Kinetic Aspects of Suzuki Cross-Coupling Using Ligandless Pd Nanoparticles Embedded in Aromatic Polymeric Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis Tools
2.2.1. Gas Chromatography-Mass Spectrometry
2.2.2. X-ray Fluorescence Analysis
2.2.3. X-ray Photoelectron Spectroscopy
2.2.4. Scanning Transmission Electron Microscopy
2.3. Experimental Procedures
2.3.1. Catalysts Preparation
2.3.2. Reaction Procedure
3. Results
3.1. Catalyst Characterization
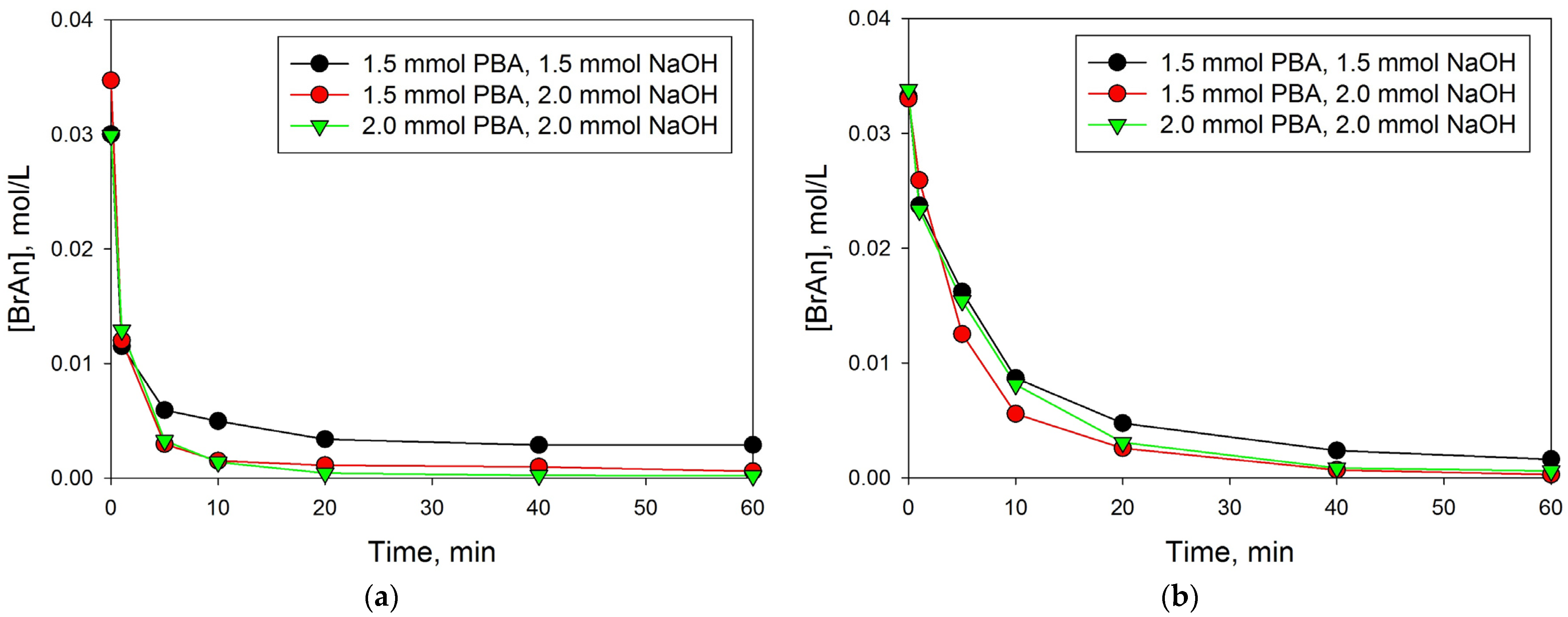
3.2. Effect of PBA-to-NaOH Ratio
3.3. Kinetic Studies and Modeling
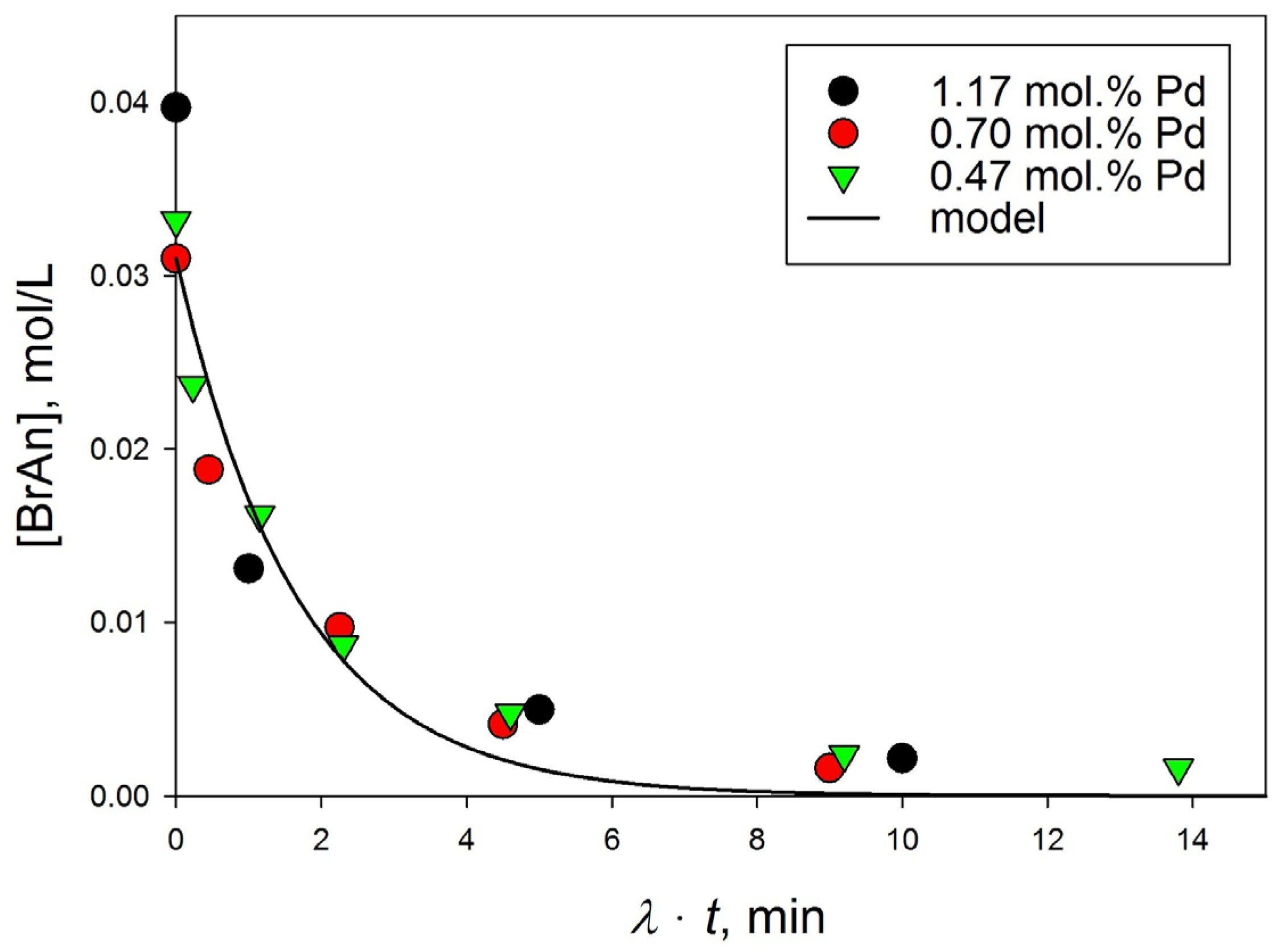
3.3.1. Diffusion Effect
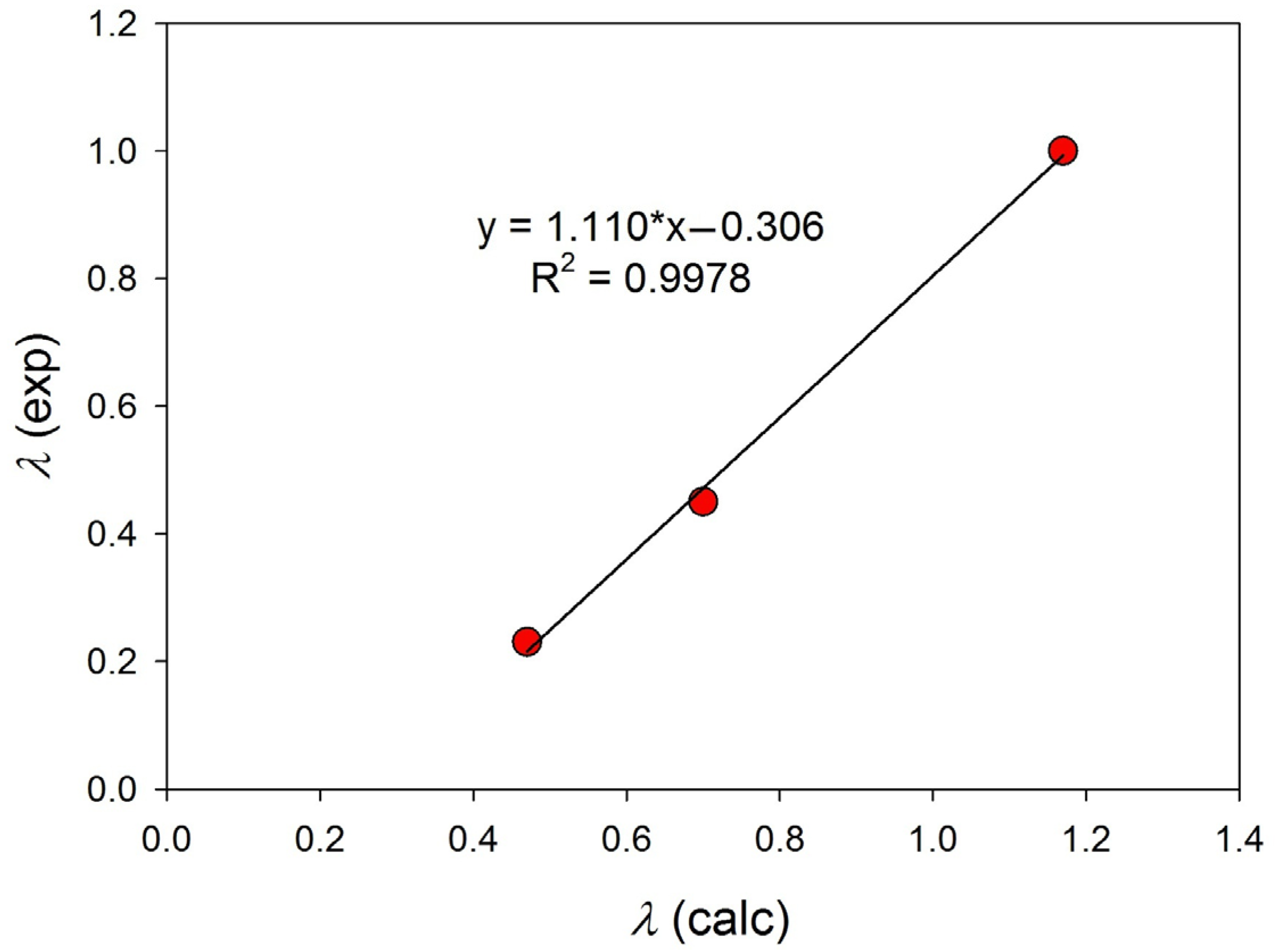
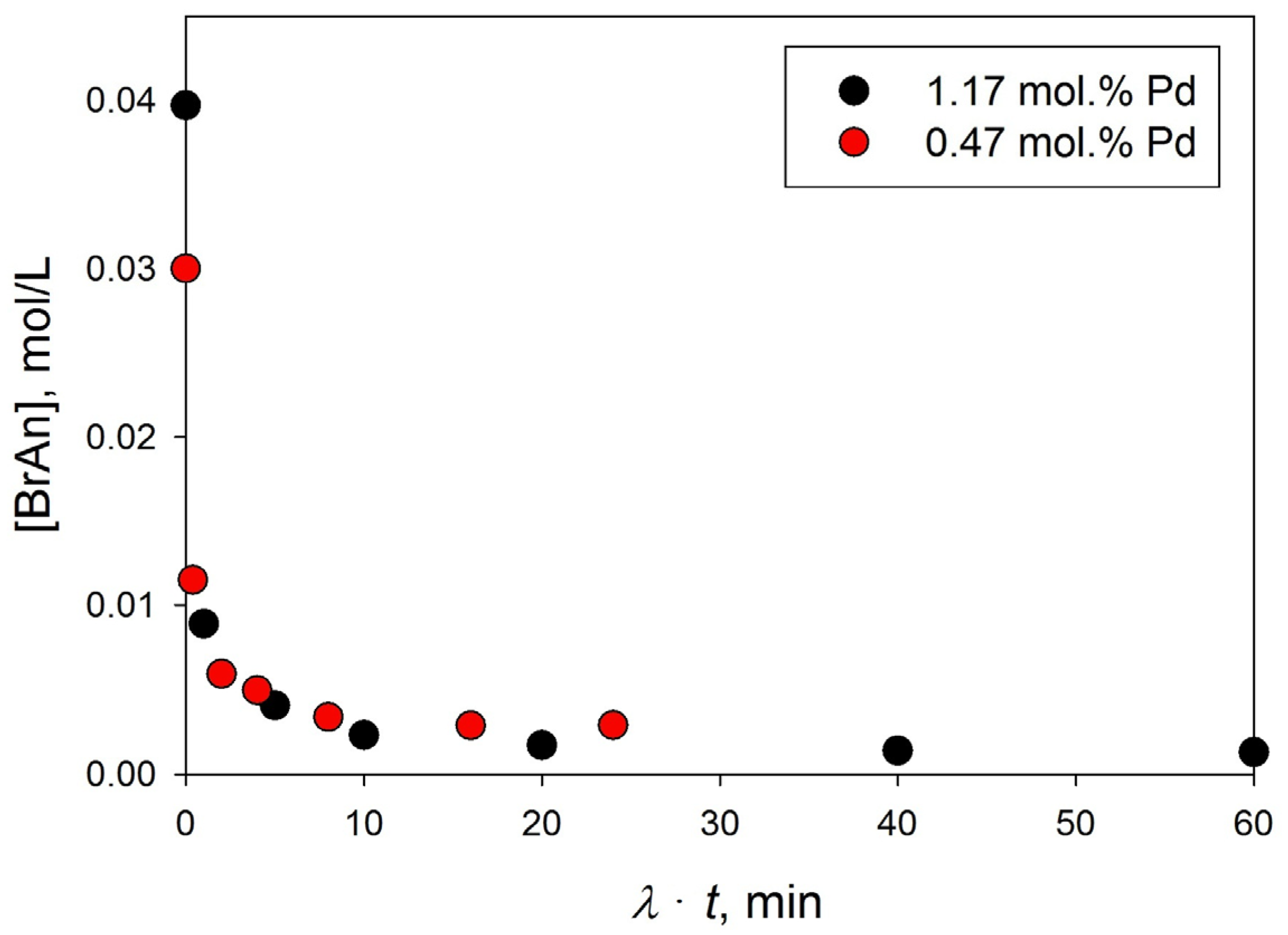
3.3.2. Influence of the Amount of Catalysts
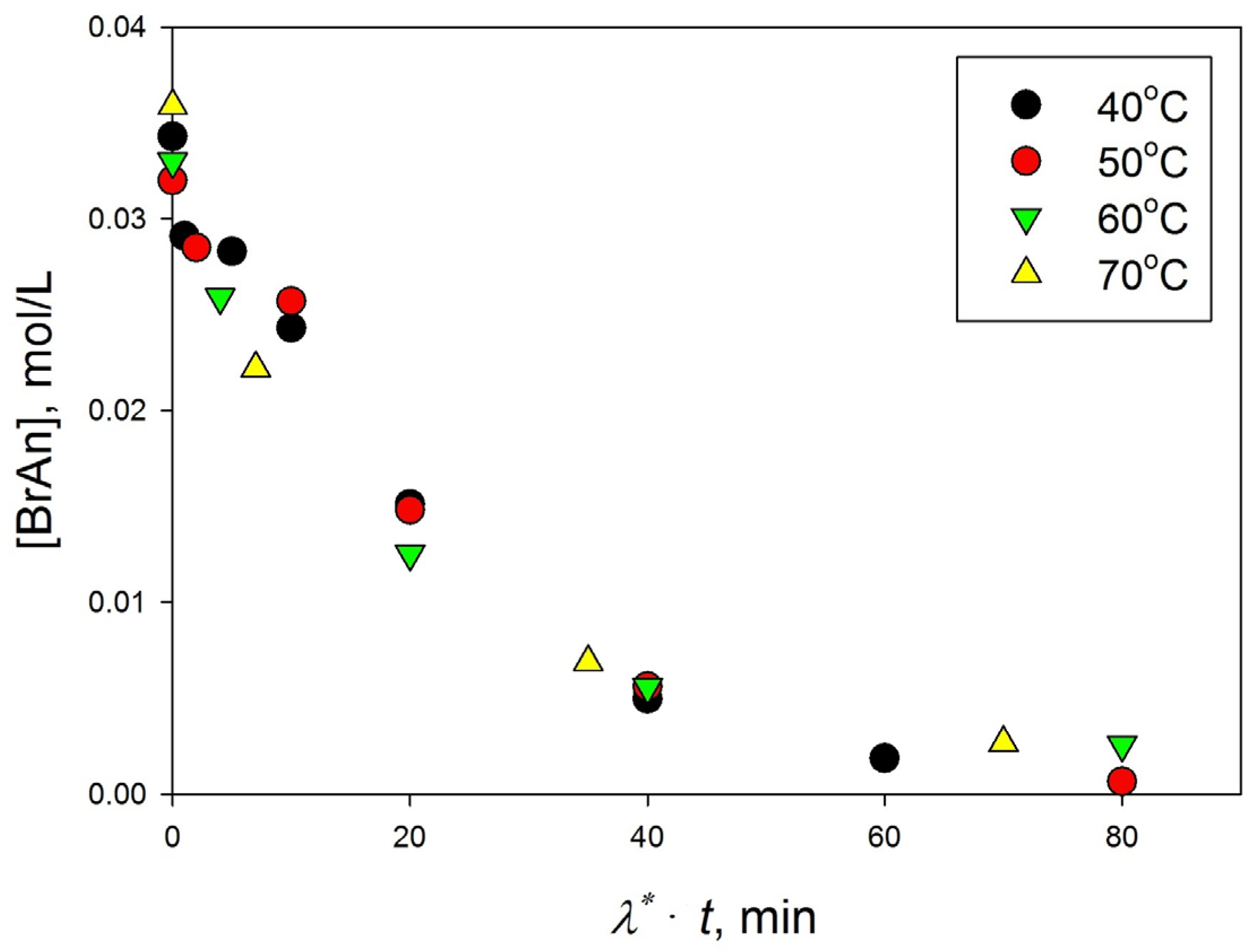
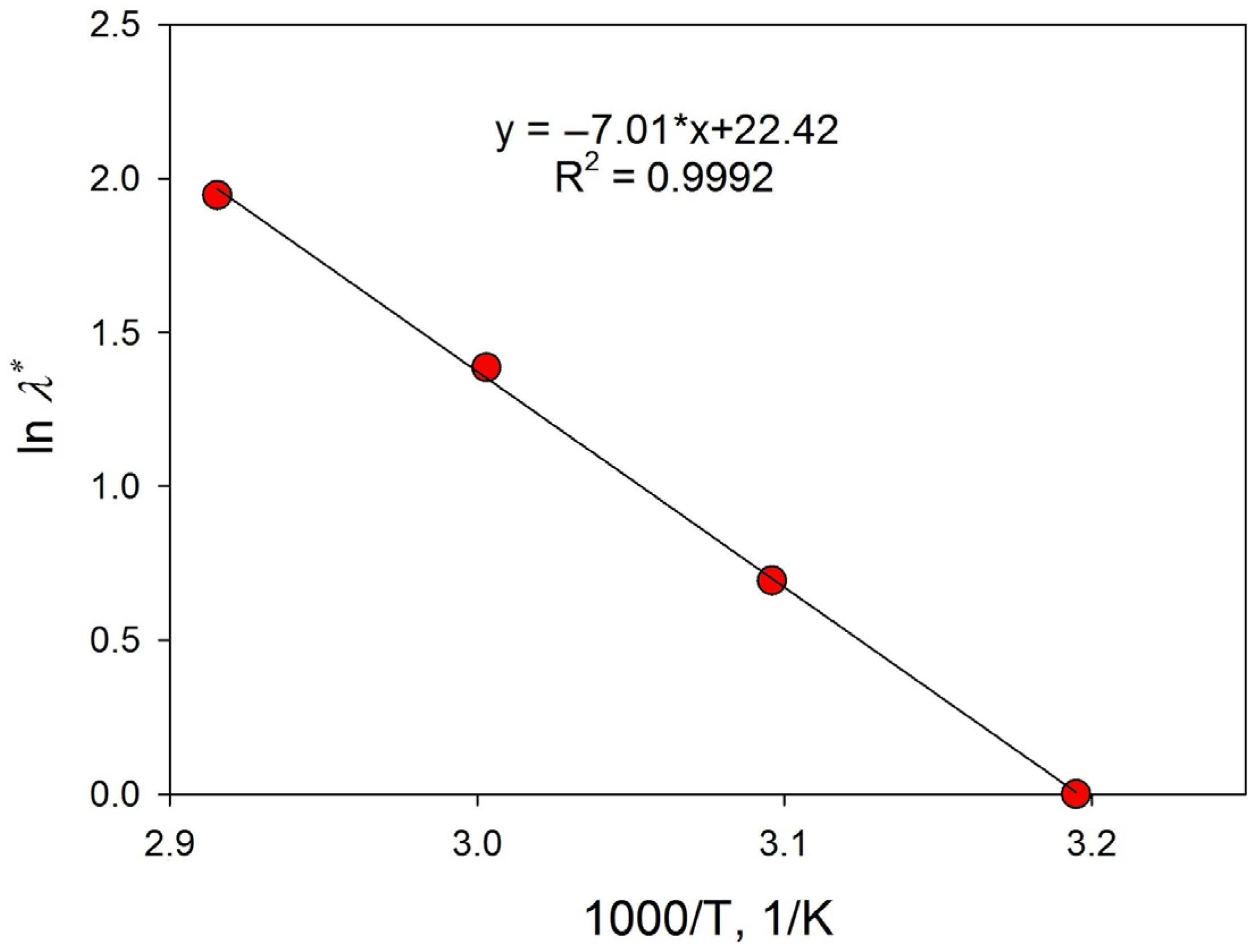
3.3.3. Effect of Temperature
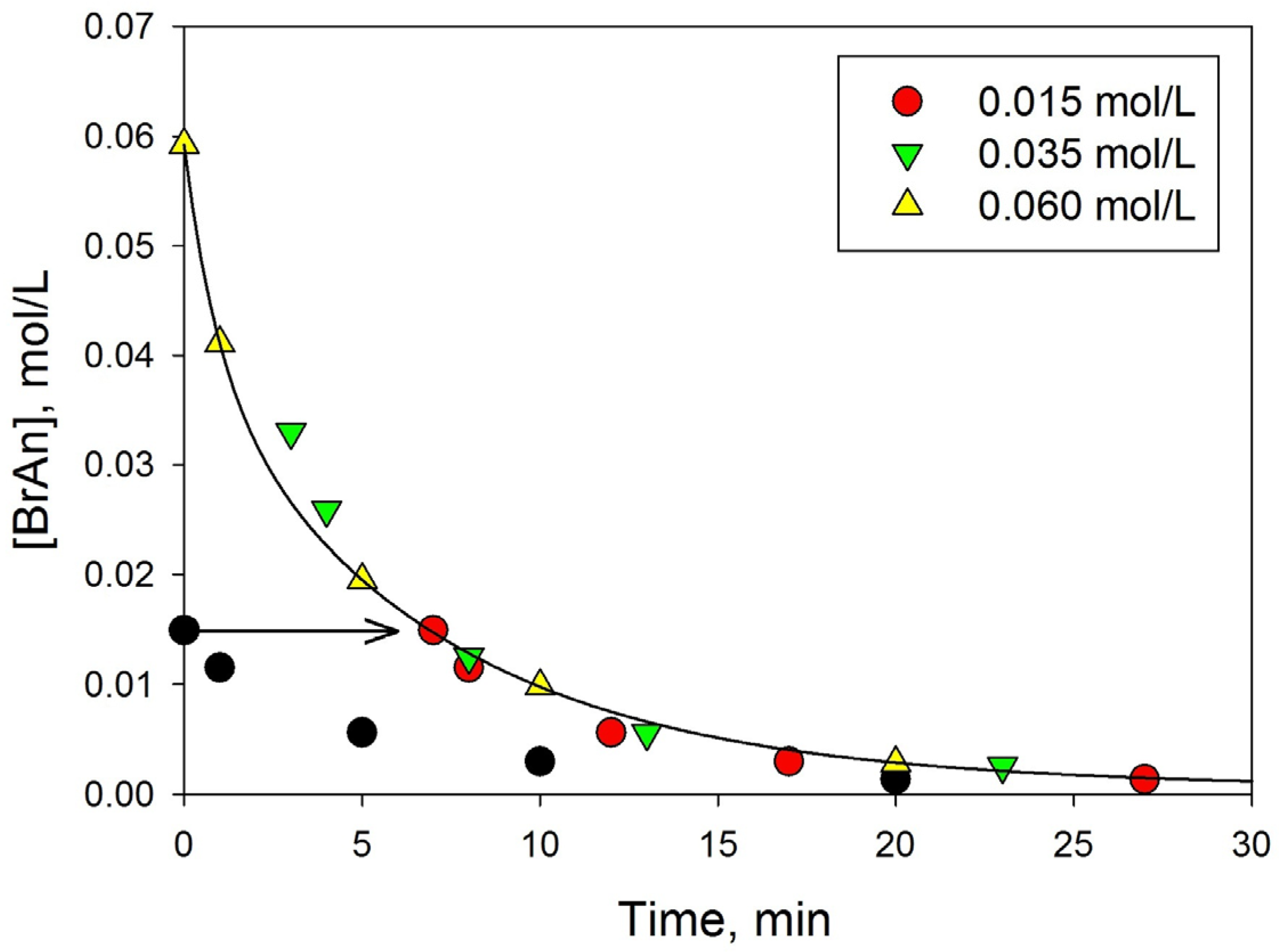
3.3.4. Influence of the Initial Concentration of BrAn
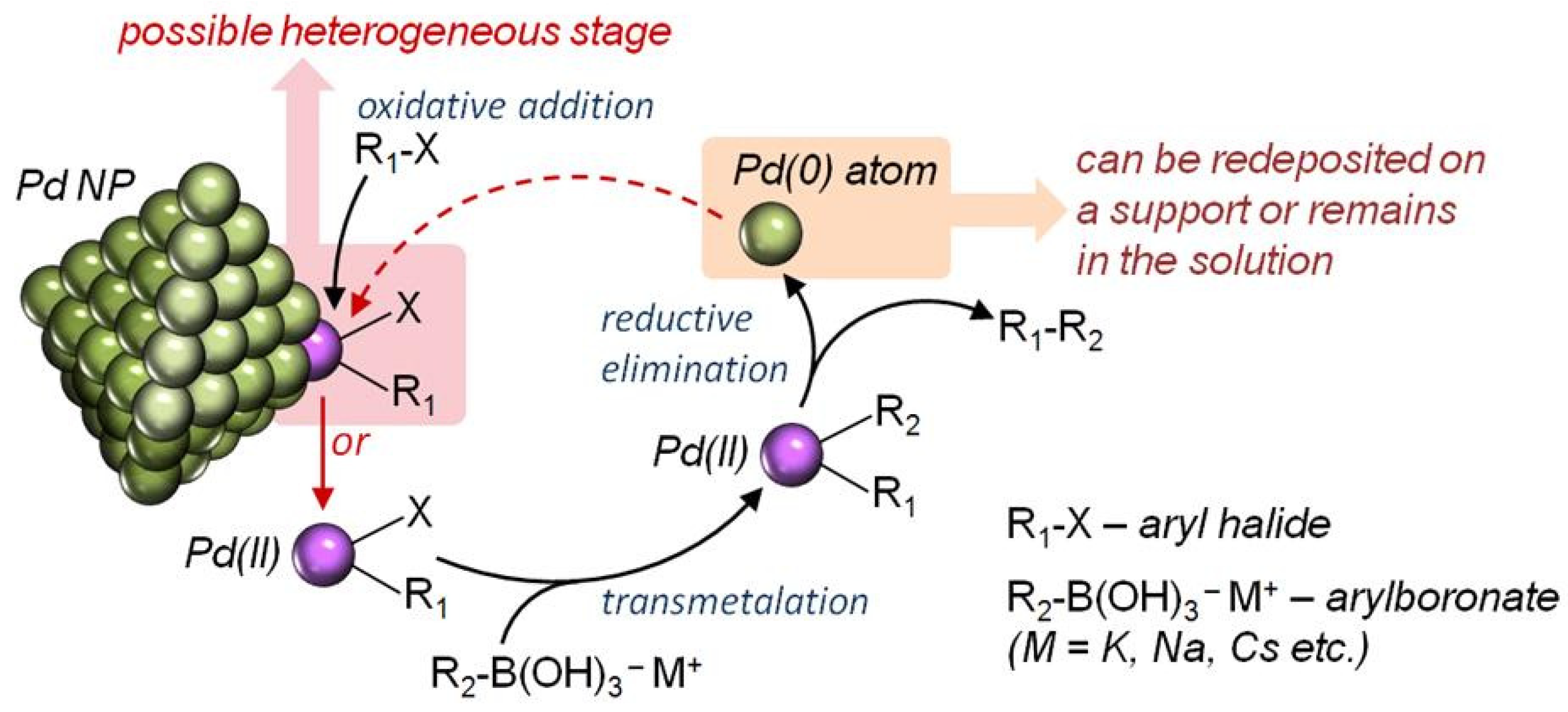
3.3.5. Mathematical Model of the Process and Hypothesis on the Reaction Mechanism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jung, J.-Y.; Taher, A.; Hossain, S.; Jin, M.-J. Highly active heterogeneous palladium catalyst for the Suzuki reaction of heteroaryl chlorides. Bull. Korean Chem. Soc. 2010, 31, 3010–3012. [Google Scholar] [CrossRef]
- Pan, C.; Liu, M.; Zhang, L.; Wu, H.; Ding, J.; Cheng, J. Palladium Catalyzed Ligand-Free Suzuki Cross-Coupling Reaction. Catal. Commun. 2008, 9, 321–323. [Google Scholar] [CrossRef]
- Kylmälä, T.; Tois, J.; Xu, Y.; Franzén, R. One Step Synthesis of Diflunisal Using a Pd-Diamine Complex. Cent. Eur. J. Chem. 2009, 7, 818–826. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Li, H.-Y.; Shi, M.-M.; Jiang, H.; Hu, X.-L.; Li, W.-Q.; Fu, L.; Chen, H.-Z. Pd/C as a Clean and Effective Heterogeneous Catalyst for C–C Couplings toward Highly Pure Semiconducting Polymers. Macromolecules 2012, 45, 9004–9009. [Google Scholar] [CrossRef]
- Soomro, S.S. C-C Coupling Reactions Catalyzed by Supported Palladium in Liquid Phase. Ph.D. Thesis, Technische Universität Münc, Munich, Germany, 2009; 134p. [Google Scholar]
- D’Alterio, M.C.; Casals-Cruañas, È.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Chem. Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef] [PubMed]
- Sołoducho, J.; Olech, K.; Świst, A.; Zając, D.; Cabaj, J. Recent Advances of Modern Protocol for C-C Bonds—The Suzuki Cross-Coupling. Adv. Chem. Eng. Sci. 2013, 3, 19–32. [Google Scholar] [CrossRef]
- Devendar, P.; Qu, R.-Y.; Kang, W.-M.; He, B.; Yang, G.-F. Palladium-Catalyzed Cross-Coupling Reactions: A Powerful Tool for the Synthesis of Agrochemicals. J. Agric. Food Chem. 2018, 66, 8914–8934. [Google Scholar] [CrossRef]
- Thomas, A.A.; Wang, H.; Zahrt, A.F.; Denmark, S.E. Structural, Kinetic, and Computational Characterization of the Elusive Arylpalladium(II)boronate Complexes in the Suzuki-Miyaura Reaction. J. Am. Chem. Soc. 2017, 139, 3805–3821. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Lu, C.; Zhou, S.; Li, X.; Li, Y.; Yang, Y.; Li, Y.; Liu, Z.; Yang, J.; et al. Unveiling the full reaction path of the Suzuki–Miyaura cross-coupling in a single-molecule junction. Nat. Nanotechnol. 2021, 16, 1214–1223. [Google Scholar] [CrossRef]
- Somorjai, G.A. On the Move. Nature 2004, 430, 730. [Google Scholar] [CrossRef]
- Kann, N. Recent Applications of Polymer Supported Organometallic Catalysts in Organic Synthesis. Molecules 2010, 15, 6306–6331. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Z.; Lee, S.D.; Bhatnagar, R.S. Toxicity of Palladium. Toxicol. Lett. 1979, 4, 469–473. [Google Scholar] [CrossRef]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2011; pp. 384–387. [Google Scholar]
- Hosseini, M.-J.; Jafarian, I.; Farahani, S.; Khodadadi, R.; Tagavi, S.H.; Naserzadeh, P.; Mohammadi-Bardbori, A.; Arghavanifard, N. New Mechanistic Approach of Inorganic Palladium Toxicity: Impairment in Mitochondrial Electron Transfer. Metallomics 2016, 8, 252–259. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Ciriminna, R.; Béland, F.; Carà, P.D. Heterogeneous versus Homogeneous Palladium Catalysts for Cross-Coupling Reactions. ChemCatChem 2012, 4, 432–445. [Google Scholar] [CrossRef]
- Nikoshvili, L.; Bakhvalova, E.S.; Bykov, A.V.; Sidorov, A.I.; Vasiliev, A.L.; Matveeva, V.G.; Sulman, M.G.; Sapunov, V.N.; Kiwi-Minsker, L. Study of Deactivation in Suzuki Reaction of Polymer-Stabilized Pd Nanocatalysts. Processes 2020, 8, 1653. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Kurokhtina, A.A.; Larina, E.V. Simple Kinetic Method for Distinguishing Between Homogeneous and Heterogeneous Mechanisms of Catalysis, Illustrated by the Example of “Ligand-Free” Suzuki and Heck Reactions of Aryl Iodides and Aryl Bromides. Kinet. Catal. 2012, 53, 84–90. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Kurokhtina, A.A. Distinguishing Between the Homogeneous and Heterogeneous Mechanisms of Catalysis in the Mizoroki-Heck and Suzuki-Miyaura Reactions: Problems and Prospects. Kinet. Catal. 2012, 53, 714–730. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. Catalytic C-C and C-Heteroatom Bond Formation Reactions: In Situ Generated or Preformed Catalysts? Complicated Mechanistic Picture behind Well-Known Experimental Procedures. J. Org. Chem. 2013, 78, 11117–11125. [Google Scholar] [CrossRef]
- Eremin, D.B.; Ananikov, V.P. Understanding Active Species in Catalytic Transformations: From Molecular Catalysis to Nanoparticles, Leaching, “Cocktails” of Catalysts and Dynamic Systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Reay, A.J.; Fairlamb, I.J.S. Catalytic C–H Bond Functionalisation Chemistry: The Case for Quasi-Heterogeneous Catalysis. Chem. Commun. 2015, 51, 16289–16307. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of Catalysis on the Stability of Metallic Nanoparticles: Suzuki Reaction Catalyzed by PVP-Palladium Nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lei, P.; Szostak, M.; Casals-Cruañas, E.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanistic Study of Suzuki–Miyaura Cross-Coupling Reactions of Amides Mediated by [Pd(NHC)(allyl)Cl] Precatalysts. ChemCatChem 2018, 10, 3096–3106. [Google Scholar] [CrossRef]
- Balanta, A.; Godard, C.; Claver, C. Pd Nanoparticles for C–C Coupling Reactions. Chem. Soc. Rev. 2011, 40, 4973–4985. [Google Scholar] [CrossRef] [PubMed]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Bouleghlimat, A.; Othman, M.A.; Lagrave, L.V.; Matsuzawa, S.; Nakamura, Y.; Fujii, S.; Buurma, N.J. Halide-Enhanced Catalytic Activity of Palladium Nanoparticles Comes at the Expense of Catalyst Recovery. Catalysts 2017, 7, 280. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Gürbüz, N.; Özdemir, İ. The Kinetics and Mechanism of Polymer-Based NHC-Pd-Pyridine Catalyzed Heterogeneous Suzuki Reaction in Aqueous Media. Int. J. Chem. Kin. 2019, 51, 931–942. [Google Scholar] [CrossRef]
- Mohanty, S.; Punji, B.; Maravanji, B. Synthesis and Reaction Kinetics of Pd(1,5-cyclooctadiene)Cl2 with N,N′-Methylene-bis(2-aminopyridyl): An Efficient Catalyst for Suzuki-Cross-Coupling Reactions. Polyhedron 2006, 25, 815–820. [Google Scholar] [CrossRef]
- Liu, Y.; Hartman, R.L. Reaction Kinetics of a Water-Soluble Palladium-β-Cyclodextrin Catalyst for a Suzuki-Miyaura Cross-Coupling in Continuous Flow. React. Chem. Eng. 2019, 4, 1341–1346. [Google Scholar] [CrossRef]
- Fyfe, J.W.B.; Fazakerley, N.J.; Watson, A.J.B. Chemoselective Suzuki–Miyaura Cross-Coupling via Kinetic Transmetallation. Angew. Chem. Int. Ed. 2017, 129, 1269–1273. [Google Scholar] [CrossRef]
- Sulman, E.M.; Nikoshvili, L.Z.; Matveeva, V.G.; Tyamina, I.Y.; Sidorov, A.I.; Bykov, A.V.; Demidenko, G.N.; Stein, B.D.; Bronstein, L.M. Palladium Containing Catalysts Based on Hypercrosslinked Polystyrene for Selective Hydrogenation of Acetylene Alcohols. Top. Catal. 2012, 55, 492–497. [Google Scholar] [CrossRef]
- Nemygina, N.; Nikoshvili, L.; Bykov, A.; Sidorov, A.; Molchanov, V.; Sulman, M.; Tiamina, I.; Stein, B.; Matveeva, V.; Sulman, E.; et al. Catalysts of Suzuki cross-coupling based on functionalized hyper-cross-linked polystyrene: Influence of precursor nature. Org. Process Res. Dev. 2016, 20, 1453–1460. [Google Scholar] [CrossRef]
- Adrio, L.A.; Nguyen, B.N.; Guilera, G.; Livingston, A.G.; Hii, K.K.M. Speciation of Pd(OAc)2 in Ligandless Suzuki-Miyaura Reactions. Catal. Sci. Technol. 2012, 2, 316–323. [Google Scholar] [CrossRef]
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent Effects in Palladium Catalysed Cross-Coupling Reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST X-ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 21 November 2020).
- Amatore, C.; Jutand, A.; Le Duc, G. Kinetic Data for the Transmetalation/Reductive Elimination in Palladium-Catalyzed Suzuki-Miyaura Reactions: Unexpected Triple Role of Hydroxide Ions Used as Base. Chem. Eur. J. 2011, 17, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Carrow, B.P.; Hartwig, J.F. Distinguishing between Pathways for Transmetalation in Suzuki−Miyaura Reactions. J. Am. Chem. Soc. 2011, 133, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Kurokhtina, A.A.; Larina, E.V. Role of a base in Suzuki-Miyaura reaction. Russ. J. Gen. Chem. 2011, 81, 1573–1574. [Google Scholar] [CrossRef]
- Schmid, R.; Sapunov, V. Non-Formal Kinetics: In Search for Chemical Reaction Pathways (Monographs in Modern Chemistry), 14th ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1982; 199p. [Google Scholar]
- Polynski, M.V.; Ananikov, V.P. Modeling Key Pathways Proposed for the Formation and Evolution of “Cocktail”-Type Systems in Pd-Catalyzed Reactions Involving ArX Reagents. ACS Catal. 2019, 9, 3991–4005. [Google Scholar] [CrossRef]
- Collins, G.; Schmidt, M.; O’Dwyer, C.; Holmes, J.D.; McGlacken, G.P. The Origin of Shape Sensitivity in Palladium-Catalyzed Suzuki-Miyaura Cross Coupling Reactions. Angew. Chem. Int. Ed. 2014, 53, 4142–4145. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapunov, V.N.; Nikoshvili, L.Z.; Bakhvalova, E.S.; Sulman, M.G.; Matveeva, V.G. Kinetic Aspects of Suzuki Cross-Coupling Using Ligandless Pd Nanoparticles Embedded in Aromatic Polymeric Matrix. Processes 2023, 11, 878. https://doi.org/10.3390/pr11030878
Sapunov VN, Nikoshvili LZ, Bakhvalova ES, Sulman MG, Matveeva VG. Kinetic Aspects of Suzuki Cross-Coupling Using Ligandless Pd Nanoparticles Embedded in Aromatic Polymeric Matrix. Processes. 2023; 11(3):878. https://doi.org/10.3390/pr11030878
Chicago/Turabian StyleSapunov, Valentin N., Linda Z. Nikoshvili, Elena S. Bakhvalova, Mikhail G. Sulman, and Valentina G. Matveeva. 2023. "Kinetic Aspects of Suzuki Cross-Coupling Using Ligandless Pd Nanoparticles Embedded in Aromatic Polymeric Matrix" Processes 11, no. 3: 878. https://doi.org/10.3390/pr11030878
APA StyleSapunov, V. N., Nikoshvili, L. Z., Bakhvalova, E. S., Sulman, M. G., & Matveeva, V. G. (2023). Kinetic Aspects of Suzuki Cross-Coupling Using Ligandless Pd Nanoparticles Embedded in Aromatic Polymeric Matrix. Processes, 11(3), 878. https://doi.org/10.3390/pr11030878







