Breastfeeding, Mother–Child Dyads of Interaction, and Neurodevelopment of Preterm Children: A Longitudinal Study of Feeding Methods During the First Two Years
Highlights
- Exclusive breastfeeding was associated with consistently higher motor, cognitive, language, and social scores in preterm children during the first two years of life compared to formula feeding; mixed feeding showed intermediate outcomes.
- The quality of mother–child interactions was a stronger predictor of better developmental trajectories, while clinical vulnerabilities (e.g., prolonged NICU stay, BPD, leukomalacia) negatively influenced outcomes.
- Breastfeeding functions as both a biological and relational protective factor, supporting neurodevelopment while strengthening sensitive mother–child interactions.
- Interventions that promote breastfeeding and responsive caregiving are crucial strategies to buffer clinical risks and optimize developmental outcomes in preterm children.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedures
2.4. Data Analysis
3. Results
3.1. Clinical and Environmental Outcomes: Group Comparisons
3.2. Cognitive, Motor, Language, and Social Development: Group Comparisons
3.3. Maternal Parenting Skills and Mother/Child Dyads of Interactions: Groups Comparisons
3.4. Longitudinal Associations Between Risk and Protective Factors and Developmental Outcomes
4. Discussion
4.1. Group Characteristics: Neonatal Clinical and Environmental Factors
4.2. Child Development, Mother Parenting Skills, and Mother–Child Interactions over Time
4.3. Associations Between Risks and Protective Factors: Motor, Cognitive, Language, and Social Development of Preterm Children
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministério da Saúde do Brasil. II Pesquisa de Prevalência de Aleitamento Materno Nas Capitais Brasileiras E Distrito Federal; Ministério da Saúde do Brasil: Brasília, Brazil, 2009.
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015, 2015, CD005495. [Google Scholar] [CrossRef] [PubMed]
- Panceri, C.; Valentini, N.C.; Silveira, R.C.; Smith, B.A.; Procianoy, R.S. Neonatal adverse outcomes, neonatal birth risks, and socioeconomic status: Combined influence on preterm infants’ cognitive, language, and motor development in Brazil. J. Child Neurol. 2020, 35, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Panceri, C.; Silveira, R.C.; Procianoy, R.S.; Valentini, N.C. Motor development in the first year of life predicts impairments in cognition and language at 3 years old in a Brazilian preterm cohort of low-income families. Front. Neurosci. 2022, 16, 1764. [Google Scholar] [CrossRef] [PubMed]
- Hass, J.V.; Panceri, C.; Procianoy, R.S.; Silveira, R.C.; Valentini, N.C. Risk Factors for cognitive, motor and language development of preterm children in the first year of life. Rev. Paul. Pediatr. 2022, 41, e2021165. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.A.C.F.; Silva, F.P.S.; Santos, M.M.; Dusing, S.C. Impact of mother–infant interaction on development during the first year of life: A systematic review. J. Child Health Care 2019, 24, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Valentini, N.C.; de Borba, L.S.; Panceri, C.; Smith, B.A.; Procianoy, R.S.; Silveira, R.C. Early detection of cognitive, language, and motor delays for low-income preterm infants: A Brazilian cohort longitudinal study on infants’ neurodevelopment and maternal practice. Front. Psychol. 2021, 12, 753551. [Google Scholar] [CrossRef] [PubMed]
- Caldas, I.F.R.; Garotti, M.F.; Shiramizu, V.K.M.; Pereira, A. The socio-communicative development of preterm infants is resistant to the negative effects of parity on maternal responsiveness. Front. Psychol. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Bronfenbrenner, U. Foreword. In Extending Families: The Social Networks of Parents and Their Children; Cochran, M., Larner, M., Riley, D., Gunnarsson, L., Henderson, C.R., Jr., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. vii–xii. [Google Scholar]
- Bronfenbrenner, U. Foreword. In Parenting: An Ecological Perspective; Luster, T., Okagaki, L., Eds.; Lawrence Erlbaum: Mahwah, NJ, USA, 1993; pp. vii–xi. [Google Scholar]
- McCrory, C.; Murray, A. The effect of breastfeeding on neuro-development in infancy. Matern. Child Health J. 2013, 17, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Riboldi, L.; Coscia, A. Role of the biological active components of human milk on long-term growth and neurodevelop-mental outcome. Ital. J. Pediatr. 2024, 50, 201. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C.; Beauchemin, J.; D’Sa, V.; Bonham, K.; Klepac-Ceraj, V. Enhanced Brain Myelination and Cognitive Development in Young Children Associated with Milk Fat Globule Membrane (MFGM) Intake: A Temporal Cohort Study. Res. Sq. 2024, rs.3.rs-4999582. [Google Scholar] [CrossRef]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, I.K.; Ueland, P.M.; Markestad, T.; Midttun, Ø.; Monsen, A.L.B. Motor development related to duration of exclusive breastfeeding, B vitamin status and B12 supplementation in infants with a birth weight between 2000–3000 g, results from a randomized intervention trial. BMC Pediatr. 2015, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Vohr, B.R.; Poindexter, B.B.; Dusick, A.M.; McKinley, L.T.; Higgins, R.D.; Langer, J.C.; Poole, W.K. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics 2006, 118, e115–e123. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Strategy for Infant and Young Child Feeding: The Optimal Duration of Exclusive Breastfeeding; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Gianni, M.L.; Bezze, E.N.; Sannino, P.; Baro, M.; Roggero, P.; Muscolo, S.; Plevani, L.; Mosca, F. Maternal views on facilitators of and barriers to breastfeeding preterm infants. BMC Pediatr. 2018, 18, 283. [Google Scholar] [CrossRef]
- Ericson, J.; Lampa, E.; Flacking, R. Breastfeeding satisfaction post hospital discharge and associated factors: A longitudinal cohort study of mothers of preterm infants. Int. Breastfeed. J. 2021, 16, 28. [Google Scholar] [CrossRef]
- Gomes, A.L.M.; Balamint, T.; Magesti, B.N.; Querido, D.L.; Scochi, C.G.S.; Christoffel, M.M. Práticas de incentivo e apoio à amamentação de recém-nascidos prematuros na perspectiva da mãe. Adv. Nurs. Health 2019, 1, 98–112. [Google Scholar] [CrossRef]
- Victora, C.G.; Horta, B.L.; de Mola, C.L.; Quevedo, L.; Pinheiro, R.T.; Gigante, D.P.; Barros, F.C. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: A prospective birth cohort study from Brazil. Lancet Glob. Health 2015, 3, e199–e205. [Google Scholar] [CrossRef]
- Pickler, R.H.; Wetzel, P.A.; Meinzen-Derr, J.; Tubbs-Cooley, H.L.; Moore, M. Patterned feeding experience for preterm infants: Study protocol for a randomized controlled trial. Trials 2015, 16, 255. [Google Scholar] [CrossRef]
- Alanazi, M.; Altawili, M.A.; Khayyal, A.I.; Alahmari, A.S.; Alhakami, A.A.; Alshehri, A.M.A. Impact of early nutrition interventions on the growth and development of preterm infants: A narrative review. Cureus 2024, 16, e54888. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.R.; Erickson, S.J.; MacLean, P.C.; Duvall, S.W.; Fuller, J.; Caplan, R. Behavioral problems are associated with cognitive and language scores in toddlers born extremely preterm. Early Hum. Dev. 2019, 128, 48–54. [Google Scholar] [CrossRef] [PubMed]
| Clinical Factors | Premature Children Groups (N = 116) | Group Comparisons p | ||
|---|---|---|---|---|
| Exclusive Breastfeeding (N = 26) | Mixed Feeding (N = 46) | Formula (N = 44) | ||
| M (SD) | ||||
| Gestational age (weeks) | 31.05 (3.64) | 29.67 (2.84) | 29.87 (3.27) | 0.086 |
| Weight at birth (grams) | 1458.46 (778.12) | 1301.47 (754.18) | 1295.65 (560.82) | 0.561 |
| Length at birth (cm) | 39.61 (4.58) | 37.78 (4.71) | 38.43 (4.70) | 0.114 |
| Cephalic perimeter at birth (cm) | 27.56 (2.90) | 26.65 (3.05) | 27.03 (2.70) | 0.221 |
| APGAR 5th minute | 8.19 (1.13) | 7.43 (1.82) | 7.41 (1.94) | 0.145 |
| NICU stay (days) | 44.85 (25.90) | 62.87 (29.45) | 64.59 (32.78) | 0.025 |
| Mechanical ventilation (days) | 1.15 (4.80) | 5.63 (11.35) | 7.52 (16.16) | 0.135 |
| CPAP (days) | 2.11 (4.72) | 3.89 (6.21) | 2.84 (4.78) | 0.414 |
| Parenteral nutrition | 5.64 (5.42) | 12.41 (12.51) | 12.59 (16.62) | 0.077 |
| N (%) | ||||
| Sex Boys | 11 (42.3) | 25 (54.3) | 21 (45.7) | 0.600 |
| Girls | 15 (57.7) | 21 (45.7) | 23 (52.3) | |
| Small for gestational age | 6 (23.1) | 10 (21.7) | 10 (22.7) | 0.472 |
| Bronchopulmonary dysplasia | 3 (11.5) | 11 (23.9) | 13 (29.5) | 0.225 |
| Late sepsis | 6 (23.1) | 19 (41.3) | 16 (36.4) | 0.294 |
| Periventricular hemorrhage | ||||
| Grade I | 3 (11.5) | 9 (19.6) | 9 (20.5) | 0.710 |
| Grade II | 2 (7.7) | 3 (6.5) | 4 (9.1) | |
| Grade III | -- | 2 (4.3) | 2 (4.5) | |
| Grade IV | 1 (3.8) | 3 (6.5) | -- | |
| Leukomalacia | 1 (3.8) | 4 (8.7) | 4 (9.1) | 0.851 |
| Environmental and Familiar Factors | Premature Children Groups (N = 116) | Group Comparisons p | ||
|---|---|---|---|---|
| Exclusive Breastfeeding (N = 26) | Mixed Feeding (N = 46) | Formula (N = 44) | ||
| M (SD) | ||||
| Breastfeeding length (months) | 13.65 (6.40) | 11.52 (10.35) | -- | 0.345 |
| Mother’s age at birth (years) | 30.08 (6.25) | 29.45 (5.52) | 29.52 (6.89) | 0.931 |
| Father’s age at birth (years) | 32.40 (6.66) | 32.09 (6.49) | 31.96 (6.75) | 0.973 |
| Family income (BRL) | 2756.25 (729.35) | 2690.90 (1894.95) | 2678.12 (2026.55) | 0.978 |
| Number of previous gestations | 1.23 (1.45) | 1.56 (142) | 1.48 (1.63) | 0.751 |
| Number of prenatal visits (n) | 5.27 (2.66) | 5.59 (2.33) | 5.34 (2.37) | 0.600 |
| MBFES—Breastfeeding experience | 105.42 (11.43) | 100.57 (11.29) | -- | 0.082 |
| DAIS—Maternal practices | 14.81 (9.08) | 9.76 (8.67) | 11.93 (7.61) | 0.052 |
| KIDI—Parental knowledge | 0.51 (0.26) | 0.38 (0.29) | 0.47 (0.26) | 0.112 |
| AHEMD-IS opportunities | ||||
| Home physical space | 2.58 (1.60) | 2.13 (1.50) | 2.64 (1.57) | 0.260 |
| Stimuli variety | 13.78 (1.55) | 14.31 (1.21) | 13.92 (1.46) | 0.216 |
| Fine motor toys | 10.92 (6.60) | 16.65 (8.76) | 11.20 (6.20) | 0.001 |
| Gross motor toys | 8.00 (1.91) | 9.13 (2.72) | 8.16 (2.83) | 0.117 |
| Total score | 30.53 (9.08) | 32.15 (10.82) | 28.66 (9.73) | 0.574 |
| N (%) | ||||
| Mother’s formal education | ||||
| Primary school | -- | 3 (8.1) | 5 (14.3) | 0.293 |
| Elementary school | 3 (20.0) | 9 (24.3) | 10 (28.6) | |
| High school | 10 (66.6) | 24 (64.9) | 15 (42.8) | |
| Higher education | 2 (13.3) | 1 (2.7) | 5 (14.3) | |
| Father’s formal education | ||||
| Primary school | 2 (13.3) | 4 (11.4) | 2 (6.5) | 0.505 |
| Elementary school | 4 (26.7) | 12 (34.3) | 11 (35.5) | |
| High school | 9 (60.0) | 14 (40.0) | 12 (38.7) | |
| Higher education | -- | 5 (14.3) | 6 (19.3) | |
| Previous premature births | 3 (11.5) | 2 (4.3) | 2 (4.5) | 0.491 |
| Type of delivery | ||||
| Natural | 8 (30.8) | 15 (32.6) | 16 (36.4) | 0.876 |
| Cesarean | 18 (69.2) | 31 (67.4) | 28 (63.6) | |
| Preeclampsia (yes) | 8 (30.8) | 17 (37.0) | 11 (25.0) | 0.472 |
| M (SD) Premature Children Groups (N = 116) | Group Factor Post Hoc Tests p | |||
|---|---|---|---|---|
| BSITD-III | Exclusive Breastfeeding (N = 26) | Mixed Feeding (N = 46) | Formula (N = 44) | |
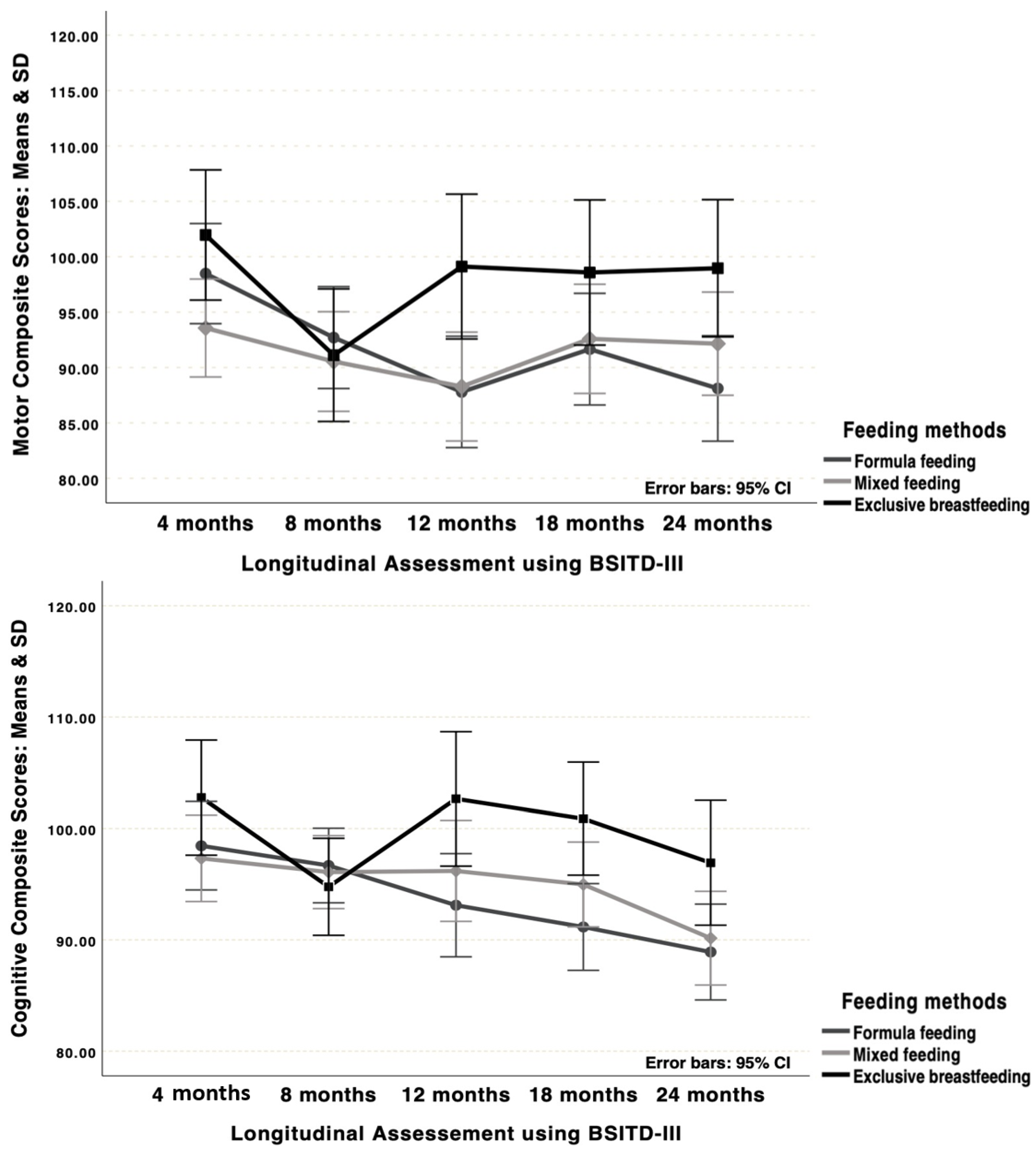
| Motor Scores | ||||
| 4 months (time 1) | 101.96 (11.44) | 93.56 (14.58) | 98.48 (17.40) t1#3 and 5 | 0.067 |
| 8 months (time 2) | 91.11 (12.49) | 90.54 (13.74) | 92.70 (18.29) | 0.794 |
| 12 months (time 3) | 99.11 (9.97) gA#B and C | 88.28 (16.26) gB | 87.79 (20.22) gC,t3 | 0.015 |
| 18 months (time 4) | 98.58 (14.98) | 92.58 (15.62) | 91.66 (19.01) | 0.226 |
| 24 months (time 5) | 98.96 (11.11) gA#C | 92.15 (16.73) | 88.11 (17.40) gC,t5 | 0.026 |
| Time Factor Post Hoc Tests p | 0.100 | 0.191 | 0.002 | |
| Cognitive Scores | ||||
| 4 months (time 1) | 102.77 (10.25) | 97.33 (10.60) t1#5 | 98.45 (16.88) t1#4,5 | 0.239 |
| 8 months (time 2) | 94.77 (9.13) | 95.09 (10.32) | 96.68 (13.06) t2#5 | 0.788 |
| 12 months (time 3) | 102.65 (10.99) gA#C | 96.19 (14.91) t3#5 | 93.11 (18.16) gC | 0.049 |
| 18 months (time 4) | 100.88 (15.55) gA#C | 94.98 (11.79) | 91.16 (12.70) t4,gC | 0.013 |
| 24 months (time 5) | 96.92 (6.34) | 90.15 (15.03) t5 | 88.90 (16.93) t5 | 0.070 |
| Time Factor Post Hoc Tests p | 0.250 | 0.021 | 0.002 | |
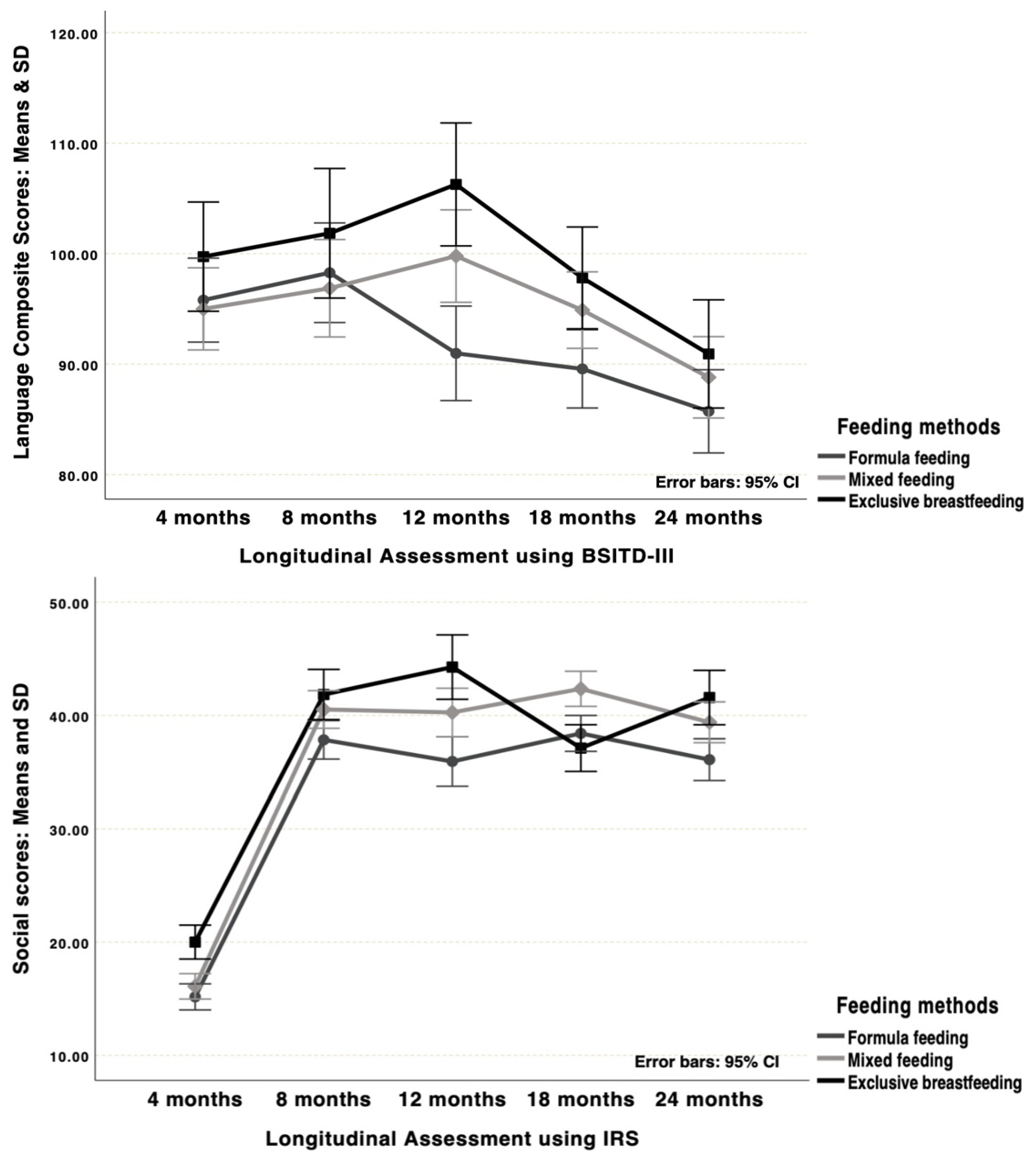
| Language Scores | ||||
| 4 months (time 1) | 99.73 (10.35) | 95.00 (12.64) | 95.79 (14.00) t1#5 | 0.300 |
| 8 months (time 2) | 101.85 (14.95) t2#5 | 96.87 (15.23) t2#5 | 98.27 (15.04) t2#4,5 | 0.405 |
| 12 months (time 3) | 106.27 (18.04) gA#C;t3#5 | 99.78 (11.60) gB#C;t3#5 | 90.98 (14.45) gC | <0.001 |
| 18 months (time 4) | 97.81 (16.38) gA#C | 94.89 (11.50) t4#5 | 89.57 (8.64) t4,gC | 0.014 |
| 24 months (time 5) | 90.92 (6.01) t5 | 88.80 (13.02) t5 | 85.72 (14.81) t5 | 0.227 |
| Time Factor Post Hoc Tests p | <0.001 | <0.001 | <0.001 | |
| Social Scores | ||||
| 4 months (time 1) | 20.00 (2.19) gA#B,C | 16.09 (3.14) gB | 15.16(5.08) gC,t1#2,3,4,5 | <0.001 |
| 8 months (time 2) | 41.85 (2.33) gA#C | 40.52 (6.28) | 37.84 (6.40) gC | 0.012 |
| 12 months (time 3) | 44.27 (2.55) gA#C | 40.26 (7.76) gB#C | 35.93 (8.62) gC | <0.001 |
| 18 months (time 4) | 37.11 (6.32 )gA#B | 42.35 (5.75) gB#C | 38.41 (4.02) gC | <0.001 |
| 24 months (time 5) | 41.58 (3.02) gA#C | 39.39 (6.48) gB#C | 36.09 (7.14) gC | 0.001 |
| Time Factor Post Hoc Test p | <0.001 | <0.001 | <0.001 | |
| M (SD) Premature Children Groups (N = 116) | Group Factor Post Hoc Test p | |||
|---|---|---|---|---|
| Exclusive Breastfeeding (N = 26) | Mixed Feeding (N = 46) | Formula (N = 44) | ||
| Mother’s Social Skills | ||||
| 4 months (time 1) | 40.65 (3.17) gA#B,C, t1#2,3,4,5 | 35.98 (6.10) gB#C, t1#2,3,4,5 | 29.27 (10.22) gC,t1#2,3,4,5 | <0.001 |
| 8 months (time 2) | 62.92 (4.24) gA#C, t2 | 59.26 (9.59) gB#C,t2 | 53.95 (11.02) gC,t2 | <0.001 |
| 12 months (time 3) | 61.00 (4.37) gA#C,t3 | 56.65 (11.23) t3 | 53.89 (12.88) gC,t3 | 0.033 |
| 18 months (time 4) | 61.46 (5.87) gA#C,t4 | 60.89 (7.22) gB#C,t4 | 55.59 (7.15) gC,t4 | <0.001 |
| 24 months (time 5) | 58.50 (6.07) t5 | 60.02 (10.22) t5 | 56.98 (10.22) t5 | 0.316 |
| Time Factor Post Hoc Test (p) | <0.001 | <0.001 | <0.001 | |
| Mother/Child Dyads | ||||
| 4 months (time 1) | 109.30 (8.54) gA#B,C, t1#2,3,4,5 | 93.31 (14.70) gB#C,t1#2,3,4,5 | 80.91 (24.06) gC, t1#2,3,4,5 | <0.001 |
| 8 months (time 2) | 159.46 (8.23) gA#B,C,t2#t4 | 144.60 (20.46) gB,t2 | 134.98 (23.35) gC,t2 | <0.001 |
| 12 months (time 3) | 153.54 (9.82) gA#C,t3 | 141.38 (23.08) t3 | 132.07 (27.99) gC,t3 | <0.001 |
| 18 months (time 4) | 144.35 (16.30) t4 | 151.69 (17.50) gB#C,t4 | 137.66 (14.05) gC,t4 | <0.001 |
| 24 months (time 5) | 145.04 (10.27) t5 | 145.34 (21.78) t5 | 136.64 (22.27) t5 | 0.086 |
| Time Factor Post Hoc Test (p) | <0.001 | <0.001 | <0.001 | |
| Motor | Linear Regression Analysis: Formula Group/Longitudinal by Months | Unstandardized B | Standardized B | p | Partial Correlation | |
|---|---|---|---|---|---|---|
| Exclusive breastfeeding | 4-month model: R = 0.46, Adjusted R2 = 0.18, Durbin Watson = 1.50, ANOVA F(1,24) = 6.61, p = 0.017 | |||||
| Predictor | Father’s age | 1.05 | 0.46 | 0.017 | 0.46 | |
| 8-month model: R = 0.37, Adjusted R2 = 0.10, Durbin Watson = 1.73, ANOVA F(1,24) = 3.92, p = 0.050 | ||||||
| Predictor | Family income | −0.008 | 0.37 | 0.050 | 0.37 | |
| 12-month model: R = 0.48, Adjusted R2 = 0.20, Durbin Watson = 2.09, ANOVA F(1,24) = 7.35, p = 0.012 | ||||||
| Predictor | BPD yes | −14.83 | −0.48 | 0.012 | −0.48 | |
| 18-month model: R = 0.56, Adjusted R2 = 0.29, Durbin Watson = 1.92, ANOVA F(1,24) = 11.26, p = 0.003 | ||||||
| Predictor | Mother–Child dyads | 0.52 | 0.56 | 0.003 | 0.56 | |
| 24-month model: R = 0.52, Adjusted R2 = 0.20, Durbin Watson = 1.92, ANOVA F(2,23) = 4.20, p = 0.028 | ||||||
| Predictors | Mother–Child dyads | 0.51 | 0.47 | 0.016 | 0.47 | |
| SGAyes | −8.65 | −0.33 | 0.080 | −0.36 | ||
| Mixed feeding | 4-month model: R = 0.59, Adjusted R2 = 0.28, Durbin Watson = 1.72, ANOVA F(4,41) = 5.44, p < 0.001 | |||||
| Predictors | Siblings number | 4.05 | 0.39 | 0.008 | 0.40 | |
| Mothers breastfeeding perceptions | 0.50 | 0.398 | 0.004 | 0.43 | ||
| Mother’s age | −1.01 | −0.37 | 0.011 | −0.38 | ||
| Mother–Child dyads | 0.22 | 0.22 | 0.097 | 0.26 | ||
| 8-month model: R = 0.44, Adjusted R2 = 0.16, Durbin Watson = 2.00, ANOVA F(2,43) = 5.30, p = 0.009 | ||||||
| Predictors | Leukomalacia yes | −15.99 | −0.33 | 0.020 | −0.34 | |
| Mother–Child dyads | 0.17 | 0.26 | 0.069 | 0.27 | ||
| 12-month model: R = 0.63, Adjusted R2 = 0.37 Durbin Watson = 1.67, ANOVA F(2,42) = 13.96, p < 0.001 | ||||||
| Predictors | Periventricular hemorrhage I–IV | −6.14 | −0.45 | 0.001 | −0.47 | |
| Mother–Child dyads | 0.22 | 0.31 | 0.022 | 0.34 | ||
| 18-month model: R = 0.68, Adjusted R2 = 0.41, Durbin Watson = 2.44, ANOVA F(4,41) = 8.74, p < 0.001 | ||||||
| Predictors | Mother’s formal education | 2.15 | 0.35 | 0.007 | 0.40 | |
| NICU stay yes | −0.18 | −0.33 | 0.012 | −0.38 | ||
| Periventricular hemorrhage I–IV | −3.43 | −0.26 | 0.048 | −0.30 | ||
| DBP yes | −11.93 | −0.22 | 0.088 | −0.26 | ||
| 24-month model: R = 0.67, Adjusted R2 = 0.39, Durbin Watson = 1.69, ANOVA F(4,41) = 8.24, p < 0.001 | ||||||
| Predictors | Periventricular hemorrhage I–IV | −4.78 | −0.34 | 0.012 | −0.38 | |
| Mechanical ventilation days | −0.47 | −0.32 | 0.015 | −0.37 | ||
| Mother–Child dyads | 0.20 | 0.26 | 0.031 | 0.33 | ||
| Breastfeeding length | 0.37 | 0.24 | 0.047 | 0.30 | ||
| Formula | 4-month model: R = 0.57, Adjusted R2 = 0.24, Durbin Watson = 1.87, ANOVA F(5,38) = 3.73, p = 0.008 | |||||
| Predictors | Mother’s age | −2.25 | −0.85 | <0.001 | −0.53 | |
| Father’s age | 2.04 | 0.69 | 0.001 | 0.49 | ||
| Siblings number | 3.33 | 0.31 | 0.050 | 0.31 | ||
| Mother–Child dyads | 0.19 | 0.26 | 0.069 | 0.29 | ||
| NICU stay days | −0.13 | −0.24 | 0.092 | −0.27 | ||
| 8-month model: R = 0.29, Adjusted R2 = 0.06, Durbin Watson = 2.07, ANOVA F(1,42) = 3.93, p = 0.050 | ||||||
| Predictor | BPD yes | −11.59 | −0.29 | 0.050 | −0.29 | |
| 12-month model: R = 0.54, Adjusted R2 = 0.26, Durbin Watson = 1.51, ANOVA F(2,41) = 8.62, p < 0.001 | ||||||
| Predictors | Mechanical ventilation days | −0.56 | −0.45 | 0.001 | −0.47 | |
| BPD yes | −20.93 | −0.30 | 0.027 | −0.34 | ||
| 18-month model: R = 0.37, Adjusted R2 = 0.09, Durbin Watson = 1.83, ANOVA F(2,41) = 3.22, p = 0.050 | ||||||
| Predictors | Mother–Child dyads | 0.40 | 0.29 | 0.050 | 0.30 | |
| Periventricular hemorrhage I–IV | −5.29 | −0.24 | 0.112 | −0.25 | ||
| 24-month model: R = 0.56, Adjusted R2 = 0.27, Durbin Watson = 1.97, ANOVA F(3,40) = 6.25, p = 0.001 | ||||||
| Predictors | Leukomalacia yes | −21.57 | −0.36 | 0.009 | −0.40 | |
| Mother–Child dyads | 0.25 | 0.32 | 0.021 | 0.36 | ||
| Periventricular hemorrhage I–IV | −5.21 | −0.25 | 0.058 | −0.29 | ||
| Cognitive | Linear Regression Analysis: Formula Group/Longitudinal by Months | Unstandardized B | Standardized B | p | Partial Correlation | |
|---|---|---|---|---|---|---|
| Exclusive breastfeeding | 4-month model: R = 0.52, Adjusted R2 = 0.24, Durbin Watson = 1.50, ANOVA F(1,24) = 8.82, p = 0.007 | |||||
| Predictor | Father’s age | 1.05 | 0.52 | <0.001 | 0.52 | |
| 8-month model: R = 0.59, Adjusted R2 = 0.26, Durbin Watson = 2.05, ANOVA F(3,22) = 3.95, p = 0.021 | ||||||
| Predictors | Mother’s formal education | 1.68 | 0.39 | 0.044 | 0.37 | |
| Father’s age | 0.67 | 0.37 | 0.049 | 0.36 | ||
| BPD yes | −9.27 | −0.33 | 0.072 | −0.32 | ||
| 12-month model: R = 0.74, Adjusted R2 = 0.49, Durbin Watson = 2.09, ANOVA F(3,22) = 9.08, p < 0.001 | ||||||
| Predictors | BPD yes | −19.38 | −0.57 | <0.001 | −0.56 | |
| Mother’s age | 0.77 | 0.41 | 0.009 | 0.41 | ||
| Mother–Child dyads | 0.28 | 0.25 | 1.00 | 0.24 | ||
| 18-month model: R = 0.71, Adjusted R2 = 0.46, Durbin Watson = 2.20, ANOVA F(2,23) = 11.50, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.46 | 0.48 | 0.004 | 0.47 | |
| BPD yes | −18.86 | −0.39 | 0.017 | −0.38 | ||
| 24-month model: R = 0.21, Adjusted R2 = 0.004, Durbin Watson = --, ANOVA F(1,24) = 1.09, p = 0.306 | ||||||
| Predictor | DBP yes | −4.06 | −0.21 | 0.306 | −0.21 | |
| Mixed feeding | 4-month model: R = 0.67, Adjusted R2 = 0.37, Durbin Watson = 2.01, ANOVA F(6,39) = 5.34, p < 0.001 | |||||
| Predictors | Siblings number | 3.06 | 0.41 | 0.004 | 0.44 | |
| Mother’s breastfeeding perceptions | 0.30 | 0.32 | 0.021 | 0.36 | ||
| Mother’s age | −0.67 | −0.34 | 0.014 | −0.38 | ||
| NICU stay yes | −0.08 | −0.23 | 0.090 | −0.27 | ||
| Father’s formal education | 0.84 | 0.24 | 0.051 | 0.31 | ||
| Mother–Child dyads | 0.18 | 0.24 | 0.060 | 0.30 | ||
| 8-month model: R = 0.63, Adjusted R2 = 0.35, Durbin Watson = 2.42, ANOVA F(3,42) = 9.27, p < 0.001 | ||||||
| Predictors | NICU stay days | −0.12 | −0.35 | 0.009 | −0.39 | |
| DBP yes | −11.97 | −0.33 | 0.009 | −0.39 | ||
| Mother–Child dyads | 0.11 | 0.23 | 0.085 | 0.26 | ||
| 12-month model: R = 0.63, Adjusted R2 = 0.35, Durbin Watson = 2.01, ANOVA F(3,41) = 9.05, p < 0.001 | ||||||
| Predictors | Periventricular hemorrhage I–IV | −4.99 | −0.40 | 0.004 | −0.43 | |
| Mother–Child dyads | 0.18 | 0.28 | 0.039 | 0.32 | ||
| Siblings number | 2.40 | 0.23 | 0.068 | 0.28 | ||
| 18-month model: R = 0.70, Adjusted R2 = 0.44, Durbin Watson = 2.31, ANOVA F(4,41) = 9.73, p < 0.001 | ||||||
| Predictors | NICU stay yes | −0.14 | −0.34 | 0.008 | −0.40 | |
| Periventricular hemorrhage I–IV | −2.85 | −0.29 | 0.026 | −0.34 | ||
| Leukomalacia yes | −11.36 | −0.27 | 0.029 | −0.33 | ||
| Mother’s formal education | 1.30 | 0.28 | 0.026 | 0.34 | ||
| 24-month model: R = 0.66, Adjusted R2 = 0.39, Durbin Watson = 1.96, ANOVA F(4,41) = 8.15, p < 0.001 | ||||||
| Predictors | Periventricular hemorrhage I–IV | −3.92 | −0.31 | 0.018 | −0.36 | |
| Mother–Child dyads | 0.24 | 0.34 | 0.006 | 0.41 | ||
| Mother’s breastfeeding perception | 0.35 | 0.26 | 0.042 | 0.31 | ||
| Mother’s formal education | 1.72 | 0.29 | 0.020 | 0.36 | ||
| Formula | 4-month model: R = 0.50, Adjusted R2 = 0.17, Durbin Watson = 2.13, ANOVA F(4,39) = 3.28, p = 0.021 | |||||
| Predictors | Mother’s age | −1.43 | −0.56 | 0.014 | −0.38 | |
| Father’s age | 1.51 | 0.53 | 0.014 | 0.38 | ||
| Mother–Child dyads | 0.23 | 0.33 | 0.028 | 0.34 | ||
| Siblings number | 3.29 | 0.32 | 0.055 | 0.30 | ||
| 8-month model: R = 0.41, Adjusted R2 = 0.13, Durbin Watson = 2.33, ANOVA F(2,41) = 4.25, p = 0.021 | ||||||
| Predictors | Mother’s formal education | −1.28 | −0.33 | 0.026 | −0.34 | |
| Periventricular hemorrhage I–IV | −4.29 | −0.28 | 0.058 | −0.29 | ||
| 12-month model: R = 0.55, Adjusted R2 = 0.26, Durbin Watson = 2.09, ANOVA F(2,41) = 8.76, p < 0.001 | ||||||
| Predictors | Mechanical ventilation yes | −0.53 | −0.47 | <0.001 | −0.49 | |
| Leukomalacia yes | −17.17 | −0.27 | 0.042 | −0.31 | ||
| 18-month model: R = 0.50, Adjusted R2 = 0.22, Durbin Watson = 1.77, ANOVA F(2,41) = 6.98, p = 0.002 | ||||||
| Predictors | Mother–Child dyads | 0.41 | 0.45 | 0.002 | 0.46 | |
| Periventricular hemorrhage I–IV | −3.57 | −0.24 | 0.084 | −0.27 | ||
| 24-month model: R = 0.64, Adjusted R2 = 0.34, Durbin Watson = 1.93, ANOVA F(4390) = 6.61, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.33 | 0.43 | 0.002 | 0.48 | |
| DBP yes | −22.59 | −0.39 | 0.004 | −0.44 | ||
| Periventricular hemorrhage I–IV | −4.69 | −0.23 | 0.065 | −0.29 | ||
| Siblings number | 2.39 | 0.23 | 0.081 | 0.27 | ||
| Language | Linear Regression Analysis: Formula Group/Longitudinal by Months | Unstandardized B | Standardized B | p | Partial Correlation | |
|---|---|---|---|---|---|---|
| Exclusive breastfeeding | 4-month model: R = 0.41, Adjusted R2 = 0.13, Durbin Watson = 1.68, ANOVA F(1,24) = 4.84, p = 0.038 | |||||
| Predictor | NICU stay days | −0.16 | −0.41 | 0.038 | −0.41 | |
| 8-month model: R = 0.38, Adjusted R2 = 0.11, Durbin Watson = 1.80, ANOVA F(1,24) = 3.98, p = 0.050 | ||||||
| Predictor | Family income | −0.01 | −0.38 | 0.050 | −0.38 | |
| 12-month model: R = 0.57, Adjusted R2 = 0.23, Durbin Watson = 1.85, ANOVA F(3,22) = 3.55, p = 0.031 | ||||||
| Predictors | Mother’s formal education | 3.34 | 0.39 | 0.046 | 0.37 | |
| Father’s age | 1.15 | 0.32 | 0.091 | 0.31 | ||
| DBP yes | −18.03 | −0.33 | 0.082 | −0.32 | ||
| 18-month model: R = 0.62, Adjusted R2 = 0.33, Durbin Watson = 2.27, ANOVA F(2,23) = 7.25, p = 0.004 | ||||||
| Predictors | Mother–Child dyads | 0.56 | 0.56 | 0.002 | 0.56 | |
| Father’s age | 1.12 | 0.35 | 0.046 | 0.34 | ||
| 24-month model: R = 0.15, Adjusted R2 = 0.02, Durbin Watson = --, ANOVA F(1,24) = 0.52, p = 0.478 | ||||||
| Predictor | Siblings number | 0.64 | 0.15 | 0.478 | 0.15 | |
| Mixed feeding | 4-month model: R = 0.77, Adjusted R2 = 0.59, Durbin Watson = 2.16, ANOVA F(6,39) = 9.57, p < 0.001 | |||||
| Predictors | Father’s formal education | 1.89 | 0.45 | <0.001 | 0.57 | |
| Mother–Child dyads | 0.39 | 0.45 | <0.001 | 0.56 | ||
| Leukomalacia yes | −9.71 | −0.22 | 0.048 | −0.31 | ||
| Breastfeeding length | 0.27 | 0.24 | 0.030 | 0.34 | ||
| Mother’s age | −0.54 | −0.23 | 0.031 | −0.34 | ||
| NICU stay days | −0.08 | −0.19 | 0.069 | −0.29 | ||
| 8-month model: R = 0.45, Adjusted R2 = 0.18, Durbin Watson = 2.50, ANOVA F(1,44) = 11.12, p = 0.002 | ||||||
| Predictor | Mother–Child dyads | 0.33 | 0.45 | 0.002 | 0.45 | |
| 12-month model: R = 0.36, Adjusted R2 = 0.11, Durbin Watson = 1.70, ANOVA F(1,43) = 6.63, p = 0.014 | ||||||
| Predictor | Mechanical ventilation days | −0.35 | −0.36 | 0.014 | −0.36 | |
| 18-month model: R = 0.49, Adjusted R2 = 0.22, Durbin Watson = 2.09, ANOVA F(1,44) = 13.73, p < 0.001 | ||||||
| Predictor | NICVU stay days | −0.19 | −0.49 | <0.001 | −0.49 | |
| 24-month model: R = 0.56, Adjusted R2 = 0.28, Durbin Watson = 2.13, ANOVA F(2,43) = 9.90, p < 0.001 | ||||||
| Predictors | Mother’s breastfeeding perceptions | 0.45 | 0.39 | 0.004 | 0.42 | |
| Mother–Child dyads | 0.22 | 0.36 | 0.007 | 0.40 | ||
| Formula | 4-month model: R = 0.50, Adjusted R2 = 0.18, Durbin Watson = 2.50, ANOVA F(1,39) = 3.32, p = 0.020 | |||||
| Predictors | Father’s age | 1.21 | 0.51 | 0.019 | 0.36 | |
| Mother’s age | −1.06 | −0.50 | 0.025 | −0.35 | ||
| Mother’s formal education | 1.42 | 0.34 | 0.025 | 0.35 | ||
| NICU stay days | −0.11 | −0.26 | 0.087 | −0.27 | ||
| 8-month model: R = 0.41, Adjusted R2 = 0.13, Durbin Watson = 2.35, ANOVA F(2,41) = 4.28, p = 0.021 | ||||||
| Predictors | DBP yes | −10.28 | −0.31 | 0.032 | −0.33 | |
| Father’s age | −0.76 | −0.29 | 0.045 | −0.27 | ||
| 12-month model: R = 0.42, Adjusted R2 = 0.14, Durbin Watson = 2.50, ANOVA F(2,41) = 4.48, p = 0.017 | ||||||
| Predictors | Mother–Child dyads | 0.18 | 0.34 | 0.021 | 0.35 | |
| DBP yes | −9.43 | −0.30 | 0.041 | −0.25 | ||
| 18-month model: R = 0.49, Adjusted R2 = 0.20, Durbin Watson = 1.72, ANOVA F(2,41) = 6.49, p = 0.004 | ||||||
| Predictors | Mother’s age | −0.72 | −0.55 | <0.001 | −0.49 | |
| Siblings number | 1.68 | 0.32 | 0.045 | 0.31 | ||
| 24-month model: R = 0.59, Adjusted R2 = 0.31, Durbin Watson = 1.56, ANOVA F(2,41) = 10.86, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.36 | 0.54 | <0.001 | 0.56 | |
| Mechanical ventilation yes | −0.23 | −0.25 | 0.056 | −0.29 | ||
| Social | Linear Regression Analysis: Formula Group/Longitudinal by Months | Unstandardized B | Standardized B | p | Partial Correlation | |
|---|---|---|---|---|---|---|
| Exclusive breastfeeding | 4-month model: R = 0.98, Adjusted R2 = 0.95, Durbin Watson = 1.40, ANOVA F(4,21) = 116.7, p < 0.001 | |||||
| Predictors | Mother–Child dyads | 0.25 | 0.99 | <0.001 | 0.84 | |
| Father’s age | 0.07 | 0.16 | 0.010 | 0.13 | ||
| BPD yes | −0.71 | −0.11 | 0.031 | 0.10 | ||
| Siblings yes | 0.19 | 0.12 | 0.065 | 0.09 | ||
| 8-month model: R = 0.90, Adjusted R2 = 0.79, Durbin Watson = 1.14, ANOVA F(3,22) = 31.94, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.22 | 0.79 | <0.001 | 0.77 | |
| BPD yes | −1.56 | −0.22 | 0.032 | −0.21 | ||
| Mother’s formal education | 0.19 | 0.17 | 0.081 | 0.17 | ||
| 12-month model: R = 0.93, Adjusted R2 = 0.87, Durbin Watson = 1.95, ANOVA F(1,24) = 165.4, p < 0.001 | ||||||
| Predictor | Mother–Child dyads | 0.24 | 0.93 | <0.001 | 0.93 | |
| 18-month model: R = 0.78, Adjusted R2 = 0.58, Durbin Watson = 1.75, ANOVA F(2,23) = 18.09, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.25 | 0.62 | <0.001 | 0.60 | |
| BPD yes | −6.44 | −0.32 | 0.025 | −0.31 | ||
| 24-month model: R = 0.70, Adjusted R2 = 0.45, Durbin Watson = 2.04, ANOVA F(2,23) = 11.23, p < 0.001 | ||||||
| Predictors | Family income | 0.03 | 0.55 | 0.001 | 0.55 | |
| BPD yes | −4.62 | −0.50 | 0.003 | 0.55 | ||
| Mixed feeding | 4-month model: R = 0.92, Adjusted R2 = 0.84, Durbin Watson = 1.98, ANOVA F(1,44) = 241.7, p < 0.001 | |||||
| Predictor | Mother–Child dyads | 0.20 | 0.92 | <0.001 | 0.92 | |
| 8-month model: R = 0.88, Adjusted R2 = 0.77, Durbin Watson = 1.95, ANOVA F(4,41) = 148.8, p < 0.001 | ||||||
| Predictor | Mother–Child dyads | 0.26 | 0.88 | <0.001 | 0.88 | |
| 12-month model: R = 0.85 Adjusted R2 = 0.71, Durbin Watson = 1.99, ANOVA F(2,42) = 54.49, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.23 | 0.698 | <0.001 | 0.77 | |
| Mechanical ventilation days | −1.96 | −0.29 | 0.002 | −0.45 | ||
| 18-month model: R = 0.93, Adjusted R2 = 0.86, Durbin Watson = 1.98, ANOVA F(2,43) = 138.8, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.31 | 0.93 | <0.001 | 0.93 | |
| Breastfeeding length | 0.08 | 0.15 | 0.011 | 0.37 | ||
| 24-month model: R = 0.91, Adjusted R2 = 0.82, Durbin Watson = 1.92, ANOVA F(3,42) = 69.82, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.27 | 0.89 | <0.001 | 0.90 | |
| NICU stay days | −0.04 | −0.17 | 0.012 | 0.38 | ||
| Breastfeeding length | 0.09 | 0.16 | 0.019 | 0.35 | ||
| Formula | 4-month model: R = 0.90, Adjusted R2 = 0.80, Durbin Watson = 2.17, ANOVA F(1,42) = 177.7, p < 0.001 | |||||
| Predictor | Mother–Child dyads | 0.19 | 0.90 | <0.001 | 0.90 | |
| 8-month model: R = 0.92, Adjusted R2 = 0.84, Durbin Watson = 2.09, ANOVA F(2,41) = 113.5, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.25 | 0.92 | <0.001 | 0.92 | |
| Father’s age | −0.12 | −0.11 | 0.072 | −0.28 | ||
| 12-month model: R = 0.88, Adjusted R2 = 0.76, Durbin Watson = 1.88, ANOVA F(1,42) = 138.4, p < 0.001 | ||||||
| Predictor | Mother–Child dyads | 0.27 | 0.88 | <0.001 | 0.88 | |
| 18-month model: R = 0.84, Adjusted R2 = 0.69, Durbin Watson = 2.16, ANOVA F(2,41) = 47.95, p < 0.001 | ||||||
| Predictors | Mother–Child dyads | 0.22 | 0.78 | <0.001 | 0.81 | |
| Mother’s age | −0.10 | −0.16 | 0.070 | −28 | ||
| 24-month model: R = 0.90, Adjusted R2 = 0.80, Durbin Watson = 1.52, ANOVA F(1,42) = 173.6, p < 0.001 | ||||||
| Predictor | Mother–Child dyads | 0.29 | 0.90 | <0.001 | 0.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hass, J.V.; Panceri, C.; Silveira, R.C.; Valentini, N.C. Breastfeeding, Mother–Child Dyads of Interaction, and Neurodevelopment of Preterm Children: A Longitudinal Study of Feeding Methods During the First Two Years. Children 2025, 12, 1480. https://doi.org/10.3390/children12111480
Hass JV, Panceri C, Silveira RC, Valentini NC. Breastfeeding, Mother–Child Dyads of Interaction, and Neurodevelopment of Preterm Children: A Longitudinal Study of Feeding Methods During the First Two Years. Children. 2025; 12(11):1480. https://doi.org/10.3390/children12111480
Chicago/Turabian StyleHass, Júlia Vicente, Carolina Panceri, Rita C. Silveira, and Nadia Cristina Valentini. 2025. "Breastfeeding, Mother–Child Dyads of Interaction, and Neurodevelopment of Preterm Children: A Longitudinal Study of Feeding Methods During the First Two Years" Children 12, no. 11: 1480. https://doi.org/10.3390/children12111480
APA StyleHass, J. V., Panceri, C., Silveira, R. C., & Valentini, N. C. (2025). Breastfeeding, Mother–Child Dyads of Interaction, and Neurodevelopment of Preterm Children: A Longitudinal Study of Feeding Methods During the First Two Years. Children, 12(11), 1480. https://doi.org/10.3390/children12111480






