Prenatal Cocaine Exposure, Perinatal Risks, and Mediators to Preadolescent Attention Deficit Hyperactivity Disorder (ADHD)
Highlights
- Prenatal cocaine exposure increases the risk of ADHD in preadolescence, with effects largely mediated through attention problems and impulsivity at 4–5 years of age.
- Male sex is an independent risk factor with a direct path to the ADHD diagnosis.
- Screening for behavioral problems before 4 years of age in prenatal cocaine exposure would make possible early intervention to mitigate later diagnosis or severity of ADHD.
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Descriptive, Regression, and Path Analyses
3.1.1. Demographic Characteristics (Table 1)
3.1.2. ADHD Diagnosis by Demographics (Table 2)
| Characteristic | All | DISC Diagnosis at 11/14 Years | |||
|---|---|---|---|---|---|
| N | ADHD N (%) | No ADHD N (%) | Unadjusted OR (95% CI) | p | |
| Demographics | 853 | 106 (12.4) | 747 (87.6) | - | - |
| Prenatal drug exposure | |||||
| High PCE/OD | 95 | 18 (19) | 77 (81) | 2.94 (1.43, 6.05) | 0.004 |
| Some PCE/OD | 198 | 29 (15) | 169 (85) | 2.16 (1.13, 4.10) | 0.019 |
| PCE−/OD+ | 343 | 43 (13) | 300 (87) | 1.80 (0.99, 3.29) | 0.055 |
| PCE−/OD− | 217 | 16 (7) | 201 (93) | REF | |
| Gender | |||||
| Male | 434 | 69 (16) | 365 (84) | 1.95 (1.28, 2.98) | 0.002 |
| Female | 419 | 37 (9) | 382 (91) | REF | |
| Race/Ethnicity | |||||
| Black | 683 | 78 (11) | 605 (89) | 0.53 (0.31, 0.90) | 0.019 |
| White | 107 | 21 (20) | 86 (80) | REF | |
| Other | 63 | 7 (11) | 56 (89) | 0.51 (0.20, 1.28) | 0.153 |
| Birth weight | |||||
| <2500 g | 342 | 47 (14) | 295 (86) | 1.22 (0.81, 1.84) | 0.341 |
| ≥2500 g | 511 | 59 (12) | 452 (88) | REF | |
| Maternal age at birth | 853 | 27.8 ± 5.5 | 27.7 ± 5.9 | 1.00 (0.97, 1.04) | 0.954 |
| Education | |||||
| Less than high school | 312 | 43 (14) | 269 (86) | 1.09 (0.64, 1.86) | 0.764 |
| High school | 354 | 39 (11) | 315 (89) | 0.84 (0.49, 1.45) | 0.531 |
| More than high school | 187 | 24 (13) | 163 (87) | REF | |
| Caretaker psychopathology (BSI) (4 months to 5 years) | 828 | 0.7 ± 0.6 | 0.6 ± 0.5 | 1.99 (1.37, 2.88) | <0.001 |
| Relationship with child: Conflict score at 5 years | 602 | 2.7 ± 0.7 | 2.4 ± 0.7 | 1.81 (1.31, 2.50) | <0.001 |
| CBQ child impulsivity score at 4 years | 658 | 2.5 ± 0.3 | 2.3 ± 0.3 | 6.41 (2.83, 14.50) | <0.001 |
| CBCL attention problems at 5 years | 61 | 27(44) | 34 (56) | 7.09 (3.98,12.64) | <0.001 |
| 536 | 54 (10) | 482 (90) | REF | ||
3.1.3. ADHD Diagnosis According to Levels of PCE (Figure 1)
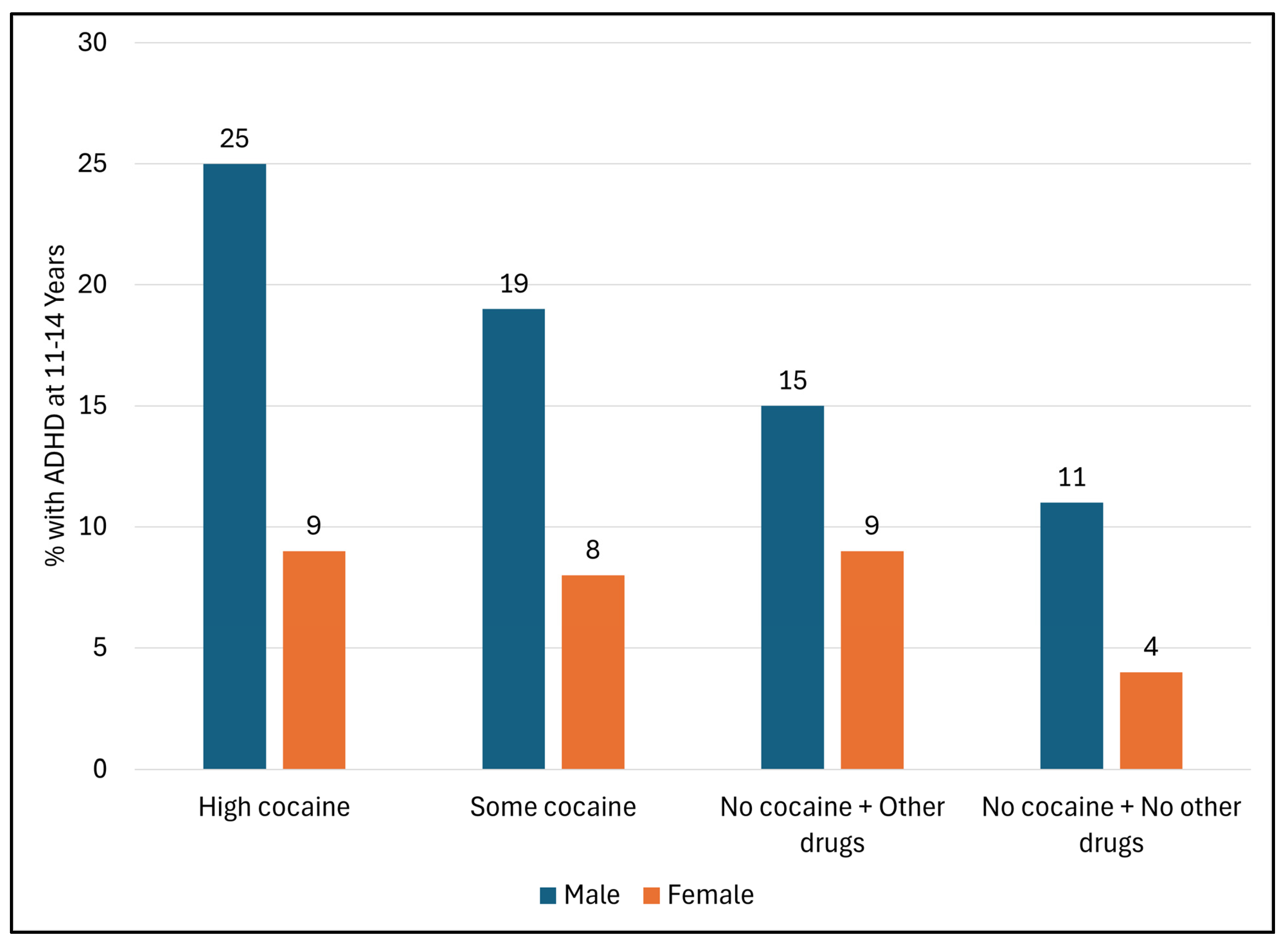
3.1.4. Logistic Regression Analyses (Table 3)
| Variable | N (%) with ADHD | Adjusted OR (95%CI) | p |
|---|---|---|---|
| Prenatal drug exposure | |||
| High PCE/OD | 18 (19) | 2.56 (1.02, 6.41) | 0.045 |
| Some PCE/OD | 29 (15) | 1.96 (0.90, 4.27) | 0.092 |
| PCE−/OD+ | 43 (13) | 1.70 (0.83, 3.48) | 0.148 |
| PCE−/OD− | 16 (7) | REF | |
| Sex | |||
| Male | 69 (16) | 2.38 (1.41, 4.03) | 0.001 |
| Female | 37 (9) | REF | |
| Race/Ethnicity | |||
| Black | 78 (11) | 0.58 (0.33, 1.02) | 0.060 |
| White/Other | 28 (16) | REF | |
| Birth weight | |||
| <2500 g | 47 (14) | 1.76 (1.06, 2.91) | 0.029 |
| ≥2500 g | 59 (12) | REF | |
| Education | |||
| Less than high school | 43 (14) | 1.01 (0.60, 1.70) | 0.976 |
| High school or more | 63 (12) | REF | |
| Caretaker BSI (4 months to 5 years) | -- | 1.74 (1.10, 2.75) | 0.018 |
| Relationship with child—Conflict score (5 years) | -- | 1.04 (1.01, 1.07) | 0.008 |
3.1.5. Path Analysis (Figure 2)

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention Deficit Hyperactivity Disorder |
| PCE | Prenatal Cocaine Exposure |
| CBCL | Child Behavioral Checklist |
| BSI | Brief Symptom Inventory |
References
- Faraone, S.V.; Biederman, J.; Mick, E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychol. Med. 2006, 36, 159–165. [Google Scholar] [CrossRef]
- Hartman, C.A.; Larsson, H.; Vos, M.; Bellato, A.; Libutzki, B.; Solberg, B.S.; Chen, Q.; Du Rietz, E.; Mostert, J.C.; Kittel-Schneider, S.; et al. Anxiety, mood, and substance use disorders in adult men and women with and without attention-deficit/hyperactivity disorder: A substantive and methodological overview. Neurosci. Biobehav. Rev. 2023, 151, 105209. [Google Scholar] [CrossRef]
- Cortese, S.; Song, M.; Farhat, L.C.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Park, S.; Oh, J.W.; Lee, S.; Cheon, K.A.; et al. Incidence, prevalence, and global burden of ADHD from 1990 to 2019 across 204 countries: Data, with critical re-analysis, from the Global Burden of Disease study. Mol. Psychiatry 2023, 28, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- NSCH. National Survey of Children’s Health: Indicator 2.7a: Severity of ADD/ADHD, Age 3–17 Years. Available online: https://www.childhealthdata.org/browse/survey/results?q=11077&r=1 (accessed on 1 May 2025).
- NSCH. National Survey of Children’s Health: Indicator 2.7: Prevalence of ADD/ADHD, Age 3–17 Years. Available online: https://www.childhealthdata.org/browse/survey/results?q=11076&r=1 (accessed on 1 May 2025).
- Danielson, M.L.; Claussen, A.H.; Bitsko, R.H.; Katz, S.M.; Newsome, K.; Blumberg, S.J.; Kogan, M.D.; Ghandour, R. ADHD Prevalence Among U.S. Children and Adolescents in 2022: Diagnosis, Severity, Co-Occurring Disorders, and Treatment. J. Clin. Child Adolesc. Psychol. 2024, 53, 343–360. [Google Scholar] [CrossRef]
- CDC. Data and Statistics on ADHD. Available online: https://www.cdc.gov/adhd/data/?CDC_AAref_Val=https://www.cdc.gov/ncbddd/adhd/data.html (accessed on 1 May 2025).
- Kirova, A.M.; Kelberman, C.; Storch, B.; DiSalvo, M.; Woodworth, K.Y.; Faraone, S.V.; Biederman, J. Are subsyndromal manifestations of attention deficit hyperactivity disorder morbid in children? A systematic qualitative review of the literature with meta-analysis. Psychiatry Res. 2019, 274, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Bellgrove, M.A.; Brikell, I.; Cortese, S.; Hartman, C.A.; Hollis, C.; Newcorn, J.H.; Philipsen, A.; Polanczyk, G.V.; Rubia, K.; et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2024, 10, 11. [Google Scholar] [CrossRef]
- Schoenfelder, E.N.; Kollins, S.H. Topical Review: ADHD and Health-Risk Behaviors: Toward Prevention and Health Promotion. J. Pediatr. Psychol. 2016, 41, 735–740. [Google Scholar] [CrossRef]
- Tso, W.W.; Ho, F.K.W.; Coghill, D.; Lee, T.M.; Wang, Y.; Lee, S.L.; Wong, M.S.; Yam, J.C.S.; Wong, I.C.K.; Ip, P. Preterm postnatal complications and risk of attention-deficit/hyperactivity disorder. Dev. Med. Child Neurol. 2023, 65, 358–366. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.Y.; Lee, J.; Jeong, G.H.; Lee, E.; Lee, S.; Lee, K.H.; Kronbichler, A.; Stubbs, B.; Solmi, M.; et al. Environmental risk factors, protective factors, and peripheral biomarkers for ADHD: An umbrella review. Lancet Psychiatry 2020, 7, 955–970. [Google Scholar] [CrossRef]
- Cecil, C.A.M.; Nigg, J.T. Epigenetics and ADHD: Reflections on Current Knowledge, Research Priorities and Translational Potential. Mol. Diagn. Ther. 2022, 26, 581–606. [Google Scholar] [CrossRef]
- Garrison-Desany, H.M.; Hong, X.; Maher, B.S.; Beaty, T.H.; Wang, G.; Pearson, C.; Liang, L.; Wang, X.; Ladd-Acosta, C. Individual and Combined Association Between Prenatal Polysubstance Exposure and Childhood Risk of Attention-Deficit/Hyperactivity Disorder. JAMA Netw. Open 2022, 5, e221957. [Google Scholar] [CrossRef]
- Bada, H.S.; Das, A.; Bauer, C.R.; Shankaran, S.; Lester, B.; LaGasse, L.; Hammond, J.; Wright, L.L.; Higgins, R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics 2007, 119, e348–e359. [Google Scholar] [CrossRef]
- Bada, H.S.; Bann, C.M.; Bauer, C.R.; Shankaran, S.; Lester, B.; LaGasse, L.; Hammond, J.; Whitaker, T.; Das, A.; Tan, S.; et al. Preadolescent behavior problems after prenatal cocaine exposure: Relationship between teacher and caretaker ratings (Maternal Lifestyle Study). Neurotoxicol. Teratol. 2011, 33, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, M.S.; Murphy, J.M. Screening for psychosocial disorders in pediatric practice. Am. J. Dis. Child. 1988, 142, 1153–1157. [Google Scholar] [CrossRef]
- Jellinek, M.S.; Murphy, J.M.; Little, M.; Pagano, M.E.; Comer, D.M.; Kelleher, K.J. Use of the Pediatric Symptom Checklist to screen for psychosocial problems in pediatric primary care: A national feasibility study. Arch. Pediatr. Adolesc. Med. 1999, 153, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, T.M.; Bada, H.S.; Bann, C.M.; Shankaran, S.; LaGasse, L.; Lester, B.M.; Bauer, C.R.; Hammond, J.; Higgins, R. Serial pediatric symptom checklist screening in children with prenatal drug exposure. J. Dev. Behav. Pediatr. 2011, 32, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Anderson, J.G. Factors in children with a history of neonatal abstinence syndrome at 10 years of age: Evidence from the maternal lifestyle study. J. Spec. Pediatr. Nurs. 2022, 27, e12358. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.; Fisher, P.; Lucas, C.P.; Dulcan, M.K.; Schwab-Stone, M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences from Previous Versions, and Reliability of Some Common Diagnoses. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 28–38. [Google Scholar] [CrossRef]
- Lester, B.M.; Tronick, E.Z.; LaGasse, L.; Seifer, R.; Bauer, C.R.; Shankaran, S.; Bada, H.S.; Wright, L.L.; Smeriglio, V.L.; Lu, J.; et al. The maternal lifestyle study: Effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics 2002, 110, 1182–1192. [Google Scholar] [CrossRef]
- Bada, H.S.; Bann, C.M.; Whitaker, T.M.; Bauer, C.R.; Shankaran, S.; Lagasse, L.; Lester, B.M.; Hammond, J.; Higgins, R. Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics 2012, 130, e1479–e1488. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Bihun, J.T.; Chiodo, L.M. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev. Psychopathol. 1999, 11, 195–208. [Google Scholar] [CrossRef]
- Derogatis, L.R. BSI Brief Symptom Inventory, Administration, Scoring, Procedures Manual; National Computer Systems: Minneapolis, MN, USA, 1993. [Google Scholar]
- Pianta, R.C. Patterns of Relationships Between Children and Kindergarten Teachers. J. Sch. Psychol. 1994, 32, 15–31. [Google Scholar] [CrossRef]
- Sroufe, L.A. The Coherence of Individual Development: Early Care, Attachment, and Subsequent Developmental Issues. Am. Psychol. 1979, 34, 834–841. [Google Scholar] [CrossRef]
- Rothbart, M. Measurement of temperament in infancy. Child Dev. 1981, 52, 569–578. [Google Scholar] [CrossRef]
- Rothbart, M.K.; Ahadi, S.A.; Hershey, K.L.; Fisher, P. Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Dev. 2001, 72, 1394–1408. [Google Scholar] [CrossRef]
- Achenbach, T.M. Manual for the Child Behavior Checklist/4-18 and 1991 Profile; Department of Psychiatry, University of Vermont: Burlington, VT, USA, 1991. [Google Scholar]
- Pauli-Pott, U.; Becker, K. Impulsivity as Early Emerging Vulnerability Factor-Prediction of ADHD by a Preschool Neuropsychological Measure. Brain Sci. 2021, 11, 60. [Google Scholar] [CrossRef]
- Biederman, J.; DiSalvo, M.; Vaudreuil, C.; Wozniak, J.; Uchida, M.; Woodworth, K.Y.; Green, A.; Farrell, A.; Faraone, S.V. The child behavior checklist can aid in characterizing suspected comorbid psychopathology in clinically referred youth with ADHD. J. Psychiatr. Res. 2021, 138, 477–484. [Google Scholar] [CrossRef]
- Accornero, V.H.; Amado, A.J.; Morrow, C.E.; Xue, L.; Anthony, J.C.; Bandstra, E.S. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J. Dev. Behav. Pediatr. 2007, 28, 195–205. [Google Scholar] [CrossRef]
- Richardson, G.A.; Conroy, M.L.; Day, N.L. Prenatal cocaine exposure: Effects on the development of school-age children. Neurotoxicol. Teratol. 1996, 18, 627–634. [Google Scholar] [CrossRef]
- Nygaard, E.; Slinning, K.; Moe, V.; Walhovd, K.B. Behavior and Attention Problems in Eight-Year-Old Children with Prenatal Opiate and Poly-Substance Exposure: A Longitudinal Study. PLoS ONE 2016, 11, e0158054. [Google Scholar] [CrossRef]
- Richardson, G.A.; Goldschmidt, L.; Leech, S.; Willford, J. Prenatal cocaine exposure: Effects on mother- and teacher-rated behavior problems and growth in school-age children. Neurotoxicol. Teratol. 2011, 33, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.J.; Graham, D.L.; Money, K.M.; Stanwood, G.D. Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.; Maymi, V.; Pawlyszyn, S.; Yu, L.; Zhdanova, I.V. Prenatal cocaine exposure disrupts the dopaminergic system and its postnatal responses to cocaine. Genes Brain Behav. 2018, 17, e12436. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, J.D.; Morrow, B.A.; Nguyen, V.T.; Mitra, J.; Picciotto, M.R.; Roth, R.H. Prenatal cocaine exposure enhances responsivity of locus coeruleus norepinephrine neurons: Role of autoreceptors. Neuroscience 2007, 147, 419–427. [Google Scholar] [CrossRef][Green Version]
- Rando, K.; Chaplin, T.M.; Potenza, M.N.; Mayes, L.; Sinha, R. Prenatal cocaine exposure and gray matter volume in adolescent boys and girls: Relationship to substance use initiation. Biol. Psychiatry 2013, 74, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lester, B.M.; Neyzi, N.; Sheinkopf, S.J.; Gracia, L.; Kekatpure, M.; Kosofsky, B.E. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr. 2013, 167, 348–354. [Google Scholar] [CrossRef]
- Warner, T.D.; Behnke, M.; Eyler, F.D.; Padgett, K.; Leonard, C.; Hou, W.; Garvan, C.W.; Schmalfuss, I.M.; Blackband, S.J. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics 2006, 118, 2014–2024. [Google Scholar] [CrossRef]
- Lent, A.; Dunn, A.; Eldawy, N.; Jhumkhawala, V.; Rao, M.; Sohmer, J.; Sacca, L. Trends in Childhood Behavioral, Mental, and Developmental Problems (2019–2022) Using the National Survey of Children’s Health. Pediatr. Rep. 2024, 16, 983–1000. [Google Scholar] [CrossRef]
- Tully, L.A.; Arseneault, L.; Caspi, A.; Moffitt, T.E.; Morgan, J. Does maternal warmth moderate the effects of birth weight on twins’ attention-deficit/hyperactivity disorder (ADHD) symptoms and low IQ? J. Consult. Clin. Psychol. 2004, 72, 218–226. [Google Scholar] [CrossRef]
- Mick, E.; Biederman, J.; Prince, J.; Fischer, M.J.; Faraone, S.V. Impact of low birth weight on attention-deficit hyperactivity disorder. J. Dev. Behav. Pediatr. 2002, 23, 16–22. [Google Scholar] [CrossRef]
- Jackson, D.B.; Beaver, K.M. Sibling differences in low birth weight, dopaminergic polymorphisms, and ADHD symptomatology: Evidence of GxE. Psychiatry Res. 2015, 226, 467–473. [Google Scholar] [CrossRef]
- Waddell, J.; McCarthy, M.M. Sexual Differentiation of the Brain and ADHD: What Is a Sex Difference in Prevalence Telling Us? In Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment; Stanford, C., Tannock, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 341–360. [Google Scholar]
- Martin, J. Why are females less likely to be diagnosed with ADHD in childhood than males? Lancet Psychiatry 2024, 11, 303–310. [Google Scholar] [CrossRef]
- Skoglund, C.; Sundström Poromaa, I.; Leksell, D.; Ekholm Selling, K.; Cars, T.; Giacobini, M.; Young, S.; Kopp Kallner, H. Time after time: Failure to identify and support females with ADHD—A Swedish population register study. J. Child Psychol. Psychiatry 2024, 65, 832–844. [Google Scholar] [CrossRef]
- Martin, J.; Langley, K.; Cooper, M.; Rouquette, O.Y.; John, A.; Sayal, K.; Ford, T.; Thapar, A. Sex differences in attention-deficit hyperactivity disorder diagnosis and clinical care: A national study of population healthcare records in Wales. J. Child Psychol. Psychiatry 2024, 65, 1648–1658. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hagan, J.F., Jr.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef]
- Shaw, M.; Hodgkins, P.; Caci, H.; Young, S.; Kahle, J.; Woods, A.G.; Arnold, L.E. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Med. 2012, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Eickmann, S.H.; Emond, A.M.; Lima, M. Evaluation of child development: Beyond the neuromotor aspect. J. Pediatr. 2016, 92, S71–S83. [Google Scholar] [CrossRef][Green Version]
- Carter, A.S.; Briggs-Gowan, M.J.; Davis, N.O. Assessment of young children’s social-emotional development and psychopathology: Recent advances and recommendations for practice. J. Child Psychol. Psychiatry 2004, 45, 109–134. [Google Scholar] [CrossRef]
- Sim, F.; Thompson, L.; Marryat, L.; Ramparsad, N.; Wilson, P. Predictive validity of preschool screening tools for language and behavioural difficulties: A PRISMA systematic review. PLoS ONE 2019, 14, e0211409. [Google Scholar] [CrossRef]
- Dutch, D.; Bell, L.; Zarnowiecki, D.; Johnson, B.J.; Denney-Wilson, E.; Byrne, R.; Cheng, H.; Rossiter, C.; Manson, A.; House, E.; et al. Screening tools used in primary health care settings to identify health behaviours in children (birth-16 years); A systematic review of their effectiveness, feasibility and acceptability. Obes. Rev. 2024, 25, e13694. [Google Scholar] [CrossRef]
- Marks, K.P.; Page Glascoe, F.; Macias, M.M. Enhancing the algorithm for developmental-behavioral surveillance and screening in children 0 to 5 years. Clin. Pediatr. 2011, 50, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Resorla, L.A. The Child Behavior Checklist (CBCL/1.5-5); Research Center for Children, Youth, & Families, Univeristy of Vermont: Burlington, VT, USA, 2000. [Google Scholar]
- Williford, A.P.; Shelton, T.L. Behavior management for preschool-aged children. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.C.; Epstein, J.N.; Hinshaw, S.P.; Conners, C.K.; Klaric, J.; Abikoff, H.B.; Abramowitz, A.; Arnold, L.E.; Elliott, G.; Greenhill, L.L.; et al. Parenting and family stress treatment outcomes in attention deficit hyperactivity disorder (ADHD): An empirical analysis in the MTA study. J. Abnorm. Child Psychol. 2000, 28, 543–553. [Google Scholar] [CrossRef]
- Pfiffner, L.J.; Haack, L.M. Behavior management for school-aged children with ADHD. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 731–746. [Google Scholar] [CrossRef]
- Doffer, D.P.A.; Dekkers, T.J.; Hornstra, R.; van der Oord, S.; Luman, M.; Leijten, P.; Hoekstra, P.J.; van den Hoofdakker, B.J.; Groenman, A.P. Sustained improvements by behavioural parent training for children with attention-deficit/hyperactivity disorder: A meta-analytic review of longer-term child and parental outcomes. JCPP Adv. 2023, 3, e12196. [Google Scholar] [CrossRef]
- Arendt, R.E.; Singer, L.T.; Minnes, S.; Salvator, A. Accuracy in Detecting Prenatal Drug Exposure. J. Drug Issues 1999, 29, 203–214. [Google Scholar] [CrossRef]
| Characteristic | All N = 853 | High PCE/OD N= 95 (11.1) | Some PCE/OD N = 198 (23.2) | PCE−/OD+ N = 343 (40.2) | PCE−/OD− N = 217 (25.4) |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Demographics | |||||
| Gender | |||||
| Male | 434 (51) | 46 (48) | 101 (51) | 175 (51) | 112 (52) |
| Female | 419 (49) | 49 (52) | 97 (49) | 168 (49) | 105 (48) |
| Race/Ethnicity | |||||
| Black | 683 (80) | 77 (81) | 166 (84) | 257 (75) | 183 (84) |
| White | 107 (13) | 12 (13) | 18 (9) | 60 (17) | 17 (8) |
| Other | 63 (7) | 6 (6) | 14 (7) | 26 (8) | 17 (8) |
| Birth weight | |||||
| <2500 g | 342 (40) | 38 (40) | 83 (42) | 121 (35) | 100 (46) |
| ≥2500 g | 511 (60) | 57 (60) | 115 (58) | 222 (65) | 117 (54) |
| Less than high school education | 312 (37) | 50 (53) | 91 (46) | 117 (34) | 54 (25) |
| Caretaker psychopathology (BSI) (4 months to 5 years), mean (SD) | 0.6 (0.5) | 0.7 (0.5) | 0.6 (0.5) | 0.6 (0.5) | 0.5 (0.5) |
| Relationship with child: Conflict score at 5 years), mean (SD) | 28.9 (8.7) | 31.1 (8.8) | 30.5 (8.8) | 28.9 (8.1) | 26.9 (9.1) |
| Mediators | |||||
| CBQ child impulsivity score at 4 years, mean (SD) | 2.3 (0.3) | 2.4 (0.3) | 2.4 (0.3) | 2.4 (0.3) | 2.3 (0.3) |
| CBCL Attention problems at 5 years | 61 (10) | 8 (12) | 25 (19) | 22 (9) | 6 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sithisarn, T.; Bann, C.M.; Lester, B.; Shankaran, S.; Whitaker, T.; Higgins, R.D.; Bada, H. Prenatal Cocaine Exposure, Perinatal Risks, and Mediators to Preadolescent Attention Deficit Hyperactivity Disorder (ADHD). Children 2025, 12, 1570. https://doi.org/10.3390/children12111570
Sithisarn T, Bann CM, Lester B, Shankaran S, Whitaker T, Higgins RD, Bada H. Prenatal Cocaine Exposure, Perinatal Risks, and Mediators to Preadolescent Attention Deficit Hyperactivity Disorder (ADHD). Children. 2025; 12(11):1570. https://doi.org/10.3390/children12111570
Chicago/Turabian StyleSithisarn, Thitinart, Carla M. Bann, Barry Lester, Seetha Shankaran, Toni Whitaker, Rosemary D. Higgins, and Henrietta Bada. 2025. "Prenatal Cocaine Exposure, Perinatal Risks, and Mediators to Preadolescent Attention Deficit Hyperactivity Disorder (ADHD)" Children 12, no. 11: 1570. https://doi.org/10.3390/children12111570
APA StyleSithisarn, T., Bann, C. M., Lester, B., Shankaran, S., Whitaker, T., Higgins, R. D., & Bada, H. (2025). Prenatal Cocaine Exposure, Perinatal Risks, and Mediators to Preadolescent Attention Deficit Hyperactivity Disorder (ADHD). Children, 12(11), 1570. https://doi.org/10.3390/children12111570






