Changes in Fetal Hemoglobin in Very Preterm Infants Born Small for Gestational Age: A Retrospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary and Secondary Endpoints
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | appropriate-for-gestational age |
| BPD | bronchopulmonary dysplasia |
| HbA | adult hemoglobin |
| HbF | fetal hemoglobin |
| IVH | intraventricular hemorrhage |
| NEC | necrotizing enterocolitis |
| RBC | red blood cell |
| ROP | retinopathy of prematurity |
| SGA | small-for-gestational age |
References
- Jiramongkolchai, K.; Repka, M.X.; Tian, J.; Aucott, S.W.; Shepard, J.; Collins, M.; Kraus, C.; Clemens, J.; Feller, M.; Burd, I.; et al. Lower foetal haemoglobin levels at 31- and 34-weeks post menstrual age is associated with the development of retinopathy of prematurity: PacIFiHER Report No. 1 PacIFiHER Study Group (Preterm Infants and Fetal Haemoglobin in ROP). Eye 2021, 35, 659–664. [Google Scholar] [CrossRef]
- Jiramongkolchai, K.; Repka, M.X.; Tian, J.; Aucott, S.W.; Shepard, J.; Collins, M.; Clemens, J.; Feller, M.; Burd, I.; Roizenblatt, M.; et al. Effects of fetal haemoglobin on systemic oxygenation in preterm infants and the development of retinopathy of prematurity PacIFiHER Report No. 2. Br. J. Ophthalmol. 2023, 107, 380–383. [Google Scholar]
- Hellström, W.; Forssell, L.; Morsing, E.; Sävman, K.; Ley, D. Neonatal clinical blood sampling led to major blood loss and was associated with bronchopulmonary dysplasia. Acta Paediatr. 2020, 109, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Bard, H.; Widness, J.A. The life span of erythrocytes transfused to preterm infants. Pediatr. Res. 1997, 42, 9–11. [Google Scholar] [CrossRef] [PubMed]
- De Halleux, V.; Truttmann, A.; Gagnon, C.; Bard, H. The effect of blood transfusion on the hemoglobin oxygen dissociation curve of very early preterm infants during the first week of life. Semin. Perinatol. 2002, 26, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Reeder, B.J. The redox activity of hemoglobins: From physiologic functions to pathologic mechanisms. Antioxid. Redox Signal. 2010, 13, 1087–1123. [Google Scholar] [CrossRef]
- Ratanasopa, K.; Strader, M.B.; Alayash, A.I.; Bulow, L. Dissection of the radical reactions linked to fetal hemoglobin reveals enhanced pseudoperoxidase activity. Front. Physiol. 2015, 6, 3. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar]
- Hellström, W.; Martinsson, T.; Morsing, E.; Gränse, L.; Ley, D.; Hellström, A. Low fraction of fetal haemoglobin is associated with retinopathy of prematurity in the very preterm infant. Br. J. Ophthalmol. 2022, 106, 970–974. [Google Scholar] [PubMed]
- Hellström, W.; Martinsson, T.; Hellstrom, A.; Morsing, E.; Ley, D. Fetal haemoglobin and bronchopulmonary dysplasia in neonates: An observational study. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 88–92. [Google Scholar]
- Prasad, N.; Dubey, A.; Kumar, K.; Shrivastava, J. Role of fetal hemoglobin in the development and progression of retinopathy of prematurity in preterm infants. Indian J. Ophthalmol. 2023, 71, 3478–3483. [Google Scholar] [CrossRef]
- Nobile, S.; Marchionni, P.; Carnielli, V.P. Neonatal outcome of small for gestational age preterm infants. Eur. J. Pediatr. 2017, 176, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Haglund, B.; Odlind, V.; Altman, M.; Ewald, U.; Kieler, H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015, 104, 259–263. [Google Scholar] [CrossRef]
- Razak, A.; Faden, M. Association of small for gestational age with retinopathy of prematurity: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 270–278. [Google Scholar] [CrossRef]
- Alda, M.G.; Holberton, J.; MacDonald, T.M.; Charlton, J.K. Small for gestational age at preterm birth identifies adverse neonatal outcomes more reliably than antenatal suspicion of fetal growth restriction. J. Matern. Fetal Neonatal Med. 2023, 36, 2279017. [Google Scholar] [CrossRef]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.I. Neonatal morbidities of fetal growth restriction: Pathophysiology and impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Kato, T.; Mandai, T.; Iwatani, S.; Koda, T.; Nagasaka, M.; Fujita, K.; Kurokawa, D.; Yamana, K.; Nishida, K.; Taniguchi-Ikeda, M.; et al. Extremely preterm infants small for gestational age are at risk for motor impairment at 3 years corrected age. Brain Dev. 2016, 38, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: A systematic review and meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef]
- Motte-Signoret, E.; Shankar-Aguilera, S.; Brailly-Tabard, S.; Soreze, Y.; Dell’Orto, V.; Ben Ammar, R.; De Luca, D.; Boileau, P. Small for gestational age preterm neonates exhibit defective GH/IGF1 signaling pathway. Front. Pediatr. 2021, 9, 711400. [Google Scholar] [CrossRef]
- Villar, J.; Giuliani, F.; Fenton, T.R.; Ohuma, E.O.; Ismail, L.C.; Kennedy, S.H. INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016, 387, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Poggi, C.; Fontanelli, G. Relationship between platelet count and volume and spontaneous and pharmacological closure of ductus arteriosus in preterm infants. Am. J. Perinatol. 2013, 30, 359–364. [Google Scholar]
- Ehrenkranz, R.A.; Walsh, M.C.; Vohr, B.R.; Jobe, A.H.; Wright, L.L.; Fanaroff, A.A.; Wrage, L.A.; Poole, K. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef]
- Papile, L.S.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of the sub-ependymal intraventricular hemorrhage; a study of infants weighing less than 1500 grams. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis: Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Girelli, G.; Antoncecchi, S.; Casadei, A.M.; Del Vecchio, A.; Isernia, P.; Motta, M.; Regoli, D.; Romagnoli, C.; Tripodi, G.; Velati, C. Recommendations for transfusion therapy in neonatology. Blood Transfus. 2015, 13, 484–497. [Google Scholar] [PubMed]
- Dani, C.; Remaschi, G.; Ulivi, M.; Monti, N.; Pratesi, S. Fetal hemoglobin in preterm infants after resuscitation with immediate cord clamping, delayed cord clamping, or cord milking. Children 2025, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Shrivastava, A.K.; Choudhary, P.R.S. tudy of fetal hemoglobin with different gestational age and birth weight of the newborn. Int. J. Contemp. Pediatr. 2021, 8, 434–439. [Google Scholar] [CrossRef]
- Wilson, K.; Hawken, S.; Murphy, M.S.Q.; Atkinson, K.M.; Potter, B.K.; Sprague, A.; Walker, M.; Chakraborty, P.; Little, J. Postnatal prediction of gestational age using newborn fetal hemoglobin levels. EBioMedicine 2017, 15, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Bard, H.; Lachance, C.; Widness, J.A.; Gagnon, C. The reactivation of fetal hemoglobin synthesis during anemia of prematurity. Pediatr. Res. 1994, 36, 253–256. [Google Scholar] [CrossRef][Green Version]
- Bard, H.; Prosmanne, J. Elevated levels of fetal hemoglobin synthesis in infants with bronchopulmonary dysplasia. Pediatrics 1990, 86, 193–196. [Google Scholar] [CrossRef]
- Bard, H. Postnatal fetal and adult hemoglobin synthesis in early preterm newborn infants. J. Clin. Investig. 1973, 52, 1789–1795. [Google Scholar] [CrossRef]
- Bard, H.; Makowski, E.L.; Meschia, G.; Battaglia, F.C. The relative rates of synthesis of hemoglobins A and F in immature red cells of newborn infants. Pediatrics 1970, 45, 766–777. [Google Scholar] [CrossRef]
- Cochran-Black, D.L.; Cowan, L.D.; Neas, B.R. The relation between newborn hemoglobin F fractions and risk factors for sudden infant death syndrome. Arch. Pathol. Lab. Med. 2001, 125, 211–217. [Google Scholar] [CrossRef]
- Park, E.A.; Kim, G.H. The changes of fetal hemoglobin in preterm and small for gestational age newborn infants. J. Korean Pediatr. Soc. 1993, 36, 919–927. [Google Scholar]
- Khandros, E.; Blobel, G.A. Elevating fetal hemoglobin: Recently discovered regulators and mechanisms. Blood 2024, 144, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Orkin, S.H. The fetal-to-adult hemoglobin switch-mechanism and therapy. N. Engl. J. Med. 2025, 392, 2135–2149. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Mayuranathan, T.; Huang, P.; Doerfler, P.A.; Li, Y.; Yao, Y. Activation of γ-globin expression by hypoxia-inducible factor 1α. Nature 2022, 610, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Patidar, S.; Shrivastava, J.; Agrawal, A.; Dwivedi, R. Assessment of iron status and red cell parameters in healthy full term small for gestational age neonates at birth. J. Clin. Neonatol. 2013, 2, 121–124. [Google Scholar] [CrossRef]
- Bard, H.; Fouron, J.C.; Prosmanne, J.; Gagnon, J. Effect of hypoxemia on fetal hemoglobin synthesis during late gestation. Pediatr. Res. 1992, 31, 483–485. [Google Scholar] [CrossRef][Green Version]
- Ara, J.; Fekete, S.; Frank, M.; Golden, J.A.; Pleasure, D.; Valencia, I. Hypoxic-preconditioning induces neuroprotection against hypoxia-ischemia in newborn piglet brain. Neurobiol. Dis. 2011, 43, 473–485. [Google Scholar] [CrossRef]
- Deng, Q.; Parker, E.; Duan, R.; Yang, L. Preconditioning and posttreatment strategies in neonatal hypoxic-ischemic encephalopathy: Recent advances and clinical challenges. Mol. Neurobiol. 2025, 62, 10020–10044. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A. Fetal responses to placental insufficiency: An update. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Park, S.H.; Lee, E.J. Iron status in small for gestational age and appropriate for gestational age infants at birth. Korean J. Pediatr. 2019, 62, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, V.; Tortora, D.; Parotto, M.; Severino, L.; Simbi, A.; Guerrini, P.; Straface, G. Determinants and effects of fluid status changes in caesarean delivered neonates. Acta Paediatr. 2020, 109, 1545–1550. [Google Scholar] [CrossRef]
| SGA Infants (n = 30) | AGA Infants (n = 60) | p | |
|---|---|---|---|
| Gestational age (wks) | 27.7 ± 1.4 | 27.9 ± 0.7 | 0.367 |
| Birth weight (g) | 625 ± 143 | 1127 ± 192 | <0.001 |
| Female | 15 (50) | 28 (47) | 0.765 |
| Antenatal steroids | 30 (100) | 58 (97) | 0.312 |
| Cesarean section | 27 (90) | 40 (67) | 0.017 |
| Maternal clinical chorioamnionitis | 2 (7) | 2 (3) | 0.470 |
| Apgar score at 5 min | 8 (7–8) | 8 (8–9) | 0.306 |
| Noninvasive ventilation | 27 (90) | 59 (98) | 0.071 |
| Duration (days) | 34 (8–50) | 20 (6–28) | <0.001 |
| Mechanical ventilation | 16 (53) | 13 (22) | 0.002 |
| Duration (days) | 19 (6–31) | 4 (3–20) | <0.001 |
| Patent ductus arteriosus | 12 (40) | 23 (38) | 0.521 |
| Sepsis | 11 (37) | 16 (27) | 0.329 |
| Bronchopulmonary dysplasia | 19 (63) | 30 (50) | 0.231 |
| Intraventricular hemorrhage | 7 (23) | 20 (33) | 0.329 |
| Grade 1 | 3 (10) | 5 (8) | |
| Grade 2 | 1 (3) | 2 (3) | |
| Grade 3 | 2 (7) | 13 (22) | |
| Grade 4 | 1 (3) | 0 | |
| Retinopathy of prematurity | 6 (20) | 5 (8) | 0.170 |
| Grade 1 | 0 | 4 (7) | |
| Grade 2 | 5 (17) | 1 (2) | |
| Grade 3 | 1 (3) | 0 | |
| Necrotizing enterocolitis | 2 (7) | 3 (5) | 0.745 |
| Stage 1 | 2 (7) | 1 (2) | |
| Stage 2 | 0 | 0 | |
| Stage 3 | 0 | 2 (3) | |
| Duration of hospital stay (d) | 89 ± 41 | 72 ± 22 | 0.012 |
| SGA Infants (n = 30) | AGA Infants (n = 60) | p | |
|---|---|---|---|
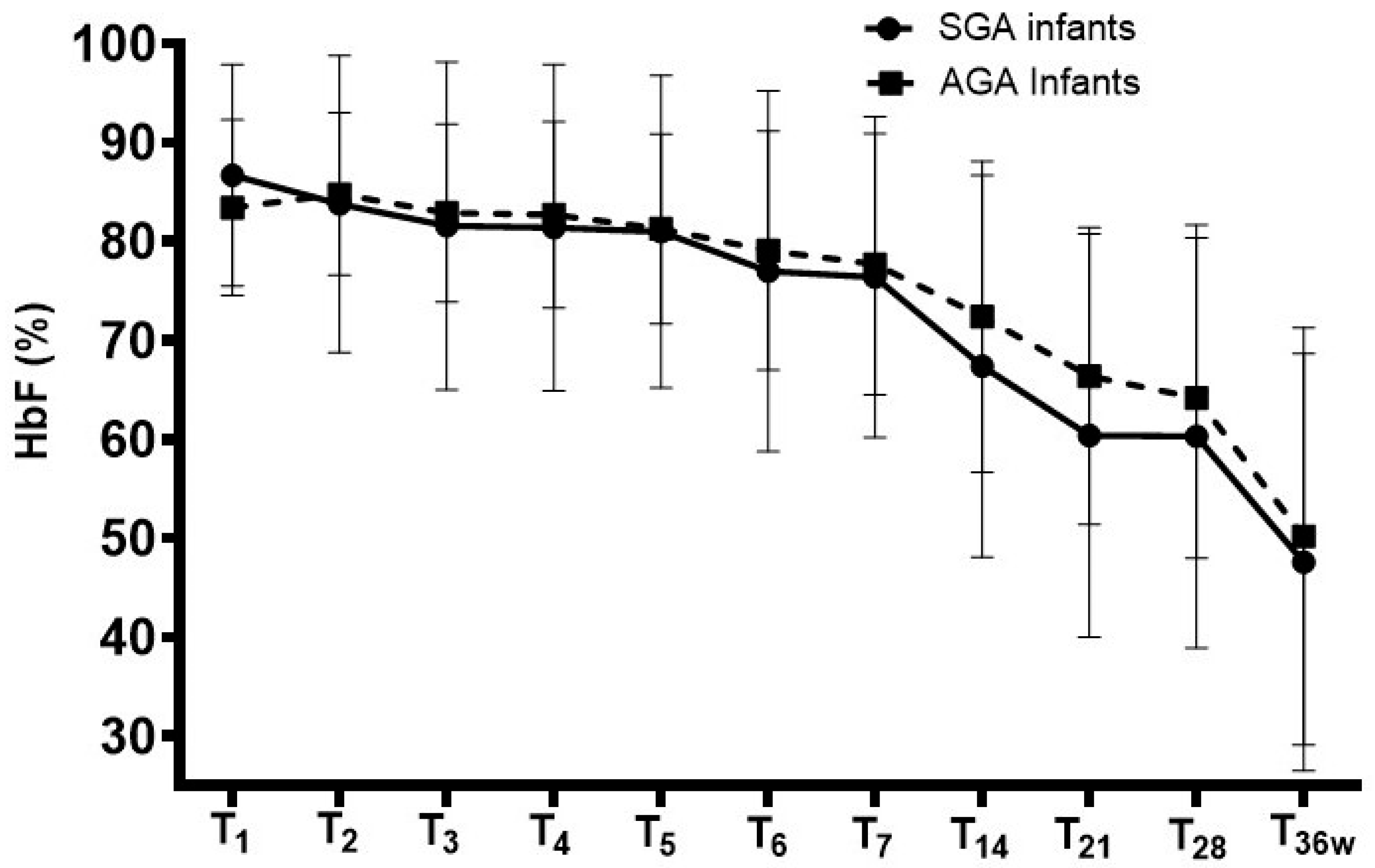
| 1st day of life | 86.7 ± 11.2 | 83.4 ± 8.9 | 0.133 |
| 2nd day of life | 83.8 ± 15.0 | 84.8 ± 8.2 | 0.683 |
| 3rd day of life | 81.6 ± 16.6 | 82.9 ± 9.0 | 0.631 |
| 4th day of life | 81.4 ± 16.5 | 82.7 ± 9.4 | 0.635 |
| 5th day of life | 81.0 ± 15.8 | 81.3 ± 9.6 | 0.911 |
| 6th day of life | 77.0 ± 18.2 | 79.1 ± 12.1 | 0.516 |
| 7th day of life | 76.4 ± 16.2 | 77.7 ± 13.2 | 0.685 |
| p * | <0.001 | <0.001 | |
| 14th day of life | 67.4 ± 19.3 | 72.4 ± 15.7 | 0.191 |
| 21st day of life | 60.4 ± 20.4 | 66.4 ± 15.0 | 0.194 |
| 28th day of life | 60.3 ± 21.4 | 64.2 ± 16.2 | 0.316 |
| 36th weeks of postmenstrual age | 47.6 ± 21.1 | 50.2 ± 21.1 | 0.685 |
| p ** | <0.001 | <0.001 | |
| Nadir level within 36 wks of postmenstrual age | 35.0 ± 16.8 | 38.9 ± 17.4 | 0.314 |
| Age at lowest level (days) | 41 ± 11 | 41 ± 14 | 1.000 |
| Patients transfused with RBCs Number of RBC transfusions Transfusion-to-patient ratio Volume of RBC (mL/kg) per infant | 24 (80) 81 2 (1–3) 32.4 ± 46.7 | 26 (43) 55 1 (1–2) 32.9 ± 22.5 | 0.001 0.491 0.960 |
| Hb (g/L) | ||||||
| Hb 1st Day | Hb 7th Day | Hb 28th day | Hb 31st wks | Hb 36 wks | p | |
| SGA infants | 15.5 ± 4.0 | 13.8 ± 2.2 | 11.3 ± 1.6 | 11.6 ± 2.9 | 10.1 ± 2.2 | <0.001 |
| AGA infants | 17.2 ± 2.4 | 15.8 ± 2.4 | 11.7 ± 1.8 | 12.8 ± 2.5 | 9.8 ± 1.3 | <0.001 |
| p | 0.014 | <0.001 | 0.306 | 0.612 | 0.419 | |
| HbF (g/L) | ||||||
| HbF 1st day | HbF 7th day | HbF 28th day | HbF 31st wks | HbF 36 wks | p | |
| SGA infants | 13.6 ± 4.1 | 10.7 ± 3.3 | 6.5 ± 2.9 | 7.2 ± 3.4 | 4.2 ± 2.6 | <0.001 |
| AGA infants | 14.5 ± 2.9 | 12.5 ± 3.4 | 7.6 ± 2.7 | 8.3 ± 3.4 | 4.6 ± 2.1 | <0.001 |
| p | 0.232 | 0.019 | 0.079 | 0.152 | 0.434 | |
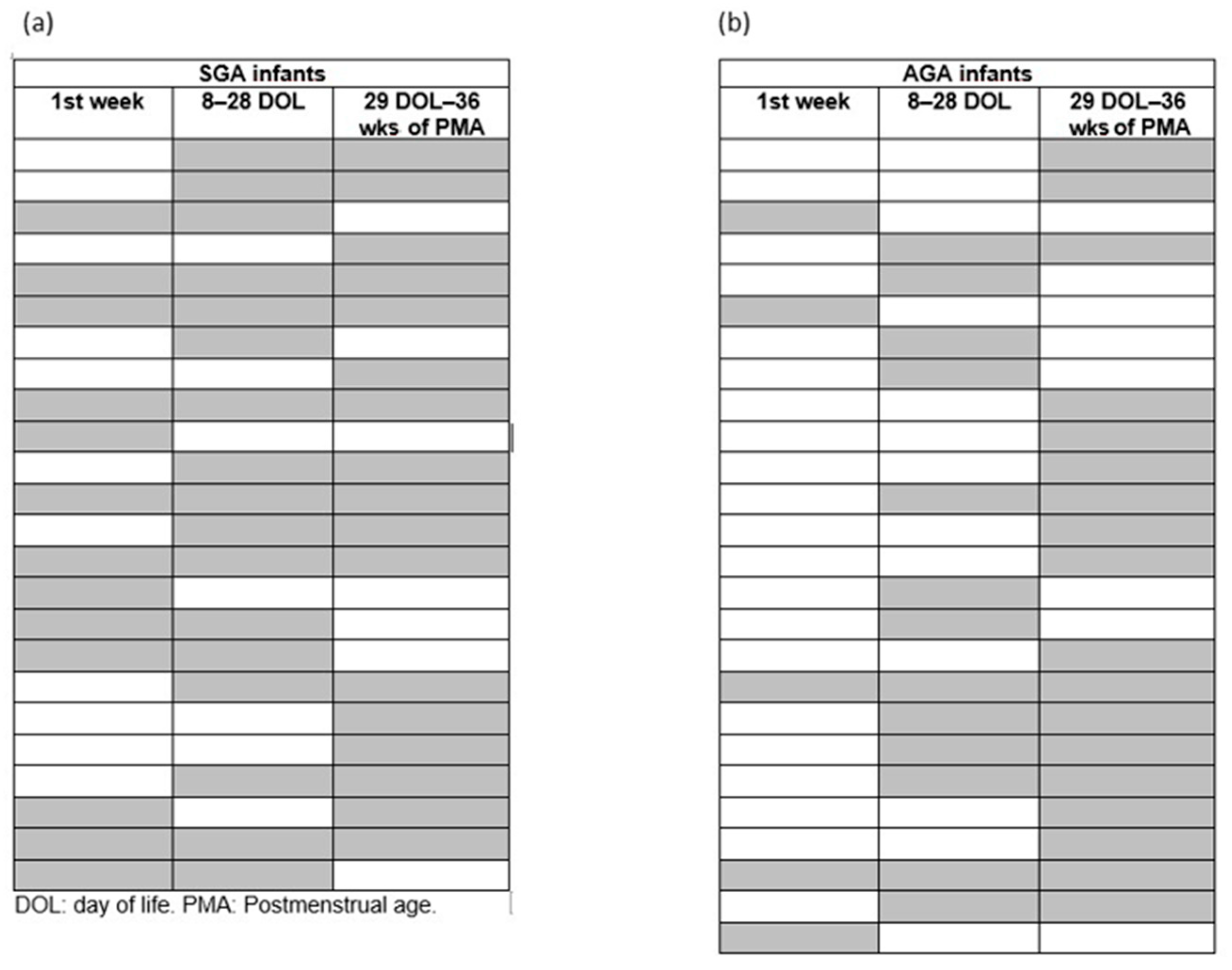
| 1st Week | 8–28 Days of Life | 29 DOL-36 Wks of PCA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SGA | AGA | p | SGA | AGA | p | SGA | AGA | p | |
| Patients | 13 (43) | 5 (8) | <0.001 | 17 (57) | 13 (22) | <0.001 | 17(57) | 18 (30) | 0.014 |
| Number of transfusions | 23 | 6 | 30 | 25 | 28 | 24 | |||
| Transfusion-to-patient ratio | 2 (1–3) | 1 (1–1) | 0.238 | 1 (1–2) | 2 (1–3) | 0.337 | 2 (1–2) | 2 (1–2) | 0.254 |
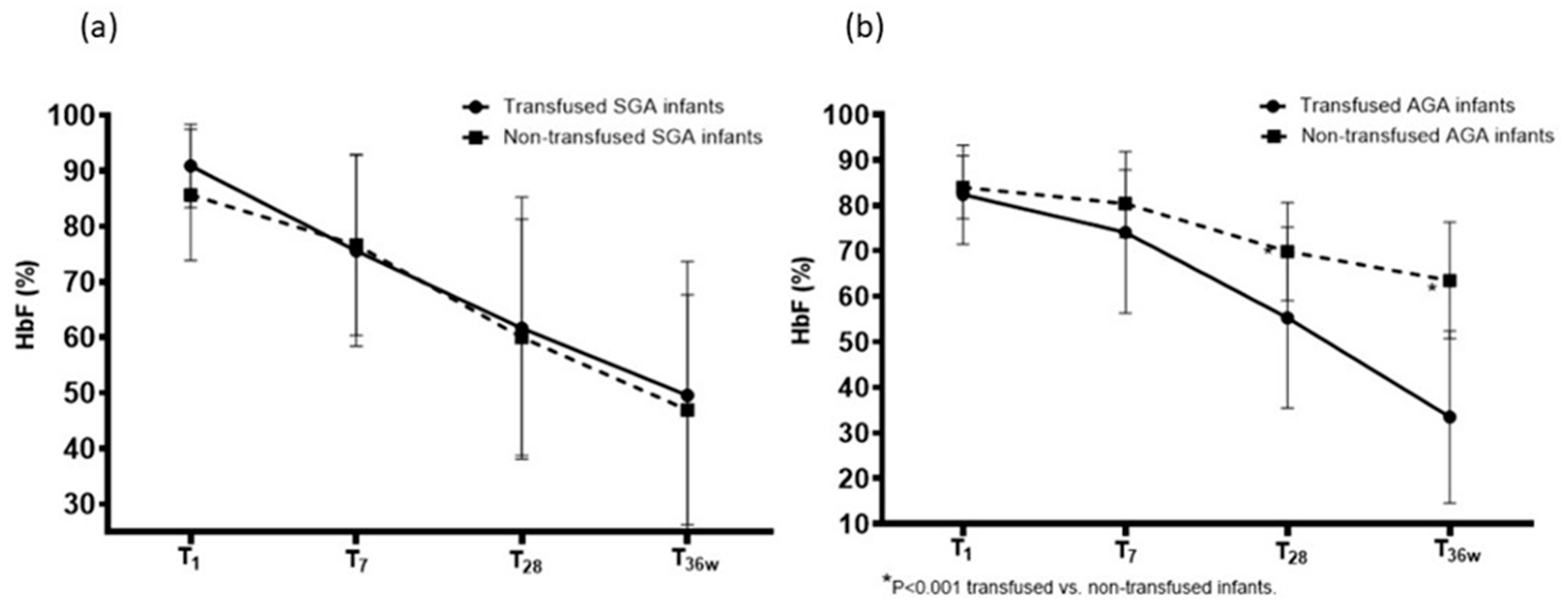
| SGA Infants | |||||
|---|---|---|---|---|---|
| HbF 1st Day | HbF 7th Day | HbF 28th Day | HbF 36 Wks | p | |
| Transfused (n = 24) | 90.9 ± 7.5 a | 75.6 ± 17.2 | 61.7 ± 23.6 | 49.6 ± 24.1 b | <0.001 |
| Non-transfused (n = 6) | 85.7 ± 11.8 | 76.7 ± 16.3 | 60.0 ± 21.3 | 47.0 ± 20.7 c | <0.001 |
| p | 0.316 | 0.885 | 0.865 | 0.792 | |
| AGA infants | |||||
| Transfused (n = 26) | 82.4 ± 10.9 | 74.1 ± 17.8 | 55.3 ± 19.9 | 33.5 ± 18.9 | <0.001 |
| Non-transfused (n = 34) | 84.0 ± 6.9 | 80.4 ± 7.4 | 69.9 ± 10.8 | 63.5 ± 12.8 | <0.001 |
| p | 0.490 | 0.067 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dani, C.; Cipriani, F.; Ciavotta, M.; Remaschi, G. Changes in Fetal Hemoglobin in Very Preterm Infants Born Small for Gestational Age: A Retrospective Observational Study. Children 2026, 13, 117. https://doi.org/10.3390/children13010117
Dani C, Cipriani F, Ciavotta M, Remaschi G. Changes in Fetal Hemoglobin in Very Preterm Infants Born Small for Gestational Age: A Retrospective Observational Study. Children. 2026; 13(1):117. https://doi.org/10.3390/children13010117
Chicago/Turabian StyleDani, Carlo, Federico Cipriani, Maria Ciavotta, and Giulia Remaschi. 2026. "Changes in Fetal Hemoglobin in Very Preterm Infants Born Small for Gestational Age: A Retrospective Observational Study" Children 13, no. 1: 117. https://doi.org/10.3390/children13010117
APA StyleDani, C., Cipriani, F., Ciavotta, M., & Remaschi, G. (2026). Changes in Fetal Hemoglobin in Very Preterm Infants Born Small for Gestational Age: A Retrospective Observational Study. Children, 13(1), 117. https://doi.org/10.3390/children13010117







