Functional Benefit and Orthotic Effect of Dorsiflexion-FES in Children with Hemiplegic Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
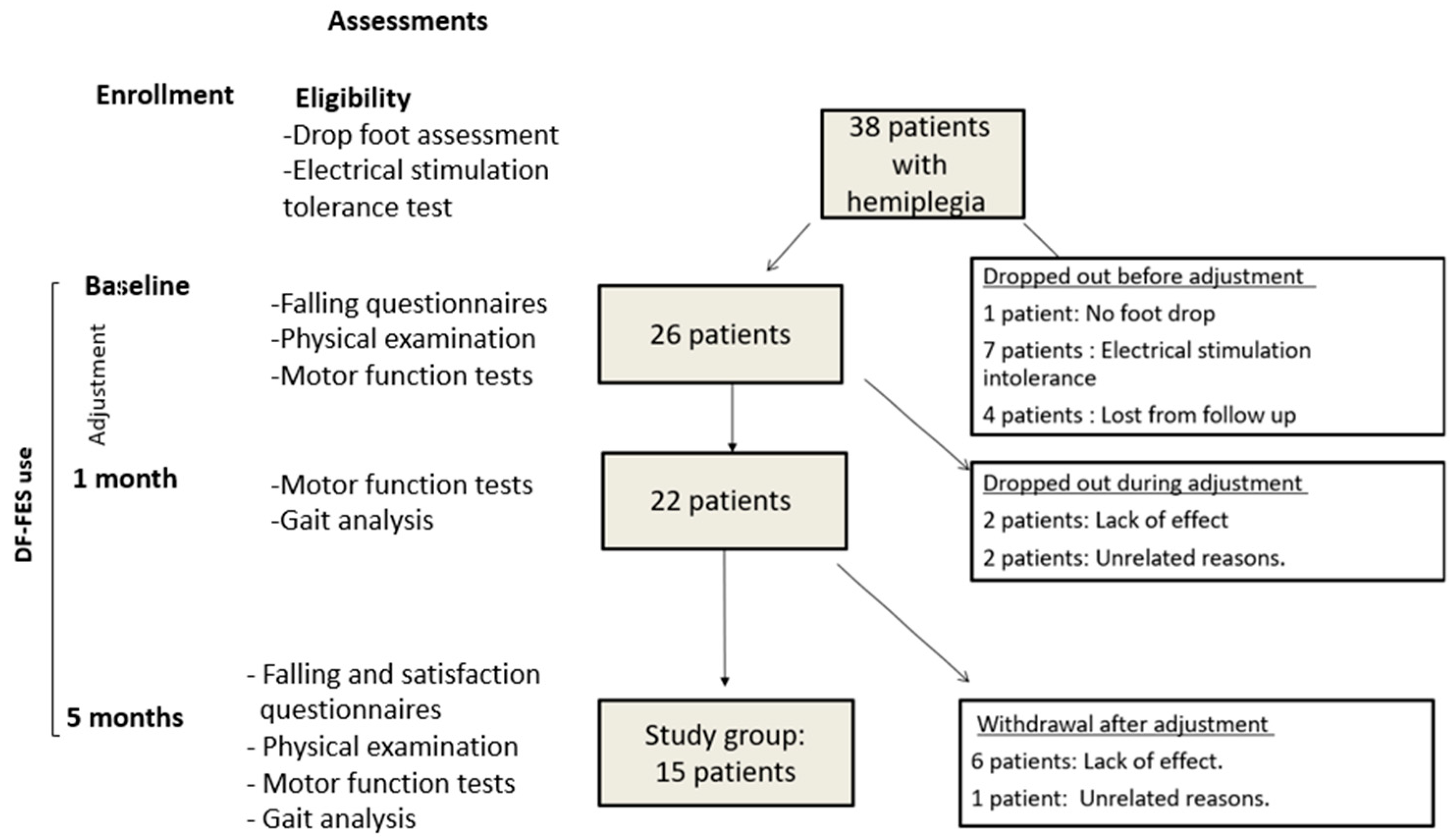
2.3. Enrollment
2.4. Motor Function Tests
2.4.1. Community Balance and Mobility Scale (CB&M)
2.4.2. Timed up and down Stairs Test (TUDS)
2.4.3. Six-Minute Walk Test (6MWT)
2.5. Falling and Satisfaction Questionnaires
2.6. Ankle Biomechanical Assessments
- Passive ankle range of motion with knee flexion and extension (with the leg supported on a bed), with the subtalar joint maintained in a neutral position. The measurement was conducted using a goniometer aligned at one end with the fibula and at the other end with the fifth metatarsal bone [34]. Foot deformities such as midfoot break were accounted for by accurately measuring calcaneal dorsiflexion with the foot held in supination.
- Plantar-flexor muscle spasticity with knee flexion and extension (for soleus and gastrocnemius muscle assessment, respectively). The measurement was conducted by dorsiflexion of the foot from maximum possible plantarflexion to maximum possible dorsiflexion. Spasticity was scored using the modified Ashworth scale [35].
- Muscle selectivity. Ankle joint selectivity was measured using the Selective Control Assessment of the Lower Extremity (SCALE), with patients in a sitting position with the knee extended. Patients were asked to move their foot up, down, and up again. Selectivity was scored based on a three-point scale (zero points = unable, one point = impaired, two points = normal) [36].
- Dorsiflexor muscle strength. Strength was evaluated in side-lying and seated positions using Kendall’s manual muscle testing scale [37].
2.7. Dorsiflexion-Functional Electrical Stimulation Device (DF-FES)
2.8. Gait Analysis
2.9. Orthotic Effect
2.10. Statistical Analyses
3. Results
3.1. Clinical and Demographic Variables
3.2. Falling
3.3. Motor Function Tests
3.3.1. Community Balance and Mobility Scale (CB&M)
3.3.2. Timed up and down Stairs Test (TUDS)
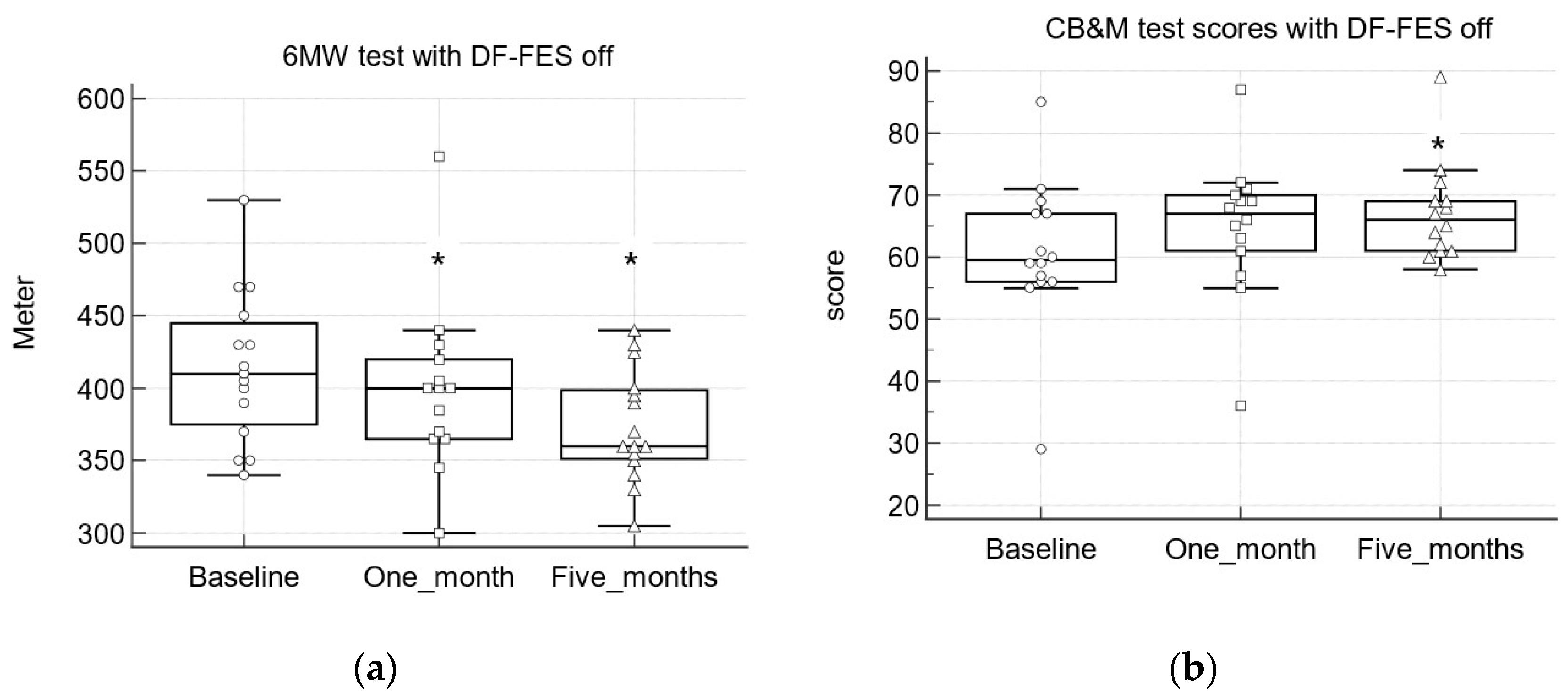
3.3.3. Six-Minute Walk Test (6MWT)
3.4. Kinematic, Spatiotemporal, and Biomechanical Parameters
3.4.1. Orthotic Effect
3.4.2. Predictors of OE+ and OE− at First Gait Analysis
3.4.3. Kinematic Parameters
3.4.4. Spatiotemporal Parameters
3.4.5. Biomechanical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wimalasundera, N.; Stevenson, V.L. Cerebral palsy. Pract. Neurol. 2016, 16, 184–194. [Google Scholar] [CrossRef]
- Odding, E.; Roebroeck, M.E.; Stam, H.J. The epidemiology of cerebral palsy: Incidence, impairments and risk factors. Disabil. Rehabil. 2006, 28, 183–191. [Google Scholar] [CrossRef]
- Pakula, A.T.; Van Naarden Braun, K.; Yeargin-Allsopp, M. Cerebral palsy: Classification and epidemiology. Phys. Med. Rehabil. Clin. N. Am. 2009, 20, 425–452. [Google Scholar] [CrossRef] [PubMed]
- Kenis-Coskun, O.; Giray, E.; Eren, B.; Ozkok, O.; Karadag-Saygi, E. Evaluation of postural stability in children with hemiplegic cerebral palsy. J. Phys. Ther. Sci. 2016, 28, 1398–1402. [Google Scholar] [CrossRef]
- Boulard, C.; Gross, R.; Gautheron, V.; Lapole, T. What causes increased passive stiffness of plantarflexor muscle-tendon unit in children with spastic cerebral palsy? Eur. J. Appl. Physiol. 2019, 119, 2151–2165. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y. Gait analysis of children with spastic hemiplegic cerebral palsy. Neural Regen. Res. 2012, 15, 1578–1584. [Google Scholar]
- Zonta, M.B.; Ramalho Júnior, A.; Camargo, R.M.; Dias, F.H.; Santos, L.H. Two-dimensional analysis of gait asymmetry in spastic hemiplegia. Einstein 2010, 8, 343–349. [Google Scholar] [CrossRef]
- Morris, C.; Bowers, R.; Ross, K.; Stevens, P.; Phillips, D. Orthotic management of cerebral palsy: Recommendations from a consensus conference. NeuroRehabilitation 2011, 28, 37–46. [Google Scholar] [CrossRef]
- Wingstrand, M.; Hagglund, G.; Rodby-Bousquet, E. Ankle-foot orthoses in children with cerebral palsy: A cross sectional population based study of 2200 children. BMC Musculoskelet. Disord. 2014, 15, 327. [Google Scholar] [CrossRef]
- Mooney, J.A.; Rose, J. A scoping review of neuromuscular electrical stimulation to improve gait in cerebral palsy: The arc of progress and future strategies. Front. Neurol. 2019, 10, 887. [Google Scholar] [CrossRef]
- Damiano, D.L.; Prosser, L.A.; Curatalo, L.A.; Alter, K.E. Muscle plasticity and ankle control after repetitive use of a functional electrical stimulation device for foot drop in cerebral palsy. Neurorehabil. Neural Repair 2013, 27, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Geboers, J.F.; van Tuijl, J.H.; Seelen, H.A.; Drost, M.R. Effect of immobilization on ankle dorsiflexion strength. Scand. J. Rehabil. Med. 2000, 32, 66–71. [Google Scholar]
- Danino, B.; Khamis, S.; Hemo, Y.; Batt, R.; Snir, E.; Wientroub, S.; Hayek, S. The efficacy of neuroprosthesis in young hemiplegic patients, measured by three different gait indices: Early results. J. Child. Orthop. 2013, 7, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Postans, N.J.; Granat, M.H. Effect of functional electrical stimulation, applied during walking, on gait in spastic cerebral palsy. Dev. Med. Child. Neurol. 2005, 47, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Khamis, S.; Herman, T.; Krimus, S.; Danino, B. Is functional electrical stimulation an alternative for orthotics in patients with cerebral palsy? A literature review. Eur. J. Paediatr. Neurol. 2018, 22, 7–16. [Google Scholar] [CrossRef]
- Pool, D.; Blackmore, A.M.; Bear, N.; Valentine, J. Effects of short-term daily community walk aide use on children with unilateral spastic cerebral palsy. Pediatr. Phys. Ther. 2014, 26, 308–317. [Google Scholar] [CrossRef]
- Pool, D.; Elliott, C.; Bear, N.; Donnelly, C.J.; Davis, C.; Stannage, K.; Valentine, J. Neuromuscular electrical stimulation-assisted gait increases muscle strength and volume in children with unilateral spastic cerebral palsy. Dev. Med. Child. Neurol. 2016, 58, 492–501. [Google Scholar] [CrossRef]
- Pool, D.; Valentine, J.; Bear, N.; Donnelly, C.J.; Elliott, C.; Stannage, K. The orthotic and therapeutic effects following daily community applied functional electrical stimulation in children with unilateral spastic cerebral palsy: A randomised controlled trial. BMC Pediatr. 2015, 15, 154. [Google Scholar] [CrossRef]
- Bailes, A.F.; Caldwell, C.; Clay, M.; Tremper, M.; Dunning, K.; Long, J. An exploratory study of gait and functional outcomes after neuroprosthesis use in children with hemiplegic cerebral palsy. Disabil. Rehabil. 2017, 39, 2277–2285. [Google Scholar] [CrossRef]
- Kottink, A.I.R.; Nikamp, C.D.; Bos, F.P.; van der Sluis, C.K.; van den Broek, M.; Onneweer, B.; Stolwijk-Swüste, J.M.; Brink, S.M.; Voet, N.B.M.; Buurke, J.B.; et al. Therapeutic effect of a soft robotic glove for activities of daily living in people with impaired hand strength: Protocol for a multicenter clinical trial (iHand). JMIR Res. Protoc. 2022, 11, e34200. [Google Scholar] [CrossRef]
- Laufer, Y.; Ring, H.; Sprecher, E.; Hausdorff, J.M. Gait in individuals with chronic hemiparesis: One-year follow-up of the effects of a neuroprosthesis that ameliorates foot drop. J. Neurol. Phys. Ther. 2009, 33, 104–110. [Google Scholar] [CrossRef]
- Robbins, S.M.; Houghton, P.E.; Woodbury, M.G.; Brown, J.L. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: A meta-analysis. Arch. Phys. Med. Rehabil. 2006, 87, 853–859. [Google Scholar] [CrossRef]
- Meilahn, J.R. Tolerability and effectiveness of a neuroprosthesis for the treatment of footdrop in pediatric patients with hemiparetic cerebral palsy. PM R 2013, 5, 503–509. [Google Scholar] [CrossRef]
- Prosser, L.A.; Curatalo, L.A.; Alter, K.E.; Damiano, D.L. Acceptability and potential effectiveness of a foot drop stimulator in children and adolescents with cerebral palsy. Dev. Med. Child. Neurol. 2012, 54, 1044–1049. [Google Scholar] [CrossRef]
- van der Linden, M.L.; Hazlewood, M.E.; Hillman, S.J.; Robb, J.E. Functional electrical stimulation to the dorsiflexors and quadriceps in children with cerebral palsy. Pediatr. Phys. Ther. 2008, 20, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bosques, G.; Martin, R.; McGee, L.; Sadowsky, C. Does therapeutic electrical stimulation improve function in children with disabilities? A comprehensive literature review. J. Pediatr. Rehabil. Med. 2016, 9, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Shieh, G.; Jan, S.L.; Randles, R.H. Power and sample size determinations for the Wilcoxon signed-rank test. J. Stat. Comput. Simul. 2007, 77, 717–724. [Google Scholar] [CrossRef]
- Schwesig, R.; Leuchte, S.; Fischer, D.; Ullmann, R.; Kluttig, A. Inertial sensor based reference gait data for healthy subjects. Gait Posture 2011, 33, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Nuesch, C.; Roos, E.; Pagenstert, G.; Mundermann, A. Measuring joint kinematics of treadmill walking and running: Comparison between an inertial sensor based system and a camera-based system. J. Biomech. 2017, 57, 32–38. [Google Scholar] [CrossRef]
- Wright, M.J.; Bos, C. Performance of children on the Community Balance and Mobility Scale. Phys. Occup. Ther. Pediatr. 2012, 32, 416–429. [Google Scholar] [CrossRef]
- Maher, C.A.; Williams, M.T.; Olds, T.S. The six-minute walk test for children with cerebral palsy. Int. J. Rehabil. Res. 2008, 31, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Zaino, C.A.; Marchese, V.G.; Westcott, S.L. Timed up and down stairs test: Preliminary reliability and validity of a new measure of functional mobility. Pediatr. Phys. Ther. 2004, 16, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.; Beath, T.; Bell, J.; Jacobson, G.; Phair, T.; Salbach, N.M.; Wright, F.V. Test-retest reliability of the 10-metre fast walk test and 6-minute walk test in ambulatory school-aged children with cerebral palsy. Dev. Med. Child. Neurol. 2008, 50, 370–376. [Google Scholar] [CrossRef]
- Charles, J.; Scutter, S.D.; Buckley, J. Static ankle joint equinus: Toward a standard definition and diagnosis. J. Am. Podiatr. Med. Assoc. 2010, 100, 195–203. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.G.; Staudt, L.A.; Greenberg, M.B.; Oppenheim, W.L. Selective Control Assessment of the Lower Extremity (SCALE): Development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Dev. Med. Child. Neurol. 2009, 51, 607–614. [Google Scholar]
- Conable, K.M.; Rosner, A.L. A narrative review of manual muscle testing and implications for muscle testing research. J. Chiropr. Med. 2011, 10, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kadaba, M.P.; Ramakrishnan, H.K.; Wootten, M.E. Measurement of lower extremity kinematics during level walking. J. Orthop. Res. 1990, 8, 383–392. [Google Scholar] [CrossRef]
- Williams, D.S.; Martin, A.E. Gait modification when decreasing double support percentage. J. Biomech. 2019, 92, 76–83. [Google Scholar] [CrossRef]
- Alnajjar, F.; Zaier, R.; Khalid, S.; Gochoo, M. Trends and technologies in rehabilitation of foot drop: A systematic review. Expert Rev. Med. Devices 2021, 18, 31–46. [Google Scholar] [CrossRef]
- Armand, S.; Attias, M. Contracture and gait deviations. In Handbook of Human Motion; Springer: Cham, Switzerland, 2019; pp. 1–21. [Google Scholar]
- Kim, D.H.; An, D.H.; Yoo, W.G. Validity and reliability of ankle dorsiflexion measures in children with cerebral palsy. J. Back Musculoskelet. Rehabil. 2018, 31, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Konor, M.M.; Morton, S.; Eckerson, J.M.; Grindstaff, T.L. Reliability of three measures of ankle dorsiflexion range of motion. Int. J. Sports Phys. Ther. 2012, 7, 279–287. [Google Scholar] [PubMed]
| Parameters | Study Group (N = 15) | Withdrawn Patients (N = 11) |
|---|---|---|
| Age (y) | 8.2 (7, 10.5) | 8 (6.8, 8.8) |
| M:F | 10:5 | 5:6 |
| Term (≥37 w) | 7 (47%) | 6 (55%) |
| GMFCS I:II | 14:1 | 8:3 |
| Current AFO use | 7 (46%) | 7 (63%) |
| Botulinum toxin—LL | ||
| No | 12 (80%) | 3 (27%) |
| Yes | 3 (20%) | 8 (73%) * |
| Surgery to LL (n) | ||
| No | 14 (93%) | 9 (82%) |
| Yes | 1(7%) | 2 (18%) |
| MAS | ||
| 1 | 2 (13%) | 3 (27%) |
| 1+ | 4 (27%) | 2 (18%) |
| 2 | 9 (60%) | 6 (55%) |
| Passive ankle ROM | ||
| Knee flexion (degrees) | 10° (5, 15) | 10° (7.5, 15) |
| Knee extension (degrees) | 5° (2, 9) | 5° (1, 10) |
| Tests | Median Difference (95% CI) * | |||
|---|---|---|---|---|
| Baseline to 1 Month | 1 Month to 5 Months | Baseline to 5 Months | ||
| CB&M (score) (N = 14) | DF-FES off | 3 $ | 3 | 6 $ |
| (1, 7.2) | (−3.2, 4.1) | (1.89, 8.1) | ||
| DF-FES on | 4.5 $ | 1 | 6.5 $ | |
| (0.89, 8.3) | (−4, 7.1) | (2.79, 10) | ||
| 6 MWT (meter) (N = 15) | DF-FES off | −17.5 | −17.5 | −30 # |
| (−67.08, 15) | (−73.12, 16.04) | (−83.67,−2.6) | ||
| DF-FES on | −30 # | −12.5 | −35 # | |
| (−55, −4.47) | (−45.5, 11.04) | (−99.67, −3.97) | ||
| TUDS (sec) (N = 15) | DF-FES off | −0.19 | −0.19 | −0.83 |
| (−2.34, 0.4) | (−0.76, 0.7) | (−2.28, 0.42) | ||
| DF-FES on | −0.7 | −0.68 | −0.41 | |
| (−1.71, 1.19) | (−1.71, 0.4) | (−2.7, 0.28) | ||
| DF-FES off (N = 15) | DF-FES on (N = 15) | |||
|---|---|---|---|---|
| FES | First | Final | First | Final |
| Maximal dorsiflexion—mid swing (degrees) | −4.57° | −3.3° | 3.13° * | 3.2° # |
| (−9.1, 4.63) | (−10.05, 4.06) | (−5.97, 6.01) | (−4.04, 6.64) | |
| Maximal dorsiflexion—terminal swing (degrees) | −3.52° | −3.86° | 3.97° * | 3.36° # |
| (−7.53, 2.05) | (−7.77, 1.79) | (−0.39, 6.49) | (1.64, 7.62) | |
| Minimal dorsiflexion—mid swing (degrees) | −11.68° | −6.72° | −0.97° * | −0.58° # |
| (−15.07, 0.74) | (−16.5, −0.99) | (−11.62, 1.63) | (−9.95, 3.28) | |
| Minimal dorsiflexion—terminal swing (degrees) | −11.46° | −11.24° | −1.88° * | −1.6° # |
| (−14.5, −4.74) | (−15.59, −7.68) | (−6.28, 1.46) | (−6.82, 0.94) | |
| Initial contact (degrees) | −7.08° | −7.2° | −0.49° * | 0.81° # |
| (−9.1, −1.02) | (−11.78, −3.79) | (−4.32, 2.99) | (−2.33, 2.7) | |
| Peak swing dorsiflexion (degrees) | −0.82° | −2.18° | 4.51° * | 4.27° # |
| (−6.59, 4.89) | (−4.3, 4.22) | (0.08, 7.18) | (1.94, 8.03) | |
| Stance time (sec) | 0.53 | 0.56 | 0.52 | 0.56 |
| (0.46, 0.57) | (0.53, 0.63) | (0.49, 0.61) | (0.51, 0.59) | |
| Stance—%gait cycle | 56.88 | 57.42 | 56.29 | 56.54 |
| (55.65, 57.59) | (56.59, 57.72) | (54.76, 56.96) | (55.61, 57.23) | |
| Walking speed (meter/sec) | 1.09 | 1.04 | 1.1 | 1.07 |
| (0.955, 1.260) | (0.97, 1.13) | (0.95, 1.2) | (0.96, 1.17) | |
| Cadence (steps/min) | 127.9 | 122.4 | 126.32 | 118.44 |
| (120.12, 147.16) | (111.42, 127.54) | (115.91, 134.91) | (112.69, 131.87) | |
| Double/single support time ratio | 0.42 | 0.47 | 0.42 | 0.4 |
| (0.35 to 0.46) | (0.4 to 0.53) | (0.36 to 0.45) | (0.36 to 0.44) | |
| Step length (cm) | 0.49 | 0.51 | 0.51 | 0.5 |
| (0.42, 0.52) | (0.46, 0.53) | (0.48, 0.57) | (0.46, 0.53) | |
| Step time (sec) | 0.49 | 0.53 $ | 0.52 | 0.54 |
| (0.43, 0.54) | (0.5, 0.59) | (0.48, 0.54) | (0.48, 0.5) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segal, I.; Khamis, S.; Sagie, L.; Genizi, J.; Azriel, D.; Katzenelenbogen, S.; Fattal-Valevski, A. Functional Benefit and Orthotic Effect of Dorsiflexion-FES in Children with Hemiplegic Cerebral Palsy. Children 2023, 10, 531. https://doi.org/10.3390/children10030531
Segal I, Khamis S, Sagie L, Genizi J, Azriel D, Katzenelenbogen S, Fattal-Valevski A. Functional Benefit and Orthotic Effect of Dorsiflexion-FES in Children with Hemiplegic Cerebral Palsy. Children. 2023; 10(3):531. https://doi.org/10.3390/children10030531
Chicago/Turabian StyleSegal, Idan, Sam Khamis, Liora Sagie, Jacob Genizi, David Azriel, Sharona Katzenelenbogen, and Aviva Fattal-Valevski. 2023. "Functional Benefit and Orthotic Effect of Dorsiflexion-FES in Children with Hemiplegic Cerebral Palsy" Children 10, no. 3: 531. https://doi.org/10.3390/children10030531
APA StyleSegal, I., Khamis, S., Sagie, L., Genizi, J., Azriel, D., Katzenelenbogen, S., & Fattal-Valevski, A. (2023). Functional Benefit and Orthotic Effect of Dorsiflexion-FES in Children with Hemiplegic Cerebral Palsy. Children, 10(3), 531. https://doi.org/10.3390/children10030531






