Abstract
Crude oil is one of the major pollutants present. Its extraction and processing generate processing waters contaminated by hydrocarbons which are harmful to both human health and the flora and fauna that come into contact with it. Hydrocarbon contamination can involve soil and water, and several technologies are used for recovery. The most used techniques for the recovery of spilt oil involve chemical-physical methods that can remove most of the pollutants. Among these, must consider the bioremediation by microorganisms, mostly bacterial capable of degrading many of the toxic compounds contained within the petroleum. Microalgae participate in bioremediation indirectly, supporting the growth of degrading bacteria, and directly acting on contaminants. Their direct contribution is based on the activation of various mechanisms ranging from the production of enzymes capable of degrading hydrocarbons, such as lipoxygenases, to the attack through the liberation of free radicals. The following review analyzed all the works published in the last ten years concerning the ability of microalgae to remove hydrocarbons, intending to identify in these microorganisms an alternative technology to the use of bacteria. The advantages of using microalgae concern not only their ability to remove toxic compounds and release oxygen into the atmosphere but their biomass could then be used in a circular economy process to produce biofuels.
1. Introduction
Today, the world economy is based on fossil fuels to obtain energy and, specifically, coal and petroleum. Petroleum consumption in 2020 increased by 0.9 million barrels per day while the demand for liquid fuels reached historic highs reaching 100 million barrels per day. The use of oil governs stock exchanges and world markets. For this reason, the extraction and refining of crude oil remain an extremely intense activity. This massive extraction causes numerous problems critical for the environmental pollution of soil and water. Furthermore, there are many accidents recorded over the years related to the transport of petroleum that have caused environmental problems. By focusing primarily on spills in aquatic environments, oil has a major impact on flora and fauna healthy. For this reason, in recent years, a solution has been sought that allows the removal and degradation of oil in a green way, limiting the use of chemical dispersants, which are themselves toxic. Microalgae are unicellular, photosynthetic microorganisms that constitute phytoplankton in the aquatic environment. In the last decade, microalgae have been studied for their nutraceutical, pharmaceutical and industrial applications, being producers of many metabolites such as carotenoids, antioxidants and lipids; these useful for biofuel production [1]. In this review, we analyzed the literature about the ability of microalgae, with particular attention to green microalgae, to remove contaminants deriving from pure crude oil. Microalgae can represent an excellent solution given their ability to metabolize various pollutants, using them as carbon sources, in a green process, releasing oxygen into the atmosphere and subtracting CO2. To do that, we search all the manuscripts about microalgae and crude oil treatment published from 2010 to 2022. Papers concerning the bioremediation of other contaminants (i.e., municipal wastewaters or heavy metal) have not been included in this manuscript. The search, carried out on the main search databases (PubMed, Scopus, and Web of Science), using as keywords “microalgae and petroleum”, “microalgae and crude oil”, “microalgae and petroleum and bioremediation”, “microalgae and crude oil and bioremediation”. A comparation between the different microorganism involved in the process has made to highlight the benefits of using microalgae.
Petroleum Composition
Usually, methane, ethane and propane, which represent the lightest hydrocarbons in natural conditions, are present in the gaseous state; the heavier ones are present in the solid or liquid. However, this can vary by oil field [2]. Petroleum is mainly composed of aliphatic and non-aliphatic compounds, but sulfur, nitrogen and oxygen atoms are also present. Linear hydrocarbons vary their state based on the number of carbon atoms that constitute them [3]. Alkanes with a number of carbon atoms greater than five are present in a liquid state, such as heptadecane (C17H36), while compounds with less than five carbon atoms, in a gaseous state [4]. Cycloalkanes, on the other hand, are formed starting from compounds, such as cyclopentane and cyclohexane, from which, in rare cases, cyclopropane and cyclobutane originated [5]. It is not difficult to find compounds such as polycyclic naphthenes within the crude oil, including pregnane and dinosterane [6]. There are aromatic compounds among the hydrocarbons present in the liquid state. The most famous, also for their toxicity, are the BTEX compounds (benzene, toluene, ethylbenzene and xylene) and makeup up to 60% of the light fraction of petroleum, where they do not have substituents in their chemical composition, both in the heavier fraction in which have one or more alkyl substituents or other connected cycloalkane rings [7]. PAHs are formed by multiple aromatic rings fused together and are divided into soluble resins such as anthracene, phenanthrene and pyrene, or as non-soluble asphaltenes [8]. The heteroatoms in the crude oil, present for less than 1% of the total composition, are mainly oxygen and sulfur. Phenols, carboxylic acids, alcohols, esters and ketones contain oxygen [9]. Carboxylic acids also contain fatty acids and naphthalenic acid, which come to a weight of around 1000 Da [10]. The amount of sulfur present affects the properties of the crude oil. It can be more or less acidic, depending on the amount of sulfur present. It is not uncommon to also find ionic compounds such as sodium chlorite or metal porphyrins such as nickel or vanadium in petroleum [11].
2. Current Bioremediation Techniques
Currently, there are several technologies for oil recovery that differ according to the matrix to be purified. Usually, multi-step protocols involve the use of chemical agents and the action of bacterial microorganisms (Table 1) [12].

Table 1.
Principal enzyme involved in bacteria crude oil bioremediation.
2.1. Bacteria Biodegradation
The bacteria are also used in the recovery of the crude oil lost during the extraction process, exploiting the ability of various species of bacteria and archaea to metabolise organic carbon and to produce biosurfactant solvents which improve the chemical-physical characteristics of the oil to recover [13,14]. The most used bacterial strains are Clostridium, Zymomonas, Klebsiella, Enterobacter and the archaeon Methanobacterium [15]. Oil and its constituents have existed in nature for millions of years, and consequently, there are organisms capable of using them as a source of nourishment and energy. Among the microorganisms that can grow in the presence of hydrocarbons, there are about 175 bacterial genera, many archaea and some eukaryotic microorganisms [16]. However, the bioremediation implemented by microorganisms is a complex mechanism that requires numerous steps and cooperation between different species capable of acting on hydrocarbons synergistically. Furthermore, it must be considered that there are numerous factors such as temperature and nutrient concentration that play a fundamental role in the remediation process [17]. Bioremediation generally begins with some bacterial genera capable of attacking straight-chain and branched alkanes present in high quantities. Between these Oceanispirillales order (class gammaproteobacteria; phylum proteobacteria), and specifically the genre Alcanivorax spp. intervene on n-alkanes and cycloalkanes [18]. To generate energy from alkanes, Alcanivorax spp. uses different hydrolases (a non-haem diiron monooxygenase AlkB1 and AlkB2) and three cytochrome P450-dependent alkane monooxygenases [19]. Given the different conditions in which these bacteria operate, some gammaproteobacteria activate special monooxygenases to survive in the presence of ultraviolet light. For example, they use the monooxygenase capable of binding flavin (AlmA) to metabolize the long-chain C22 and C36 n-alkanes as an energy source [20], instead Cycloclasticus spp., Colwellia and Pseudoalteromonas (class gammaproteobacteria; phylum proteobacteria), degrade aromatic hydrocarbons when, in a second phase, they are found in larger quantities [21,22]. Heterotrophic bacteria degrade exopolymer by-products thanks to peptidase and hydrolase. These enzymes are more expressed in contaminated environments. Halomonas bacteria fall into this category by producing exopolysaccharides. They reduce the solubilization of PAHs in an aqueous environment, making them more vulnerable to biodegradation and the formation of aggregates. [14,23]. However, the bioremediation processes mediated by microorganisms are in the balance between the increase of bacteria due to the degradation of toxic compounds and the lack of nutrients which decrease indirectly in proportion to the growth of bacterial biomass [24]. For this reason, it is sometimes necessary to add nutrients, and in particular, nitrogen, to improve performance. In a protected environment, this aspect is easy to solve, but in nature, the consumption of hydrocarbons by microorganisms causes a high degradation of the oxygen necessary for the sustenance of the other species present in the environment [25]. The bioremediation processes can also take place in the absence of oxygen in anaerobic conditions, where specialized bacteria use an alternative metabolism [26]. For example, some species of archaea can decompose methane through a process of reverse methanogenesis under anaerobic conditions. This process involves the use of different terminal electron acceptors [27]. Although the metabolic pathway is not entirely clear, the anaerobic methanotrophic archaea may use methyl-coenzyme M reductase as a key enzyme, exploiting its reverse reaction. In addition to the domain of the archaea, also the bacterium Methylomirabilis oxyfera can attack methane. It can convert nitrogen oxides (NO) from reduced nitrite into N2 and O2, thus activating methane monooxygenases [28].
2.2. Different Bacteria Consortium
It is evident that there is a collaboration between the different domains for the bioremediation process. In fact, in contaminated waters, phytoplankton and zooplankton collaborate synergistically due to the degradation of hydrocarbons, very often forming agglomerates that settle on the seabed. These agglomerates are rich in crude oil and are formed thanks to the coagulation of phytoplankton, which incorporates oil droplets and precipitates on the seabed. [29]. In the vicinity of oil spills, the indigenous microbial community increases the expression of genes, which are involved in the biodegradation process. It improves bacterial motility, chemotaxis and enzymes involved in aliphatic degradation. Even the very action of the currents favors bacterial blooms and accelerate the degradation [30]. Furthermore, the degradation of the various oil components involves different plasmid genes, depending on the hydrocarbons involved. For the metabolism of alkanes, aerobic microorganisms mainly use various monooxygenases, rubredoxin and rubredoxin reductase to convert alkanes into alcohol by increasing the expression of several alk genes. The PAHs metabolism, on the other hand, is more complex given the size of the hydrocarbons. The genes involved are mainly naphthalene dioxygenase (nah) genes [31], naphthalene dioxygenase (ndo) [32], doxycycline-inducible system (dox) [33].
3. Microalgae and Petroleum Bioremediation
Microalgae constitute a fundamental element in the treatment of water contaminated by crude oil and hydrocarbons. Ugya et al. evaluated the ability of some microalgae grown on a biofilm to remove contaminants of petroleum origin, including PAHs and total petroleum hydrocarbon (TPH). The results showed a significant reduction of phytochemical parameters such as sulphate −17.5%, chloride −14.65%, nitrates −33% total suspended solids (TSS) −26%, total dissolved solids (TDS) −7.9%, and chemical oxygen demand (COD) and biochemical oxygen demand (BODs) reduced by 8% and 16.7% respectively. Although not in high percentages, the removal of TPH was equal to 15% after 14 days of exposure [34]. Kuttiyathil et al., on the other hand, analysed not only the removal of crude oil by the microalga Chlorella spp. but also how, in nature, the mechanical action of sea waves contributes to creating an emulsion of water and crude oil that could favour the removal of pollutants making them more available. Their results show that following an initial period of adaptation, the Total Organic Carbon (TOC) of the solution was drastically reduced and that, after 5 days, Chlorella removed 80% of the emulsified oil [35]. Water mixing and how it can alter bioremediation was also studied in 2014 by Özhan et al., which demonstrated how the bioavailability of crude oil is altered by physical mixing applied in the laboratory. The mixing of the water column containing crude oil does not significantly affect the concentration of total petroleum hydrocarbons (TPH) but increases the concentration of some alkanes and PAHs and causes the formation of colloidal micro-particles (1–70 μm), which improve the degradation of hydrocarbons. [36]. Chlorella spp. has been the subject of several studies precisely because of its ability to survive in contaminated media. Znad et al., reported that the treatment of petroleum effluent (PE) with Chlorella spp. completely removed phosphorus after 13 days, reduced nitrogen by 78% and reduced COD from 504 mg/L to 144 mg/L. However, treatment of petroleum effluent with Chlorella spp. initially increased the biomass, but in the long term, start to be toxic and inhibites cell growth [37]. The nature and concentration of the crude oil, and its constituents, greatly influence the growth and removal of Chlorella. For example, the use of Water-Accommodated Fraction (WAF) deriving from diesel is more toxic for Chlorella than diesel as it is containing many low molecular weight hydrocarbons (LMW-HC), which can cause damage to cell membranes and affect the production of protective pigments, as reported by Ramadass et al. in its 2017 study [38]. Further studies carried out on Chlorella have confirmed its ability to remove various compounds contained in crude oil. For example, Xaaldi Kalhor et al., in both of their studies [39,40] tested different concentrations of crude oil (10 and 20 g/L) on Chlorella vulgaris for two intervals (7 and 14 days). The results were encouraging, and the best removal of low molecular weight hydrocarbons (LW), equal to 100%, was achieved with 10 g/L for 14 days, while at higher concentrations (20 g/L), after 14 days, the LW were reduced by 82%. The removal of heavy molecular weight hydrocarbons (HW) followed the same trend as the light ones, reaching higher values for the 14-day intervals and at a concentration of 10 g/L (reduction of HW equal to approximately 78%) [39]. Hamouda et al. (2016) and El-Sheekh et al. (2013) evaluated how the addition of crude oil to the Chlorella culture affected its metabolism and, specifically, whether the microalgae preferred a mixotrophic and heterotrophic mechanism rather than the classic autotrophic one. Hamouda et al. tested the growth of Chlorella in mixotrophic conditions using 1% crude oil, and the results on the hydrocarbons concentrations, present after 30 days of incubation, showed that the following aliphatic compounds: 3-methyl decane, heptadecane, octadecane, nonadecane, docosane, and tetracosane were removed, while decane, undecane, tridecane, hexadecane, tricosane were significantly reduced compared to the control [41]. El-Sheekh et al. instead, tested Chlorella’s bioremediation capacity using up to 2% crude oil. The results obtained by gas chromatography-mass spectrometry (GC-MS) showed that, after 15 days of incubation, Indole-3-acetic acid was removed at all tested concentrations, while decane, Indole-3-acetic acid, p-Phenyltoluene, Naphthalene, 3-ethyl, Tridecane, phenanthracene, 1-methyl, Benzene, decyl, phenanthracene, 2-methyl, cyclohexane undecyl, b-pregnane and Octacosane were removed at a concentration of 2% crude oil [42]. One of the most interesting aspects concerning the El-Sheekh study is that PAHs were reduced more efficiently in heterotrophic conditions. This supports the hypothesis that eukaryotic microalgae, such as Chlorella, use organic carbon, present in solution, improving their growth range and biomass using a heterotrophic metabolism that allows them to use, split and/or convert hydrocarbons into intermediate metabolites. Confirming this hypothesis is also the study conducted by Das et al. in 2019, which demonstrated how Chlorella reached the highest biomass yield (1.72 g/L) in mixotrophic conditions with the addition of pre-treated produced water (PPW) of petroleum origin and removed 92% of the total nitrogen (TN) and 73% of the TOC [43].
4. Mechanism of Action
From the studies analysed, it is evident that green microalgae, in particular Chlorophyceae, are excellent candidates to remove crude oil pollutants (Table 2). The mechanism of action with which this happens is not yet completely known, but the principal hypotheses are two: either they use organic carbon deriving from hydrocarbons, or they accumulate them inside by carrying out a defence mechanism and treating them as real contaminants. Ugya et al. analysed both hypotheses, and their results show that, in the microalgae, there was a net increase of saponins after the treatment of petroleum contaminants [34]. Saponins usually play a protective role thanks to their glycosidic-terpenic nature, lowering the surface tension and forming colloidal and foamy solutions [44]. Their amphipathic and surfactant nature increases the bioavailability of petroleum contaminants which are easier to “attack”. Ugya et al., demonstrated that the production of ROS increased, highlighting cellular stress induced by crude oil after the treatment. This is related also to the increase of alkaloids, flavonoids and carotenoids within the algae after treatment, suggesting that the ROS produced by the microalgae have degraded the hydrocarbons, protecting them from their toxic action. Furthermore, analyzes carried out by scanning electron microscopic show how the oil has affected the morphology and the surface of the microalgae [34]. If the cell surface was rough before the treatment, it was smooth and polished afterwards; moreover, an analysis of some elements such as silicones, aluminium and iron, showed how these have accumulated on the biofilm thanks to the production of extracellular polymeric substances (EPS) by the microalgae and that have accumulated thanks to the presence of groups functional such as OH, C = O, CO, as also confirmed by the study of the composition of the polysaccharide produced by Chlorella spp. conducted by El-Naggar et al. [45]. Ghodrati et al., instead, focused on the genetic nature underlying the bioremediation mechanism. At the basis of their study, there is the idea that PAHs could be a source of ROS, alkoxyl (RO °) and hydroxyls (OH °) inside of cells. Starting from the knowledge on degrading bacteria [46], Ghodrati et al., hypothesized that green algae, being aerobic, could also use dioxygenases to remove and degrade PAHs, focusing specifically on lipoxygenases (LOXs) which oxidize PAHs through the insertion of two oxygen atoms which lead to the rupture of the aromatic ring through ortho-cleavages or meta-cleavages. The addition of oxygen in the hydrocarbon skeleton generates the formation of hydroperoxydes activated by becoming oxylipins. The molecular mechanism in microalgae has not yet been studied, but it would seem that lipoxygenases have both lipoxygenase and hydroperoxidase activity. Consequently, the results of Ghodrati et al. show that exposure to 1% crude oil for 21 days induced the expression of the LOX genes, ultimately leading to the decomposition of hydrocarbons and the production of hydroperoxy acids, fats and oxylipins which are useful to the algae for growth and sustenance, as well as for the resistance to stress-induced by crude oil [47]. SureshKumar et al. hypothesized that the degradation mechanism of PAHs in microalgae could be similar to that implemented by prokaryotes, turning an eye to the bacterial world. Starting from the idea that higher plants and animals share enzymatic and genetic pathways in the removal of exogenous substances, the group of researchers carried out a non-laboratory predictive analysis, considering as a metabolizing mechanism the oxidative system of cytochrome P450 (CYP450), which intervenes in the degradation of those molecules resistant to dioxygenases. Several parameters were analysed to create a model that could simulate the link between PAHs and CYP450 of Haematococcus pluvialis. Thirty-eight PAHs formed from 1 to 6 benzene rings were involved in the analysis, and the results showed that hydrogen, hydrophobic, electrostatic, π-π, and Van der Waals interaction occurred in the active site of CYP450. Specifically, 18 PAHs interacted with Threonine282 (Thr282), Alanine337 (Ala337), Serine404 (Ser404) and Lisyne407 (Lys407) via hydrogen bonds. However, in this study, it is evident that only LMW-PAHs were able to bind CYP450, while HMW-PAHs did not [48]. Therefore, there is an antioxidant mechanism for the degradation of petroleum pollutions, in particular, hydrocarbons. It acts in a double capacity, removing the toxic agent and producing nutrients useful for cell growth. Low doses of toxins could activate mechanisms to repair not only the damage induced by the toxin but also other damages previously accumulated by the cell, according to hormesis hypothesis [49]. The hormesis hypothesis claims that an organism responds to small doses of stress adaptively to survive [50]. However, some studies show that the ability of microalgae to remove contaminants continues even after the cell is dead as the microalgae can adsorb micro-drops of crude oil on their surface and, consequently, TOC, removing it from the solution as demonstrated by Kuttiyathil et al. [35].

Table 2.
Principal enzymes and molecules involved in bioremediation process.
5. Consortium Microalgae and Bacteria
Nowadays, bacteria are widely studied as bioremediators, and several species suitable for this process are known. On the other hand, microalgae could be valid substitutes. For this reason, many studies have focused on bacteria and microalgae collaboration to degrade crude oil and its pollutants. This collaboration can be of various types, but the basic principle sees the microorganisms work synergistically to obtain a better result (Figure 1). For example, Ashwaniy et al. found that the microalga grown in petroleum refinery effluent (PRE) can reduce the concentration of COD, 81% of BOD, 61% of sulphide, 61% of TSS by 70%. 67% phosphorous and TDS and can act as a substrate for bacterial growth in a microbial desalination cell (MDC) to produce clean energy [51]. Chernikova et al. described how microalgae and bacteria collaborate continuously in nature. The microalgae provide oxygen, exopolymers and organic-material useful for bacterial growth. In turn, bacteria support microalgae growth, producing vitamins, micronutrients, iron and carbon dioxide. Furthermore, Chernikova et al., in their work, demonstrated that in two petroleum-enriched microalgae cultures, P. lutheri and N. oculata, there was a selection of hydrocarbonoclastic alpha and gammaproteobacteria, especially Alcanivorax and Marinobacter spp., identifying in total 48 non-redundant bacterial strains also belonging to the genera Thalassospira, Hyphomonas, Halomonas, Marinovum and Roseovarius. These results are interesting as they candidate microalgae as possible host organisms for these bacteria whose housing niches are ignored [52]. Das et al. found that the ability of Chlorella spp. to remove various contaminants supported the growth of aerobic bacteria present in the unsterilized pretreated waters deriving from petroleum processing (PPW). In addition, the bacteria made nitrogen more available by promoting the microalgae biomass [38]. These results confirm the studies conducted by Mahdavi et al. in 2015 where algae produce oxygen through photosynthesis, which is necessary for aerobic bacteria for toxic compounds biodegradation. But the results support the ability of some algal strains to degrade directly and completely, some compounds such as naphthenic acids. In their study, a sample of freshwater taken directly from a pond in northern Alberta was tested for removal. Various conditions were tested, such as the absence of oxygen, presence of a Navicula pelliculosa diatom, and light variations. Only bacteria were tested, and bacteria with algae. The results showed how the algae-bacteria consortium led to an increase in the removal of toxic compounds given by the increase in microbial biomass in the algae-bacteria consortium. The higher rate of detoxification, obtained with bacteria alone, was improved by microalgae, which improved bacterial growth [53]. The coexistence of bacteria and microalgae was also observed by Hodges et al. where filamentous cyanobacteria dominated the reactor used for the decontamination and bio-removal of nutrients and suspended solids petrochemical wastewater [54]. So far, it has been analyzed how algae have been supporting bacterial growth in bioremediation, but Abid et al. have conducted a study in which the opposite occurs. A double-chamber bioreactor was built in which in one the bacteria biodegraded petroleum wastewater and the CO2 produced was channelled into the chamber containing the microalga Spongiochloris sp, which used it to increase its growth, sequestering the CO2 produced by the bacteria from the atmosphere [55]. However, these two paths of mutual exchange are accompanied by a third possibility. Tang demonstrated how a microalgae-bacteria consortium, artificially created, can optimize the removal of different petroleum constituents. In his study conducted in 2010, he separately tested four bacterial strains known for their ability to degrade PAHs (Shingomonas GY2B, Burkholderia capacia GS3C, Pseudomonas GP3A and Pandoraea pnomenusa GP3B) and the microalga Scenedesmus obliquus GH2, both as unialgal and axenic algae. Unialgal GH2 alone was able to remove various contaminants even with high percentages, such as 46% of alkanes and 51% of alkylcycloalkanes, or by reducing PAHs and alkylated naphtalenes by 81%, while axenic GH2 did not show potential for removal. However, these results were disproved by the union of microalgae with bacterial strains. Unialgal GH2, added with the various strains, has not increased its degradative properties, indeed in some cases, it has reduced its efficiency; axenic GH2 in conjunction with the different bacteria, on the other hand, has shown an increase in all degradation rates, completely removing toxic compounds such as PAHs, naphthalene, fluorene and phenanthrene [56]. Although there are not many studies in this regard, Ozhan et al., have shown how the oil spill in southern Louisiana has created dysfunctions in the phytoplankton, which is a valid indicator of toxicity for the health of the compromised marine ecosystem [36]. Jung et al. confirm this and argues that the dose of oil with which the phytoplankton comes into contact is responsible for the imbalance between bacteria and microalgae, reporting that concentrations greater than 1000 ppm inhibit the growth of microalgae by stimulating the bacterial one instead [57].
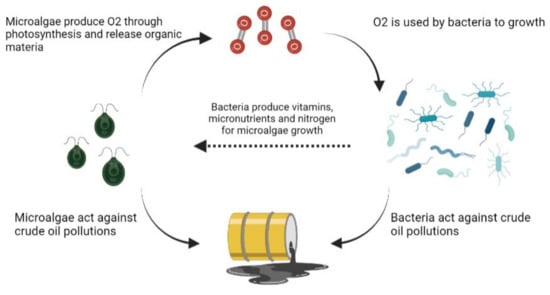
Figure 1.
Microalgae and bacteria consortium: mechanisms of action.
6. Conclusions
The bibliography shows how microalgae are a valid alternative for the bioremediation of hydrocarbons and contaminants from crude oil. However, the current techniques used, through the action of specific bacteria, create waste material that must be disposed of, represent an additional cost, and release carbon dioxide into the atmosphere, resulting from their metabolism. Microalgae release oxygen into the atmosphere, sequestering carbon dioxide, being great bioremediators and carrying out a double purification action. Furthermore, in recent years microalgae have been a source of study for their application in the production of biofuels thanks to the quality of their fatty acids; it is possible to enrich the lipids used for conversion using alternative organic carbon-containing media, such as contaminated water [58]. This chain begins with the recovery and degradation of fossil fuel by a microorganism, which in itself constitutes the basis for the production of alternative biofuel [59]. The biofuel deriving from microalgae is extremely interesting as it is part of a circular economy mechanism that allows the reuse of a polluting matrix such as oil to form a new efficient and economical fuel. [60]. Furthermore, microalgal biomass can also be used in various fields, in addition to energy, as microalgae are excellent natural sources of nutrients such as vitamins, proteins, fatty acids and antioxidants. Some microalgae mentioned in this work (such as Chlorella and Haematococcus pluvialis), after bioremediation, are used in the nutraceutical field, thanks to important elements such as lipids and astaxanthin, respectively [61,62]. The results of this review demonstrate how microalgae could be used in the direct removal of petroleum hydrocarbons and lay the foundations for further specific studies to investigate the pathways involved in bioremediation to carry out any work of selecting the most performing strains. In conclusion, we believe that green microalgae, such as those described in this review, which already find application in various fields, can become a new biotechnological tool to solve a problem of global interest.
Author Contributions
Conceptualization, G.M. and R.P.R.; methodology, A.S.; investigation, R.P.R.; writing—original draft preparation, R.P.R.; writing—review and editing, V.D.F. and A.D.; visualization, R.P.R., V.D.F. and A.D.; supervision, G.M.; project administration, G.M. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferreira Mota, G.; Germano de Sousa, I.; Luiz Barros de Oliveira, A.; Luthierre Gama Cavalcante, A.; da Silva Moreira, K.; Thálysson Tavares Cavalcante, F.; Erick da Silva Souza, J.; Rafael de Aguiar Falcão, Í.; Guimarães Rocha, T.; Bussons Rodrigues Valério, R.; et al. Biodiesel Production from Microalgae Using Lipase-Based Catalysts: Current Challenges and Prospects. Algal. Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Hsu, C.S.; Robinson, P.R. Springer Handbook of Petroleum Technology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Walters, C. Petroleum. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–44. [Google Scholar] [CrossRef]
- Kissin, Y. Catagenesis and Composition of Petroleum: Origin of n-Alkanes and Isoalkanes in Petroleum Crudes. Geochim. Cosmochim. Acta 1987, 51, 2445–2457. [Google Scholar] [CrossRef]
- Dooley, S.; Heyne, J.; Won, S.H.; Dievart, P.; Ju, Y.; Dryer, F.L. Importance of a Cycloalkane Functionality in the Oxidation of a Real Fuel. Energy Fuels 2014, 28, 7649–7661. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, G.; Zhang, M. Distribution Difference and Significance of Short-Chain Steranes in Humic Coal and Coal-Measure Mudstone of Triassic Xujiahe Formation in Sichuan Basin, SW China. Arab. J. Geosci. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Heibati, B.; Pollitt, K.J.G.; Karimi, A.; Yazdani Charati, J.; Ducatman, A.; Shokrzadeh, M.; Mohammadyan, M. BTEX Exposure Assessment and Quantitative Risk Assessment among Petroleum Product Distributors. Ecotoxicol. Environ. Saf. 2017, 144, 445–449. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Palacio Lozano, D.C.; Ramírez, C.X.; Sarmiento Chaparro, J.A.; Thomas, M.J.; Gavard, R.; Jones, H.E.; Cabanzo Hernández, R.; Mejia-Ospino, E.; Barrow, M.P. Characterization of Bio-Crude Components Derived from Pyrolysis of Soft Wood and Its Esterified Product by Ultrahigh Resolution Mass Spectrometry and Spectroscopic Techniques. Fuel 2020, 259, 116085. [Google Scholar] [CrossRef]
- Ni, W.; Zhu, G.; Liu, F.; Li, Z.; Xie, C.; Han, Y. Carboxylic Acids in Petroleum: Separation, Analysis, and Geochemical Significance. Energy Fuels 2021, 35, 12828–12844. [Google Scholar] [CrossRef]
- Gab-Allah, M.A.; Goda, E.S.; Shehata, A.B.; Gamal, H. Critical Review on the Analytical Methods for the Determination of Sulfur and Trace Elements in Crude Oil. Crit. Rev. Anal. Chem. 2019, 50, 161–178. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Use of Microorganisms in the Recovery of Oil from Recalcitrant Oil Reservoirs: Current State of Knowledge, Technological Advances and Future Perspectives. Front Microbiol. 2020, 10, 2996. [Google Scholar] [CrossRef]
- Tourova, T.P.; Sokolova, D.S.; Semenova, E.M.; Ershov, A.P.; Grouzdev, D.S.; Nazina, T.N. Genomic and Physiological Characterization of Halophilic Bacteria of the Genera Halomonas and Marinobacter from Petroleum Reservoirs. Microbiology 2022, 91, 235–248. [Google Scholar] [CrossRef]
- Gutierrez, T.; Berry, D.; Yang, T.; Mishamandani, S.; McKay, L.; Teske, A.; Aitken, M.D. Role of Bacterial Exopolysaccharides (EPS) in the Fate of the Oil Released during the Deepwater Horizon Oil Spill. PLoS ONE 2013, 8, e67717. [Google Scholar] [CrossRef] [PubMed]
- Nwidee, L.N.; Theophilus, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S.; Nwidee, L.N.; Theophilus, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. EOR Processes, Opportunities and Technological Advancements. In Chemical Enhanced Oil Recovery (cEOR)—A Practical Overview; InTechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Prince, R.C.; Gramain, A.; McGenity, T.J. Prokaryotic Hydrocarbon Degraders. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1669–1692. [Google Scholar]
- Kebede, G.; Tafese, T.; Abda, E.M.; Kamaraj, M.; Assefa, F. Factors Influencing the Bacterial Bioremediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Dong, C.; Bai, X.; Sheng, H.; Jiao, L.; Zhou, H.; Shao, Z. Distribution of PAHs and the PAH-Degrading Bacteria in the Deep-Sea Sediments of the High-Latitude Arctic Ocean. Biogeosciences Discuss 2014, 11, 13985–14021. [Google Scholar] [CrossRef]
- Mcgenity, T.J.; Folwell, B.D.; Mckew, B.A.; Sanni, G.O. Marine Crude-Oil Biodegradation: A Central Role for Interspecies Interactions. Saline Syst. 2012, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Wu, Y.; Zhou, Z.; Lai, Q.; Shao, Z. Multiple Alkane Hydroxylase Systems in a Marine Alkane Degrader, Alcanivorax Dieselolei B-5. Env. Microbiol. 2011, 13, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Niepceron, M.; Portet-Koltalo, F.; Merlin, C.; Motelay-Massei, A.; Barray, S.; Bodilis, J. Both Cycloclasticus Spp. and Pseudomonas Spp. as PAH-Degrading Bacteria in the Seine Estuary (France). FEMS Microbiol. Ecol. 2010, 71, 137–147. [Google Scholar] [CrossRef]
- Dubinsky, E.A.; Conrad, M.E.; Chakraborty, R.; Bill, M.; Borglin, S.E.; Hollibaugh, J.T.; Mason, O.U.; Piceno, Y.M.; Reid, F.C.; Stringfellow, W.T.; et al. Succession of Hydrocarbon-Degrading Bacteria in the Aftermath of the Deepwater Horizon Oil Spill in the Gulf of Mexico. Environ. Sci. Technol. 2013, 47, 10860–10867. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.L.; Kessler, J.D.; Redmond, M.C.; Mendes, S.D.; Heintz, M.B.; Farwell, C.; Hu, L.; Kinnaman, F.S.; Yvon-Lewis, S.; Du, M.; et al. Propane Respiration Jump-Starts Microbial Response to a Deep Oil Spill. Science 2010, 330, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial Degradation of Hydrocarbons—Basic Principles for Bioremediation: A Review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Enhanced Bioremediation of Oil Spills in the Sea. Curr. Opin. Biotechnol. 2014, 27, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Dong, C.D.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Biohydrogen Production from Microalgae—Major Bottlenecks and Future Research Perspectives. Biotechnol. J. 2021, 16, 2000124. [Google Scholar] [CrossRef]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum Hydrocarbon Contamination in Terrestrial Ecosystems—Fate and Microbial Responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Reynolds, D.; Liu, M.; Stark, M.; Kjelleberg, S.; Webster, N.S.; Thomas, T. Functional Equivalence and Evolutionary Convergence in Complex Communities of Microbial Sponge Symbionts. Proc. Natl. Acad. Sci. USA 2012, 109, E1878–E1887. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Trannum, H.C.; Bakke, T.; Hodson, P.V.; Collier, T.K. Environmental Effects of the Deepwater Horizon Oil Spill: A Review. Mar. Pollut. Bull. 2016, 110, 28–51. [Google Scholar] [CrossRef]
- Mason, O.U.; Hazen, T.C.; Borglin, S.; Chain, P.S.G.; Dubinsky, E.A.; Fortney, J.L.; Han, J.; Holman, H.Y.N.; Hultman, J.; Lamendella, R.; et al. Metagenome, Metatranscriptome and Single-Cell Sequencing Reveal Microbial Response to Deepwater Horizon Oil Spill. ISME J. 2012, 6, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Haritash, A.K. Bacterial Degradation of Mixed-PAHs and Expression of PAH-Catabolic Genes. World J. Microbiol. Biotechnol. 2022, 39, 1–13. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, H.; Lee, A.H.; Kwon, B.O.; Khim, J.S.; Yim, U.H.; Kim, B.S.; Kim, J.J. Microbial Community Composition and PAHs Removal Potential of Indigenous Bacteria in Oil Contaminated Sediment of Taean Coast, Korea. Environ. Pollut. 2018, 234, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Hong, Y.; Odinga, E.S.; Liu, J.; Tsang, D.C.W.; Gao, Y. Bacterial Community and PAH-Degrading Genes in Paddy Soil and Rice Grain from PAH-Contaminated Area. Appl. Soil Ecol. 2021, 158, 103789. [Google Scholar] [CrossRef]
- Ugya, Y.A.; Hasan, D.B.; Tahir, S.M.; Imam, T.S.; Ari, H.A.; Hua, X. Microalgae Biofilm Cultured in Nutrient-Rich Water as a Tool for the Phycoremediation of Petroleum-Contaminated Water. Int. J. Phytoremediation 2021, 23, 1175–1183. [Google Scholar] [CrossRef]
- Kuttiyathil, M.S.; Mohamed, M.M.; Al-Zuhair, S. Using Microalgae for Remediation of Crude Petroleum Oil-Water Emulsion. Biotechnol. Prog. 2020, 37, e309. [Google Scholar] [CrossRef] [PubMed]
- Özhan, K.; Miles, S.M.; Gao, H.; Bargu, S. Relative Phytoplankton Growth Responses to Physically and Chemically Dispersed South Louisiana Sweet Crude Oil. Environ. Monit. Assess 2014, 186, 3941–3956. [Google Scholar] [CrossRef] [PubMed]
- Znad, H.; Al Ketife, A.M.D.; Judd, S.; AlMomani, F.; Vuthaluru, H.B. Bioremediation and Nutrient Removal from Wastewater by Chlorella Vulgaris. Ecol. Eng. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Toxicity of Diesel Water Accommodated Fraction toward Microalgae, Pseudokirchneriella Subcapitata and Chlorella Sp. MM3. Ecotoxicol. Environ. Saf. 2017, 142, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Xaaldi Kalhor, A.; Movafeghi, A.; Mohammadi-Nassab, A.D.; Abedi, E.; Bahrami, A. Potential of the Green Alga Chlorella Vulgaris for Biodegradation of Crude Oil Hydrocarbons. Mar. Pollut. Bull. 2017, 123, 286–290. [Google Scholar] [CrossRef]
- Xaaldi Kalhor, A.; Mohammadi Nassab, A.D.; Abedi, E.; Bahrami, A.; Movafeghi, A. Biodiesel Production in Crude Oil Contaminated Environment Using Chlorella Vulgaris. Bioresour. Technol. 2016, 222, 190–194. [Google Scholar] [CrossRef]
- Hamouda, R.A.E.F.; Sorour, N.M.; Yeheia, D.S. Biodegradation of Crude Oil by Anabaena Oryzae, Chlorella Kessleri and Its Consortium under Mixotrophic Conditions. Int. Biodeterior. Biodegrad. 2016, 112, 128–134. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Hamouda, R.A.; Nizam, A.A. Biodegradation of Crude Oil by Scenedesmus Obliquus and Chlorella Vulgaris Growing under Heterotrophic Conditions. Int. Biodeterior. Biodegrad. 2013, 82, 67–72. [Google Scholar] [CrossRef]
- Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Chaudhary, A.K.; Alghasal, G.; Al-Jabri, H.M.S.J. Microalgal Bioremediation of Petroleum-Derived Low Salinity and Low PH Produced Water. J. Appl. Phycol. 2019, 31, 435–444. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-Based, Biological-Active Surfactants from Plants. In Application and Characterization of Surfactants; InTechOpen: London, UK, 2017. [Google Scholar]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, Extraction and Characterization of Chlorella Vulgaris Soluble Polysaccharides and Their Applications in AgNPs Biosynthesis and Biostimulation of Plant Growth. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Haritash, A.K. A Comprehensive Review of Metabolic and Genomic Aspects of PAH-Degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar] [CrossRef]
- Ghodrati, M.; Kosari-Nasab, M.; Zarrini, G.; Movafeghi, A. Crude Oil Contamination Enhances the Lipoxygenase Gene Expression in the Green Microalga Scenedesmus Dimorphus. Biointerface Res. Appl. Chem. 2021, 11, 11431–11439. [Google Scholar] [CrossRef]
- SureshKumar, P.; Thomas, J.; Poornima, V. Structural Insights on Bioremediation of Polycyclic Aromatic Hydrocarbons Using Microalgae: A Modelling-Based Computational Study. Environ. Monit. Assess 2018, 190, 92. [Google Scholar] [CrossRef]
- Stebbing, A.R.D. Hormesis—The Stimulation of Growth by Low Levels of Inhibitors. Sci. Total Environ. 1982, 22, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Burbano, M.S.J.; Gilson, E. The Power of Stress: The Telo-Hormesis Hypothesis. Cells 2021, 10, 1156. [Google Scholar] [CrossRef]
- Ashwaniy, V.R.V.; Perumalsamy, M.; Pandian, S. Enhancing the Synergistic Interaction of Microalgae and Bacteria for the Reduction of Organic Compounds in Petroleum Refinery Effluent. Environ. Technol. Innov. 2020, 19, 100926. [Google Scholar] [CrossRef]
- Chernikova, T.N.; Bargiela, R.; Toshchakov, S.V.; Shivaraman, V.; Lunev, E.A.; Yakimov, M.M.; Thomas, D.N.; Golyshin, P.N. Hydrocarbon-Degrading Bacteria Alcanivorax and Marinobacter Associated with Microalgae Pavlova Lutheri and Nannochloropsis Oculata. Front. Microbiol. 2020, 11, 572931. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Prasad, V.; Liu, Y.; Ulrich, A.C. In Situ Biodegradation of Naphthenic Acids in Oil Sands Tailings Pond Water Using Indigenous Algae-Bacteria Consortium. Bioresour. Technol. 2015, 187, 97–105. [Google Scholar] [CrossRef]
- Hodges, A.; Fica, Z.; Wanlass, J.; VanDarlin, J.; Sims, R. Nutrient and Suspended Solids Removal from Petrochemical Wastewater via Microalgal Biofilm Cultivation. Chemosphere 2017, 174, 46–48. [Google Scholar] [CrossRef]
- Abid, A.; Saidane, F.; Hamdi, M. Feasibility of Carbon Dioxide Sequestration by Spongiochloris Sp Microalgae during Petroleum Wastewater Treatment in Airlift Bioreactor. Bioresour. Technol. 2017, 234, 297–302. [Google Scholar] [CrossRef]
- Tang, X.; He, L.Y.; Tao, X.Q.; Dang, Z.; Guo, C.L.; Lu, G.N.; Yi, X.Y. Construction of an Artificial Microalgal-Bacterial Consortium That Efficiently Degrades Crude Oil. J. Hazard Mater. 2010, 181, 1158–1162. [Google Scholar] [CrossRef]
- Jung, S.W.; Park, J.S.; Kown, O.Y.; Kang, J.H.; Shim, W.J.; Kim, Y.O. Effects of Crude Oil on Marine Microbial Communities in Short Term Outdoor Microcosms. J. Microbiol. 2010, 48, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, L.; Qiu, S.; Ge, S. Determination of Microalgal Lipid Content and Fatty Acid for Biofuel Production. Biomed Res. Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A Comprehensive Review on Carbon Source Effect of Microalgae Lipid Accumulation for Biofuel Production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Agrawal, S.; Nawaz, T.; Pan, S.; Selvaratnam, T. A Review of Algae-Based Produced Water Treatment for Biomass and Biofuel Production. Water 2020, 12, 2351. [Google Scholar] [CrossRef]
- Feng, Y.; Li, C.; Zhang, D. Lipid Production of Chlorella Vulgaris Cultured in Artificial Wastewater Medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef]
- Nishshanka, G.K.S.H.; Liyanaarachchi, V.C.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Chang, J.-S. Haematococcus Pluvialis: A Potential Feedstock for Multiple-Product Biorefining. J. Clean. Prod. 2022, 344, 131103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).