Abstract
Bronchial hyperreactivity, reversible airflow limitation and chronic airway inflammation characterize asthma pathophysiology. Personalized medicine, i.e., a tailored management approach, is appropriate for asthma management and is based on the identification of peculiar phenotypes and endotypes. Biomarkers are necessary for defining phenotypes and endotypes. Several biomarkers have been described in asthma, but most of them are experimental and/or not commonly available. The current paper will, therefore, present pragmatic biomarkers useful for asthma management that are available in daily clinical practice. In this regard, eosinophil assessment and serum allergen-specific IgE assay are the most reliable biomarkers. Lung function, mainly concerning forced expiratory flow at 25-755 of vital capacity (FEF25-75), and nasal cytology may be envisaged as ancillary biomarkers in asthma management. In conclusion, biomarkers have clinical relevance in asthma concerning both the endotype definition and the personalization of the therapy.
1. Introduction
Asthma represents a common disease in childhood and adolescence because of the high prevalence (about 5%–10%), chronic nature, the potentially severe symptoms, and the relevant burden on healthcare resources. The definition of asthma, provided by the Global Initiative for Asthma (GINA) guidelines, describes it as a heterogeneous disease [1]. Different phenotypes and endotypes characterize distinct clinical characteristics and specific pathophysiological mechanisms [2,3]. Airway inflammation and hyperresponsiveness are the mainstay of asthma and govern the clinical scenario. In this regard, type 2 inflammation is the predominant phenotype in asthmatic children and adolescents [4]. Type 2 inflammation occurs when at least one factor is decisive, including blood eosinophils > 300 cells/µL, fractioned exhaled nitric oxide (FeNO) > 35 ppb (in children), total IgE > 100 IU/mL, and allergy [5,6]. Type 2 airway inflammation is characterized by an eosinophilic infiltrate closely associated with asthma exacerbations [7,8]. Therefore, abating eosinophilic inflammation could be an ideal therapeutic strategy for asthmatic patients. Personalized medicine is a tailored treatment strategy, taking into account the phenotype/endotype of the single subject to identify the more appropriate individual treatment [9,10]. To this purpose, the precision medicine approach is mandatory and consists of a thorough workup of asthmatic patients to precisely define the individual pheno/endotype [11]. Endotyping requires the availability of reliable markers able to identify the immunopathological characteristics that are specific in every subject [12,13].
On the other hand, the control of asthma represents the goal in the management of asthmatic patients [14]. GINA guidelines identify three levels of asthma control: well-controlled, partly controlled, and uncontrolled [1]. The assessment of asthma control considers some clinical variables, such as symptom frequency and severity [15]. Moreover, the asthma control also considers the future risk of adverse outcomes, including ≥1 exacerbation in the past year, poor adherence, incorrect inhaler technique, impaired lung function, smoking, and eosinophilia [1]. It is well known that uncontrolled asthma is a relevant risk factor for severe asthma exacerbation, impairs the quality of life of children, adolescents, and also their parents, and increases healthcare costs [15]. A control-based approach should be, therefore, preferred to manage asthmatic children and adolescents.
Notably, the availability of new treatments, namely biologics, has changed the prescriptive attitude [16]. As a consequence, asthma management should include asthma control assessment and pheno/endotyping. However, there are very few available markers in clinical practice [17]. Many of the proposed biomarkers are still experimental and cannot be used in daily activity. Recently, it has been proposed that blood eosinophils, serum allergen-specific IgE, and lung function can be pragmatic markers to personalize asthma management [18]. As new evidence has been provided in the last years, the present review updates the list of pragmatic markers, evaluated in the real-world setting, that may be useful in the care of asthmatic children and adolescents. The real-world setting concerns the medical experience occurring in clinical practice, such as considering all patients examined. In particular, the information derived from a randomized controlled trial that involves selected patient populations rarely mirrors the real situation occurring in clinical practice [19]. We will, therefore, present pragmatic biomarkers useful for asthma management that are available in daily clinical practice.
2. Clinical Markers
In addition to conventional clinical variables, including symptoms, allergy, infections, and response to medications, some other parameters could be considered for integration into the assessment of asthma control.
2.1. Visual Analog Scale (VAS)
The perception of symptoms and drug use, measured by the visual analog scale (VAS), could be recognized as a very useful, simple, quick, and handy tool in clinical practice [20]. Formerly, a real-life pediatric study has demonstrated that the perception of breathlessness, assessed by VAS, was related with forced expiratory volume at 1 s (FEV1) and, in particular, a VAS value of 6 was found to be a reliable cutoff (area under the curve = 0.83; Odds Ratio = 9.4) for discriminating children with bronchial obstruction [21]. The clinical relevance of this study was that the VAS assessment, everywhere feasible, may quickly provide an approximative idea of lung function and suggest the necessary measures. The pragmatic proposal was a four-colored scale: (i) green zone (children with VAS value between 8 and 10) means that you do not worry; (ii) yellow zone (VAS 6–8) means to see and wait; (iii) orange zone (VAS 4–6) means to refer to the pediatrician and consider spirometry; and (iv) red zone (VAS < 4) means to treat with relievers [21] immediately. A second study evidenced that VAS could also be considered a primary tool, for example, at home or in the pediatrician’s office, to predict the bronchial reversibility [22]. A further study suggested that VAS could be used to estimate the patient’s perception of short-acting β2-agonists used in clinical practice [23]. The awareness of relievers use could, therefore, improve the belief of asthma severity and potentially improve the self-management. More recently, it has been reported that the VAS assessment of asthma symptoms was linked with the asthma control grade [24]. Asthmatic children with uncontrolled, but also with partly controlled, asthma had the lowest VAS scores.
These outcomes suggest, therefore, that VAS assessment of asthma symptoms could be used in the management of asthmatic children and adolescents, even though VAS concerns the subjective perception of symptoms and should be integrated by objective measurements.
2.2. Emotional Aspects
There is consensus that the emotional disorders, namely anxiety and depression, frequently affect adolescents who have asthma [25] and are common also in their parents [26]. A recent systematic review and meta-analysis concluded that caregivers of asthmatic children are more anxious and depressed than caregivers of healthy children [27]. Consequently, emotional disorders of the parents and anxiety and/or depression in their asthmatic children may affect asthma outcomes [28]. Parental emotional problems, mainly maternal, worsen the asthma severity and control, and increase asthma medication use in their children [29,30,31]. Moreover, adolescence is a critical age from an emotional point of view, as the adolescent is defining his/her identity and personality, maturing into a person and experiencing a range of new emotions [32]. Asthmatic adolescents have a weak acceptance of the asthma diagnosis, underestimate symptoms, have scarce compliance and adherence to the prescribed treatment, and self-management of asthma, mainly concerning the decision to take reliever drugs, is inadequate [33,34]. As a consequence, asthma control becomes a complicated task. In this regard, a real-world study conducted in asthmatic adults showed that anxiety and depression were common and significantly associated with uncontrolled asthma [35]. These outcomes were consistent with a recent real-world study showing that children and adolescents with uncontrolled and partly controlled asthma experienced more frequent anxiety and depression than their well-controlled peers [24]. A further study reported that anxiety and depression were common in asthmatic adolescents and their parents, mainly in their mothers [36]. Mostly, emotional disorders significantly affected asthma control, so that only 29% of adolescents had well-controlled asthma and, consistently, the lowest rate of emotional problems. Moreover, maternal anxiety was frequent in adolescents with uncontrolled asthma. A longitudinal study demonstrated that the standard asthma treatment improved the asthma control but did not affect the emotional pattern in their parents [37].
Therefore, these studies suggest that the assessment of emotional issues should be considered in the management of asthmatic children, adolescents, and their caregivers. However, confounding aspects, including literacy and socio-economic status, should be considered.
2.3. Type 2 Inflammation
Asthma in children and adolescents is predominantly characterized by type 2 inflammation, mainly by the allergic phenotype. Therefore, the assessment of type 2 markers is useful in asthma management. The primarily available markers for type 2 inflammation are serum IgE, blood eosinophils, FeNO, and periostin.
Total serum IgE has no clinical value in monitoring asthma control as there is evidence that it does not correlate with asthma control grade in follow-up studies [24,38,39]. However, a study provided conflicting results [40]. On the other hand, total IgE evaluation is required for prescribing and titrating omalizumab: adult and adolescent patients should have ≥76 IU/mL and children >200 IU/mL to be included in the treatment.
Allergen-specific IgE assessment is useful for phenotyping asthma, such as defining allergic asthma. The presence of sensitization, such as the production of allergen-specific IgE, allows for discriminating allergic asthma from non-allergic asthma [17]. In other words, the documentation of sensitization is essential to phenotype a patient and, if indicated, to identify the more appropriate biologic [9,10]. In particular, it is essential to distinguish allergic asthma and eosinophilic asthma, as different pathways are involved [11,12,13].
FeNO has been proposed as a reliable marker to measure eosinophilic airway inflammation. A longitudinal study first showed its utility to predict and diagnose poorly controlled asthma [41]. FeNO forecasted loss of control with a positive predictive value between 80% and 90%. These data showed that an absolute FeNO value of ≥15 ppb or 60% over baseline was a useful inception for ongoing airway inflammation detection and positively predicts breakthrough symptom arrival.
Several studies showed an increase of FeNO in asthma, further heightening when asthma control declines or when exacerbation occurs [42]. During corticosteroid therapy, FeNO changes come before symptoms, FEV1, and sputum eosinophilia improvement. Thus, FeNO can be considered a sensitive predictor of loss of asthma control [43]. Another study concluded that FeNO value > 300% of predicted identifies subjects at risk of excessive use of rescue medication and needing oral corticosteroids within one year [44]. However, a recent pediatric real-world study showed that FeNO did not discriminate children based on asthma control [24]. Therefore, FeNO assessment could have some clinical relevance, but its use is still controversial.
Blood eosinophils steadily correlate with sputum recovered from bronchial lavage or biopsic eosinophils [11]. Therefore, blood eosinophil count is commonly used in clinical practice to assess type 2 inflammation [45,46]. Moreover, blood eosinophil count is a predictive parameter that identifies responders to biologics [47,48,49]. Therefore, blood eosinophil count should be considered a useful marker in the management of asthmatic children and adolescents.
Serum periostin: there are conflicting findings concerning the real value of periostin as a reliable asthma biomarker. Wagener showed that serum periostin was not related to sputum eosinophils [50]. Mansur demonstrated that FeNO had a stronger correlation with asthma exacerbations than blood eosinophils or periostin [51]. Consistently, a real-world pediatric study demonstrated that serum periostin was not associated with the asthma control grade [52]. El Basha reported conflicting results showing that serum periostin was linked with severe asthma and asthma exacerbation [53]. From a practical point of view, the use of serum periostin is still experimental, and its assessment should not be performed in clinical practice.
2.4. Lung Function
Lung function evaluation, mainly concerning FEV1 and the rate between FEV1 and forced vital capacity (FVC), is mandatory for asthma diagnosis and monitoring. In particular, FEV1 has always been considered the gold standard in the interpretation of spirometry in asthmatic patients [54]. However, FEV1 values may frequently be in the normal range when evaluating children and adolescents with asthma [55]. Therefore, there is a growing interest in the definition of the practical role exerted by the forced expiratory flow between 25% and 75% of forced vital capacity (FEF25-75). FEF25-75 may have clinical relevance, especially when FEV1 values are normal [56]. Indeed, it has been proposed that reduced FEF25-75 value might precede FEV1 impairment, therefore indicating early disease and poor prognosis [57]. There is a body of evidence that suggests FEF25-75 as a reliable marker to monitor asthmatic patients [58,59]. Moreover, there is evidence that FEF25-75 is significantly associated with the asthma control grade [60,61,62,63]. Therefore, FEF25-75 should be included in the spirometry parameters evaluated in daily practice.
2.5. Asthma Control Questionnaire (ACT)
Asthma control may also be assessed by the Asthma Control Test (ACT) questionnaire developed for use in clinical practice [64]. The ACT questionnaire consists of five questions with five possible responses, exploring the patient’s perception of his/her asthma control. The result could range between 0 and 25, where 25 is the optimal asthma control. Many studies confirmed that ACT correlated well with the asthma control grade [65,66,67]. A meta-analysis also provided convincing evidence concerning its reliability in assessing the asthma control grade [68]. Moreover, ACT can predict future asthma risks [69]. Interestingly, a pediatric version is also available, the children ACT (cACT), that provides fruitful information in asthma management [70]. A real-life study underlined its benefit in assessing asthmatic patients [71]. However, it has been reported that ACT scores did not correlate with the asthma control grade proposed by GINA guidelines [72]. This outcome was confirmed in a pediatric study showing that the frequencies of the two categorizing methods did not agree between them [24]. Therefore, both ways should be pursued in asthma control grading. ACT is a reliable tool to assess and monitor asthma control and provides quick and straightforward information about the patient’s perception of asthma control. On the other hand, ACT reflects the perception of asthma control and consequently could be influenced by confounding factors, including emotional issues.
3. Conclusions
At present, very few inflammatory biomarkers are validated and reliable. In the common practice, such as in primary and secondary care levels, but also tertiary levels in some geographical areas, the use of biomarkers is minimal for financial reasons. We would like to propose a pragmatic workup using popular markers that are routinely evaluated in a real-life setting and are available everywhere (Figure 1). Blood eosinophils and allergen-specific IgE should be measured in all children and adolescents with asthma. They may give information about allergy presence, type 2-driven bronchial inflammation, asthma severity, and responsiveness to both steroids and allergen-immunotherapy (AIT). With these two simple biomarkers, it is possible to manage asthmatics, following the criteria of precision medicine, in every real-life setting. Blood eosinophils and allergen-specific IgE are fundamental to differentiate the allergic phenotype (both markers are positive) from the eosinophilic phenotype (absence of allergen-specific IgE). Phenotyping/endotyping is useful to identify the most appropriate therapy for every patient. On the other hand, other phenotypes and endotypes exist, including neutrophilic and non-allergic asthma. As a consequence, additional investigations may be required in some patients.
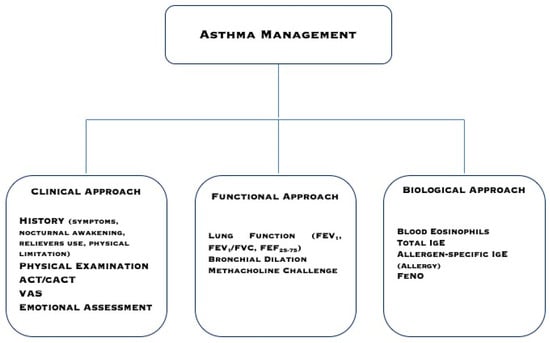
Figure 1.
A pragmatic approach to manage asthmatic patients.
ACT, VAS, and emotional assessments are straightforward and quick tools that allow for obtaining relevant information. Spirometry is mandatory to diagnose asthma and correctly manage the patient. However, as FEV1 may be frequently normal and bronchodilation testing and the methacholine challenge are complex exams, FEF25-75 is a simple parameter that could give relevant information concerning early bronchial airflow limitation.
Therefore, it seems to be an acceptable pragmatic approach to consider these parameters as common markers. Therefore, the daily clinical practice suggests considering a simple pathway that should be based on symptom history, functional assessment, response to conventional treatment, and measurement of basilar inflammatory biomarkers. However, further studies are needed to validate this integrated approach.
In conclusion, pragmatic markers have clinical relevance in asthma management concerning the assessment of asthma control, endotype definition, and personalization of the therapy.
Author Contributions
All authors reviewed the literature and discussed the outcomes, G.C. wrote the article, G.L.M., F.L.M.R., and M.A.T. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Initiative for Asthma. GINA Guidelines. Global Strategy for Asthma Management and Prevention. 2019. Available online: htpp://www.ginasthma.org (accessed on 4 May 2020).
- Licari, A.; Castagnoli, R.; Brambilla, I.; Marseglia, A.; Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. Asthma endotyping and biomarkers in childhood asthma. Pediatr. Allergy Immunol. Pulmonol. 2018, 31, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Nakagome, K.; Nagata, M. Involvement and possible role of eosinophils in asthma exacerbation. Front. Immunol. 2018, 9, 2220. [Google Scholar] [CrossRef] [PubMed]
- Lebold, K.M.; Jacoby, D.B.; Drake, M.G. Inflammatory Mechanisms Linking Maternal and Childhood Asthma. J. Leokoc. Biol. 2020. [Google Scholar] [CrossRef]
- Froidure, A.; Mouthuy, J.; Durham, S.R.; Chanez, P.; Sibille, Y.; Pilette, C. Asthma phenotypes and IgE responses. Eur. Respir. J. 2016, 47, 304–319. [Google Scholar] [CrossRef]
- Zervas, E.; Samitas, K.; Papaioannou, A.I.; Bakakos, P.; Loukides, P. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res. 2018, 4, 00125–02017. [Google Scholar] [CrossRef]
- Price, D.B.; Rigazio, A.; Campbell, J.D.; Bleecker, E.R.; Corrigan, C.J.; Thomas, M.; Wenzel, S.E.; Wilson, A.M.; Buatti Small, M.; Gopalan, G.; et al. Blood eosinophil count and prospective annual asthma disease burden: A UK cohort study. Lancet Respir. Med. 2015, 3, 849–858. [Google Scholar] [CrossRef]
- Denlinger, L.C.; Phillips, B.R.; Ramratnam, S.; Ross, K.; Bhakta, N.R.; Cardet, J.C.; Castro, M.; Peters, S.P.; Phipatanakul, W.; Aujla, S.; et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am. J. Respir. Crit. Care Med. 2017, 195, 302–313. [Google Scholar] [CrossRef]
- Agache, I.; Beltran, J.; Akdis, C.; Akdis, M.; Canelp-Aybar, C.; Caconica, W.; Casale, T.; Chivato, T.; Corren, J.; Del Giacco, S.; et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, and omalizumab) for severe eosinophilic asthma. Allergy 2020, in press. [Google Scholar] [CrossRef]
- Agache, I.; Rocha, C.; Beltran, J.; Song, Y.; Posso, M.; Solà, I.; Alonso-Coello, P.A.; Akdis, C.; Akdis, M.; Caconica, W.; et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, and omalizumab) for severe allergic asthma. Allergy 2020. [Google Scholar] [CrossRef]
- Slob, E.M.; Maitland-Van der Zee, A.H.; Koppelman, G.H.; Pijnenburg, M.W. Precision Medicine in Childhood Asthma. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 141–147. [Google Scholar] [CrossRef]
- Li, W.; Glaum, M.K. Biomarkers in severe asthma. In Severe Asthma; Lee, Y.C., Akdis, C., Eds.; Springer Nature: Singapore, 2018; pp. 59–88. [Google Scholar]
- Schoettler, N.; Strek, M.E. Recent Advances in Severe Asthma: From Phenotypes to Personalized Medicine. Chest 2020, 157, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.S.; Bailey, C.M.; Qin, X.; Johnson, C. Long-term control medication use and asthma control status among children and adults with asthma. J. Asthma 2017, 54, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, S.J.; Cowan, K.J.; Robinson, K.J.; Pellegrino, C.A.; Frankowski, B.L.; Chmielewski, M.V.; Shaw, J.S.; Harder, V.S. A primary care learning collaborative to improve office systems and clinical management of pediatric asthma. J. Asthma 2020. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, E.M.; O’Connor, G.T. New Therapeutic Strategies for Asthma. JAMA 2020, 323, 517–518. [Google Scholar] [CrossRef]
- Ciprandi, G.; Tosca, M.A.; Silvestri, M.; Ricciardolo, F.L.M. Inflammatory biomarkers for asthma endotyping and personalized therapy. Exp. Rev. Clin. Immunol. 2017, 13, 715–721. [Google Scholar] [CrossRef]
- Niespodziana, K.; Borochova, K.; Pazderova, P.; Schlederer, T.; Astafveva, N.; Baramovskaya, T.; Barbouche, M.R.; Beltyukov, E.; Berger, A.; Borzova, E.; et al. Towards Personalization of Asthma Treatment According to Trigger Factors. J. Allergy Clin. Immunol. 2020. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.w.; Robb, M.A.; et al. Real-world evidence—What Is It And What can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Ciprandi, G.; Tosca, M.A.; Silvestri, M. Measuring the perception of symptom, drug use, and allergen immunotherapy efficacy using the visual analog scale. Exp. Rev. Clin. Immunol. 2014, 10, 179–182. [Google Scholar] [CrossRef]
- Tosca, M.A.; Silvestri, M.; Olcese, R.; Pistorio, A.; Rossi, G.A.; Ciprandi, G. Breathlessness perception assessed by visual analogue scale and lung function in children with asthma: A real-life study. Pediatr. Allergy Immunol. 2012, 23, 537–542. [Google Scholar] [CrossRef]
- Tosca, M.A.; Silvestri, M.; Rossi, G.A.; Ciprandi, G. Perception of bronchodilation assessed by Visual Analogue Scale in children with asthma. Allergol Immunopathol. 2013, 41, 359–363. [Google Scholar] [CrossRef]
- Ciprandi, G.; Silvestri, M.; Tosca, M.A. VAS for assessing the perception of short-acting B2 agonist use in clinical practice. Lung India 2019, 36, 82–83. [Google Scholar] [CrossRef]
- Licari, A.; Marseglia, G.L.; Tosca, M.A.; Ciprandi, G. Asthma control in children and adolescents: A study in clinical practice. J. Asthma 2020, 57, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Vila, G.; Nollet-Clemencon, C.; de Blic, J.; Falissard, B.; Mouren-Simeoni, M.C.; Scheinmann, P. Assessment of anxiety disorders in asthmatic children. Psychosom 1999, 40, 404–413. [Google Scholar] [CrossRef]
- Brew, B.K.; Lundholm, C.; Gong, T.; Larsson, H.; Almqvist, C. The familial aggregation of atopic diseases and depression or anxiety in children. Clin. Exp. Allergy 2018, 48, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Easter, G.; Sharpe, L.; Hunt, C.J. Systematic review and meta-analysis of anxious and depressive symptoms in caregivers of children with asthma. J. Pediatri. Psychol. 2015, 40, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Rioseco, A.; Serrano, C.; Celedon, J.C.; Padilla, O.; Puschel, K.; Castro-Rodriguez, J.A. Caregiver’s depressive symptoms and asthma control in children from an underserved community. J. Asthma 2017, 54, 1059–1064. [Google Scholar] [CrossRef]
- Ozdogan, S.; Kurtaraner, T.; Gencer, H.; Kabakci-Kaya, D.; Celik-Erden, S. Association between maternal depression and wheezing in preschool children. Turk. J. Pediatri. 2016, 58, 632–640. [Google Scholar] [CrossRef]
- Sleath, B.; Carpenter, D.M.; Walsh, K.E.; Davis, S.A.; Watson, C.H.; Lee, C.; Corrigan, G.; Marrel, C. Factors associated with adolescent and caregiver reported problems in using asthma medications. J. Asthma 2019, 56, 451–457. [Google Scholar] [CrossRef]
- Holley, S.; Walker, D.; Knibb, R.; Latter, S.; Liossi, C.; Mitchell, F.; Radley, R.; Roberts, G. Barriers and facilitators to self-management of asthma in adolescents: An interview study to inform development of a novel intervention. Clin. Exp. Allergy 2018, 48, 944–956. [Google Scholar] [CrossRef]
- Bechard, M.; VanderLaan, D.P.; Wood, H.; Wasserman, L.; Zucker, K.J. Psychosocial and Psychological Vulnerability in Adolescents with Gender Dysphoria: A "Proof of Principle" Study. J. Sex Marital Ther. 2017, 43, 678–688. [Google Scholar] [CrossRef]
- Sundbom, F.; Malinovschi, A.; Lindberg, E.; Alving, K.; Janson, C. Effects of poor asthma control, insomnia, anxiety, and depression on quality of life in young asthmatics. J. Asthma 2016, 53, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.R.; Thompson, L.A.; Gross, H.E.; Reeve, B.B.; Huang, S.W.; DeWalt, D.A.; Huang, I.C. Association of consistently suboptimal quality of life with consistently poor asthma control in children with asthma. Ann. Allergy Asthma Immunol. 2017, 119, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Schiavetti, I.; Riciardolo, F. The impact of anxiety and depression on outpatients with asthma. Ann. Allergy Asthma Immunol. 2015, 115, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Ciprandi, R.; Marseglia, G.; Ciprandi, G. Anxiety and depression in adolescents with asthma and their parents: A study in clinical practice. Monaldi. Arch. Chest Dis. 2019, 89, 3. [Google Scholar] [CrossRef]
- Licari, A.; Ciprandi, R.; Marseglia, G.L.; Ciprandi, G. Anxiety and depression in adolescents with severe asthma and their parents: Preliminary results after 1-year treatment. Behav. Sci. 2019, 9, 78. [Google Scholar] [CrossRef]
- Galiniak, S.; Biesiadecki, M.; Aebisher, D.; Rachel, M. Nasal Nitric Oxide in Upper Airways in Children with Asthma and Allergic Rhinitis. Adv. Med. Sci. 2020, 65, 127–133. [Google Scholar] [CrossRef]
- Zhu, Z.; Xia, S.; Chen, X.; Guan, W.J.; Guo, Z.J.; Sun, B.Q. Factors Associated with Exhaled Nitric Oxide in Children with Asthma and Allergic Rhinitis. Clin. Respir. J. 2020, 14, 9–15. [Google Scholar] [CrossRef]
- Tanaka, A.; Jinno, M.; Hirai, K.; Miyata, Y.; Mizuma, H.; Yamaguchi, M.; Ohta, S.; Watanabe, Y.; Yamamoto, M.; Suzuki, S.; et al. Longitudinal Increase in Total IgE Levels in Patients with Adult Asthma: An Association with Poor Asthma Control. Respir. Res. 2014, 15, 144. [Google Scholar] [CrossRef]
- Jones, S.L.; Kittelson, J.; Cowan, J.O.; Flannery, E.M.; Hancox, R.J.; McLachlan, C.R.; taylor, D.L. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am. J. Respir. Crit. Care Med. 2001, 164, 738–743. [Google Scholar] [CrossRef]
- Sandrini, A.; Taylor, D.R.; Thomas, P.S.; Yates, D.H. Fractional exhaled nitric oxide in asthma: An update. Respirology 2010, 15, 57–70. [Google Scholar] [CrossRef]
- Michils, A.; Baldassarre, S.; Van Muylem, A. Exhaled nitric oxide and asthma control: A longitudinal study in unselected patients. Eur. Respir. J. 2008, 31, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, R.S.; Schatz, M.; Zhang, F.; Crawford, W.W.; Kaplan, M.S.; Roth, R.M.; Chen, W. Elevated exhaled nitric oxide is a clinical indicator of future uncontrolled asthma in asthmatic patients on inhaled corticosteroids. J. Allergy Clin. Immunol. 2011, 128, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Thi-Bich, H.; Duong-Thi-Ly, H.; Thom, V.T.; Pham-Thi-Hong, N.; Dinh, L.D.; Le-Thi-Minh, H.; Craig, T.J.; Duong-Quy, S. Study of the Correlations Between Fractional Exhaled Nitric Oxide in Exhaled Breath and Atopic Status, Blood Eosinophils, FCER2 Mutation, and Asthma Control in Vietnamese Children. J. Asthma Allergy 2016, 9, 163–170. [Google Scholar] [PubMed]
- Coskun, O.; Ercan, N.; Bostanci, I. The Peripheral Blood Inflammatory Patterns in the Control Levels of Asthma in Children. J. Asthma 2020. [Google Scholar] [CrossRef]
- Bel, E.H.; Ten Brinke, A. New Anti-Eosinophil Drugs for Asthma and COPD: Targeting the Trait! Chest 2017, 152, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Sur, S. IgE and eosinophils as therapeutic targets in asthma. Curr. Opin Allergy Clin. Immunol. 2017, 17, 42–49. [Google Scholar] [CrossRef]
- Casciano, J.; Krishnan, J.; Dotiwala, Z.; Li, C.; Sun, S.X. Clinical and Economic Burden of Elevated Blood Eosinophils in Patients With and Without Uncontrolled Asthma. J. Manag. Care Spec. Pharm. 2017, 23, 85–91. [Google Scholar] [CrossRef]
- Wagener, A.H.; de Nijs, S.B.; Lutter, R.; Sousa, A.R.; Weersink, E.J.M.; Bel, E.H.; Sterk, P.J. External validation of blood eosinophils, FeNO and serum periostin as surrogates for sputum eosinophils in asthma. Thorax 2015, 70, 115–120. [Google Scholar] [CrossRef]
- Mansur, A.H.; Srivastava, S.; Sahal, A. Disconnect of type 2 biomarkers in severe asthma; dominated by FeNO as a predictor of exacerbations and periostin as predictor of reduced lung function. Resp. Med. 2018, 143, 31–38. [Google Scholar] [CrossRef]
- Licari, A.; Brambilla, I.; Sacchi, L.; Marseglia, G.L.; Ciprandi, G. Periostin, type 2 biomarker, is not associated with asthma control grade in asthmatic allergic children. Resp. Med. 2019, 151, 118–120. [Google Scholar] [CrossRef]
- El Basha, N.R.; Osman, H.M.; Abdelaal, A.A.; Saed, S.M.; Shaaban, H.H. Increased Expression of Serum Periostin and YK. L40 in Children with Severe Asthma and Asthma Exacerbation. J. Investig. Med. 2018, 66, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van dr Grinten, C.P.M.; Gistafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.R.; Chinchilli, V.M.; Phillips, B.R.; Sorkness, C.A.; Lemanske, R.F.; Szefler, S.L.; Taussig, L.; Bacharier, L.B. Forced expiratory flow between 25% and 75% of vital capacity and, F.E. V1/forced vital capacity ratio concerning clinical and physiological parameters in asthmatic children with normal FE. V1 values. J. Allergy Clin. Immunol. 2010, 126, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.B.; Smith, E.O.; Schecter, M.G.; Mallory, G.B.; Elidemir, O. Decline in 25% to 75% forced expiratory flow as an early predictor of chronic airway rejection in pediatric lung transplant recipients. J. Heart Lung Transpl. 2012, 31, 1288–1292. [Google Scholar] [CrossRef]
- Riley, C.M.; Wenzel, S.E.; Castro, M.; Erzurum, S.C.; Chung, K.F.; Fitzpatrick, A.M.; Gaston, B.; Israel, E.; Moore, W.; Bleecker, E.R.; et al. Clinical implication for having reduced mild forced expiratory flow rates (FEF25-75), independently of FE. V1, in adult patients with asthma. PLoS ONE 2015, 10, e0145476. [Google Scholar] [CrossRef]
- Siroux, V.; Boudier, A.; Dolgopoloff, M.; Chanoine, S.; Bousquet, J.; Gormand, F.; Just, J.; Le Moual, N.; Nadif, R.; Pison, C.; et al. Forced mid expiratory flow between 25% and 75% of forced vital capacity is associated with long-term persistence of asthma and poor asthma outcomes. J. Allergy Clin. Immunol. 2016, 137, 1709–1716. [Google Scholar] [CrossRef]
- Cirillo, I.; Gallo, F.; Ciprandi, G. Impaired spirometry may predict bronchial hyper-responsiveness. JACI Pract. 2018, 6, 2127–2129. [Google Scholar]
- Ciprandi, G.; Cirillo, I. The pragmatic role of FEF25-75 in asymptomatic subjects, allergic rhinitis, asthma, and in military setting. Exp. Rev. Resp. Med. 2019, 13, 1147–1151. [Google Scholar] [CrossRef]
- Wei, J.; Ma, L.; Wang, J.; Xu, Q.; Chen, M.; Jiang, M.; Luo, M.; Wu, J.; She, W.; Chu, S.; et al. Airway Reversibility in Asthma and Phenotypes of Th2-biomarkers, Lung Function and Disease Control. Allergy Asthma Clin. Immunol. 2018, 14, 89. [Google Scholar] [CrossRef]
- Uppala, R.; Kaenpugdee, P.; Srisutthjkamol, S.; Teeratakulpisarn, J. Assessment of Small Airway Function and Reversibility in Symptom-Controlled Asthma in Pediatric Patients. Asian Pac. J. Allergy Immunol. 2019, 37, 25–29. [Google Scholar]
- Kanchongkittiphon, W.; Gaffin, J.M.; Kopel, L.; Petty, C.R.; Bollinger, M.E.; Miller, R.L.; Perzanowski, M.; Matsui, E.C.; Phpatanakul, W. Association of, FEF25%-75% and Bronchodilator Reversibility with Asthma Control and Asthma Morbidity in Inner-City Children with Asthma. Ann. Allergy Asthma Immunol. 2016, 117, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A. Development of the asthma control test: A survey form assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Kay, S.; Pike, J.; Williams, A.; Rosenzweig, J.R.; Hillyer, E.V.; Price, D. The Asthma Control Test (ACT) as a predictor of, G.I.NA guideline-defined asthma control: Analysis of a multinational cross-sectional survey. Prim. Car. Respir. J. 2009, 18, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Chavannes, N.; Le, L.T.; Price, D. The Asthma Control Test (ACT) as an alternative tool to Global Initiative for Asthma (GINA) guideline criteria for assessing asthma control in Vietnamese outpatients. Prim. Care Respir. J. 2012, 21, 85–89. [Google Scholar] [CrossRef]
- Miedinger, D.; Neukomm, E.; Chhajed, P.N.; Schnyder, A.; Naef, M.; Ackermann, M.; Leuppi, J.D. The use of the Asthma Control Test in general practice and its correlation with asthma control according to the, G.I.NA guidelines. Curr. Med. Res. Opin. 2011, 27, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, H.P.; Ly, Y.; Liang, R.; Jiang, Y.Q.; Powell, H.; Fu, J.J.; Wang, L.; Gibson, P.G.; Wang, G. The asthma control test and asthma control questionnaire for assessing asthma control: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2013, 131, 695–703. [Google Scholar] [CrossRef]
- Bateman, E.D.; Reddel, H.K.; Eriksson, G.; Petrson, S.; Ostlund, O.; Sears, M.R.; Jenkins, C.; Humbert, M.; Buhl, R.; Harrison, T.W.; et al. Overall asthma control: The relationship between current control and future risk. J. Allergy Clin. Immunol. 2010, 125, 600–608. [Google Scholar] [CrossRef]
- Deschildre, A.; Pin, I.; El Abd, K.; Belmin-Larrar, S.; Mourad, S.; Thumerelle, C.; Le Roux, P.; Langlois, C.; de Blic, J. Asthma control assessment in a pediatric population: Comparison between, G.I.NA/NAEPP guidelines, Childhood Asthma Control Test (C-ACT), and physician’s rating. Allergy 2014, 69, 784–790. [Google Scholar] [CrossRef]
- Ciprandi, G.; Gallo, F.; Ricciardolo, F. Asthma Control Test in the real life. J. Asthma 2017, 54, 114–115. [Google Scholar] [CrossRef]
- Ciprandi, G.; Gallo, F.; Ricciardolo, F. A real-life comparison between Asthma Control Test and, G.I.NA asthma control grading. Ann. Allergy Asthma Immunol. 2016, 117, 725–727. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).