Abstract
Sickle cell disease (SCD) is the most common inherited single-gene disease. Complications include chronic anaemia, reduced oxygen-carrying capability, and cerebral vasculopathy, resulting in silent cerebral infarction, stroke, and cognitive dysfunction with impairments in measures of executive function, attention, reasoning, language, memory, and IQ. This systematic review aims to investigate the association between neuroimaging findings and cognition in children with SCD. Searches of PubMed and Embase were conducted in March 2022. Studies were included if participants were <18 years, if original data were published in English between 1960 and 2022, if any genotype of SCD was included, and if the relationship between cognition and neuroimaging was examined. Exclusion criteria included case studies, editorials, and reviews. Quality was assessed using the Critical Appraisal Skills Programme Case Control Checklist. A total of 303 articles were retrieved; 33 met the eligibility criteria. The presence of overt or silent strokes, elevated blood flow velocities, abnormal functional connectivity, and decreased fMRI activation were associated with neuropsychological deficits in children with SCD when compared to controls. There is a critical need to address the disease manifestations of SCD early, as damage appears to begin at a young age. Most studies were cross-sectional, restricting the interpretation of the directionality of relationships. Future research employing longitudinal neuroimaging and neuropsychological assessments could improve our understanding of the cumulative consequences of SCD on the developing brain.
1. Introduction
Sickle cell disease (SCD) refers to a group of genetic disorders characterised by misshapen red blood cells caused by the polymerisation of abnormal haemoglobin in hypoxic conditions, with approximately 300,000 babies born each year worldwide suffering from these disorders [1,2]. SCD-related complications result in anaemia, cerebrovascular disease [3], brain infarction [4], ischaemia-reperfusion injury [5,6], and increased risk of cognitive impairments [7] (Figure 1). Research has suggested most individuals with SCD experience cognitive impairments as a result of overt strokes or clinically silent cerebral infarctions (SCIs) [8]. For instance, specific impairments in writing and reading abilities have been observed in children with SCD who have experienced strokes [9], and receptive and expressive language developmental delays are frequent [10]. Studies have also shown that children with SCD and SCIs performed worse on tests of vocabulary, coordination, visual-motor speed, and arithmetic when compared to children with SCD with no imaging abnormalities [11,12]. The most common SCD genotype is haemoglobin SS (HbSS) [13]. The relationship between cerebrovascular complications and cognitive function (Figure 2) can be investigated using transcranial Doppler ultrasonography (TCD), and, in addition to structural magnetic resonance imaging (MRI), other MRI modalities can be used, including functional magnetic resonance imaging (fMRI) and diffusion magnetic resonance imaging (dMRI).
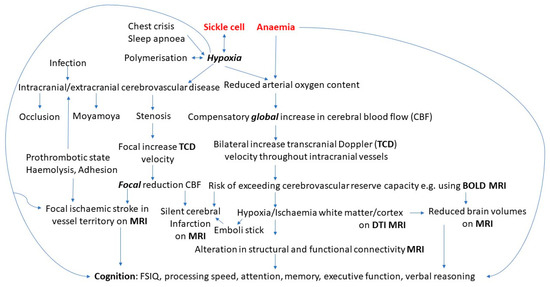
Figure 1.
Pathophysiology of cognitive difficulties in sickle cell disease.
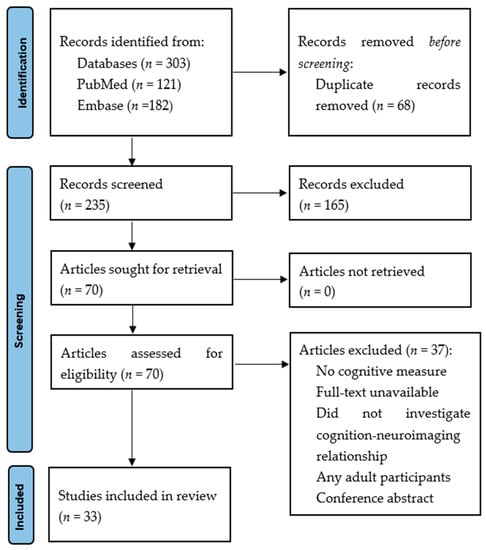
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Vaso-occlusion arises when sickled red blood cells obstruct blood flow to the extent that tissues are oxygen-deprived [14]. In response, an inflammatory reaction is triggered, which may lead to the narrowing of vessels, including intracranial and extracranial arteries (Figure 2). This increases the risk of vascular nitric oxide release, endothelial activation, and adherence of red and white cells, platelets and microparticles, especially as contemporaneous reduction of protein S and C shifts the blood towards a more prothrombotic condition [15,16,17], increasing the risk of arterial, as well as venous, thrombosis [18].
An estimated 10% of children with HbSS will have an overt stroke without screening and preventative treatment [19,20]. Transcranial Doppler is used to measure the velocity in the intracranial arteries, typically the middle cerebral (MCA) but also the anterior cerebral (ACA) and distal internal carotid arteries (dICA). Abnormal ICA/MCA velocities ≥200 cm/s, secondary to narrowing of the arteries or high cerebral blood flow, are associated with a 40% risk of stroke over the subsequent 3 years, while conditional velocities (170–199 cm/s) predict a 7% risk. Regular blood transfusion or hydroxyurea treatment for those with abnormal velocities reduces the stroke risk substantially. Cognitive difficulties may also be related to high cerebral blood flow velocities.
Neuroimaging plays a crucial role in the screening, diagnosis, treatment, and prevention of brain damage in children with SCD, as around one-third sustain SCIs in childhood [19,20], with more than half affected by young adulthood [21]. SCIs may go undiscovered, but they can have life-altering implications, as there is evidence for association with reduced global intellectual functioning [22], poor educational performance [22], and decreased quality of life [23], as well as potentially impeding the normal development of brain structure and function. There have been several systematic reviews that have examined the neurological correlates of the cognitive deficits identified in individuals with SCD [24,25,26]. Kawadler et al. found that children with SCD and SCI had lower IQ scores than children with SCD with no SCI [25], but those with no MRI abnormalities displayed lower IQs than healthy participants, suggesting that other factors (biological, environmental, and socio-economic) play a more substantial role in cognitive function [25]. Similarly, a previous meta-analysis concluded that children with SCD without MRI abnormalities were at risk of cognitive difficulties [24]. Prussien et al. found participants with SCD, including those without SCI or overt strokes, showed significant impairments in attention, FSIQ, executive function, and verbal reasoning [26], positing that anaemia and altered cerebral haemodynamics [27] impact neurological functioning [28].
MRI is a reliable, safe, and practical technique for the detection and management of SCD-related neurological damage [29]. In addition to the detection of SCI, quantitative MRI can be used to examine volume reduction in white matter (WM) [30,31], deep grey matter [32] and the cortex [33], which has, in SCD, been associated with impaired cognition, even in the absence of focal brain injuries. Additionally, arterial vasculopathy may induces persistent focal ischaemia in the brain tissue of individuals with SCD, which results in microstructural alterations such as increased intercellular water content and WM fibre degradation [34]. Diffusion-weighted imaging, which detects acute ischaemia, has reported damage to WM tract integrity and density in SCD, which is related to cognitive deficits [35]. There has not yet been a systematic review examining the relationship between quantitative neuroimaging modalities and cognitive function in children with SCD.
fMRI can be used to assess blood oxygen level-dependent (BOLD) signals in distinct brain areas during resting states or task performance in SCD [36]. The BOLD signal changes in the brain are temporally associated across functionally related regions [37,38]. However, aberrant cerebral haemodynamics and anaemia may make it challenging to detect fMRI activation in patients with SCD [39,40], even if the brain activity itself is undisturbed by the disease. Studies of patients with SCD have demonstrated that abnormal resting cerebral blood flow (CBF) reduces the BOLD response due to a diminished capability to increase blood flow in response to an increase in brain activity [41].
Given the importance of cerebrovascular disease and brain structure and function in cognition and the contributing role of neuroimaging in diagnosing and identifying those at greater risk for complications in SCD, the purpose of this review was to examine how quantitative imaging modalities relate to cognition in children with SCD, identify measures that relate to neuropsychological functioning, and ascertain key regions for future studies. Further insight into the pathophysiology of SCD will also allow for the implementation of different targets for intervention (Figure 1).
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
This review synthesises research on neuroimaging and cognition in paediatric patients with SCD using electronic searches of the PubMed and Embase databases conducted in March 2022. Table 1 illustrates the search strategy that was employed in one of the databases. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for this review [42].

Table 1.
Search strategy in PubMed.
Inclusion and exclusion criteria for articles were created and are summarised in Table 2. References of excluded articles were examined to identify any appropriate articles for inclusion. The search was conducted from 1960 to 2022 to provide a complete and exhaustive list of the neuroimaging research in paediatric SCD. No limits were applied for foreign articles, but studies were only included if they had been translated into English due to time constraints.

Table 2.
Inclusion and exclusion criteria.
EndNote X9 (The EndNote Team, 2013) was used to store results, with all duplicates omitted. One reviewer examined titles and abstracts, and articles were retained if they collected neuroimaging data and cognitive performance in SCD. The remaining papers’ full texts were obtained, and eligibility was assessed using inclusion and exclusion criteria (Table 2).
2.2. Quality Assessment
To assess quality, the Critical Appraisal Skills Programme Case Control Checklist was used. Articles were evaluated, which included questions on the suitability of controls (data from normative databases, community/sibling control) and the validity of neuropsychological assessments and neuroimaging used in articles of interest. The checklist included 3 categories with quality scores of 0 (No) or 1 (Yes), from which a total score was calculated. Articles were classified into good quality (66% or higher), satisfactory quality (36–65%), and poor quality (0–35%).
3. Results
The search retrieved 303 articles. A total of 68 duplicates were removed, and 235 articles were assessed by title and abstract. A total of 165 articles were excluded as they did not meet the eligibility criteria. Full texts of 70 articles were analysed, of which 33 met the inclusion criteria. The PRISMA flow diagram (Figure 2) outlines the specific selection process.
3.1. Data Extraction
The methodological characteristics of included studies presented by imaging modality are summarised in Table 3 (A), (B), (C), (D) and (E).

Table 3.
(A). Characteristics of transcranial Doppler (TCD) articles included in the systematic review. (B). Characteristics of SCI on Structural MRI articles included in the systematic review. (C). Characteristics of volume on structural MRI articles included in the systematic review. (D). Characteristics of diffusion tensor imaging articles included in the systematic review. (E). Characteristics of functional MRI articles included in the systematic review.
Quality appraisal scores for each paper are summarised in Table 4. Thirteen articles (39.3%) were of good quality, and 20 articles (60.6%) were of satisfactory quality.

Table 4.
Quality appraisal of studies included in the systematic review.
3.2. Characteristics of Articles in Review
Most studies used cross-sectional designs (n = 29; 87.8%), with two longitudinal studies and two retrospective cohort studies. Publication years ranged from 1993 to 2022, with seven (21.2%) from the 1990s, fourteen (42.4%) from the 2000s and twelve (36.3%) from 2011–2022. Articles’ sample sizes varied from 14 to 373, with mean ages spanning 3 months to 16.34 years. All SCD genotypes were included, with HbSS represented in all studies, sickle beta-thalassaemia in 14 (42.4%), and HbSC in 12 (36.3%). Of the 33 studies included, 23 (69.6%) included SCI+ and/or stroke groups, and 13 (39.3%) included healthy controls. Most studies were conducted in the USA (n = 21; 63.6%), with 3 studies (9.1%) in Italy, 2 studies (6.1%) in the Netherlands, 3 studies (9.1%) in the UK, 1 study (3%) in Canada, 1 study (3%) in Tanzania, 1 study (3%) in Nigeria, and 1 study (3%) in France.
3.3. Study Outcomes
- A.
- TCD
CBFV measured using TCD was investigated in 11 (33.3%) studies of infants, toddlers, preschoolers, and school-age children and adolescents with SCD (Table 3 (A)). Hogan et al. [43] and Schatz et al. [44] reported that in 9-month-old infants with SCD and in 26-month-old children with SCD, high CBF velocities were related to a moderate to high risk of developmental delay when compared to controls. Although narrowing of the intracranial arteries plays a role, the increase in CBF velocity is mainly related to the anaemia and may affect brain development over a longer period. However, Aygun et al. [45] found no relationship between TCD velocities and scores on an academic screening measure in children with SCD (mean age of 3.5 years). Differing family and environmental factors may partially explain these contradictory TCD findings.
Sanchez et al. [46] reported that higher TCD velocities were negatively related to phonological processing and syntactical ability in children with HbSS genotypes of SCD. Kral et al. [49] found that children with abnormal velocities showed greater verbal intelligence impairments than those with lower conditional TCD values. Conversely, Kral et al. [50] reported most subjects (n = 22) displayed elevated TCD velocities and found a positive relationship between better verbal memory and raised TCD values. The abnormal TCD group’s performance in both studies may have been influenced by the chronic blood transfusions patients underwent. Prussien et al. [52] found participants not receiving chronic transfusion with higher TCD velocity had poorer performance on tests of executive function and perceptual reasoning.
Onofri et al. [51] and Strouse et al. [53] included younger participants (mean age 8–9 years) and did not find any relationship between cognition and TCD velocities. Similarly, Hijmans et al. [48] found no correlation between neuropsychological measures (including sustained attention, IQ, and inhibition) and TCD values. Hijmans et al. reported children with asymmetries in CBF scored higher on 8 of 13 neuropsychological assessments than those without asymmetries. The researchers found only one test of sustained attention statistically significant; children with right-left asymmetries had lower mean reaction times than those without asymmetries [48]. Lastly, Bernaudin et al. [47] found that elevated TCD measures were related to poorer neuropsychological outcomes and lower IQ scores in children with SCD. However, there were no significant differences between the children with abnormal or normal TCD values after the patients with stroke were excluded.
- B.
- Structural MRI: infarction
Cognitive impairments were reported in children with SCD using validated assessments of global intellectual function (intelligence quotient (IQ)) and academic achievement. Twenty-one studies (63.6%) assessed brain structure (Table 3 (B)), specifically SCI presence, with most including number and volume as well. Fourteen (66.6%) MRI studies found children with SCD and overt stroke or SCI performed more poorly on neuropsychological tests, including full-scale IQ and measures of executive function, visuomotor skills, mathematical ability, working memory, verbal IQ, and attention, than children with SCD and normal-appearing MRI [11,12,28,47,54,55,56,60,61,62,64,66,68].
Seven (33.3%) studies did not find a significant association between MRI abnormalities and impaired cognition [48,51,57,58,59,63,65] (Table 3 (B)). Grueneich et al. [57] suggested these null findings were related to MRI abnormalities being associated with greater variability in cognition. The authors posited that children with pathopsychological changes develop a pattern of strengthening certain cognitive abilities to accommodate for weak abilities that have been negatively impacted by these changes. This results in uneven neuropsychological profiles while maintaining an overall level of functioning within normal limits on assessments [57]. Hijmans et al. [48] and Montanaro et al. [59] found there were no significant differences in neuropsychological function and intelligence between children with SCI on MRI and children with normal-appearing MRI. The discrepancy between these results and previous studies may be due to confounding variables (e.g., socio-cultural) as well as technical MRI differences. Onofri et al. [51] found no relationship between impaired IQ and SCI, despite 40% of the patients presenting with SCI on MRI. However, participants were younger than in the other studies.
- C.
- Structural MRI: volume (Table 3 (C))
Chen et al. [67] reported that, compared to their counterparts in the high-IQ group, children with SCD in the low-IQ group had reduced grey matter volume in the frontal, parietal, and temporal lobes (Table 3 (C)). Schatz and Buzan [68] found that the size of the CC was associated with working memory, speed of production, and distractibility.
- D.
- Structural MRI: diffusion tensor imaging for microstructure (Table 3 (D))
Scantlebury et al. [69] found the structural integrity of WM pathways was affected in children with SCD and reported increased apparent diffusion coefficient values in several brain areas associated with deficits in processing speed and working memory. Stotesbury et al. [70] found widespread WM anomalies were significantly correlated with slower processing speed, even when there was no evidence of infarct on MRI.
- E.
- Functional MRI for connectivity (Table 3 (E)).
Colombatti et al. [71] reported that patients with SCD exhibited greater connectivity in the precuneus than controls, which was pronounced in children with poorer neuropsychological functioning. The connectivity within the default-mode network may be reduced in patients with severe cognitive deficits, as it is involved in speculative processes such as planning and memory. Zou et al. [41] found children with SCD had diminished BOLD responses within the visual cortex during black-and-white visual stimulation tests compared to non-SCD children with posterior fossa tumours, which was associated with lower scores on a test of IQ [41].
4. Discussion
This systematic review found that children with SCD showed poorer cognition when compared to controls. The following characteristics related to poorer neuropsychological functioning in children with SCD: elevated blood flow velocities, presence of overt stroke or SCI, decreased fMRI activation, and abnormal functional connectivity. The consistent finding of neuropsychological deficits is notable considering the variability across articles in neuroimaging modalities, patient criteria for inclusion, and cognitive assessments. The discrepant findings reported by some MRI studies [57] might be explained by differences in their study samples and methodology. For example, some studies excluded participants based on chronic transfusion [48], others had small samples [51], and some included mixed immigrant cohorts from various socio-economic backgrounds including bilingual or multilingual speakers [48,59], making generalisation more difficult.
Findings indicated that haemoglobin may be a marker for decreased oxygen delivery and that reduced oxygen saturation in children with SCD may indicate cerebral hypoxia, resulting in neuropsychological deficits. Hijmans et al. [48] reported haemoglobin was a significant predictor of verbal memory and that anaemia was a greater predictor of neuropsychological impairments than SCI on MRI. Similarly, Steen et al. [28] reported that severity of chronic anaemia accounted for 23% of the variance in full-scale IQ in children with SCD without strokes.
A review of the research on cognition and TCD velocities in SCD also revealed mixed findings. For example, Kral et al. [50] found that children with SCD with the highest TCD velocities receiving chronic blood transfusions had better verbal memory than those with moderately high velocities who remained untreated. These results imply that blood transfusions may affect cognition by improving oxygen saturation and haemoglobin. Conversely, some TCD studies had null findings but provided limited information related to potential confounding variables (e.g., medications and blood transfusion), which limits the ability to interpret neuropsychological results [45,48,51,53].
Research has also shown children with SCD have higher resting CBF velocity than controls [72]. Therefore, as CBF velocities in these patients may be at maximum during resting states, the need for increased CBF during executive function and working memory processes in the prefrontal cortex may go unfulfilled [52]. According to interactive specialisation theory, executive function development requires interconnectivity between multiple brain areas [73]. This theory may be especially relevant to SCD. Even in the absence of stroke, blood flow and WM integrity are impaired in children. WM pathways and sufficient blood flow are critical for integrating information between different brain areas in circuits connected to executive functioning [70,73,74]. Increasing global CBF may be a risk factor and a response to cerebral hypoxia; however, these compensatory strategies may be inadequate when there is a further demand for an increase in CBF, raising the risk of cognitive decline.
Few studies combine resting-state fMRI with task-based results, partly because the assumptions used to calculate the BOLD response may not be accurate in anaemia; despite this, differences between children may still be clinically significant. Zou et al. [41] reported that children with SCD had reduced visual cortical (V1) activity, which is associated with lower IQ [41]. Because of the small sample, these results must be carefully interpreted; however, patients with SCD not receiving a disease-modifying treatment exhibited no V1 activity under the same stimuli and poorer IQ scores, suggesting an association between neuropsychological deficits and untreated SCD.
Increases in the mean diffusivity of several brain areas, indicating damage to myelin integrity and neural microstructures, are correlated with poor processing speed in patients with SCD [69,70]. Recently, Chai et al. [74] speculated that the maintenance of CBF to the GM is prioritised over WM to maintain the health of neurons essential for survival, as infarcts in GM are immediately devastating. In contrast, infarcts in the WM are ‘silent’ because they merely inhibit fast information processing. However, WM strokes can substantially hinder crucial aspects of the day-to-day lives of patients with SCD, even if they do not result in major motor impairments.
4.1. Methodological Issues and Future Directions
There are several methodological issues in the SCD literature. First, apart from two longitudinal studies, all articles investigating neuroimaging and cognition were cross-sectional, limiting any interpretation of the directionality of relationships. Moreover, because of the challenges of determining when an SCI has occurred, few studies can explore the role of time since cerebrovascular injury is a potential mediating variable related to neuropsychological impairments in children. Studies including neuroimaging rarely assess other possible contributors such as sleep apnoea and numerous school absences on cognition. Research shows environmental factors, in addition to biological risks, are significantly related to executive function and IQ in SCD [22]. Children with SCD frequently grow up in low socio-economic status households, and factors including home environment, parental education, and family income are associated with neuropsychological functioning [22]. More studies with larger sample sizes are required to evaluate the mechanisms and risk factors for neuropsychological deficits in SCD in the presence and absence of overt stroke and SCI. For these reasons, it is crucial to differentiate between socio-economic and psychological aspects, the indirect impact of chronic illness, and the disease-related effect on brain function [75]. This necessitates the use of both neuropsychological studies and longitudinal neuroimaging, as well as clinical and demographic data.
4.2. Clinical Implications
This review aims to raise awareness of the need for more research focusing on the mediating role of environmental/psychosocial factors in the relationship between neuroimaging and neuropsychological deficits in SCD. In clinical settings, objective, standardised assessments should be given to children, caregivers, and teachers to ensure multiple sources of information are collected, followed by carefully formulating targeted interventions for children with SCD and their families. This information is critical in identifying vulnerable children with SCD and families who require additional support. The paediatric neurocognitive interventions model is a comprehensive framework that could aid in the development of appropriate treatments for children with SCD [76]. The model emphasises the importance of a developmental, multi-dimensional treatment that focuses on behaviour, emotions, and cognition. The foundation of this approach involves tackling the psychosocial and systemic requirements of children. Therapies are individually tailored to identify neuropsychological impairments, progressing from externally supported compensatory tactics to self-directed compensatory approaches. In children with SCD, there is a critical need for holistic treatments that address foundational needs that are not yet met due to living with a chronic illness (e.g., mental health, social isolation, hospitalisations), as well as targeted interventions for cognitive deficits.
A significant problem within this patient group is that decreased cognitive functioning may have a negative impact on medication adherence, self-care, and clinical attendance, particularly as children with SCD get older [77], with executive function deficits predicting hydroxyurea non-adherence [78]; clinician monitoring of adherence is, therefore, essential [79]. Moreover, recall barriers (forgetfulness) were identified by Fogarty et al. [80] as the biggest challenge of medication adherence for Irish teenagers, who are, however, amenable to the use of mobile apps to monitor, be notified of, or obtain information on SCD treatments [80].
4.3. Limitations
There are some limitations to this review. Non-English language published studies were not reviewed, and only two databases were searched. One reviewer carried out the review and quality appraisal. As with other reviews of retrospectively published findings, the results may be biased as significant correlations are more likely to be reported than non-significant relationships [81]. Another limitation of this review is the exclusion of other MRI measures, including cerebral blood flow and oxygen extraction fraction (OEF), and their relationship with cognitive function, recently reviewed by Ramos et al. [27]. Lastly, although articles used standardised neuropsychological assessments, they differed significantly, making direct comparisons challenging. Therefore, it was difficult to determine how certain neuroimaging findings were differentially related to neuropsychological domains.
5. Conclusions
This review highlights the prevalence of cerebrovascular disease in children with SCD and finds that more research is needed as neuroimaging findings do not fully explain cognitive deficits. Results suggest macro-and microscopic brain damage occurring due to insufficient blood supply and energy-intensive processes, with decreased blood flow leading to atrophy of cortical areas and neuronal cell death. This review also emphasises the need to address the disease manifestations of SCD at a young age. Finally, this review indicates there is great promise in utilising neuroradiological markers for early risk stratification so that children with SCD at the highest risk for cognitive deficits can be targeted early for interventions. With successful early risk stratification, interventions and treatments can also be tailored to specific patterns of strengths and weaknesses.
Author Contributions
Conceptualization, S.S.A., M.D.H. and F.J.K.; methodology, S.S.A., M.D.H. and F.J.K.; formal analysis, S.S.A., M.D.H. and F.J.K.; writing—original draft preparation, S.S.A.; writing—review and editing, M.D.H. and F.J.K.; supervision, M.D.H. and F.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available on direct request to the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Piel, F.B.; Hay, S.I.; Gupta, S.; Weatherall, D.J.; Williams, T.N. Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions. PLOS Med. 2013, 10, e1001484. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A. Do we need to test blood donors for sickle cell anaemia? Blood Transfusion = Trasfusione del sangue. Blood Transfus. 2010, 8, 137–138. [Google Scholar] [PubMed]
- el Gammal, T.; Adams, R.J.; Nichols, F.T.; McKie, V.; Milner, P.; McKie, K.; Brooks, B.S. MR and CT investigation of cerebrovascular disease in sickle cell patients. Am. J. Neuroradiol. 1986, 7, 1043–1049. [Google Scholar] [PubMed]
- Pavlakis, S.G.; Bello, J.; Prohovnik, I.; Sutton, M.; Ince, C.; Mohr, J.P.; Piomelli, S.; Hilal, S.; De Vivo, D.C. Brain infarction in sickle cell anemia: Magnetic resonance imaging correlates. Ann. Neurol. 1988, 23, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Coloigner, J.; Kim, Y.; Bush, A.; Choi, S.Y.; Balderrama, M.C.; Coates, T.D.; O’Neil, S.H.; Lepore, N.; Wood, J.C. Contrasting resting-state fMRI abnormalities from sickle and non-sickle anemia. PLoS ONE 2017, 12, e0184860. [Google Scholar] [CrossRef] [PubMed]
- Mallon, D.; Doig, D.; Dixon, L.; Gontsarova, A.; Jan, W.; Tona, F. Neuroimaging in Sickle Cell Disease: A Review. J. Neuroimaging 2020, 30, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Steen, R.G.; Fineberg-Buchner, C.; Hankins, G.; Weiss, L.; Prifitera, A.; Mulhern, R.K. Cognitive Deficits in Children With Sickle Cell Disease. J. Child Neurol. 2005, 20, 102–107. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Schatz, J.; Siegel, M.J.; Koby, M.; Craft, S.; Resar, L.; Chu, J.-Y.; Launius, G.; Dadash-Zadeh, M.; Lee, R.B.; et al. Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology 1998, 50, 1678–1682. [Google Scholar] [CrossRef]
- Sanders, C.; Gentry, B.; Dancer, J.; Jackson, J.; Saccente, S.; Davis, P. Reading, Writing, and Vocabulary Skills of Children with Strokes Due to Sickle Cell Disease. Percept. Mot. Ski. 1997, 85, 477–478. [Google Scholar] [CrossRef]
- Davis, P.; Landers, A.; Gentry, B.; Montague, J.; Dancer, J.; Jackson, J.; Williams, L. Speech and Language Characteristics of Children with Strokes Due to Sickle Cell Disease. Percept. Mot. Ski. 1997, 85, 809–810. [Google Scholar] [CrossRef]
- Armstrong, F.D.; Thompson, R.J.; Wang, W.; Zimmerman, R.; Pegelow, C.H.; Miller, S.; Moser, F.; Bello, J.; Hurtig, A.; Vass, K. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease Cognitive Functioning and Brain Magnetic Resonance Imaging in Children With Sickle Cell Disease. Pediatrics 1996, 97 Pt 1, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Enos, L.; Gallagher, D.; Thompson, R.; Guarini, L.; Vichinsky, E.; Wright, E.; Zimmerman, R.; Armstrong, F. Neuropsychologic performance in school-aged children with sickle cell disease: A report from the Cooperative Study of Sickle Cell Disease. J. Pediatr. 2001, 139, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Prim. 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.; Poplawska, M.; Cimpeanu, E.; Mo, G.; Dutta, D.; Lim, S.H. Vaso-occlusive crisis in sickle cell disease: A vicious cycle of secondary events. J. Transl. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Murphy, C.; Eakins, E.; Kinsella, A.; McMahon, C.; Smith, O.P.; Murphy, W.G. Protein C and free protein S in children with sickle cell anemia. Ann. Hematol. 2012, 91, 1669–1671. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Murphy, C.; Eakins, E.; Kunde, J.; Corvetta, D.; Di Pierro, A.; Negri, G.; Guido, M.; Sainati, L.; Mc Mahon, C.; et al. Circulating microparticles, protein C, free protein S and endothelial vascular markers in children with sickle cell anaemia. J. Extracell. Vesicles 2015, 4, 28414. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Sartori, M.T.; Bisogno, G.; Van Schilfgaarde, M.; Saggiorato, G.; Pierro, A.M.D.; Corvetta, D.; Marcheselli, L.; Mega, A.; Gastl, G.; et al. New insights into sinusoidal obstruction syndrome. Intern. Med. J. 2017, 47, 1173–1183. [Google Scholar] [CrossRef]
- Shet, A.S.; Lizarralde-Iragorri, M.A.; Naik, R.P. The molecular basis for the prothrombotic state in sickle cell disease. Haematologica 2020, 105, 2368–2379. [Google Scholar] [CrossRef]
- Dowling, M.M.; Lee, N.; Quinn, C.; Rogers, Z.R.; Boger, D.; Ahmad, N.; Ramaciotti, C.; Buchanan, G.R. Prevalence of Intracardiac Shunting in Children with Sickle Cell Disease and Stroke. J. Pediatr. 2010, 156, 645–650. [Google Scholar] [CrossRef]
- Farooq, S.; Testai, F.D. Neurologic Complications of Sickle Cell Disease. Curr. Neurol. Neurosci. Rep. 2019, 19, 17. [Google Scholar] [CrossRef]
- Kassim, A.A.; Pruthi, S.; Day, M.; Rodeghier, M.; Gindville, M.C.; Brodsky, M.A.; DeBaun, M.R.; Jordan, L.C. Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood 2016, 127, 2038–2040. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Strouse, J.J.; Rodeghier, M.J.; Compas, B.E.; Casella, J.F.; McKinstry, R.C.; Noetzel, M.J.; Quinn, C.T.; Ichord, R.; Dowling, M.M.; et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am. J. Hematol. 2013, 89, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Schatz, J.; Finke, R.; Roberts, C.W. Interactions of Biomedical and Environmental Risk Factors for Cognitive Development. J. Dev. Behav. Pediatr. 2004, 25, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Schatz, J.; Finke, R.L.; Kellett, J.M.; Kramer, J.H. Cognitive Functioning in Children With Sickle Cell Disease: A Meta-Analysis. J. Pediatr. Psychol. 2002, 27, 739–748. [Google Scholar] [CrossRef]
- Kawadler, J.M.; Clayden, J.D.; A Clark, C.; Kirkham, F.J. Intelligence quotient in paediatric sickle cell disease: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2016, 58, 672–679. [Google Scholar] [CrossRef]
- Prussien, K.V.; Jordan, L.C.; DeBaun, M.R.; E Compas, B. Cognitive Function in Sickle Cell Disease Across Domains, Cerebral Infarct Status, and the Lifespan: A Meta-Analysis. J. Pediatr. Psychol. 2019, 44, 948–958. [Google Scholar] [CrossRef]
- Ramos, K.; Guilliams, K.P.; Fields, M.E. The Development of Neuroimaging Biomarkers for Cognitive Decline in Sickle Cell Disease. Hematol. Clin. North Am. 2022, 36, 1167–1186. [Google Scholar] [CrossRef]
- Steen, R.G.; Miles, M.A.; Helton, K.J.; Strawn, S.; Wang, W.; Xiong, X.; Mulhern, R.K. Cognitive Impairment in Children with Hemoglobin SS Sickle Cell Disease: Relationship to MR Imaging Findings and Hematocrit. Am. J. Neuroradiol. 2003, 24, 382–389. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7973593/pdf/0484.pdf (accessed on 7 February 2023).
- Zhang, X.; Li, C.; Li, Q. Magnetic resonance imaging in pediatric sickle cell anemia. Exp. Ther. Med. 2016, 12, 555–558. [Google Scholar] [CrossRef]
- Chen, R.; Arkuszewski, M.; Krejza, J.; Zimmerman, R.A.; Herskovits, E.H.; Melhem, E.R. A Prospective Longitudinal Brain Morphometry Study of Children with Sickle Cell Disease. Am. J. Neuroradiol. 2014, 36, 403–410. [Google Scholar] [CrossRef]
- Baldeweg, T.; Hogan, A.M.; Saunders, D.E.; Telfer, P.; Gadian, D.G.; Vargha-Khadem, F.; Kirkham, F. Detecting white matter injury in sickle cell disease using voxel-based morphometry. Ann. Neurol. 2006, 59, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Mackin, R.S.; Insel, P.; Truran, D.; Vichinsky, E.P.; Neumayr, L.D.; Armstrong, F.D.; Gold, J.I.; Kesler, K.; Brewer, J.; Weiner, M.W.; et al. Neuroimaging abnormalities in adults with sickle cell anemia: Associations with cognition. Neurology 2014, 82, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Kirk, G.R.; Haynes, M.R.; Palasis, S.; Brown, C.; Burns, T.G.; McCormick, M.; Jones, R.A. Regionally Specific Cortical Thinning in Children with Sickle Cell Disease. Cereb. Cortex 2008, 19, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Balci, A.; Karazincir, S.; Beyoglu, Y.; Cingiz, C.; Davran, R.; Gali, E.; Okuyucu, E.; Egilmez, E. Quantitative Brain Diffusion-Tensor MRI Findings in Patients With Sickle Cell Disease. Am. J. Roentgenol. 2012, 198, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kawadler, J.M.; Kirkham, F.J.; Clayden, J.D.; Hollocks, M.; Seymour, E.L.; Edey, R.; Telfer, P.; Robins, A.; Wilkey, O.; Barker, S.; et al. White Matter Damage Relates to Oxygen Saturation in Children With Sickle Cell Anemia Without Silent Cerebral Infarcts. Stroke 2015, 46, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Darbari, D.S.; Hampson, J.P.; Ichesco, E.; Kadom, N.; Vezina, G.; Evangelou, I.; Clauw, D.J.; Vi, J.G.T.; Harris, R.E. Frequency of Hospitalizations for Pain and Association With Altered Brain Network Connectivity in Sickle Cell Disease. J. Pain 2015, 16, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Mackay, C.E.; Filippini, N.; Watkins, K.; Toro, R.; Laird, A.; Beckmann, C.F. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon, R.O.; Schechter, A.N.; Gladwin, M.T. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef]
- Prohovnik, I.; Hurlet-Jensen, A.; Adams, R.; De Vivo, D.; Pavlakis, S.G. Hemodynamic Etiology of Elevated Flow Velocity and Stroke in Sickle-Cell Disease. J. Cereb. Blood Flow Metab. 2009, 29, 803–810. [Google Scholar] [CrossRef]
- Zou, P.; Helton, K.J.; Smeltzer, M.; Li, C.-S.; Conklin, H.M.; Gajjar, A.; Wang, W.C.; Ware, R.E.; Ogg, R.J. Hemodynamic responses to visual stimulation in children with sickle cell anemia. Brain Imaging Behav. 2011, 5, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2020, 372, 2. [Google Scholar] [CrossRef]
- Hogan, A.M.; Kirkham, F.J.; Prengler, M.; Telfer, P.; Lane, R.; Vargha-Khadem, F.; Haan, M. An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anaemia. Br. J. Haematol. 2006, 132, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Schatz, J.; McClellan, C.B.; Puffer, E.S.; Johnson, K.; Roberts, C.W. Neurodevelopmental Screening in Toddlers and Early Preschoolers With Sickle Cell Disease. J. Child Neurol. 2007, 23, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Aygun, B.; Parker, J.; Freeman, M.B.; Stephens, A.L.; Smeltzer, M.P.; Wu, S.; Hankins, J.S.; Wang, W.C. Neurocognitive screening with the Brigance Preschool screen-II in 3-year-old children with sickle cell disease. Pediatr. Blood Cancer 2010, 56, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Schatz, J.; Roberts, C.W. Cerebral blood flow velocity and language functioning in pediatric sickle cell disease. J. Int. Neuropsychol. Soc. 2010, 16, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Bernaudin, F.; Verlhac, S.; Fréard, F.; Roudot-Thoraval, F.; Benkerrou, M.; Thuret, I.; Mardini, R.; Vannier, J.; Ploix, E.; Romero, M.; et al. Multicenter Prospective Study of Children With Sickle Cell Disease: Radiographic and Psychometric Correlation. J. Child Neurol. 2000, 15, 333–343. [Google Scholar] [CrossRef]
- Hijmans, C.T.; Grootenhuis, M.A.; Oosterlaan, J.; Heijboer, H.; Peters, M.; Fijnvandraat, K. Neurocognitive deficits in children with sickle cell disease are associated with the severity of anemia. Pediatr. Blood Cancer 2010, 57, 297–302. [Google Scholar] [CrossRef]
- Kral, M.C.; Brown, R.T.; Nietert, P.J.; Abboud, M.R.; Jackson, S.M.; Hynd, G.W. Transcranial Doppler Ultrasonography and Neurocognitive Functioning in Children With Sickle Cell Disease. Pediatrics 2003, 112, 324–331. [Google Scholar] [CrossRef]
- Kral, M.C.; Brown, R.T.; Curé, J.K.; Besenski, N.; Jackson, S.M.; Abboud, M.R. Radiographic Predictors of Neurocognitive Functioning in Pediatric Sickle Cell Disease. J. Child Neurol. 2006, 21, 37–44. [Google Scholar] [CrossRef]
- Onofri, A.; Montanaro, M.; Rampazzo, P.; Colombatti, R.; Farina, F.M.; Manara, R.; Sainati, L.; Ermani, M.; Baracchini, C.; Meneghetti, G. Intellectual impairment and TCD evaluation in children with sickle cell disease and silent stroke. Perspect. Med. 2012, 1, 272–274. [Google Scholar] [CrossRef]
- Prussien, K.V.; Salihu, A.; Abdullahi, S.U.; Galadanci, N.A.; Bulama, K.; Belonwu, R.O.; Kirkham, F.J.; Yarboi, J.; Bemis, H.; DeBaun, M.R.; et al. Associations of transcranial doppler velocity, age, and gender with cognitive function in children with sickle cell anemia in Nigeria. Child Neuropsychol. 2018, 25, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Strouse, J.J.; Cox, C.S.; Melhem, E.R.; Lu, H.; Kraut, M.A.; Razumovsky, A.; Yohay, K.; van Zijl, P.C.; Casella, J.F. Inverse correlation between cerebral blood flow measured by continuous arterial spin-labeling (CASL) MRI and neurocognitive function in children with sickle cell anemia (SCA). Blood 2006, 108, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.T.; Davis, P.C.; Lambert, R.; Hsu, L.; Hopkins, K.; Eckman, J. Neurocognitive Functioning and Magnetic Resonance Imaging in Children With Sickle Cell Disease. J. Pediatr. Psychol. 2000, 25, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Schatz, J.; Glauser, T.A.; Lee, B.; DeBaun, M.R. Neuropsychologic effects of stroke in children with sickle cell anemia. J. Pediatr. 1993, 123, 712–717. [Google Scholar] [CrossRef]
- Gold, J.I.; Johnson, C.B.; Treadwell, M.J.; Hans, N.; Vichinsky, E. Detection and Assessment of Stroke in patients With Sickle Cell Disease: Neuropsychological Functioning and Magnetic Resonance Imaging. Pediatr. Hematol. Oncol. 2008, 25, 409–421. [Google Scholar] [CrossRef]
- Grueneich, R.; Ris, M.D.; Ball, W.; Kalinyak, K.A.; Noll, R.; Vannatta, K.; Wells, R. Relationship of Structural Magnetic Resonance Imaging, Magnetic Resonance Perfusion, and Other Disease Factors to Neuropsychological Outcome in Sickle Cell Disease. J. Pediatr. Psychol. 2004, 29, 83–92. [Google Scholar] [CrossRef]
- Jacob, M.; Stotesbury, H.; Kija, E.; Saunders, D.; Mtei, R.J.; Tutuba, H.; Masanu, U.; Kilonzo, M.; Kazema, R.; Hood, A.M.; et al. Effect of age, cerebral infarcts, vasculopathy and haemoglobin on cognitive function, in Tanzanian children with sickle cell anaemia. Eur. J. Paediatr. Neurol. 2022, 37, 105–113. [Google Scholar] [CrossRef]
- Montanaro, M.; Colombatti, R.; Pugliese, M.; Migliozzi, C.; Zani, F.; Guerzoni, M.E.; Manoli, S.; Manara, R.; Meneghetti, G.; Rampazzo, P.; et al. Intellectual function evaluation of first generation immigrant children with sickle cell disease: The role of language and sociodemographic factors. Ital. J. Pediatr. 2013, 39, 36. [Google Scholar] [CrossRef]
- Craft, J.S.S.; Koby, M.; Siegel, M.J.; Resar, L.; Lee, R.R.; Chu, J.-Y.; Launius, G.; Dadash-Zadehm, M.; Le DeBaun, M.R. Neuropsychologic Deficits in Children with Sickle Cell Disease and Cerebral Infarction: Role of Lesion Site and Volume. Child Neuropsychol. 1999, 5, 92–103. [Google Scholar] [CrossRef]
- Schatz, J.; White, D.A.; Moinuddin, A.; Armstrong, M.; DeBaun, M.R. Lesion Burden and Cognitive Morbidity in Children With Sickle Cell Disease. J. Child Neurol. 2002, 17, 890–894. [Google Scholar] [CrossRef]
- Steen, R.G.; Reddick, W.E.; Mulhern, R.K.; Langston, J.W.; Ogg, R.J.; Bieberich, A.A.; Kingsley, P.B.; Wang, W.C. Quantitative MRI of the brain in children with sickle cell disease reveals abnormalities unseen by conventional MRI. J. Magn. Reson. Imaging 1998, 8, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Steen, R.G.; Xiong, X.; Mulhern, R.K.; Langston, J.W.; Wang, W.C. Subtle brain abnormalities in children with sickle cell disease: Relationship to blood hematocrit. Ann. Neurol. 1999, 45, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Van Der Land, V.; Hijmans, C.T.; De Ruiter, M.; Mutsaerts, H.J.M.M.; Cnossen, M.H.; Engelen, M.; Majoie, C.B.L.M.; Nederveen, A.J.; Grootenhuis, M.A.; Fijnvandraat, K. Volume of white matter hyperintensities is an independent predictor of intelligence quotient and processing speed in children with sickle cell disease. Br. J. Haematol. 2014, 168, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Langston, J.W.; Steen, R.G.; Wynn, L.W.; Mulhern, R.K.; Wilimas, J.A.; Kim, F.M.; Figueroa, R.E. Abnormalities of the central nervous system in very young children with sickle cell anemia. J. Pediatr. 1998, 132, 994–998. [Google Scholar] [CrossRef]
- Watkins, K.; Hewes, D.E.M.; Connelly, A.; Kendall, B.; E Kingsley, D.P.; Evans, J.E.P.; Gadian, D.; Vargha-Khadem, F.; Kirkham, F. Cognitive deficits associated with frontal-lobe infarction in children with sickle cell disease. Dev. Med. Child Neurol. 2008, 40, 536–543. [Google Scholar] [CrossRef]
- Chen, R.; Pawlak, M.A.; Flynn, T.B.; Krejza, J.; Herskovits, E.H.; Melhem, E.R. Brain Morphometry and Intelligence Quotient Measurements in Children With Sickle Cell Disease. J. Dev. Behav. Pediatr. 2009, 30, 509–517. [Google Scholar] [CrossRef]
- Schatz, J.; Buzan, R. Decreased corpus callosum size in sickle cell disease: Relationship with cerebral infarcts and cognitive functioning. J. Int. Neuropsychol. Soc. 2006, 12, 24–33. [Google Scholar] [CrossRef]
- Scantlebury, N.; Mabbott, D.; Janzen, L.; Rockel, C.; Widjaja, E.; Jones, G.; Kirby, M.; Odame, I. White Matter Integrity and Core Cognitive Function in Children Diagnosed With Sickle Cell Disease. J. Pediatr. Hematol. 2011, 33, 163–171. [Google Scholar] [CrossRef]
- Stotesbury, H.; Kirkham, F.J.; Kölbel, M.; Balfour, P.; Clayden, J.D.; Sahota, S.; Sakaria, S.; Saunders, D.E.; Howard, J.; Kesse-Adu, R.; et al. White matter integrity and processing speed in sickle cell anemia. Neurology 2018, 90, e2042–e2050. [Google Scholar] [CrossRef]
- Colombatti, R.; Lucchetta, M.; Montanaro, M.; Rampazzo, P.; Ermani, M.; Talenti, G.; Baracchini, C.; Favero, A.; Basso, G.; Manara, R.; et al. Cognition and the Default Mode Network in Children with Sickle Cell Disease: A Resting State Functional MRI Study. PLoS ONE 2016, 11, e0157090. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.J.; Hofmann, J.; Churches, O.F.; A Badcock, N.; Kohler, M.; Keage, H.A. Cerebrovascular function and cognition in childhood: A systematic review of transcranial doppler studies. BMC Neurol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H. Interactive Specialization: A domain-general framework for human functional brain development? Dev. Cogn. Neurosci. 2011, 1, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Bush, A.M.; Coloigner, J.; Nederveen, A.J.; Tamrazi, B.; Vu, C.; Choi, S.; Coates, T.D.; Lepore, N.; Wood, J.C. White matter has impaired resting oxygen delivery in sickle cell patients. Am. J. Hematol. 2019, 94, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Houwing, M.E.; Grohssteiner, R.L.; Dremmen, M.H.G.; Atiq, F.; Bramer, W.M.; de Pagter, A.P.J.; Zwaan, C.M.; White, T.J.H.; Vernooij, M.W.; Cnossen, M.H. Silent cerebral infarcts in patients with sickle cell disease: A systematic review and meta-analysis. BMC Med. 2020, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Limond, J.; Adlam, A.-L.; Cormack, M. A Model for Pediatric Neurocognitive Interventions: Considering the Role of Development and Maturation in Rehabilitation Planning. Clin. Neuropsychol. 2014, 28, 181–198. [Google Scholar] [CrossRef]
- Merkhofer, C.; Sylvester, S.; Zmuda, M.; Jonassaint, J.; De Castro, L.M.; Kato, G.; Butters, M.A.; Novelli, E.M. The Impact of Cognitive Function on Adherence to Hydroxyurea Therapy in Patients with Sickle Cell Disease. Blood 2016, 128, 2493. [Google Scholar] [CrossRef]
- Longoria, J.N.; Pugh, N.L.; Gordeuk, V.; Hsu, L.L.; Treadwell, M.; King, A.A.; Gibson, R.; Kayle, M.; Crego, N.; Glassberg, J.; et al. Patient-reported neurocognitive symptoms influence instrumental activities of daily living in sickle cell disease. Am. J. Hematol. 2021, 96, 1396–1406. [Google Scholar] [CrossRef]
- Walsh, K.E.; Cutrona, S.L.; Kavanagh, P.L.; Crosby, L.E.; Malone, C.; Lobner, K.; Bundy, D.G. Medication Adherence Among Pediatric Patients With Sickle Cell Disease: A Systematic Review. Pediatrics 2014, 134, 1175–1183. [Google Scholar] [CrossRef]
- Fogarty, H.; Gaul, A.; Syed, S.; Aleksejenko, N.; Geoghegan, R.; Conroy, H.; Crampton, E.; Ngwenya, N.; Tuohy, E.; McMahon, C. Adherence to hydroxyurea, health-related quality of life domains and attitudes towards a smartphone app among Irish adolescents and young adults with sickle cell disease. Ir. J. Med. Sci. 2021, 191, 809–816. [Google Scholar] [CrossRef]
- Easterbrook, P.; Gopalan, R.; Berlin, J.; Matthews, D. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).