Abstract
The search for novel highly effective materials with target properties for different electrochemical purposes is active for now. Ceramic materials with high levels of ionic conductivity can be applied as electrolytic materials in solid oxide fuel cells and in electrolyzers. Layered perovskites are a novel class of ionic conductors demonstrating almost-pure proton transportation at mid-temperatures. Gadolinium-doped ceramic materials based on layered perovskite BaLa2In2O7 were obtained and investigated for the first time in this study. The effect of the dopant concentrations on the hydration processes and on ionic conductivity was revealed. It was shown that compositions 0 ≤ x ≤ 0.15 of BaLa2–xGdxIn2O7 exhibited proton conductivity when under wet air and at mid-temperatures (lower than ~450 °C). Gadolinium doping led to an increase in the conductivity values up to an order of magnitude of ~0.5. The protonic conductivity of the most conductive composition BaLa1.85Gd0.15In2O7 was 2.7∙10−6 S/cm at 400 °C under wet air. The rare earth doping of layered perovskites is a prospective approach for the design of ceramics for electrochemical devices for energy applications.
1. Introduction
The search of novel highly effective materials with target properties for different electrochemical purposes is active for now. One of the main goals of modern humanity is the creation of highly effective, low-cost, eco-friendly and safe energy sources [1,2,3]. Hydrogen energy perfectly satisfies these characteristics, and its development is now a very high priority [4,5,6,7]. For full function in hydrogen energy systems, the creation of devices for the production, storage and transportation of hydrogen is required [8,9,10]. Devices such as protonic ceramic electrolysis cells and protonic ceramic fuel cells use electrochemical technologies to obtain hydrogen and for clean energy production [11,12,13,14,15,16]. Ceramic materials with a high level of ionic conductivity can be applied as electrolytic materials in solid oxide fuel cells and in electrolyzers [17,18,19,20,21,22,23]. The proton-conducting materials used for these purposes must have a high chemical resistance to carbon dioxide and water vapor and must exhibit high values of proton conductivity at the same time. Achieving a combination of all these characteristics in one material is a difficult task, so the material search continues.
Hexagonal perovskites [24,25] and layered perovskites [26,27] have been studied as proton-conducting materials in recent years. Layered perovskites may be represented by the formula AA’nBnO3n+1, where A is a bivalent metal (alkali-earth metal), A’ is a trivalent metal (rare-earth metal) and B is a trivalent metal (indium, scandium) with a smaller ionic radius compared with the radius of the A’ cation. The protonic conductivities of these materials, such as BaNdInO4 [28,29,30,31,32], SrLaInO4 [33,34,35,36,37], BaNdScO4 [38], BaLaInO4, is realized due to the possibility of dissociative water intercalation into the interlayer space of the layered structure. The monolayer barium-lanthanum indate BaLaInO4 was described as a nearly pure proton conductor below 400 °C, and an increase in the conductivity values up to 1.5 orders of magnitude caused by heterovalent [39,40,41,42] and isovalent [43,44,45,46] doping was revealed. The two-layer composition BaLa2In2O7 of this homologous series AA’nBnO3n+1 was also described as a protonic conductor [47]. The possibility of water uptake was proved, and the use of the acceptor-doping strategy made it possible to significantly increase the proton conductivity [48,49,50]. However, the isovalent-doping strategy for the modification of the structure and transportation properties has not been applied to the two-layer perovskite BaLa2In2O7 yet. In this work, gadolinium-doped ceramic materials based on the layered perovskite BaLa2In2O7 were obtained and investigated for the first time. The effect of the dopant concentrations on the hydration processes and on ionic conductivity was revealed.
2. Materials and Methods
The samples of BaLa2–xGdxIn2O7 were synthesized using solid state method. The starting reagents BaCO3, La2O3, In2O3 and Gd2O3 were used. The final temperature of calcination was 1300 °C.
The XRD investigations were performed using a Bruker D8 Advance Cu Kα diffractometer (step of 0.01°, scanning rate of 0.5°/min). The thermogravimetry (TG) was performed using STA 409 PC NETZSCH analyzer. The heating of initially hydrated samples was performed in the temperature range of 40–1100 °C at the rate of 10 °C/min under a flow of dry Ar. The hydrated samples were obtained during slow cooling (1 °C/min) from 1100 to 150 °C under a flow of wet Ar.
The electrical conductivity was measured using impedance spectrometer Z-1000P, Elins, RF. The investigations were performed from 1000 to 200 °C with 1°/min cooling rate under dry air or dry Ar conditions. The dry gas (air or Ar) was produced by circulating the gas through P2O5 (pH2O = 3.5·10−5 atm). The wet gas (air or Ar) was obtained by bubbling the gas at room temperature first through distilled water and then through saturated solution of KBr (pH2O = 2·10−2 atm).
3. Results
The phase attestation of the solid solution of BaLa2–xGdxIn2O7 was performed using the XRD method. It was shown that the compositions at the dopant concentrations 0 ≤ x ≤ 0.15 were in a single phase and were isostructural to the matrix composition BaLa2In2O7 (Figure 1a). The samples from the solid solution’s homogeneity region had a tetragonal symmetry and belonged to the space group P42/mnm. The XRD-patterns of the obtained compositions are presented in Figure 1b–d. Table 1 contains the lattice parameters and unit cell volumes of the compositions.
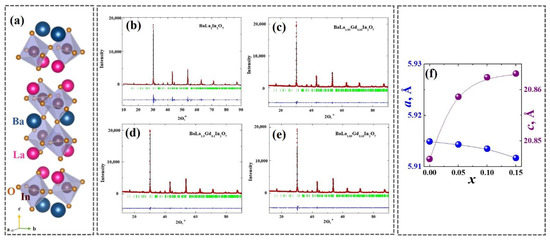
Figure 1.
(a) Crystal structure of BaLa2In2O7 and (f) the concentration dependencies of the lattice parameters. XRD-patterns of (b) BaLa2In2O7, (c) BaLa1.95Gd0.05In2O7, (d) BaLa1.9Gd0.1In2O7 and (e) BaLa1.85Gd0.15In2O7 compositions.

Table 1.
The lattice parameters and unit cell volumes of investigated compositions of BaLa2–xGdxIn2O7.
As can be seen, the introduction of an ion with a slightly smaller ionic radius ( = 1.216 Å; = 1.107 Å [51]) led to a decrease in lattice parameter a but also to an increase in lattice parameter c (Figure 1e). Therefore, the unit cell volume almost did not change. It was obvious that the reason for these changes was the interatomic distance during doping, which is not only the difference in the ionic radii of the ions but also the difference in their electronegativity. The electronegativities of lanthanum and gadolinium were different ( = 1.10; = 1.20 [52]), which caused the occurrence of additional repulsion effects between these cations when in the same crystallographic positions of the crystal lattice. This could be probable because of the increase in the c lattice parameter during doping. It should be noted that the same increase in the lattice parameters during gadolinium doping occurred for the monolayer composition BaLaInO4 [45].
The possibility of water uptake from the gas phase was investigated using the thermogravimetry (TG) method. The TG-curves for all of the investigated samples had the same shape, and the results for the composition BaLa1.9Gd0.1In2O7 are presented in Figure 2 as an example. Water loss occurred in several steps and ended at 600–700 °C. The mass spectroscopy (MS) results (the green line in Figure 2) confirmed the TG-data. The values of the water uptake of the doped compositions were in the range 0.10–0.13 mol per formula unit, which was comparable to the water uptake of the undoped composition (0.17 mol [47]).
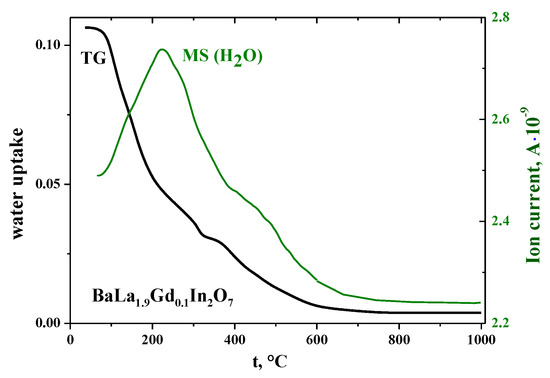
Figure 2.
Thermogravimetry (TG) and mass spectrometry (MS) results for the composition BaLa1.9Gd0.1In2O7.
We could suppose that very small changes in the unit cell volume that occurred during doping caused these small changes in the water uptake. Despite the relatively small water uptake, the possibility for the dissociative incorporation of water into the crystal lattice of the gadolinium-doped compositions indicated the possibility of protonic transport. The interaction of the investigated compositions with water molecules can be described as:
The electrical conductivity values were obtained using the impedance spectroscopy method in the atmospheres with controlled humidity (pH2O) and an oxygen partial pressure (pO2). Figure 3 represents the temperature dependencies of the conductivities obtained under dry air (Figure 3a), dry Ar (Figure 3b), wet air (Figure 3c) and wet Ar (Figure 3d).
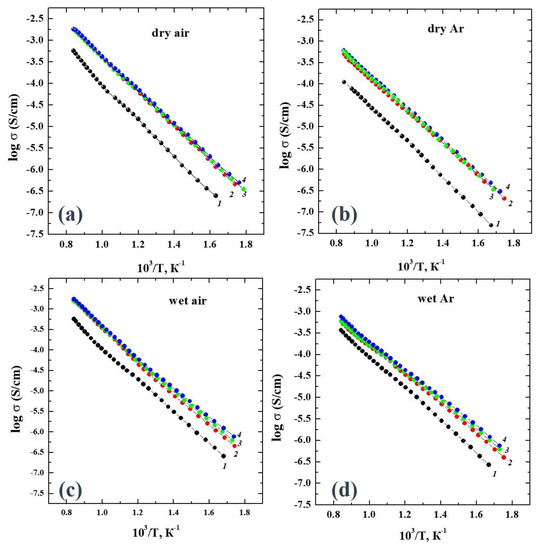
Figure 3.
The temperature dependencies of conductivities for the compositions of BaLa2–xGdxIn2O7 at x = 0 (1), x = 0.05 (2), x = 0.10 (3) and x = 0.15 (4) obtained under (a) dry air, (b) dry Ar, (c) wet air and (d) wet Ar.
As can be seen, an increase in the gadolinium concentration led to an increase in the electrical conductivity regardless of the values of pH2O and pO2. The concentration dependencies (Figure 4a) were well illustrated with this regularity. The effect of the changes in the pH2O and pO2 on the conductivity values at the same dopant concentrations is presented in Figure 4b. The values obtained under dry Ar (pO2 ~ 10−5 atm) were lower than those obtained under dry air (pO2 = 0.21 atm), which indicated the mixed oxygen-hole nature of the conductivity. The effect of the humidity changes was more visible in the Ar atmosphere, where the conductivity values under wet conditions (pH2O = 2·10−2 atm) were significantly increased compared with those under dry conditions (pH2O = 3.5·10−5 atm). This indicated the appearance of a proton contribution to the conductivity.
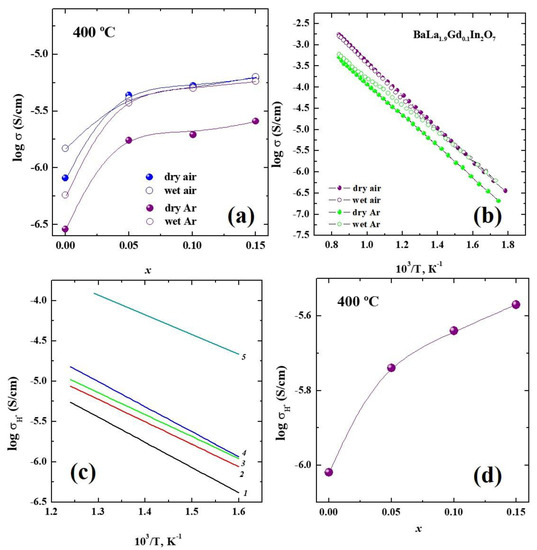
Figure 4.
(a) The concentration dependencies and (b) temperature dependencies of conductivities for the compositions of BaLa2–xGdxIn2O7 obtained under different conditions. (c) The temperature dependencies and (d) concentration dependency of protonic conductivities of the compositions of BaLa2−xGdxIn2O7 with x = 0 (1), x = 0.05 (2), x = 0.10 (3) and x = 0.15 (4) and for the composition BaLa1.7Ba0.3In2O6.85 (5).
The protonic conductivity can be calculated as the differences between the ionic conductivities under wet and dry conditions, i.e., between conductivities obtained under wet and dry Ar. The temperature dependencies of the protonic conductivities of the BaLa2–xGdxIn2O7 compositions are presented in Figure 4c. Doping led to an increase in the conductivity values (Figure 4d) up to an order of magnitude of ~ 0.5 for the mostly conductive composition BaLa1.85Gd0.15In2O7. In general, this increase in the electrical conductivity values was due to the increase in the concentration of the current carriers and their mobility. In the case of oxygen-ionic conductivity, the concentration of the oxygen point defects did not change during the gadolinium doping of the lanthanum sublattice (isovalent doping). However, the oxygen-ionic conductivity values (the conductivities obtained under dry Ar) increased with the increase of the dopant concentrations. The most reasonable cause was the increase in the oxygen mobility with the increase of the gadolinium content. The lattice parameter c increased, which indicated the increase in the interlayer space in the crystal structure, so the free migration volume increased, which could facilitate ion transportation, i.e., the increase in the oxygen mobility. In the case of the protonic conductivity, the water uptake (i.e., the proton concentration) was almost the same for all the compositions of BaLa2–xGdxIn2O7; thus, the main reason for the protonic conductivity increase during doping was the increase in the proton mobility. Due to the fact that proton transportation is carried out by the jumping of protons onto oxygen atoms, an increase in the oxygen mobility should have led to the increase in the protonic mobility.
The comparison of the protonic conductivities of the investigated composition obtained by isovalent doping and of the acceptor-doped composition BaLa1.7Ba0.3In2O6.85 is presented in Figure 4c. The composition BaLa1.7Ba0.3In2O6.85 was chosen as the most proton-conductive compound obtained by the heterovalent doping of the matrix composition BaLa2In2O7 [48]. As can be seen, the protonic conductivity values for the acceptor-doped composition were higher than those for the isovalent-doped compositions by about one order of magnitude. Because the values of the water uptake for the acceptor-doped (~0.2 mol) and isovalent-doped (~0.13 mol) compositions were comparable to each other, we could suggest that the different proton mobilities were the most reasonable explanation for the significant difference in the protonic conductivity values. The lattice parameter c (20.954(9) Å) and the unit cell volume (743.50(2) Å3) of the acceptor-doped composition BaLa1.7Ba0.3In2O6.85 were much larger than those of the isovalent-doped composition BaLa1.85Gd0.15In2O7 (20.863(1) Å and 729.00(0) Å3). Obviously, this increase provided the facilitation of proton transportation, which led to the increase in the protonic mobility and the conductivity. We suggested that choosing the isovalent dopant with a larger ionic radius than lanthanum would increase the conductivity more significantly.
Summarizing all the obtained results, we could say that the isovalent-doping strategy of the layered perovskite BaLa2In2O7 was a successful way to improve the proton conductivity. Gadolinium doping led to an increase in the lattice parameter c, which led to an increase in the ionic transportation in the layered structure. The protonic conductivity increased with the increasing dopant content. The protonic conductivity of the most conductive composition BaLa1.85Gd0.15In2O7 was 2.7∙10−6 S/cm at 400 °C under wet air.
4. Conclusions
The isovalent-doping strategy for the modification of the structure and transportation properties of the two-layer perovskite BaLa2In2O7 was applied for the first time. The gadolinium-doped ceramic materials BaLa2–xGdxIn2O7 were obtained and investigated. The effect of the dopant concentrations on the hydration processes and the ionic conductivity was revealed. It was shown that all of the compositions exhibited proton conductivity under wet air and at mid-temperatures (lower than ~450 °C). Gadolinium doping led to an increase in the conductivity values up to an order of magnitude of ~0.5. The protonic conductivity of the most conductive composition BaLa1.85Gd0.15In2O7 was 2.7·10−6 S/cm at 400 °C under wet air. The rare earth doping of layered perovskites is a prospective approach for the design of ceramics for electrochemical devices for energy applications.
Author Contributions
Conceptualization, I.A. and N.T.; methodology, I.A. and N.T.; investigation, A.B. and E.V.; data curation, A.B.; writing—original draft preparation, N.T.; writing—review and editing, N.T. and I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malerba, D. Poverty-energy-emissions pathways: Recent trends and future sustainable development goals. Int. J. Sustain. Energy Dev. 2019, 49, 109–124. [Google Scholar] [CrossRef]
- Buonomano, A.; Barone, G.; Forzano, C. Advanced energy technologies, methods, and policies to support the sustainable development of energy, water and environment systems. Energy Rep. 2022, 8, 4844–4853. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Mikulcic, H.; Kalogirou, S. Sustainable development using renewable energy technology. Renew. Energy 2020, 146, 2430–2437. [Google Scholar] [CrossRef]
- International Energy Agency. The Future of Hydrogen: Seizing Today’s Opportunities; OECD: Paris, French, 2019. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Scovell, M.D. Explaining hydrogen energy technology acceptance: A critical review. Int. J. Hydrog. Energy 2022, 47, 10441–104591. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development—A technological and geopolitical overview. Int. J. Hydrog. Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Mittaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrog. Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef]
- Medvedev, D. Trends in research and development of protonic ceramic electrolysis cells. Int. J. Hydrog. Energy 2019, 44, 26711–26740. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Zvonareva, I.; Fu, X.-Z.; Medvedev, D.; Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 2022, 15, 439–465. [Google Scholar] [CrossRef]
- Chiara, A.; Giannici, F.; Pipitone, C.; Longo, A.; Aliotta, C.; Gambino, M.; Martorana, A. Solid-Solid Interfaces in Protonic Ceramic Devices: A Critical Review. ACS Appl. Mater. Interfaces 2020, 12, 55537–55553. [Google Scholar] [CrossRef]
- Cao, J.; Ji, Y.; Shao, Z. New Insights into the Proton-Conducting Solid Oxide Fuel Cells. J. Chin. Ceram. Soc. 2021, 49, 83–92. [Google Scholar] [CrossRef]
- Shim, J.H. Ceramics breakthrough. Nat. Energy 2018, 3, 168–169. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; He, Q.; Cheng, C.; Dai, Y.; Chen, B.; Zhang, Y.; Ni, M. Materials development and prospective for protonic ceramic fuel cells. Int. J. Energy Res. 2021, 46, 2212–2240. [Google Scholar] [CrossRef]
- Irvine, J.; Rupp, J.L.M.; Liu, G.; Xu, X.; Haile, S.; Qian, X.; Snyder, A.; Freer, R.; Ekren, D.; Skinner, S.; et al. Roadmap on inorganic perovskites for energy applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Kim, J.; Sengodan, S.; Kim, S.; Kwon, O.; Bu, Y.; Kim, G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2019, 109, 606–618. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Zvonareva, I.A.; Tarasova, N.A.; Bi, L.; Medvedev, D.A.; Shao, Z. Electrolyte materials for protonic ceramic electrochemical cells: Main limitations and potential solutions. Mater. Rep. Energy, 2022; 100158, in press. [Google Scholar] [CrossRef]
- Fop, S.; McCombie, K.S.; Wildman, E.J.; Skakle, J.M.S.; Irvine, J.T.S.; Connor, P.A.; Savaniu, C.; Ritter, C.; McLaughlin, A.C. High oxide ion and proton conductivity in a disordered hexagonal perovskite. Nat. Mater. 2020, 19, 752–757. [Google Scholar] [CrossRef]
- Yashima, M.; Tsujiguchi, T.; Sakuda, Y.; Yasui, Y.; Zhou, Y.; Fujii, K.; Torii, S.; Kamiyama, T.; Skinner, S.J. High oxide-ion conductivity through the interstitial oxygen site in Ba7Nb4MoO20-based hexagonal perovskite related oxides. Nat. Comm. 2021, 12, 556. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: Relationship between ion (oxygen-ion, proton) conductivity, water uptake and structural changes. Materials 2022, 15, 114. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Medvedev, D. Layered and hexagonal perovskites as novel classes of proton-conducting solid electrolytes: A focus review. Electrochem. Mater. Technol. 2022, 1, 20221004. [Google Scholar] [CrossRef]
- Fujii, K.; Shiraiwa, M.; Esaki, Y.; Yashima, M.; Kim, S.J.; Lee, S. Improved oxide-ion conductivity of NdBaInO4 by Sr doping. J. Mater. Chem. A 2015, 3, 11985–11990. [Google Scholar] [CrossRef]
- Ishihara, T.; Yan, Y.; Sakai, T.; Ida, S. Oxide ion conductivity in doped NdBaInO4. Solid State Ion. 2016, 288, 262–265. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Lu, F.; Xu, J.; Kuang, X. Acceptor doping and oxygen vacancy migration in layered perovskite NdBaInO4- based mixed conductors. J. Phys. Chem. C 2016, 12, 6416–6426. [Google Scholar] [CrossRef]
- Fujii, K.; Yashima, M. Discovery and development of BaNdInO4—A brief review. J. Ceram. Soc. Jpn. 2018, 126, 852–859. [Google Scholar] [CrossRef]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ogasawara, M.; Sugai, M.; Nakata, S. Synthesis and oxide ion conductivity of new layered perovskite La1−xSr1+xInO4−d. Solid State Ion. 2002, 149, 53–57. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1−xInO4+d. J. Mater. Chem. A 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Aguadero, A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1−xBxO4+δ system (B = Zr, Ti). Solid State Ion. 2015, 282, 82–87. [Google Scholar] [CrossRef]
- Troncoso, L.; Mariño, C.; Arce, M.D.; Alonso, J.A. Dual oxygen defects in layered La1.2Sr0.8−xBaxInO4+d (x = 0.2, 0.3) oxide-ion conductors: A neutron diffraction study. Materials 2019, 12, 1624. [Google Scholar] [CrossRef]
- Troncoso, L.; Arce, M.D.; Fernández-Díaz, M.T.; Mogni, L.V.; Alonso, J.A. Water insertion and combined interstitial-vacancy oxygen conduction in the layered perovskites La1.2Sr0.8−xBaxInO4+d. New J. Chem. 2019, 43, 6087–6094. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Kido, T.; Fujii, K.; Yashima, M. High-temperature proton conductors based on the (110) layered perovskite BaNdScO4. J. Mat. Chem. A 2021, 9, 8607–8619. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A. Effect of acceptor and donor doping on the state of protons in block-layered structures based on BaLaInO4. Solid State Comm. 2021, 323, 114093. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Improvement of oxygen-ionic and protonic conductivity of BaLaInO4 through Ti doping. Ionics 2020, 26, 5075–5088. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I. Ba2+/Ti4+—Co-doped layered perovskite BaLaInO4: The structure and ionic (O2−, H+) conductivity. Int. J. Hydrog. Energy 2021, 46, 16868–16877. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Belova, K. Simultaneous hetero- and isovalent doping as the strategy for improving transport properties of proton conductors based on BaLaInO4. Materials 2021, 14, 6240. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Davletbaev, K. Novel proton-conducting layered perovskite based on BaLaInO4 with two different cations in B-sublattice: Synthesis, hydration, ionic (O2+, H+) conductivity. Int. J. Hydrog. Energy 2022, 47, 18972–18982. [Google Scholar] [CrossRef]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Anokhina, I.; Gilev, A.; Cheremisina, P. Novel mid-temperature Y3+ → In3+ doped proton conductors based on the layered perovskite BaLaInO4. Ceram. Int. 2022, 48, 15677–15685. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I. Proton transport in the gadolinium-doped layered perovskite BaLaInO4. Materials 2022, 15, 7351. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Bedarkova, A. Advanced proton-conducting ceramics based on layered perovskite BaLaInO4 for energy conversion technologies and devices. Materials 2022, 15, 6841. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, N.; Galisheva, A.; Animitsa, I.; Korona, D.; Kreimesh, H.; Fedorova, I. Protonic transport in layered perovskites BaLanInnO3n+1 (n = 1, 2) with Ruddlesden-Popper structure. Appl. Sci. 2022, 12, 4082. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Belova, K.; Abakumova, E.; Cheremisina, P.; Medvedev, D. Oxygen Ion and Proton Transport in Alkali-Earth Doped Layered Perovskites Based on BaLa2In2O7. Inorganics 2022, 10, 161. [Google Scholar] [CrossRef]
- Tarasova, N.A. Local structure and ionic transport in acceptor-doped layered perovskite BaLa2In2O7. Chim. Techno Acta 2022, 9, 20229415. [Google Scholar] [CrossRef]
- Tarasova, N.; Bedarkova, A.; Animitsa, I.; Abakumova, E.; Belova, K.; Kreimesh, H. Novel high conductive ceramic materials based on two-layer perovskite BaLa2In2O7. Int. J. Mol. Sci. 2022, 23, 12813. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Allred, A.L. Electronegativity values from thermochemical data. J. Inorg. Nucl. Chem. 1961, 17, 215–221. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).