Highly Efficient and Rapid Removal of Methylene Blue from Aqueous Solution Using Folic Acid-Conjugated Dendritic Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
Material
2.2. Methods
2.2.1. Preparation of Dendritic Mesoporous Silica Nanoparticles (DMSNs)
2.2.2. Amine-Surface Functionalization of DMSNs (AP-DMSN)
2.2.3. Folic Acid (FA-) Conjugated DMSNs (FA-DMSN)
2.3. Material Characterization
2.4. Adsorption Experiments
2.4.1. Adsorption Kinetics
Pseudo-First-Order Reaction Kinetic Model
Pseudo-Second-Order Reaction Kinetic Model
The Intraparticle Diffusion Model
2.4.2. Adsorption Isotherms
Langmuir Adsorption Isotherm Model
Freundlich Adsorption Isotherm Model
3. Results
3.1. Properties of Synthesized and Surface-Modified DMSNs
3.2. Adsorption Studies
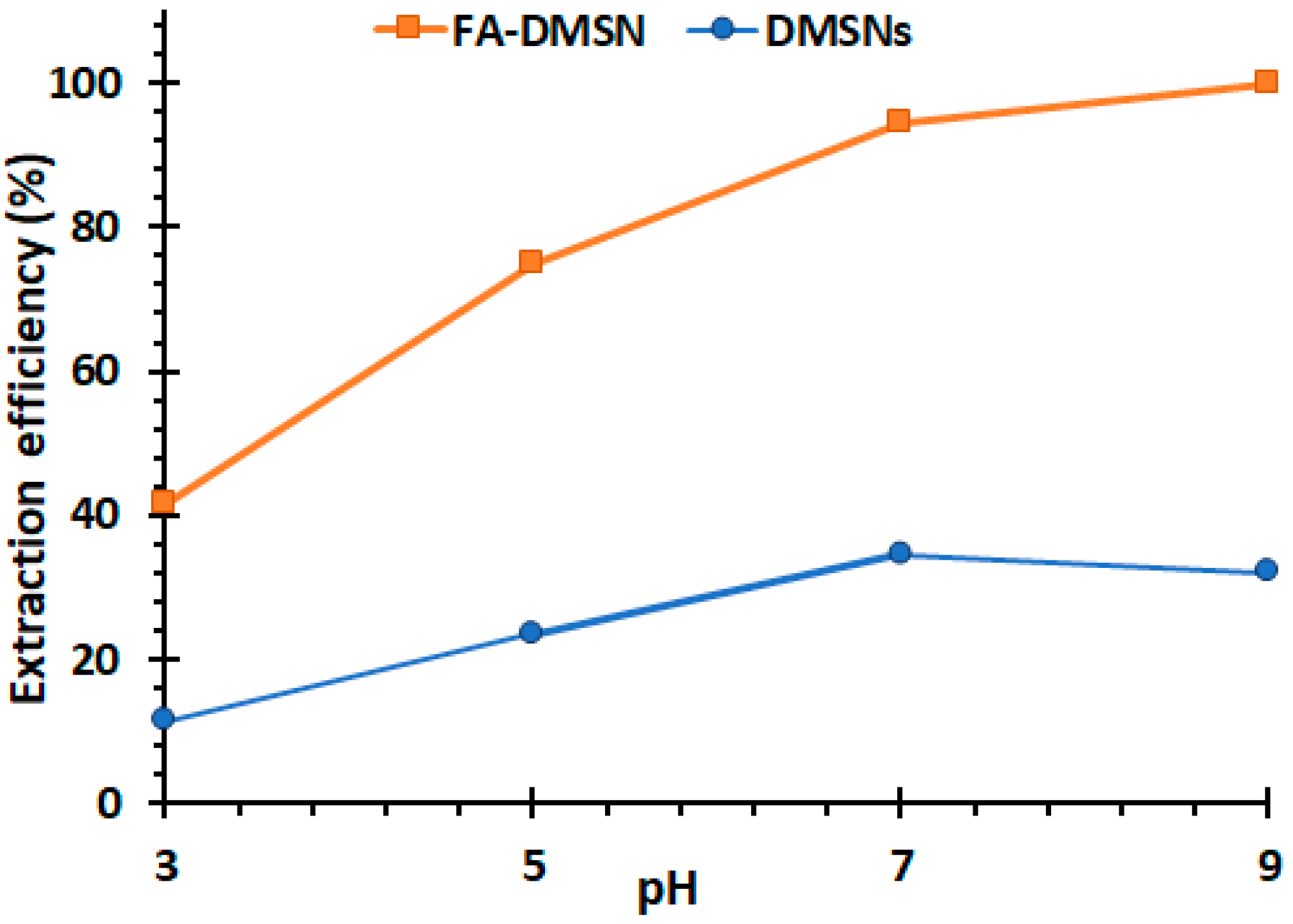
3.2.1. The Effect of pH on MB Removal
3.2.2. Equilibrium Isotherms
3.2.3. Effect of Adsorption Time and Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.C. Pollution Control in Textile Industry, 1st ed.; Devraj, S., Ed.; Woodhead Publishing India Pvt. Ltd.: New Delhi, India, 2017; Volume 4, ISBN 978-1-351-37306-7. [Google Scholar]
- Hassan, M.M.; Carr, C.M. A Critical Review on Recent Advancements of the Removal of Reactive Dyes from Dyehouse Effluent by Ion-Exchange Adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Crowley, D.E.; Khalid, A.; Hussain, S.; Mumtaz, M.W.; Arshad, M. Microbial Biotechnology for Decolorization of Textile Wastewaters. Rev. Environ. Sci. Biotechnol. 2015, 14, 73–92. [Google Scholar] [CrossRef]
- Nippatla, N.; Philip, L. Electrocoagulation-Floatation Assisted Pulsed Power Plasma Technology for the Complete Mineralization of Potentially Toxic Dyes and Real Textile Wastewater. Process. Saf. Environ. Prot. 2019, 125, 143–156. [Google Scholar] [CrossRef]
- Meerbergen, K.; Crauwels, S.; Willems, K.A.; Dewil, R.; Van Impe, J.; Appels, L.; Lievens, B. Decolorization of Reactive Azo Dyes Using a Sequential Chemical and Activated Sludge Treatment. J. Biosci. Bioeng. 2017, 124, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Chen, H.; Yang, Y.; He, Q.; Dai, X. Treatment of Printing and Dyeing Wastewater Using MBBR Followed by Membrane Separation Process. Desalin. Water Treat. 2014, 52, 4562–4567. [Google Scholar] [CrossRef]
- Rachna, K.; Agarwal, A.; Singh, N. Rice Husk and Sodium Hydroxide Activated Rice Husk for Removal of Reactive Yellow Dye from Water. Mater. Today Proc. 2019, 12, 573–580. [Google Scholar] [CrossRef]
- Alotaibi, K.M. Mesoporous Silica Nanoparticles Modified with Stimuli-Responsive Polymer Brush as an Efficient Adsorbent for Chlorophenoxy Herbicides Removal from Contaminated Water. Int. J. Environ. Anal. Chem. 2021, 1–14. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Shukla, A.K.; Mrad, M.H.; Alswieleh, A.M.; Alotaibi, K.M. Fabrication of Polysulfone-Surface Functionalized Mesoporous Silica Nanocomposite Membranes for Removal of Heavy Metal Ions from Wastewater. Membranes 2021, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Beagan, A.; Alotaibi, K.; Almakhlafi, M.; Algarabli, W.; Alajmi, N.; Alanazi, M.; Alwaalah, H.; Alharbi, F.; Alshammari, R.; Alswieleh, A. Amine and Sulfonic Acid Functionalized Mesoporous Silica as an Effective Adsorbent for Removal of Methylene Blue from Contaminated Water. J. King Saud Univ. Sci. 2022, 34, 101762. [Google Scholar] [CrossRef]
- Standeker, S.; Novak, Z.; Knez, Z. Adsorption of Toxic Organic Compounds from Water with Hydrophobic Silica Aerogels. J. Colloid Interface Sci. 2007, 310, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Caputo, G.; De Marco, I.; Reverchon, E. Silica Aerogel–Metal Composites Produced by Supercritical Adsorption. J. Supercrit. Fluids 2010, 54, 243–249. [Google Scholar] [CrossRef]
- Alotaibi, K.M.; Shiels, L.; Lacaze, L.; Peshkur, T.A.; Anderson, P.; Machala, L.; Critchley, K.; Patwardhan, S.V.; Gibson, L.T. Iron Supported on Bioinspired Green Silica for Water Remediation. Chem. Sci. 2016, 8, 567–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, S.A.; Alotaibi, K.; Peshkur, T.A.; Anderson, P.; Gibson, L.T. Preconcentration and Selective Extraction of Chromium Species in Water Samples Using Amino Modified Mesoporous Silica. J. Colloid Interface Sci. 2012, 386, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Cha, D.; Zhang, X.; Basset, J.M. High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology. Angew. Chemie 2010, 122, 9846–9850. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Basset, J.-M. High Surface Area Fibrous Silica Nanoparticles. U.S. Patant US 8,883,308 B2, 11 November 2014. [Google Scholar]
- Kienzle, A.; Kurch, S.; Schlöder, J.; Berges, C.; Ose, R.; Schupp, J.; Tuettenberg, A.; Weiss, H.; Schultze, J.; Winzen, S.; et al. Dendritic Mesoporous Silica Nanoparticles for PH-Stimuli-Responsive Drug Delivery of TNF-Alpha. Adv. Healthc. Mater. 2017, 6, 1700012. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Colilla, M. Structure and Functionalization of Mesoporous Bioceramics for Bone Tissue Regeneration and Local Drug Delivery. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef] [PubMed]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Mahmudi, M.; Shadjou, N.; Hasanzadeh, M. Synthesis and Adsorption Behavior of Dendritic Fibrous Nano-Silica (DFNS) Grafted by d-Penicillamine as an Advanced Nanomaterial for the Removal of Some Metal Ions from Contaminated Water. J. Electroanal. Chem. 2019, 848, 113272. [Google Scholar] [CrossRef]
- Nia, M.H.; Kiasat, A.R.; van de Ven, T.G.M. Dendritic Fibrous Colloidal Silica Internally Cross-Linked by Bivalent Organic Cations: An Efficient Support for Dye Removal and the Reduction of Nitrobenzene Derivatives. Langmuir 2021, 37, 13676–13688. [Google Scholar] [CrossRef] [PubMed]
- Abbasvash, L.; Shadjou, N. Synthesize of β-Cyclodextrin Functionalized Dendritic Fibrous Nanosilica and Its Application for the Removal of Organic Dye (Malachite Green). J. Mol. Recognit. 2020, 33, e2850. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.F.; Zamboni, W.C.; Crom, W.R.; Gajjar, A.; Heideman, R.L.; Furman, W.L.; Meyer, W.H.; Houghton, P.J.; Pratt, C.B. Topoisomerase I Interactive Drugs in Children with Cancer. Invest. New Drugs 1996, 14, 37–47. [Google Scholar] [CrossRef] [PubMed]
- AbouAitah, K.; Swiderska-Sroda, A.; Farghali, A.A.; Wojnarowicz, J.; Stefanek, A.; Gierlotka, S.; Opalinska, A.; Allayeh, A.K.; Ciach, T.; Lojkowski, W. Folic Acid-Conjugated Mesoporous Silica Particles as Nanocarriers of Natural Prodrugs for Cancer Targeting and Antioxidant Action. Oncotarget 2018, 9, 26466–26490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic Acid-Conjugated Mesoporous Silica Nanoparticles for Enhanced Therapeutic Efficacy of Topotecan in Retina Cancers. Int. J. Nanomed. 2018, 13, 4379–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, X.; Miao, G.; Liu, H.; Mao, C.; Yuan, G.; Liang, Q.; Shen, X.; Ning, C.; Fu, X. Synthesis of Radial Mesoporous Bioactive Glass Particles to Deliver Osteoactivin Gene. J. Mater. Chem. B 2014, 2, 7045–7054. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic Acid-Terminated Poly(2-Diethyl Amino Ethyl Methacrylate) Brush-Gated Magnetic Mesoporous Nanoparticles as a Smart Drug Delivery System. Polymers 2021, 13, 59. [Google Scholar] [CrossRef]
- Ji, J.; Wu, D.; Liu, L.; Chen, J.; Xu, Y. Preparation, Characterization, and in Vitro Release of Folic Acid-Conjugated Chitosan Nanoparticles Loaded with Methotrexate for Targeted Delivery. Polym. Bull. 2012, 68, 1707–1720. [Google Scholar] [CrossRef]
- Alotaibi, K.M.; Almethen, A.A.; Beagan, A.M.; Al-Swaidan, H.M.; Ahmad, A.; Bhawani, S.A.; Alswieleh, A.M. Quaternization of Poly (2-Diethyl Aminoethyl Methacrylate) Brush-Grafted Magnetic Mesoporous Nanoparticles Using 2-Iodoethanol for Removing Anionic Dyes. Appl. Sci. 2021, 11, 10451. [Google Scholar] [CrossRef]
- Abdeen, Z.; Mohammad, S.G. Study of the Adsorption Efficiency of an Eco-Friendly Carbohydrate Polymer for Contaminated Aqueous Solution by Organophosphorus Pesticide. Open J. Org. Polym. Mater. 2014, 4, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Kiurski, J.; Adamovic, S.; Krstic, J.; Oros, I.; Vojinovic Miloradov, M. Adsorption Efficiency of Low-Cost Materials in the Removal of Zn (II) Ions from Printing Developer. Acta Tech. Corviniensis Bull. Eng. 2011, 4, 61–66. [Google Scholar]
- Alaqarbeh, M. Adsorption Phenomena: Definition, Mechanisms, and Adsorption Types: Short Review. RHAZES Green Appl. Chem. 2021, 13, 43–51. [Google Scholar]
- Ho, Y.S.; McKay, G. Application of Kinetic Models to the Sorption of Copper(II) on to Peat. Adsorpt. Sci. Technol. 2002, 20, 797–815. [Google Scholar] [CrossRef]
- I IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres and Dusts; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100, ISBN 978 92 832 1320 8. [Google Scholar]
- Ho, Y.-S. Second-Order Kinetic Model for the Sorption of Cadmium onto Tree Fern: A Comparison of Linear and Non-Linear Methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of Adsorption of Metal Ions on Inorganic Materials: A Review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indraswati, N.; Ismadji, S. Equilibrium and Kinetic Studies in Adsorption of Heavy Metals Using Biosorbent: A Summary of Recent Studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.; Yang, J.; Li, X.; Zhou, L.; Zhang, R.; Li, W.; Chen, L.; Wang, R.; Zhang, F.; Zhao, D. Biphase Stratification Approach to Three-Dimensional Dendritic Biodegradable Mesoporous Silica Nanospheres. Nano Lett. 2014, 14, 923–932. [Google Scholar] [CrossRef]
- Alswieleh, A.M.; Beagan, A.M.; Alsheheri, B.M.; Alotaibi, K.M.; Alharthi, M.D.; Almeataq, M.S. Hybrid Mesoporous Silica Nanoparticles Grafted with 2-(Tert-Butylamino)Ethyl Methacrylate-b-Poly(Ethylene Glycol) Methyl Ether Methacrylate Diblock Brushes as Drug Nanocarrier. Molecules 2020, 25, 195. [Google Scholar] [CrossRef] [Green Version]
- Beagan, A.M. Investigating Methylene Blue Removal from Aqueous Solution by Cysteine-Functionalized Mesoporous Silica. J. Chem. 2021, 2021, 8839864. [Google Scholar] [CrossRef]
- Trukawka, M.; Cendrowski, K.; Konicki, W.; Mijowska, E. Folic Acid/Methotrexate Functionalized Mesoporous Silica Nanoflakes from Different Supports: Comparative Study. Appl. Sci. 2020, 10, 6465. [Google Scholar] [CrossRef]
- Bandara, S.; Carnegie, C.-a.; Johnson, C.; Akindoju, F.; Williams, E.; Swaby, J.M.; Oki, A.; Carson, L.E. Synthesis and Characterization of Zinc/Chitosan-Folic Acid Complex. Heliyon 2018, 4, e00737. [Google Scholar] [CrossRef] [Green Version]
- Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Zhu, J.; Lu, M. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Xiong, Z.; Zhang, J.; Wu, C. The Strengthening Role of the Amino Group in Metal–Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption. J. Chem. Eng. Data 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Zhang, G.; Wo, R.; Sun, Z.; Hao, G.; Liu, G.; Zhang, Y.; Guo, H.; Jiang, W. Effective Magnetic Mofs Adsorbent for the Removal of Bisphenol a, Tetracycline, Congo Red and Methylene Blue Pollutions. Nanomaterials 2021, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Al-Absi, R.S. Mechanistic Understanding of the Adsorption and Thermodynamic Aspects of Cationic Methylene Blue Dye onto Cellulosic Olive Stones Biomass from Wastewater. Sci. Rep. 2020, 10, 15928. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Highly Efficient Methylene Blue Dyes Removal from Aqueous Systems by Chitosan Coated Magnetic Mesoporous Silica Nanoparticles. J. Porous Mater. 2015, 22, 1383–1392. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, Z.; Sun, T.; Shi, W.; Cui, F. Enhanced Adsorption of the Cationic Dyes in the Spherical CuO/Meso-Silica Nano Composite and Impact of Solution Chemistry. J. Colloid Interface Sci. 2017, 485, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater: The Perspective Role of Nanomaterials and Nanotechnology. In Micro and Nano Technologies; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R.B.T.-N., Eds.; Chapter 7; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. ISBN 978-0-12-818489-9. [Google Scholar]
- Lee, S.Y.; Seader, J.D.; Tsai, C.H.; Massoth, F.E. Restrictive Liquid-Phase Diffusion and Reaction in Bidisperse Catalysts. Ind. Eng. Chem. Res. 1991, 30, 1683–1693. [Google Scholar] [CrossRef]
- PAPPENHEIMER, J.R. Passage of Molecules through Capillary Wals. Physiol. Rev. 1953, 33, 387–423. [Google Scholar] [CrossRef] [PubMed]
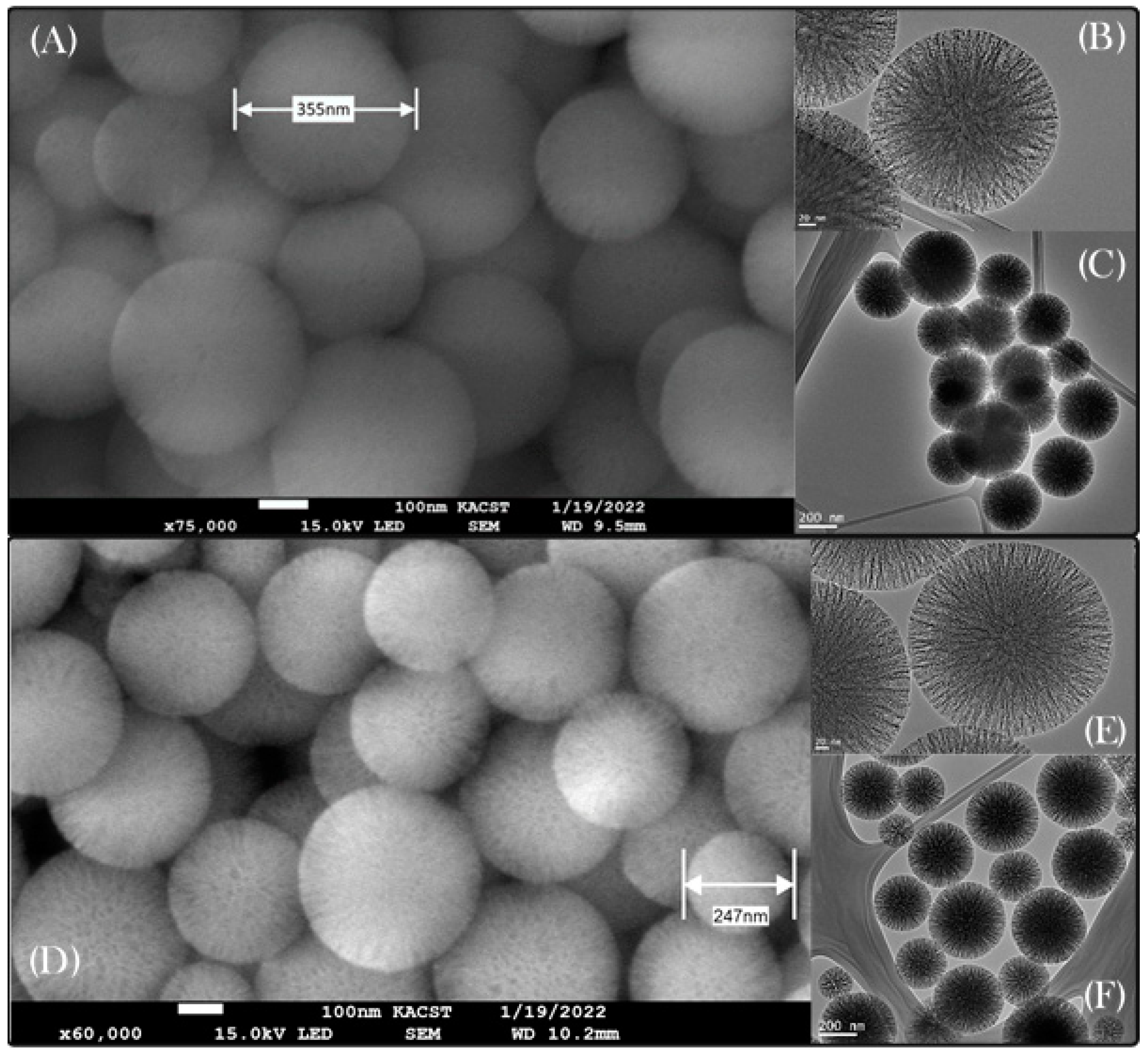

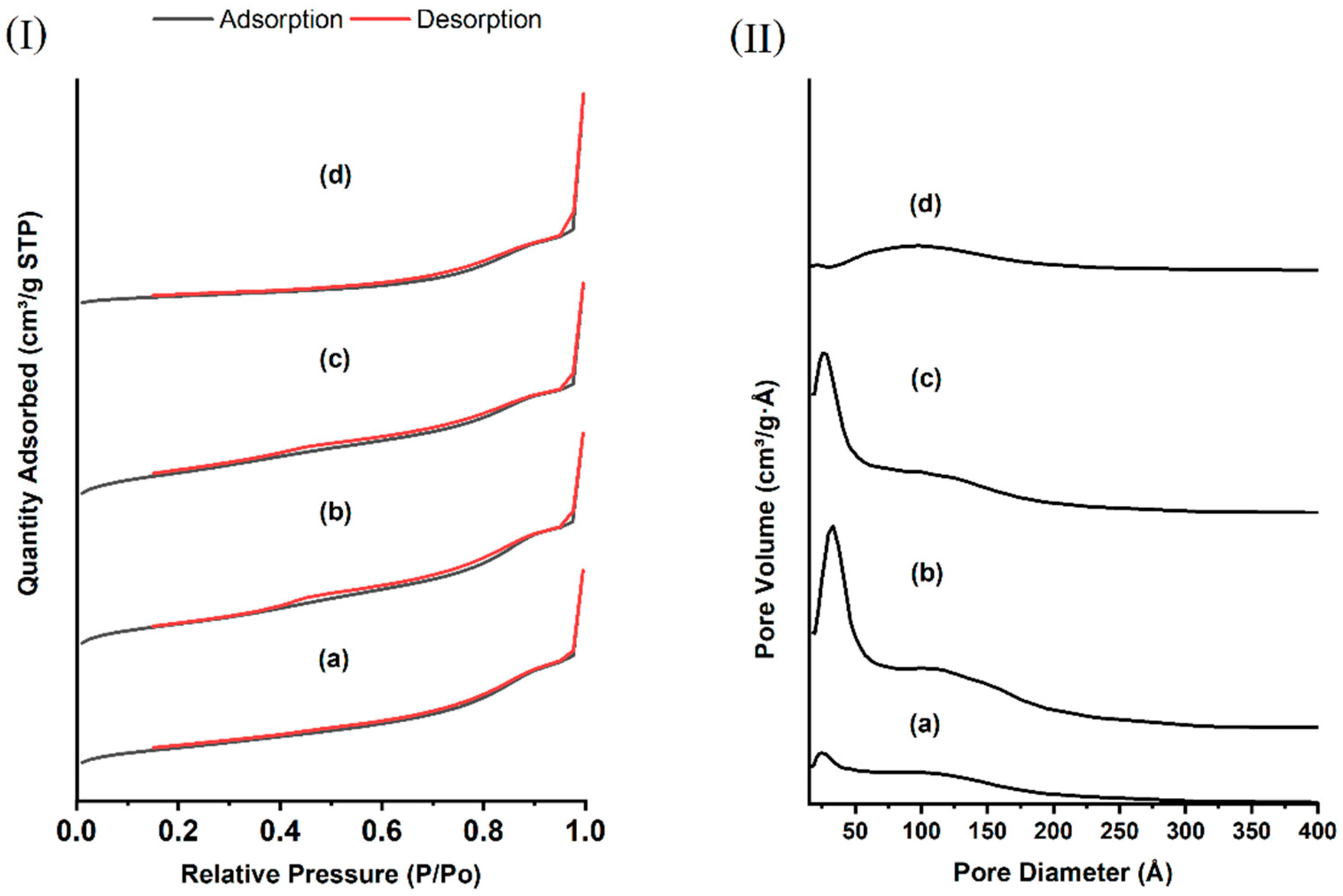
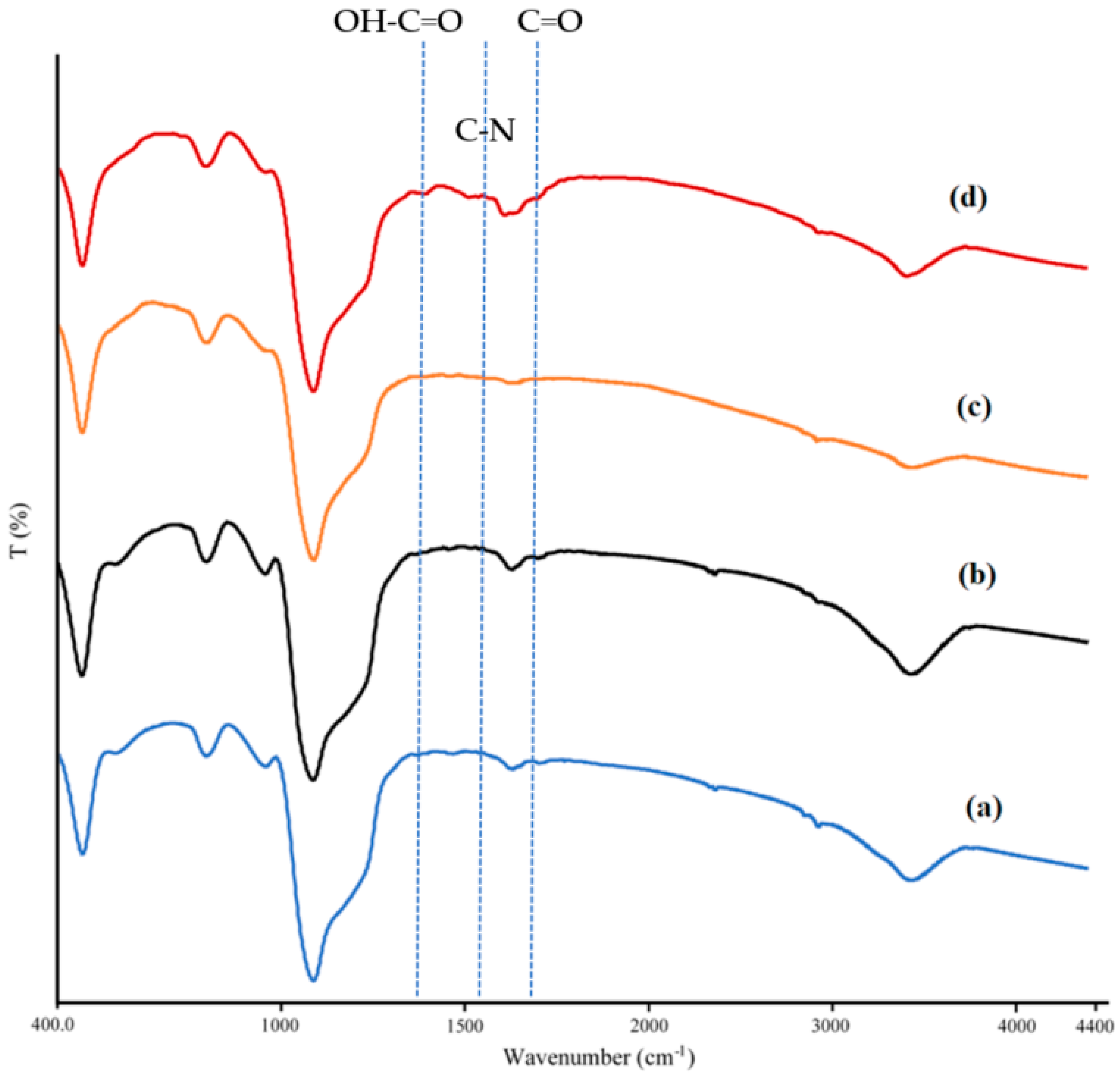
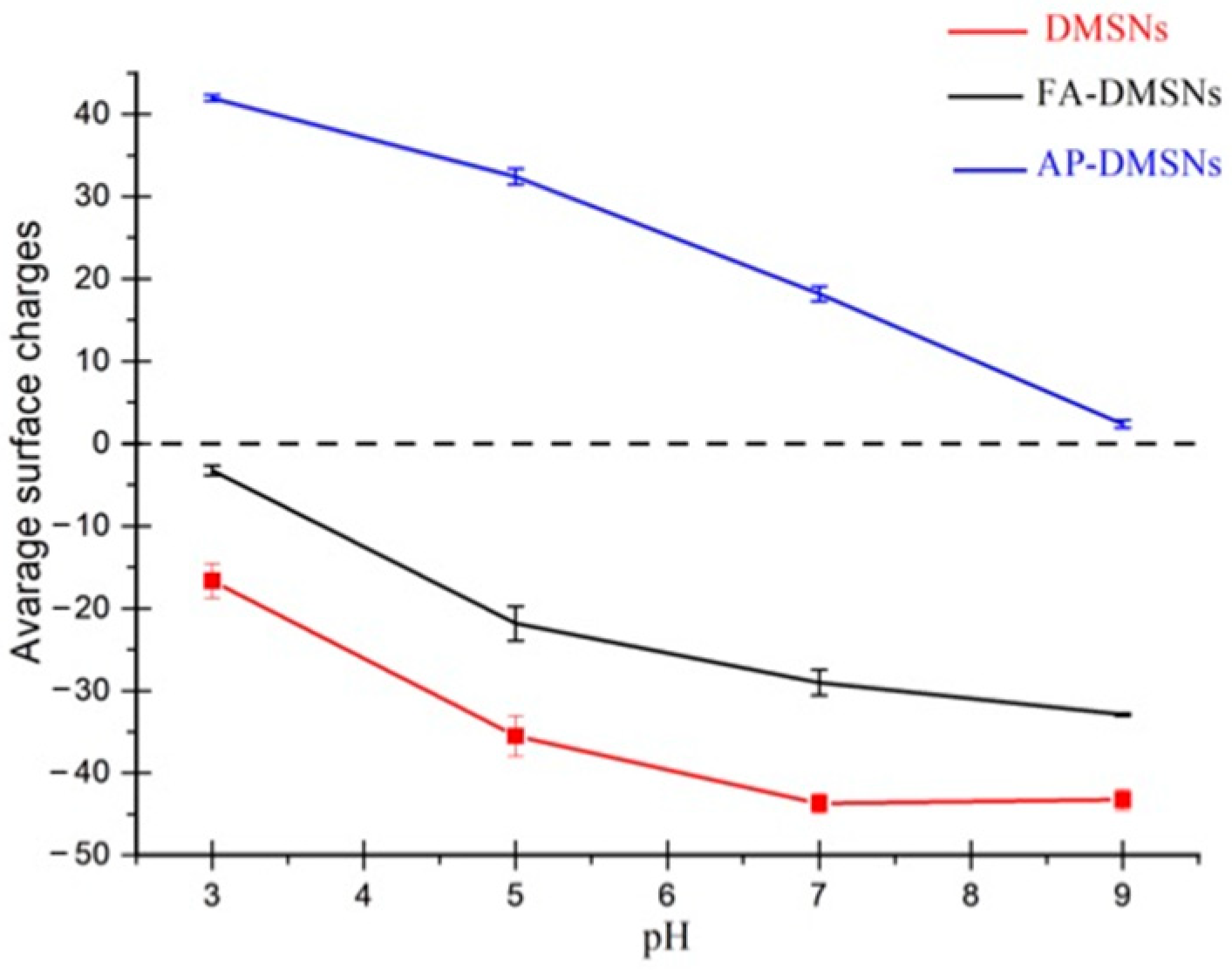
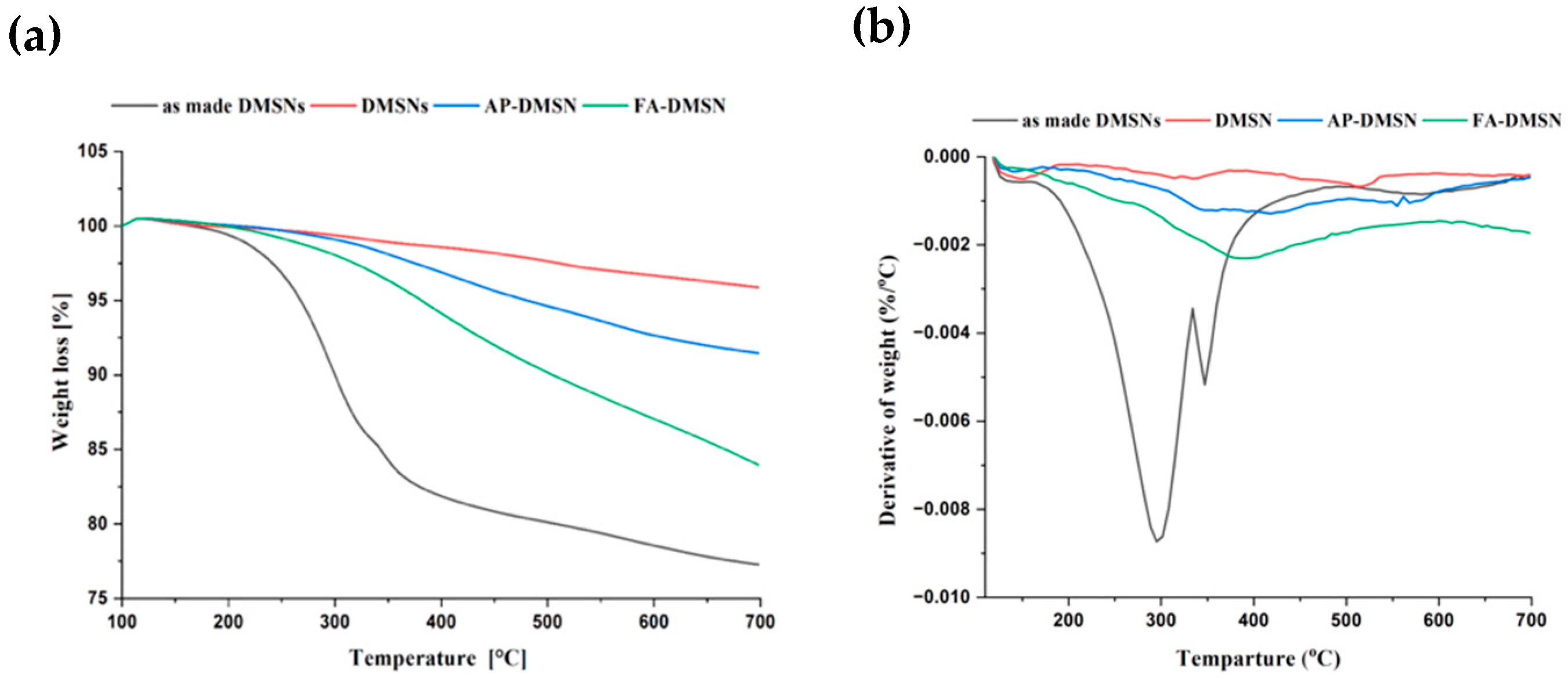


| Sample | BET Surface Area (m2·g−1) | Pore Volume (cm3·g−1) |
|---|---|---|
| As made−DMSN | 160.16 | 0.55 |
| DMSN | 520.93 | 1.21 |
| AP-DMSN | 382.54 | 0.97 |
| FA-DMSN | 53.14 | 0.56 |
| Sorbent | Equilibrium Time | Qm (mg/g) | Ref. |
|---|---|---|---|
| Monodispersed MSNs | within 6 min | 38.17 | [45] |
| MIL-53(Al)-NH2 | 300 min | 188.60 | [46] |
| Fe3O4@MIL-53(Al) | 180 min | 148.80 | [47] |
| Green olive stones | 24 h | 588.20 | [48] |
| Black olive stones | 24 h | 476.20 | |
| CMMSNs | 300 min | 120.0 | [49] |
| MCM-41 | 60 min | 65.70 | [50] |
| CuO/MCM-41 | 60 min | 87.80 | |
| FA-DMSN | within 3 min | 90.66 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almethen, A.A.; Alotaibi, K.M.; Alhumud, H.S.; Alswieleh, A.M. Highly Efficient and Rapid Removal of Methylene Blue from Aqueous Solution Using Folic Acid-Conjugated Dendritic Mesoporous Silica Nanoparticles. Processes 2022, 10, 705. https://doi.org/10.3390/pr10040705
Almethen AA, Alotaibi KM, Alhumud HS, Alswieleh AM. Highly Efficient and Rapid Removal of Methylene Blue from Aqueous Solution Using Folic Acid-Conjugated Dendritic Mesoporous Silica Nanoparticles. Processes. 2022; 10(4):705. https://doi.org/10.3390/pr10040705
Chicago/Turabian StyleAlmethen, Abdurrahman A., Khalid Mohammed Alotaibi, Haitham S. Alhumud, and Abdullah M. Alswieleh. 2022. "Highly Efficient and Rapid Removal of Methylene Blue from Aqueous Solution Using Folic Acid-Conjugated Dendritic Mesoporous Silica Nanoparticles" Processes 10, no. 4: 705. https://doi.org/10.3390/pr10040705
APA StyleAlmethen, A. A., Alotaibi, K. M., Alhumud, H. S., & Alswieleh, A. M. (2022). Highly Efficient and Rapid Removal of Methylene Blue from Aqueous Solution Using Folic Acid-Conjugated Dendritic Mesoporous Silica Nanoparticles. Processes, 10(4), 705. https://doi.org/10.3390/pr10040705






