Abstract
Background: There is considerable evidence to support the association between exposure to environmental tobacco smoke (ETS) and children’s burden of disease. However, the literature on the health outcomes of prenatal ETS exposure among Chinese children has not yet been comprehensively reviewed. Objective: This systematic review examines the currently available evidence and identifies gaps for further research on the health consequences of prenatal ETS exposure on Chinese children. Methods: Following the JBI systematic-scoping review methodological framework, we conducted a computer-aided search of three electronic databases—PubMed, EBSCOhost, and ProQuest to include studies from January 2011 to May 2023 that addressed the health outcomes of Chinese children whose mothers were exposed to ETS at any stage of pregnancy. Furthermore, a methodological quality assessment of the selected articles was conducted using JBI critical appraisal checklists. Results: A total of 30 articles were reviewed, including eleven high-quality studies and nineteen moderate-quality studies. Five main themes, including hypertension, fetal and children’s development, behavioural disorders, respiratory outcomes, and “other health outcomes”, were encompassed. The majority of the studies showed a positive link between prenatal ETS exposure and an increased risk of preterm birth, and moderate risk of fetal growth restriction. A few studies explored other potential adverse outcomes of ETS, including hypertension, respiratory morbidity, lung function, and asthma in children. Conclusions: The currently available evidence on prenatal ETS exposure in Chinese children has unveiled a wide range of health outcomes, including preterm birth, fetal development, behavioural disorders, and much more. However, Chinese studies in this area are still lacking and a gap still exists in relation to the strength of association between prenatal ETS exposure and some health risks. Efficient anti-smoking policies and smoking cessation programs should be developed to promote maternal and child health. Further research is also needed to provide better evidence in this field.
1. Background
Environmental tobacco smoke (ETS), also known as second-hand smoke (SHS), contains more than 40 known or suspected carcinogens and various cardiovascular toxicants [1]. Early in the second half of the 1980s, evidence from several major international reports, including landmark publications issued by The International Agency for Research on Cancer, Australia’s National Health and Medical Research Council, the US Surgeon General, the US National Research Council, and the UK’s Scientific Committee on Tobacco and Health, showed that exposure to second-hand smoke increased the risk of illness and death in non-smokers from infancy to adulthood [2]. It is now well established that ETS exposure causes adverse health effects in thousands of passive smokers, including respiratory disease, cardiovascular disease, cancer, and mental and behavioural disorders [3,4,5].
Worldwide, approximately one-third of adult non-smokers and approximately 40% of children have been exposed to ETS at home, causing significant morbidity and mortality [6]. Pregnant women and children are particularly vulnerable to the harmful effects of second-hand smoke, as their bodies are undergoing developmental processes and they are more susceptible to the harmful substances in ETS and are at particular risk of serious health consequences [7,8]. Moreover, children are also generally unable to control their environment and have a lower ability to detoxify cancer-causing chemicals from smoke [7,8]. With numerous studies conducted to establish a strong association between ETS and adverse health outcomes in children, consistent and increasing evidence has shown that exposure to ETS during childhood and prenatally can contribute to adverse physical, psychological, and behavioural outcomes in children, such as respiratory tract infections (RTIs), asthma, low birth weight, orofacial clefts, childhood cancer, psychological symptoms, attention deficit/hyperactivity disorder (ADHD), and cognitive and language impairments [4,5,9,10,11,12].
China is the world’s largest consumer of tobacco, and exposure to ETS remains a significant public health problem [13]. Approximately 55.19% of non-smokers have been exposed to ETS in public places or at home [14]. Although policies prohibiting smoking in public places have been promoted globally over the past few decades with intensive research on the health effects of ETS, the household environment remains a high-risk setting for ETS exposure [13]. Non-smoking reproductive-age females are one of the high-risk groups, with 65.1% of Chinese women aged 15–49 years having been exposed to ETS in their homes according to the 2010 Global Adult Tobacco Survey (GATS) [15]. Moreover, subsequent and extended epidemiological studies have found that approximately 31.5% of children and 54.6% of pregnant women have been exposed to ETS at home in China [13,16]. It is well-established that in order to reduce the burden of disease attributable to second-hand smoke, an urgent need to reduce the prevalence of ETS exposure, especially among pregnant women and women of reproductive age, is warranted [6,8].
Despite all this, research on the association between children’s health outcomes and prenatal ETS exposure in China remains inadequate, and the lack of high-quality data from relevant studies hinders the development of more effective policies to prevent ETS exposure in children and pregnant women [13]. Therefore, a systematic scoping review was conducted to address this broad research question and represent the complex and heterogenous evidence [17]. This review aimed to identify, analyze, and summarize the literature on the adverse effects of fetal exposure to ETS on the health of children in China and identify gaps for further research.
2. Materials and Methods
A systematic scoping review was performed following the steps of the scoping review methodological framework developed by the Joanna Briggs Institute (JBI): (1) developing the research question; (2) identifying relevant studies; (3) selecting eligible studies; (4) extracting the results; and (5) presenting the results [18]. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) checklist [19], the reviewers presented the review results in a narrative format.
2.1. Eligibility Criteria
This study focused on children’s health outcomes with prenatal ETS exposure. Therefore, we included studies in which the population was fetuses or children ages 0–18 years whose mothers were exposed to ETS during pregnancy. ETS exposure was defined as indoor or outdoor exposure times daily more than 0 min/d. The studies included ETS exposure from various sites—at home, at the workplace, and in public places at any stage of the mother’s pregnancy. Additionally, since we were focusing on the context of China, only studies conducted in China were included. To avoid confusion of different terminology used and inaccuracy due to translation, we excluded studies published in languages other than English.
The specific inclusion criteria were as follow:
- Publication type: peer-reviewed journal article/report
- Article type: systematic reviews, meta-analyses, scoping reviews, cohort studies, cross-sectional studies, case-control studies, non-RCTs, case series, individual case reports
- Text availability: full text
- Publication date: since 2011
- Language: English
- Location: Mainland China, Hong Kong, Macau, and Taiwan
- Study population: pregnant women, children ages 0–18 years, and fetus
2.2. Type of Resources
The literature search on the research topic was conducted using three electronic databases—PubMed, EBSCOhost, and ProQuest—and supplemented by other extensive search techniques, such as screening the reference lists of selected articles and checking the ‘cited by’ and ‘similar articles’ options in PubMed. This was done to include relevant articles not included in the primary search strategy.
To obtain the best and latest evidence and maximize the available data, we included all types of journal articles published since 2011. Firstly, experimental and quasi-experimental study designs were considered. However, randomized controlled trials were not be possible, as people cannot be randomly allocated to exposures. Moreover, epidemiological observational studies, such as prospective and retrospective cohort studies, case-control studies, and analytical cross-sectional studies, were the main types of sources that were included [20]. Furthermore, descriptive observational studies including case series, individual case reports, and systematic reviews that met the inclusion criteria were also considered [20]. However, this scoping review did not include qualitative studies. Finally, other reliable resources, such as government reports, were also considered for inclusion.
2.3. Search Strategy
A full search strategy was developed based on the initial limited search using the keywords contained in the titles and abstracts of relevant articles. Boolean operators were used to increase the sensitivity of the search. The keywords we applied included ‘environmental tobacco smoke’, ‘second-hand smoke’, ‘child*’, ‘foetal’, and ‘Chin*’. Moreover, search terms related to ‘prenatal exposure’ were added but then removed before the search process, as the search results were too limited when this keyword was applied. We refine the final search string as: (((environmental tobacco smoke) OR second-hand smoke) AND (((child*) OR foetal) OR fetal)) AND (Chin*). Then, in the filters area, “free full text”, “full text”, “10 years”, ‘English’, and ‘Humans’ were selected to further narrow the search results. The search process was carried out from June 2022 and revised again in June 2023 for the purpose of this submission.
2.4. Selection of Eligible Studies
The reviewers followed the PRISMA flow diagrams for study selection to identify the final included articles [19]. Firstly, as the search proceeded, articles retrieved from different databases that met the inclusion criteria were synchronized in Mendeley, then duplicate studies were removed. Then, the titles and abstracts were screened against the eligibility criteria by a reviewer (HE), while a second reviewer (XY) screened a random sample of 10% of the titles and abstracts. The results were in 100% agreement with the inclusion/exclusion decisions. Next, the study proceeded to a full-text review. Full texts of the selected articles were read through to exclude those that were not relevant to the research objective and those that did not meet the inclusion criteria. Finally, the reference lists of the retrieved articles were searched to include additional potentially relevant resources. Moreover, where no consensus was reached on inclusion/exclusion decisions, the two reviewers discussed it until an agreement was reached, or the senior author (FH) was asked to adjudicate.
An overview of the selection process is given in Figure 1.
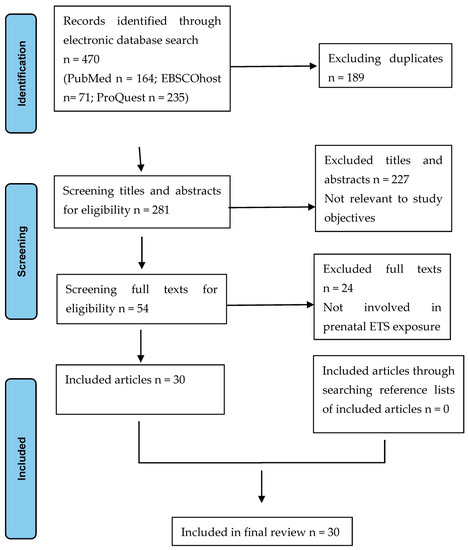
Figure 1.
PRISMA flow chart of the study selection process.
2.5. Data Charting
The researchers divided the articles equally and extracted data from them, then re-examined the articles reviewed by the other, and finally summarized all the information. The extracted data were categorized and summarized in Excel and later exported into tables and graphs. The data extraction categories included the author(s), publication year, location, aims/purpose, methodology, study population, sample size, data collection and measurement, key findings that related to the research question, and research quality.
2.6. Critical Appraisal
All the included studies were assessed using the JBI Critical Appraisal tools for Systematic Reviews. Three different types of methodologies were identified in the selected articles, namely cohort, case-control, and cross-sectional studies. Consequently, the JBI Critical Appraisal Checklist for Cohort Studies, Checklist for Case-Control Studies, and Checklist for Cross-Sectional Studies were utilized to assess the methodological quality of various study methods. The process of quality appraisal was done by one reviewer (HY) and then reassessed by the second reviewer (XY) until a consensus was reached. No study was excluded in the quality assessment process. Each study received a score based on specific criteria in the checklists. The score was given as follows: (NO or unclear or not applicable) = 0 and (YES) = 1. Later, the total scores and the percentage of scores were calculated to evaluate the methodological quality. The maximum score was 11, and the low, moderate, and high quality scores fell within the ranges of 0–50%, 50–75%, and 76–100%, respectively.
2.7. Synthesis of Results
The study results were summarized and reported in narrative formats in two parts:
- A descriptive analysis, mapping the data and showing the distribution of studies by year of publication, origin, study method, aims, and quality.
- A thematic summary, narratively describing how the identified research relates to the systematic-scoping review research question and objectives, as well as the main findings from these organized by theme.
2.8. Characteristics and Quality of the Included Studies
The main characteristics of the selected articles are summarized in Table 1.

Table 1.
Characteristics and quality of the included studies.
We included thirty journal articles, all of which were primary research. The majority (60%) were cross-sectional studies, five were cohort studies, and seven were case-control studies. The quality scores (%) of these studies, appraised using the JBI Critical Appraisal Checklists, ranged from 55% to 82%. The number of studies with high qualities, moderate qualities, and low qualities were 11, 19, and 0, respectively. The results of the critical appraisal are presented in Table 2.

Table 2.
Quality scores of the included studies.
2.9. Health Outcomes
Table 3 shows the main health outcomes of prenatal ETS exposure in Chinese children that were found in the twenty-two of the included articles.

Table 3.
Main health outcomes of prenatal ETS exposure in children.
2.10. Hypertension
One study by Zhang et al., (2020) [21] found a significant association between maternal ETS exposure during pregnancy and hypertension in their offspring. The research results showed that prenatal ETS exposure led to higher odds of hypertension in children, even after excluding the effects of potential confounders such as the age of the mother, prematurity, and low birth weight.
2.11. Foetal and Children’s Development
Seventeen studies reported the health impact of prenatal ETS exposure on fetal and child development.
2.12. Birth Outcomes
Eight articles examined the association between prenatal ETS exposure and children’s birth outcomes.
A cross-sectional study conducted in Shanghai investigated the association between LBW and mothers’ prenatal tobacco smoke exposure [22]. The results showed that the incidence of LBW was higher in children whose mothers were prenatally exposed to ETS, and their mean birth weight was 66.1 g lower than those without an exposure history. Furthermore, Huang et al. [33] found that prenatal ETS exposure was linked to a higher risk of giving birth to full-term low birth weight (FT-LBW) children, and the association remained consistent in subcategories of symmetric FT-LBW but not asymmetric FT-LBW. Moreover, another longitudinal prospective study suggested that maternal ETS exposure was likely to be an independent risk factor for fetal growth restriction and could reduce fetal birth length by more than 1 cm [48]. Additionally, exposure to ETS during pregnancy was associated with an increased risk of preterm birth (PTB) both before and after adjusting for potential confounders. Liu et al. [39] also found an increased risk of PTB among mothers with prenatal exposure to ETS, and the risk increased with the average level of daily ETS exposure. Surprisingly, the increased risk of PTB due to ETS during pregnancy was observed only among mothers who were more educated [28]. This might be because most participating mothers were well-educated, and there were significant differences in maternal educational level between PTBs and FTBs [39]. Liu et al. [32] also pointed out that paternal smoking during gestation had a significant association with PTB, and the association was more obvious in boys and children with old (≥34-year-old) mothers. Recent findings also suggested paternal smoking and preconception paternal smoking was independently positively associated with PTB risk, and the HRs increased with the increment of paternal smoking and preconception paternal smoking categories [46].
However, inconsistent findings were found in the association between ETS during pregnancy and PTB, LBW, and “small for gestational age” (SGA). One study reported a positive but non-significant effect of father smoking during pregnancy on infant birth outcomes [32], while another pointed out that the association between ETS during pregnancy and PTB was only found to be consistent in medically indicated PTB and late PTB, but not in spontaneous PTB and early PTB [28]. In addition, Chen et al. [48] stated that there was no evidence linking ETS to low birth weight or SGA based on their findings. Similarly, Lee et al. [47] found no significant association between infant birth weight and prenatal ETS exposure in their study. Chen et al. [28] also reported no significant association between ETS during pregnancy and the risk of LBW or SGA, and they suggested the inconsistent results could be due to different rates of LBW and SGA, differences in ETS definitions, and the exposure magnitude.
2.13. Orofacial Clefts (OFCs)
Two studies reported the health impact of prenatal ETS exposure on orofacial clefts. As Pi et al. [26] stated, prenatal ETS exposure among children of non-smoking mothers was significantly associated with an increased risk of developing OFCs. The association was dose-dependent; when mothers were exposed to ETS more than six days per week. There was an increased risk of OFCs in their offspring. Sakran et al. [44] also reported maternal passive smoking during early gestation to be a significant risk factor for nonsyndromic cleft lip and/or palate (NSCLP) incidence, with OR = 4.349.
2.14. Neural Tube Defects (NTDs)
One case-control study by Chen et al. [40] found a significant association between passive smoking and NTD occurrence, with a significant dose–response relationship between NTD risk and an exposure index of ETS or other household air pollution.
2.15. Congenital Heart Disease (CHD)
Three articles examined the association between prenatal ETS exposure and CHD, mostly focusing on exposure during the three months before pregnancy and in the first trimester of pregnancy. Both Song et al. [42] and Wang et al. [41] reported an increased risk of CHD in offspring whose mothers were exposed to SHS during the three months before pregnancy. All three identified articles showed significant associations between maternal exposure to ETS and CHDs in their offspring [42,45,46] also found a dose–response gradient between the risk of CHDs and maternal exposure to ETS in first trimester of pregnancy.
2.16. Developmental Coordination Disorder (DCD)
One case-control study conducted by Wu et al. [43] reported strong negative association between scores of the Chinese version of the Little Developmental Coordination Disorder Questionnaire (LDCDQ) and an increased risk of suspected DCD. Additionally, the prevalence of the suspected DCD was significantly higher in the prenatal SHS-exposed group, and the prevalence of suspected DCD in girls was higher than that in boys in the same age group.
2.17. Developmental Delay
Two studies explored the relationship between ETS exposure and developmental delay.
Ren et al. [27] noted that there was a higher rate of maternal exposure to ETS during pregnancy in children with cerebral palsy compared to the general population. After adjusting for confounding factors such as the delivery mode and birth weight, the results still revealed that children born to mothers exposed to ETS during pregnancy had a higher risk of cerebral palsy than the children of unexposed mothers, and this risk increased with increasing durations of ETS exposure [27]. Moreover, He et al. [4] study indicated that prenatal ETS exposure led to impairment in children’s early cognitive and language development in their first two years of life. Additionally, the level of language development was negatively correlated with the frequency of prenatal ETS exposure, with each additional pack of cigarettes smoked per week by a member of the household potentially associated with a 0.48-point decrease in early childhood language scores [4].
2.18. Behavioural Disorders
Six studies investigated the association between prenatal ETS exposure and children’s behavioural disorders.
2.19. ADHD
Three studies linked prenatal ETS exposure to the development of ADHD in children.
Wang et al. [12] examined prenatal tobacco smoking exposure (PSE) as a moderator in the association between genetic variants and ADHD. They reported that the genetic risk of ADHD may be affected by environmental factors, and the risk of genetic variation in ADHD is significantly increased if the child has been exposed to ETS prenatally. Thus, PSE was a potential risk factor for ADHD, which was significantly associated with all ADHD subtypes in children [12]. Lin, et al. [31] also found ETS exposure from pregnancy to childhood was associated with higher chances of having ADHD symptoms and subtypes, and the associations were stronger in the prenatal periods. Moreover, a significant positive association between prenatal ETS exposure and the risk of hyperactivity disorder in children was evident after adjusting for potential confounders in another cross-sectional study [24]. Women who have been exposed to ETS during any trimesters of pregnancy are more likely to have children who display hyperactive behaviours [24]. Furthermore, this relationship was dose-dependent; as the dose of ETS exposure increased, children were more likely to exhibit hyperactivity behaviours [24].
2.20. Autism Behaviour
One study showed that children with early life ETS exposure were more likely to exhibit autistic-like behaviours, and those who were exposed to ETS during gestation had a significantly increased risk of developing autistic-like behaviours [37]. This association persisted only in further analysis of the combined effect of children’s ETS exposure in three stages of early life—during pregnancy, from birth to one year, and from one to three years [37]. In addition, as the duration of exposure and the average number of cigarettes smoked in the child’s immediate environment increased, the risk of autistic-like behaviours also increased [37].
2.21. Other Disorders
Two articles pointed out the association between prenatal ETS exposure and behavioural disorders other than ADHD and autism. Liu et al. [23] investigated the association between prenatal ETS exposure and externalizing behaviours in Chinese children. Children born to mothers who had been exposed to ETS during pregnancy were at higher risk for externalizing behavioural problems, but no dose–response relationship was identified. In addition, a cohort study that estimated the associations between early ETS exposure during the prenatal and postnatal periods and several aspects of adolescent mental health found that prenatal ETS exposure from non-parental sources was associated with behavioural problems in children after adjusting for potential confounders [34]. While paternal smoking and maternal smoking were associated with more mental health problems, prenatal ETS exposure from non-parental sources, both occasional and daily, was associated with several behavioural problems but not with lower self-esteem or depressive symptoms [34].
2.22. Respiratory Outcomes
Four studies explored the association between prenatal ETS exposure and children’s respiratory outcomes.
In a study investigating the associations between children’s exposure to tobacco smoke in utero and in the first year of life and childhood and respiratory outcomes, Zhuge et al. [38] reported that 14.3% of children ages 3–8 years had at least one respiratory symptom. Pneumonia was the most frequently reported respiratory outcome, with a 32.3% lifetime incidence, followed by dry night cough (17.1%), frequent common colds (9.5%), and croup (6.0%). Except for frequent common colds, the crude odd ratios showed a stronger association between most respiratory health outcomes and paternal smoking only during pregnancy, and the effect of maternal smoking on respiratory outcomes was insignificant, possibly due to the low maternal smoking rate [38].
Similarly, Dong et al. [29] pointed out that compared to unexposed children, the prevalence of respiratory morbidities (including a history of asthma, current asthma, current wheeze, persistent cough, persistent phlegm, and allergic rhinitis) was higher in children with ETS exposure in utero, and increased with the increasing numbers of cigarettes smoked. Lee et al. [25] also suggested that perinatal and postnatal problems were more prevalent in the children whose mothers passively smoked during pregnancy than those whose mothers actively smoked during pregnancy and those that were unexposed. Fetal exposure to maternal passive smoking was significantly associated with having ever experienced wheezing, currently experiencing wheeze, and having ever experienced allergic rhinitis or eczema [25]. A dose–response relationship between having ever experienced wheezing and currently experiencing wheezing and increasing exposure to maternal passive smoking was also observed [25].
Furthermore, while the percentage of ETS exposure in utero among children with allergic predispositions was higher than that among children without allergic predispositions, children without allergic predispositions were more susceptible to ETS [29]. For children without allergic predispositions, ETS exposure in utero was associated with a history of asthma and current asthma only among boys [29].
Additionally, it was suggested that the adverse effects of maternal passive smoking or maternal active smoking on fetuses is due to their effects on lung function [25]. Hu et al. [30] found that in utero exposure to ETS was independently associated with decreased lung function. A significant association between ETS exposure in utero and decreased maximal mid-expiratory flow (MMEF) was found in children with asthma, but not in those without asthma, and in females but not in males [30]. No significant associations were observed between ETS exposure in utero, decreased forced vital capacity (FVC), and decreased absolute forced expiratory volume in 1 s (FEV1). However, it was reported that 66.4% and 57.2% of cases of prenatal ETS exposure with decreased FVC and decreased FEV1, respectively, were mediated by childhood asthma [30].
However, the association between children’s exposure to tobacco smoke in utero and respiratory outcomes were insignificant after adjusting for potential confounders [38]. The above study found that indoor smoke odor was clearly and strongly associated with most investigated respiratory outcomes. They argued that the perceived indoor smoke odor could be a more direct indicator of ETS exposure than parental smoking. This might be because parents who smoke may avoid smoking in the presence of children, which can be supported by the weak relationship between parental smoking and tobacco smoke odor [38].
3. Others
3.1. Astigmatism
One study investigating the association between ETS exposure during early life and early-onset astigmatism, showing no significant increased risk of astigmatism when children were exposed to ETS only during pregnancy. However, significant combined effects were observed—children were more likely to exhibit astigmatism when they were exposed to ETS during both pregnancy and from one to three years, or during both pregnancy and the first three years of life [35].
3.2. Sleep Disorders
According to Lin and his colleagues [36], ETS exposure during pregnancy was associated with higher total T-scores of the Sleep Disturbance Scale for Children (SDSC) and higher T-scores in six domains of sleep disturbance. ETS exposure during both pregnancy and the first two years of life had the highest total T-scores of SDSC and higher odds of increased sleep problems, with the strongest associations found in sleep–wake transition disorders (SWTD), as well as higher odds of long sleep latency in disorders of initiating and maintaining sleep (DIMS) [36].
4. Discussion
This systematic scoping review, which included thirty well-designed studies, has provided vital insights into the health impacts of ETS on Chinese children. Several studies have indicated that Chinese women have a low prevalence of passive smoking but a high incidence of ETS exposure at home and in public places. This special exposure profile reduces the interference of active smoking over passive smoking when investigating the health effects of tobacco exposure. Thus, our findings could provide convincing evidence for examining the harmful effects of ETS exposure during pregnancy on fetuses and children.
Prenatal ETS exposure has been reported to cause LBW, congenital birth defects, and infant mortality. According to Leonardi-Bee et al. [49], prenatal ETS exposure increases the risk of a fetus having congenital birth defects, such as cardiovascular, reproductive, musculoskeletal, and facial defects, by 10–50%. Maternal exposure to ETS during pregnancy was approximately twice as likely to result in LBW infants compared to non-exposed mothers [50]. Similar findings were shown in our study. Moreover, our review found that prenatal ETS exposure significantly increases Chinese children’s risks of developing respiratory diseases. Research has suggested that infant prenatal exposure to ETS increased the susceptibility to childhood respiratory diseases by impairing immune function in the early years of life [5]. As a result, ETS exposure affected lung development in infants and was associated with respiratory infections, wheezing, and asthma in children. In addition, it increased the risk of lifelong poor lung health and was associated with more serious respiratory diseases, such as lung cancer, in adulthood [5]. Our findings corroborated this conclusion. Furthermore, findings from this review also revealed that after adjusting for potential confounders, such as parental intelligence, parental literacy, and socioeconomic status, prenatal or childhood ETS exposure remained a significant contributor to impaired cognitive function and the onset of behavioural problems such as ADHD. These findings were consistent with similar findings from a systematic review by Zhou and colleagues [10].
By conducting this systematic review, we found numerous studies worldwide over the past decade investigating the health effects of prenatal ETS exposure on children. The currently available evidence on prenatal ETS exposure in Chinese children also has addressed a wide range of health outcomes, including respiratory diseases, hypertension, fetal and child development, behavioural disorders, sleep disorders, and astigmatism. However, relevant Chinese studies are still inadequate. For example, the research examining the association between ETS exposure, childhood cancers, and infant mortality in China remains almost nonexistent. Therefore, researchers need to focus on these gaps to design and conduct studies to further improve the well-being of children and pregnant women.
Evidence has shown a promising decline in the ETS exposure rate, from 46.8% to 30.8% in recent years, compared to data from the previous two decades [26]. However, the prevalence of ETS exposure in the household environment was still high [51]. Chen et al. [48] stated that although nearly half of women experienced passive smoking before pregnancy in a household environment, fewer women (36.5%) were exposed to ETS during their pregnancy from their family members. Meanwhile, ETS exposure before or during pregnancy is more common among women who are younger, less educated, multiparous, and have lower average personal income. Those women are also significantly more likely to drink alcohol, compounding their risk of poor pregnancy outcomes [28]. Therefore, all stakeholders must better understand and act effectively on ETS exposure in pregnant women.
The underlying biological mechanisms of how prenatal ETS leads to birth defects are still not fully understood. Tobacco smoke contains thousands of compounds, some of which are known to generally have toxic effects on reproductive health, such as carbon monoxide, nicotine, and metals. ETS contains similar constituents and may even have higher concentrations of some toxicants. A recent comprehensive Australian review has demonstrated how developing fetuses are particularly susceptible to the toxic compounds in tobacco [2]. Studies exploring the mechanisms of how smoking affects developmental outcomes mostly focus on the effects of those compounds, such as carbon monoxide, nicotine, and the carcinogens polycyclic aromatic hydrocarbon nitrosamines and aromatic amines.
To protect pregnant women from ETS exposure from their husbands or other household members, policymakers and public health professionals in the Chinese health system need to employ an evidence-based framework to assess and identify the health services needed for children and pregnant women and to develop more effective and tailored anti-smoking policies and smoking cessation programs for their family members. Moreover, healthcare providers, such as doctors and nurses, should educate both pregnant women and their husbands on the importance of tobacco control, raise public awareness about the harms of ETS exposure during pregnancy, and engage vulnerable populations in actively participating in smoking cessation programs.
5. Limitations
Our study had some limitations. Firstly, the number of included studies was small. Additionally, research articles published in Chinese were not included in this systematic scoping review, which may have led to data missing from potentially high-quality articles written in Chinese. Although we included only twenty-two articles, we carried out a well-structured screening process, and we included all the resources available to us under our screening criteria. This review involved studies conducted in urban and rural areas of Mainland of China, Hong Kong, and Taiwan. Thus, the findings can be broadly applied to the general Chinese population because the survey data were drawn from a diverse geographic and socioeconomic population in China.
Secondly, most studies measured the extent of ETS exposure by collecting self-reported data through questionnaires. However, people are often biased when they report their own experiences. For example, many individuals are either consciously or unconsciously influenced by “social desirability.” That is, they are more likely to report experiences that are socially acceptable or preferred rather than being truthful. In addition, the validity of self-reported data may be limited by the participants’ recall bias and introspective ability. Therefore, future studies on the association between ETS exposure and children’s health outcomes should use more objective exposure measurements to quantify exposure levels and reduce bias caused by data collection.
Finally, even though our systematic-scoping review study performed a quality appraisal for the included articles, which made the results of the review more reliable, further systematic reviews with meta-analyses are required to provide a better level of evidence and confirm some of the findings of this review.
6. Conclusions
This systematic scoping review provided a comprehensive summary of the adverse health effects of prenatal ETS exposure on Chinese children and identified a number of health issues in this context, including LBW, respiratory diseases, hypertension, fetal and child development, behavioural disorders, sleep disorders and astigmatism. Our findings may provide a strong framework for the development and implementation of smoking bans in indoor and public settings to minimize the harmful effects of ETS exposure on our offspring. In addition, the findings may also support health care providers in raising awareness about the above public health issues. A meta-analysis may be required to confirm these findings.
Author Contributions
F.H., H.Y. and X.Y. developed the topic, concept and design of the review. HY. and X.Y. conducted the search and reviewed studies for final selection with F.H. approval. Data extraction and analysis was conducted by H.Y. and X.Y. with support and guidance from F.H. All authors contributed to the interpretation of findings and final write up and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was a result of student capstone research project and no funding was required.
Institutional Review Board Statement
Not applicable for a review study of publicly available data.
Informed Consent Statement
Not applicable for a review study of publicly available data.
Data Availability Statement
Data including studies being analysed in this review are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Air Quality Guidelines for Europe, 2nd ed.; World Health Organization. Regional Office for Europe: Geneva, Switzerland, 2000. [Google Scholar]
- Greenhalgh, E.M.; Scollo, M.M.; Winstanley, M.H. Tobacco in Australia: Facts and Issues; Cancer Council Victoria: Melbourne, Australia, 2022; Available online: www.TobaccoInAustralia.org.au (accessed on 23 May 2023).
- DiGiacomo, S.I.; Jazayeri, M.-A.; Barua, R.S.; Ambrose, J.A. Environmental Tobacco Smoke and Cardiovascular Disease. Int. J. Environ. Res. Public Health 2018, 16, 96. [Google Scholar] [CrossRef]
- He, Y.; Luo, R.; Wang, T.; Gao, J.; Liu, C. Prenatal Exposure to Environmental Tobacco Smoke and Early Development of Children in Rural Guizhou Province, China. Int. J. Environ. Res. Public Health 2018, 15, 2866. [Google Scholar] [CrossRef]
- Vanker, A.; Gie, R.P.; Zar, H.J. The association between environmental tobacco smoke exposure and childhood respiratory disease: A review. Expert Rev. Respir. Med. 2017, 11, 661–673. [Google Scholar] [CrossRef]
- Öberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Chao, M.-R.; Cooke, M.S.; Kuo, C.-Y.; Pan, C.-H.; Liu, H.-H.; Yang, H.-J.; Chen, S.-C.; Chiang, Y.-C.; Hu, C.-W. Children are particularly vulnerable to environmental tobacco smoke exposure: Evidence from biomarkers of tobacco-specific nitrosamines and oxidative stress. Environ. Int. 2018, 120, 238–245. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; U.S. Department of Health and Human Services: Washington, DC, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf (accessed on 23 May 2023).
- Snodgrass, A.M.; Tan, P.T.; E Soh, S.; Goh, A.; Shek, L.P.; van Bever, H.P.; Gluckman, P.D.; Godfrey, K.M.; Chong, Y.S.; Saw, S.M.; et al. Tobacco smoke exposure and respiratory morbidity in young children. Tob. Control 2016, 25, e75–e82. [Google Scholar] [CrossRef]
- Zhou, S.; Rosenthal, D.G.; Sherman, S.; Zelikoff, J.; Gordon, T.; Weitzman, M. Physical, behavioral, and cognitive effects of prenatal tobacco and postnatal secondhand smoke exposure. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 219–241. [Google Scholar] [CrossRef]
- Sabbagh, H.J.; Hassan, M.H.A.; Innes, N.P.T.; Elkodary, H.M.; Little, J.; Mossey, P.A. Passive smoking in the etiology of non-syndromic orofacial clefts: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0116963. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, D.; Chen, W.; Xue, H.; Du, Y. Prenatal Tobacco Exposure Modulated the Association of Genetic variants with Diagnosed ADHD and its symptom domain in children: A Community Based Case-Control Study. Sci. Rep. 2019, 9, 4274. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Fang, P. Influence of family resources on secondhand smoking in pregnant women: A cross-sectional study in the border and minority urban areas of Northwest China. BMC Pregnancy Childbirth 2020, 20, 642. [Google Scholar] [CrossRef]
- Li, Q.; Hsia, J.; Yang, G. Prevalence of Smoking in China in 2010. N. Engl. J. Med. 2011, 364, 2469–2470. [Google Scholar] [CrossRef]
- World Health Organization. Global Adult Tobacco Survey (GATS)—China Fact Sheet 2010; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.tobaccofreekids.org/assets/global/pdfs/en/GATS_china_2010.pdf (accessed on 3 May 2023).
- Dai, S.; Chan, K.C.C. Household environmental tobacco smoke exposure in healthy young children in Hong Kong: Prevalence and risk factors. PLoS ONE 2020, 15, e0227733. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute. The Joanna Briggs Institute Reviewers’ Manual 2015, Methodology for JBI Scoping Reviews. 2015. Available online: https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/Scoping-.pdf (accessed on 16 December 2022).
- Rethlefsen, M.L.; Page, M.J. PRISMA 2020 and PRISMA-S: Common questions on tracking records and the flow diagram. J. Med. Libr. Assoc. JMLA 2022, 110, 253. [Google Scholar] [CrossRef]
- The Joanna Briggs Institute. Template for Scoping Review Protocols; The Joanna Briggs Institute: Adelaide, Australia, 2020; Available online: https://jbi.global/scoping-review-network/resources (accessed on 17 December 2022).
- Zhang, H.; Yu, L.; Wang, Q.; Tao, Y.; Li, J.; Sun, T.; Zhang, Y.; Zhang, H. In utero and postnatal exposure to environmental tobacco smoke, blood pressure, and hypertension in children: The Seven Northeastern Cities study. Int. J. Environ. Health Res. 2020, 30, 618–629. [Google Scholar] [CrossRef]
- Wang, R.; Sun, T.; Yang, Q.; Yang, Q.; Wang, J.; Li, H.; Tang, Y.; Yang, L.; Sun, J. Low birthweight of children is positively associated with mother’s prenatal tobacco smoke exposure in Shanghai: A cross-sectional study. BMC Pregnancy Childbirth 2020, 20, 603. [Google Scholar] [CrossRef]
- Liu, J.; Leung, P.W.; McCauley, L.; Ai, Y.; Pinto-Martin, J. Mother’s environmental tobacco smoke exposure during pregnancy and externalizing behavior problems in children. Neurotoxicology 2013, 34, 167–174. [Google Scholar] [CrossRef]
- Lin, Q.; Hou, X.-Y.; Yin, X.-N.; Wen, G.-M.; Sun, D.; Xian, D.-X.; Fan, L.; Jiang, H.; Jing, J.; Jin, Y.; et al. Prenatal Exposure to Environmental Tobacco Smoke and Hyperactivity Behavior in Chinese Young Children. Int. J. Environ. Res. Public Health 2017, 14, 1132. [Google Scholar] [CrossRef]
- Lee, S.L.; Lam, T.H.; Leung, T.H.; Wong, W.H.S.; Schooling, M.; Leung, G.M.; Lau, Y.L. Foetal exposure to maternal passive smoking is associated with childhood asthma, allergic rhinitis, and eczema. Sci. World J. 2012, 2012, 542983. [Google Scholar] [CrossRef]
- Pi, X.; Li, Z.; Jin, L.; Liu, J.; Zhang, Y.; Zhang, L.; Wang, L.; Ren, A. Secondhand smoke during the periconceptional period increases the risk for orofacial clefts in offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 423–427. [Google Scholar] [CrossRef]
- Ren, S.; Xie, S.; Li, X.; Li, G.; Wang, Y.; Liu, W.; Wang, L. The association between maternal exposure to secondhand smoke during pregnancy and their children’s cerebral palsy, Shandong, China. Tob. Induc. Dis. 2020, 18, 87. [Google Scholar] [CrossRef]
- Chen, X.; Huang, L.; Zhong, C.; Li, Q.; Chen, R.; Sun, G.; Jin, Z.; Yang, X.; Hao, L.; Yang, H.; et al. Association between environmental tobacco smoke before and during pregnancy and the risk of adverse birth outcomes: A birth cohort study in Wuhan, China. Environ. Sci. Pollut. Res. 2021, 28, 27230–27237. [Google Scholar] [CrossRef]
- Dong, G.-H.; Wang, D.; Yang, Z.-H.; Zhang, P.-F.; Ren, W.-H.; Zhao, Y.-D.; He, Q.-C. Gender-specific differences in effects of prenatal and postnatal environmental tobacco smoke exposure on respiratory symptoms in 23,474 children with and without allergic predisposition: Results from 25 districts of northeast China. Int. J. Environ. Health Res. 2011, 21, 173–188. [Google Scholar] [CrossRef]
- Hu, L.-W.; Yang, M.; Chen, S.; Shah, K.; Hailegiorgis, Y.; Burgens, R.; Vaughn, M.; Huang, J.; Xaverius, P.; Paul, G.; et al. Effects of in utero and Postnatal Exposure to Secondhand Smoke on Lung Function by Gender and Asthma Status: The Seven Northeastern Cities (SNEC) Study. Respiration 2017, 93, 189–197. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Xu, S.-L.; Wu, Q.-Z.; Zhou, Y.; Ma, H.-M.; Chen, D.-H.; Chen, G.-B.; Yu, H.-Y.; Yang, B.-Y.; Zeng, X.-W.; et al. Association of Prenatal, Early Postnatal, or Current Exposure to Secondhand Smoke With Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Netw. Open 2021, 4, e2110931. [Google Scholar] [CrossRef]
- Liu, W.; Huang, C.; Cai, J.; Wang, X.; Zou, Z.; Sun, C. Household environmental exposures during gestation and birth outcomes: A cross-sectional study in Shanghai, China. Sci. Total Environ. 2018, 615, 1110–1118. [Google Scholar] [CrossRef]
- Huang, L.; Tian, F.-Y.; Fan, L.; He, Y.-H.; Peng, D.; Xie, C.; Tao, L.; Yuan, S.-X.; Jia, D.-Q.; Chen, W.-Q. Appetite during the second and third trimesters mediates the impact of prenatal environmental tobacco smoke exposure on symmetric full-term low birth weight. J. Matern. Fetal Neonatal Med. 2020, 33, 1544–1553. [Google Scholar] [CrossRef]
- Leung, C.Y.; Leung, G.M.; Schooling, C.M. Early second-hand smoke exposure and child and adolescent mental health: Evidence from Hong Kong’s ‘Children of 1997’ birth cohort. Addiction 2015, 110, 1811–1824. [Google Scholar] [CrossRef]
- Li, C.-G.; Yang, G.-Y.; Schmid, K.L.; Huang, L.-H.; He, G.-H.; Liu, L.; Ruan, Z.-L.; Chen, W.-Q. Associations between Environmental Tobacco Smoke Exposure in Early Life and Astigmatism among Chinese Preschool Children. Int. J. Environ. Res. Public Health 2019, 16, 3725. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Xu, S.-L.; Wu, Q.-Z.; Zhou, Y.; Ma, H.-M.; Chen, D.-H.; Dong, P.-X.; Xiong, S.-M.; Shen, X.-B.; Zhou, P.-E.; et al. Exposure to second-hand smoke during early life and subsequent sleep problems in children: A population-based cross-sectional study. Environ. Health 2021, 20, 127. [Google Scholar] [CrossRef]
- Yang, J.-H.; Strodl, E.; Wu, C.-A.; Yin, X.-N.; Wen, G.-M.; Sun, D.-L.; Xian, D.-X.; Chen, J.-Y.; Chen, Y.-J.; Chen, W.-Q. Association between environmental tobacco smoke exposure in early life and autistic-like behaviors in Chinese preschoolers. J. Psychosom. Res. 2021, 152, 110680. [Google Scholar] [CrossRef]
- Zhuge, Y.; Qian, H.; Zheng, X.; Huang, C.; Zhang, Y.; Li, B.; Zhao, Z.; Deng, Q.; Yang, X.; Sun, Y.; et al. Effects of parental smoking and indoor tobacco smoke exposure on respiratory outcomes in children. Sci. Rep. 2020, 10, 4311. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-C.; Strodl, E.; Huang, L.-H.; Hu, B.-J.; Chen, W.-Q. Effect of prenatal exposure to household air pollution from multiple sources on risk of preterm birth. Atmosphere 2022, 13, 2022. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhang, L.; Liu, J.; Jin, L.; Ren, A.; Li, Z. Indoor air pollution from coal combustion and tobacco smoke during the periconceptional period and risk for neural tube defects in offspring in five rural counties of Shanxi province, China, 2010–2016. Environ. Int. 2023, 171, 107728. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, Y.; Yang, Y.; Liu, F.; Xu, Q.; Peng, Z.; He, Y.; Wang, Y.; Xu, J.; Zhang, H.; et al. Paternal smoking and preterm birth: A population-based retrospective cohort study among non-smoking women aged 20–49 years in rural China. Reprod. Health 2022, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Q.; Diao, J.; Li, J.; Li, Y.; Zhang, S.; Zhao, L.; Chen, L.; Wei, J.; Shu, J.; et al. Association of mthfd1 gene polymorphisms and maternal smoking with risk of congenital heart disease: A hospital-based case-control study. BMC Pregnancy Childbirth 2022, 22, 88. [Google Scholar] [CrossRef]
- Wu, M.; Williams, G.J.; Chen, G.; Zhang, L.; Hu, C.; Dai, X.; Du, W.; Hua, J. Prenatal second-hand smoke exposure and the risk of suspected developmental coordination disorder in preschoolers: A nationwide retrospective cohort study in China. Front. Public Health 2022, 10, 993471. [Google Scholar] [CrossRef]
- Sakran, K.A.; Abotaleb, B.M.; Al-Rokhami, R.K.; Hsieh, T.-Y.; Al-Wesabi, M.A.; Mohammed, A.A.; Al-Sharani, H.M.; Shi, P.; He, D. Analysis of environmental exposures for nonsyndromic cleft lip and/or palate: A case-control study. Iran. J. Public Health 2022, 51, 578. [Google Scholar] [CrossRef]
- Deng, C.; Pu, J.; Deng, Y.; Xie, L.; Yu, L.; Liu, L.; Guo, X.; Sandin, S.; Liu, H.; Dai, L. Association between maternal smoke exposure and congenital heart defects from a case–control study in China. Sci. Rep. 2022, 12, 14973. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Ni, B.; Sheng, X.; Huang, P.; Zhang, S.; Qin, J. Maternal pre-pregnancy/early-pregnancy smoking and risk of congenital heart diseases in offspring: A prospective cohort study in central china. J. Glob. Health 2022, 12, 11009. [Google Scholar] [CrossRef]
- Lee, N.L.; Samet, J.M.; Yang, G.; Zhou, M.; Yang, J.; Correa, A.; Lees, P.S.J. Prenatal Secondhand Smoke Exposure and Infant Birth Weight in China. Int. J. Environ. Res. Public Health 2012, 9, 3398–3420. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Chiu, C.-H.; Yuan, C.-P.; Liao, Y.-C.; Guo, S.-E. Influence of Environmental Tobacco Smoke and Air Pollution on Fetal Growth: A Prospective Study. Int. J. Environ. Res. Public Health 2020, 17, 5319. [Google Scholar] [CrossRef] [PubMed]
- Leonardi-Bee, J.; Britton, J.; Venn, A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: A meta-analysis. Pediatrics 2011, 127, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.P.D.S.; Da Mata, F.A.; Figueiredo, A.C.G.; de Andrade, K.R.C.; Pereira, M.G. Maternal Active Smoking During Pregnancy and Low Birth Weight in the Americas: A Systematic Review and Meta-analysis. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2017, 19, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Jiang, Y.; Zhang, J.; Parascandola, M. Secondhand Smoke Exposure among Nonsmokers in China. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 17–22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).