Abstract
Non-Hodgkin B-cell lymphomas are a heterogeneous group of lymphoid malignancies with variable biological behavior, clinical presentation and treatment response. While chemoimmunotherapy remains the cornerstone of their management, growing evidence implicates the gut microbiota as a critical modulator of both lymphomagenesis and therapeutic efficacy. Gut microbiota dysbiosis, characterized by reduced microbial diversity and pathogenic taxonomic shifts, has been observed also in newly diagnosed patients and not just after therapy. This microbial imbalance contributes to mucosal barrier disruption, systemic inflammation, and altered immune responses, affecting treatment outcomes and toxicity profiles. Antibiotic exposure, especially broad-spectrum agents, exacerbates dysbiosis and has been associated with inferior responses to immunochemotherapy and CAR T-cell therapy. Conversely, certain commensal taxa, like Faecalibacterium prausnitzii and Lactobacillus johnsonii, may exert protective effects by preserving mucosal homeostasis and promoting antitumor immunity. Targeted interventions, including prudent antibiotic stewardship, prebiotics, probiotics, dietary modulation, and fecal microbiota transplantation, are under investigation to restore eubiosis and improve clinical outcomes. Preliminary clinical trials suggest a strong correlation between baseline microbiome composition and therapeutic response. Further mechanistic studies and randomized trials are warranted to define the causal role of the microbiome in non-Hodgkin B-cell lymphomas pathophysiology and to develop personalized microbiome-modulating strategies as adjuncts to standard treatment.
1. General Background About Non-Hodgkin Lymphomas
Non-Hodgkin lymphomas (NHLs) represent a heterogeneous group of lymphoid malignancies. The global incidence of NHL has increased over the past few decades, with significant geographic variation influenced by environmental, genetic, and infectious factors. According to data from the Global Cancer Observatory (GLOBOCAN) database (2020), NHL is among the ten most common cancers worldwide, with an estimated 544,000 new cases and approximately 260,000 deaths annually. The median age at diagnosis is around 67 years, although specific subtypes may present at younger ages [1,2,3].
NHLs encompass over 60 distinct entities as classified by the World Health Organization (WHO), based on morphological, immunophenotypic, genetic, and clinical criteria. They are broadly categorized into B-cell and T/NK-cell neoplasms, with B-cell lymphomas accounting for approximately 85–90% of all cases [4,5]. Among B-cell NHLs, the most prevalent subtype is diffuse large B-cell lymphoma (DLBCL), constituting about 30–40% of cases [6,7,8,9].
For aggressive B-cell NHLs, particularly DLBCL, the standard first-line therapy is immunochemotherapy with the R-CHOP regimen, which combines rituximab (an anti-CD20 monoclonal antibody) with cyclophosphamide, doxorubicin, vincristine, and prednisone. This regimen induces complete response (CR) rates of approximately 60–75% and overall response rates (ORRs) exceeding 80%. Long-term disease-free survival is achievable in a significant proportion of patients, particularly those with limited-stage disease or favorable prognostic features. Intensified regimens such as dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) may be used in high-risk cases or specific subtypes such as primary mediastinal B-cell lymphoma, often with improved outcomes [10,11,12,13].
Indolent lymphomas, such as follicular lymphoma (FL), typically follow a relapsing–remitting course. First-line treatment is generally initiated based on symptoms or disease burden, and regimens include rituximab alone for low tumor burden cases or combination therapies such as R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone) or R-CHOP. Bendamustine combined with rituximab (BR) has emerged as a preferred regimen due to comparable efficacy and improved tolerability. In FL, CR rates range from 30 to 50%, with ORR often exceeding 90%; however, long-term remissions are uncommon, and most patients eventually relapse [7]. Mantle cell lymphoma (MCL), due to its aggressive nature, is typically treated with intensive immunochemotherapy, often including high-dose cytarabine followed by autologous stem cell transplantation (ASCT) in eligible patients. BR may also be used in older or less fit individuals. Response rates are generally high (ORR 70–90%), but relapses are frequent [14].
For T-cell lymphomas, CHOP-based regimens remain standard, although responses are often suboptimal, with CR rates below 50% and poor long-term survival. Novel agents and clinical trials are increasingly important in these subtypes [15]. Overall, the first-line treatment of NHL aims to maximize response and prolong survival while minimizing toxicity. Advances in immunotherapy and precision medicine continue to refine therapeutic strategies and improve patient outcomes.
The aim of this narrative review is to explore the topic of gut microbiota dysbiosis in patients with NHL, finding possible dietary interventions and trying to understand if this alteration can have a role on the response to treatments.
In order to reach this target, we searched PubMed, Scopus, and Web of Science databases for articles published in English between January 2015 and June 2025. The following combinations of Medical Subject Headings (MeSH) and keywords were used: “non-Hodgkin lymphoma”; “chemotherapy”; “immunotherapy”; “response rates”; “gut microbiota”; “gut microbiota dysbiosis”; “probiotics”; “prebiotics”; “diet”; “fecal transplantation”. Additional references were identified through manual screening of bibliographies from relevant articles and reviews.
2. Adverse Events Related to Treatments: What About Gastrointestinal Toxicity?
Chemotherapy and immunotherapy represent cornerstone treatments in the management of NHL, leading to significant improvements in survival outcomes [16]. However, these modalities are frequently associated with a broad spectrum of adverse effects, both hematological toxicities such as neutropenia, anemia, and thrombocytopenia and non-hematological ones such as gastrointestinal (GI) toxicity [17,18].
GI toxicity encompasses a range of symptoms including nausea, vomiting, mucositis, diarrhea, abdominal pain, and anorexia. These effects may result from direct cytotoxic damage to the rapidly proliferating epithelial lining of the gastrointestinal tract. Regimens such as CHOP or R-CHOP (with rituximab) are commonly used in NHL and have well-documented GI side effects. Diarrhea may also be exacerbated by antibiotic exposure or infectious causes during immunosuppression. Moreover, agents like vincristine can cause autonomic neuropathy leading to ileus or severe constipation [19,20].
Immunotherapy, particularly monoclonal antibodies like rituximab and checkpoint inhibitors used in refractory or relapsed NHL, may compound toxicity by inducing immune-mediated adverse events. Although rituximab is generally well tolerated, it can sometimes induce colitis or hepatitis due to immune dysregulation. Additionally, novel agents such as Chimeric Antigen Receptor (CAR) T-cell therapies and bispecific antibodies introduce unique toxicity profiles, including cytokine release syndrome and immune effector cell-associated neurotoxicity, but also contribute to GI symptoms like diarrhea, nausea, and mucosal inflammation [21].
The interplay between chemotherapy, immunotherapy, and the gut microbiota is gaining attention as a potential modifier of treatment-related GI toxicity. Disruption of microbial diversity—known as intestinal dysbiosis—has been increasingly associated with mucosal injury, inflammation, and exacerbation of gastrointestinal symptoms in patients receiving chemoimmunotherapy. Emerging evidence suggests that maintaining microbiome homeostasis could mitigate toxicity and improve treatment tolerability [22].
In clinical practice, managing GI toxicity involves a combination of prophylactic and supportive strategies, including antiemetics, antidiarrheals, nutritional support, and in some cases, dose adjustments or treatment delays. Close monitoring and early intervention are essential to prevent complications such as dehydration, electrolyte imbalance, or malnutrition. Given the impact of GI and other toxicities on patient outcomes, ongoing research is focused on predictive biomarkers, microbiome modulation, and toxicity-reduction strategies to optimize NHL treatment safety and efficacy [23].
3. What Is Gut Microbiota?
The human gut microbiota refers to the diverse community of microorganisms, encompassing bacteria, viruses, fungi, archaea, and protists that reside in various areas of the human body, including the gastrointestinal tract, oral cavity, skin and respiratory tract [24,25].
This complex ecosystem, predominantly localized in the colon, comprises an estimated 1013–1014 microbial cells and includes over 1,000 different bacterial species, with the majority belonging to the Firmicutes and Bacteroidetes phyla [12]. Bacteria constitute the most extensively studied component, contributing approximately 99% of the gut microbiota. These microbes are engaged in a symbiotic relationship with the host, influencing numerous physiological functions including nutrient metabolism, vitamin synthesis, protection against pathogens, and maintenance of the intestinal epithelial barrier [13].
The gut microbiota exhibits a highly individualized composition that evolves over the course of a person’s life and is influenced by intrinsic factors like genetic background and immune system activity [14]. External variables—including dietary habits, use of antibiotics and other medications, infections, circadian rhythms, and environmental exposures—also significantly influence the structure and function of the intestinal microbiota [18].
The gut microbiota is essential for maintaining intestinal and systemic homeostasis. By fermenting indigestible carbohydrates, gut bacteria produce short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate. These SCFAs not only nourish colonic epithelial cells but also exert systemic effects, modulating host metabolism and immune responses. Furthermore, the gut flora supports the maturation and integrity of the gut-associated lymphoid tissue (GALT), which is crucial for immune surveillance and tolerance [19].
In terms of immunomodulation, gut microbiota plays a critical role in both innate and adaptive immune responses. It induces the development of regulatory T cells (Tregs), promotes IgA production by B cells, and influences the secretion of anti-inflammatory cytokines like IL-10. Certain microbial strains, such as Bacteroides fragilis and Clostridium spp., are particularly effective at promoting an anti-inflammatory milieu, while others, like segmented filamentous bacteria (SFB), drive pro-inflammatory Th17 responses. Dysregulation of this balance—known as dysbiosis—can compromise the intestinal barrier, facilitate the translocation of bacterial components like lipopolysaccharides (LPSs) into systemic circulation, and provoke chronic inflammation.
Dysbiosis has been increasingly recognized as a contributing factor in the pathogenesis of several malignancies, including NHLs. Evidence indicates that microbial imbalance can disrupt immune homeostasis and activate oncogenic pathways. Specifically, microbial products can engage pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), leading to activation of the NF-κB signaling pathway. This cascade promotes the expression of pro-inflammatory cytokines like TNF-α, IL-1, and IL-6, which contribute to the pro-tumorigenic microenvironment through persistent inflammation, inhibition of apoptosis, and promotion of cellular proliferation [23].
The cumulative effect of microbiota-induced chronic inflammation may alter the equilibrium between cell proliferation and apoptosis, fostering genomic instability and contributing to lymphomagenesis. These immune perturbations provide a fertile ground for the emergence of lymphoid neoplasms, especially in genetically predisposed individuals.
Comparative gut microbiota analysis, utilizing high-throughput sequencing (e.g., 16S rRNA and metagenomic profiling), enables the discrimination between healthy subjects and those with NHL. Patients with B-cell NHL exhibit significant reductions in alpha diversity and a marked shift in microbial composition—specifically, increased Bacteroidetes and decreased Firmicutes, particularly in aggressive subtypes such as diffuse large B-cell lymphoma [26]. Taxa such as Barnesiellaceae, Coriobacteriaceae, Faecalibacterium, Christensenella, and Sutterella have been inversely correlated with bloodstream infections in NHL patients. Functional metagenomic evidence further reveals that depletion of Eubacterium rectale—a butyrate producer—is a distinguishing hallmark in lymphoma patients, with potential mechanistic roles in attenuating intestinal inflammation and B-cell NF-κB activation [27].
Evidence from preclinical studies supports this association. A landmark investigation by Yamamoto and colleagues utilized a murine model of ataxia–telangiectasia (AT)—a condition marked by defective DNA repair due to mutations in the ATM gene—to examine microbiota-mediated modulation of lymphoma risk [28]. Mice colonized with a limited microbial repertoire exhibited accelerated lymphoma onset, increased oxidative stress, and systemic genotoxicity. Conversely, administration of Lactobacillus johnsonii reduced inflammatory and genotoxic markers, suggesting a protective effect against lymphoma development in susceptible hosts [28] (Figure 1).
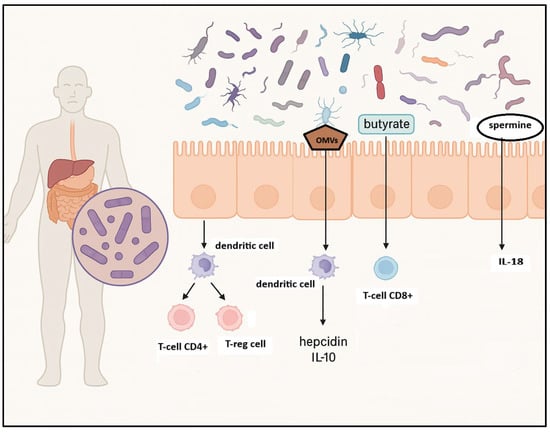
Figure 1.
Influence of gut microbiota on innate and adaptive immune response. Gut microbiota influences innate immune responses by regulating the secretion of antimicrobial peptides and modulating natural killer (NK) cell activity; microbial metabolites, such as spermine, lead to IL-18 production; outer membrane vesicles (OMVs) induce IL-10 and hepcidin production. Gut microbiota also shapes adaptive immune responses by encouraging the development of regulatory T cells (T-regs), which promote immune tolerance and activate CD8+ cytotoxic T cells that are critical for B-cell maturation. Created in BioRender. Santino Caserta. (2025) https://app.biorender.com/illustrations/68b5c21594f73e7a5f30080d.
In vivo investigations in animal models have increasingly elucidated the contributory role of gut microbiota dysbiosis in lymphomagenesis. A notable veterinary study demonstrated that intestinal dysbiosis in dogs and cats—characterized by an increased prevalence of Gram-negative facultative anaerobes such as Parabacteroides—is associated with chronic mucosal inflammation and the onset of gastrointestinal lymphoma. In a pilot study of dogs with stage IV multicentric lymphoma, distinct alterations in the fecal microbiota were reported, indicating that lymphoid malignancy correlates with microbiota imbalance [29]. Complementary evidence from chemotherapy studies further supports that interventions aimed at restoring eubiosis can attenuate gastrointestinal damage and systemic immune dysregulation. Although not lymphoma-specific, numerous preclinical models of chemotherapy-induced mucositis—an inflammation-driven gut injury—have shown beneficial effects of probiotics, synbiotics, and prebiotics in mitigating dysbiosis, preserving epithelial integrity, and reducing inflammatory responses [30].
4. Gut Microbiota Dysbiosis Due to Chemoimmunotherapy
Chemoimmunotherapy remains the standard of care for B-cell NHL, significantly improving survival outcomes through the synergistic effects of cytotoxic agents and monoclonal antibodies [31]. Despite its clinical efficacy, this therapeutic approach is accompanied by substantial off-target toxicities, particularly within the GI tract [32,33]. A growing body of preclinical and clinical evidence highlights that GI toxicity is not merely a collateral effect but a key driver of intestinal dysbiosis in this patient population [34,35,36]. The integrity of the gut mucosa, a critical interface between the host immune system and the microbiota, is profoundly affected by chemotherapeutic regimens [37,38,39].
The commonly used agents in B-cell NHL—contribute to cumulative damage of the intestinal epithelial lining, inducing direct cytotoxicity to rapidly proliferating epithelial cells, compromising tight junction integrity, and increasing intestinal permeability. Chemotherapy-induced epithelial injury disrupts barrier homeostasis, shifts nutrient availability, and promotes translocation of microbial products—conditions that favor dysbiosis and amplify mucosal inflammation.
Multiple studies have documented a decline in beneficial microbial populations—including Bifidobacterium spp., Lactobacillus, and Faecalibacterium prausnitzii—during and after chemoimmunotherapy. These taxa are known to exert anti-inflammatory effects, support mucosal healing, and maintain regulatory immune responses. Their depletion is paralleled by the overrepresentation of pro-inflammatory species such as Enterococcus, Escherichia coli, and Clostridioides difficile, which further exacerbate GI toxicity. Additionally, rituximab-mediated B-cell depletion reduces secretory IgA levels and impairs antigen-specific immune responses in the gut-associated lymphoid tissue, weakening the mucosal defense against microbial invasion and perpetuating dysbiotic changes [40].
Importantly, intestinal dysbiosis has implications beyond local GI injury, in fact bile acid derivatives, SCFAs and tryptophan metabolites produced by gut microbiota influence systemic immune tone and modulate the tumor microenvironment. Dysregulation of these microbial products may alter immune checkpoint expression, T-cell polarization, and cytokine release, thereby affecting the efficacy of anticancer therapies. Reduced microbial diversity during treatment has been associated with increased GI complications, heightened susceptibility to systemic infections, and inferior clinical outcomes in B-NHL patients [41] (Table 1).

Table 1.
Clinical studies reporting microbiome alterations associated with chemoimmunotherapy in B-cell non-Hodgkin lymphomas.
This table summarizes key clinical studies that have evaluated gut microbiome changes in patients with B-cell non-Hodgkin lymphomas undergoing chemotherapy or chemoimmunotherapy. Reported alterations include loss of commensal taxa (Bifidobacterium, Lactobacillus, Faecalibacterium prausnitzii) and expansion of potentially pathogenic species (Enterococcus, Escherichia coli, Clostridioides difficile). These shifts have been associated with gastrointestinal mucosal injury, systemic infections, increased treatment-related toxicities, and inferior therapeutic responses. Abbreviations: R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; SCFAs, short-chain fatty acids; GvHD, graft-versus-host disease.
Chemotherapy induces mucosal injury, alters bile acid flux, and reduces luminal carbohydrates, while broad-spectrum and anti-anaerobic antibiotics (e.g., carbapenems, piperacillin–tazobactam, some cephalosporins) deplete SCFA, producing commensals such as Faecalibacterium, Blautia, and other Clostridiales. The resulting loss of microbial diversity and SCFA signaling compromises epithelial barrier integrity, tight junction expression, and colonocyte bioenergetics, amplifying endotoxin translocation and systemic inflammation. In this context, dysbiosis has been associated with higher rates of febrile neutropenia, Clostridioides difficile infection, and bloodstream infections, as well as with attenuated responses to chemoimmunotherapy and cellular therapies in selected cohorts.
The timing and spectrum of antibiotic exposure appear critical. Peri-induction regimens that unnecessarily suppress obligateanaerobes accelerate community collapse (“domination”) by pathobionts such as Enterococcus or Proteobacteria, which correlates with infectious complications and, in transplant candidates, with more severe graft-versus-host disease (GvHD). Conversely, gut-sparing strategies—early de-escalation when cultures are negative, avoidance of redundant double coverage, preference of agents with narrower anaerobic activity when clinically appropriate, and strict duration controls—are consistently linked to preserved diversity. Prophylaxis also matters: while fluoroquinolones or trimethoprim–sulfamethoxazole may reduce specific infections, they can expand the intestinal resistome and select for taxa that sustain dysbiosis; decisions should therefore be individualized to local epidemiology and patient risk.
These antimicrobial effects interact with diet and supportive care. Enteral nutrition favors SCFA recovery and mucosal healing more than parenteral nutrition, and resistant-starch/fermentable-fiber intake can support butyrate-producing consortia when mucosal integrity allows. Probiotics and postbiotics remain promising but require cautious, protocolized use in immunocompromised hosts; the risk–benefit profile varies by strain, preparation, and neutropenic status. In heavily pretreated or transplant settings, microbiome-restorative approaches (e.g., fecal microbiota transplantation under stringent screening) are under investigation for steroid-refractory gastrointestinal GvHD and for decolonization of multidrug-resistant organisms, but they should be confined to experienced centers [42].
Understanding the bidirectional relationship between chemoimmunotherapy-induced GI toxicity and intestinal dysbiosis is crucial for the development of supportive care strategies. Emerging interventions—including dietary modulation, selective prebiotic and probiotic formulations, and fecal microbiota transplantation (FMT)—are under investigation for their potential to preserve mucosal integrity and restore microbial equilibrium. Future research should focus on delineating the molecular mechanisms underlying host-microbiota interactions during therapy, identifying predictive biomarkers of microbiome-related toxicity, and personalizing microbiome-supportive interventions to improve treatment tolerability and efficacy in B-NHL (Table 2).

Table 2.
Chemoimmunotherapy related mechanisms leading to gut microbiota dysbiosis in non-Hodgkin B-cell lymphomas: microbial metabolites involved and consequences on immune response.
5. Targeted Interventions for Dysbiosis and Influence on Chemoimmunotherapy Response
Disruption of the gut microbial equilibrium, known as dysbiosis, has emerged as a critical factor in both the pathogenesis and treatment responsiveness of NHLs.
Patients newly diagnosed with DLBCL often exhibit distinct alterations in their gut microbiota compared to healthy individuals, characterized by a reduction in alpha diversity and a significantly divergent microbial composition [43]. Notably, this dysbiotic state frequently involves an overrepresentation of Enterobacteriaceae, including species such as Escherichia coli and Citrobacter freundii, and Enterococcaceae such as Enterococcus faecium, whereas healthy individuals demonstrate a higher abundance of beneficial taxa such as Lachnospiraceae, Prevotellaceae, and Coriobacteriaceae. Functional metagenomic analyses suggest that the predominance of Enterobacteriaceae may contribute to opportunistic pathogenesis, including enhanced biofilm formation and mechanisms of antibiotic resistance [44]. Mendelian randomization studies have provided further support for a causal relationship between specific microbial taxa and the risk of DLBCL, implicating genera such as Ruminococcaceae UCG-002 and Coprobacter as potential risk factors, while Alistipes and Turicibacter may exert protective effects. Similar patterns of microbial disruption, including reduced diversity and specific taxonomic shifts, have been documented in FL, particularly in its gastrointestinal manifestations [45,46,47].
The gut microbiota plays a pivotal role in modulating therapeutic responses across various oncologic treatments, including conventional chemotherapy and advanced therapies such as CAR T-cell therapy, R-CHOP and allogenic hematopoietic stem cell transplantation (HSCT). In patients with DLBCL undergoing R-CHOP chemotherapy, an increased abundance of Enterobacteriaceae correlates with a higher incidence of febrile neutropenia, a complication that is independently associated with reduced progression-free survival, and inferior treatment outcomes; in detail, the 1-year PFS is 70% in patients with high Enterobacteriaceae versus 95% in those with low levels [48]. This microbial pattern may potentiate systemic inflammation, as reflected by elevated circulating levels of interleukin-6 (IL-6) and interferon-gamma (IFN-γ). Conversely, the presence of certain commensal bacteria, such as Lactobacillus fermentum, has been associated with a more favorable clinical response, in fact it can enhance host immunity by promoting anti-inflammatory cytokine production (e.g., IL-10) and supporting regulatory T cell activity; these effects may help mitigate systemic inflammation and enhance the tumor-directed immune response. Importantly, chemotherapy itself is a known driver of dysbiosis, leading to marked reductions in microbial richness and diversity [49,50].
In the context of CAR T-cell therapy, the gut microbiota exerts a dual influence on both therapeutic efficacy and toxicity profiles. Research indicates a strong correlation between gut microbial composition and CAR-T cell therapy outcomes; in fact, a study [51] found that specific species of Clostridia were associated with achieving a complete response by day 100 following CAR-T treatment.
Accumulating evidence highlights three key domains: antibiotic associations, mechanistic rationale, and exploratory signals with oral vancomycin.
In patients with non-Hodgkin B-cell lymphoma, those who received high-risk antibiotics such as meropenem, cefepime, ceftazidime, and piperacillin–tazobactam during therapy exhibited poorer treatment responses. This adverse outcome was linked to higher abundances of opportunistic pathogens like Prevotella, Veillonella, and Enterococcus species. Conversely, patients who did not receive these high-risk antibiotics demonstrated better treatment responses, which correlated with higher abundances of beneficial bacteria, including Roseburia, Bifidobacterium, Lactobacillus, and Eubacterium species [52,53].
CAR-T cell therapy commonly induces significant alterations in the gut microbiota. One study revealed a notable decrease in overall bacterial diversity after CAR-T therapy, with Firmicutes becoming more abundant and Bacteroidetes decreasing. Additionally, there was an increase in Enterococcus, Lactobacillus, and Actinomyces, while Bifidobacterium and Lachnospira levels declined. Regarding toxicity, lower levels of Bifidobacterium have been significantly correlated with the severity of cytokine release syndrome (CRS), a common toxic effect of CAR-T therapy affecting approximately 80% of patients [40].
Interestingly, oral vancomycin—an antibiotic with minimal systemic absorption—has been shown in preclinical studies to enhance CAR T-cell efficacy and is correlated with increased CAR T-cell expansion in the 100% of patients demonstrated significantly higher CAR T-cell expansion relative to unexposed patients. This phenomenon may be partially explained by the microbiota-modulating effects of vancomycin, which promote tumor-associated antigen (TAA) cross-presentation and endogenous CD8+ T-cell activation. Moreover, FMT experiments involving human-to-mouse transfer have demonstrated that vancomycin-induced shifts in the microbiota, including decreased alpha diversity and enrichment of species such as Enterobacteriaceae and Akkermansia muciniphila, can potentiate both CAR and non-CAR T-cell-mediated antitumor immunity [51].
Given these findings, targeted modulation of the gut microbiota represents a promising therapeutic avenue to improve outcomes in NHL.
Prudent antibiotic stewardship is essential, with emphasis on minimizing unnecessary use of broad-spectrum antibiotics during critical phases of treatment to preserve microbial integrity and avoid complications such as febrile neutropenia or diminished CAR T-cell efficacy. Personalized approaches to antimicrobial use, informed by microbial profiling, are likely to enhance therapeutic precision [54].
Additionally, probiotic and prebiotic interventions are under investigation for their potential to enrich beneficial taxa. Certain commensals—such as Faecalibacterium, Prevotella, Paraprevotella, and Bifidobacterium—may counterbalance the pathogenic effects of Enterobacteriaceae, while Lactobacillus johnsonii has demonstrated the capacity to reduce systemic inflammation and lymphoma incidence in genetically susceptible mouse models. Notably, the strength of evidence varies across these taxa: while Bifidobacterium and Faecalibacterium have shown emerging supportive data in B-cell NHL and other hematological malignancies, where their depletion has been associated with higher infection risk and inferior clinical outcomes, the evidence for Prevotella and Paraprevotella remains preliminary and is mainly derived from studies in solid tumors or broader oncology cohorts, thus requiring cautious interpretation in the context of NHL [55,56].
Dietary interventions, including the adoption of Mediterranean-style diets rich in plant-based fibers, have been shown to promote a more favorable microbial profile. While direct evidence of their impact on NHL outcomes remains limited, dietary modulation may represent a supportive measure for maintaining gut eubiosis during treatment [57,58].
Among the most promising strategies is FMT, a therapeutic approach involving the transfer of fecal microbiota from healthy donors to restore microbial balance in recipients. FMT is well-established in the treatment of recurrent Clostridioides difficile infection and is being increasingly explored in oncology. In NHL, FMT has shown efficacy in resolving therapy-resistant colitis, particularly cases induced by immune checkpoint inhibitors, which often mimic inflammatory bowel disease and are associated with microbiota dysregulation. In preclinical models and select clinical settings, FMT has also been associated with improved CAR T-cell therapy outcomes. The “donor effect”—the observation that specific donor microbiota engrafts more successfully and yield better clinical responses—highlights the necessity of rigorous donor screening and characterization [34].
Looking ahead, while current data provide compelling associations between microbiota composition and NHL pathophysiology, further large-scale clinical trials are essential to establish causality and define standardized therapeutic protocols. Research should expand beyond bacterial communities to include the mycobiome and virome, which may also play significant roles in immune modulation and tumor biology. These efforts will be instrumental in translating microbiome science into safe, personalized, and mechanistically informed therapies for NHL patients.
Dietary and probiotic interventions aimed at ameliorating chemotherapy-induced dysbiosis in lymphoma patients face multiple limitations. First, chemotherapy-associated mucositis, nausea, anorexia, and gustatory alterations frequently hinder patients’ adherence to prescribed dietary regimens or supplement intake. Second, the profound immunosuppression and compromised mucosal barrier integrity in these patients elevate the risk of bacterial translocation, rendering the safety of probiotic administration—especially strains like Lactobacillus and Bifidobacterium—a concern in this vulnerable population. Although meta-analyses in cancer patients more broadly have demonstrated that probiotics can reduce the incidence and severity of oral mucositis and diarrhea, these studies often involve heterogeneous cancer types and do not focus specifically on lymphoma patients, limiting generalizability [34]. Moreover, current clinical guidelines (e.g., MASCC/ISOO) for gastrointestinal mucositis prevention emphasize that evidence for dietary and probiotic interventions remains inadequate or conflicting, and no new recommendations have been established due to methodological limitations and low-quality data. Finally, interindividual variability in baseline microbiota composition, tumor biology, and chemotherapy regimens further complicates the prediction of therapeutic response, underscoring the need for personalized intervention strategies that are yet to be validated in rigorous lymphoma-specific trials.
HSCT represents a curative strategy for several hematological malignancies, including selected cases of B-cell non-Hodgkin lymphomas, but its success is frequently limited by infectious complications and graft-versus-host disease (GvHD). Increasing evidence indicates that the intestinal microbiome plays a pivotal role in modulating these outcomes. Conditioning regimens, prophylactic or therapeutic antibiotics, and nutritional alterations profoundly disrupt microbial diversity, leading to a state of dysbiosis that predisposes to systemic inflammation and immune dysregulation [49]. Clinical studies have shown that a marked reduction in microbial richness at the time of engraftment correlates with increased transplant-related mortality and higher incidence of both acute and chronic GvHD. In particular, the loss of commensal taxa such as Blautia, Faecalibacterium, and other Clostridiales members is consistently associated with a more aggressive GvHD course, whereas intestinal domination by pathogenic organisms, notably Enterococcus and Enterobacteriaceae, predicts adverse survival outcomes
The microbiota protects the epithelial barrier by producing SCFAs, particularly butyrate and propionate, which drive colonocyte metabolism and promote regulatory T cell development. Reduced SCFA availability after allo-HSCT contributes to epithelial damage, impaired mucosal healing, and a pro-inflammatory milieu favoring GvHD onset. Experimental data further highlight the role of microbial-derived metabolites in modulating donor T-cell alloreactivity, with signaling pathways involving G-protein-coupled receptors and epigenetic regulation of immune effector functions [59].
From a therapeutic perspective, several microbiome-directed interventions are under investigation. Antibiotic stewardship, aimed at limiting unnecessary exposure to broad-spectrum anaerobic agents, is associated with preserved microbial diversity and reduced GvHD incidence. Moreover, fecal microbiota transplantation (FMT) has emerged as a promising strategy, with preliminary reports documenting restoration of microbial complexity and clinical improvement in patients with steroid-refractory intestinal GvHD [60] (Figure 2).
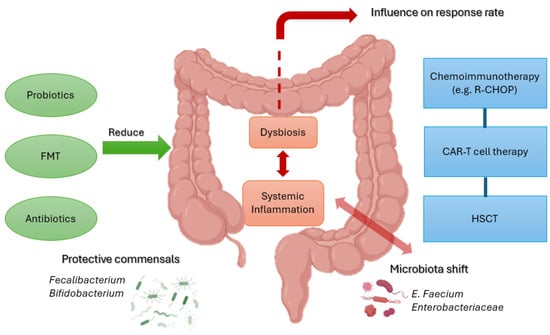
Figure 2.
Schematic representation of the interplay between gut microbiota and treatment responses in B-cell NHL. Protective taxa (Faecalibacterium, Bifidobacterium) promote barrier integrity and immune regulation, while dysbiosis with Enterococcus and Enterobacteriaceae drives inflammation and toxicity. Interventions such as probiotics, FMT, and antibiotic stewardship may restore balance and improve outcomes with chemoimmunotherapy, CAR-T therapy, and HSCT. Created with BioRender. Santino Caserta. (2025) https://app.biorender.com/illustrations/68b5c44de87b2863610e44c6.
Overall, the integration of microbiota monitoring and supportive interventions into transplant practice holds the potential to improve outcomes in HSCT recipients. For patients with B-cell lymphomas undergoing allogeneic transplantation, tailoring peri-transplant strategies to preserve or restore microbial equilibrium could mitigate the risk of GvHD while maintaining graft-versus-lymphoma effects. These insights reinforce the importance of considering the gut microbiota as a critical, modifiable factor influencing both treatment-related toxicity and long-term survival in the transplant setting.
6. Clinical Trials
Clinical trials exploring the relationship between the gut microbiome and hematologic malignancies have gained momentum in recent years, highlighting the potential influence of microbial composition on treatment response, immune recovery, and therapy-related toxicity. Among these studies, the clinical trial NCT06161896 represents a prospective, observational, single-center cohort, recruiting, study in Europe, enrolling approximately 200 patients newly diagnosed with DLBCL. Its primary purpose is to characterize the baseline gut microbiota and to investigate possible correlations with clinical outcomes, treatment response, and prognosis. Participants provide stool and blood samples, undergo bioelectrical impedance analysis for the assessment of body composition, and complete detailed dietary and lifestyle questionnaires. The study focuses on microbiome diversity and taxonomic composition, correlating these data with treatment efficacy, toxicity, and survival, while also evaluating the impact of potential confounders such as antibiotic exposure, comorbidities, and diet. Recruitment is ongoing, with study completion expected in July 2026 (NCT06161896).
In contrast, the PRIMAL trial (NCT05135351) is a pilot, randomized, double-blind, placebo-controlled, recruiting, interventional study conducted on a small cohort of 30 adult patients with multiple myeloma or lymphoma undergoing ASCT. Its main objective is to assess the feasibility and impact of prebiotic supplementation with resistant starch compared to a maltodextrin placebo, starting ten days before transplantation and continuing until neutrophil engraftment. Unlike NCT06161896, which is purely observational, PRIMAL actively tests a microbiome-modulating intervention and evaluates its effect on gut microbiome diversity, the abundance of beneficial taxa, and serum markers of gut permeability. Secondary outcomes include hospitalization duration, incidence of neutropenic fever, antibiotic exposure, and gastrointestinal symptoms, with exploratory analyses focused on the correlation between dietary patterns and microbial shifts, particularly in taxa such as Faecalibacterium prausnitzii, Ruminococcus, and Akkermansia muciniphila. The trial is actively recruiting at the University of Nebraska Medical Center and includes a dedicated arm for CAR T-cell therapy recipients.
Together, these two studies exemplify complementary strategies for investigating the role of the gut microbiota in lymphoid malignancies. NCT06161896 focuses on describing microbiome–disease associations in a large, newly diagnosed DLBCL cohort without intervention, whereas PRIMAL tests a specific prebiotic approach in a smaller, post-transplant population, emphasizing feasibility and the generation of early efficacy signals. The differences in study design, objectives, and patient populations reflect the diverse approaches currently being used to elucidate the potential therapeutic relevance of the gut microbiome in lymphoma therapy (Table 3).

Table 3.
Key aspects of NCT06161896 and NCT05135351 clinical trials about the evaluation of gut microbiota influence on the response to treatments in non-Hodgkin B-cell lymphomas (https://clinicaltrials.gov/, accessed on 27 July 2025).
7. Conclusions
The growing body of evidence underscores the gut microbiota as a key player in the pathogenesis, progression, and treatment responsiveness of NHL. Dysbiosis—characterized by reduced microbial diversity and shifts toward pathogenic taxa—has been consistently observed in newly diagnosed NHL patients, particularly those with DLBCL and gastrointestinal follicular lymphoma [1,2,3]. This altered microbial landscape contributes not only to gastrointestinal toxicity and systemic inflammation but also to diminished therapeutic efficacy, notably in the context of chemoimmunotherapy and CAR T-cell therapy [8,12,13].
Chemoimmunotherapeutic agents exacerbate dysbiosis by disrupting epithelial integrity, depleting beneficial commensals, and promoting expansion of opportunistic pathogens such as Enterobacteriaceae and Clostridioides difficile. These changes have been correlated with increased inflammatory cytokines (e.g., IL-6, IFN-γ), febrile neutropenia, and impaired treatment response. Importantly, rituximab-mediated B-cell depletion further impairs mucosal immunity through a reduction in secretory IgA. Similarly, in CAR T-cell therapy, prior exposure to broad-spectrum antibiotics is associated with reduced CAR T-cell expansion, increased toxicity, and inferior overall survival, highlighting the detrimental impact of microbiota disruption on cellular immunotherapy [20,21].
In contrast, a balanced gut microbiome enriched with taxa such as Faecalibacterium prausnitzii, Lactobacillus johnsonii, Paraprevotella, and Akkermansia muciniphila appears to enhance antitumor immunity, support mucosal recovery, and improve therapeutic outcomes. Preclinical and early-phase clinical data suggest that selective antibiotic use (e.g., oral vancomycin) and microbiota-modulating strategies can potentiate CAR T-cell responses by enhancing antigen presentation and endogenous CD8+ T-cell activation. Moreover, the gut microbiota is emerging as a potential prognostic biomarker of treatment response in NHL, with specific microbial signatures correlating with therapeutic success, risk of relapse, and incidence of severe toxicities [52,53].
These insights have spurred the development of microbiota-targeted interventions as adjuncts in NHL management. Strategies under investigation include antibiotic stewardship protocols to minimize unnecessary broad-spectrum exposure, microbial profiling to personalize antimicrobial use, dietary interventions promoting fiber-rich, microbiota-supportive diets, and supplementation with prebiotics and probiotics. FMT is also emerging as a promising therapeutic modality, particularly for managing immune-mediated toxicities and potentially enhancing cellular immunotherapy outcomes: studies emphasize the “donor effect,” underscoring the importance of careful donor selection and microbial characterization to achieve consistent clinical benefit [51].
Clinical trials (NCT0616189 and NCT05135351) are currently exploring correlations between baseline microbiome composition and treatment outcomes, as well as the feasibility of prebiotic supplementation during hematopoietic stem cell transplantation.
In this evolving landscape, artificial intelligence (AI) and machine learning are expected to play a transformative role by enabling integration of high-dimensional microbiome datasets with clinical and molecular variables, improving the ability to predict treatment response, stratify patients by risk, and identify actionable microbial targets. AI-driven models could facilitate the development of microbiota-based prognostic tools and guide real-time decision-making in precision oncology [61].
Moving forward, large-scale prospective trials and mechanistic studies are essential to validate these preliminary findings and establish causality. Furthermore, expanding research beyond bacterial taxa to include the virome and mycobiome may uncover additional dimensions of host–microbiota–tumor interaction [62,63]. Ultimately, integrating microbiome-derived prognostic markers with AI-powered predictive models may optimize therapeutic efficacy, reduce toxicity, and transform the management of non-Hodgkin B-cell lymphomas into a holistic, data-driven, precision-based approach.
Author Contributions
Conceptualization, M.E.A. and S.C.; Data Curation S.C. and M.E.A.; Formal Analysis A.A. and F.S.; Investigation M.E.A., S.C. and G.P.; Methodology S.C., M.E.A. and M.F.; Supervision A.A. and F.S.; Validation A.A. and F.S.; Writing—original draft preparation M.E.A. and S.C.; Writing—review and editing S.C. and M.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Valvano, L.; Vilella, R.; D’Auria, F.; D’Arena, G.; Libonati, R.; Soda, M.; Telesca, A.; Pietrantuono, G.; Mansueto, G.R.; Villani, O.; et al. Prognostic relevance of bone marrow immune cell fractions in newly diagnosed B-cell non-Hodgkin lymphoma patients. Ann. Med. 2025, 57, 2490825. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caserta, S.; Cancemi, G.; Loreta, S.; Allegra, A.; Stagno, F. Hematological Malignancies in Older Patients: Focus on the Potential Role of a Geriatric Assessment Management. Diagnostics 2024, 14, 1390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, F.; Lu, L.; Zang, H.; Yue, Y.; Cao, Y.; Chen, M.; Weiying, G.; He, B. Malnutrition defined by Controlling Nutritional Status score was independently associated with prognosis of diffuse large B-cell lymphoma primarily on elderly patients. Hematology 2024, 30, 2434276. [Google Scholar] [CrossRef]
- Cancemi, G.; Campo, C.; Caserta, S.; Rizzotti, I.; Mannina, D. Single-Agent and Associated Therapies with Monoclonal Antibodies: What About Follicular Lymphoma? Cancers 2025, 17, 1602. [Google Scholar] [CrossRef]
- Patil, S.; Rajput, S.; Patil, S.; Mhaiskar, A. B-cell lymphoma: Advances in pathogenesis, diagnosis, and targeted therapies. Pathol. Res. Pract. 2025, 271, 156036. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, P.; Zhang, W.; Liu, H.; Sun, X.; Xiao, X.; Wang, J.; Li, Z.; Li, L.; Wang, S.; et al. Clinical outcomes and therapeutic modalities in older Chinese patients with MCL: A multi-center real-world retrospective study. Ann. Med. 2025, 57, 2482013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miao, M.; Chen, Y.; Wang, X.; Li, S.; Hu, R. The critical role of ferroptosis in virus-associated hematologic malignancies and its potential value in antiviral-antitumor therapy. Virulence 2025, 16, 2497908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, D. The role of non-malignant B cells in malignant hematologic diseases. Hematology 2025, 30, 2466261. [Google Scholar] [CrossRef]
- Hough, B.; Albitar, M. mRNA Expression in DLBCL Patients who are CD20 Dim With an Eye Towards More Thoughtful Design of R/R Trials, Possibly Incorporating BCMA Bite Therapy. Clin. Lymphoma Myeloma Leuk. 2025, 25, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Caserta, S.; Campo, C.; Cancemi, G.; Neri, S.; Stagno, F.; Mannina, D.; Allegra, A. Bispecific Antibodies and Antibody–Drug Conjugates in Relapsed/Refractory Aggressive non-Hodgkin Lymphoma, Focusing on Diffuse Large B-Cell Lymphoma. Cancers 2025, 17, 2479. [Google Scholar] [CrossRef]
- Guan, J.; Sun, F.; Wang, J.; Huang, J.; Lu, S.; Zhu, J.; Zhu, X.; Huang, H.; Xia, Z.; Que, Y.; et al. Efficacy and safety comparison between R-CHOP and modified NHL-BFM-90 regimens in children and adolescents with diffuse large B-cell lymphoma. Ann. Hematol. 2022, 101, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Pavlovsky, M.; Cubero, D.; Agreda-Vásquez, G.P.; Enrico, A.; Mela-Osorio, M.J.; Sebastián, J.A.S.; Fogliatto, L.; Ovilla, R.; Avendano, O.; Machnicki, G.; et al. Clinical Outcomes of Patients With B-Cell non-Hodgkin Lymphoma in Real-World Settings: Findings From the Hemato-Oncology Latin America Observational Registry Study. JCO Glob. Oncol. 2022, 8, e2100265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Xu, J.; Li, J.; Wei, Z.; Shi, M.; Tao, R.; Chen, B.; Tian, Y.; Zhang, W.; Ma, Y.; et al. Rituximab plus cladribine versus R-CHOP in frontline management of marginal zone lymphoma in China: A propensity-score matched multicenter study. Ann. Hematol. 2022, 101, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, S.J.; Bouzani, M.; Symeonidis, A.; Angelopoulou, M.K.; Papageorgiou, S.G.; Michail, M.; Gainaru, G.; Kourti, G.; Sachanas, S.; Kalpadakis, C.; et al. Real-life Experience With Rituximab-CHOP Every 21 or 14 Days in Primary Mediastinal Large B-cell Lymphoma. In Vivo 2022, 36, 1302–1315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alnassfan, T.; Cox-Pridmore, M.J.; Taktak, A.; Till, K.J. Mantle cell lymphoma treatment options for elderly/unfit patients: A systematic review. EJHaem 2021, 3, 276–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yannakou, C.K.; Wu, S.; Rajah, K.; Abeyakoon, C.; Nguyen-Ngo, C.; Yap, Y.Z.; Sheldon, J.; Blombery, P.; Prince, H.M. Circulating Tumour DNA Is a Biomarker of Response in Angioimmunoblastic T-Cell Lymphoma. Int. J. Mol. Sci. 2025, 26, 6719. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Caserta, S.; Mirabile, G.; Gangemi, S. Aging and Age-Related Epigenetic Drift in the Pathogenesis of Leukemia and Lymphomas: New Therapeutic Targets. Cells 2023, 12, 2392. [Google Scholar] [CrossRef]
- Belsky, J.A.; Smith, C.M.; Alexander, S. Current Treatment Strategies and Supportive Care Practices in the Care of Children and Adolescents With Mature B-Cell non-Hodgkin Lymphoma: A Survey of Children’s Oncology Group Institutions. Pediatr. Blood Cancer 2025, 72, e31687. [Google Scholar] [CrossRef]
- Algiraigri, A.H. Optimizing Outcomes in Childhood Mature B-cell non-Hodgkin Lymphoma: Insights Into Staging, Risk Stratification, and Response Evaluation. J. Pediatr. Hematol. 2025, 47, e138–e143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dahal, P.K.; Mosharaf, P.; Shahjalal, M.; Mahumud, R.A. Assessing the Clinical Effectiveness of Radioimmunotherapy with Combined Radionuclide/Monoclonal Antibody Conjugates in Cancer Treatment: Insights from Randomised Clinical Trials. Cancers 2025, 17, 1413. [Google Scholar] [CrossRef]
- Rao, U.K.; Majhail, N.S.; Blunk, B.; Abernathy, K.; Bachier, C.; Bhushan, V.; Cruz, J.C.; Elayan, M.; Gregory, T.; LeMaistre, C.F.; et al. Comparative Efficacy of Bendamustine Versus Fludarabine/Cyclophosphamide for Lymphodepletion Before Chimeric Antigen Receptor T-Cell Therapy in Lymphoma. Transplant. Cell Ther. 2025, 31, 549.e1–549.e11. [Google Scholar] [CrossRef] [PubMed]
- Jameel, M.; Wali, R.; Zaidi, S.M.J.; Shaheen, N.; Sindhu, I.I. Impact of Filgrastim on Mortality During Induction Chemotherapy in Childhood B-Cell non-Hodgkin Lymphoma. Cureus 2025, 17, e77320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Łyko, M.; Maj, J.; Jankowska-Konsur, A. The Role of the Gut Microbiome in non-Hodgkin Lymphoma (NHL): A Focus on Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, Cutaneous T-Cell Lymphoma, and NK/T-Cell Lymphoma. Cancers 2025, 17, 1709. [Google Scholar] [CrossRef]
- Fish, K.; Gao, D.; Raji, M.; Balducci, L.; Kuo, Y.-F. Trends in the use of granulocyte colony stimulating factors for older patients with cancer, 2010 to 2019. J. Geriatr. Oncol. 2024, 15, 102049. [Google Scholar] [CrossRef] [PubMed]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Zhang, B.; Jiang, L.; Cheng, T.; Cheng, H.; Qian, P. Gut microbiota plays pivotal roles in benign and malignant hematopoiesis. Blood Sci. 2024, 6, e00200. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Yang, Q.; He, Y.; Zhou, W. Intestinal Microbes and Hematological Malignancies. Cancers 2023, 15, 2284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, M.L.; Maier, I.; Dang, A.T.; Berry, D.; Liu, J.; Ruegger, P.M.; Yang, J.-I.; Soto, P.A.; Presley, L.L.; Reliene, R.; et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res. 2013, 73, 4222–4232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breczko, W.J.; Bubak, J.; Miszczak, M. The Importance of Intestinal Microbiota and Dysbiosis in the Context of the Development of Intestinal Lymphoma in Dogs and Cats. Cancers 2024, 16, 2255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Gómez, L.; Alcorta, A.; Abalo, R. Probiotics and Probiotic-like Agents against Chemotherapy-Induced Intestinal Mucositis: A Narrative Review. J. Pers. Med. 2023, 13, 1487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Charbonnier, L.-M.; Noval Rivas, M.; Georgiev, P.; Li, N.; Gerber, G.; Bry, L.; Chatila, T.A. MyD88 Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces Commensalism. Immunity 2015, 43, 289–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahl, W.J.; Mendoza, D.R.; Lambert, J.M. Diet, nutrients and the microbiome. Prog. Mol. Biol. Transl. Sci. 2020, 171, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mikkelsen, H.; Jungersen, G. Intracellular Pathogens: Host Immunity and Microbial Persistence Strategies. J. Immunol. Res. 2019, 2019, 1356540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toffoli, E.C.; van Vliet, A.A.; Forbes, C.; Arns, A.J.; Verheul, H.W.M.; Tuynman, J.; van der Vliet, H.J.; Spanholtz, J.; de Gruijl, T.D. Allogeneic NK cells induce the in vitro activation of monocyte-derived and conventional type-2 dendritic cells and trigger an inflammatory response under cancer-associated conditions. Clin. Exp. Immunol. 2024, 216, 159–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Santomasso, B.; Bachier, C.; Westin, J.; Rezvani, K.; Shpall, E.J. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2019; pp. 433–444. [Google Scholar]
- Birebent, R.; Drubay, D.; Alves Costa Silva, C.; Marmorino, F.; Vitali, G.; Piccinno, G.; Hurtado, Y.; Bonato, A.; Belluomini, L.; Messaoudene, M.; et al. Surrogate markers of intestinal dysfunction associated with survival in advanced cancers. OncoImmunology 2025, 14, 2484880. [Google Scholar] [CrossRef]
- Tian, M.; Fan, D.; Liu, Z.; Mu, X.; Tao, Q.; Yu, C.; Zhang, S. Oral Supramolecular Adsorbent for Preventing Chemo-Induced Gastrointestinal Mucositis and Microbial Dysbiosis and for Enhancing Chemoimmunotherapy. Adv. Mater. 2022, 34, e2205299. [Google Scholar] [CrossRef] [PubMed]
- Schmiester, M.; Maier, R.; Riedel, R.; Durek, P.; Frentsch, M.; Kolling, S.; Mashreghi, M.-F.; Jenq, R.; Zhang, L.; Peterson, C.B.; et al. Flow cytometry can reliably capture gut microbial composition in healthy adults as well as dysbiosis dynamics in patients with aggressive B-cell non-Hodgkin lymphoma. Gut Microbes 2022, 14, 2081475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2020, 14, 547–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diefenbach, C.S.; Peters, B.A.; Li, H.; Raphael, B.; Moskovits, T.; Hymes, K.; Schluter, J.; Chen, J.; Bennani, N.N.; Witzig, T.E.; et al. Microbial dysbiosis is associated with aggressive histology and adverse clinical outcome in B-cell non-Hodgkin lymphoma. Blood Adv. 2021, 5, 1194–1198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, J.; Liu, G.; Wang, W.; Xue, H. Causal relationships between gut microbiota and lymphoma: A bidirectional Mendelian randomization study. Front. Cell. Infect. Microbiol. 2024, 14, 1374775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bigenwald, C.; Zitvogel, L. Light shed from the gut in large B-cell lymphoma. Blood 2023, 141, 2165–2166. [Google Scholar] [CrossRef] [PubMed]
- Peled, J.U.; Gomes, A.L.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aarnoutse, R.; Ziemons, J.; Penders, J.; Rensen, S.S.; de Vos-Geelen, J.; Smidt, M.L. The Clinical Link between Human Intestinal Microbiota and Systemic Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 4145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef]
- Madhavan, S.; Nagarajan, S. GRP78 and next generation cancer hallmarks: An underexplored molecular target in cancer chemoprevention research. Biochimie 2020, 175, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, I.G.; Todor, S.B.; Ichim, C.; Helgiu, C.; Helgiu, A. A Literature Review on the Impact of the Gut Microbiome on Cancer Treatment Efficacy, Disease Evolution and Toxicity: The Implications for Hematological Malignancies. J. Clin. Med. 2025, 14, 2982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.H.; Whiteson, K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. mSystems 2021, 6, e00115–e00121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayase, E.; Hayase, T.; Jamal, M.A.; Miyama, T.; Chang, C.-C.; Ortega, M.R.; Ahmed, S.S.; Karmouch, J.L.; Sanchez, C.A.; Brown, A.N.; et al. Mucus-degrading Bacteroides link carbapenems to aggravated graft-versus-host disease. Cell 2022, 185, 3705–3719.e14. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, C.R.; Huang, T.W. Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 13268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO); Bowen, J.M.; Gibson, R.J.; Coller, J.K.; Blijlevens, N.; Bossi, P.; Al-Dasooqi, N.; Bateman, E.H.; Chiang, K.; de Mooij, C.; et al. Systematic review of agents for the management of cancer treatment-related gastrointestinal mucositis and clinical practice guidelines. Support. Care Cancer 2019, 27, 4011–4022. [Google Scholar] [CrossRef]
- Mizuno, S.; Masaoka, T.; Naganuma, M.; Kishimoto, T.; Kitazawa, M.; Kurokawa, S.; Nakashima, M.; Takeshita, K.; Suda, W.; Mimura, M.; et al. Bifidobacterium-Rich Fecal Donor May Be a Positive Predictor for Successful Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Digestion 2017, 96, 29–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Todor, S.B.; Ichim, C. Microbiome Modulation in Pediatric Leukemia: Impact on Graft-Versus-Host Disease and Treatment Outcomes: A Narrative Review. Children 2025, 12, 166. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, Z.; Hu, G.; Li, M.W.; Zhang, L.; Li, X.; Li, L.; Wang, X.; Fu, X.; Sun, Z.; Zhang, X.; et al. Gut microbiota as non-invasive diagnostic and prognostic biomarkers for natural killer/T-cell lymphoma. Gut 2023, 72, 1999–2002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, A.; Jiang, A.; Huang, L.; Li, Y.; Zhang, C.; Zhu, L.; Mou, W.; Liu, Z.; Zhang, J.; Cheng, Q.; et al. From chaos to order: Optimizing fecal microbiota transplantation for enhanced immune checkpoint inhibitors efficacy. Gut Microbes 2025, 17, 2452277. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Musolino, C.; Tonacci, A.; Pioggia, G.; Gangemi, S. Interactions between the MicroRNAs and Microbiota in Cancer Development: Roles and Therapeutic Opportunities. Cancers 2020, 12, 805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).