Abstract
Reduction in food waste, as well as non-invasive methods for extending the shelf-life of perishable fruits, are important global challenges. To achieve these objectives, in this paper, the use of natural compounds, chitosan films (CS) incorporated with essential oils from leaves, for postharvest fungal protection of strawberries is proposed. In the present study, the CS films incorporated with the essential oil from Perilla frutescens leaves (PFEO) at different concentrations were prepared and employed for packaging strawberries infected by B. cinerea during refrigerated storage at 4 °C for 10 days. Interestingly, the strawberries coated with CS films containing PFEO at 1.0% during this period possessed an effective antimicrobial effect against B. cinerea infection in potato dextrose agar (PDA). Moreover, the quality properties of the strawberries, (i.e., weight loss, firmness index, decay percentage, yeasts/molds, pH value, total soluble solids, titrable acidity, and maturity index), together with the sensory attributes (i.e., appearance, flavor, taste, and overall acceptability (p < 0.05 or p < 0.01)) were improved. These results demonstrated that (i) PFEO displayed a significant inhibitory effect against B. cinerea infection in strawberries, (ii) CS films containing PFEO at 1.0% could be a sustainable active food packaging for the refrigerated storage of strawberries.
1. Introduction
The vast majority of mature strawberries (Fragaria x ananassa Duch., Rosaceae family), one of the most common fruits, is largely consumed because of their sweet taste, unique flavor, delicious aroma, and juicy texture. In addition, they have long been applied in the production processes of strawberry juices, flavored yogurts, and ice creams [1]. Quite interestingly, strawberries are also recognized as an important source of bioactive ingredients, such as vitamins, minerals, carotenoids, and anthocyanins, which are responsible for their attractive appearance, seductive fragrance, and high nutrition value. These bioactive ingredients displayed synergistic and cumulative effects in the disease prevention of plants and health promotion of humans [2]. Unfortunately, during their regular preservation and transportation periods, they are easily susceptible to mechanical damage, moisture reduction, microbial spoilage, and physiological deterioration due to the delicate external structure and high metabolic rate. For this reason, strawberries are characterized by a short guarantee period and an easily perishable quality [3]. Among them, gray-mold spoilage, resulting from the infection of Botrytis cinerea, is one of the most destructive and hazardous postharvest diseases of strawberries. Gray-mold spoilage may bring about a 30–40% loss of the harvested strawberries if no fungicides are applied, and the loss may reach 50–60% in acute infestations, with the consequent economic loss of almost 100% [4]. Actually, gray-mold spoilage of strawberries can be inhibited or even controlled by some synthetic fungicides. Nevertheless, the employment of the fungicides such as cyprodinil and fludioxonil in the infection of B. cinerea is always accompanied by some intractable problems, such as environmental contamination, the development of resistant fungal strains, and even potential dangers to human health. Consequently, the search for alternative antimicrobial agents against B. cinerea infection of strawberries so as to improve their quality and extend their shelf-life is an important challenge [5].
Over recent decades, the essential oils from spice and herb plants have been found to have a potential effective inhibitory effect against the B. cinerea infection of strawberries. For example, the effect of the vapors of clove and/or mustard essential oils against the B. cinerea infection of strawberries was both demonstrated in vitro and in vivo, together with their synergistic effect [6]. The essential oils of Mentha piperita, Lavandula angustifolia, Foeniculum vulgare, and Cuminum cyminum could suppress the postharvest decay of strawberries resulting from the infections of B. cinerea, Rhizopus stolonifera, and Aspergillus niger [7]. The essential oils of M. piperita, Moringa oleifera, and Eucalyptus camaldulensis could improve the postharvest quality of strawberries during the refrigerated storage periods, however seriously they were infected by B. cinerea [8]. However, although the essential oils of spice and herb plants are considered as generally recognized as safe (GRAS), their employment as antimicrobial agents is drastically limited because of some objective effects. Among these, their effective antimicrobial doses always exceed the organoleptic acceptance levels of consumers, so the direct addition of essential oils to strawberries could lead to inappropriate flavors and supererogatory smells. In addition, the active compounds in essential oils are volatile and unstable when they are exposed to heat, light, and oxygen [9]. Meanwhile, the fabrication of biodegradable films, especially chitosan-(CS) based films, incorporated with essential oils of spice and herb plants provides one feasible method for their application as antimicrobial agents to dissimulate their negative effects [10,11].
As one cationic natural high-polymer and one linear binary heteropolysaccharide consisting of β-(1-4)-2-acetamido-D-glucose and β-(1-4)-2-amino-D-glucose units, which are broadly distributed in living organism including insects, reptiles and crustaceans, CS is derived from the deacetylation of the major ingredients of crustacean shells, chitin [12]. It is one non-toxic, biodegradable, and biocompatible polymer that has an immense potential employment in the manufacture of packaging material owing to its antioxidant and antimicrobial effects, acceptable permeability to carbon dioxide and oxygen, excellent mechanical properties, and, above all, film-generation capacity [13]. Nevertheless, its application is also restricted because of its hydrophilic peculiarity and high water-vapor permeability; in fact, in order to increase its water-barrier properties, hydrophobic compounds are contained in the polymer matrix [14]. Among them, the incorporation of essential oils of spice and herb plants into CS films appears to be a concise but effective method [15]. For instance, along with pomegranate juice dipping, it has been reported that CS enriched with Zataria multiflora essential oil could prolong the shelf-life of chicken meat in the refrigerated storage period [16]. For strawberries, CS incorporated with T. capitatus essential oil could increase their shelf-life during the refrigerated storage process, while CS incorporated with Citrus limon essential oil could improve the sensory properties of strawberries during room-temperature storage procedure [17,18]. However, until now, to our knowledge, there is no research article about the application of the CS-essential oil films in the control of B. cinerea infection of strawberries. In the meantime, the fabrication and characterization of CS films containing essential oil of Perilla frutescens (PFEO), a medicinal plant used as spice and herb in the Henan province of China, were investigated, and the antimicrobial activity of these CS films was demonstrated in detail [19].
Reduction in food waste, as well as non-invasive methods for extending the shelf-life of perishable fruits, is an important global challenge. To achieve these objectives, in the present study, the inhibitory effect of CS films incorporated with PFEO against B. cinerea infection of strawberries during refrigerated storage was investigated. Additionally, the influence of CS films incorporated with PFEO on the physicochemical quality and sensory properties of these strawberries was investigated.
2. Materials and Methods
2.1. Materials and Chemicals
Glycerol (99.7%), CS with a molecular weight of 2000 (≥98%) was purchased from Wengjiang Co., Ltd. (Shaoguan, China). The leaves of Perilla frutescens and the fruits of strawberries were harvested and purchased from the Beilei Organic Farm (Zhengzhou, China) at their harvest time. Using agar, potatoes, potassium dihydrogen phosphate, and magnesium sulfate heptahydrate supplied by Xiangde Co., Ltd. (Xinxiang, China), the potato dextrose agar (PDA) was manufactured by The Affiliated Academy for Chinese Medicine of the Henan University of Chinese Medicine, (Zhengzhou, China). In addition, all other materials and chemicals employed in this investigation were of analytical grade and bought from Xinfeng Co., Ltd. (Zhengzhou, China).
2.2. Chemical Analysis of Essential Oil of Perilla Frutescens Leaves (PFEO)
PFEO was extracted using a steam distillation apparatus (XH-1000, Xinhu Co., Ltd., Shangai, China), using a previously published method [20,21]. By means of gas chromatography–flame ionization detection (GC-FID) and gas chromatography–mass spectrometer (GC-MS), the chemical analysis of PFEO was carried out according to Zhang et al.’s method [19]. Briefly, one PerkinElmer Clarus GC 600 system coupled to a Clarus 600 MS was employed, equipped with an ELITE-5 (30 m × 0.25 mm × 0.25 μm) capillary column. The injector was 250 °C, while the temperature of the oven was programmed: increased from 55 °C at 3 °C/min to 250 °C and stayed for 5 min. The injected volume was 0.1 mL of 1% solution. The carrier gas was He at a flow rate of 1.0 mL/min. The identification of the compounds was obtained by the comparison of their retention indices with that of literature.
2.3. Fabrication of CS Films
The fabrication of CS films was performed in accordance with Priyadarshi et al.’s method, with slight modifications [22]. Briefly, 100.0 g of CS powder was adequately dissolved in 5.0 L of acetic acid aqueous solution (0.5%, v/v) to prepare the CS solution (2.0%, w/v). After one agitation process triggered by a magnetic blender (MS7-H550-Pro, Dalong, China) of 48 h, the CS solution was filtered using strainers (pore size 1.0 μm) at 25 °C to dispose of the insoluble substances. Together with Tween 80 (0.2%, v/v) used as an emulsifier, glycerol used as a plasticizer was poured into the CS solution at 0.15 g glycerol/1.0 g CS powder, and then the blended solution was stirred for 30 min. Soon afterward, PFEO was appended into the blended solution at the concentrations 0.0%, 0.2%, 0.6%, and 1.0% (v/v) to fabricate the CS film-forming solutions CS/PFEO-0.0, CS/PFEO-0.2, CS/PFEO-0.6 and CS/PFEO-1.0, respectively. In order to make sure PFEO was uniformly distributed, all the film-forming solutions were homogenized via a homogenizer (LPH60, AC Serendip, Wollerau, Switzerland) at 1.6 × 104 r/min for 10 min and subjected to one ultrasonic treatment for 20 min to eliminate bubbles. In the fabrication of CS films, 30.0 mL of the film-forming solutions CS/PFEO-0.0, CS/PFEO-0.2, CS/PFEO-0.6, and CS/PFEO-1.0 was poured onto polymethyl methacrylate (PMMA) plates (24 × 32 cm) to generate CS films CS-0.0, CS-0.2, CS-0.6, and CS-1.0, respectively. After drying in an oven (F202, Shuli, Shanghai, China) at 38 °C for 48 h to obtain a uniform thickness, all the whole CS films were removed and disposed of under controlled conditions in one specific carton room with a temperature of 25 ± 1 °C and a humidity of 60 ± 5% for 48 h.
2.4. Inhibitory Effect of CS Films against B. cinerea Infection
The inhibitory effect of CS films against B. cinerea was performed in accordance with Sangsuwan et al.’s method with slight modifications [23]. Briefly, after isolation from strawberries with a B. cinerea infection, the gray mycelium was immediately transferred to the PDA using a sterile needle and incubated in an incubator at 25 °C for 5 days, so that the mycelium could grow to approximate 4.0 cm and the conidia could be observed by means of an optical microscope (CX-31, Olympus, Guangdong, China). Soon afterward, the mycelium was smashed and cultured on PDA for four spots (radius: 2.5 cm), and the CS films CS-0.0, CS-0.2, CS-0.6, and CS-1.0 were smashed into pieces (diameter: 1.0 mm) and placed in sterile plastic cups that were located in the center of the cultured plates. At the same time, one cultured plate without CS films was applied as the control. Subsequently, all the cultured plates were separately incubated in an incubator (BJPX-200, Bohua, China) at 25 °C for 10 days. The continual expansion of the colony size of B. cinerea was measured every other day.
2.5. Strawberry Administration
For the administration, 100.0 kg of strawberries without visible fungal infection and external physical damage, which were about 2.4 cm in diameter and about 3/4 surface in redness, were harvested and purchased from Beilei Organic Farm and transported immediately to Laboratory for the Preservation of Fruits and Vegetables (Henan University of Chinese Medicine, Zhengzhou, China). After the calyxes were stripped, all the strawberries were immediately refrigerated at 4 °C. Thereafter, they were divided into five groups (20.0 kg each) at random as follow: Control group: strawberries packed without CS films (Control); CS-0.0 group: strawberries packed with CS film CS-0.0 (CS-0.0); CS-0.2 group: strawberries packed with CS film CS-0.2 (CS-0.2); CS-0.6 group: strawberries packed with CS film CS-0.6 (CS-0.6); CS-1.0 group: strawberries packed with CS film CS-1.0 (CS-1.0).
After being sterilized by means of 70% ethanol solution for 5 s, every 4 strawberries of all the groups were washed by distilled water for 5 min, dried by natural air-drying for 5 min, and preserved in a polyethylene terephthalate (PET) container (JSYXL190402, Jinshuo, China) with a rigorous cover. For the CS-0.0, CS-0.2, CS-0.6, and CS-1.0, the CS film CS-0.0, CS-0.2, CS-0.6, and CS-1.0 groups were attached to the internal surface of the covers to avoid physical contact with the strawberries, respectively, and the strawberries preserved in containers were refrigerated at 4 °C for 10 days. Meanwhile, they were photographed, and the determination of their shelf-life indices and sensory indices were carried out every other day.
2.6. Physicochemical Measurements of Strawberries
2.6.1. Determination of Weight Loss, Firmness Index, Decay Percentage, and Yeasts/Molds
The weight loss of strawberries was evaluated using the gravimetric analysis as reported by Liu et al. [24]. The firmness index of strawberries was evaluated via a firmness tester with a probe (GY-1, Tuopu, Guangzhou, China). The decay percentage of strawberries was visually inspected by a 3-point scale from 10 semi-trained panelists, where no decay = 0, slight decay = 1 (<25% of surface decay), and moderate decay (≥25% of surface decay) = 2. The decay percentage was calculated according to the equation: ((0 × N0 + 1 × N1 + 2 × N2) × 100/(2 × N)), where N0, N1, and N2 were the number of strawberries of the point scale 0, 1, and 2, respectively [25]. The yeast/mold of strawberries was evaluated in accordance with the Chinese National Standard SN/T 2566-2010. Briefly, 25.0 g of strawberries was immediately immersed into 250.0 mL (10%, m/v) of phosphate buffer, completely homogenized using a fruit homogenizer (S2-A818, Jiuyang, Jinan, China) at 8000 rpm for 2 min, and absolutely percolated through one piece of filtration fabric (HW-2, Hongwei, Laiwu, China) to acquire the strawberry juice. Subsequently, 10.0 μL of the strawberry juice was dropwise appended to one piece of examination slide for PetrifilmTM yeast/mold count plate (6477, Chuqi, Zhengzhou, China) deposited onto the laboratory table under aseptic condition. After incubated in the incubator at 25 °C for 72 h, the count for the colony of examination slides was calculated using visual inspection.
2.6.2. Determination of pH Value, Total Soluble Solids, Titrable Acidity, and MATURITY INDEX
As mentioned above, the juice of 25.0 g of strawberries was obtained once again. Immediately, the pH values of strawberry juices were determined with a pH meter (PHSJ-4A, INESA, Shanghai, China). The total soluble solids of strawberry juices was determined with a digital refractometer (CM-800α, Atago, Tokyo, Japan). Additionally, as reported by Hussain et al., the titrable acidity of strawberry juices was determined by the titration method and expressed as the percentage of citric acid. Finally, the maturity index was determined as the quotient of total solution solids and titrable acidity, i.e., maturity index = total solution solids/titrable acidity [26].
2.6.3. Colorimetric Measurement
The colorimetric measurement of strawberries was carried out by a Hunterlab Spectro colorimeter (ScanVis, VA, USA). CIELAB coordinates, including L*, a*, b*, were obtained using 65 illuminants and one 10° observer angle as a reference system. Among them, L* stands for the overall brightness (0) or darkness (100), while a* represents the overall greenness (−) or redness (+) and b* represents the blueness (−) or yellowness (+). For the measurement, two readings were taken in different locations of each strawberry, and six strawberries from each administrated group were analyzed one after another [27].
2.6.4. Measurements of Total Phenolic Content (TPC), Total Flavonoid Content (TFC), Anthocyanin Content (ACC), and Ascorbic Acid Content (AAC)
The measurements of TPC, TFC, ACC, and AAC of strawberries from every administrated group were carried out according to Khalifa et al.’s method [28]. In brief, 200 mL of each extract was mixed with 1 mL of 10-fold diluted Folin–Ciocalteu reagent; after 5 min, the reaction was stopped by 1 mL of 7.5% Na2CO3, then 1.5 mL distilled water was added. The mixture was incubated in the dark for 60 min then the absorbance at 760 nm was measured. For TFC, a 0.5 mL aliquot of 2% AlCl3 ethanolic solution was added to 0.5 mL of extract and mixed well, then kept for 1 h, and the absorbance at 420 nm was measured. Moreover, a 5.0 g strawberry sample was extracted with 45 mL of acidified ethanol (95% ethanol: HCl 1.5N 85:15) for 2 h in the dark and then filtered through Whatman No.1 filter paper. Absorbance was measured at 535 nm to obtain the ACC. The ascorbic acid (AA) content in strawberry fruits during storage periods was determined by using the 2,6-dichlorophenol-indophenol titrimetric method.
2.7. Sensorial Analysis
In the sensorial analysis, 40 undergraduate students aged 18–22 (20 males/20 females) from the Institute of Chinese Medicine Health Care, Guangdong Food and Drug Vocational College, Guangzhou, China, who possessed the Qualification Certificate from the Sensorial Evaluation Training Center, Foodmate Information Technology, Yantai, China were requested to constitute the sensorial analysis group. In order to guarantee them to be quite familiar with the entire correlative procedure and understand the attribute identification of the sensorial descriptors of strawberries, such as appearance, flavor, taste, and overall acceptability, all were technically tutored for 6 h and personally instructed for 6 h before the sensorial analysis. In the sensorial analysis, each strawberry was individually numbered in 3-digital numbers and evaluated using the quantitative descriptive analysis. One 10-point hedonic scale from 1 (dislike extremely) to 10 (like extremely) was employed to indicate the sensorial descriptors of strawberries.
2.8. Statistical Analysis
The experimental data were expressed as mean values when presented in the manuscript, while expressed as mean values ± standard deviations (SD) when presented in Figures and Tables. For statistical analysis, the significance analysis (ANOVA test) of experimental data from different groups was carried out by means of GraphPad Prism 8 (GraphPad, San Diego, CA, USA). The values of p < 0.01 and p < 0.05 were considered to be highly significant and statistically significant, respectively.
3. Results and Discussion
3.1. Chemical Composition of PFEO
The determination of the chemical composition of the essential oils from spice and herb plants has always played an important role in explaining the antioxidant and antimicrobial effects of the main compounds responsible for these biological activities [29,30]. Therefore, before the investigation of its antimicrobial effect, the chemical analysis of the essential oil was performed. First, 91.1 g of PFEO was extracted from 5.0 kg of P. frutescens leaves (extraction yield: 1.82%). Table 1 shows the retention index and area percentage for each compound, together with a total of 23 identified compounds with more than 90% compatibility, accounting for 98.60% of PFEO. Among them, the highest amounts belonged to thymoquinone (32.26%), 2-hexanoylfuran (19.69%), trans-bergamotene (10.06%), 1,1,3,6-tetramethyl-3-vinyl-8-hexahydro-1H-isochromene (8.71%), and diisooctyl adipate (8.9%). Despite the area percentages of these compounds not agreeing, the identified results of PFEO were of similar to a previous investigation [18]. This similarity in the chemical composition of PFEO may be attributed to the duplicate cultivated region and analogical survival climate of the harvested plants [31,32]. The former plants were collected from Taiyuan, China (112°26′30″ E, long., 38°0′51″ N, lat.), while the latter plants were collected from Zhengzhou, China (114°02′04″ E, long., 34°37′19″ N, lat.), indicating that they were harvested in two close regions with quite similar climatic conditions [19]. Above all, the similar chemical compositions of PFEO in the two investigations demonstrated that the CS films incorporating PFEO manufactured in the experiment should possess a commendable antimicrobial effect as well.

Table 1.
Chemical composition of PFEO using GC-FID/GC-MS a.
3.2. Inhibition of CS Films against B. cinerea Infection
Strawberries are an easily perishable fruits, and their shelf-life is always terminated due to the decay triggered by the fungal infection, including B. cinerea, with obvious variation in their appearance [33]. As shown in Figure 1, after 10 days storage of strawberries coated with CS films, a B. cinerea growth over untreated fruits was significantly presented. Essential oils from spice and herb plants have been reported to be effective in gray mold reduction, quality maintenance, and essential improvement in the postharvest life of many fruits, including strawberries [34]. The results for the inhibition efficacy of CS films incorporating PFEO against B. cinerea infection in strawberries were determined on PDA and reported in Table 2. From the 4th day, the area for B. cinerea mycelium of the Control and CS-0.0 groups was prominently increased, while the area for B. cinerea mycelium of CS-0.2, CS-0.6, and CS-1.0 groups gradually increased, so that the different values between them were quite remarkable (p < 0.05 or p < 0.01). Furthermore, although the different values between the inhibition effects against B. cinerea infection of CS films were not remarkable, there was a trend that CS films containing higher amounts of PFEO were more effective. This was also confirmed by Sangsuwan et al.’s investigation, where the CS beads containing lavender or red thyme essential oils revealed an inhibitory effect against B. cinerea infection [23]. As reported, essential oils can be employed in the storage of strawberries only if the released compounds show biological effects over time. Until now, despite the reaction mechanism for the antimicrobial effects of essential oils not being absolutely illustrated, this activity of essential oils was attributed to their phenolic compounds, such as thymol, eugenol, and carvacrol. After all, their antimicrobial effects may have something to do with their self-capabilities to dissolve or restrain the integrity of cell walls and membranes [35]. However, the reaction mechanism for the antimicrobial effect of PFEO needs to be further investigated in another way. In terms of CS films, to our knowledge, this exploration is the first concerning its application in the inhibition of gray mold in strawberries.
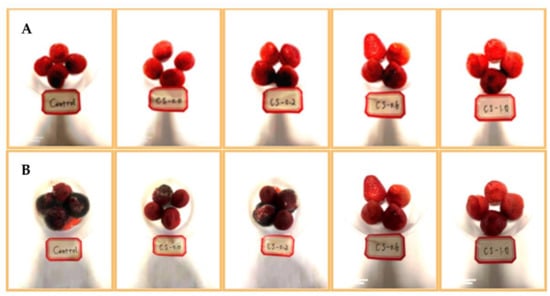
Figure 1.
Photographs of strawberries during the refrigeration storage. (A): Day 0, (B) Day 10. Control, strawberries packed without CS films; CS-0.0, strawberries packed with CS film CS-0.0; CS-0.2, strawberries packed with CS film CS-0.2; CS-0.6, strawberries packed with CS film CS-0.6; CS-1.0, strawberries packed with CS film CS-1.0.

Table 2.
Inhibition efficacy of CS films containing PFEO against B. cinerea on PDA over 10 days a.
3.3. Physicochemical Analysis of Strawberries in Refrigeration
For strawberries, the respiration rate and moisture evaporation between strawberry tissue and the surrounding air usually leads to the weight loss and firmness decrease. Meanwhile, the infection of yeasts/molds always brings about decay [36].
In the present study, as revealed in Figure 2, the weight loss, decay percentage, and yeasts/molds for the strawberries in the Control group were significantly improved during the refrigeration period (p < 0.01), while the firmness index was significantly lowered (p < 0.05). When deposited in PET containers with lids pasted with CS films, the variations in weight loss, decay percentage, yeasts/molds, and firmness index of the strawberries were all effectively restrained, especially for the strawberries in the CS-1.0 group. For this group, on day 10, the weight loss, decay percentage, and yeasts/molds were prominently decreased from 45.0%, 44.1%, and 12.5 Log UFC/g to 16.1% (p < 0.01), 4.8% (p < 0.01), and 5.7 Log UFC/g (p < 0.01), respectively, compared with that of the strawberries in the Control group, while the firmness index was prominently increased from 2.04 N to 2.65 N (p < 0.05). The results showed that the CS film containing PFEO at 1.0% was a favorable selective O2 and CO2 barrier, and therefore could change the internal environment, reduce the respiration rate, and affect the growth atmosphere of yeasts/molds growing on strawberries. This result was in agreement with Valenzuela et al.’s study, where the edible quinoa protein-CS-based films were demonstrated to lengthen the shelf-life of refrigerated strawberries by means of inhibiting the variation in their weight loss, decay percentage, yeasts/molds, and firmness index [37].
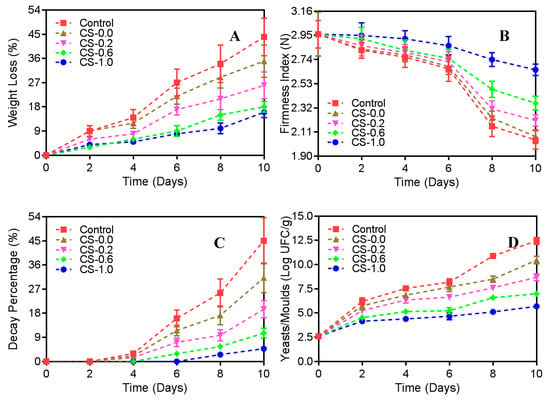
Figure 2.
Weight loss (A), firmness index (B), decay percentage (C), and yeasts/molds (D) of strawberries during refrigeration storage (10 days). Control, strawberries packed without CS films; CS-0.0, strawberries packed with CS film CS-0.0; CS-0.2, strawberries packed with CS film CS-0.2; CS-0.6, strawberries packed with CS film CS-0.6; CS-1.0, strawberries packed with CS film CS-1.0.
During the refrigeration of strawberries, organic acids adopted as an energy source to maintain the fruit-ripening process are reduced, because they are applied as respiratory substrates and carbon skeleton for the synthesis of new compounds [28]. In the meantime, the degradation of internal starch into soluble sugars and the hydrolysis of cell-wall polysaccharides happens [26]. In the present study, as revealed in Figure 3, the pH values, total soluble solids, and maturity index of strawberries from the Control group were improved during the refrigeration period (p < 0.01), while their titrable acidity dropped (p < 0.05). When deposited in PET containers with lids pasted by CS films, the variation in pH values, total soluble solids, maturity index, and titrable acidity of the strawberries were effectually suppressed, in particular, for the strawberries from the CS-1.0 group, where the pH values, total soluble solids, and maturity index were dramatically reduced on day 10 from 3.79, 8.13%, and 11.54 to 3.42 (p < 0.05), 6.74% (p < 0.05), and 8.32 (p < 0.05), respectively, while the titrable acidity dramatically rose from 0.72 to 0.77 (p < 0.05). The results described that the CS film containing PFEO at 1.0% was able to inhibit strawberry metabolism, including the respiration procedure where organic acids were consumed and internal starch was degraded, which was consistent with that of previous investigations concerning the impression of CS-coated administration on the different commodities, including shrimp, chicken, frankfurters, and silver carp fillets [12].
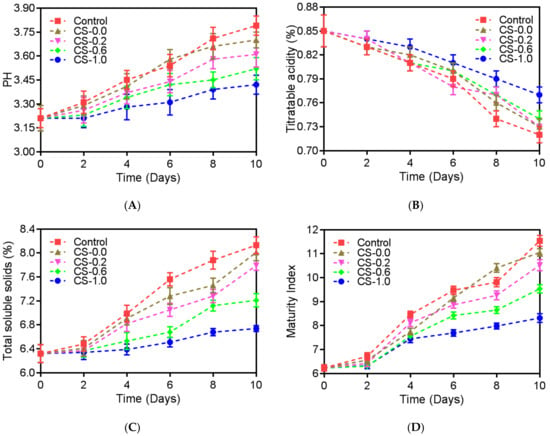
Figure 3.
pH values (A), titrable acidity (B), total soluble solids (C), and maturity index (D) of strawberries during refrigeration storage (10 days). Control, strawberries packed without CS films; CS-0.0, strawberries packed with CS film CS-0.0; CS-0.2, strawberries packed with CS film CS-0.2; CS-0.6, strawberries packed with CS film CS-0.6; CS-1.0, strawberries packed with CS film CS-1.0.
The other significant characteristics of strawberries that can be influenced by the administration of CS films were also external-shape and, in particular, surface color [38]. As exhibited in Table 3, there were no significant differences between the color parameters (L*, a*, and b*) for the strawberries of all groups, whether they were deposited in PET containers with lids pasted by CS films or not (p > 0.05). For all these strawberries, both the lightness (L*) value and yellow (b*) value possessed a moderating trend from day 0 to day 4, whereas the red (a*) value possessed an escalating trend from day 0 to day 4 and then a moderating trend from day 4 to day 10. The results displayed that the color values of the strawberries were not influenced by the PET containers with lids pasted by means of CS films. For the present study, this result was very interesting and satisfactory, since plenty of investigations have shown that the application of the emulsified film-forming solutions including CS on strawberries was able to prevent the external changes and induce their texture opacity [39].

Table 3.
The influence of CS films containing PFEO on the colors (L*, a* and b*) of strawberries refrigerated at 4 °C for 10 days a.
Table 4 shows the values of TPC, TFC, ACC, and AAC of strawberries during refrigerated storage (10 days). The TPC, TFC, ACC parameters of the Control group showed a variation tendency with a decreasing trend, while the ACC content ascended from day 0 to day 6 and descended from day 6 to day 10. For the strawberries preserved in the PET containers with lids pasted by CS films, the contents for TPC, TFC, and AAC were observed to ascend, especially for CS-1.0 group, where the contents for TPC, TFC, and AAC ascended from 0.24 mg/100 g, 0.15 mg/100 g, and 12.45 mg/100 g to 0.69 mg/100 g (p < 0.01), 1.25 mg/100 g (p < 0.01), and 36.14 mg/100 g (p < 0.01), respectively. The reason for the variation in TPC content during refrigeration was linked to the structural breakdown of strawberry cells [40]. Additionally, the increased TFC and AAC contents of the CS-1.0 group during refrigeration were on account of the CS films [28,39].

Table 4.
The influence of CS films containing PFEO on the total phenolic content (TPC), total flavonoid content (TFC), anthocyanin content (ACC), and ascorbic acid content (AAC) of strawberries refrigerated at 4 °C for 10 days a.
As an attractive and flavorable fruit with a short postharvest life because of their physiological deterioration, susceptibility to mechanical breakdown, and the absence of protective rinds that exhibit symptoms of pathogens, strawberries face a troublesome problem, quality loss [4]. Compared with the physical treatments (i.e., heat application, osmotic application, hypobaric processing, irradiation processing, and modified atmosphere packaging), and the chemical treatments (i.e., the addition of synthetic and natural fungicides), the employment of CS films containing essential oils with an antimicrobial effect not only overcomes the drawbacks of the indirect destructive effect on the strawberry quality and the inherent dangerous effect on human health performance but also improves their sensory attributes.
3.4. Sensorial Analysis of Strawberries in Refrigeration
As important factors that influence the selectivity for fruit enjoyment, the sensorial properties of strawberries are increasingly appreciated and recognized by consumers. The stage of “being able to eat enough” for people has passed and the stage of “being able to choose” has arrived, so the sensorial properties are the first impression in the case of fruits, including strawberries, when people see them at a glance [41]. In the present study, with the extension of refrigeration time, the sensorial attributes, including appearance, flavor, taste, and overall acceptability of strawberries, from the Control group were memorably lowered because of the infection of B. cinerea (Figure 1 and Table 5). When packaged with the CS film CS-1.0, the appearance, flavor, taste, and overall acceptability of the strawberries were memorably driven up (on day 10) from 5.29, 5.23, 4.26, and 4.29 to 7.55 (p < 0.05), 7.22 (p < 0.05), 7.48 (p < 0.05), and 7.49 (p < 0.05), respectively. The results illustrated that the CS-1.0 film package could ameliorate the sensorial attributes of strawberries. This interesting result was similar to that of Huang et al.’s exploration [42]. The changes in these sensorial attributes were due to the metabolic processes that occur during the refrigeration, such as oxidative degradation of ascorbic acid, which could produce visual obscuring, change extensive peel, and soften intensive pulp [43].

Table 5.
The influence of CS films containing PFEO on the sensory attributes (appearance, flavor, taste, and overall acceptability) of strawberries refrigerated at 4 °C for 10 days a.
4. Conclusions
The research confirmed that CS films containing essential oils from spice and herb plants are a commendable biodegradable polymer with an effective antimicrobial effect for refrigeration storage of fruits and vegetables. During this investigation, CS films incorporated with 1.0% PFEO possessed an effective antimicrobial effect against B. cinerea for the packaging of strawberries during refrigerated storage at 4 °C for 10 days. Interestingly, strawberries preserved by CS films incorporated with 1.0% PFEO exhibited improved sensorial attributes, including appearance, flavor, taste, and overall acceptability. The compound/compounds which exerted antimicrobial effects against B. cinerea has/have been explored in our laboratory by bioassay-guided fractionation using preparative TLC, and the Chinese patents for the application of CS films containing PFEO in refrigeration storage of strawberries have been completed. In addition, this interesting approach lays a solid foundation for the performance of similar investigations using CS films containing essential oils from other spice and herb plants in the preservation of other perishable fruits and vegetables.
Author Contributions
Conceptualization, D.W. and F.B.; methodology, D.W.; software, D.W. and H.Y.; validation, D.W. and H.Y.; investigation, D.W. and X.L.; data curation, D.W. and X.L.; writing—original draft preparation, D.W. and F.B.; writing—review and editing, D.W. and F.B.; visualization, D.W. and Y.W.; supervision, D.W. and F.B.; project administration, D.W.; funding acquisition, D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Fundamental Research Funds for the Henan Provincial Colleges and Universities of Henan University of Technology (2018QNJH20).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Velde, F.V.; Méndez-Galarraga, M.P.; Grace, M.H.; Fenoglio, C.; Lila, M.A.; Pirovani, M.É. Changes due to high oxygen and high carbon dioxide atmospheres on the general quality and the polyphenolic profile of strawberries. Postharvest Biol. Technol. 2019, 148, 49–57. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Zubik, M.; Mazurek, W.; Sosnowska, B. Detection of fungal infections in strawberry fruit by VNIR/SWIR hyperspectral imaging. Postharvest Biol. Technol. 2018, 139, 115–126. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saha, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Villa-Rojas, R.; Sosa-Morales, M.E.; López-Malo, A.; Tang, J. Thermal inactivation of Botrytis cinerea conidia in synthetic medium and strawberry puree. Int. J. Food Microbiol. 2012, 155, 269–272. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Keulemans, J.; Geeraerd, A.; Nicolai, B.M.; Hertog, M.L.A.T.M. Evaluation of fast volatile analysis for detection of Botrytis cinerea infections in strawberry. Food Microbiol. 2012, 32, 406–414. [Google Scholar] [CrossRef]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Hadian, J.; Ghasemnezhad, M.; Ranjbar, H.; Frazane, M.; Ghorbanpour, M. Antifungal potency of some essential oils in control of postharvest decay of strawberry caused by Botrytis cinerea, Rhizopus stolonifer and Aspergillus niger. J. Essent. Oil Bear. Plants 2018, 11, 533–562. [Google Scholar]
- Abd-Elkader, D.Y.; Salem, M.Z.M.; Komeil, D.A.; Al-Huqail, A.A.; Ali, H.M.; Salah, A.H.; Akrami, M.; Hassan, H.S. Post-harvest enhancing and Botrytis cinerea control of strawberry fruits using low cost and eco-friendly natural oils. Agronomy 2021, 11, 1246. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involved in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active edible films fortified with natural extracts: Case study with fresh-cut apple pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Jahed, E.; Khaledabad, M.A.; Almasi, H.; Hasanzadeh, R. Physicochemical properties of Carum copticum essential oil loaded chitosan films containing organic nanoreinforcements. Carbohydr. Polym. 2017, 164, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Castañeda, C.G.G.; Valencia, M.E.; Tovar, C.D.G. The effect of edible chitosan coatings incorporated with Thymus capitatus essential oil on the shelf-life of strawberry (Fragaria x ananassa) during cold storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef]
- Perdones, Á.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, N.; Li, H.Z.; Li, X.J.; Cao, J.M.; Zhang, G.P.; He, D.L. Preparation and characterization of biocomposite chitosan film containing Perilla frutescens (L.) Britt. essential oil. Ind. Crops Prod. 2018, 112, 660–667. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Y.; Wang, Q.; Wang, X.; Fan, W. Limonene, the compound in essential oil of nutmeg displayed antioxidant effect in sunflower oil during the deep-frying of Chinese Maye. Food Sci. Nutr. 2020, 8, 511–520. [Google Scholar] [CrossRef]
- Wang, D.; Meng, Y.; Wang, C.; Wang, X.; Blasi, F. Antioxidant activity and sensory improvement of Angelica dahurica cv. Yubaizhi essential oil on sunflower oil during high-temperature storage. Processes 2020, 8, 403. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Pongsapakworawat, T.; Bangmo, P.; Sutthasupa, S. Effect of chitosan beads incorporated with lavender or red thyme essential oils in inhibiting Botrytis cinerea and their application in strawberry packaging system. LWT-Food Sci. Technol. 2016, 74, 14–20. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication and testing of PVA/chitosan bilayer films for strawberry packaging. Coatings 2017, 7, 109. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early detection and classification of pathogenic fungal disease in post-harvest strawberry fruit by electronic nose and gas chromatography-mass spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Hussain, P.R.; Dar, M.A.; Wani, A.M. Effect of edible coating and gamma irradiation on inhibition of mould growth and quality retention of strawberry during refrigerated storage. Int. J. Food Sci. Technol. 2012, 47, 2318–2324. [Google Scholar] [CrossRef]
- Garcia, L.C.; Pereira, L.M.; De Luca Sarantópoulos, C.I.G.; Hubinger, M.D. Effect of antimicrobial starch edible coating on shelf-life of fresh strawberries. Packag. Technol. Sci. 2012, 25, 413–425. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.S.; Soliman, S.A. Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packag. Shelf Life 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Wang, Q.; Meng, Y.; Wang, D.; Wang, X. Influence of the essential oil of Mentha spicata cv. Henanshixiang on sunflower oil during the deep-frying of Chinese Maye. LWT-Food Sci. Technol. 2020, 122, 109020. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Wang, D.; Fan, W.; Guan, Y.; Huang, H.; Yi, T.; Ji, J. Oxidative stability of sunflower oil flavored by essential oil from Coriandrum sativum L. during accelerated storage. LWT-Food Sci. Technol. 2018, 98, 268–275. [Google Scholar] [CrossRef]
- Wang, D.; Meng, Y.; Zhao, X.; Fan, W.; Yi, T.; Wang, X. Sunflower oil flavored by essential oil from Punica granatum cv. Heyinshiliu peels improved its oxidative stability and sensory properties. LWT-Food Sci. Technol. 2019, 111, 55–61. [Google Scholar] [CrossRef]
- Nadim, Z.; Ahmadi, E.; Sarikhani, H.; Chayjan, R.A. Effect of methylcellulose-based edible coating on strawberry fruit’s quality maintenance during storage. J. Food Process. Preserv. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Ye, M.; Wang, K.; Fan, L.; Su, F. Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, A.; Hassani, A.; Ghuosta, Y.; Bernousi, I.; Meshkatalsadat, M.H. In vitro Efficacy of four plant essential oils against Botrytis cinerea Pers.:Fr. and Mucor piriformis A. Fischer. J. Essent. Oil Bear. Plants 2010, 13, 97–107. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef]
- Valenzuela, C.; Tapia, C.; López, L.; Bunger, A.; Escalona, V.; Abugoch, L.L. Effect of edible quinoa protein-chitosan based films on refrigerated strawberry (Fragaria × ananassa) quality. Electron. J. Biotechnol. 2015, 18, 406–411. [Google Scholar] [CrossRef]
- Jang, S.A.; Shin, Y.J.; Song, K.B. Effect of rapeseed protein–gelatin film containing grapefruit seed extract on ‘Maehyang’ strawberry quality. Int. J. Food Sci. Technol. 2011, 46, 620–625. [Google Scholar] [CrossRef]
- Eroglu, E.; Torun, M.; Dincer, C.; Topuz, A. Influence of pullulan-based edible coating on some quality properties of strawberry during cold storage. Packag. Technol. Sci. 2012, 27, 831–838. [Google Scholar] [CrossRef]
- Dhital, R.; Joshi, P.; Becerra-Mora, N.; Umagiliyage, A.; Chai, T.; Kohli, P.; Choudhary, R. Integrity of edible nano-coatings and its effects on quality of strawberries subjected to simulated in-transit vibrations. LWT-Food Sci. Technol. 2017, 80, 257–264. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M.J. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, M.; Yan, W.Q.; Mujumdar, A.S.; Sun, D.F. Rehydration characteristics of freeze-dried strawberry pieces as affected by whey protein edible coatings. Int. J. Food Sci. Technol. 2011, 46, 671–677. [Google Scholar] [CrossRef]
- Treviño-Garza, M.Z.; García, S.; del Socorro Flores-González, M.; Arévalo-Niño, K. Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (Fragaria ananassa). J. Food Sci. 2015, 80, M1823–M1830. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).