrating that the tensioactive molecules present in the extracts did not lose their surfactant function.
In the field of cosmetics, many emulsions, such as shampoos, creams, and conditioners, are prepared with heating, and their pH is generally between 4 and 7 to maintain compatibility with skin and hair [30,31]. Therefore, all three C. quinoa, G. max, and M. emarginata extracts showed characteristics suitable for possible applications in diverse formulations.
3.2. Radical Scavenging Activity of Extracts
Antioxidant compounds are capable of inhibiting or retarding oxidative harm caused by oxidants through several mechanisms, such as the inhibition of free radicals and metal complexation [32]. Antioxidant compounds are widely used in cosmetics, especially “anti-aging” products, as they assist in restoring the skin and preventing signs of aging, promoting a reduction in wrinkles [33]. Regarding hair, antioxidants increase blood circulation, preventing hair loss and assisting in healthy hair growth [34].
Studies indicate that there is no single method capable of precisely quantifying the antioxidant properties of a given compound; thus, the use of several different methods is recommended [35]. Therefore, different assays were used in the present study to assess the radical scavenging activity of plant extracts and combinations, whose results in terms of the concentration (µg/mL) required to scavenge 50% of radicals (IC50) are compared in Table 4.

Table 4.
Radical scavenging activity of extracts of Chenopodium quinoa, Malpighia emarginata, and Glycine max expressed as the concentration required to eliminate 50% of radicals (IC50).
The results show that all the aqueous extracts of the three plants analyzed and their mixture had appreciable antioxidant activity, with that of M. emarginata standing out as it showed the lowest IC50 values in all the methods tested, except for CAT. This result, which is consistent with the excellent antioxidant activity reported for its fruit (acerola cherry) [36], is a positive feature in cosmetic formulations. Although the extracts did not present sufficient antioxidant activity to be used specifically for this purpose, they could be exploited as promising multifunctional additives.
3.3. Assessment of Ocular Irritation Potential of Extracts (HET-CAM Test)
The results of the ocular irritant potential (IP) of the extracts assessed by the hen’s egg chorioallantoic membrane test (HET-CAM) are summarized in Table 5. The C. quinoa extract was classified as a non-irritant (IP = 0), G. max as a low irritant (IP = 3.86 ± 0.19), and M. emarginata as a moderate irritant (IP = 5.84 ± 0.09), while sodium lauryl sulfate 1%, which is widely used as a surfactant in shampoos, proved to be a severe irritant (IP = 18.78 ± 0.82).

Table 5.
Results of hen’s egg chorioallantoic membrane test applied to the extracts of Chenopodium quinoa, Glycine max, and Malpighia emarginata.
Owing to the lack of and low irritation potential of C. quinoa and G. max extracts, respectively, their use in cosmetic applications would not pose any risk, enabling formulations of shampoos for individuals with sensitive skin. On the other hand, the M. emarginata extract, despite being considered a moderate irritant, showed a much lower irritation potential than sodium lauryl sulfate.
3.4. Cleaning Potential of Extracts
The analysis of the cleaning potential of the extracts alone and in mixture revealed that only that of M. emarginata and mainly the combination of C. quinoa and G. max at 4% concentration allowed detectable sebum removal yields of 4.4% and 7.6%, respectively (Figure 4).
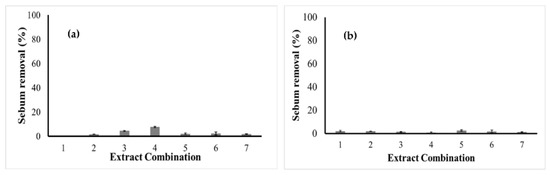
Figure 4.
Cleaning potential of plant extracts at concentrations of (a) 4% and (b) 10%. Extract combinations: 1. C. quinoa; 2. G. max; 3. M. emarginata; 4. C. quinoa + G. max; 5. C. quinoa + M. emarginata; 6. G. max + M. emarginata; and 7. C. quinoa + G. max + M. emarginata.
Regarding the extracts at the same concentration but containing 10% disodium cocoyl glutamate (DCG) (Figure 5a), the best result was achieved with the same combination (DCG + C. quinoa and G. max), which allowed a cleaning yield (80.79%) 2.3-fold greater than that of DCG alone. However, the presence of extracts increased the cleaning potential compared with DCG alone of only two other combinations (DCG + M. emarginata and DCG + C. quinoa + M. emarginata), with the worst performance (1.29%) being detected for DCG + C. quinoa.
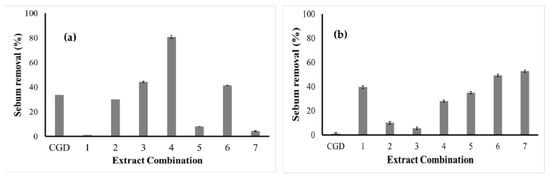
Figure 5.
Cleaning potential of plant extracts at concentrations of (a) 4% + 10% disodium cocoyl glutamate and (b) 10% + 4% disodium cocoyl glutamate. 1. C. Quinoa; 2. G. Max; 3. M. Emarginata; 4. C. Quinoa + G. Max; 5. C. Quinoa + M. Emarginata; 6. G. Max + M. Emarginata; and 7. C. quinoa + G. max + M. emarginata.
As for the formulations containing the extracts at concentrations of 10% and 4% DCG, the cleaning yield of DCG alone was much lower (0.94%) than that of all extract combinations, with the best performance (52.73%, 56-fold greater) being obtained with the mixture of the three extracts (Figure 5b). As the aim of the present study was to reduce the quantity of synthetic surfactants in formulations as much as possible, and the extracts increased the cleaning capacity of 4% DCG, shampoo formulations could be prepared using the extracts as primary surfactants and DCG as a secondary surfactant. It should be remembered that DCG was selected in the present study because it is a milder synthetic surfactant than sulfates and the favorite when the goal is to formulate products that are less likely to cause an allergic reaction [37].
3.5. Determination of Hydrophilic/Lipophilic Balance of Extracts
The determination of the hydrophilic/lipophilic balance (HLB) of extracts is important, as emulsion is the most widely used physical form to integrate liposoluble and water-soluble ingredients in cosmetic formulations. In particular, knowing the HLB of emulsifying agents is fundamental to knowing what liposoluble ingredients these agents can emulsify. The emulsifying capacity of the extracts studied here was confirmed for some vegetable oils in a previous study [11]. The C. quinoa extract exhibited a milky, stable dispersion in contact with water, with HLB in the range of 8–10, whereas those of G. max and M. emarginata formed a translucent dispersion with HLB in the range of 10–13.
3.6. Characterization of Biosurfactants in Plant Extracts
The 1H NMR spectrum of the biosurfactants isolated from the extracts suggested the presence of hydrogens of the methyl group (0.8–1.0 ppm), aliphatic carbons (1.2–1.4 ppm, 1.4–1.6 ppm, and 1.6 ppm), and carbonyl groups (2.2–2.4 ppm). The region between 3 and 4 ppm from the biosurfactants isolated from G. max and M. emarginata is an indication of the presence of hydrogens bonded to atoms close to oxygen, quite common in alcohol functions, probably from the sugar fraction present in the biosurfactant molecule. The 5.2–5.4 ppm region was attributed to hydrogens near double bonds. The 1H NMR spectra of standard saponin showed signals in regions similar to those of the biosurfactants isolated from the extracts, confirming the presence of hydrogens both in the methyl group and near double bonds, aliphatic carbons, and carbonyl groups. Ester groups were also identified in the biosurfactants isolated from C. quinoa and M. emarginata (Figure 6, Figure 7, Figure 8 and Figure 9).
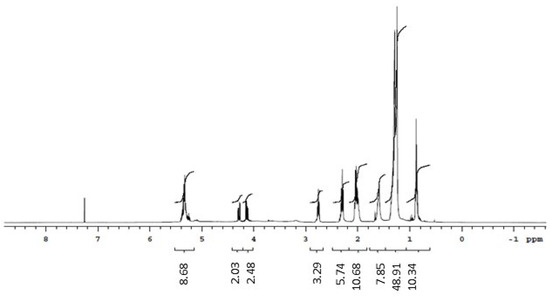
Figure 6.
1H NMR spectrum (CDCl3, 300 MHz) of the biosurfactant from Chenopodium quinoa.
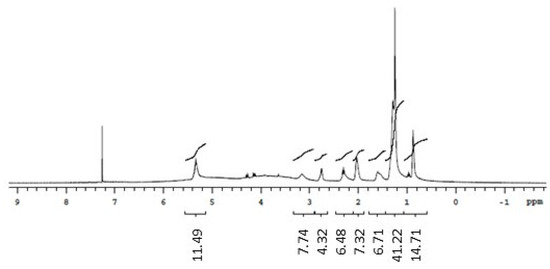
Figure 7.
1H NMR spectrum (CDCl3, 300 MHz) of the biosurfactant from Glycine max.
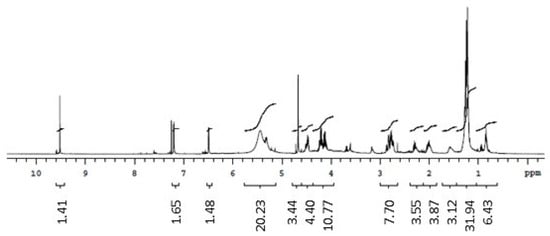
Figure 8.
1H NMR spectrum (CDCl3, 300 MHz) of the biosurfactant from Malpighia emarginata.
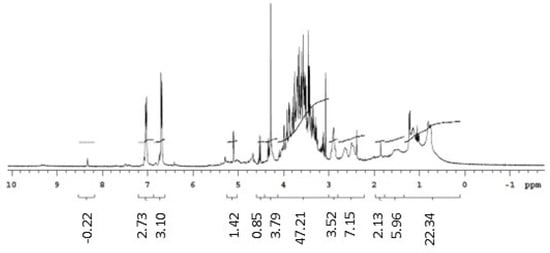
Figure 9.
1H NMR spectrum (D2O, 300 MHz) of the standard saponin from Sigma.
Doublets from anomeric protons of sugar residues bonded to aglycone triterpenoid, which constitute one of the most characteristic NMR signals corresponding to saponins [38], were identified in all isolated biosurfactants between 4.1 and 4.3 ppm and in the standard at 4.5 ppm.
The 13C NMR spectra revealed double bond peaks between 128 and 130 ppm for all samples, as well as methyl signals attributed to triterpene saponins both in the standard (20 ppm) and the isolated biosurfactants (20–30 ppm). Signals characteristic of oxygenated carbons were also found between 60 and 62 ppm. Peaks of aromatic carbons (152–154 ppm) were detected in both the standard and biosurfactants isolated from C. quinoa and M. emarginata (Figures S1–S4). These results are consistent with the findings of Nascimento et al. [39]. No characterization of saponins from M. emarginata was found in the literature, which makes this study the first such report.
In the UV spectra, all samples showed a maximum absorption peak at 220 nm and a second peak between 270 and 290 nm, demonstrating a consistent tendency (Figure 10). These peaks may be related to proteins, sugars, and flavonoids in saponins [40].
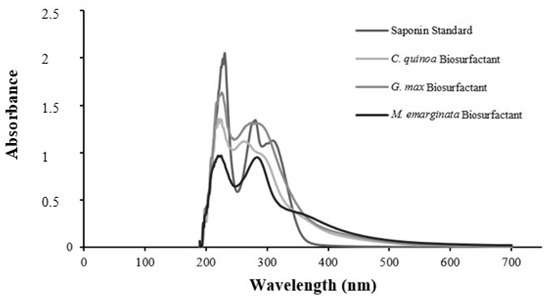
Figure 10.
UV spectra of standard saponin (Sigma) (λmax 220 and 285 nm) and biosurfactants from Chenopodium quinoa (λ max 220 and 270 nm), Glycine max (λmax 220 and 290 nm), and Malpighia emarginata (λmax 220 and 285 nm).
The FTIR spectra of the standard and isolated, partially purified plant-derived surfactants indicated characteristic absorbance peaks of saponins (Figure 11). The long, sharp peak at 3402 cm−1 indicates the presence of the hydroxyl groups (–OH), the peak at 2940 cm−1 indicates the characteristic alkyl groups (C–H) of alkanes, and the peaks at 1634 and 1063 cm−1 indicate the C=C and C–O–C bonds, respectively, with –OH, C–H, and C=C being characteristic of saponins, while the C–O–C of glycosidic bonds is characteristic of sapogenins. The FTIR spectra correspond to signals indicative of saponins in the extracts and are in agreement with the results of similar studies found in the literature [41,42].
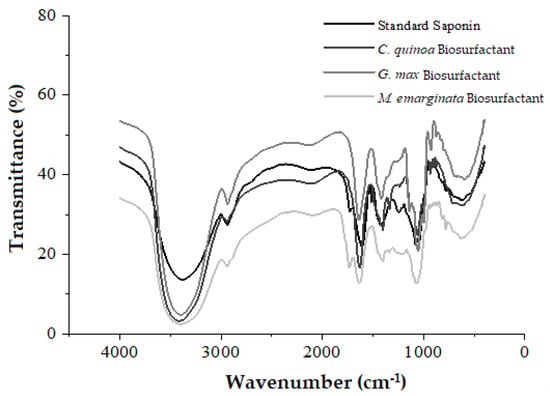
Figure 11.
FTIR spectra of standard saponin (Sigma) and biosurfactants from Chenopodium quinoa, Glycine max, and Malpighia emarginata.
3.7. Development of Shampoo Formulations
The components used in shampoo formulations are listed in Table 6. Synthetic thickeners, wetting agents, and moisturizers were replaced with natural ingredients, i.e., xanthan gum, plant-derived glycerin, and coconut oil, respectively, and the quantity of synthetic surfactants was reduced by the use of natural surfactants in the extract, which is the focus of the present study.

Table 6.
Shampoo formulations developed using criteria established in the present study.
The combination of surfactant agents, concentration, and primary and secondary surfactants were selected based on the results of the cleaning potential test performed on plant extracts. As shown in Table 7, formulation F1 was developed without the addition of any surfactant, F2 with 4% DCG, and the other formulations using the extracts alone or in combination at a concentration of 10% of primary surfactants and 4% DCG as a secondary surfactant.

Table 7.
Composition of formulations, showing primary and secondary surfactants.
3.8. Characterization of Formulations
3.8.1. Determination of Surface and Interfacial Tension
The determination of surface and interfacial tension in shampoo formulations is fundamental, as these aspects perform important functions in terms of product quality and stability. The detergency of a shampoo is directly related to the surface tension, i.e., the lower the surface tension, the greater its cleansing action [43]. On the other hand, interfacial tension is related to the affinity among molecules of different components and their mixing capacity, i.e., the lower the interfacial tension, the greater the affinity, mixing capacity, and stability [44]. The results of surface and interfacial tensions of the shampoo formulations developed in this study are presented in Figure 12.
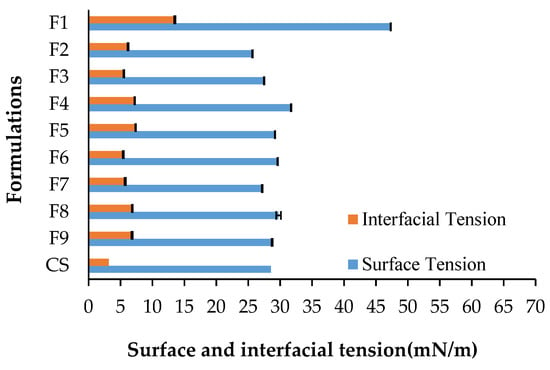
Figure 12.
Surface and interfacial tensions of formulations tested in this study. The codes of the formulations are given in Table 7.
The surface tension was 47.3 ± 0.08 mN/m in the absence of any surfactant (F1), 25.6 ± 0.36 mN/m using DCG alone (F2), and 28.5 ± 0.04 mN/m in the commercial shampoo (CS) taken as a reference, while it ranged from 27.1 ± 0.04 (F7: CGD + C. quinoa + M. emarginata) to 31.7 ± 0.06 mN/m (F4: CGD + G. max) in the other formulations (F3 to F9). On the other hand, interfacial tension was the highest in the absence of a surfactant (13.5 ± 0.1 mN/m) and the lowest in the commercial shampoo (3.1 ± 0.05 mN/m), was 6.1 ± 0.07 mN/m using DCG alone, and ranged from 5.4 ± 0.05 mN/m (F3: DCG + C. quinoa) to 7.3 ± 0.04 mN/m (F5: DCG + M. emarginata) in the other formulations. These results suggest that the C. quinoa extract enhanced the action of DCG in F1, F3, and F7, which had the lowest interfacial tensions among the extract-containing formulations.
According to AlQuadeib et al. [45], a shampoo is considered to have good quality when it reduces the surface tension of water from 72 to 40 mN/m or less. All the formulations with plant extracts tested here had surface tension values lower than 32 mN/m, showing potential for the development of quality shampoo products. Bhatt et al. [46] prepared a shampoo using G. max seeds, whose surface tension (33.3 mN/m) was close to the range found in this study, while no recent studies reported the surface tension of shampoos formulated with C. quinoa or M. emarginata.
3.8.2. Determination of pH, Wettability, Solids Content, and Density
The results of pH, wettability, percentage of solids, and density of tested formulations are listed in Table 8.

Table 8.
pH, wettability, percentage of solids content, and density of formulations. The codes of the formulations are given in Table 7.
Due to the naturally acidic pH of the scalp, which serves as a barrier to viruses, bacteria, and other contaminants, slightly acidic shampoos are generally recommended [47]. Among the formulations, the pH ranged from 5.10 ± 0.00 (F7: DCG + C. quinoa + M. emarginata) to 5.96 ± 0.00 (F4: DCG + G. max), which is within the natural range of the scalp (4.5–6.2).
Wettability is the capacity of a liquid to spread on a surface. At times, however, this is not possible due to the hydrophobicity of the surface and the high surface tension of water. For a shampoo to come into contact with the scalp and hair fibers, a significant amount of surfactants must be incorporated into its formulation [48]. Surfactant molecules are, in fact, able to absorb at the hair/water and scalp/water interface, leading to a reduction in interfacial tension, as well as the promotion of wetting and, consequently, cleaning [49].
The wetting of the formulation without the addition of surfactant (F1) took more than 24 h, which means that it was almost negligible. The presence of DCG alone in the formulation (F2) reduced the wetting time to 11.52 s, and the addition also of any of the extracts further improved the wettability, achieving a minimum value (3.22 s) in F9 (DCG + C. quinoa + G. max + M. emarginata), which was quite close to that of the commercial shampoo. According to AlQuadeib et al. [45], the shorter the time until the disk’s submergence, the greater the detergency of a shampoo and its ability to remove dirt and sebum.
The solid content of shampoos has an effect on rinsing, i.e., the lower the solid content, the faster and easier the rinse [19]; therefore, a good shampoo should have a maximum content of solids of 30% [23,50]. All formulations developed in the present study had a solids content within the range of 13.4% (F6: DCG + C. quinoa + G. max) to 17.6% (F8: CGD + G. max + M. emarginata), while that of the commercial shampoo was 12%. As all these values are lower than the recommended threshold value (<30%), the proposed formulations can easily be applied and rinsed from the hair.
The importance of determining the density of a shampoo resides in the fact that this parameter indicates the loss of volatile ingredients or the incorporation of air. The mean density of formulations tested in the present study (1.112 ± 0.04 g/cm3) was very close to that of the commercial shampoo taken as reference in this study (1.053 ± 0.01 g/cm3) and within the range reported for commercial shampoos (0.950–1.100 g/cm3) [51].
3.8.3. Foam Formation and Dirt Dispersion Test
Although the generation of foam has little to do with the cleaning capacity of shampoos, it is of the utmost importance to consumers and therefore an important criterion in the evaluation of shampoos. All formulations exhibited stable foam-forming characteristics, with a mean foam volume of 19.5 ± 2.1 mL, with the exception of F4 (DCG + G. max) and F6 (DCG + C. quinoa + G. max), whose foam decreased over time. All formulations with extracts produced more foam than that with DCG alone (F2), confirming the foam-forming potential of the extracts, the one without surfactant (F1) produced no foam, and the commercial shampoo (CS) produced no less than 34 mL of stable foam.
Regarding dirt dispersion, a shampoo that allows dye to concentrate in the foam is considered to have poor quality, as the dirt should stay in the water. Dirt that remains in the foam is difficult to remove and can be redeposited on the hair [45]. In the present study, all formulations, including the commercial shampoo, provided similar results, with the amount of dye in the foam considered light. These results indicate that almost no dirt would remain in the foam. Considering the commercial shampoo as the standard, the formulations exhibited satisfactory results (Table 9).

Table 9.
Foam stability and dirt dispersion of formulations. The codes of formulations are given in Table 7.
3.8.4. Cleaning Potential of Formulations
The F4 (DCG + G. max), F6 (DCG + C. quinoa + G. max), and F5 (DCG + M. emarginata) formulations exhibited the greatest cleaning potential, with sebum removal yields (98 ± 4.2%, 87 ± 3.1%, and 74 ± 0.5%, respectively) significantly higher than that found for the commercial shampoo (63 ± 2.3%). Moreover, the formulation with DCG alone as the surfactant (F2) showed a sebum removal yield of 57 ± 0.2%, which means that the cleaning action of the formulations was enhanced by the addition of those plant extracts. On the contrary, the F3, F7, F8, and F9 formulations had lower mean sebum removal yields (51 ± 3.8%) and the one without the addition of surfactants (F1) had no cleaning potential (Figure 13).
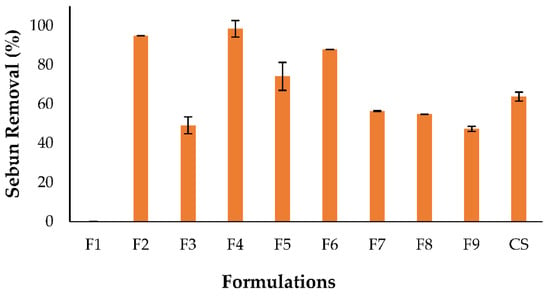
Figure 13.
Cleaning potential of formulations: F1: no surfactant; F2: DCG; F3: DCG + C. quinoa; F4: DCG + G. max; F5: DCG + M. emarginata; F6: DCG + C. quinoa + G. max; F7: DCG + C. quinoa + M. emarginata; F8: DCG + G. max + M. emarginata; and F9: DCG + C. quinoa + G. max + M. emarginata; CS: commercial shampoo.
3.8.5. Preliminary Stability Test
The first step of preliminary stability tests consists of subjecting the product to centrifugation for 30 min at 3000 rpm, after which all formulations remained stable and were, therefore, approved for the stability test.
The appearance, color, and pH of the formulations underwent no alterations when submitted to heating and cooling cycles. However, the odor of F6 (DCG + C. quinoa + G. max) was altered after the first cycle under all temperatures, becoming pungent, likely due to contamination. In contrast, all other formulations kept their fragrant aroma (Table 10). A non-uniform appearance was found for F4 (DCG + G. max) prior to the stability test, likely due to the non-emulsifying feature of coconut oil used as a moisturizer, which separated from the formulation and appeared in the form of small, whitish spheres. This occurrence may have been due to the higher HLB of G. max extract (10–13) compared with coconut oil (8), which may have hindered the formation of a stable emulsion.

Table 10.
Organoleptic characteristics of the formulations submitted to alternating heating and cooling cycles. The codes of the formulations are given in Table 7.
The preliminary stability study consists of a test in the initial phase of product development, the duration of which is short. Extreme temperature conditions are employed to accelerate possible reactions among the components and the emergence of signs that can be analyzed with regard to specific characteristics. Owing to the conditions by which it is conducted, the purpose of this test is not to estimate the useful life of the product, but rather to assist in the screening of formulations. Thus, the F4 and F6 formulations needed to undergo a reassessment process regarding their sterility during production and storage (F6) and the effectiveness of the conservative used, with the choice of another vegetable oil that has an ideal HLB to serve as a moisturizer (F4).
4. Conclusions
The present study showed that formulating shampoos with a lower concentration of synthetic surfactants than the current practice would be possible and that plant-derived surfactants have properties suitable for obtaining good alternative natural products.
The structural characterization of the biosurfactants present in the extracts of Chenopodium quinoa, Glycine max, and Malpighia emarginata confirmed the presence of saponin biosurfactant. The plant surfactants had an excellent performance in terms of stability, maintaining their surface-active and emulsifying properties when exposed to pH ranges compatible with the pH used in shampoo formulations, as well as remaining stable at the temperatures used to form emulsions in cosmetic products. The extracts also showed excellent performance in terms of toxicity, showing no or low toxicity compared with sodium lauryl sulfate, proving to be good primary surfactants.
Among the formulations produced, five of them (F4, F5, F6, F7, and F8) have the potential to undergo subsequent quality-control tests and become commercial formulations, as the results of the reduction in surface and interfacial tensions, wettability, foaming capacity, dirt dispersion, and cleaning capacity were within the standards of formulations already available on the market.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11030879/s1, Figure S1: 13C NMR spectrum (D2O, 300 MHz) of standard saponin from Sigma.; Figure S2: 13C NMR spectrum (CDCl3, 300 MHz) of saponin from Chenopodium quinoa.; Figure S3: 13C NMR spectrum (CDCl3, 300 MHz) of saponin from Glycine max; Figure S4: 13C NMR spectrum (CDCl3, 300 MHz) of saponin from Malpighia emarginata.
Author Contributions
Conceptualization, L.A.S. and K.G.O.B.; methodology, K.G.O.B., H.M.M., B.O.V., T.C.M.S. and E.L.F.; validation, L.A.S. and R.D.R.; formal analysis, L.A.S.; investigation, K.G.O.B. and R.D.R.; resources, L.A.S.; data curation, K.G.O.B. and A.C.; writing—original draft preparation, K.G.O.B. and B.O.V.; writing—review and editing, K.G.O.B., L.A.S. and A.C.; visualization, L.A.S. and A.C.; supervision, L.A.S. and R.D.R.; project administration, L.A.S.; funding acquisition, L.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian fostering agencies Fundação de Apoio à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Finance Code 001).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The authors are grateful to the laboratories of Universidade Católica de Pernambuco (UNICAP), Universidade Federal de Pernambuco (UFPE), Instituto Avançado de Tecnologia e Inovação (IATI), and Finnofarma Farmácia de Manipulação, Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gama, R.M.; França-Stefoni, S.A.; Sá-Dias, T.C.; Bedin, V.; Baby, A.R.; Velasco, M.V.R. Protective effect of conditioner agents on hair treated with oxidative hair dye. J. Cosmet. Dermatol. 2018, 17, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Zeragui, B.; Hachem, K.; Halla, N.; Kahloula, K. Essential oil from Artemisia judaica L. (ssp. sahariensis) flowers as a natural cosmetic preservative: Chemical composition, and antioxidant and antibacterial activities. J. Essent. Oil-Bear. Plants 2019, 22, 685–694. [Google Scholar] [CrossRef]
- Leite, M.G.A.; Maia Campos, P.M.B.G. Development of shampoo formulations with guarana extract: Influence of thickening agents in the texture profile. Int. J. Phytocosmet. Nat. Ingred. 2020, 7, e1. [Google Scholar] [CrossRef]
- Milanović, I.; Bašić, J.; Pecarski, D.; Dragaš Milovanović, D. Potential irritants and allergens in shampoos-type preparations. Maced. Pharm. Bull. 2020, 66, 109–110. [Google Scholar] [CrossRef]
- Cosmetics Europe Socio-Economic Contribution of the European Cosmetics Industry. Available online: https://www.cosmeticseurope.eu/files/4715/6023/8405/Socio-Economic_Contribution_of_the_European_Cosmetics_Industry_Report_2019.pdf (accessed on 30 October 2022).
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities anddistribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. J. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Chen, C.; Zhu, L. Enhanced soil washing ofphenanthrene by a plant-derived natural biosurfactant, Sapindus saponin. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 122–128. [Google Scholar] [CrossRef]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Mannosylerythritol lipids: Microbial production and their applications. J. Oleo Sci. 2015, 20, 145–177. [Google Scholar] [CrossRef]
- Du, K.; Li, J.; Wang, L.; Hao, J.; Yang, X.; Gao, X.; Chang, Y. Biosurfactant trehalose lipid-enhanced ultrasound-assisted micellar extraction and determination of the main antioxidant compounds from functional plant tea. J. Sep. Sci. 2020, 43, 799–807. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Silva, I.G.S.; Almeida, F.C.G.; Rufino, R.D.; Sarubbo, L.A. Plant-derived biosurfactants: Extraction, characteristics and properties for application in cosmetics. Biocatal. Agric. Biotechnol. 2021, 34, 102036. [Google Scholar] [CrossRef]
- Cavalcanti, M.H.C.; Magalhães, V.M.; Rocha e Silva, N.M.P.; Farias, C.B.B.; Almeida, F.C.G.; Sarubbo, L.A. Maximization of biosurfactant production by Bacillus invictae using agroindustrial residues for application in the removal of hydrophobic pollutants. Chem. Eng. Trans. 2020, 79, 55–60. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active agents from two Bacillus species. Microbiology 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Prazeres, L.D.K.T.; Aragão, T.P.; Brito, S.A.; Almeida, C.L.F.; Silva, A.D.; De Paula, M.M.F.; Farias, J.S.; Vieira, L.D.; Damasceno, B.P.G.L.; Rolim, L.A.; et al. Antioxidant and antiulcerogenic activity of the dry extract of pods of Libidibia ferrea Mart. Ex Tul. (Fabaceae). Oxid. Med. Cell. Longev. 2019, 2019, 1983137. [Google Scholar] [CrossRef] [PubMed]
- de Veras, B.O.; de Oliveira, M.B.M.; Oliveira, F.G.d.S.; dos Santos, Y.Q.; de Oliveira, J.R.S.; Lima, V.L.d.M.; Almeida, J.R.G.d.S.; Navarro, D.M.d.A.F.; de Aguiar, J.C.R.d.O.F.; Aguiar, J.d.S.; et al. Chemical composition and evaluation of the antinociceptive, antioxidant and antimicrobial effects of essential oil from Hymenaea cangaceira (Pinto, Mansano & Azevedo) native to Brazil: A natural medicine. J. Ethnopharmacol. 2020, 247, 112265. [Google Scholar] [CrossRef]
- de Veras, B.O.; de Oliveira, J.R.S.; Lima, V.L.d.M.; Navarro, D.M.d.A.F.; de Oliveira, J.C.R.d.O.F.; Moura, G.M.d.M.; da Silva, J.W.; de Assis, C.R.D.; Gorlach-Lira, K.; de Assis, P.A.C.; et al. The essential oil of the leaves of Verbesina macrophylla (Cass.) S.F.Blake has antimicrobial, anti-inflammatory and antipyretic activities and is toxicologically safe. J. Ethnopharmacol. 2021, 265, 113248. [Google Scholar] [CrossRef]
- Wilson, T.D.; Steck, W.F. A modified HET-CAM assay approach to the assessment of anti-irritant properties of plant extract. Food Chem. Toxicol. 2000, 38, 867–872. [Google Scholar] [CrossRef]
- Thompson, D.; Lemaster, C.; Allen, R.; Whittam, J. Evaluation of relative shampoo detergency. J. Soc. Cosmet. Chem. 1985, 286, 271–286. [Google Scholar]
- Azadbakht, M.; Monadi, T.; Esmaeili, Z.; Chabra, A.; Tavakoli, N. Formulation and evaluation of licorice shampoo in comparison with commercial shampoo. J. Pharm. Bioallied Sci. 2018, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, A. Determination of hydrophilic-lipophilic balance value. Int. J. Sci. Res. 2014, 3, 573–575. [Google Scholar]
- Obasi, T.C.; Moldovan, R.; Toiu, A.; Braicu, C.; Bodoki, E.; Berindan-Neagoe, I.; Oniga, I.; Sandulescu, R.; Oprean, R. Molecular-trapping in emulsion’s monolayer: A new strategy for production and purification of bioactive saponins. Sci. Rep. 2017, 7, 14511. [Google Scholar] [CrossRef]
- Durval, I.J.B.; Mendonça, A.H.R.; Rocha, I.V.; Luna, J.M.; Rufino, R.D.; Converti, A.; Sarubbo, L.A. Production, characterization, evaluation and toxicity assessment of a Bacillus cereus UCP 1615 biosurfactant for marine oil spills bioremediation. Mar. Pollut. Bull. 2020, 157, 111357. [Google Scholar] [CrossRef]
- Al Badi, K.; Khan, S.A. Formulation, evaluation and comparison of the herbal shampoo with the commercial shampoos. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 301–305. [Google Scholar] [CrossRef]
- Moghimipour, E.; Jasemnezhad, M.; Soleymani, S.M.; Salimi, A. Preparation and evaluation of a free surfactant herbal shampoo with Acanthophyllum squarrosum saponins. J. Cosmet. Dermatol. 2021, 20, 181–187. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária. Guia de Estabilidade de Produtos Cosméticos—Séries Temáticas, 1st ed.; Agência Nacional de Vigilância Sanitária: Brasilia, Brazil, 2004; p. 52. ISBN 8588233150.
- Cosmetics Europe Guidelines on Stability of Cosmetic Products. Available online: https://www.cosmeticseurope.eu/files/5914/6407/8121/Guidelines_on_Stability_Testing_of_Cosmetics_CE-CTFA_-_2004.pdf (accessed on 24 January 2022).
- Patil, A.; Bhide, S.; Bookwala, M.; Soneta, B.; Shankar, V.; Almotairy, A.; Almutairi, M.; Narasimha Murthy, S. Mini-review theme: Stability of pharmaceutical excipients stability of organoleptic agents in pharmaceuticals and cosmetics. AAPS PharmSciTech 2018, 19, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hajimohammadi, R.; Hosseini, M.; Amani, H.; Najafpour, G.D. Production of saponin biosurfactant from Glycyrrhiza glabra as an agent for upgrading heavy crude oil. J. Surfactants Deterg. 2016, 19, 1251–1261. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Malinauskyte, E.; Cornwell, P.A.; Reay, L.; Shaw, N.; Petkov, J. Effect of equilibrium on the structure and properties of bleach-damaged human hair fibers. Biopolymers 2020, 111, 23401. [Google Scholar] [CrossRef] [PubMed]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2017, 45, 347–351. [Google Scholar] [CrossRef]
- Apak, R.; Mustafa, Ö.; Kubilay, G.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable antioxidant chitosan films useful as an anti-aging skin mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef]
- Joshi, N.; Patidar, K.; Solanki, R.; Mahawar, V. Preparation and evaluation of herbal hair growth promoting shampoo formulation containing Piper betle and Psidium guajava leaves extract. Int. J. Green Pharm. 2018, 2018, S835–S839. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Papa, F.; Vittori, S.; Maggi, F.; et al. Chemopreventive and antioxidant activity of the chamazulene-rich essential oil obtained from Artemisia arborescens L. growing on the isle of La Maddalena, Sardinia, Italy. Chem. Biodivers. 2013, 10, 1464–1474. [Google Scholar] [CrossRef]
- Leffa, D.D.; Da Silva, J.; Daumann, F.; Luiza, A.; Dajori, F.; Longaretti, L.M.; Damiani, A.P.; De Lira, F.; Campos, F.; Ferraz, A.B.F.; et al. Corrective effects of acerola (Malpighia emarginata DC.) juice intake on biochemical and genotoxical parameters in mice fed on a high-fat diet. Mutat. Res. 2014, 770, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Pharma, I. Amisoft ECS-22SB Tensoativo Aniônico Derivado de Aminoácidos. Available online: https://infinitypharma.com.br/wp-content/uploads/2020/05/AmisoftECS-22SB.pdf (accessed on 4 January 2022).
- Khakimov, B.; Tseng, L.H.; Godeljohann, M.; Bak, S.; Engelsen, S.B. Screening for triterpenoid saponins in plants using hyphenated analytical platforms. Molecules 2016, 21, 1614. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, Y.M.; Abreu, L.S.; Lima, R.L.; Costa, V.C.O.; de Melo, J.I.M.; Braz-Filho, R.; Silva, M.S.; Tavares, J.F. Rapid characterization of triterpene saponins from Zornia brasiliensis by HPLC-ESI-MS/MS. Molecules 2019, 24, 2519. [Google Scholar] [CrossRef]
- Yu, X.-L.; He, Y. Development of a rapid and simple method for preparing tea-leaf saponins and investigation on their surface tension differences compared with tea-seed saponins. Molecules 2018, 23, 1796. [Google Scholar] [CrossRef] [PubMed]
- El-Keiy, M.M.; Radwan, A.M.; Mohamed, T.M. Cytotoxic effect of soy bean saponin against colon cancer. J. Biosci. Med. 2019, 7, 70–86. [Google Scholar] [CrossRef]
- Norouzpour, M.; Nabipour, M.; Azdarpour, A.; Akhondzadeh, H.; Santos, R.M.; Keshavarz, A. Experimental investigation of the effect of a quinoa-derived saponin-based green natural surfactant on enhanced oil recovery. Fuel 2022, 318, 123652. [Google Scholar] [CrossRef]
- Meduri, T.S.; Munnangi, L.D.; Potharaju, S.; Suravarapu, S.T.; Swami, V.R.D.; Uppala, V.; Yepuri, D.; Vadlamudi, P.; Nadendla, R.R. Formulation and evaluation of fermented rice water herbal shampoo. J. Drug Deliv. Ther. 2021, 11, 127–130. [Google Scholar] [CrossRef]
- Acosta, E. Engineering cosmetics using the net-average-curvature (NAC) model. Curr. Opin. Colloid Interface Sci. 2020, 48, 149–167. [Google Scholar] [CrossRef]
- AlQuadeib, B.T.; Eltahir, E.K.D.; Banafa, R.A.; Al-Hadhairi, L.A. Pharmaceutical evaluation of different shampoo brands in local Saudi market. Saudi Pharm. J. 2018, 26, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Kumar, A.; Sharma, A. Formulation and evaluation of shampoo formulated by Glycine max seeds. Indian Res. J. Pharm. Sci. 2017, 4, 1232–1238. [Google Scholar] [CrossRef]
- Cline, A.; Uwakwe, L.N.; McMichael, A.J. No sulfates, no parabens, and the “No-Poo” method: A new patient perspective on common shampoo ingredients. Cutis 2018, 101, 22–26. [Google Scholar] [PubMed]
- Tsujii, K. Wetting and surface characterization. In Cosmetic Science and Technology: Theoretical Principles and Applications, 1st ed.; Sakamoto, K., Lochhead, R., Maibach, H., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 373–388. [Google Scholar]
- Akbari, S.; Nour, A.H.; Yunus, R.M.; Farhan, A.H. Biosurfactants as promising multifunctional agent: A mini review. Int. J. Innov. Res. Adv. Stud. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Wasu, M.; Ramarau, N.K.; Yahya, M.H.; Mahmood, S.; Halim, N.A.; Kutty, R.V. Formulation and evaluation of propolis extracts based shampoo on dandruff causing bacteria. Malays. J. Microbiol. 2020, 16, 49–57. [Google Scholar] [CrossRef]
- Do Canto Pereira, G.; de Moura Murat, S.C.; de Souza Magalhães, B.; Dionísio da Silva, L.; Dos Santos Garcia Ribeiro, R.; Ribeiro Benevenuto, B.; de Souza Siqueira Pereira, C. Análise da estabilidade de um shampoo produzido com adição de óleo essencial de alecrim (Rosmarinus officinalis). Rev. Eletron. TECCEN 2020, 13, 2–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).