Abstract
Slaughterhouse wastewater (SHWW) is classified as industrial waste, which is exceptionally harmful to the environment due to its high content of biological oxygen demand (BOD), chemical oxygen demand (COD), and suspended solids, which result from high organic and nutrient loading. This study used a pilot system to treat SHWW from the Kafrelsheikh Governorate slaughterhouse, which includes a three-step process. It started with sedimentation, then coagulation and flocculation using different concentrations of each: natural zeolites (Z) and Psidium guajava-leaf powder (GLP) as green and environmentally friendly agents, and alum (A) as an inorganic coagulant. The final step was filtration with physically treated rice straw (RS). Each step was judged separately by measuring the removal percentages of each analyzed pollutant, and finally, the overall process was evaluated using the same method. A jar test was used to determine the best concentration of each coagulant used. The measured pollutants were physico-chemical, such as COD, BOD, TSS, TKN, and turbidity. The bacteriological examination included TBC, TCC, and FC. The jar-test results determined that Z 1200 mg/L SHWW, GLP 1 g/L, and A 6 g/L were the best concentrations for each coagulant used. In the coagulation step, GLP 1 g/L gave the highest removal percentage of TSS, TKN, EC, and turbidity, while Z 1200 mg/L gave the highest removal percentage of COD, TDS, TBC, and TCC. From these results, it was concluded that a natural coagulant performs better than a chemical one. Finally, judging the overall pilot test system after applying the filtration with physically treated RS, we found that the best removal efficiencies were obtained from Z 1200 mg/L combined with RS. This combination resulted in 90.58, 83.47, 88.75, 54.89, 21.39, 34.49, 84.16, 99.98, and 99.93 removal percentages for BOD, COD, TSS, TKN, EC, turbidity, TBC, and TCC, respectively.
1. Introduction
Slaughterhouse wastewater (SHWW) is classified as industrial waste in the agricultural and food industries. Due to its elevated blood, protein, and fat content, it is very harmful to the environment, with profound amounts of biological oxygen demand (BOD), chemical oxygen demand (COD), and suspended solids resulting from high organic and nutrient loading. Its discharge into surface water in an untreated form will lead to the eutrophication of rivers [1] and groundwater contamination [2].
Blood, one of the significant, dissolved pollutants in slaughterhouse wastewater, has a chemical oxygen demand (COD) of 375,000 mg/L [3]. Slaughterhouse wastewater also contains high concentrations of suspended solids (SS), including pieces of fat, grease, hair, feathers, flesh, manure, grit, and undigested feed [4]. According to Egyptian law No. 44/2000 (Executive Regulation of Law No. 93 of 1962 as amended by Decree No. 44 of 2000 for the discharge of wastewater, the disposal of abattoir wastewater into municipal wastewater is prohibited, so it must be treated separately before disposal.
A large amount of fresh water is consumed by slaughterhouses, which represents a tremendous stress on water sources, especially in combination with water crises [5], as the ordinary water utilization for a European steer slaughterhouse ranges somewhere between 2500 and 40,000 L/1000 kg of live weight killed (LW), while the average water utilization of a medium-size U.S. slaughterhouse is roughly 3000 L/1000 kg of live weight killed [5]. SHWW quality will depend on the amount of blood resulting from bleeding (the primary source of BOD) and whether it is captured separately or not, the amount of water used during slaughtering, and the type of animal slaughtered [6]. Thus, the proper treatment and disposal of SHWW pose an economic and public health necessity for its safe and sustainable release into the environment [7]. Several methods used to treat slaughterhouse wastewater have been studied intensively, of which biological treatments includes aerobic, anaerobic, and hybrid systems [8]. However, these methods were associated with either high energy consumption and high sludge production in the case of aerobic treatment [9], or with impairment and inhibition by the accumulation of suspended solids, fats, and high levels of organic matter, which inhibit methanogenic bacteria [10]. The most common methods used for treating slaughterhouse wastewater are fine screening, sedimentation, coagulation−flocculation, trickling filters, and activated sludge processes [10]. The physicochemical method is another system used for the treatment of SHWW, which includes dissolved air flotation (DAF) and coagulation−flocculation units, which are used mainly for the removal of total suspended solids (TSS), colloids, turbidity, color, and fats [11]. Coagulation−flocculation is the most commonly used, relatively cheap, and promising new technology in wastewater treatment systems [12], especially in terms of being naturally available and affordable. The traditional coagulation process includes the addition of divalent, positively charged chemical compounds, such as aluminum sulfate and ferric chloride, which have diverse impacts on both health and the environment. The most common environmental impacts of using chemical coagulants are high levels of chemical residuals, toxic sludge, and diseases upon prolonged use [13]. Substituting natural coagulants for chemicals can limit the ecological contamination and hazards to well-being brought about by using chemical coagulants [14]. Natural coagulants, sourced mainly from plants that are consistently abundant and accessible, are notable as being nontoxic [15].
Psidium guajava (guava) is a traditional medicinal plant. It is widely cultivated in more than 50 countries, particularly in the tropics, subtropics, and in some Mediterranean areas [16]. Psidium guajava-leaf extract is utilized as a natural coagulant and has been observed to be harmless to the ecosystem, accessible, economical, and easy to plant when contrasted with other coagulant substances. It is an effective and inexpensive agent in water treatment, especially in the removal of heavy metals, suspended solids, and coagulants [17].
The inhibitory effects of Psidium guajava extract on S. aureus, and E. coli have been reported to different degrees [18]. Additionally, a study has explained that Psidium guajava contains several organic constituents with antimicrobial activities belonging mainly to phenolic, flavonoid, carotenoid, terpenoid, and triterpene compounds [19].
Zeolites (Zs) are a group of microporous sodium or calcium aluminosilicates with cavities of less than 2 nm. They are low-cost adsorbents, naturally occurring in the environment, and they can also be synthesized in the laboratory [20]. Natural zeolites (such as clinoptilolite) are among the most-studied groups of adsorbents used for wastewater purification and the removal of different pollutants, such as heavy metals, oil, and organic contaminants [21]. Nontoxic, exchangeable cations in zeolite structures, such as Na+, K+, Mg2+, and Ca2+, make zeolites suitable for wastewater treatment [22]. Many forms of rural waste, such as rice straw, wheat straw, rice husks, maize straw, tree leaves, wood chips, and so on, are agricultural byproducts. This rural waste matter is frequently burned as a method of disposal, which is ecologically damaging and harmful to the atmosphere [23]. To tackle this issue, these waste products might be utilized in wastewater treatment.
Rice straw (RS) is a byproduct of rice processing; it is mainly composed of 28–48% cellulose, 26.40% hemicelluloses, and 12.26% lignin; and presented as the cell wall structure, 12.26% ash, 2.18% wax, and 9% silica [24]. In Egypt, about 3.5 million tons of rice straw is produced; every autumn, a considerable quantity of straw from rice cultivation is disposed of, traditionally by burning in situ, causing real environmental problems [25].
Using rice straw in combination with different water coagulants and adsorbents was previously studied, with zeolite [26] and with cement kiln dust [27], proving to be an efficient and low-cost technology for wastewater treatment and reuse.
To the best of the authors’ knowledge, the comparison between the efficiency of using alum (aluminum sulfate), as an example of a chemical coagulant, and Psidium guajava-leaf extract and zeolite, as examples of natural coagulants, in the treatment of SHWW, followed by filtration with physically treated rice straw, has not been investigated previously. Therefore, this study primarily aimed to assess the efficiency of using zeolites and Psidium guajava as green and environmentally friendly agents in treating SHWW as compared with alum as an inorganic coagulant. Secondly, we wished to evaluate the efficacy of treating SHWW using rice straw in combination with zeolite and Psidium guajava.
2. Materials and Methods
2.1. Slaughterhouse Wastewater (SHWW) Collection and Characterization
The SHWW used in this study was obtained from the Kafrelshiekh slaughterhouse located in the Kafrelshiekh governorate (31°6′22.752″ N, and longitude of 30°56′31.11″ E), Egypt. SHWW samples were collected as grab samples from the drain present at the slaughter hall discharge point, stored in 25 L polyvinyl chloride (PVC) containers, and refrigerated at 4 °C. The characteristics of the untreated (unfiltered) SHWW used in this study are listed in Table 1.

Table 1.
Characteristics of the untreated SHWW used in this study after 24 h of settling and standards and specifications of sewage which are licensed to be discharged into brackish or saline surface-water bodies.
2.2. Lab-Scale SHWW Treatment System
In this study, the lab-scale SHWW treatment system consisted of (1) sedimentation for the removal of suspended solids; (2) coagulation processes for the removal of suspended and dissolved solids and decreasing COD and BOD using natural coagulants (Psidium guajava-leaf powder (GLP) and natural zeolite (Z)) and a chemical coagulant (alum) used in different concentrations using a jar test, and (3) the final treatment step of natural filtration using physically activated rice straw (Figure 1).
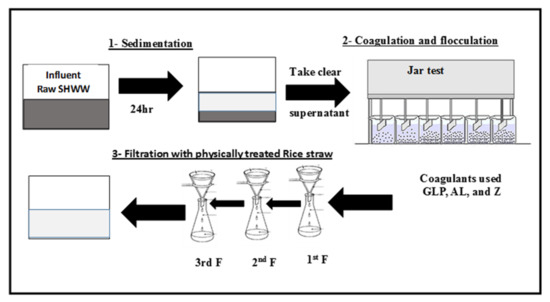
Figure 1.
Lab-scale of SHWW treatment system.
2.3. Sedimentation
A total of 15 L of collected raw SHWW was allowed to settle in a low-edge plastic pot with a 20 L capacity and allowed to stand for 24 h. Samples were ordered before and after sedimentation to measure COD, BOD, turbidity, TDS, and SS. Results are illustrated in Table 1.
2.4. Coagulation
Alum (aluminum sulfate, Al2(SO4)3) was purchased from ASCE—Egyptian Aluminum Sulphate Co. (Cairo, Egypt)—Misr El Gedeida Egypt. It was used as a chemical coagulant for this study because it has been used extensively at water and wastewater treatment plants to remove solids and may function as an effective and less expensive coagulant [10,28]. To determine the optimum alum (A) coagulant dosage, varying dosages from 2 g, 3 g, 4 g, 5 g, and 6 g/L [12] were tested. Zeolite (Z) and Psidium guajava-leaf powder (GLP) were used as natural coagulants. Zeolite was purchased from (Alix zeolite, Cairo, Egypt). The characterization of zeolite is illustrated in Table 2. Different concentrations of zeolite, 0.4 g, 0.6 g, 0.8 g, 1 g, and 1.2 g/L, were used to determine the best one. Leaves of Psidium guajava (guava) were purchased from the local market of the city of Kafrelsheikh and were authenticated by a botanist from the Faculty of Agriculture, Kafrelsheikh University. The leaves were washed in a salt solution to remove possible microbial contaminants, rinsed in clean water, and then spread to dry under shade at room temperature for one week. The dried leaves were crushed into a coarse powder using an electric grinder, dried again under shade at room temperature, and then ground into a fine powder. The resultant fine powder was then stored in nylon bags at 4 °C until required for use [29]. The used dosages of GLP are illustrated in Table 3.
2.5. Jar Test
To test the efficiency of the coagulation process of SHWW and investigate different types and dosages of coagulants, the standard jar-test device, illustrated in Figure 2, was used. was used. The SHWW samples were homogeneously mixed and distributed into beakers, each containing 500 mL of suspension, and they were initially sampled for the measurement of pH, COD, BOD, TSS, TP, TN, EC, TDS, turbidity, TBC, TCC, and FCC. The coagulation experiment conducted with a jar test began with coagulation, flocculation, and settling, respectively, using two stages: rapid mixing and slow mixing. Rapid mixing (170 rpm for 3 min) at the beginning expedites interactions with a colloidal coagulant, known as the destabilization of colloids. Slow mixing (20 rpm for 20 min) is necessary to allow the combined particles to form larger particles and settle quickly (Rui, 2013). Settling is then allowed to occur for 1 h.

Figure 2.
(a) Jar test used in the coagulation and filtration of SHWW; (b) materials used in the coagulation and filtration of SHWW.
To determine the optimum dose of various coagulants, 5 quantities of aluminum sulfate coagulant (2000, 3000, 4000, 5000, and 6000 mg/L) were poured into beakers containing raw SHWW and rapidly mixed at 170 rpm for 3 min, and slow mixed at 20 rpm and 20 min. The mixture was then allowed to settle for 1 h [12]. After settling, triplicate samples were withdrawn from the top of the supernatant from each beaker to measure pH, COD, BOD, TSS, TP, TN, EC, TDS, turbidity, TBC, TCC, and FCC. The same procedures were repeated using the other coagulants according to Table 3.

Table 2.
Chemical composition and physical properties of natural zeolite sample (wt%).
Table 2.
Chemical composition and physical properties of natural zeolite sample (wt%).
| Major Oxides | % | Physical Properties | |
|---|---|---|---|
| SiO2 | 70.5 | Overall surface area | 89.82 m2 gm−1 |
| Al2O3 | 11.72 | Porosity % | 27.8% |
| Na2O | 0.35 | Total pore area | 35.836 m2 gm−1 |
| K2O | 4.57 | Average pore diameter | 0.0181 µm |
| CaO | 1.01 | Bulk density | 1.83 gm cm−3 |
| MgO | 0.48 | Humidity | 6.75% |
| Fe2O3 | 2.56 | Solubility | 7.38% |
| P2O5 | 0.02 | Swelling index | 2.52 |
| TiO2 | 0.16 | pH | 6.8 |
| MnO | 0.09 | Apparent density | 2.37gm cm−3 |
| ZnO | 0.01 | ||
| ZrO2 | 0.055 | ||
| SO3 | 0.007 | ||
Zeolite samples were crushed (using a secondary crusher) and ground to less than 200 mesh for chemical analysis. Chemical analysis was carried out using PANalytical Axios Advanced XRF at the Center for Metallurgical Research and Development, Egypt.

Table 3.
Coagulants and coagulant dosages.
Table 3.
Coagulants and coagulant dosages.
| Coagulants | Coagulant Dosages (mg/L) | ||||
|---|---|---|---|---|---|
| Alum (A) | 2000 | 3000 | 4000 | 5000 | 6000 |
| Zeolite (Z) | 400 | 600 | 800 | 1000 | 1200 |
| Guava-leaf powder (GLP) | 1000 | 1500 | 2000 | 2500 | 3000 |
2.6. Filtration with Physically Activated Rice Straw (RS)
Rice straw (RS) is 28–48% cellulose, 26.40% hemicelluloses and lignin, and presented as the cell wall structure: 12.26% ash, 2.18% wax, and 9% silica (Shaban et al., 2009). RS was cut into small pieces, washed with distilled water, and subjected to physical activation by boiling in distilled water for 2 h. The treated samples were drained on filter paper and then dried at 105 °C until reaching a constant weight and preserved in desiccators [30].
After coagulation, flocculation, and sedimentation, the resultant effluent was subjected to three stages of rice-straw filtration using physically activated rice straw. Each stage of rice straw filtration was achieved by pressed the straw into a plastic funnel with a diameter and depth of 10 cm (Figure 1). The best concentration of each coagulant was chosen according to the removal percentage of COD and BOD after the jar test. The desired concentrations were as follows; a zeolite concentration of 1200 mg/L, a GLP concentration of 1 g/L, and an alum dose of 6 g/L. (Samples were taken before and after filtration and subjected to analysis as described in Section 2.5).
2.7. Analytical Methods
Methods used to measure pH, COD, BOD, TSS, TP, TN, EC, TDS, turbidity, TBC, TCC, and FCC are listed in Table 4.

Table 4.
Analytical methods.
3. Results
3.1. Wastewater Characterization
Table 1 shows the SHWW characteristics preceding any treatment, after 24 h of settling time, the pollutant removal percentage after 24 h settlement, and the standards and specifications of sewage licensed to be discharged into brackish or saline surface-water bodies. The upper limits of the contamination boundaries were lowered after 24 h of primer settling time. Additionally, the correlation of these qualities showed that the COD, BOD, microbial pointers (total and coliforms), and suspended solids were significantly higher than those mandated by Egyptian standards (Law 48, 1982).
3.2. Effect of 24 h Pretreatment Sedimentation of SHWW
In the present study, the SHWW used was raw without any treatment, which means it had a higher content of pollutants. The settlement process allowed the SHWW to settle for 24 h before coagulant addition. It was performed at room temperature. The process resulted in a decrease in BOD from 5094 ± 821 to 1634 ± 149 mg/L, with 67.9% removal. Additionally, COD was decreased by 20.6%, and TSS was reduced by 33%.
3.3. Effect of the Coagulation Process
Coagulation experiments were performed using alum (aluminum sulfate, Al2(SO4)3) as a chemical coagulant and zeolite (Z) and Psidium guajava leaf-powder (GLP) as natural coagulants. These experiments were performed using a jar test to investigate the effect of the coagulation process on the removal efficiencies of COD, BOD, TSS, TP, TN, EC, TDS, turbidity, TBC, TCC, and FC to compare the efficiency of chemical and green coagulant agents in the treatment of SHWW. For this purpose, 3 experiments were performed using different concentrations of alum, zeolite, and GLP, as explained in Section 2.4 and Section 2.5; the results are shown in Figure 3, Figure 4 and Figure 5. The idea of adding a coagulant to the slaughterhouse wastewater is to achieve particle instability and increase particle size, consequently performing the effective removal of organic substances present as COD and BOD. Different concentrations of Z, GLP, and alum were used to determine the best concentration of each coagulant and the best coagulant type used.
3.3.1. Effect of Coagulation Process Using Natural Zeolite (Z)
The zeolite doses added to SHWW after settling for 24 h were 0, 400, 600, 800, 1000, and 1200 mg/L. It was noticed that with increases in the Z dose, the removal efficiency for pollutants increased, with the best obtained at 1200 mg/L concentration, which achieved 62.06, 60.42, 40.63, 74.5, 31.25, 97.34, 82, and 93.3% for BOD, COD, TSS, TP, TKN, TBC, TCC, and FC, respectively (Figure 3a, Figure 4a, and Figure 5a). The reverse was found in the case of the EC and TDS removal percentages, which decreased with an increase in the Z dose, with the highest removal percentages being 5.64.1 and 8.8%, respectively, at 400 mg/L Z. The highest turbidity removal percentage of 28.57% was obtained from Z at 1000 mg/L.
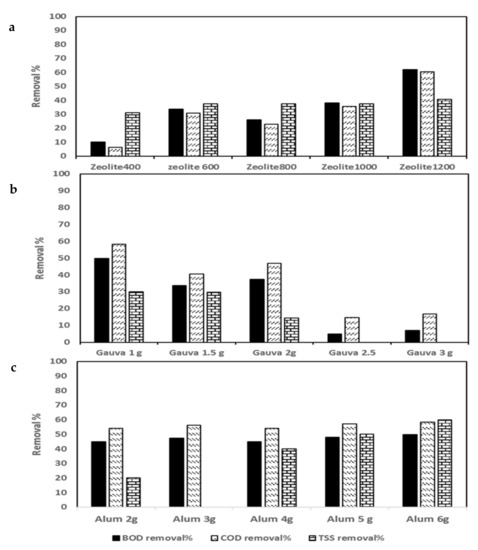
Figure 3.
Chemical oxygen demand (COD), biological oxygen demand (BOD), and total suspended solids (TSS): Contaminant removal efficiencies of wastewater by coagulation processes using: (a) zeolite, (b) guava-leaf powder (GLP), and (c) alum.
3.3.2. Effect of Coagulation Process Using Psidium Guajava-Leaf Powder (GLP)
The results are illustrated in Figure 3b and Figure 5b. The results denote the highest removal efficiencies of 49.82, 58.33, 30, 94.47, 82, and 16.67 for BOD, COD, and TSS. TBC, TCC, and FC were obtained at the lowest dose of GLP (1000 mg/L). On the other hand, the highest removal efficiency percentages of TP, EC, and TDS of 66.67, 17.5, and 12.58% were obtained using 3000 mg/L (Figure 4b). On the other hand, the highest TKN removal percentage of 37.5% was obtained using 1500 mg/L GLP.
3.3.3. Effect of Coagulation Process Using Alum
Alum doses ranging from 0–6000 mg/L were used. The highest removal percentages of 49.8, 58.33, 60, 17.58, 99.5, and 100% for BOD, COD, TSS, turbidity, TCC, and FC, respectively, were obtained at a dose of 6000 mg/L (Figure 3c and Figure 5c). An alum dose of 4000 mg/L resulted in higher removal percentages of TP and TKN (54.9 and 37.5%) (Figure 4c).
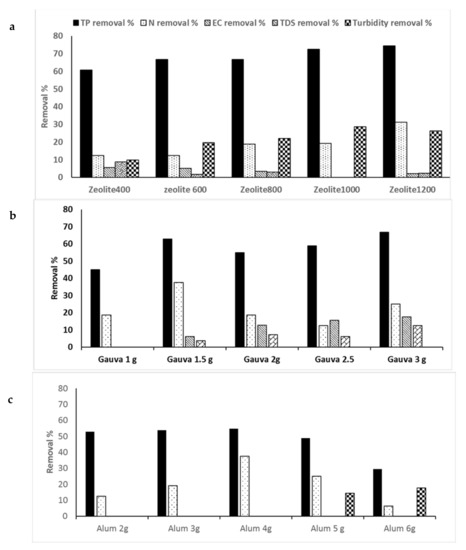
Figure 4.
Contaminant removal efficiencies of wastewater by coagulation processes using: (a) zeolite, (b) guava-leaf powder (GLP), and (c) alum. Total phosphorus (TP), Nitrogen Removal (TKN), Electric Conductivity, (EC), and total dissolved solids (TDS).
On the other hand, the current results for the TP removal percentage were higher than those reported by Omotayo and Abass [31], who reported a removal percentage for TP of 45% for abattoir wastewater treated by 750 mg/L alum. In addition, the TN removal percentage was higher than those reported by Lubna et al. [32] The discrepancy could be attributed to the current study’s higher alum dose (4000 mg/L).
3.4. Effect of Filtration with Physically Activated Rice Straw (RS)
Coagulant concentrations of Z at 1200 mg/L, GLP at 1 g/L, and alum at 6000 mg/L were used as influents for the three-step RS filtration process, and the character of the effluent produced is illustrated in Table 5 and Figure 6. The best concentrations, which gave the best BOD, COD, and TSS removal percentages for each coagulant were chosen for this step. The data in Table 5 show that the highest BOD removal value (31.71%) was obtained from alum 6 g/L + RS, followed by GLP 1 g/L (30.49), and the lowest was Z 1200 mg/L. For COD, the filtration step with RS resulted in 90, 30, and 13.8% from Z 1200 mg/L, GLP 1 g/L, and alum 6 g/L, respectively. TSS was mostly removed with 82.14% by filtration with RS following coagulation with GLP 1 g/L.

Table 5.
Effect of filtration process with physically treated rice straw (RS) on the physicochemical and bacteriological characters of SHWW after coagulation with Z 1200 mg, GLP 1 g, and alum 6 g (±SD).
TKN, EC, and turbidity were mainly removed using the natural coagulant GLP + RS compared with Z 1200 + RS or A 6g + RS, as illustrated in Table 5. In the present step, the highest TBC and TCC removal percentage was obtained by filtration of RS following coagulation with Z 1200 (Z 1200 + RS). This result shows that the filtration with RS increased TBC and TCC removal percentages by Z 1200 more than using it alone. On the other hand, A 6g + RS showed the highest removal percentage for FC.
3.5. Evaluation of the Current Pilot Test Performance
To evaluate the current system used for the treatment of SHWW by natural and chemical coagulants, the removal percentages of all tested parameters are illustrated in Table 6. Data in Table 6 summarize the comparison between the efficiency of the biological and chemical coagulants used. It was found that a natural coagulant (zeolite with a concentration of 1200 mg/L) showed the best removal percentage for physicochemical and bacteriological parameters. Using Z 1200 for the treatment of SHWW achieved removal percentages of 90.58, 83.47, 88.75, 54.89, 21.39, 34.49, 84.16, 99.98, and 99.93% for BOD, COD, TSS, TKN, EC, turbidity, TBC, and TCC, respectively.

Table 6.
Average physicochemical and bacteriological characteristics of untreated (USHWW) and treated slaughterhouse wastewater (TSHWW) (±SD).
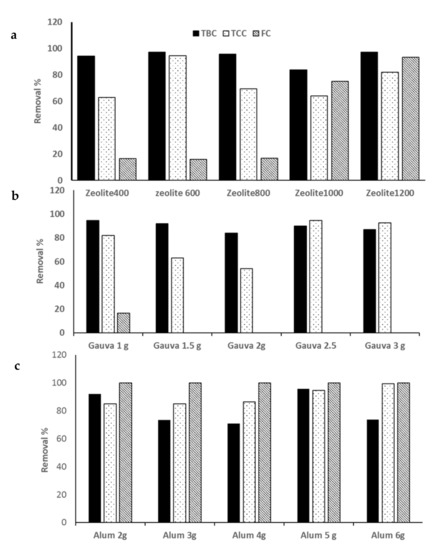
Figure 5.
Microbiological contaminant removal efficiencies from wastewater by coagulation processes using (a)zeolite, (b) guava-leaf powder (GLP), and (c) Alum. Total bacterial count (TBC), total coliform count (TCC), fecal coliform (FC).
This system successfully removed TBC, TCC, and FC with a maximum removal percentage of 100% for all. In comparison, El-Sesy and Mahran [16] recorded 88–92% removal of total viable bacterial counts, using an ethanolic extract of Psidium guajava (1.5 g), from treated wastewater samples.
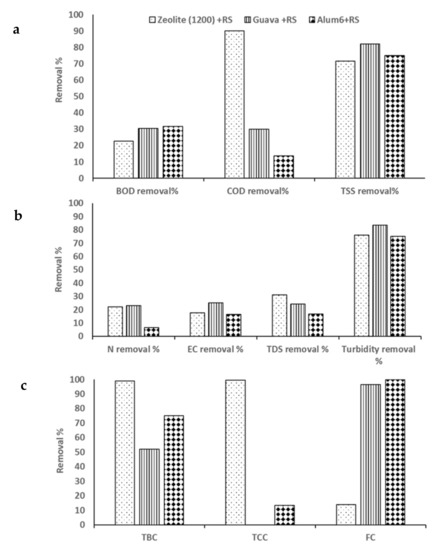
Figure 6.
Contaminant removal efficiencies by the most effective dose of coagulants followed by filtration with physically treated rice straw (RS): Z 1200 mg; GLP 1000 mg; alum 6000 mg: (a) Chemical oxygen demand (COD), biological oxygen demand (BOD), and total suspended solids (TSS); (b) Total phosphorus (TP), nitrogen removal (N), electric conductivity (EC), and total dissolved solids (TDS); (c) Total bacterial count (TBC), total coliform count (TCC), and fecal coliform (FC).
4. Discussion
The upper limits of the contamination boundaries were brought down after 24 h of primer settling time. Additionally, the correlation of these qualities showed that the COD, BOD, microbial pointers (total and coliforms), and suspended solids were significantly higher than those mandated by Egyptian standards (Law 48, 1982). Thus, the SHWW should have been treated before release. The concentration of COD and BOD were higher than those obtained by [10] (with ranges of 5817 ± 473 and 2543 ± 362 mg/L for COD and BOD, respectively). On the other hand, the obtained results were lower than those obtained by Sunder and Satyanarayan [33], showing COD, BOD, and suspended solids in the ranges of 22,000–27,500 mg/L, 10,800–14,600 mg/L, and 1280–1500 mg/L, respectively. Finally, the current results are similar to those of Bustillo-Lecompte and Mehrvar [34], who obtained a BOD range of 150–8500 mg/L, a COD range of 500–16000 mg/L, and a TSS range of 0.1–10,000 mg/L. TKN recorded in this study (see Table 1) was lower than that obtained by Bazrafshan et al. [10] (137 mg/L) and Husam and Nassar [35] (154 ± 12 mg/L), but higher than that obtained by Bustillo-Lecompte and Mehrvar [34]. For the bacteriological character, the total coliform count and fecal coliform found in the examined SHWW were lower than those obtained by [10].
The primer settling measure is a characteristic treatment strategy that requires no added chemicals, and it only depends on gravity, making it a low-cost step [36]. Although a few specialists understood the significance of the regular settling measure, there are few data accessible in the previous works. Most past studies used pre-settled, diluted SHWW [37]. However, in the present study, the SHWW used was raw, without any treatment, which means it had a higher pollutant content. The settlement process allowed the SHWW to settle for 24 h before coagulant addition. It was performed at room temperature. The process resulted in a decrease in BOD from 5094 ± 821 to 1634 ± 149 mg/L with a 67.9% removal percentage. Additionally, COD was decreased by 20.6%, and TSS was reduced by 33%. The BOD removal percentage was much higher than that found by Bazrafshan et al. [10], who obtained a 13% reduction, and Mittal [36] reported a BOD removal percentage of 25–40%. On the other hand, they obtained a higher COD removal percentage (28%) than did the present study. This difference in BOD removal percentage may be attributed to the higher content of blood in the used SHWW and the absence of any blood removal practice [38]. Few data are available on the efficiency of pretreatment sedimentation in removing different pollutants, such as TSS. In the current study, 33% of the initial TSS was released, which is less than that obtained by Bazrafshan et al. [10] and Amuda and Alade [39], who obtained 64% and 65% removal, respectively, as the initial TSS (480 mg/L) was much lower than in case of the SHWW used by Bazrafshan et al. [10], which contained 3247 mg/L, as the SHWW used in the present study contains fewer suspended solids but more dissolved solids (Table 1). The effluent parameter after the 24 h sedimentation period revealed higher organic COD and BOD; however, the BOD/COD ratio was 0.2, which means that 80% of this COD needed physicochemical treatment and the other 20% needed biological treatment [7]. On the other hand, the 24 h sedimentation period resulted in a low nutrient removal percentage, as illustrated by a TP of 17.7 and a TKN of 15.79%. The obtained result of the TKN removal percentage was lower than that obtained by Bazrafshan et al. [9], who obtained a 33% TKN removal percentage from SHWW after a 24 h settling period.
This step resulted in a 38.1, 25.9, and 20% removal percentage of TBC, TCC, and FC for bacteriological examination. These results were much higher than those reported by Bazrafshan et al. [12], who reported a 17.86 and 10.5% removal percentage for TCC and FC.
Natural zeolite is a suitable ion exchanger that can remove and recover cations [40]. Khotimah et al. [41] obtained a BOD removal percentage range of 26–29%, which is lower than that obtained currently by Z 1200 (62.06%) using a different soil mixture box (SMB) mixed with natural zeolite from domestic wastewater in contrast with [42], who obtained higher BOD (66.33%) and turbidity removal percentages (71.45%) but a lower COD removal percentage of 43.78%, and then-current levels from oil refinery wastewater, using Fe3O4/mordenite zeolite, a hydrothermal process.
The results in Figure 3 show that Z in a dose of 1200 mg/L had a higher removal efficiency for COD (60.42%) with an optimum pH of 7.59 [43] than that reported by Samkutty and Gough [44], who obtained a 30% removal percentage from dairy wastewater; Lakdawala and Patel [45], who obtained 1–26.6% from sugar industry wastewater; and Kotoulas et al. [46], who obtained up to a 40% removal percentage from second cheese whey. Kolakovic et al. [47] reported a lower COD reduction range of 30–50% than did the present study from dairy wastewater using organo-zeolite and a filter column. They mentioned that the capability of COD removal might be attributed to the zeolite’s ability to absorb both organic and inorganic substances. In the current study, zeolite used in a dose of 1200 mg/L resulted in 74.5% TP removal, which is much higher than that obtained by Aly et al. [48], who used natural zeolite in the treatment of olive-mill wastewater; in contrast, they obtained a higher removal percentage for turbidity (96.8%) and EC (48.4%) than the present study. This difference could be attributable to our study’s higher initial turbidity, as the SHWW was not diluted with water, so it had a high content of blood with a strong color and a high level of iron (dissolved, especially under the low pH), which has a positive correlation with turbidity [49] (202 ± 9.16) and lower initial EC (3.46 ± 0.31 ds/m). Compared with the current results, Ospanov et al. (2016) obtained a higher TSS removal efficiency of 93.7–96.9% and for ammonia-nitrogen, 79.7–92.7% from a wastewater treatment plant and treated sewage after preliminary mechanical treatment using natural zeolite. They used two stages of treatments and used activated sludge with natural zeolite, but in the current study, only natural zeolite was used. In this study, the initial TKN (the sum of ammonia-nitrogen plus organically bound nitrogen) was 532 mg/L of the SHWW. The recorded removal percentage was 31.25%, which is much lower than that obtained by Ospanov et al. (2016), who obtained an ammonia-nitrogen removal percentage of 79.7–92.7%) from wastewater treatment plant and treated sewage after preliminary mechanical treatment using natural zeolite [50] and 99% removal after 1 day of contact time and an initial ammonia –N of 45 mg/L, which is much lower than the initial level in the current study, and the contact time applied in this experiment was 93 min. Pirsaheb et al. [51] obtained a lower TKN removal percentage of 21.21% in a filling ratio of 10%, with an initial COD concentration of 30,000 mg/L in compost leachate wastewater, and an HRT of 3 days.
Total and fecal coliforms (TCC and FC) have long been recommended as excellent microbiological indicators of drinking-water and treated-wastewater quality. They are simple to detect [52]. Garcia et al. [53] obtained higher removal percentages of 100% for TCC and FC on the tenth day for fecal coliforms using natural phillipsite (zeolite) columns in a percolation reactor at a constant solution flowrate than did this study, and this may be attributed to the shorter contact time of only 83 min. Using synthesized zeolite and copper-modified zeolite led to complete elimination of total coliforms (100%) after 90 and 50 min contact time, respectively [54], from wastewater from the Akaki River. This also has a higher removal percentage than obtained in the current study, resulting from natural zeolite’s use without any modification. The removal percentage obtained in this study was in line with that obtained by Hrenović et al. [55], who reported a 95.1% reduction in FC and a 96.48% reduction in TC using natural zeolite as a pig slurry.
Several studies have used plant extracts as natural coagulants to preserve a healthy environment and lower costs. This is the first time Psidium guajava leaves have been applied in form powder, not as an extract, and it is easy to prepare and does require any chemicals or additional costs. Upon treatment with Psidium guajava-leaf powder, the BOD was decreased by 49.82% at a concentration of 1 g/L. This value is lower than that obtained by El-Sesy and Mahran’s [17] 77% removal percentage obtained using 1.5 g GLP ethanol extract. GLP has an efficient adsorption effect, as mentioned by Aly et al. [56] who used it to remove Nile blue dye from wastewater.
El-Sesy and Mahran [13] recorded an 88%–92% removal of total viable bacteria, using an ethanolic extract of Psidium guajava (1.5 g), from treated wastewater samples. This result was lower than that obtained in the current study as TBC was removed at a rate of 94.47%, using 1 g/L GLP, from SHWW, while they obtained a higher FC removal percentage than the current study did. The antimicrobial effect of Psidium guajava-leaf powder is due to its containing an essential oil rich in cineol, tannins, and triterpenes, which is capable of damaging enteric bacteria [57], in addition to the presence of flavonoids and phenols [58]. Flavonoids and tannins, which are present in GLP, are considered as coagulating agents [59]
Alum doses ranging from 0–6000 mg/L were used. The highest removal percentages of 49.8, 58.33, 60,17.58, 99.5, and 100% for BOD, COD, TSS, turbidity, TCC, and FC, respectively, were obtained at a dose of 6000 mg/L (Figure 3c and Figure 5c). An alum dose of 4000 mg/L resulted in higher removal percentages of TP and TKN (54.9 and 37.5%) (Figure 4c).
The findings within the range reported by Al-Mutairi et al. [37] and Amuda and Alade [39] noted COD removal efficiency of 45–75% from slaughterhouse wastewater treated by aluminum salts and polymer compounds. However, these results are lower than the removal percentages reported for palm-oil mill effluent (POME) treated by alum at 4000 mg/L obtained by Jagaba et al. [12], who recorded removal percentages for TSS, turbidity, NH3-N, and COD as 98.72%, 98.68%, 98.46%, and 75.01%, respectively, and the recorded results are lower than those documented for alum coagulation for simulated dairy wastewater performed by Loloei et al. [60], who obtained removal percentages of 95% and 68% for turbidity and COD, respectively. The issue may be attributed to the lower initial COD in their study (3200 mg/L) compared with the initial COD recorded for the current analysis (9680 mg/L) and different wastewater characteristics.
On the other hand, the current results for TP removal percentage were higher than those reported by Omotayo and Abass [31], who reported a removal percentage for TP of 45% for abattoir wastewater treated with 750 mg/L alum. Beyond that, the TN removal percentage was higher than that reported by Lubna et al. [32]. The discrepancy could be attributed to the current study’s usage of a higher alum dose (4000 mg/L).
Alum achieved superior removal percentages for both TCC and FC for the microbial load of the examined wastewater, inconsistent with those reported by Ewida and Ibrahim [61]. They recorded a 99.8% removal rate of TCC in treated wastewater collected from the El-Rahway drain after treatment with alum. The discrepancy could be attributed to the high content of natural organic matter and other chemical pollutants, which can generate massive flocs in treated water. As a result, most bacterial cells would be adsorbed b6y these flocs, which are typically eliminated during filtration [62].
Rice straw (RS), which is physically activated through boiling, was used in the final step of this pilot-scale treatment for the filtration of SHWW, which was treated with sedimentation, followed by coagulation and flocculation with zeolite, GLP, or alum. So, in the current section, the results of the filtration step are presented and discussed so as to judge its effect in combination with both chemical and natural coagulants. In their study, [63] stated that the physical activation of RS through boiling slightly increases its removal efficiency.
The obtained reductions in BOD, COD, and TSS by using physically treated RS infiltration was much higher than those obtained by Hegazy et al. [27], who obtained a 50% and a 32% reduction in TSS and COD when using rice straw (as a first step) followed by treatment with 2 g cement dust for the treatment of raw sewage from a wastewater treatment plant. At the same time, they obtained a slightly higher BOD (35.3%) removal percentage than did the current study.
Rahman et al. (2018) recorded a lower turbidity removal percentage than did the current study at 82% using Artocarpus Heterophyllus (Jackfruit seed) powder as a coagulant from dairy wastewater. In the present step, the highest TBC and TCC removal percentages were obtained by filtration with RS following coagulation with Z 1200 (Z 1200 + RS). This result demonstrates that the filtration with RS increased the TBC and TCC removal percentages more than did using Z 1200 alone. On the other hand, A 6g + RS showed the highest removal percentage for FC.
Comparing the obtained results with previous studies, Bazrafshan et al. [10] obtained COD and BOD removal percentages of 99%, which are slightly higher than those obtained currently, but via chemical coagulation and electrocoagulation techniques (100 mg/L PACl and applied voltage 40 V) from cattle SHWW. The COD removal percentage in the current study was much higher than that obtained by Al-Mutairi et al. [37] and Amuda and Alade [39]. They achieved 45–75% via the chemical coagulation of slaughterhouse wastewater by adding aluminum salts and polymer compounds. The current COD removal percentage was also in the same range as that of Rajakumar et al. [64], who achieved TCOD and SCOD removal efficiencies of 70–86% and 80–92%, respectively, from the treatment of poultry slaughterhouse wastewater in a hybrid up-flow anaerobic sludge blanket reactor. In addition, COD and BOD removal percentages lie in the same range as those achieved by Sunder and Satyanarayan [33], who obtained 86.0–93.58% and 88.9–95.71%, respectively, via the anaerobic treatment of SHWW.
Pirsaheb et al. [65] obtained a 48% reduction in TKN from compost leachate in strong wastewater using an anaerobic baffled reactor containing natural zeolite. The compost leachate wastewater percentage was much lower than that obtained by the current treatment system (54.89%). They were in line with our result, which found that TKN removal efficiency was enhanced by increasing the amount of zeolite. On the other hand, the TKN removal percentage lies in the same range as that obtained by Cai et al. [66] (39–65.9%) and Pirsaheb et al. (2021) (48–100%). The current system resulted in a maximum turbidity percentage of 84.16%, which is higher than that obtained by El-Sesy and Mahran [17], who recorded 73%, and Khotimah et al. (2021), who obtained 71.45%.
This system successfully removed TBC, TCC, and FC with a maximum removal percentage of 100% for all. This removal percentages were similar to those that El-Sesy and Mahran [16] recorded: an 88–92% removal percentage of total viable bacterial counts using an ethanolic extract of Psidium guajava (1.5 g) from treated wastewater samples.
Despite the successful removal of pollutants from SHWW by using this pilot-scale process, the obtained TSHWW still does not meet the standard requirements for wastewater disposal for any of the measured parameters except for TSS, as shown in Table 1 and Table 6 (according to Egyptian Standard Law 48, 1982 and Decree 8, 1993). This may be attributable to raw SHWW containing a high amount of blood, without dilution or pretreatment, as the sampling point was the bleeding hall, and sampling was performed without blood removal. Thus, further studies are needed to improve this process.
5. Conclusions
This study used a pilot system to treat SHWW, which included a three-step process which started with sedimentation and then included coagulation and flocculation using natural zeolites and Psidium guajava as green and environmentally friendly agents and alum as an inorganic coagulant. The final step was filtration with physically treated rice straw. Each step was judged separately by measuring the removal percentage of each analyzed pollutant. The overall process was evaluated by the same method. The following conclusions can be reached from the results obtained in this work: 24 h pretreatment sedimentation of SHWW resulted in 67.9, 20.66, and 33.3% removal of BOD, COD, and TSS. Additionally, 38.1, 25.9 and 20% removal percentages were achieved for TBC, TCC, and FC. According to the current results, the best-obtained pollutant removal percentage for each coagulant used was zeolite at 1200 mg/L, GLP at 1000 mg/L, and alum at 6000 mg/L. GLP 1 g/L gives the highest removal percentage of TSS, TKN, EC, and turbidity in the coagulation step. Z 1200 offers the highest removal percentage of COD, TDS, TBC, and TCC. From these results, it was concluded that natural coagulants performed better than the chemical coagulant.
Finally, judging the overall pilot test system after applying the filtration with physically treated RS, we found that the best removal efficiencies were obtained from Z 1200 + RS. It resulted in 90.58, 83.47, 88.75, 54.89, 21.39, 34.49, 84.16, 99.98, and 99.93% removal percentages of BOD, COD, TSS, TKN, EC, turbidity, TBC, and TCC, respectively.
Author Contributions
Conceptualization: F.A., Y.A.T., M.S., N.R.E. and S.S.; data curation: F.A., Y.A.T., M.S. and N.R.E.; formal analysis: M.S., S.S., M.E.-S. and A.G.; funding acquisition: F.A., Y.A.T., M.S., N.R.E., S.S., M.E.-S. and A.G; methodology: F.A., Y.A.T., M.S. and N.R.E.; investigation: F.A., Y.A.T., M.S. and N.R.E.; supervision: F.A., Y.A.T., M.S., N.R.E., M.E.-S. and A.G.; writing—original draft: F.A., Y.A.T. and N.R.E.; writing—review and editing: M.S., S.S., A.G. and M.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The current work was funded by the Taif University Researchers Supporting Project number (TURSP-2020/139), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to, and the appropriate ethical review committee approval has been received. The authors confirm that they have followed EU standards to protect animals used for scientific purposes and feed legislation.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data sets during the current study are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Alum | Aluminum sulfate, Al2(SO4)3 |
| BOD | Biological oxygen demand |
| COD | Chemical oxygen demand |
| EC | Electerical conductivity |
| FC | Fecal coliform |
| GLP | Guava-leaf powder |
| PVC | Polyvinyl chloride |
| POME | Palm-oil mill effluent |
| RS | Rice straw |
| SCOD | Soluble chemical oxygen demand |
| SHWW | Slaughterhouse wastewater |
| TBC | Total bacterial count |
| TCC | Total coliform count |
| TCOD | Total chemical oxygen demand |
| TDS | Total dissolved solids |
| TKN | Total Kieldahl nitrogen |
| TN | Total nitrogen |
| TP | Total phosphorus |
| TSS | Total suspended solids |
| Z | Zeolites |
References
- Kundu, P.; Debsarkar, A.; Mukherjee, S. Treatment of slaughter house wastewater in a sequencing batch reactor: Performance evaluation and biodegradation kinetics. BioMed Res. Int. 2013, 2013, 134872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elemile, O.O.; Raphael, D.O.; Omole, D.O.; Oloruntoba, E.O.; Ajayi, E.O.; Ohwavborua, N.A. Assessment of the impact of abattoir effluent on the quality of groundwater in a residential area of Omu-Aran, Nigeria. Environ. Sci. Eur. 2019, 31, 16. [Google Scholar] [CrossRef] [Green Version]
- Tritt, W.; Schuchardt, F. Materials flow and possibilities of treating liquid and solid wastes from slaughterhouses in Germany. A review. Bioresour. Technol. 1992, 41, 235–245. [Google Scholar] [CrossRef]
- Cristian, O. Characteristics of the untreated wastewater produced by food industry. An. Univ. Din Oradea Fasc. Protecţia Mediu. 2010, 15, 709–714. [Google Scholar]
- Ziara, R.M.; Li, S.; Dvorak, B.I.; Subbiah, J. Water and energy use of antimicrobial interventions in a mid-size beef packing plant. Appl. Eng. Agric. 2016, 32, 873–879. [Google Scholar]
- Massé, D.; Masse, L. Characterization of wastewater from hog slaughterhouses in Eastern Canada and evaluation of their in-plant wastewater treatment systems. Can. Agric. Eng. 2000, 42, 139–146. [Google Scholar]
- Bustillo-Lecompte, C.; Mehrvar, M.; Quiñones-Bolaños, E. Slaughterhouse wastewater characterization and treatment: An economic and public health necessity of the meat processing industry in Ontario, Canada. J. Geosci. Environ. Prot. 2016, 4, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Ün, Ü.T.; Koparal, A.S.; Öğütveren, Ü.B. Hybrid processes for the treatment of cattle-slaughterhouse wastewater using aluminum and iron electrodes. J. Hazard. Mater. 2009, 164, 580–586. [Google Scholar]
- Suman, A.; Ahmad, T.; Ahmad, K. Dairy wastewater treatment using water treatment sludge as coagulant: A novel treatment approach. Environ. Dev. Sustain. 2018, 20, 1615–1625. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Kord Mostafapour, F.; Farzadkia, M.; Ownagh, K.A.; Mahvi, A.H. Slaughterhouse wastewater treatment by combined chemical coagulation and electrocoagulation process. PLoS ONE 2012, 7, e40108. [Google Scholar] [CrossRef]
- Kakoi, B.; Kaluli, J.W.; Ndiba, P.; Thiong’o, G. Banana pith as a natural coagulant for polluted river water. Ecol. Eng. 2016, 95, 699–705. [Google Scholar] [CrossRef]
- Jagaba, A.; Kutty, S.; Hayder, G.; Latiff, A.; Aziz, N.; Umaru, I.; Ghaleb, A.; Abubakar, S.; Lawal, I.; Nasara, M. Sustainable use of natural and chemical coagulants for contaminants removal from palm oil mill effluent: A comparative analysis. Ain Shams Eng. J. 2020, 11, 951–960. [Google Scholar] [CrossRef]
- Vatvani, C. The Toxic Waste That Enters Indonesia’s Citarum River, One of the World’s Most Polluted. Channel News Asia. 2018. Available online: https://www.channelnewsasia.com/news/asia/indonesia-citarum-river-worlds-most-polluted-toxic-waste-10124436 (accessed on 14 April 2018).
- Montuori, P.; Lama, P.; Aurino, S.; Naviglio, D.; Triassi, M. Metals loads into the Mediterranean Sea: Estimate of Sarno River inputs and ecological risk. Ecotoxicology 2013, 22, 295–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, M.R.; Camacho, F.P.; Sousa, V.S.; Bergamasco, R. Green technologies for cyanobacteria and natural organic matter water treatment using natural based products. J. Clean. Prod. 2017, 162, 484–490. [Google Scholar] [CrossRef]
- Mitra, T.; Das, S.K. Cr (VI) removal from aqueous solution using Psidium guajava leaves as green adsorbent: Column studies. Appl. Water Sci. 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- El-Sesy, M.E.; Mahran, B.N. The Antibacterial and Coagulant Activity of Psidium Guajava Leaves Extracts in Purification of Wastewater. Biosci. Biotechnol. Res. Asia 2020, 17, 191–203. [Google Scholar] [CrossRef]
- Alamin, M.; Samia, M.; Alqurashi, A.; Elsheikh, A. Bactericidal activity of Psidium guajava leaves against some pathogenic microbes. IOSR J. Dent. Med. Sci. 2016, 15, 61–70. [Google Scholar]
- Naili, M.; Errayes, A.; Alghazeer, R.; Mohammed, W.A.; Darwish, M. Evaluation of Antimicrobial and Antioxidant Activities of Psidium guajava L growing in Libya. Int. J. Adv. Biol. Biomed. Res. 2020, 8, 419–428. [Google Scholar]
- Abouelenien, F.A.; Elshahawy, I.S.; Hamad, M.E.; Elsaidy, N.R. Biosecurity assessment in relation to the occurrence of some coccidian parasites in poultry farms, with in vitro evaluation of Psidium gujava as coccidia sporulation inhibitor. Emir. J. Food Agric. 2021, 33, 532–543. [Google Scholar]
- Tasić, Ž.; Bogdanović, G.; Antonijević, M. Application of natural zeolite in wastewater treatment: A review. J. Min. Metall. A Min. 2019, 55, 67–79. [Google Scholar] [CrossRef]
- Madej, J. Sorption of Heavy Metal Ions of Chromium, Manganese, Selenium, Nickel, Cobalt, Iron from Aqueous Acidic Solutions in Batch and Dynamic Conditions on Natural and Synthetic Aluminosilicate Sorbents. Materials 2020, 13, 5271. [Google Scholar]
- Somvanshi, S.S.; Kumari, M. Comparative analysis of different vegetation indices with respect to atmospheric particulate pollution using sentinel data. Appl. Comput. Geosci. 2020, 7, 100032. [Google Scholar] [CrossRef]
- Bakar, S.A.S.A. Binding Agent for Biodegradable Compression Molded Rice Straw Filled Rice Bran Packaging Products. Ph.D. Thesis, Universiti Teknologi Malaysia, Iskandar Puteri, Malaysia, 2009. [Google Scholar]
- Abdelsalam, N.R.; Fouda, M.M.; Abdel-Megeed, A.; Ajarem, J.; Allam, A.A.; El-Naggar, M.E. Assessment of silver nanoparticles decorated starch and commercial zinc nanoparticles with respect to their genotoxicity on onion. Int. J. Biol. Macromol. 2019, 133, 1008–1018. [Google Scholar] [CrossRef]
- Luo, X.; Shen, M.; Huang, Z.; Chen, Z.; Chen, Z.; Lin, B.; Cui, L. Efficient removal of Pb (II) through recycled biochar-mineral composite from the coagulation sludge of swine wastewater. Environ. Res. 2020, 190, 110014. [Google Scholar] [CrossRef]
- Hegazy, B.; El-Khateeb, M.; El-adly Amira, A.; Kamel, M. Low-cost wastewater treatment technology. J. Appl. Sci. 2007, 7, 815–819. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; Volume 529. [Google Scholar]
- Mohammed, M.; Shitu, A.; Ibrahim, A. Removal of methylene blue using low cost adsorbent: A review. Res. J. Chem. Sci. ISSN 2014, 2231, 606X. [Google Scholar]
- Sarker, N.; Fakhruddin, A. Removal of phenol from aqueous solution using rice straw as adsorbent. Appl. Water Sci. 2017, 7, 1459–1465. [Google Scholar] [CrossRef] [Green Version]
- Omotayo, A.; Hammad, M.A.; Barker, K. Efficient data harvesting for tracing phenomena in sensor networks. In Proceedings of the 18th International Conference on Scientific and Statistical Database Management (SSDBM’06), Vienna, Austria, 3–5 July 2006; pp. 59–70. [Google Scholar]
- Ibrahim, L.A.; Asaad, A.A.; Khalifa, E.A. Laboratory approach for wastewater treatment utilizing chemical addition case study: El-Rahway Drain, Egypt. Life Sci. J. 2019, 16, 56–67. [Google Scholar]
- Sunder, G.C.; Satyanarayan, S. Efficient treatment of slaughter house wastewater by anaerobic hybrid reactor packed with special floating media. Int. J. Chem. Phys. Sci. 2013, 2, 73–81. [Google Scholar]
- Bustillo-Lecompte, C.F.; Mehrvar, M. Treatment of actual slaughterhouse wastewater by combined anaerobic–aerobic processes for biogas generation and removal of organics and nutrients: An optimization study towards a cleaner production in the meat processing industry. J. Clean. Prod. 2017, 141, 278–289. [Google Scholar] [CrossRef]
- Husam, A.-N.; Nassar, A. Slaughterhouses wastewater characteristics in the Gaza strip. J. Water Resour. Prot. 2019, 11, 844. [Google Scholar] [CrossRef] [Green Version]
- Mittal, G.S. Treatment of wastewater from abattoirs before land application—a review. Bioresour. Technol. 2006, 97, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, N.; Hamoda, M.; Al-Ghusain, I. Coagulant selection and sludge conditioning in a slaughterhouse wastewater treatment plant. Bioresour. Technol. 2004, 95, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Johns, M. Developments in wastewater treatment in the meat processing industry: A review. Bioresour. Technol. 1995, 54, 203–216. [Google Scholar] [CrossRef]
- Amuda, O.; Alade, A. Coagulation/flocculation process in the treatment of abattoir wastewater. Desalination 2006, 196, 22–31. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, L.; Zhu, Z.; Wei, M. Study on potassium extraction by static adsorption using zeolite. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, Chian, 24–26 June 2011; pp. 4494–4497. [Google Scholar]
- Khotimah, C.; Handayani, D.; Hadiwidodo, M.; Wardhana, I. The efficiency of biological oxygen demand removal in domestic wastewater treatment using multi soil layering. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kitakyushu, Japan, 25–26 April 2021; p. 012006. [Google Scholar]
- Gil-San-Millan, R.; Grau-Atienza, A.; Johnson, D.T.; Rico-Francés, S.; Serrano, E.; Linares, N.; Garcia-Martinez, J. Improving hydrogen production from the hydrolysis of ammonia borane by using multifunctional catalysts. Int. J. Hydrog. Energy 2018, 43, 17100–17111. [Google Scholar] [CrossRef]
- Syafalni, S.; Johari, N.; Satrio, S. Pre Treatment of River Water By Using Bentonite and Modified Zeolite. Int. J. Appl. Eng. Res. 2015, 10, 14515–14528. [Google Scholar]
- Samkutty, P.J.; Gough, R.H. Filtration treatment of dairy processing wastewater. J. Environ. Sci. Health Part A 2002, 37, 195–199. [Google Scholar] [CrossRef]
- Lakdawala, M.M.; Patel, Y.S. Studies on adsorption capacity of zeolite for removal of chemical and bio-chemical oxygen demands. Chem. J. 2015, 1, 139–143. [Google Scholar]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Zeolite as a potential medium for ammonium recovery and second cheese whey treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Kolaković, S.; Stefanović, D.; Milićević, D.; Trajković, S.; Milenković, S.; Kolaković, S.S.; Anđelković, L. Effects of reactive filters based on modified zeolite in dairy industry wastewater treatment process. Chem. Ind. Chem. Eng. Q. CICEQ 2013, 19, 583–592. [Google Scholar] [CrossRef]
- Aly, A.A.; Hasan, Y.N.; Al-Farraj, A.S. Olive mill wastewater treatment using a simple zeolite-based low-cost method. J. Environ. Manag. 2014, 145, 341–348. [Google Scholar] [CrossRef]
- Amfo-Out, R.; Agyenim, J.; Nimba-Bumah, G. Correlation Analysis of Groundwater Coloration from Mountainous Areas. Environ. Res. Eng. Manag. 2014, 1, 16–24. [Google Scholar]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Verma, P. Current perspective on wastewater treatment using photobioreactor for Tetraselmis sp.: An emerging and foreseeable sustainable approach. Environ. Sci. Pollut. Res. 2021, 2021, 1–33. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hadei, M.; Sharafi, K. Human health risk assessment by Monte Carlo simulation method for heavy metals of commonly consumed cereals in Iran-Uncertainty and sensitivity analysis. J. Food Compos. Anal. 2021, 96, 103697. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, M.; Zhang, D.-k. The potential application of natural zeolite for greywater treatment. Desalination 2008, 218, 271–280. [Google Scholar] [CrossRef]
- Garcia, J.; Gonzalez, M.; Notario, J. Removal of bacterial indicators of pollution and organic matter by phillipsite-rich tuff columns. Appl. Clay Sci. 1992, 7, 323–333. [Google Scholar] [CrossRef]
- Fanta, F.T.; Dubale, A.A.; Bebizuh, D.F.; Atlabachew, M. Copper doped zeolite composite for antimicrobial activity and heavy metal removal from waste water. BMC Chem. 2019, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Hrenović, J.; Tofant, A.; Ostović, M.; Milić, D. Effect of clinoptilolite addition on bacterial counts in pig slurry. Folia Microbiol 1999, 44, 729–734. [Google Scholar]
- Aly, S.; El-Sayed, A.M.; Tharwat, K.M.; Mahmoud, M.A.; Khaled, A.M.; Gad, A.M.; Mahmoud, A.T. Adsorption of Nile Blue Dye using Guava Leaf Powder. Int. J. Eng. Res. 2019, 8, 69–72. [Google Scholar]
- Doughari, J.; Manzara, S. In vitro antibacterial activity of crude leaf extracts of Mangifera indica Linn. Afr J Microbiol Res 2008, 2, 67–72. [Google Scholar]
- Abouelenien, F.; Miura, T.; Nakashimada, Y.; Elleboudy, N.S.; Al-Harbi, M.S.; Ali, E.F.; Shukry, M. Optimization of Biomethane Production via Fermentation of Chicken Manure Using Marine Sediment: A Modeling Approach Using Response Surface Methodology. Int. J. Environ. Res. Public Health 2021, 18, 11988. [Google Scholar] [CrossRef] [PubMed]
- Aweng, E.; Jessuta, J.; Prawit, K.; Liyana, A. Potential of the concoction of Champereia manillana and Psidium guajava shoot extracts as coagulant for drinking water treatment. Malay. Nat. J. 2015, 67, 419–426. [Google Scholar]
- Loloei, M.; Alidadi, H.; Nekonam, G.; Kor, Y. Study of the coagulation process in wastewater treatment of dairy industries. Int. J. Environ. Health Eng. 2014, 3, 12. [Google Scholar] [CrossRef]
- Ewida, A.; Ibrahim, L. Comparative Performance Studies of Different Chemical Additives In Water Treatment On Variety Of Water Sources. Off. J. Arab Water Counc. 2014, 5, 50–69. [Google Scholar]
- Ibrahim, L.A.; ElSayed, E.E. Performance evaluation of Suez Bay industrial wastewater treatment plant case study: Ataqa Region, Egypt. World 2019, 8, 1–11. [Google Scholar]
- Goodman, B.A. Utilization of waste straw and husks from rice production: A review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Rajakumar, R.; Meenambal, T.; Saravanan, P.; Ananthanarayanan, P. Treatment of poultry slaughterhouse wastewater in hybrid upflow anaerobic sludge blanket reactor packed with pleated poly vinyl chloride rings. Bioresour. Technol. 2012, 103, 116–122. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Amini, J.; Hossaini, H. Application of ABR/zeolite for TKN removal from compost leachate. Environ. Technol. Innov. 2021, 24, 102020. [Google Scholar] [CrossRef]
- Cai, L.; Sun, X.; Hao, D.; Li, S.; Gong, X.; Ding, H.; Yu, K. Sugarcane bagasse amendment improves the quality of green waste vermicompost and the growth of Eisenia fetida. Front. Environ. Sci. Eng. 2020, 14, 61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).