Abstract
Reactive oxygen species (ROS) have been the focus of redox research in the realm of oxidative neonatal respiratory diseases such as bronchopulmonary dysplasia (BPD). Over the years, nitric oxide (NO) and carbon monoxide (CO) have been identified as important gaseous signaling molecules involved in modulating the redox homeostasis in the developing lung. While animal data targeting aspects of these redox pathways have been promising in treating and/or preventing experimental models of neonatal lung disease, none are particularly effective in human neonatal clinical trials. In recent years, hydrogen sulfide (H2S) has emerged as a novel gasotransmitter involved in a magnitude of cellular signaling pathways and functions. The importance of H2S signaling may lie in the fact that early life-forms evolved in a nearly anoxic, sulfur-rich environment and were dependent on H2S for energy. Recent studies have demonstrated an important role of H2S and its synthesizing enzymes in lung development, which normally takes place in a relatively hypoxic intrauterine environment. In this review, we look at clues from evolution and explore the important role that the H2S signaling pathway may play in oxidative neonatal respiratory diseases and discuss future opportunities to explore this phenomenon in the context of neonatal chronic lung disease.
1. Introduction
Organisms function in a tightly balanced redox environment influenced by the reactivities of oxidants and antioxidants. At steady state, slightly more oxidants (termed ‘oxidative eustress’) are necessary for critical cellular processes to occur []. If, however, the balance is shifted further towards oxidants, the phenomenon is termed ‘oxidative stress’ and can trigger an array of signaling and compensatory mechanisms []. Babies who are born prematurely encounter oxidative stress in several forms []. Under normal circumstances, the human fetus develops in a relatively hypoxic environment in the womb when compared to the outside world. The sudden increase in the partial pressure of oxygen presents an uphill battle against the detrimental effects of reactive oxygen species (ROS) []. Additionally, ROS can develop secondarily to infection, inflammation, and reperfusion. Several antioxidant mechanisms are active in the lungs of premature babies to counteract the effects of oxidants, which include the glutathione (GSH) and thioredoxin (Trx) systems, superoxide dismutase (SOD), and catalase, among others. Many of these antioxidant systems develop in a similar timeline to that of pulmonary surfactants, which means that they are underdeveloped in prematurely born babies, making them further vulnerable to oxidative stress-related damage []. While the physiological influence of these redox perturbations has been well documented in premature babies, no single antioxidant treatment has been proven to be particularly effective in treating or preventing neonatal respiratory diseases []. While antioxidant specificity and tissue delivery have been major hurdles that we have yet to overcome, the current situation also raises the possibility that perhaps regulation of redox homeostasis in the premature and developing lung requires further exploration.
Recent advances in H2S research have shed some light on the anti-inflammatory, antiapoptotic, antioxidant, and other potential beneficial effects of H2S []. Furthermore, H2S and its synthesizing enzymes have been shown to play an important role in lung and airway development [,,], thus raising the question: ‘Is H2S the missing link in the lung redox homeostasis?’. To answer this intriguing question, we will have to look at evolutionary clues and understand the context in which these various redox systems developed and evolved under different oxidative environments. In this review, we will attempt to address this overarching question and hopefully also raise novel questions in the context of oxidative neonatal respiratory diseases with a focus on bronchopulmonary dysplasia (BPD).
2. Evolution of Oxidant and Antioxidant Pathways and the Importance of H2S
A vast majority of organisms now depend on the sun for their energy. Plants and many bacteria use oxygenic photosynthesis to transfer electrons from water to carbon dioxide, generating oxygen as a byproduct []. Aerobic organisms essentially reverse these chemical reactions in aerobic metabolism. It is interesting to note that millions of years ago, when life first started on this planet, the atmosphere was nearly anoxic. Consequently, oxygenic photosynthesis was not the predominant source of energy for early life-forms. In this context, the sulfur cycle and hydrogen sulfide were essential for the survival of these species. The remnants of this evolutionary process persist in anaerobic microorganisms such as sulfate-reducing bacteria that can breathe without oxygen [,]. These bacteria can survive in extreme and anoxic environments such as near volcanos or hydrothermal vents because of their unique ability to use sulfate as their terminal electron acceptor instead of oxygen. Cyanobacteria were the first organisms to demonstrate oxygenic photosynthesis through four-electron oxidation of two water molecules []. As cyanobacteria and plants synthesized more oxygen, oxygen levels slowly rose over millions of years (termed the ‘Great Oxidation Event’) to levels that are comparable to today [,]. During this time, there was a paradigm shift from sulfide being a major source of energy to oxygenic photosynthesis becoming the principal source. However, this significant increase in oxygen also posed an existential threat to the extant organisms. Those who were able to evolve and develop antioxidant systems to counter ROS had a significant survival advantage in this new environment.
One school of thought is that a vast number of organisms were able to develop antioxidant systems to counter ROS because they already had enzyme systems to counter reactive sulfur species (RSS) []. RSS are intermediary molecules produced through the stepwise one-electron oxidation of H2S producing thiyl (HS•) and supersulfide (HS2•−) radicals, hydrogen persulfide (H2S2), and elemental sulfur []. While there are several ROS that have been described, hydrogen peroxide (H2O2) is generally believed as the principal ROS signaling molecule as it is more stable than superoxide (O2•−) or the hydroxyl radical (HO·), which rapidly dissolves into peroxide and water [,]. H2O2, however, is promptly scavenged once produced by cellular antioxidant systems []. There are many aspects of ROS and RSS metabolism that are similar, but RSS signaling is generally considered more versatile [,]. A possible reason for this is that H2S signaling and RSS are ancient, and most extant organisms evolved to develop enzyme systems to scavenge them and eventually use them as important signaling molecules []. As atmospheric levels of oxygen rose (and the H2S level decreased) and ROS-mediated damage posed a considerable existential threat, organisms made minor modifications in their enzyme systems to counter ROS [,] (Figure 1). This phenomenon may explain the chemical similarity between peroxide and H2S or RSS and the interaction between their enzyme systems.
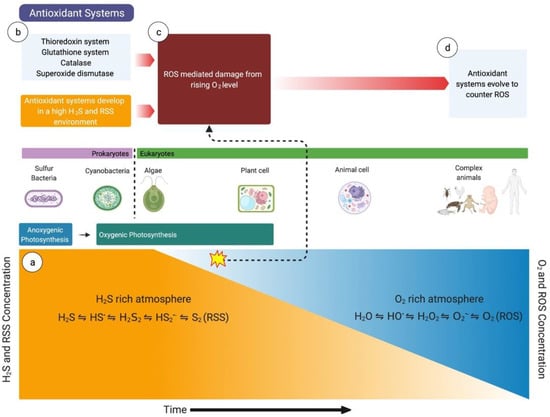
Figure 1.
The importance of hydrogen sulfide (H2S) in evolution. (a) Life started in an atmosphere rich in H2S and reactive sulfur species (RSS), where H2S was used to convert carbon dioxide to water. (b) The antioxidant systems (e.g., glutathione system, thioredoxin system) also developed in H2S- and RSS-rich environments. The antioxidant systems initially developed to counter RSS-mediated damage. (c) As the oxygen level slowly rose (the ‘Great Oxidation Event’ secondary to oxygenic photosynthesis by cyanobacteria and plants), the extant species were at increased risk of extinction from reactive oxygen species (ROS)-mediated damage. (d) To counter the increased ROS-mediated damage, the antioxidant systems evolved over the years to neutralize ROS. HS•, thiyl radical; H2S2, hydrogen persulfide; HS2•−, supersulfide; S2, elemental sulfur; H2O, water; HO•, hydroxyl radical; H2O2, hydrogen peroxide; O2•−, superoxide, O2, oxygen.
Wang et al. first coined the term ‘gasotransmitters’ to denote gaseous molecules that are produced and regulated endogenously, permeate through lipid membranes, and affect multitudes of cellular functions []. Over the years, important cardiopulmonary functions were attributed to NO and CO signaling. Only in the last two decades has H2S, which used to be known for its pungent and poisonous nature, been reinvented as the third important gasotransmitter. Since then, H2S, RSS, and their downstream signaling pathways have been studied in many tissues [,]. H2S has been shown to have cytoprotective, anti-inflammatory, and redox-regulatory effects in various tissues and cell types and its perturbation has been associated with multiple disease phenotypes [,,,]. Consequently, there has been a heightened interest in H2S and its signaling in recent years.
3. Sulfur Homeostasis and Metabolism
H2S levels are dynamically controlled within a very narrow range that represents the aggregate of its rate of formation and degradation []. Intracellular levels can range from <1 µM to >100 µM with the steady-state concentration in the nanomolar range []. H2S easily dissolves in water and dissociates into H+, HS−, and elemental sulfur. Endogenous H2S production can occur through enzymatic or nonenzymatic pathways. Enzymatic production is generally considered as the principal source of H2S; however, a recent study has shown that nonenzymatic production may be an important source in several different tissues (e.g., lung, brain, gut) []. The study also found that the primary substrate for nonenzymatic production of H2S is cysteine (Cys). This pathway is, however, understudied and underappreciated. The other nonenzymatic pathway of H2S generation involves the reduction of sulfur from a persulfide or polysulfide in the presence of an electron acceptor such as NADPH [].
3.1. H2S Biogenesis
Enzymatic production of H2S is controlled through the transsulfuration pathway []. Three key enzymes are involved in the production of H2S: cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) (Figure 2). The expression of these enzymes differs depending on the tissue and cell type. Two of these enzymes, CBS and CSE, mainly reside in the cytosol, while 3-MST localizes both in cytosolic and mitochondrial compartments [,].
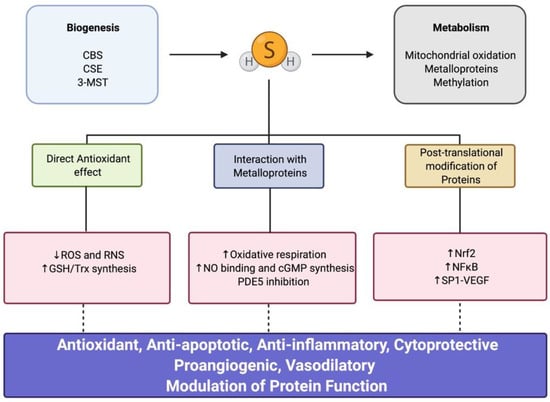
Figure 2.
Hydrogen sulfide (H2S) biogenesis, metabolism, and downstream signaling. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; 3-MST, 3-mercaptopyruvate sulfurtransferase; ROS, reactive oxygen species; RNS, reactive nitrogen species; GSH, glutathione; Trx, thioredoxin; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; PDE5, phosphodiesterase 5; Nrf2, nuclear factor erythroid 2-related factor; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; SP1, specificity protein 1; VEGF, vascular endothelial growth factor.
CBS serves as a gatekeeper between the methionine cycle and the transsulfuration pathway by catalyzing the first irreversible reaction from homocysteine to cystathionine, thus committing sulfur towards cysteine production and metabolism []. The reaction generates H2S as a byproduct and thus CBS is an important regulator of H2S biosynthesis. CBS is the most common enzyme deficiency seen in the autosomal recessive disorder hereditary homocystinuria []. CBS can also serve as a redox sensor and interact with CO and NO, making it an important molecule in the intersection of the three gaseous signaling systems []. The enzyme is constitutively expressed in tissues and its expression can be modified through several post-translational modifications, such as sumoylation, glutathionylation, and phosphorylation [].
CSE, the second enzyme in the transsulfuration pathway, primarily catalyzes the conversion of cystathionine to cysteine [,]. This reaction serves as an important source for the amino acid cysteine, which is essential for the glutathione pool in various tissues []. CSE is not expressed constitutively and its expression can be induced by a range of oxidative stressors []. The promoter site for CSE has a binding site for nuclear factor erythroid 2-related factor (Nrf2), which is the master regulator for oxidative stress [,]. CSE can also undergo post-translational modifications similar to CBS, resulting in changes to its localization (e.g., nuclear localization) and function; however, the predominant control occurs at the transcriptional level []. Both CBS and CSE can catabolize cysteine to produce H2S as a byproduct.
3-MST is located both in the cytoplasmic and mitochondrial compartments of most mammalian tissues []. It is a sulfurtransferase enzyme that contains several redox-sensitive cysteine residues, which serve as important regulators of its function, and is unlike CBS and CSE, which are primarily regulated at the translational or post-translational level []. 3-MST is also an important H2S-generating enzyme. In the 3-MST-related pathway of H2S production, cysteine is initially converted through the enzyme cysteine aminotransferase to 3-mercaptopyruvate (3-MP), which acts as a substrate for 3-MST to produce an enzyme-bound persulfide, which in turn can give rise to H2S [].
3.2. H2S Metabolism
H2S metabolism is tightly controlled through the mitochondrial sulfide oxidation pathway, which acts as a bridge to the electron transport chain (ETC) at the level of complex III on the inner mitochondrial membrane []. H2S toxicity leads to uncoupling of the ETC through inhibition of cytochrome c oxidase (complex IV) []. Thus, H2S concentration inside the cell is maintained in a narrow range through an intricate balance between its biogenesis and degradation. H2S is initially oxidized to a persulfide inside the mitochondria by the enzyme sulfide quinone oxidoreductase (SQR) []. The persulfide is further oxidized by the enzyme persulfide dioxygenase (PDO) or ethylmalonic encephalopathy 1 protein (ETHE1) to further produce sulfite. The sulfite is then oxidized by rhodanese or sulfite oxidase in a tissue-specific manner to produce either thiosulfate (lung), sulfate (liver), or a mixture of thiosulfate and sulfate (kidney) [,]. Electrons produced through these reactions are transferred to complex III of the ETC through ubiquinone. H2S metabolism can thus drive oxidative phosphorylation and ATP synthesis in the mitochondria.
In the extracellular and vascular space, the interaction of H2S with metalloproteins, such as methemoglobin and metmyoglobin, can result in its clearance from the circulation by forming sulfheme products [,]. This pathway of H2S metabolism also marks an intersection between other gasotransmitter signaling pathways []. Another alternative mechanism for H2S clearance is methylation through the enzyme thiol-s-methyltransferase and mostly occurs in the cytoplasm; however, it is not as important as the other two mechanisms [].
4. Mechanisms of H2S Signaling
Over the years, several studies have characterized the versatile role of H2S as an important modulator of redox signaling pathways, having antiapoptotic and cytoprotective properties and being a regulator of the inflammatory response in several tissues and cell types [,]. Three main underlying mechanisms have been described for H2S: (a) direct antioxidant effect, (b) interaction with metalloproteins, and (c) post-translational modification of proteins (Figure 2). In recent years, additional novel pathways of H2S and its downstream signaling have been described; however, they are beyond the scope of this review [].
4.1. Direct Antioxidant Effect
Numerous studies have demonstrated that H2S can effectively interact with ROS and reactive nitrogen species (RNS) []. In fact, in many situations, H2S can scavenge reactive intermediates more efficiently than other antioxidants such as cysteine or GSH. H2S is particularly effective in juxtacrine signaling mechanisms given its gaseous state, allowing it to freely diffuse through lipid bilayers []. H2S also plays a role in GSH synthesis through positive feedback in several tissues (e.g., lung, brain, liver, and kidney) to protect against oxidative stress [,]. Furthermore, H2S can also increase the intracellular production of thioredoxin 1 (Trx1), which protects cells from oxidative injury and promotes peroxidase-dependent detoxification of hydrogen peroxide []. Nicholson et al. showed that H2S demonstrated cardioprotective effects through the upregulation of Trx1 []. H2S is a weak reductant and can scavenge free radicals such as superoxide (ROS) or peroxynitrite (RNS) directly []. Additionally, H2S can dissociate to form HS•−, which is a powerful reductant and can scavenge ROS/RNS effectively [,]. It is, however, worth mentioning that even though H2S can scavenge free radicals effectively, its low nanomolar concentration inside the cells does not compare to the impact of classical antioxidants such as GSH, which are present in micromolar concentrations [].
4.2. Interaction with Metalloproteins
H2S can interact with metal centers of metalloproteins, resulting in a reduction or covalent modification []. An important example of H2S–metalloprotein interaction is in the ETC with the enzyme cytochrome c oxidase (complex IV), which is the final electron acceptor. Cytochrome c oxidase uses electrons provided through cytochrome c to reduce oxygen into water []. The enzyme contains two copper and two iron centers. CO and NO can inhibit the enzyme through interaction with its metal centers, thus making them a catalytic site for crosstalk between these gasotransmitter pathways. H2S, on the other hand, has a biphasic dose-dependent interaction with cytochrome c oxidase such that at low concentrations (~3 µM), it promotes cellular respiration while irreversibly inhibiting it at higher levels (30–100 µM) []. H2S can also interact with cytochrome c at a low concentration, resulting in its reduction and subsequent formation of RSS, which can drive further redox reactions downstream []. The activity of soluble guanylate cyclase, which is essential for NO signaling, can be modified by H2S []. The heme iron in soluble guanylate cyclase is reduced by H2S, thus promoting NO binding and subsequent cyclic guanosine monophosphate (cGMP) synthesis, which is critical for vasodilation. As mentioned above, several other metalloproteins such as methemoglobin, metmyoglobin, and metneuroglobin serve as a reservoir for scavenging excess H2S, thus protecting tissues/cells from H2S toxicity []. Sulfheme products produced have a much lower affinity for oxygen, which prevents their oxidation and may subsequently be protective against the formation of atherosclerosis []. Finally, H2S can interact with zinc-containing proteins. H2S can inhibit androgen receptor activation by interacting with its zinc finger motif []. Additionally, H2S in low concentrations can inhibit phosphodiesterase 5, which is a zinc-containing enzyme []. H2S–zinc interaction is, however, understudied.
4.3. Post-Translational Modification of Proteins
H2S can modify critical cysteine residues on proteins through a process called persulfidation []. Persulfidation is a post-translational modification of proteins and can result in the alteration of protein structure and function. There are also low-molecular-weight persulfides (e.g., cysteine persulfide, glutathione persulfide) that serve as intermediate RSS products and demonstrate strong antioxidant and cytoprotective properties []. Low-molecular-weight persulfides are found in very low concentrations inside the cell. On the other hand, persulfidation of protein cysteine residues is a relatively common phenomenon in the cellular proteome []. Persulfidation is generally driven by the enzymes CBS and CSE and can be repressed by inhibiting these enzymes []. Interestingly, persulfidation is closely related to nitrosation, as one of the studies demonstrated that several cysteine targets in the proteome had an overlap of both processes []. On numerous occasions, persulfidation and nitrosation of the same cysteine residue exerted different or even opposite effects []. Several important proteins related to redox homeostasis of the cell are persulfidated, resulting in modulation of their function. We will discuss a few of them that are potentially important in the context of neonatal lung redox homeostasis. NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), which is an essential transcription factor for antiapoptotic activity, is activated by H2S through persulfidation of its p65 subunit at Cys38 []. Another transcription factor known as SP1 (specificity protein 1), which is a regulator of endothelial function, is persulfidated at multiple cysteine residues, which in turn modulates vascular endothelial growth factor (VEGF) and neuropilin-1 expression []. Finally, Nrf2, which is the master regulator of antioxidant response inside the cell, is also modulated by H2S through persulfidation []. Kelch-like ECH-associated protein 1 (Keap1) normally binds and retains Nrf2 in the cytoplasm, rendering it inactive. Recent studies have shown that H2S can persulfidate Keap1 at Cys151, resulting in the release of Nrf2 and causing its nuclear localization []. After Nrf2 enters the nucleus, it can activate antioxidant response elements (ARE) in promoters of genes, directly supporting activities of GSH and Trx superfamilies and heme oxygenase 1. Interestingly, recent studies suggest that this pathway of Nrf2 activation may be the primary underlying mechanism for upregulation of GSH and Trx by H2S rather than direct positive feedback, as was previously described [].
5. Current State of Antioxidant Therapy in Oxidative Neonatal Respiratory Disease
Bronchopulmonary dysplasia (BPD), characterized by an arrest in alveolar and vascular development, is the most common comorbidity in preterm infants [], affecting 30–60% of infants born very prematurely []. Even preterm infants without the diagnoses of BPD endure long-term and persistent pulmonary dysfunction in the form of repeated respiratory infections, recurrent wheezing disorders, and airway hyperreactivity [,].
5.1. Treatment Modalities
Studies observing the increased concentration of ROS in premature infants who develop BPD [] and genetic association studies linking polymorphisms of key redox enzymes and outcomes [], in addition to experimental animal models of BPD, collectively demonstrate the key role of ROS as modulators of lung disease and have triggered several attempts to ameliorate lung injury with the use of antioxidants [,]. Some therapies were aimed to augment nonenzymatic and enzymatic antioxidants as well as the use of exogenous administration of vitamins and micronutrients to scavenge ROS [,,]. One of the most studied is N-acetylcysteine (NAC), which acts as a Cys precursor and thiol donor in the glutathione (GSH) system and has been used with success in many other diseases such as chronic bronchitis and chronic obstructive pulmonary disease (COPD) [,]. In preterm infants, a double-blind placebo-controlled trial showed no impact on the severity or incidence of BPD nor improved lung function when administered intravenously []. Similarly, and despite strong preclinical data, agents such as superoxide dismutase (SOD), vitamin E, and others have been administered to babies, with perhaps only vitamin A showing the most positive but still modest result of decreasing oxygen requirement at 36 weeks [].
5.2. Possible Reasons for the Antioxidant Therapy Failures
Despite mixed and somewhat underwhelming results of clinical trials, much can be learned from them, and in the light of recent laboratory research findings, several speculations can be made to design successful future interventions. First, it is well established that the first week of life is when much of the lung injury occurs in the preterm infant [,,,]. The brisk shift from the intrauterine to the rather hostile but life-sustaining NICU (Neonatal Intensive Care Unit) environment leads to an immediate and long-lasting oxidative-stress-induced injury. Despite this observation, most interventions aimed to prevent lung disease began many hours up to several days after birth, missing this key treatment window. The experience with prenatal steroids, as perhaps the single and most effective way to prevent BPD [], indicates that we should design future interventions for even before the infant is born and focus research efforts in understanding maternal–fetal effects of new or known agents.
Another important characteristic of all preterm infants is the absence of the last trimester maternal–fetal nutritional transfer. This period, which preterm-born infants lack, is fundamental for building stores and supplies of many nutrients that are pivotal for antioxidant function. For example, selenium (Se) is an essential trace element that serves as a substrate for selenoproteins, including oxidoreductases such as glutathione peroxidases (GPx) and thioredoxin reductases (TrxR) []. Very preterm infants, those most likely develop BPD, are thought to be Se-deficient. Se supplementation was not enough to prevent BPD [], but coadministration of Se with an additional agent may be an attractive alternative.
A third aspect to consider is the possibility of agents that can upregulate the infants’ endogenous response rather than administrating an exogenous antioxidant. Exogenous antioxidants lack target specificity and have unknown bioavailability, making their effects difficult to predict or interpret. Aiming therapies toward cellular mechanisms that could enhance enzymatic systems and ‘prepare’ the preterm infant to the outside world must be considered. Nrf2 is one of those potential targets that has been explored in other etiologies but not in BPD []. As described above, Nrf2 induces antioxidant response genes via the activation of antioxidant response element (ARE) in the promoter/enhancer regions of target genes. Nrf2 plays a crucial role in executing the cellular response to oxidative injury and may provide an opportunity to directly prime endogenous antioxidant systems.
Nonetheless, the apparent failure of antioxidant therapy trials has also prompted the need to explore novel antioxidant mechanisms. We believe that exploring H2S and its downstream signaling pathway can fill a critical gap and uncover promising future targets for the therapy of neonatal respiratory diseases.
6. H2S in the Developing Lung and Neonatal Respiratory Diseases
Most of the studies with H2S in the lung have been done using models of adult lung diseases; however, recent studies have demonstrated an emerging role of H2S and sulfide signaling in the developing lung [] (Figure 3). Research across many species has demonstrated the presence of important H2S enzymes in different lung tissue compartments and the lung vasculature [,].
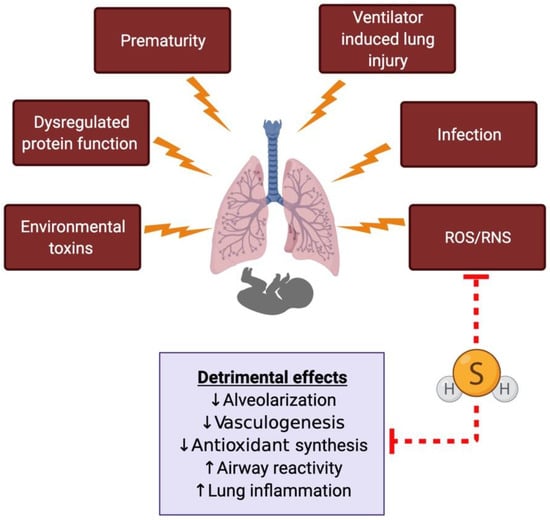
Figure 3.
The proposed role of hydrogen sulfide (H2S) in the developing lung and neonatal respiratory diseases. The developing neonatal lung is subjected to different kinds of insults, leading to oxidative stress-related damage. H2S can directly scavenge free radicals and has been shown to prevent or reverse some of the detrimental effects in animal models of neonatal respiratory diseases. ROS, reactive oxygen species; RNS, reactive nitrogen species.
Recently, Bartman et al. showed that the H2S machinery including the metabolizing enzymes (SQR, ETHE1) are present and functional in the human fetal airway smooth muscle cells []. The synthesizing enzymes (CBS and CSE) were, however, expressed in a lower amount in the fetal airway when compared to adults, suggesting decreased H2S production in the preterm airways. Decreased H2S would limit the ability of the preterm airway and lung to counter oxidative stress from hyperoxia- or ventilator-induced lung injury. Indeed, they found that the supplemental oxygen altered the expression of enzymes associated with H2S biogenesis and metabolism, which resulted in further blunted H2S production. Additionally, by using external H2S donors (e.g., NaHS or sodium hydrosulfide, GYY4137 or morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate), it was possible to reverse the detrimental effect of oxygen exposure on airway constriction response (i.e., airway reactivity), resulting in diminished intracellular calcium response to bronchoconstrictor agonists.
In an earlier study, Madurga et al. showed in a murine model of BPD that exogenous administration of H2S improved alveolarization and vascular growth following hyperoxia exposure []. In a later study, the same group showed that CBS is mainly expressed in the lung/airway epithelial cells and pulmonary vessels, whereas CSE is initially expressed predominantly in the lung parenchyma, and eventually its expression is upregulated in the airway []. Furthermore, CBS and CSE were found to play an important role in vasculogenesis during normal alveolar development. This is an intriguing finding and underscores the fact that the H2S machinery plays an important role in lung development in utero, which happens to be a relatively hypoxic environment []. Extrapolating from what we know from human fetal development and evolutionary remnants, this finding reiterates the evolutionary importance of H2S and its downstream signaling, which once used to be the predominant energy source in an environment that was severely hypoxic [].
H2S production is distinguished from the production of the other two gasotransmitters CO and NO since oxygen is not essential. H2S consumption, on the other hand, requires oxygen and is related to oxidative phosphorylation, which means H2S and oxygen concentrations in a system tend to be inversely proportional []. This was shown in a study with rat lungs in a hypoxic environment where the tissue concentration of H2S decreased swiftly as oxygen was added []. In this regard, H2S indirectly acts as an oxygen sensor in the tissues. One school of thought is that H2S is produced constitutively in tissues and its metabolism, specifically H2S clearance, depends on environmental oxygen tension []. However, it may not be that simple, as H2S-generating enzymes, especially CSE, are highly inducible and respond to various stimuli including oxidative stress []. This means normoxia (or hyperoxia) may initially upregulate H2S production, but given the rapid clearance in the presence of increased oxygen, the H2S levels in the tissues are still maintained in a low and narrow range. This is supported by a study that measured the urinary metabolite of H2S (thiosulfate) in term and preterm human infants as a measure of H2S turnover rate and found that the highest H2S turnover rate was seen in very preterm infants []. Given what is known about the relationship between H2S and oxygen, it is reasonable to assume that the H2S machinery is highly active in fetal life with a high concentration of H2S, which rapidly falls after birth following exposure to higher oxygen tension.
Several important mediators of normal lung development and angiogenesis—such as hypoxia-inducible factor-1 alpha (HIF-1α) and vascular endothelial growth factor (VEGF)—are known to be sensitive to changes in oxygen tension []. Interestingly, both these mediators are directly or indirectly modulated through H2S and its downstream signaling. An elegant study published recently demonstrated that CBS modulates HIF-1α stability through persulfidation of its inhibitor []. Further, H2S can stabilize the transcription factor SP1 through persulfidation, which in turn modulates the expression of VEGF receptor 2 []. As described above, H2S can regulate Nrf2 activation and its downstream antioxidant response inside the cell [,]. Given the emerging roles of Nrf2 and associated GSH and Trx superfamilies in BPD [,,,,], it would be interesting to explore the crosstalk between H2S and the Nrf2 signaling pathways.
H2S has also been shown to have protective effects on the lung in studies using ventilator-induced lung injury models []. Exogenous administration of an H2S donor was shown to be protective to both mouse and rat lungs when used prophylactically or during ventilation [,]. In animal models of adult COPD using environmental toxin exposure (cigarette smoke), H2S was shown to be protective (Figure 3) [,]. H2S and sulfide signaling has been explored in numerous animal models of airway hyperreactivity and asthma [,,]. In population-based studies, H2S levels in different body fluids (sputum, serum) correlated with the degree of airway inflammation in both children and adults [,]. Additionally, exhaled H2S is a marker for airway inflammation in asthmatic patients []. All these studies suggest a role of H2S as a biomarker for the severity of respiratory diseases.
Finally, loss of the H2S-generating enzyme CSE in an airway epithelial cell model for the respiratory syncytial virus (RSV) increased the severity of the infection, augmented inflammatory damage, and worsened airway reactivity [,]. H2S has also been touted as a prophylactic and/or therapeutic agent against the novel SARS-CoV-2 and a potential biomarker for COVID-19 disease severity [,,].
7. Future Direction
Preterm infants deficient in hepatic CSE activity are already prone to impaired transsulfuration [,], and human fetal airway smooth muscle cells have decreased transcript and protein expression of H2S-synthesizing (CBS, CSE) and -metabolizing (SQRDL, ETHE1) enzymes compared to adult cells []. H2S production was further blunted in fetal airway smooth muscle cells by 40% hyperoxia after two days in culture []. This raises a strong interest in therapeutic opportunities targeting H2S for oxidative neonatal respiratory disease, along with data supporting that H2S synthesis is required for proper programming of perinatal alveolarization [] and that H2S donors attenuate hyperoxic lung injury in neonatal rodents [,]. Therapeutic targeting of H2S is possible via donors, amplification of endogenous H2S synthesis, or direct delivery of the gasotransmitter to the lung via the trachea. A recent review published on this subject paints a similar picture regarding an experimental approach to neonatal respiratory diseases using H2S []. Sulfide signaling is an evolutionary conserved pathway, which presents an exciting prospect to uncover promising targets for future therapies. H2S machinery is endogenously present and active, which may suggest a lower probability of detrimental off-target effects with optimal bioavailability. However, upon reflection on lessons learned from failed antioxidant therapies, there are still several key issues that require a greater depth of investigation before considering H2S-targeted therapeutic opportunities for oxidative neonatal respiratory disease: cell-specific physiologies, identification of molecular networks modified through persulfidation of protein cysteine thiols, intersection with other redox-dedicated pathways, dynamic changes as a function of developmental timing, and influences of prematurity and/or hyperoxic injury on the aforementioned molecular and cellular events.
Systemic targeting of H2S is a common limitation for highly encouraging studies demonstrating preclinical efficacy of H2S donors in experimental hyperoxic rodent models of BPD [,] and CBS and CSE promotion of perinatal alveolarization using whole-body knockout mice []. H2S biology is complex with cell-specific and context-dependent effects. Similar spatial expression patterns of CBS and CSE in airway epithelium and vessel walls (colocalization with smooth muscle actin) were detected in neonatal mouse and human lung samples [,]. This is in relative agreement with single-cell RNA sequencing data from LungMAP demonstrating murine pulmonary epithelial, endothelial, and fibroblast expression of CBS, CSE, and 3-MST across late embryonic and neonatal ages []. The cell-specific influence of H2S biosynthesis enzymes in the contexts of mammalian lung development and experimental models of oxidative neonatal respiratory disease require further investigation, since resulting data could be critical for determination of preferred therapeutic strategy.
Furthermore, taking a systems-level approach to H2S biology is likely to reveal ubiquitous and cell-specific functions of H2S. First, H2S biosynthesis and metabolism enzymes could be differentially regulated in a cell-specific manner. CBS and CSE activities are influenced by several different post-translational modifications []. Akt increased catalytic activity of CSE through direct binding and phosphorylation in liver sinusoidal cells []. Interestingly, treatment of neonatal mice treated with an H2S donor during 85% oxygen exposure for the first ten days of life had increased Akt activation (phosphorylation) in the whole lung []. Although H2S donors caused similar Akt activation in mouse primary alveolar type II cells and MLE-12 cells, H2S-induced Akt activation was oxygen-dependent in vivo. While it is unknown if Akt activation augments endogenous H2S synthesis through CSE phosphorylation, this further underscores the need to investigate cell-specific and context-dependent H2S biology and sulfide signaling. Second, it is likely that H2S-dependent protein cysteine thiol persulfidation targets different molecular networks and/or has different kinetics across various cell types. It is possible to map H2S protein networks by identifying S-sulfhydration of protein cysteine thiols using a maleimide assay []. This biochemical approach would give better mechanistic context to understanding H2S-mediated effects and could identify additional novel therapeutic targets downstream of H2S. Lastly, it is important to consider how H2S-depenedent processes intersect with other redox-dedicated pathways to coordinate cellular and physiological outcomes. Although data are conflicting, it is clear that H2S influences NO and GSH pathways, which can in turn also regulate H2S processes [].
One powerful approach that should be employed to delineate cell-specific H2S effects is to ablate or modify genes encoding for H2S-synthesizing enzymes using Cre-mediated recombination in the mouse. There is a plethora of mouse strains that have been engineered to express Cre recombinase in lung using epithelial, endothelial, fibroblast, and smooth muscle-specific promoters []. This includes a subset of tetracycline- and tamoxifen-inducible systems for temporal control of Cre recombinase. Conditional control of Cre-dependent genetic ablation or modification of H2S-synthesizing enzymes initiated during precise embryonic and perinatal developmental windows, as well as during hyperoxic exposure, would allow for temporal mapping of systems-level H2S-dependent biochemical and molecular processes. However, there are several limitations and caveats regarding spatial expression of Cre recombinase, recombination efficiency, and off-target effects. One last limitation, specifically influencing generation of double- and triple-transgenic mouse lines, is strain-dependent redox responses. Inbred mouse strains have differential responses to hyperoxic lung injury [,], and it is possible that hyperoxic pathologies in the widely popular C57Bl/6J strain are influenced by a mutation impairing mitochondrial NADPH synthesis []. NADPH provides reducing potential for both glutathione and thioredoxin antioxidant systems. Although there is optimism and enthusiasm for H2S-mediated therapeutic opportunities in neonatal oxidative respiratory disease, additional depth of knowledge on H2S biology and factors influencing H2S activities in a cell-specific context is needed, otherwise future clinical trials could meet a similar fate of failed antioxidant therapies.
8. Conclusions
H2S and its downstream signaling pathway are emerging as novel and important players in lung development. Recent studies have demonstrated that exploring H2S signaling pathways can uncover promising therapeutic targets for acute and chronic respiratory diseases associated with prematurity. While exogenous H2S donors have primarily been used to treat or prevent lung injury, it is not completely clear how the endogenous H2S pathway and enzymes are regulated. Future studies should focus on exploring the regulation and signaling of endogenous H2S machinery in the context of oxidative neonatal respiratory disease. Additionally, sulfide signaling, being an ancient pathway, also carries importance from an evolutionary perspective. Evidence, although limited, does suggest highly active H2S machinery in premature babies, much like in the Archean era, when H2S served as the major energy source for the extant organisms. More innovative studies using novel in vitro models such as embryonic lung explant cultures and lung organoid cultures are needed to gain a clear understanding of this intriguing phenomenon.
Author Contributions
A.G. conceived the idea for the manuscript. A.G., P.F.V. and G.O. prepared the manuscript. A.G. created the figures with BioRender.com. All authors have read and agreed to the published version of the manuscript.
Funding
P.F.V. was supported by grant R01HL135112 from the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sánchez-Illana, A.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Auten, R.L. Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal. Neonatal. Med. 2010, 15, 191–195. [Google Scholar] [CrossRef]
- Berkelhamer, S.K.; Farrow, K.N. Developmental Regulation of Antioxidant Enzymes and Their Impact on Neonatal Lung Disease. Antioxid. Redox Signal. 2014, 21, 1837–1848. [Google Scholar] [CrossRef]
- Ofman, G.; Tipple, T.E. Antioxidants & bronchopulmonary dysplasia: Beating the system or beating a dead horse? Free Radic. Biol. Med. 2019, 142, 138–145. [Google Scholar] [PubMed]
- Kimura, H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Madurga, A.; Golec, A.; Pozarska, A.; Ishii, I.; Mizikova, I.; Nardiello, C.; Vadász, I.; Herold, S.; Mayer, K.; Reichenberger, F.; et al. The H2S-generating enzymes cystathionine beta-synthase and cystathionine gamma-lyase play a role in vascular development during normal lung alveolarization. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L710–L724. [Google Scholar] [CrossRef]
- Madurga, A.; Mižíková, I.; Ruiz-Camp, J.; Vadász, I.; Herold, S.; Mayer, K.; Fehrenbach, H.; Seeger, W.; Morty, R.E. Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L684–L697. [Google Scholar] [CrossRef]
- Bartman, C.M.; Schiliro, M.; Helan, M.; Prakash, Y.S.; Linden, D.; Pabelick, C. Hydrogen sulfide, oxygen, and calcium regulation in developing human airway smooth muscle. FASEB J. 2020, 34, 12991–13004. [Google Scholar] [CrossRef]
- Olson, K.R. Reactive oxygen species or reactive sulfur species: Why we should consider the latter. J. Exp. Biol. 2020, 223, jeb196352. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Genet. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Cruaud, P.; Alsop, E.; De Rezende, J.R.; Head, I.M.; Tsesmetzis, N. Beyond the tip of the iceberg; a new view of the diversity of sulfite- and sulfate-reducing microorganisms. ISME J. 2018, 12, 2096–2099. [Google Scholar] [CrossRef]
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2016, 31, 60–72. [Google Scholar] [CrossRef]
- Barley, M.; Bekker, A.; Krapez, B. Late Archean to Early Paleoproterozoic global tectonics, environmental change and the rise of atmospheric oxygen. Earth Planet. Sci. Lett. 2005, 238, 156–171. [Google Scholar] [CrossRef]
- Holland, H.D. The oxygenation of the atmosphere and oceans. Philos. Trans. R Soc. Lond. B Biol. Sci. 2006, 361, 903–915. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Kevil, C.G. Reactive Sulfur Species: A New Redox Player in Cardiovascular Pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Biological Production, Detection, and Fate of Hydrogen Peroxide. Antioxid. Redox Signal. 2018, 29, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Pluth, M.D. Reactive sulfur species (RSS): Persulfides, polysulfides, potential, and problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Nasim, M.J.; Ali, W.; Jacob, C. The Reactive Sulfur Species Concept: 15 Years On. Antioxidants 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, R.; Wu, L.; Yang, G. Hydrogen sulfide signaling in regulation of cell behaviors. Nitric Oxide 2020, 103, 9–19. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen Sulfide: From Brain to Gut. Antioxid. Redox Signal. 2010, 12, 1111–1123. [Google Scholar] [CrossRef]
- Giuffrè, A.; Tomé, C.S.; Fernandes, D.G.F.; Zuhra, K.; Vicente, J.B. Hydrogen Sulfide Metabolism and Signaling in the Tumor Microenvironment. Clin. Biol. Mol. Asp. Covid-19 2020, 1219, 335–353. [Google Scholar]
- de Cabo, R.; Diaz-Ruiz, A. A Central Role for the Gasotransmitter H2S in Aging. Cell Metab. 2020, 31, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Xu, A.; Chen, Y.; Chen, X.; Li, Y.; Wang, W. Protective effect of a hydrogen sulfide donor on balloon injury-induced restenosis via the Nrf2/HIF-1α signaling pathway. Int. J. Mol. Med. 2019, 43, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Bazhanov, N.; Ansar, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Hydrogen Sulfide: A Novel Player in Airway Development, Pathophysiology of Respiratory Diseases, and Antiviral Defenses. Am. J. Respir. Cell Mol. Biol. 2017, 57, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Kabil, O.; Banerjee, R. High Turnover Rates for Hydrogen Sulfide Allow for Rapid Regulation of Its Tissue Concentrations. Antioxid. Redox Signal. 2012, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Searcy, D.G.; Lee, S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998, 282, 310–322. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharm. 2018, 176, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Wu, B.; Zhao, K.; Yang, G.; Wu, L.; Wang, R. Oxygen-sensitive mitochondrial accumulation of cystathionine β-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA 2013, 110, 12679–12684. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Ito, T.; Kitamura, H.; Nishino, T. Tissue and subcellular distribution of mercaptopyruvate sulfurtransferase in the rat: Confocal laser fluorescence and immunoelectron microscopic studies combined with biochemical analysis. Histochem. Cell Biol. 1998, 110, 243–250. [Google Scholar] [CrossRef]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-beta-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef]
- Mudd, S.H.; Finkelstein, J.D.; Irreverre, F.; Laster, L. Homocystinuria: An Enzymatic Defect. Science 1964, 143, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Zou, C.G. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: A PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The Quantitatively Important Relationship between Homocysteine Metabolism and Glutathione Synthesis by the Transsulfuration Pathway and Its Regulation by Redox Changes. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef]
- Akiyama, M.; Unoki, T.; Shinkai, Y.; Ishii, I.; Ida, T.; Akaike, T.; Yamamoto, M.; Kumagai, Y. Environmental Electrophile-Mediated Toxicity in Mice Lacking Nrf2, CSE, or Both. Environ. Heal. Perspect. 2019, 127, 067002. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects Against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Banerjee, R. Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine beta-synthase sumoylation. PLoS ONE 2008, 3, e4032. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and Kinetic Analysis of H2S Production by Human Mercaptopyruvate Sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Yoshii, T.; Abe, Y.; Matsumura, T. Thioredoxin-dependent Enzymatic Activation of Mercaptopyruvate Sulfurtransferase. J. Biol. Chem. 2007, 282, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sulfurtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Enzymology of H2S Biogenesis, Decay and Signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef]
- Bouillaud, F.; Blachier, F. Mitochondria and Sulfide: A Very Old Story of Poisoning, Feeding, and Signaling? Antioxid. Redox Signal. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Szczesny, B.; Marcatti, M.; Zatarain, J.R.; Druzhyna, N.; Wiktorowicz, J.E.; Nagy, P.; Hellmich, M.R.; Szabo, C. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci. Rep. 2016, 6, 36125. [Google Scholar] [CrossRef]
- Merz, T.; Vogt, J.A.; Wachter, U.; Calzia, E.; Szabo, C.; Wang, R.; Radermacher, P.; McCook, O. Impact of hyperglycemia on cystathionine-γ-lyase expression during resuscitated murine septic shock. Intensiv. Care Med. Exp. 2017, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Yadav, P.K.; Kurthen, A.; Banerjee, R. Sulfide Oxidation by a Noncanonical Pathway in Red Blood Cells Generates Thiosulfate and Polysulfides. J. Biol. Chem. 2015, 290, 8310–8320. [Google Scholar] [CrossRef] [PubMed]
- Bostelaar, T.; Vitvitsky, V.; Kumutima, J.; Lewis, B.E.; Yadav, P.K.; Brunold, T.C.; Filipovic, M.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. Hydrogen Sulfide Oxidation by Myoglobin. J. Am. Chem. Soc. 2016, 138, 8476–8488. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Whiteman, M. Hydrogen sulfide signaling: Interactions with nitric oxide and reactive oxygen species. Ann. N. Y. Acad. Sci. 2015, 1365, 5–14. [Google Scholar] [CrossRef]
- Bełtowski, J. Synthesis, Metabolism, and Signaling Mechanisms of Hydrogen Sulfide: An Overview. Methods Mol. Biol. 2019, 2007, 1–8. [Google Scholar] [PubMed]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef]
- Wedmann, R.; Bertlein, S.; Macinkovic, I.; Böltz, S.; Miljkovic, J.; Muñoz, L.E.; Herrmann, M.; Filipovic, M.R. Working with “H2S”: Facts and apparent artifacts. Nitric Oxide 2014, 41, 85–96. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Miljkovic, J.; Allgäuer, A.; Chaurio, R.; Shubina, T.; Herrmann, M.; Ivanovic-Burmazovic, I. Biochemical insight into physiological effects of H₂S: Reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem. J. 2012, 441, 609–621. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.-Y.; Liu, Z.-W.; Fu, Y.; Zhao, B. Effect of endogenous hydrogen sulfide on oxidative stress in oleic acid-induced acute lung injury in rats. Chin. Med. J. 2011, 124, 3476–3480. [Google Scholar] [PubMed]
- Xie, Z.-Z.; Liu, Y.; Bian, J.-S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxidative Med. Cell. Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Nicholson, C.K.; Lambert, J.P.; Molkentin, J.D.; Sadoshima, J.; Calvert, J.W. Thioredoxin 1 Is Essential for Sodium Sulfide–Mediated Cardioprotection in the Setting of Heart Failure. Arter. Thromb. Vasc. Biol. 2013, 33, 744–751. [Google Scholar] [CrossRef]
- Xiao, L.; Dong, J.-H.; Jing-Hui, D.; Xue, H.-M.; Guo, Q.; Teng, X.; Wu, Y.-M. Hydrogen Sulfide Improves Endothelial Dysfunction via Downregulating BMP4/COX-2 Pathway in Rats with Hypertension. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Al-Magableh, M.R.; Kemp-Harper, B.K.; Ng, H.H.; Miller, A.A.; Hart, J.L. Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 387, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen Sulfide in Biochemistry and Medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Haouzi, P.; Klingerman, C.M. Fate of intracellular H2S/HS− and metallo-proteins. Respir. Physiol. Neurobiol. 2013, 188, 229–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshikawa, S.; Shimada, A. Reaction mechanism of cytochrome c oxidase. Chem. Rev. 2015, 115, 1936–1989. [Google Scholar] [CrossRef] [PubMed]
- Módis, K.; Panopoulos, P.; Coletta, C.; Papapetropoulos, A.; Szabo, C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem. Pharm. 2013, 86, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Vitvitsky, V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; et al. Cytochrome c Reduction by H2S Potentiates Sulfide Signaling. ACS Chem. Biol. 2018, 13, 2300–2307. [Google Scholar] [CrossRef]
- Zhou, Z.; Martin, E.; Sharina, I.; Esposito, I.; Szabo, C.; Bucci, M.; Cirino, G.; Papapetropoulos, A. Regulation of soluble guanylyl cyclase redox state by hydrogen sulfide. Pharm. Res. 2016, 111, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Potor, L.; Mehes, G.; Hendrik, Z.; Jeney, V.; Pethő, D.; Vasas, A.; Pálinkás, Z.; Balogh, E.; Gyetvai, A.; Whiteman, M.; et al. Hydrogen Sulfide Abrogates Hemoglobin-Lipid Interaction in Atherosclerotic Lesion. Oxidative Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Zhao, K.; Li, S.; Wu, L.; Lai, C.; Yang, G. Hydrogen Sulfide Represses Androgen Receptor Transactivation by Targeting at the Second Zinc Finger Module. J. Biol. Chem. 2014, 289, 20824–20835. [Google Scholar] [CrossRef]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen Sulfide is an Endogenous Inhibitor of Phosphodiesterase Activity. Arter. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef]
- Yang, C.-T.; Devarie-Baez, N.O.; Hamsath, A.; Fu, X.-D.; Xian, M. S-Persulfidation: Chemistry, Chemical Biology, and Significance in Health and Disease. Antioxid. Redox Signal. 2020, 33, 1092–1114. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, K.; He, J.; Tian, C.; Yu, X.; Yang, J. Direct Proteomic Mapping of Cysteine Persulfidation. Antioxid. Redox Signal. 2020, 33, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-H.; Krokowski, D.; Guan, B.-J.; Bederman, I.R.; Majumder, M.; Parisien, M.; Diatchenko, L.; Kabil, O.; Willard, B.; Banerjee, R.; et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. eLife 2015, 4, e10067. [Google Scholar] [CrossRef]
- Vandiver, M.S.; Paul, B.D.; Xu, R.; Karuppagounder, S.S.; Rao, F.; Snowman, A.M.; Ko, H.S.; Lee, Y.I.; Dawson, V.L.; Dawson, T.M.; et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell. 2012, 45, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chakraborty, P.K.; Xiong, X.; Dwivedi, S.K.; Mustafi, S.B.; Leigh, N.R.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathionine β-synthase regulates endothelial function via protein S-sulfhydration. FASEB J. 2016, 30, 441–456. [Google Scholar] [CrossRef]
- Zhao, S.; Song, T.; Gu, Y.; Zhang, Y.; Cao, S.; Miao, Q.; Zhang, X.; Chen, H.; Gao, Y.; Zhang, L.; et al. Hydrogen sulfide alleviates liver injury via S-sulfhydrated-Keap1/Nrf2/LRP1 pathway. Hepatology 2020, 73, 282–302. [Google Scholar] [CrossRef]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef]
- Filbrun, A.G.; Popova, A.P.; Linn, M.J.; McIntosh, N.A.; Hershenson, M.B. Longitudinal measures of lung function in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 2010, 46, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Martin, R.J. Vulnerability of the developing airway. Respir. Physiol. Neurobiol. 2019, 270, 103263. [Google Scholar] [CrossRef] [PubMed]
- Vento, M.; Moro, M.; Escrig, R.; Arruza, L.; Villar, G.; Izquierdo, I.; Roberts, L.J.; Arduini, A.; Escobar, J.J.; Sastre, J.; et al. Preterm Resuscitation With Low Oxygen Causes Less Oxidative Stress, Inflammation, and Chronic Lung Disease. Pediatrics 2009, 124, e439–e449. [Google Scholar] [CrossRef] [PubMed]
- Huizing, M.J.; Cavallaro, G.; Moonen, R.M.; González-Luis, G.E.; Mosca, F.; Vento, M.; Villamor, E. Is the C242T Polymorphism of the CYBA Gene Linked with Oxidative Stress-Associated Complications of Prematurity? Antioxid. Redox Signal. 2017, 27, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Parinandi, N.L.; Kleinberg, M.A.; Usatyuk, P.V.; Cummings, R.J.; Pennathur, A.; Cardounel, A.J.; Zweier, J.L.; Garcia, J.G.N.; Natarajan, V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L26–L38. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.A. DNA damage and cell cycle checkpoints in hyperoxic lung injury: Braking to facilitate repair. Am. J. Physiol. Cell. Mol. Physiol. 2001, 281, L291–L305. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.E.; Wright, L.L.; Oh, W.; Kennedy, K.A.; Mele, L.; Ehrenkranz, R.A.; Stoll, B.J.; Lemons, J.A.; Stevenson, D.K.; Bauer, C.R.; et al. Vitamin A Supplementation for Extremely-Low-Birth-Weight Infants. N. Engl. J. Med. 1999, 340, 1962–1968. [Google Scholar] [CrossRef]
- Ahola, T.; Lapatto, R.; Raivio, K.O.; Selander, B.; Stigson, L.; Jónsson, B.; Jonsbo, F.; Esberg, G.; Stövring, S.; Kjartansson, S.; et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: A randomized controlled trial. J. Pediatr. 2003, 143, 713–719. [Google Scholar] [CrossRef]
- Davis, J.M.; Rosenfeld, W.N.; Richter, S.E.; Parad, R.; Gewolb, I.H.; Spitzer, A.R.; Carlo, W.A.; Couser, R.J.; Price, A.; Flaster, E.; et al. Safety and Pharmacokinetics of Multiple Doses of Recombinant Human CuZn Superoxide Dismutase Administered Intratracheally to Premature Neonates With Respiratory Distress Syndrome. Pediatrics 1997, 100, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Stey, C.; Steurer, J.; Bachmann, S.; Medici, T.; Tramèr, M. The effect of oral N-acetylcysteine in chronic bronchitis: A quantitative systematic review. Eur. Respir. J. 2000, 16, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.N.; Tseng, C.Z.S. Update on the pathological processes, molecular biology, and clinical utility of N-acetylcysteine in chronic obstructive pulmonary disease. Int. J. Chronic. Obs. Pulm. Dis. 2014, 9, 825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chessex, P.; Watson, C.; Kaczala, G.W.; Rouleau, T.; Lavoie, M.-È.; Friel, J.; Lavoie, J.-C. Determinants of oxidant stress in extremely low birth weight premature infants. Free Radic. Biol. Med. 2010, 49, 1380–1386. [Google Scholar] [CrossRef]
- Joung, K.E.; Kim, H.-S.; Lee, J.; Shim, G.H.; Choi, C.W.; Kim, E.-K.; Kim, B.I.; Choi, J.-H. Correlation of urinary inflammatory and oxidative stress markers in very low birth weight infants with subsequent development of bronchopulmonary dysplasia. Free Radic. Res. 2011, 45, 1024–1032. [Google Scholar] [CrossRef]
- Perrone, S.; Tataranno, M.L.; Negro, S.; Longini, M.; Marzocchi, B.; Proietti, F.; Iacoponi, F.; Capitani, S.; Buonocore, G. Early identification of the risk for free radical-related diseases in preterm newborns. Early Hum. Dev. 2010, 86, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Watterberg, K.L.; Demers, L.M.; Scott, S.M.; Murphy, S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics 1996, 97, 210–215. [Google Scholar] [PubMed]
- Roberts, D.; Brown, J.; Medley, N.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2017, 3, CD004454. [Google Scholar] [CrossRef] [PubMed]
- Tindell, R.; Tipple, T. Selenium: Implications for outcomes in extremely preterm infants. J. Perinatol. 2018, 38, 197–202. [Google Scholar] [CrossRef]
- Darlow, B.A.; Austin, N. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst. Rev. 2003, CD003312. [Google Scholar] [CrossRef]
- Amata, E.; Pittalà, V.; Marrazzo, A.; Parenti, C.; Prezzavento, O.; Arena, E.; Nabavi, S.M.; Salerno, L. Role of the Nrf2/HO-1 axis in bronchopulmonary dysplasia and hyperoxic lung injuries. Clin. Sci. 2017, 131, 1701–1712. [Google Scholar] [CrossRef]
- Olson, K.R.; Whitfield, N.L.; Bearden, S.E.; Leger, J.S.; Nilson, E.; Gao, Y.; Madden, J.A. Hypoxic pulmonary vasodilation: A paradigm shift with a hydrogen sulfide mechanism. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R51–R60. [Google Scholar] [CrossRef]
- Liu, T.; Mukosera, G.T.; Blood, A.B. The role of gasotransmitters in neonatal physiology. Nitric Oxide 2020, 95, 29–44. [Google Scholar] [CrossRef]
- Olson, K.R. Hydrogen sulfide as an oxygen sensor. Antioxid. Redox Signal. 2015, 22, 377–397. [Google Scholar] [CrossRef]
- Madden, J.A.; Ahlf, S.B.; Dantuma, M.W.; Olson, K.R.; Roerig, D.L. Precursors and inhibitors of hydrogen sulfide synthesis affect acute hypoxic pulmonary vasoconstriction in the intact lung. J. Appl. Physiol. 2012, 112, 411–418. [Google Scholar] [CrossRef]
- Dyson, R.M.; Palliser, H.K.; Latter, J.L.; Chwatko, G.; Glowacki, R.; Wright, I.M.R. A Role for H2S in the Microcirculation of Newborns: The Major Metabolite of H2S (Thiosulphate) is Increased in Preterm Infants. PLoS ONE 2014, 9, e105085. [Google Scholar] [CrossRef]
- Hilgendorff, A.; Reiss, I.; Ehrhardt, H.; Eickelberg, O.; Alvira, C.M. Chronic Lung Disease in the Preterm Infant: Lessons Learned From Animal Models. Am. J. Respir. Cell Mol. Biol. 2013, 50, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Prabhudesai, S.; Zhang, Y.; Rao, G.; Thirugnanam, K.; Hossen, M.N.; Dwivedi, S.K.D.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathione beta-synthase regulates HIF-1alpha stability through persulfidation of PHD2. Sci. Adv. 2020, 6, eaaz8534. [Google Scholar] [CrossRef] [PubMed]
- Floen, M.J.; Forred, B.J.; Bloom, E.J.; Vitiello, P.F. Thioredoxin-1 redox signaling regulates cell survival in response to hyperoxia. Free Radic. Biol. Med. 2014, 75, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Forred, B.J.; Daugaard, D.R.; Titus, B.K.; Wood, R.R.; Floen, M.J.; Booze, M.L.; Vitiello, P.F. Detoxification of Mitochondrial Oxidants and Apoptotic Signaling Are Facilitated by Thioredoxin-2 and Peroxiredoxin-3 during Hyperoxic Injury. PLoS ONE 2017, 12, e0168777. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wall, S.B.; Ren, C.; Velten, M.; Hill, C.L.; Locy, M.L.; Rogers, L.K.; Tipple, T.E. Thioredoxin Reductase Inhibition Attenuates Neonatal Hyperoxic Lung Injury and Enhances Nuclear Factor E2–Related Factor 2 Activation. Am. J. Respir. Cell Mol. Biol. 2016, 55, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Tipple, T.E. The Thioredoxin System in Neonatal Lung Disease. Antioxid. Redox Signal. 2014, 21, 1916–1925. [Google Scholar] [CrossRef]
- Faller, S.; Ryter, S.W.; Choi, A.M.K.; Loop, T.; Schmidt, R.; Hoetzel, A. Inhaled Hydrogen Sulfide Protects against Ventilator-induced Lung Injury. Anesthesiology 2010, 113, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Spassov, S.; Pfeifer, D.; Strosing, K.; Ryter, S.; Hummel, M.; Faller, S.; Hoetzel, A. Genetic Targets of Hydrogen Sulfide in Ventilator-Induced Lung Injury—A Microarray Study. PLoS ONE 2014, 9, e102401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ge, X.; Sun, J.; Fei, A.; Gao, C.; Pan, S.; Wu, Z. Hydrogen sulfide treatment alleviated ventilator-induced lung injury through regulation of autophagy and endoplasmic reticulum stress. Int. J. Biol. Sci. 2019, 15, 2872–2884. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, J.; Li, D.; Li, Z.; Liu, H.; Ding, M.; Cai, Z.; Liang, X.; Yang, Q.; Long, Z.; et al. Hydrogen sulfide inhibits cigarette smoke-induced inflammation and injury in alveolar epithelial cells by suppressing PHD2/HIF-1α/MAPK signaling pathway. Int. Immunopharmacol. 2020, 81, 105979. [Google Scholar] [CrossRef]
- Lin, F.; Liao, C.; Sun, Y.; Zhang, J.; Lu, W.; Bai, Y.; Liao, Y.; Li, M.; Ni, X.; Hou, Y.; et al. Hydrogen Sulfide Inhibits Cigarette Smoke-Induced Endoplasmic Reticulum Stress and Apoptosis in Bronchial Epithelial Cells. Front. Pharm. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, P.; Yang, G.; Cao, Q.; Wang, R. The Inhibitory Role of Hydrogen Sulfide in Airway Hyperresponsiveness and Inflammation in a Mouse Model of Asthma. Am. J. Pathol. 2013, 182, 1188–1195. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, R.; Geng, B.; Qi, Y.-F.; Wang, P.-P.; Yao, W.-Z.; Tang, C.-S. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 2009, 45, 117–123. [Google Scholar] [CrossRef]
- Mendes, J.A.; Ribeiro, M.C.; Filho, G.J.R.; Rocha, T.; Muscará, M.N.; Costa, S.K.; Ferreira, H.H. Hydrogen sulfide inhibits apoptosis and protects the bronchial epithelium in an allergic inflammation mice model. Int. Immunopharmacol. 2019, 73, 435–441. [Google Scholar] [CrossRef]
- Saito, J.; Zhang, Q.; Hui, C.; Macedo, P.; Gibeon, D.; Menzies-Gow, A.; Bhavsar, P.K.; Chung, K.F. Sputum hydrogen sulfide as a novel biomarker of obstructive neutrophilic asthma. J. Allergy Clin. Immunol. 2013, 131, 232–234.e3. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, Y.; Lu, Y.-Q.; Yan, M.; Jiang, Y.-H.; Zhao, D.-Y. Correlation between serum H2S and pulmonary function in children with bronchial asthma. Mol. Med. Rep. 2012, 6, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Chen, Y.; Yao, W. Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology 2014, 19, 1165–1169. [Google Scholar] [CrossRef]
- Ivanciuc, T.; Sbrana, E.; Ansar, M.; Bazhanov, N.; Szabo, C.; Casola, A.; Garofalo, R.P. Hydrogen Sulfide Is an Antiviral and Antiinflammatory Endogenous Gasotransmitter in the Airways. Role in Respiratory Syncytial Virus Infection. Am. J. Respir. Cell Mol. Biol. 2016, 55, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.; Ma, Y.; Escaffre, O.; Ivanciuc, T.; Komaravelli, N.; Kelley, J.P.; Coletta, C.; Szabo, C.; Rockx, B.; Garofalo, R.P.; et al. Role of Hydrogen Sulfide in Paramyxovirus Infections. J. Virol. 2015, 89, 5557–5568. [Google Scholar]
- Yang, G. H2S as a potential defense against COVID-19? Am. J. Physiol. Physiol. 2020, 319, C244–C249. [Google Scholar] [CrossRef] [PubMed]
- Renieris, G.; Katrini, K.; Damoulari, C.; Akinosoglou, K.; Psarrakis, C.; Kyriakopoulou, M.; Dimopoulos, G.; Lada, M.; Koufargyris, P.; Giamarellos-Bourboulis, E.J. Serum Hydrogen Sulfide and Outcome Association in Pneumonia by the SARS-CoV-2 Coronavirus. Shock 2020, 54, 633–637. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Brancaleone, V.; Brogi, S.; Gojon, G.; Montanaro, R.; Morales, G.; Testai, L.; Calderone, V. Anti-inflammatory and antiviral roles of hydrogen sulfide: Rationale for considering H 2 S donors in COVID-19 therapy. Br. J. Pharm. 2020, 177, 4931–4941. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.H.; Anderson, G.H. The Development of Cystathionase Activity during the First Year of Life. Pediatr. Res. 1982, 16, 65–68. [Google Scholar] [CrossRef]
- Viña, J.; Vento, M.; García-Sala, F.; Puertes, I.R.; Gascó, E.; Sastre, J.; Asensi, M.; Pallardó, F.V. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am. J. Clin. Nutr. 1995, 61, 1067–1069. [Google Scholar] [CrossRef]
- Vadivel, A.; Alphonse, R.S.; Ionescu, L.; Machado, D.S.; O’Reilly, M.; Eaton, F.; Haromy, A.; Michelakis, E.D.; Thébaud, B. Exogenous Hydrogen Sulfide (H2S) Protects Alveolar Growth in Experimental O2-Induced Neonatal Lung Injury. PLoS ONE 2014, 9, e90965. [Google Scholar] [CrossRef]
- Schiliro, M.; Bartman, C.M.; Pabelick, C. Understanding hydrogen sulfide signaling in neonatal airway disease. Expert Rev. Respir. Med. 2021, 15, 351–372. [Google Scholar] [CrossRef]
- Ardini-Poleske, M.E.; Clark, R.F.; Ansong, C.; Carson, J.P.; Corley, R.A.; Deutsch, G.H.; Hagood, J.S.; Kaminski, N.; Mariani, T.J.; Potter, S.S.; et al. LungMAP: The Molecular Atlas of Lung Development Program. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L733–L740. [Google Scholar] [CrossRef]
- Renga, B.; Cipriani, S.; Carino, A.; Simonetti, M.; Zampella, A.; Fiorucci, S. Reversal of Endothelial Dysfunction by GPBAR1 Agonism in Portal Hypertension Involves a AKT/FOXOA1 Dependent Regulation of H2S Generation and Endothelin-1. PLoS ONE 2015, 10, e0141082. [Google Scholar] [CrossRef] [PubMed]
- Sen, N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017, 429, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Zhu, D. An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System. Oxidative Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Perl, A.K. The a“MAZE”ing world of lung-specific transgenic mice. Am. J. Respir. Cell Mol. Biol. 2012, 46, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-Y.; Jedlicka, A.E.; Gladwell, W.; Marzec, J.; McCaw, Z.R.; Bienstock, R.J.; Kleeberger, S.R. Association of Nrf2 polymorphism haplotypes with acute lung injury phenotypes in inbred strains of mice. Antioxid. Redox Signal. 2015, 22, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.; Das, P.; Ponnalagu, D.; Singh, H.; Bhandari, V. Genetic Strain and Sex Differences in a Hyperoxia-Induced Mouse Model of Varying Severity of Bronchopulmonary Dysplasia. Am. J. Pathol. 2019, 189, 999–1014. [Google Scholar] [CrossRef]
- Ronchi, J.A.; Figueira, T.R.; Ravagnani, F.G.; Oliveira, H.C.; Vercesi, A.E.; Castilho, R.F. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 2013, 63, 446–456. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).