Diagnostic Use of Selected Metalloproteinases in Endometrioid Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochemical Assays
2.2. Statistical Analysis
3. Results
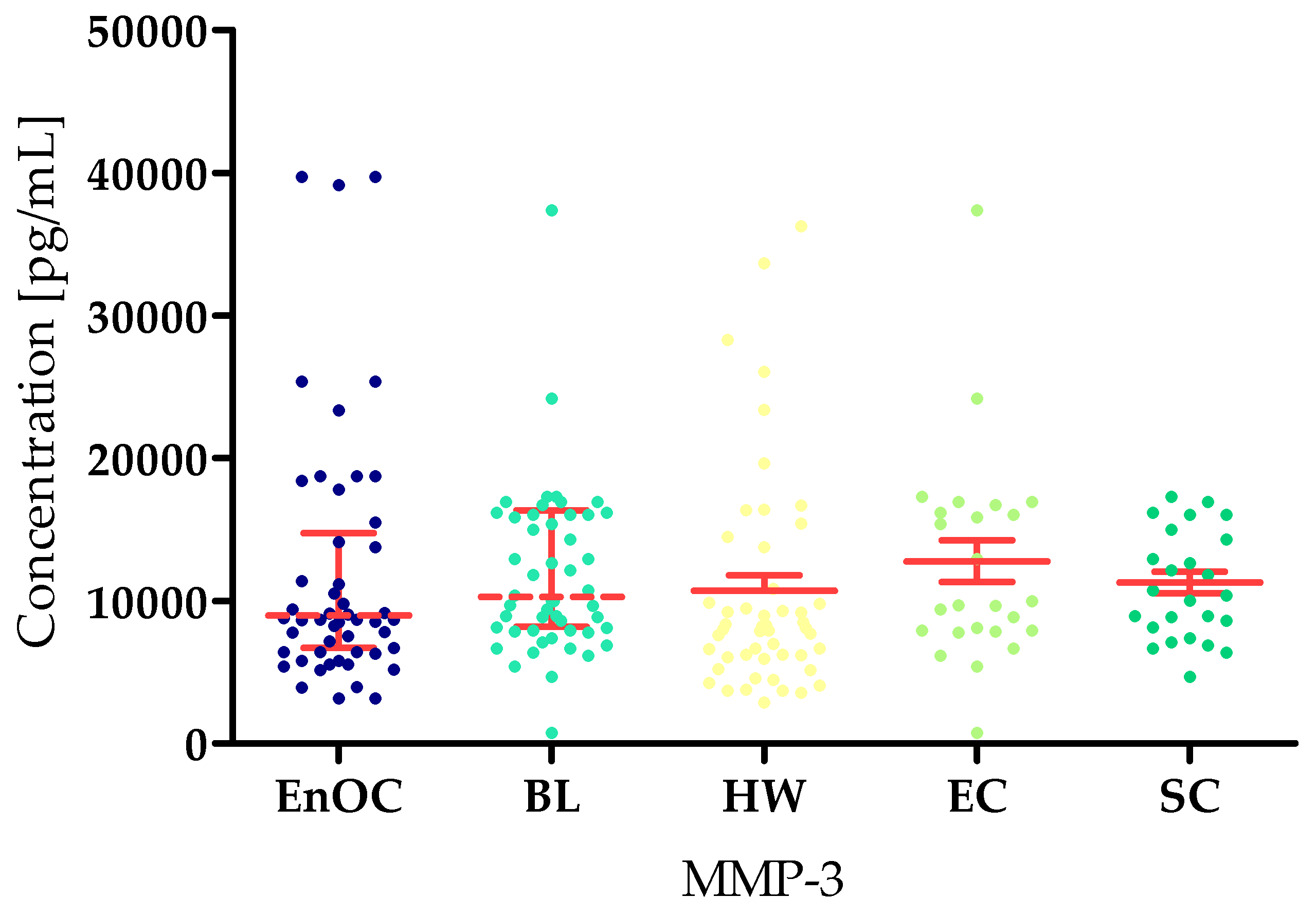
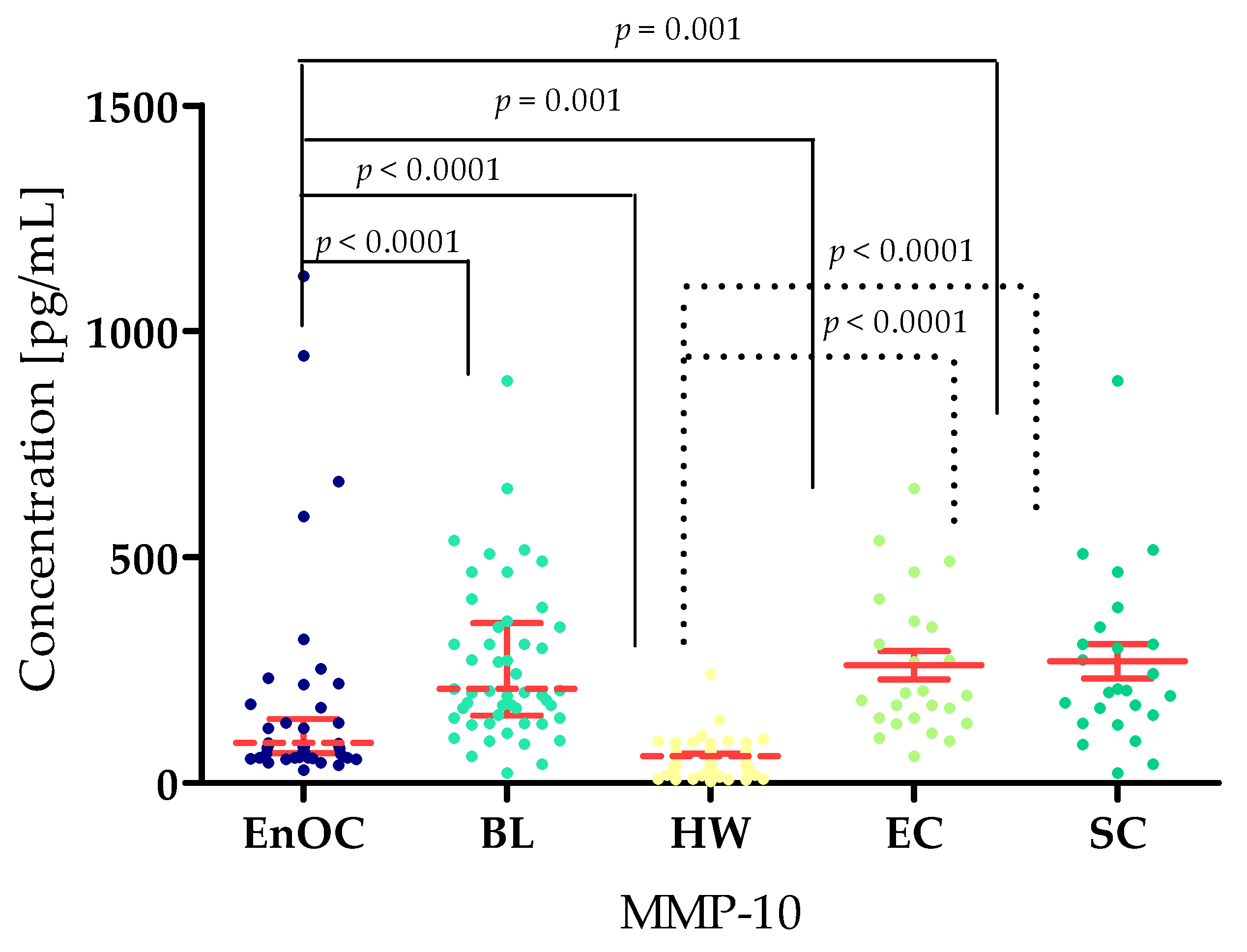
3.1. Concentrations of Selected Matrilysins and Stromelysins in Patients with Ovarian Endometrioid Carcinoma, Patients with Ovarian Cysts, and Healthy Women
3.2. Evaluation of Correlation Using Spearman’s Method
3.3. Diagnostic Criteria of Tested MMPs, CA 125, and HE4
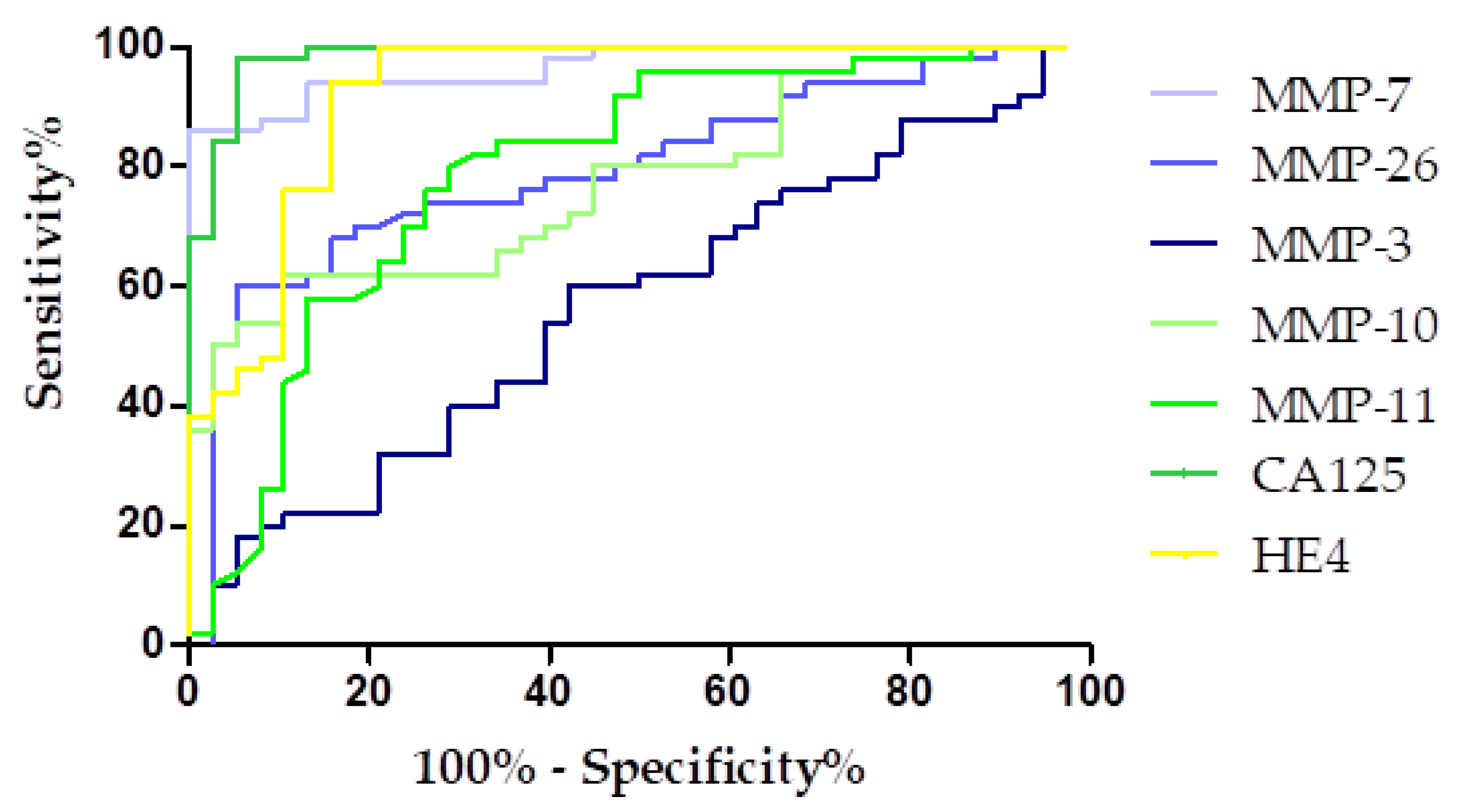
3.4. Evaluation of the Diagnostic Power of Tests by ROC Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kicman, A.; Gacuta, E.; Kulesza, M.; Będkowska, E.G.; Marecki, R.; Klank-Sokołowska, E.; Knapp, P.; Niczyporuk, M.; Ławicki, S. Diagnostic Utility of Selected Matrix Metalloproteinases (MMP-2, MMP-3, MMP-11, MMP-26), HE4, CA125 and ROMA Algorithm in Diagnosis of Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 6265. [Google Scholar] [CrossRef]
- Kicman, A.; Gacuta, E.; Marecki, R.; Kicman, M.S.; Kulesza, M.; Klank-Sokołowska, E.; Knapp, P.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Diagnostic Utility of Metalloproteinases from Collagenase Group (MMP-1, MMP-8 and MMP-13) in Biochemical Diagnosis of Ovarian Carcinoma. Cancers 2024, 16, 3969. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Gęca, K.; Litwiński, J.; Ostrowski, T.; Świetlicka, I.; Polkowski, W.P.; Skórzewska, M. Exploring the Survival Determinants in Recurrent Ovarian Cancer: The Role of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Cancers 2024, 16, 2150. [Google Scholar] [CrossRef] [PubMed]
- Dochez, V.; Caillon, H.; Vaucel, E.; Dimet, J.; Winer, N.; Ducarme, G. Biomarkers and Algorithms for Diagnosis of Ovarian Cancer: CA125, HE4, RMI and ROMA, a Review. J. Ovarian Res. 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Muñoz, B.; Aznar-Oroval, E.; García, A.G.; Peris, A.C.; Ballestero, P.P.; Yepes, M.S.; Lozano, T.G.; Ballester, C.I.; Garcia, E.G. HE4, Ca125 and ROMA Algorithm for Differential Diagnosis between Benign Gynaecological Diseases and Ovarian Cancer. Tumor Biol. 2014, 35, 7249–7258. [Google Scholar] [CrossRef]
- Karlsen, M.A.; Sandhu, N.; Høgdall, C.; Christensen, I.J.; Nedergaard, L.; Lundvall, L.; Engelholm, S.A.; Pedersen, A.T.; Hartwell, D.; Lydolph, M.; et al. Evaluation of HE4, CA125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) as Diagnostic Tools of Epithelial Ovarian Cancer in Patients with a Pelvic Mass. Gynecol. Oncol. 2012, 127, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, A.; Augusto, K.; Portela, M.; Sucupira, L.C.; Oliveira, L.A.; Pouchaim, A.J.; Mesquita Nóbrega, L.R.; de Magalhães, T.F.; Sobreira, L.R. Endometriosis and Ovarian Cancer: An Integrative Review (Endometriosis and Ovarian Cancer). Asian Pac. J. Cancer Prev. 2017, 18, 11–16. [Google Scholar] [PubMed]
- Nakamura, K.; Banno, K.; Yanokura, M.; Iida, M.; Adachi, M.; Masuda, K.; Ueki, A.; Kobayashi, Y.; Nomura, H.; Hirasawa, A.; et al. Features of Ovarian Cancer in Lynch Syndrome (Review). Mol. Clin. Oncol. 2014, 2, 909–916. [Google Scholar] [CrossRef]
- Murakami, K.; Kotani, Y.; Nakai, H.; Matsumura, N. Endometriosis-Associated Ovarian Cancer: The Origin and Targeted Therapy. Cancers 2020, 12, 1676. [Google Scholar] [CrossRef]
- Giordano, G.; Ferioli, E.; Tafuni, A. The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets. Cancers 2022, 14, 2283. [Google Scholar] [CrossRef] [PubMed]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Chudecka-Głaz, A.; Strojna, A.; Michalczyk, K.; Wieder-Huszla, S.; Safranow, K.; Skwirczyńska, E.; Jurczak, A. Evaluation of He4 Use in the Diagnosis of Ovarian Cancer: First and Second Recurrence, and an Analysis of HE4 Concentration during Second- and Third-Line Chemotherapy. Diagnostics 2023, 13, 452. [Google Scholar] [CrossRef]
- Ma, B.; Ran, R.; Liao, H.Y.; Zhang, H.H. The paradoxical role of matrix metalloproteinase-11 in cancer. Biomed. Pharmacother. 2021, 141, 111899. [Google Scholar] [CrossRef]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and Its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Piskór, B.; Gacuta, E.; Zajkowska, M.; Osada, J.; Szmitkowski, M.; Dąbrowska, M.; Ławicki, S. Diagnostic Power of Selected Cytokines, MMPs and TIMPs in Ovarian Cancer Patients—ROC Analysis. Anticancer Res. 2019, 39, 2575–2582. [Google Scholar] [CrossRef]
- Będkowska, G.E.; Gacuta, E.; Zajkowska, M.; Głażewska, E.K.; Osada, J.; Szmitkowski, M.; Chrostek, L.; Dąbrowska, M.; Ławicki, S. Plasma Levels of MMP-7 and TIMP-1 in Laboratory Diagnostics and Differentiation of Selected Histological Types of Epithelial Ovarian Cancers. J. Ovarian Res. 2017, 10, 39. [Google Scholar] [CrossRef]
- Kicman, A.; Niczyporuk, M.; Kulesza, M.; Motyka, J.; Ławicki, S. Utility of Matrix Metalloproteinases in the Diagnosis, Monitoring and Prognosis of Ovarian Cancer Patients. Cancer Manag. Res. 2022, 14, 3359–3382. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix Metalloproteinases As Novel Biomarker s and Potential Therapeutic Targets in Human Cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Al-Alem, L.; Curry, T.E. Ovarian Cancer: Involvement of the Matrix Metalloproteinases. Reproduction 2015, 150, R55–R64. [Google Scholar] [CrossRef]
- Acar, A.; Onan, A.; Coskun, U.; Uner, A.; Bagriacik, U.; Atalay, F.; Unsal, D.K.; Guner, H. Clinical Significance of Serum MMP-2 and MMP-7 in Patients with Ovarian Cancer. Med. Oncol. 2008, 25, 279–283. [Google Scholar] [CrossRef]
- Zhai, Y.; Wu, R.; Schwartz, D.R.; Darrah, D.; Reed, H.; Kolligs, F.T.; Nieman, M.T.; Fearon, E.R.; Cho, K.R. Role of beta-catenin/T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas. Am. J. Pathol. 2002, 160, 1229–1238. [Google Scholar] [CrossRef]
- Sillanpää, S.M.; Anttila, M.A.; Voutilainen, K.A.; Ropponen, K.M.; Sironen, R.K.; Saarikoski, S.V.; Kosma, V.M. Prognostic significance of matrix metalloproteinase-7 in epithelial ovarian cancer and its relation to beta-catenin expression. Int. J. Cancer 2006, 119, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Qian, J.; Zhu, F.; Wu, F.; Zhao, H.; Zhu, H. The prognostic values of matrix metalloproteinases in ovarian cancer. J. Int. Med. Res. 2020, 48, 300060519825983. [Google Scholar] [CrossRef]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Plasma Concentrations of Matrilysins MMP-7 and MMP-26 as Diagnostic Biomarkers in Breast Cancer. J. Clin. Med. 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, I.; Tacheva, T.; Mindov, I.; Petrov, B.; Aleksandrova, E.; Valkanov, S.; Gulubova, M. Serum levels of MMP-7 in primary brain cancers and brain metastases. Biotechnol. Biotechnol. Equip. 2019, 33, 881–885. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Basit Ashraf, M.A.; Parveen, G.; Iqbal, S.; Ali, I.; Qazi, M.H.; Asif, M.; Kamran, K.; Iqbal, A.; et al. Evaluation of Matrix Metalloproteinases, Cytokines and Their Potential Role in the Development of Ovarian Cancer. PLoS ONE 2016, 11, e0167149. [Google Scholar] [CrossRef]
- Périgny, M.; Bairati, I.; Harvey, I.; Beauchemin, M.; Harel, F.; Plante, M.; Têtu, B. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am. J. Clin. Pathol. 2008, 129, 226–231. [Google Scholar] [CrossRef]
- Pang, L.; Wang, D.W.; Zhang, N.; Xu, D.H.; Meng, X.W. Elevated serum levels of MMP-11 correlate with poor prognosis in colon cancer patients. Cancer Biomark. 2016, 16, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Fan, Y.; Lang, F.; Fu, F.; Liu, Q. MMP11 and MMP17 are potential biomarkers for uterine corpus endometrial carcinoma prognosis. Clin. Transl. Oncol. 2024, 26, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, L.; Li, J.; Zhu, S.; Tai, M.; Mason, C.W.; Chapman, J.A.; Reynolds, E.A.; Weiner, C.P.; Zhou, H.H. MicroRNA-205 promotes cell invasion by repressing TCF21 in human ovarian cancer. J. Ovarian Res. 2017, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Mariya, T.; Hirohashi, Y.; Torigoe, T.; Tabuchi, Y.; Asano, T.; Saijo, H.; Kuroda, T.; Yasuda, K.; Mizuuchi, M.; Saito, T.; et al. Matrix metalloproteinase-10 regulates stemness of ovarian cancer stem-like cells by activation of canonical Wnt signaling and can be a target of chemotherapy-resistant ovarian cancer. Oncotarget 2016, 7, 26806–26822. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, S.; Wu, M.; Lou, J.; Zhang, J.; Xu, T.; Huang, L.; Huang, P.; Wang, F.; Pan, S. Tumor-associated macrophages promote invasion via Toll-like receptors signaling in patients with ovarian cancer. Int. Immunopharmacol. 2016, 40, 184–195. [Google Scholar] [CrossRef]
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Plasma Level of MMP-10 May Be a Prognostic Marker in Early Stages of Breast Cancer. J. Clin. Med. 2020, 9, 4122. [Google Scholar] [CrossRef]
- Ripley, D.; Tunuguntla, R.; Susi, L.; Chegini, N. Expression of matrix metalloproteinase-26 and tissue inhibitors of metalloproteinase-3 and -4 in normal ovary and ovarian carcinoma. Int. J. Gynecol. Cancer 2006, 16, 1794–1800. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Xiao, A.Z.; Ni, J.; Man, Y.G.; Sang, Q.X. Expression of matrix metalloproteinase-26 in multiple human cancer tissues and smooth muscle cells. Ai Zheng. 2009, 28, 1168–1175. [Google Scholar] [CrossRef]
- Tunuguntla, R.; Ripley, D.; Sang, Q.X.; Chegini, N. Expression of matrix metalloproteinase-26 and tissue inhibitors of metalloproteinases TIMP-3 and -4 in benign endometrium and endometrial cancer. Gynecol. Oncol. 2003, 89, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Yunusova, N.V.; Patysheva, M.R.; Molchanov, S.V.; Zambalova, E.A.; Grigor’eva, A.E.; Kolomiets, L.A.; Ochirov, M.O.; Tamkovich, S.N.; Kondakova, I.V. Metalloproteinases at the surface of small extrcellular vesicles in advanced ovarian cancer: Relationships with ascites volume and peritoneal canceromatosis index. Clin. Chim. Acta 2019, 494, 116–122. [Google Scholar] [CrossRef] [PubMed]





| Ovarian Endometrioid Carcinoma (EnOC) | |
| Number of patients—50 (100%) Median of age: 56 Menopausal status Pre-menopause—12 Postmenopause—38 Tumor stage I–II—25 III–IV—25 | |
| Ovarian Benign Lesions (BL) | |
| Endometrial Cysts (EC) | Serous Cysts (SC) |
| Number of patients—25 (50%) Median of age: 44 Menopausal status Pre-menopause—16 Postmenopause—9 | Number of patients—25 (50%) Median of age: 56 Menopausal status Pre-menopause—5 Postmenopause—20 |
| Healthy Women (HW) | |
| Number of patients—50 (100%) Median of age: 60 Menopausal status Pre-menopause—24 Postmenopause—26 | |
| Endometrioid Ovarian Carcinoma (EnOC) | |||||||
| Parameter | MMP-7 [ng/mL] | MMP-26 [pg/mL] | MMP-3 [pg/mL] | MMP-10 [pg/mL] | MMP-11 [pg/mL] | CA125 [U/mL] | HE4 [U/mL] |
| Median | 5.64 | 9425.00 | 12,106.47 | 160.35 | 2733.13 | 149.10 | 338.01 |
| Standard deviation | 5.93 | 7332.16 | 8519.96 | 190.39 | 3138.24 | 496.78 | 410.71 |
| Minimum | 2.20 | 3578.00 | 3468.52 | 38.90 | 40.00 | 19.60 | 35.57 |
| Quartile 1 | 3.77 | 8625.00 | 6705.05 | 65.32 | 766.75 | 64.87 | 90.52 |
| Quartile 3 | 9.64 | 13,310.00 | 14,768.24 | 177.36 | 3900.00 | 609.55 | 430.30 |
| Maximum | 27.40 | 42,070.00 | 40,000.00 | 954.50 | 15,180.00 | 2100.00 | 1944.00 |
| Healthy Women (HW) | |||||||
| Parameter | MMP-7 [ng/mL] | MMP-26 [pg/mL] | MMP-3 [pg/mL] | MMP-10 [pg/mL] | MMP-11 [pg/mL] | CA125 [U/mL] | HE4 [U/mL] |
| Median | 0.95 | 7163.00 | 10,719.41 | 59.51 | 762.00 | 16.90 | 39.51 |
| Standard deviation | 1.08 | 7903.60 | 7605.36 | 41.96 | 807.22 | 7.45 | 14.68 |
| Minimum | 0.04 | 3037.55 | 3178.22 | 12.30 | 20.00 | 4.24 | 23.52 |
| Quartile 1 | 0.66 | 3920.00 | 6329.89 | 28.37 | 260.00 | 11.38 | 31.07 |
| Quartile 3 | 1.87 | 7162.50 | 11,880.69 | 77.06 | 895.00 | 22.27 | 44.53 |
| Maximum | 5.23 | 9128.75 | 36,528.70 | 249.54 | 4400.00 | 39.94 | 1944.20 |
| Benign Lesions Total Group (BL) | |||||||
| Parameter | MMP-7 [ng/mL] | MMP-26 [pg/mL] | MMP-3 [pg/mL] | MMP-10 [pg/mL] | MMP-11 [pg/mL] | CA125 [U/mL] | HE4 [U/mL] |
| Median | 2.78 | 10,985.00 | 10,298.00 | 208.80 | 190.00 | 23.38 | 52.55 |
| Standard deviation | 1.28 | 4342.00 | 10,298.00 | 170.50 | 1610.00 | 57.43 | 18.61 |
| Minimum | 1.50 | 5140.00 | 1054.00 | 30.98 | 20.00 | 8.900 | 28.20 |
| Quartile 1 | 2.27 | 9591.00 | 8211.00 | 149.70 | 55.00 | 13.13 | 43.65 |
| Quartile 3 | 3.50 | 12,539.00 | 16,332.00 | 353.90 | 440.00 | 43.98 | 62.91 |
| Maximum | 7.52 | 27,235.00 | 37,648.00 | 898.10 | 7640.00 | 410.30 | 112.30 |
| Ovarian Endometrioid Cyst Group (EC) | |||||||
| Parameter | MMP-7 [ng/mL] | MMP-26 [pg/mL] | MMP-3 [pg/mL] | MMP-10 [pg/mL] | MMP-11 [pg/mL] | CA125 [U/mL] | HE4 [U/mL] |
| Median | 2.80 | 10,825.00 | 10,235.00 | 160.35 | 233.13 | 24.98 | 53.20 |
| Standard deviation | 3.17 | 11,265.58 | 12,604.60 | 190.39 | 1338.24 | 496.78 | 410.71 |
| Minimum | 1.31 | 4122.06 | 7245.47 | 38.90 | 40.00 | 19.60 | 35.57 |
| Quartile 1 | 1.84 | 5140.00 | 1054.22 | 65.32 | 76.75 | 64.87 | 90.52 |
| Quartile 3 | 2.79 | 10,825.00 | 9968.57 | 177.36 | 390.00 | 609.55 | 430.30 |
| Maximum | 3.51 | 12,538.75 | 16,615.94 | 954.48 | 7518.00 | 2100.00 | 1944.20 |
| Ovarian Serous Cyst Group (SC) | |||||||
| Parameter | MMP-7 [ng/mL] | MMP-26 [pg/mL] | MMP-3 [pg/mL] | MMP-10 [pg/mL] | MMP-11 [pg/mL] | CA125 [U/mL] | HE4 [U/mL] |
| Median | 2.94 | 12,169.00 | 10,683.00 | 113.80 | 200.00 | 19.00 | 51.47 |
| Standard deviation | 1.25 | 4519.00 | 3774.00 | 187.10 | 1160.00 | 13.54 | 22.46 |
| Minimum | 1.50 | 5975.00 | 4972.00 | 30.98 | 20.00 | 9.00 | 35.15 |
| Quartile 1 | 2.07 | 9660.00 | 8079.00 | 150.50 | 90.00 | 12.58 | 40.86 |
| Quartile 3 | 3.39 | 13,593.00 | 14,959.00 | 335.30 | 430.00 | 37.10 | 62.95 |
| Maximum | 7.52 | 27,235.00 | 17,599.00 | 898.10 | 7640.00 | 47.30 | 112.30 |
| MMP-7 | MMP-26 | MMP-3 | MMP-10 | MMP-11 | CA125 | HE4 | |
| BL Total Group | |||||||
| MMP-3 | N/S | N/S | - | N/S | p = 0.0238 r = 0.4420 | p = 0.0210 r = −0.1591 | p = 0.0100 r = −0.2545 |
| MMP-10 | N/S | N/S | N/S | - | p = 0.0490 r = −0.2904 | N/S | N/S |
| MMP-11 | p = 0.0410 r = 0.4506 | N/S | p = 0.0238 r = 0.4420 | p = 0.0160 r = −0.4675 | - | p = 0.0145 r = −0.4358 | p = 0.0105 r = −0.4660 |
| SC Group | |||||||
| MMP-26 | N/S | - | N/S | p < 0.0001 r = −0.7065 | p < 0.0001 r = −0.6291 | p < 0.0001 r = −0.6602 | p < 0.0001 r = −0.7110 |
| MMP-3 | p < 0.0001 r = −0.7041 | p = 0.0088 r = 0.3668 | - | p < 0.0001 r = −0.6660 | p < 0.0001 r = −0.6664 | p < 0.0001 r = −0.6564 | p < 0.0001 r = 0.6743 |
| MMP-10 | N/S | N/S | p < 0.0001 r = −0.6256 | - | p < 0.0001 r = −0.6423 | p < 0.0001 r = −0.7456 | p < 0.0001 r = −0.7114 |
| MMP-11 | p = 0.0089 r = 0.3552 | N/S | p = 0.0084 r = 0.3447 | p = 0.0081 r = 0.3657 | N/S | p < 0.0001 r = −0.8542 | p < 0.0001 r = −0.5475 |
| Parameter | SE [%] | SP [%] | PPV [%] | NPV [%] | ACC [%] |
|---|---|---|---|---|---|
| MMP-7 | 98.55% | 86.00% | 87.72% | 95.41% | 57.58% |
| MMP-26 | 94.00% | 60.00% | 70.15% | 90.91% | 77.00% |
| MMP-3 | 58.00% | 60.00% | 59.18% | 58.82% | 59.00% |
| MMP-10 | 92.00% | 62.00% | 70.77% | 88.57% | 77.00% |
| MMP-11 | 32.00% | 82.00% | 64.00% | 54.67% | 57.00% |
| CA125 | 96.00% | 47.95% | 97.96% | 96.08% | 97.00% |
| HE4 | 80.00% | 52.05% | 93.02% | 82.46% | 87.00% |
| Parameters | MMP-7 | MMP-26 | MMP-3 | MMP-10 | MMP-11 | CA125 | HE4 |
|---|---|---|---|---|---|---|---|
| AUC | 0.9658 | 0.7708 | 0.5679 | 0.7763 | 0.7926 | 0.9758 | 0.9247 |
| SEAUC | 0.0165 | 0.0520 | 0.0616 | 0.0485 | 0.0509 | 0.0101 | 0.0306 |
| 95% CI | 0.9335–0.9981 | 0.6688–0.8727 | 0.4471–0.6886 | 0.6812–0.8714 | 0.6928–0.8925 | 0.9660–1.006 | 0.8647–0.9848 |
| p (AUC = 0.5) | <0.0001 | <0.0001 | 0.2772 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacuta, E.; Kicman, A.; Ławicki, P.; Ławicki, M.; Kulesza, M.; Malinowski, P.; Chlabicz, M.; Zajkowska, M.; Ławicki, S. Diagnostic Use of Selected Metalloproteinases in Endometrioid Ovarian Cancer. Biomedicines 2025, 13, 2143. https://doi.org/10.3390/biomedicines13092143
Gacuta E, Kicman A, Ławicki P, Ławicki M, Kulesza M, Malinowski P, Chlabicz M, Zajkowska M, Ławicki S. Diagnostic Use of Selected Metalloproteinases in Endometrioid Ovarian Cancer. Biomedicines. 2025; 13(9):2143. https://doi.org/10.3390/biomedicines13092143
Chicago/Turabian StyleGacuta, Ewa, Aleksandra Kicman, Paweł Ławicki, Michał Ławicki, Monika Kulesza, Paweł Malinowski, Marcin Chlabicz, Monika Zajkowska, and Sławomir Ławicki. 2025. "Diagnostic Use of Selected Metalloproteinases in Endometrioid Ovarian Cancer" Biomedicines 13, no. 9: 2143. https://doi.org/10.3390/biomedicines13092143
APA StyleGacuta, E., Kicman, A., Ławicki, P., Ławicki, M., Kulesza, M., Malinowski, P., Chlabicz, M., Zajkowska, M., & Ławicki, S. (2025). Diagnostic Use of Selected Metalloproteinases in Endometrioid Ovarian Cancer. Biomedicines, 13(9), 2143. https://doi.org/10.3390/biomedicines13092143







