A Prospective Observational Study of a 2-Week Integrative Inpatient Therapy on Patients with Fibromyalgia Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Material
2.3.1. Symptom-Specific Questionnaires
2.3.2. Psychometric Outcomes and Quality of Life
2.3.3. Further Outcomes
2.4. Procedure
2.5. Statistical Methods
3. Results
3.1. FMS Sample
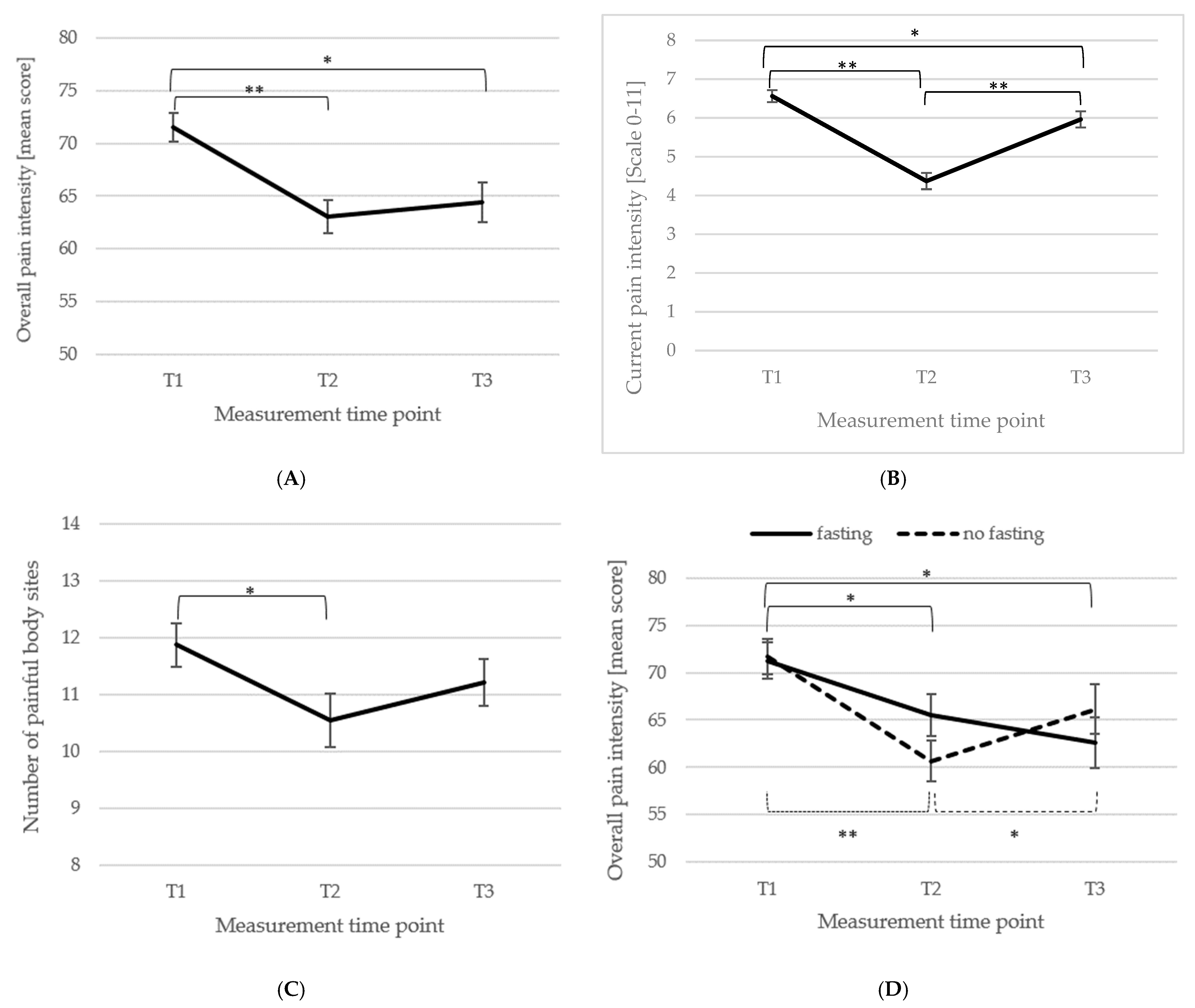
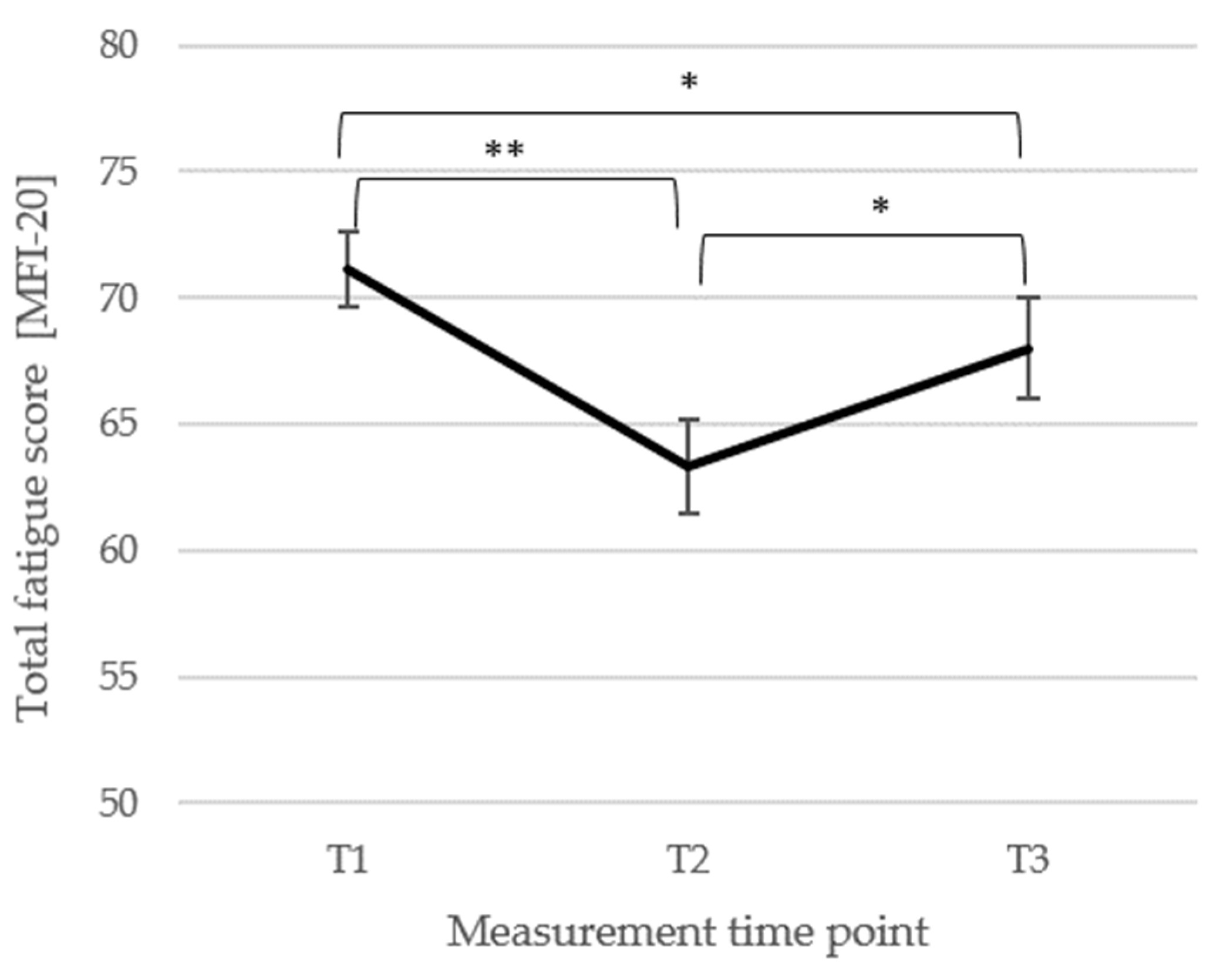
3.2. Short- and Long-Term Changes in Main FMS Symptoms of Pain and Fatigue
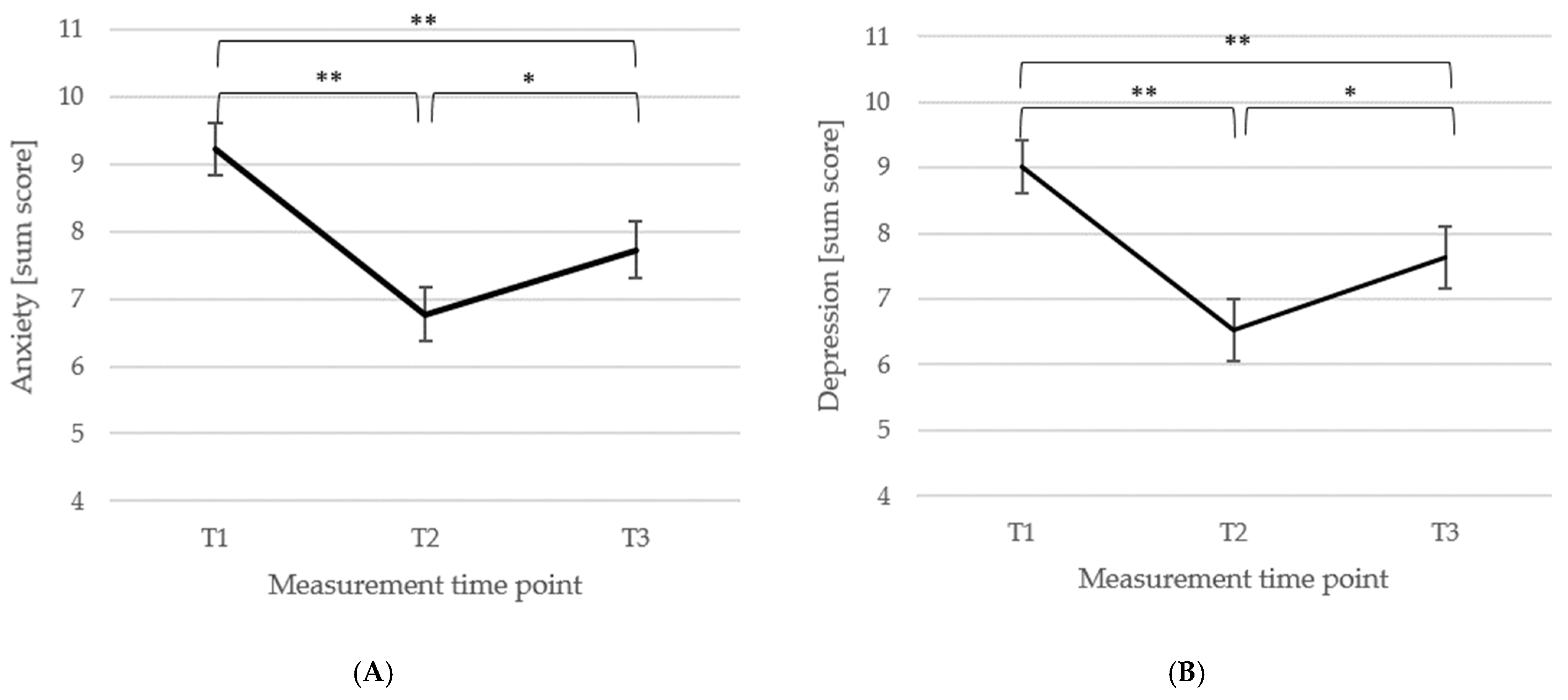
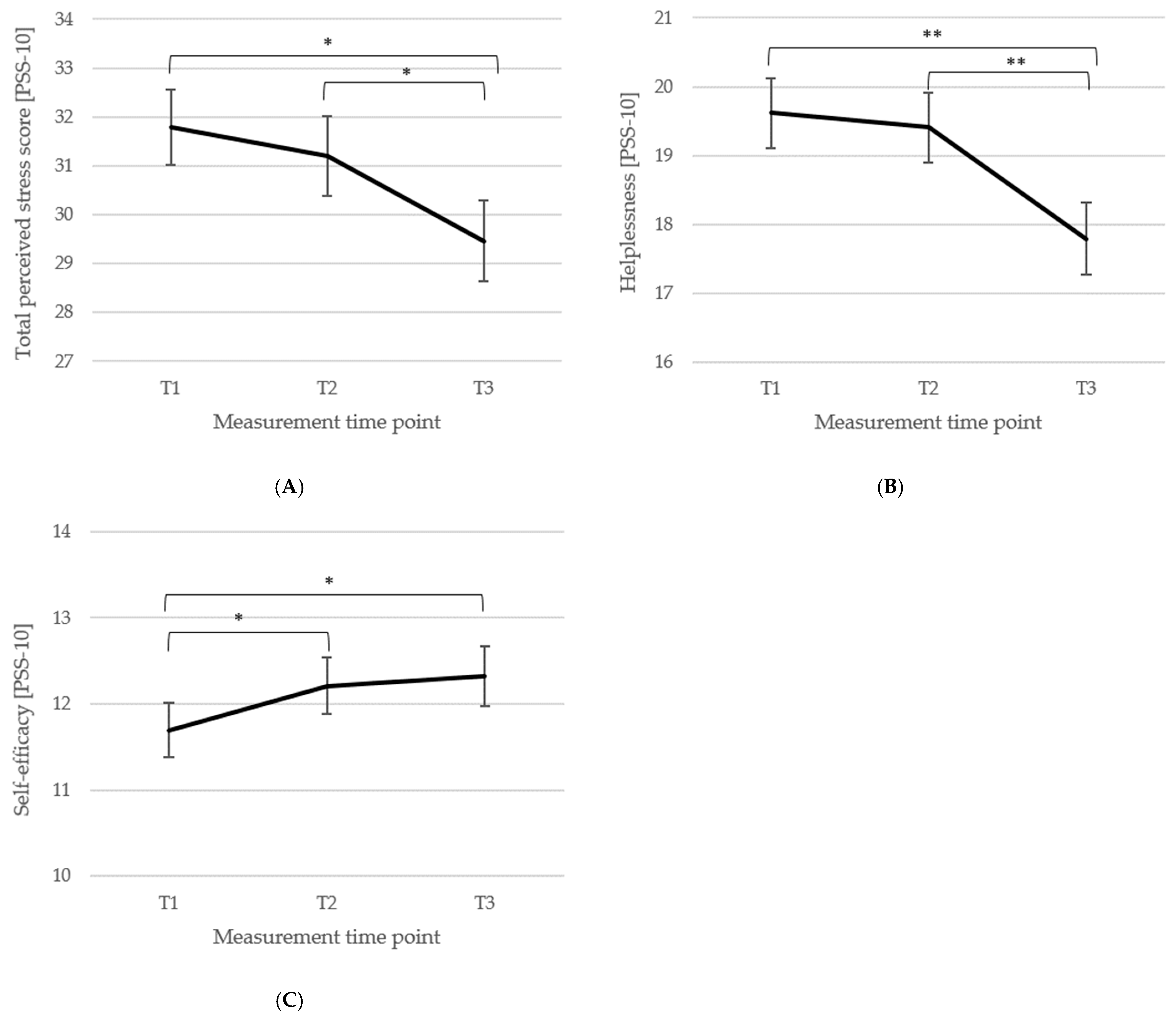
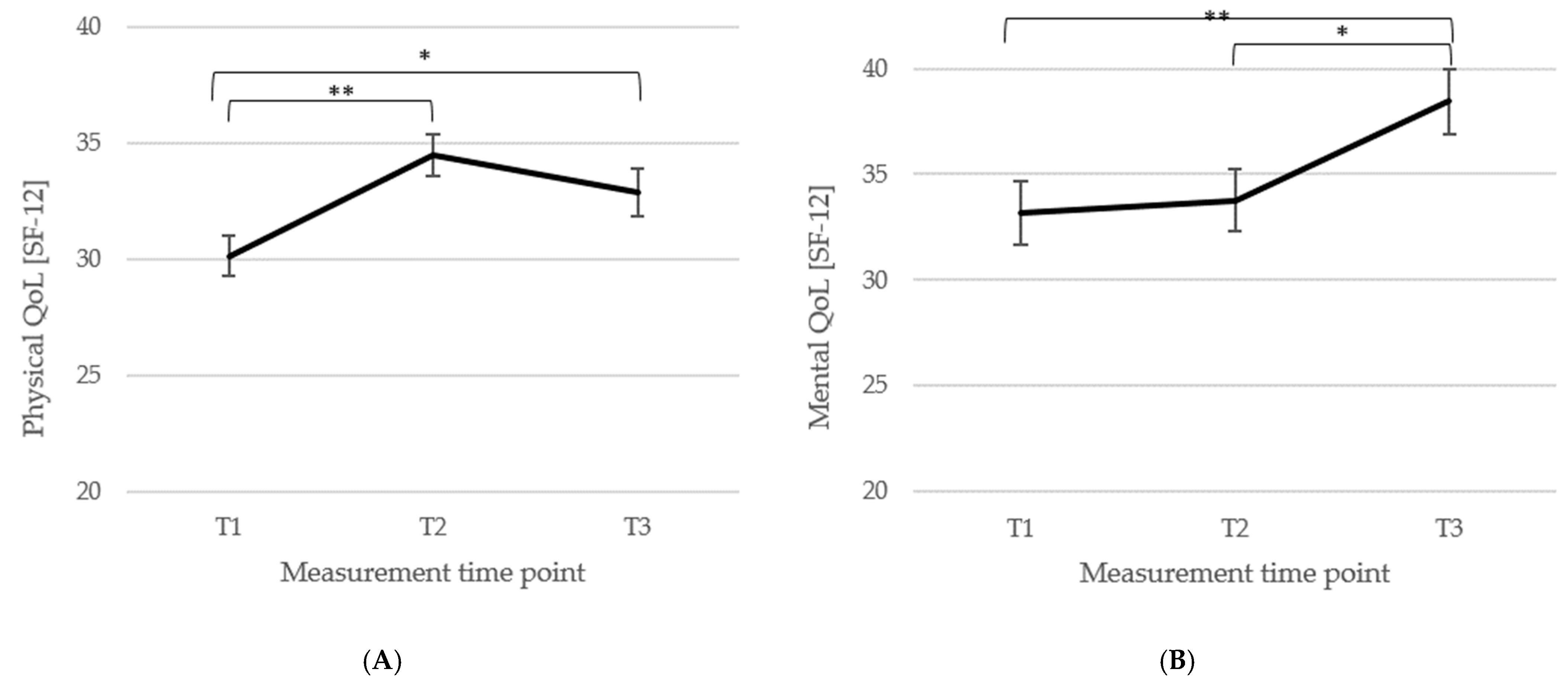
3.3. Short- and Long-Term Outcomes Regarding Psychometric Outcomes and Quality of Life
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Queiroz, L. Worldwide Epidemiology of Fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Cabo-Meseguer, A.; Cerdá-Olmedo, G.; Trillo-Mata, J.L. Fibromialgia: Prevalencia, perfiles epidemiológicos y costes económicos. Med. Clín. 2017, 149, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia Prevalence, Somatic Symptom Reporting, and the Dimensionality of Polysymptomatic Distress: Results from a Survey of the General Population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef]
- Meißner, T. Typischer Symptom-Cluster lenkt den Verdacht auf ein Fibromyalgie-Syndrom. Schmerzmedizin 2018, 34, 41. [Google Scholar] [CrossRef]
- Fitzcharles, M.-A.; Rampakakis, E.; Ste-Marie, P.A.; Sampalis, J.S.; Shir, Y. The Association of Socioeconomic Status and Symptom Severity in Persons with Fibromyalgia. J. Rheumatol. 2014, 41, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Reisine, S.; Fifield, J.; Walsh, S.; Forrest, D.D. Employment and health status changes among women with fibromyalgia: A five-year study. Arthritis Rheum. 2008, 59, 1735–1741. [Google Scholar] [CrossRef]
- Creed, F. A review of the incidence and risk factors for fibromyalgia and chronic widespread pain in population-based studies. Pain 2020, 161, 1169–1176. [Google Scholar] [CrossRef]
- van Koulil, S.; van Lankveld, W.; Kraaimaat, F.W.; van Riel, P.L.C.M.; Evers, A.W.M. Risk factors for longer term psychological distress in well-functioning fibromyalgia patients: A prospective study into prognostic factors. Patient Educ. Couns. 2010, 80, 126–129. [Google Scholar] [CrossRef]
- Skarpsno, E.S.; Nilsen, T.I.L.; Sand, T.; Hagen, K.; Mork, P.J. The joint effect of insomnia symptoms and lifestyle factors on risk of self-reported fibromyalgia in women: Longitudinal data from the HUNT Study. BMJ Open 2019, 9, e028684. [Google Scholar] [CrossRef]
- Langhorst, A.; Langhorst, J. Integrative Medizin, Naturheilkunde und Komplementärmedizin in der Therapie des Fibromyalgiesyndroms. Schmerz 2023, 37, 319–323. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Atzeni, F.; Gorla, R.; Kosek, E.; Choy, E.H.; Bazzichi, L.; Häuser, W.; Ablin, J.N.; Aloush, V.; et al. Fibromyalgia position paper. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 130), 186–193. [Google Scholar] [CrossRef]
- D’Agnelli, S.; Arendt-Nielsen, L.; Gerra, M.C.; Zatorri, K.; Boggiani, L.; Baciarello, M.; Bignami, E. Fibromyalgia: Genetics and epigenetics insights may provide the basis for the development of diagnostic biomarkers. Mol. Pain 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yepez, D.; Grandes, X.A.; Talanki Manjunatha, R.; Habib, S.; Sangaraju, S.L. Fibromyalgia and Depression: A Literature Review of Their Shared Aspects. Cureus 2022, 14, e24909. [Google Scholar] [CrossRef]
- Chang, M.-H.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Bai, Y.-M.; Li, C.-T.; Yang, A.C.; Chang, W.-H.; Chen, T.-J.; Tsai, S.-J.; et al. Bidirectional Association Between Depression and Fibromyalgia Syndrome: A Nationwide Longitudinal Study. J. Pain 2015, 16, 895–902. [Google Scholar] [CrossRef]
- Gul, Y.; Dawar, S.; Riaz, Q.; Ullah, K.; Kamal, A. Association of Depression in Patients with Fibromyalgia Syndrome. Pak. J. Med. Health Sci. 2023, 17, 762–765. [Google Scholar]
- Epstein, S.A.; Kay, G.; Clauw, D.; Heaton, R.; Klein, D.; Krupp, L.; Kuck, J.; Leslie, V.; Masur, D.; Wagner, M.; et al. Psychiatric Disorders in Patients with Fibromyalgia: A Multicenter Investigation. Psychosomatics 1999, 40, 57–63. [Google Scholar] [CrossRef]
- Henao-Pérez, M.; López-Medina, D.C.; Arboleda, A.; Bedoya Monsalve, S.; Zea, J.A. Patients with Fibromyalgia, Depression, and/or Anxiety and Sex Differences. Am. J. Men’s Health 2022, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kleykamp, B.A.; Ferguson, M.C.; McNicol, E.; Bixho, I.; Arnold, L.M.; Edwards, R.R.; Fillingim, R.; Grol-Prokopczyk, H.; Turk, D.C.; Dworkin, R.H. The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: An ACTTION systematic review. Semin. Arthritis Rheum. 2021, 51, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Fibromyalgie Vereinigung; Deutsche Rheuma-Liga; Deutsche Schmerzgesellschaft. Patientenversion der Wissenschaftlichen Leitlinie “Definition, Ursachen, Diagnostik und Therapie des Fibromyalgiesyndroms”. AWMF-Register Nr. 145/004. 2017. Available online: https://register.awmf.org/assets/guidelines/145-004p_S3_Fibromyalgiesyndrom_2017-03-abgelaufen_01.pdf (accessed on 15 February 2025).
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia Criteria and Severity Scales for Clinical and Epidemiological Studies: A Modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Wolfe, F.; Walitt, B.T.; Rasker, J.J.; Katz, R.S.; Hauser, W. The Use of Polysymptomatic Distress Categories in the Evaluation of Fibromyalgia (FM) and FM Severity. J. Rheumatol. 2015, 42, 1494–1501. [Google Scholar] [CrossRef]
- Gomides, A.P.M.; Bezerra, J.C.; do Rosário E Souza, E.J.; da Mota, L.M.H.; Santos-Neto, L.L. Work disability in fibromyalgia and other soft tissue disorders: Analysis of preventive benefits in Brazil from 2006 to 2015. Adv. Rheumatol. 2018, 58, 13. [Google Scholar] [CrossRef]
- Henriksson, C.M.; Liedberg, G.M.; Gerdle, B. Women with fibromyalgia: Work and rehabilitation. Disabil. Rehabil. 2005, 27, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Palstam, A.; Mannerkorpi, K. Work Ability in Fibromyalgia: An Update in the 21st Century. Curr. Rheumatol. Rev. 2017, 13, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Walitt, B.T.; Katz, R.S.; Häuser, W. Social security work disability and its predictors in patients with fibromyalgia. Arthritis Care Res. 2014, 66, 1354–1363. [Google Scholar] [CrossRef]
- Arnold, L.M.; Gebke, K.B.; Choy, E.H.S. Fibromyalgia: Management strategies for primary care providers. Int. J. Clin. Pract. 2016, 70, 99–112. [Google Scholar] [CrossRef]
- Deutsche Schmerzgesellschaft, e.V. (Hrsg.). S3-Leitlinie Definition, Pathophysiologie, Diagnostik und Therapie des Fibromyalgiesyndroms. AWMF-Leitlinien-Register, Nr. 145/004. 2017. Available online: https://register.awmf.org/assets/guidelines/145-004l_S3_Fibromyalgiesyndrom_2019-11_1-abgelaufen.pdf (accessed on 15 February 2025).
- Müller, A.; Müller, K.; Blumenstiel, K.; Bieber, C.; Eich, W. Das Konzept der Selbstwirksamkeit als bedeutsamer Prädiktor anhaltenden Behandlungserfolgs von Fibromyalgie-Patienten. Aktuelle Rheumatol. 2004, 29, 101–108. [Google Scholar]
- Beal, C.C.; Stuifbergen, A.K.; Brown, A. Predictors of a health promoting lifestyle in women with fibromyalgia syndrome. Psychol. Health Med. 2009, 14, 343–353. [Google Scholar] [CrossRef] [PubMed]
- López-Roig, S.; Pastor-Mira, M.-Á.; Núñez, R.; Nardi, A.; Ivorra, S.; León, E.; Peñacoba, C. Assessing Self-Efficacy for Physical Activity and Walking Exercise in Women with Fibromyalgia. Pain Manag. Nurs. 2021, 22, 571–578. [Google Scholar] [CrossRef]
- Häuser, W.; Bernardy, K.; Arnold, B.; Offenbächer, M.; Schiltenwolf, M. Efficacy of multicomponent treatment in fibromyalgia syndrome: A meta-analysis of randomized controlled clinical trials. Arthritis Care Res. 2009, 61, 216–224. [Google Scholar] [CrossRef]
- Nüesch, E.; Häuser, W.; Bernardy, K.; Barth, J.; Jüni, P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: Network meta-analysis. Ann. Rheum. Dis. 2013, 72, 955–962. [Google Scholar] [CrossRef]
- Perrot, S.; Russell, I.J. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: A meta-analysis examining six core symptoms. Eur. J. Pain 2014, 18, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Kaiser, J.M.; Novitch, M.B.; Cornett, E.M.; Urman, R.D.; Kaye, A.D. The Role of Complementary and Alternative Medicine Treatments in Fibromyalgia: A Comprehensive Review. Curr. Rheumatol. Rep. 2019, 21, 14. [Google Scholar] [CrossRef]
- Langhorst, J.; Heldmann, P.; Henningsen, P.; Kopke, K.; Krumbein, L.; Lucius, H.; Winkelmann, A.; Wolf, B.; Häuser, W. Complementary and alternative procedures for fibromyalgia syndrome: Updated guidelines 2017 and overview of systematic review articles. Schmerz 2017, 31, 289–295. [Google Scholar] [CrossRef]
- Mist, S.D.; Firestone, K.A.; Jones, K.D. Complementary and alternative exercise for fibromyalgia: A meta-analysis. J. Pain Res. 2013, 6, 247–260. [Google Scholar] [CrossRef]
- Wang, C.; Schmid, C.H.; Fielding, R.A.; Harvey, W.F.; Reid, K.F.; Price, L.L.; Driban, J.B.; Kalish, R.; Rones, R.; McAlindon, T. Effect of tai chi versus aerobic exercise for fibromyalgia: Comparative effectiveness randomized controlled trial. BMJ 2018, 360, k851. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.W.; Carson, K.M.; Jones, K.D.; Bennett, R.M.; Wright, C.L.; Mist, S.D. A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain 2010, 151, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Sawynok, J.; Lynch, M.E. Qigong and Fibromyalgia circa 2017. Medicines 2017, 4, 37. [Google Scholar] [CrossRef]
- Courtois, I.; Cools, F.; Calsius, J. Effectiveness of body awareness interventions in fibromyalgia and chronic fatigue syndrome: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2015, 19, 35–56. [Google Scholar] [CrossRef]
- Veehof, M.M.; Oskam, M.-J.; Schreurs, K.M.G.; Bohlmeijer, E.T. Acceptance-based interventions for the treatment of chronic pain: A systematic review and meta-analysis. Pain 2011, 152, 533–542. [Google Scholar] [CrossRef]
- Schönenberger, K.A.; Schüpfer, A.-C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-Braun, N.; Reber, E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4221. [Google Scholar] [CrossRef]
- Koppold, D.A.; Kandil, F.I.; Müller, A.; Güttler, O.; Steckhan, N.; Meiss, S.; Breinlinger, C.; Nelle, E.; Rajput Khokhar, A.; Jeitler, M.; et al. Effects of Prolonged Medical Fasting during an Inpatient, Multimodal, Nature-Based Treatment on Pain, Physical Function, and Psychometric Parameters in Patients with Fibromyalgia: An Observational Study. Nutrients 2024, 16, 1059. [Google Scholar] [CrossRef]
- Hussein, M.; Fathy, W.; Abdelghaffar, M.; Hegazy, M.T.; Teleb, D.A.; Adel, S.; Kassim, D.Y.; Magdy, R. Effect of first week-intermittent fasting during Ramadan on the severity of neuropsychiatric symptoms in patients with fibromyalgia: A prospective study. Egypt. Rheumatol. 2024, 46, 47–50. [Google Scholar] [CrossRef]
- Michalsen, A.; Li, C.; Kaiser, K.; Lüdtke, R.; Meier, L.; Stange, R.; Kessler, C. In-patient treatment of fibromyalgia: A controlled nonrandomized comparison of conventional medicine versus integrative medicine including fasting therapy. Evid.-Based Complement. Altern. Med. 2013, 2013, 908610. [Google Scholar] [CrossRef]
- Chen, L.; Michalsen, A. Management of chronic pain using complementary and integrative medicine. BMJ 2017, 357, j1284. [Google Scholar] [CrossRef]
- Crofford, L.J.; Appleton, B.E. Complementary and alternative therapies for fibromyalgia. Curr. Rheumatol. Rep. 2001, 3, 147–156. [Google Scholar] [CrossRef]
- Mohabbat, A.B.; Mahapatra, S.; Jenkins, S.M.; Bauer, B.A.; Vincent, A.; Wahner-Roedler, D.L. Use of Complementary and Integrative Therapies by Fibromyalgia Patients: A 14-Year Follow-up Study. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 418–428. [Google Scholar] [CrossRef]
- National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What’s in a Name? NCCIH. April 2021. Available online: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name (accessed on 15 February 2025).
- Öznur, Ö.; Schlee, C.; Utz, S.; Langhorst, J. Investigating the Influential Factors of Mild Water-Filtered Infrared-A Whole-Body Hyperthermia for Pain Relief in Fibromyalgia: A Mixed-Methods Approach Focusing on Predictors and Patient Perspectives. Biomedicines 2023, 11, 2949. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Koch, A.K.; Kehm, C.; Öznur, Ö.; Engler, H.; Häuser, W. Mild Water-Filtered Infrared-A Whole-Body Hyperthermia Reduces Pain in Patients with Fibromyalgia Syndrome—A Randomized Sham-Controlled Trial. J. Clin. Med. 2023, 12, 2945. [Google Scholar] [CrossRef]
- Steen, J.P.; Kannan, V.; Zaidi, A.; Cramer, H.; Ng, J.Y. Mind-body therapy for treating fibromyalgia: A systematic review. Pain Med. 2024, 25, 703–737. [Google Scholar] [CrossRef] [PubMed]
- Lauche, R.; Cramer, H.; Dobos, G.; Langhorst, J.; Schmidt, S. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. J. Psychosom. Res. 2013, 75, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Lauche, R.; Cramer, H.; Häuser, W.; Dobos, G.; Langhorst, J. A systematic review and meta-analysis of Qigong for the fibromyalgia syndrome. Evid.-Based Complement. Altern. Med. 2013, 2013, 635182. [Google Scholar] [CrossRef]
- Lauche, R.; Spitzer, J.; Schwahn, B.; Ostermann, T.; Bernardy, K.; Cramer, H.; Dobos, G.; Langhorst, J. Efficacy of cupping therapy in patients with the fibromyalgia syndrome-a randomised placebo controlled trial. Sci. Rep. 2016, 6, 37316. [Google Scholar] [CrossRef]
- Langhorst, J.; Klose, P.; Musial, F.; Irnich, D.; Häuser, W. Efficacy of acupuncture in fibromyalgia syndrome—A systematic review with a meta-analysis of controlled clinical trials. Rheumatology 2010, 49, 778–788. [Google Scholar] [CrossRef]
- Langhorst, J.; Klose, P.; Dobos, G.J.; Bernardy, K.; Häuser, W. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: A systematic review and meta-analysis of randomized controlled trials. Rheumatol. Int. 2013, 33, 193–207. [Google Scholar] [CrossRef]
- Häuser, W.; Klose, P.; Langhorst, J.; Moradi, B.; Steinbach, M.; Schiltenwolf, M.; Busch, A. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2010, 12, R79. [Google Scholar] [CrossRef]
- Lauche, R.; Langhorst, J.; Paul, A.; Dobos, G.; Cramer, H. Self-reported health and satisfaction of patients with chronic diseases who meditate: A case–control study. Qual. Life Res. 2014, 23, 2639–2644. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol. 2012, 30 (Suppl. 74), 10–17. [Google Scholar]
- Bauer, B.A.; Tilburt, J.C.; Sood, A.; Li, G.; Wang, S. Complementary and alternative medicine therapies for chronic pain. Chin. J. Integr. Med. 2016, 22, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Jung, E.; Erbslöh-Möller, B.; Gesmann, M.; Kühn-Becker, H.; Petermann, F.; Langhorst, J.; Thoma, R.; Weiss, T.; Wolfe, F.; et al. German fibromyalgia consumer reports: Benefits and harms of fibromyalgia syndrome therapies. Schmerz 2012, 26, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Jung, E.; Erbslöh-Möller, B.; Gesmann, M.; Kühn-Becker, H.; Petermann, F.; Langhorst, J.; Thoma, R.; Weiss, T.; Wolfe, F.; et al. The German fibromyalgia consumer reports—A cross-sectional survey. BMC Musculoskelet. Disord. 2012, 13, 74. [Google Scholar] [CrossRef]
- Lauche, R.; Häuser, W.; Jung, E.; Erbslöh-Möller, B.; Gesmann, M.; Kühn-Becker, H.; Petermann, F.; Weiss, T.; Thoma, R.; Winkelmann, A.; et al. Patient-related predictors of treatment satisfaction of patients with fibromyalgia syndrome: Results of a cross-sectional survey. Clin. Exp. Rheumatol. 2013, 31 (Suppl. 79), S34–S40. [Google Scholar]
- Langhorst, J.; Musial, F.; Klose, P.; Häuser, W. Efficacy of hydrotherapy in fibromyalgia syndrome—A meta-analysis of randomized controlled clinical trials. Rheumatology 2009, 48, 1155–1159. [Google Scholar] [CrossRef]
- Von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133. [Google Scholar] [CrossRef]
- Häuser, W.; In Leibniz-Institut für Psychologie (ZPID) (Hrsg.). Fibromyalgia Survey Questionnaire—Deutsche Fassung [Verfahrensdokumentation und Fragebogen mit Auswertung]. Open Test Archive. ZPID. 2015. Available online: https://www.psycharchives.org/en/item/78a5b4d6-4087-4612-86af-f6c260e627c1 (accessed on 20 December 2020).
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wintermann, G.-B.; Rosendahl, J.; Weidner, K.; Strauß, B.; Hinz, A.; Petrowski, K. Fatigue in chronically critically ill patients following intensive care—Reliability and validity of the multidimensional fatigue inventory (MFI-20). Health Qual. Life Outcomes 2018, 16, 37. [Google Scholar] [CrossRef]
- Ericsson, A.; Mannerkorpi, K. Assessment of fatigue in patients with fibromyalgia and chronic widespread pain. Reliability and validity of the Swedish version of the MFI-20. Disabil. Rehabil. 2007, 29, 1665–1670. [Google Scholar] [CrossRef]
- Ericsson, A.; Bremell, T.; Mannerkorpi, K. Usefulness of multiple dimensions of fatigue in fibromyalgia. J. Rehabil. Med. 2013, 45, 685–693. [Google Scholar] [CrossRef]
- Bakalidou, D.; Krommydas, G.; Abdimioti, T.; Theodorou, P.; Doskas, T.; Fillopoulos, E. The Dimensionality of the Multidimensional Fatigue Inventory (MFI-20) Derived from Healthy Adults and Patient Subpopulations: A Challenge for Clinicians. Cureus 2022, 14, e26344. [Google Scholar] [CrossRef]
- Gecaite-Stonciene, J.; Bunevicius, A.; Burkauskas, J.; Brozaitiene, J.; Neverauskas, J.; Mickuviene, N.; Kazukauskiene, N. Validation of the Multidimensional Fatigue Inventory with Coronary Artery Disease Patients. Int. J. Environ. Res. Public Health 2020, 17, 8003. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Benzing, C.; Brähler, E.; Zenger, M.; Herzberg, P.Y.; Finck, C.; Schmalbach, B.; Petrowski, K. Psychometric Properties of the Multidimensional Fatigue Inventory (MFI-20), Derived from Seven Samples. J. Pain Symptom Manag. 2020, 59, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Herrmann-Lingen, C.; Buss, U.; Snaith, R.P. Hospital Anxiety and Depression Scale—Deutsche Version (HADS-D); Verlag Hans Huber, Hogrefe AG: Bern, Switzerland, 1995. [Google Scholar]
- Hinz, A.; Brähler, E. Normative values for the hospital anxiety and depression scale (HADS) in the general German population. J. Psychosom. Res. 2011, 71, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Casale, R.; Sarzi-Puttini, P.; Botto, R.; Alciati, A.; Batticciotto, A.; Marotto, D.; Torta, R. Fibromyalgia and the concept of resilience. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 116), 105–113. [Google Scholar] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Büssing, A. Perceived Stress Scale—Deutsche 10-Item-Fassung. 2011. Available online: https://www.cmu.edu/dietrich/psychology/stress-immunity-disease-lab/scales/.doc/german_pss_10.doc (accessed on 25 April 2023).
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Morfeld, M.; Kirchberger, I.; Bullinger, M. SF-36—Fragebogen zum Gesundheitszustand; 2. Ergänzte und Überarbeitete Auflage; Hogrefe Verlag: Göttingen, Germany, 2011. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 27.0; IBM Corp: Armonk, NY, USA, 2020.
- Thieme, K.; Mathys, M.; Turk, D.C. Evidenced-Based Guidelines on the Treatment of Fibromyalgia Patients: Are They Consistent and If Not, Why Not? Have Effective Psychological Treatments Been Overlooked? J. Pain 2017, 18, 747–756. [Google Scholar] [CrossRef]
- Müller, H.; de Toledo, F.W.; Resch, K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar] [CrossRef]
- Michalsen, A.; Hoffmann, B.; Moebus, S.; Bäcker, M.; Langhorst, J.; Dobos, G.J. Incorporation of fasting therapy in an integrative medicine ward: Evaluation of outcome, safety, and effects on lifestyle adherence in a large prospective cohort study. J. Altern. Complement. Med. 2005, 11, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ring, R.M.; Eisenmann, C.; Kandil, F.I.; Steckhan, N.; Demmrich, S.; Klatte, C.; Kessler, C.S.; Jeitler, M.; Boschmann, M.; Michalsen, A.; et al. Mental and behavioural responses to Bahá’í Fasting: Looking behind the scenes of a religiously motivated intermittent fast using a mixed methods approach. Nutrients 2022, 14, 1038. [Google Scholar] [CrossRef]
- Michalsen, A. Prolonged fasting as a method of mood enhancement in chronic pain syndromes: A review of clinical evidence and mechanisms. Curr. Pain Headache Rep. 2010, 14, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Nakajima, W.; Takada, G. Short-term fasting alters neonatal rat striatal dopamine levels and serotonin metabolism: An in vivo microdialysis study. Dev. Brain Res. 1997, 104, 131–136. [Google Scholar] [CrossRef]
- Caron, J.P.; Kreher, M.A.; Mickle, A.M.; Wu, S.; Przkora, R.; Estores, I.M.; Sibille, K.T. Intermittent fasting: Potential utility in the treatment of chronic pain across the clinical spectrum. Nutrients 2022, 14, 2536. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, mechanisms, diagnosis and treatment options update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Phillips, T.M.; Cheng, A.; Morrell, C.H.; Mattson, M.P.; Wan, R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010, 67, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Rodriguez-Pintó, I.; Agmon-Levin, N.; Howard, A.; Shoenfeld, Y. Fibromyalgia and cytokines. Immunol. Lett. 2014, 161, 200–203. [Google Scholar] [CrossRef]
- Schlee, C.; Uecker, C.; Bauer, N.; Koch, A.K.; Langhorst, J. Multimodal stress reduction and lifestyle modification program for patients with ulcerative colitis: A qualitative study. BMC Complement. Med. Ther. 2022, 22, 60. [Google Scholar] [CrossRef]
- Schlee, C.; Uecker, C.; Öznur, Ö.; Bauer, N.; Langhorst, J. Participants’ perspectives on a multimodal stress management and comprehensive lifestyle modification program for patients with Crohn’s disease—A qualitative interview study. PLoS ONE 2024, 19, e0313127. [Google Scholar] [CrossRef]
- Luszczynska, A.; Schwarzer, R. Social Cognitive Theory. In Predicting Health Behaviours, 3rd ed.; Conner, M., Norman, P., Eds.; McGraw-Hill Education Open University Press: Berkshire, UK, 2015; pp. 225–251. [Google Scholar]
- Adams, L.M.; Turk, D.C. Psychosocial Factors and Central Sensitivity Syndromes. Curr. Rheumatol. Rev. 2015, 11, 96–108. [Google Scholar] [CrossRef]
- Oliver, K.; Cronan, T. Predictors of exercise behaviors among fibromyalgia patients. Prev. Med. 2002, 35, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Lauche, R.; Moebus, S.; Michalsen, A.; Langhorst, J.; Dobos, G.; Paul, A. Predictors of health behavior change after an integrative medicine inpatient program. Int. J. Behav. Med. 2014, 21, 775–783. [Google Scholar] [CrossRef]
- Marcus, B.H.; Simkin, L.R. The transtheoretical model: Applications to exercise behavior. Med. Sci. Sports Exerc. 1994, 26, 1400. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef]
- Rau, J.; Petermann, F. Motivationsförderung bei chronischen Schmerzpatienten. Schmerz 2008, 22, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C. Stages and processes of self-change of smoking: Toward an integrative model of change. J. Consult. Clin. Psychol. 1983, 51, 390–395. [Google Scholar] [CrossRef] [PubMed]

| Sample (n = 134) | |
|---|---|
| Sociodemographic characteristics | |
| Sex | |
| female | 127 (94.8%) |
| male | 7 (5.2%) |
| Age (years) | 57.2 ± 8.5 |
| Educational level | |
| no school certificate | 1 (0.7%) |
| secondary school | 113 (84.3%) |
| A-level | 17 (12.7%) |
| Professional qualification | |
| vocational training | 78 (58.2%) |
| master training | 32 (23.9%) |
| high school/university | 10 (7.4%) |
| without/still in training | 7 (5.2%) |
| Employment status | |
| full-time | 13 (9.7%) |
| part-time/occasional work | 35 (26.1%) |
| on sick leave | 16 (11.9%) |
| unemployed | 14 (10.5%) |
| retired (due to age) | 22 (16.4%) |
| retired (due to health reasons) | 32 (23.9%) |
| Health related characteristics | |
| Time since diagnosis (years) | 9.2 ± 8.5 |
| Body-Mass-Index (m/kg2) | 29.3 ± 7.3 |
| <19 | 0 (0%) |
| 19–24 | 30 (22.7%) |
| 24–29 | 53 (39.4%) |
| >30 | 51 (37.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utz, S.; Uecker, C.; Kropač, S.; Langhorst, J. A Prospective Observational Study of a 2-Week Integrative Inpatient Therapy on Patients with Fibromyalgia Syndrome. Biomedicines 2025, 13, 2144. https://doi.org/10.3390/biomedicines13092144
Utz S, Uecker C, Kropač S, Langhorst J. A Prospective Observational Study of a 2-Week Integrative Inpatient Therapy on Patients with Fibromyalgia Syndrome. Biomedicines. 2025; 13(9):2144. https://doi.org/10.3390/biomedicines13092144
Chicago/Turabian StyleUtz, Sandra, Christine Uecker, Stefanie Kropač, and Jost Langhorst. 2025. "A Prospective Observational Study of a 2-Week Integrative Inpatient Therapy on Patients with Fibromyalgia Syndrome" Biomedicines 13, no. 9: 2144. https://doi.org/10.3390/biomedicines13092144
APA StyleUtz, S., Uecker, C., Kropač, S., & Langhorst, J. (2025). A Prospective Observational Study of a 2-Week Integrative Inpatient Therapy on Patients with Fibromyalgia Syndrome. Biomedicines, 13(9), 2144. https://doi.org/10.3390/biomedicines13092144





