More than Just a Toothache: Inflammatory Mechanisms Linking Periodontal Disease to Cardiovascular Disease and the Protective Impact of Cardiorespiratory Fitness
Abstract
1. Introduction
2. Periodontitis and CVD: Evidence from Population Studies, Imaging and Animal Models
3. Periodontitis and Mortality
4. Mechanism of CVD Progression in Periodontal Disease
5. Other Inflammatory Conditions and CVD Progression
6. Inflammation and CVD Risk: Evidence from Meta-Analyses and Cohort Studies
7. The Impact of Cardiorespiratory Fitness on Periodontitis and CVD
8. Improving Cardiorespiratory Fitness
9. Future Directions and Clinical Applications
9.1. Anti-Inflammatory Therapies and Lifestyle Interventions for Periodontal and Cardiovascular Disease
9.2. Interdisciplinary Approaches: Advocate for Collaboration Between Dental, Medical, and Fitness Professionals
9.3. Public Health Implications: Stress the Importance of Awareness Campaigns for Better Oral and Cardiovascular Health
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Oral Disorders Collaborators; Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.; Gajagowni, S.; Qadeer, Y.; Wang, Z.; Virani, S.S.; Meurman, J.H.; Leischik, R.; Lavie, C.J.; Strauss, M.; Krittanawong, C. More than just teeth: How oral health can affect the heart. Am. Heart J. Plus 2024, 43, 100407. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and the pathogenesis of atherosclerosis. Vasc. Pharmacol. 2024, 154, 107255. [Google Scholar] [CrossRef]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal diseases and association with atherosclerotic disease. Periodontol. 2000 2020, 83, 66–89. [Google Scholar] [CrossRef]
- Martinsson, A.; Östling, G.; Persson, M.; Sundquist, K.; Andersson, C.; Melander, O.; Engström, G.; Hedblad, B.; Smith, J.G. Carotid Plaque, Intima-Media Thickness, and Incident Aortic Stenosis: A Prospective Cohort Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Balzer, D. Mayo Clinic News Network. Many Factors Play into Increased Heart Disease Risk for Those with RA. 2016. Available online: https://newsnetwork.mayoclinic.org/discussion/mayo-clinic-q-and-a-many-factors-play-into-increased-heart-disease-risk-for-those-with-ra-repost/ (accessed on 24 January 2025).
- Naderi, S.; Merchant, A.T. The Association Between Periodontitis and Cardiovascular Disease: An Update. Curr. Atheroscler. Rep. 2020, 22, 1–5. [Google Scholar] [CrossRef]
- Cairo, F.; Castellani, S.; Gori, A.M.; Nieri, M.; Baldelli, G.; Abbate, R.; Pini-Prato, G.P. Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J. Clin. Periodontol. 2008, 35, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Tsai, K.-Z.; Liu, P.-Y.; Huang, W.-C.; Chu, C.-C.; Sui, X.; Lavie, C.J.; Lin, G.-M. Oral health and physical performance in Asian military males: The cardiorespiratory fitness and health in armed forces. J. Dent. Sci. 2024, 19, 998–1003. [Google Scholar] [CrossRef]
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’aIuto, F. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 706432. [Google Scholar] [CrossRef]
- Luo, H.; Wu, B. Self-awareness of “Gum Disease” Among US Adults. J. Public Health Manag. Pract. 2017, 23, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Periodontal diseases—Level 4 cause|Institute for Health Metrics and Evaluation [Internet]. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-periodontal-diseases-level-4-disease (accessed on 4 November 2024).
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, E347–E913. [Google Scholar] [CrossRef] [PubMed]
- CDC. Heart Disease. Heart Disease Facts. 2024. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html (accessed on 26 November 2024).
- Leng, Y.; Hu, Q.; Ling, Q.; Yao, X.; Liu, M.; Chen, J.; Yan, Z.; Dai, Q. Periodontal disease is associated with the risk of cardiovascular disease independent of sex: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1114927. [Google Scholar] [CrossRef]
- Carra, M.C.; Rangé, H.; Caligiuri, G.; Bouchard, P. Periodontitis and atherosclerotic cardiovascular disease: A critical appraisal. Periodontology 2000, 2023; Early View. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association?: A scientific statement from the American Heart Association. Circulation 2012, 125, 2520–2544. [Google Scholar] [CrossRef] [PubMed]
- Vedin, O.; Hagström, E.; Gallup, D.; Neely, M.L.; Stewart, R.; Koenig, W.; Budaj, A.; Sritara, P.; Wallentin, L.; White, H.D.; et al. Periodontal disease in patients with chronic coronary heart disease: Prevalence and association with cardiovascular risk factors. Eur. J. Prev. Cardiol. 2015, 22, 771–778. [Google Scholar] [CrossRef]
- Sanz, M.; del Castillo, A.M.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’aIuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.; Liao, C.; Feng, X.; He, T.; Siddiqi, T.J. Periodontal disease and subsequent risk of cardiovascular outcome and all-cause mortality: A meta-analysis of prospective studies. PLoS ONE 2023, 18, e0290545. [Google Scholar] [CrossRef]
- Xu, S.; Song, M.; Xiong, Y.; Liu, X.; He, Y.; Qin, Z. The association between periodontal disease and the risk of myocardial infarction: A pooled analysis of observational studies. BMC Cardiovasc. Disord. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Guallar, E.; Qiao, Y.; Wasserman, B.A. Is Carotid Intima-Media Thickness as Predictive as Other Noninvasive Techniques for the Detection of Coronary Artery Disease? Arter. Thromb. Vasc. Biol. 2014, 34, 1341–1345. [Google Scholar] [CrossRef]
- Uriza, C.L.; Roa, N.S.; Velosa-Porras, J.; Poveda, J.C.V.; Otero, L.; Ruiz, A.J.; Arregoces, F.M.E. Relationship between Carotid Intima-Media Thickness, Periodontal Disease, and Systemic Inflammation Biomarkers in an Adult Population. Biomedicines 2024, 12, 1425. [Google Scholar] [CrossRef]
- Associations Among Tooth Loss, Periodontitis, and Carotid Intima-Media Thickness: The Nagahama Study [Internet]. Available online: https://www.jstage.jst.go.jp/article/jat/30/10/30_63801/_html/-char/en (accessed on 26 November 2024).
- Tsai, K.-Z.; Huang, W.-C.; Chang, Y.-C.; Kwon, Y.; Sui, X.; Lavie, C.J.; Lin, G.-M. Localized periodontitis severity associated with carotid intima-media thickness in young adults: CHIEF atherosclerosis study. Sci. Rep. 2023, 13, 10523. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef]
- Ishai, A.; Osborne, M.T.; El Kholy, K.; Takx, R.A.; Ali, A.; Yuan, N.; Hsue, P.; Van Dyke, T.E.; Tawakol, A. Periodontal Disease Associates With Arterial Inflammation Via Potentiation of a Hematopoietic-Arterial Axis. JACC Cardiovasc. Imaging 2019, 12 Pt 1, 2271–2273. [Google Scholar] [CrossRef]
- Fifer, K.M.; Qadir, S.; Subramanian, S.; Vijayakumar, J.; Figueroa, A.L.; Truong, Q.A.; Hoffman, U.; Brady, T.J.; Tawakol, A. Positron emission tomography measurement of periodontal 18F-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J. Am. Coll. Cardiol. 2011, 57, 971–976. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; El Kholy, K.; Ishai, A.; Takx, R.A.; Mezue, K.; Abohashem, S.M.; Ali, A.; Yuan, N.; Hsue, P.; Osborne, M.T.; et al. Inflammation of the periodontium associates with risk of future cardiovascular events. J. Periodontol. 2021, 92, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Batista, E.L.; Serhan, C.; Stahl, G.L.; Van Dyke, T.E. Role for periodontitis in the progression of lipid deposition in an animal model. Infect. Immun. 2003, 71, 6012–6018. [Google Scholar] [CrossRef] [PubMed]
- Brodala, N.; Merricks, E.P.; Bellinger, D.A.; Damrongsri, D.; Offenbacher, S.; Beck, J.; Madianos, P.; Sotres, D.; Chang, Y.-L.; Koch, G.; et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arter. Thromb. Vasc. Biol. 2005, 25, 1446–1451. [Google Scholar] [CrossRef]
- Gibson, F.C., 3rd; Hong, C.; Chou, H.-H.; Yumoto, H.; Chen, J.; Lien, E.; Wong, J.; Genco, C.A. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004, 109, 2801–2806. [Google Scholar] [CrossRef]
- Dimitriu, T.; Bolfa, P.; Daradics, Z.; Suciu, Ş.; Armencea, G.; Cătoi, C.; Dinu, C.; Băciuţ, G.; Văcăraş, S.; Bran, S.; et al. Ligature induced periodontitis causes atherosclerosis in rat descending aorta: An experimental study. Med. Pharm. Rep. 2021, 94, 106–111. [Google Scholar] [CrossRef]
- Ngamdu, K.S.; Mallawaarachchi, I.; Dunipace, E.A.; Chuang, L.-H.; Jafri, S.H.; Shah, N.R.; Jeong, Y.N.; Morrison, A.R.; Bhatt, D.L. Association Between Periodontal Disease and Cardiovascular Disease (from the NHANES). Am. J. Cardiol. 2022, 178, 163–168. [Google Scholar] [CrossRef]
- Chen, F.; Song, Y.; Li, W.; Xu, H.; Dan, H.; Chen, Q. Association between periodontitis and mortality of patients with cardiovascular diseases: A cohort study based on NHANES. J. Periodontol. 2024, 95, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Scientific World Journal. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Welch, J.L.M.; Ramírez-Puebla, S.T.; Borisy, G.G. Oral Microbiome Geography: Micron-Scale Habitat and Niche. Cell Host Microbe 2020, 28, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontology 2000 2000, 24, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Larvin, H.; Baptiste, P.J.; Gao, C.; Muirhead, V.; Donos, N.; Pavitt, S.; Kang, J.; Wu, J. All-cause and cause-specific mortality in US adults with periodontal diseases: A prospective cohort study. J. Clin. Periodontol. 2024, 51, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Parahitiyawa, N.B.; Jin, L.J.; Leung, W.K.; Yam, W.C.; Samaranayake, L.P. Microbiology of Odontogenic Bacteremia: Beyond Endocarditis. Clin. Microbiol. Rev. 2009, 22, 46–64. [Google Scholar] [CrossRef]
- Tian, S.; Ding, T.; Li, H. Oral microbiome in human health and diseases. mLife 2024, 3, 367–383. [Google Scholar] [CrossRef]
- Welch, J.L.M.; Dewhirst, F.E.; Borisy, G.G. Biogeography of the Oral Microbiome: The Site-Specialist Hypothesis. Annu. Rev. Microbiol. 2019, 73, 335–358. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Luo, M.-Y.; Liang, N.; Gong, S.-X.; Chen, W.; Huang, W.-Q.; Tian, Y.; Wang, A.-P. Interleukin-6: A Novel Target for Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2021, 12, 745061. [Google Scholar] [CrossRef]
- Mazurek-Mochol, M.; Bonsmann, T.; Mochol, M.; Poniewierska-Baran, A.; Pawlik, A. The Role of Interleukin 6 in Periodontitis and Its Complications. Int. J. Mol. Sci. 2024, 25, 2146. [Google Scholar] [CrossRef]
- Perschinka, H.; Mayr, M.; Millonig, G.; Mayerl, C.; van der Zee, R.; Morrison, S.G.; Morrison, R.P.; Xu, Q.; Wick, G. Cross-Reactive B-Cell Epitopes of Microbial and Human Heat Shock Protein 60/65 in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1060–1065. [Google Scholar] [CrossRef]
- Burke, A.P.; Farb, A.; Virmani, R. Plaque rupture and plaque erosion. Thromb. Haemost. 1999, 82 (Suppl. 1), 1–3. [Google Scholar] [CrossRef]
- Luo, X.; Lv, Y.; Bai, X.; Qi, J.; Weng, X.; Liu, S.; Bao, X.; Jia, H.; Yu, B. Plaque Erosion: A Distinctive Pathological Mechanism of Acute Coronary Syndrome. Front. Cardiovasc. Med. 2021, 8, 711453. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Libby, P.; Jang, I.-K. New Insights Into Plaque Erosion as a Mechanism of Acute Coronary Syndromes. JAMA 2021, 325, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.C.; de Araújo, B.J.; Cordeiro, C.S.; Arruda, V.M.; Faria, B.Q.; Guerra, J.F.D.C.; De Araújo, T.G.; Fürstenau, C.R. Endothelial dysfunction due to the inhibition of the synthesis of nitric oxide: Proposal and characterization of an in vitro cellular model. Front. Physiol. 2022, 13, 978378. [Google Scholar] [CrossRef]
- Heat Shock Proteins and Periodontitis—Cross-Reaction Between Bacterial and Human HSP in Periodontal Infection Linking with Cardiovascular Diseases|SpringerLink [Internet]. Available online: https://link.springer.com/chapter/10.1007/7515_2020_24 (accessed on 6 March 2025).
- Seymour, G.; Ford, P.; Cullinan, M.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13, 3–10. [Google Scholar] [CrossRef]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr. Atheroscler. Rep. 2020, 22, 1–6. [Google Scholar] [CrossRef]
- Gerasimova, E.V.; Popkova, T.V.; Kirillova, I.G.; Gerasimova, D.A.; Nasonov, E.L.; Lila, A.M. Interleukin-6: Cardiovascular Aspects of Long-Term Cytokine Suppression in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2024, 25, 12425. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.; Meyers, K.J.; Workman, J.; Hartley, L.; McMahon, M. Cardiovascular events and risk in patients with systemic lupus erythematosus: Systematic literature review and meta-analysis. Lupus 2023, 32, 325–341. [Google Scholar] [CrossRef]
- Dijkshoorn, B.; Raadsen, R.; Nurmohamed, M.T. Cardiovascular Disease Risk in Rheumatoid Arthritis Anno 2022. J. Clin. Med. 2022, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.d.O.; Corrêa, M.G.; Magno, M.B.; Almeida, A.P.C.P.S.C.; Fagundes, N.C.F.; Rosing, C.K.; Maia, L.C.; Lima, R.R. Physical Activity Reduces the Prevalence of Periodontal Disease: Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 234. [Google Scholar] [CrossRef]
- Eberhard, J.; Stiesch, M.; Kerling, A.; Bara, C.; Eulert, C.; Hilfiker-Kleiner, D.; Hilfiker, A.; Budde, E.; Bauersachs, J.; Kück, M.; et al. Moderate and severe periodontitis are independent risk factors associated with low cardiorespiratory fitness in sedentary non-smoking men aged between 45 and 65 years. J. Clin. Periodontol. 2014, 41, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, B.; Stubbe, B.; Gläser, S.; Trabandt, J.; Völzke, H.; Ewert, R.; Kocher, T.; Ittermann, T.; Schäper, C. Periodontitis Is Related to Exercise Capacity: Two Cross-sectional Studies. J. Dent. Res. 2021, 100, 824–832. [Google Scholar] [CrossRef]
- Admin, A. Periodontal disease and systemic health: An update for medical practitioners—Annals Singapore [Internet]. 2022. Available online: https://annals.edu.sg/periodontal-disease-and-systemic-health-an-update-for-medical-practitioners/ (accessed on 6 March 2025).
- Mendoza, M.F.; Suan, N.M.; Lavie, C.J. Exploring the Molecular Adaptations, Benefits, and Future Direction of Exercise Training: Updated Insights into Cardiovascular Health. J. Funct. Morphol. Kinesiol. 2024, 9, 131. [Google Scholar] [CrossRef]
- Buti, J.; Ronca, F.; Burgess, P.W.; Gallagher, J.; Ashley, P.; Needleman, I. Association between periodontitis and physical fitness in law enforcement workers. Clin. Oral Investig. 2025, 29, 1–13. [Google Scholar] [CrossRef]
- Tsai, K.Z.; Huang, C.M.; Wang, H.S.; Sui, X.; Lavie, C.J.; Lin, G.M. Does the guideline-based physical activity level for cardiovascular health also benefit periodontal health? J. Dent. Sci. 2024, 19, 46–50. [Google Scholar] [CrossRef]
- Kuo, H.-K.; Yen, C.-J.; Chen, J.-H.; Yu, Y.-H.; Bean, J.F. Association of cardiorespiratory fitness and levels of C-reactive protein: Data from the National Health and Nutrition Examination Survey 1999–2002. Int. J. Cardiol. 2007, 114, 28–33. [Google Scholar] [CrossRef]
- Su, M.-Z.; Lee, S.; Shin, D. Association of Dietary Fiber and Measures of Physical Fitness with High-Sensitivity C-Reactive Protein. Nutrients 2024, 16, 888. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.; Dupke, A.; Rohleder, N. Associations Between C-Reactive Protein Levels, Exercise Addiction, and Athlete Burnout in Endurance Athletes. Front. Psychol. 2021, 12, 615715. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pavón, D.; Lavie, C.J.; Blair, S.N. The role of cardiorespiratory fitness on the risk of sudden cardiac death at the population level: A systematic review and meta-analysis of the available evidence. Prog. Cardiovasc. Dis. 2019, 62, 279–287. [Google Scholar] [CrossRef]
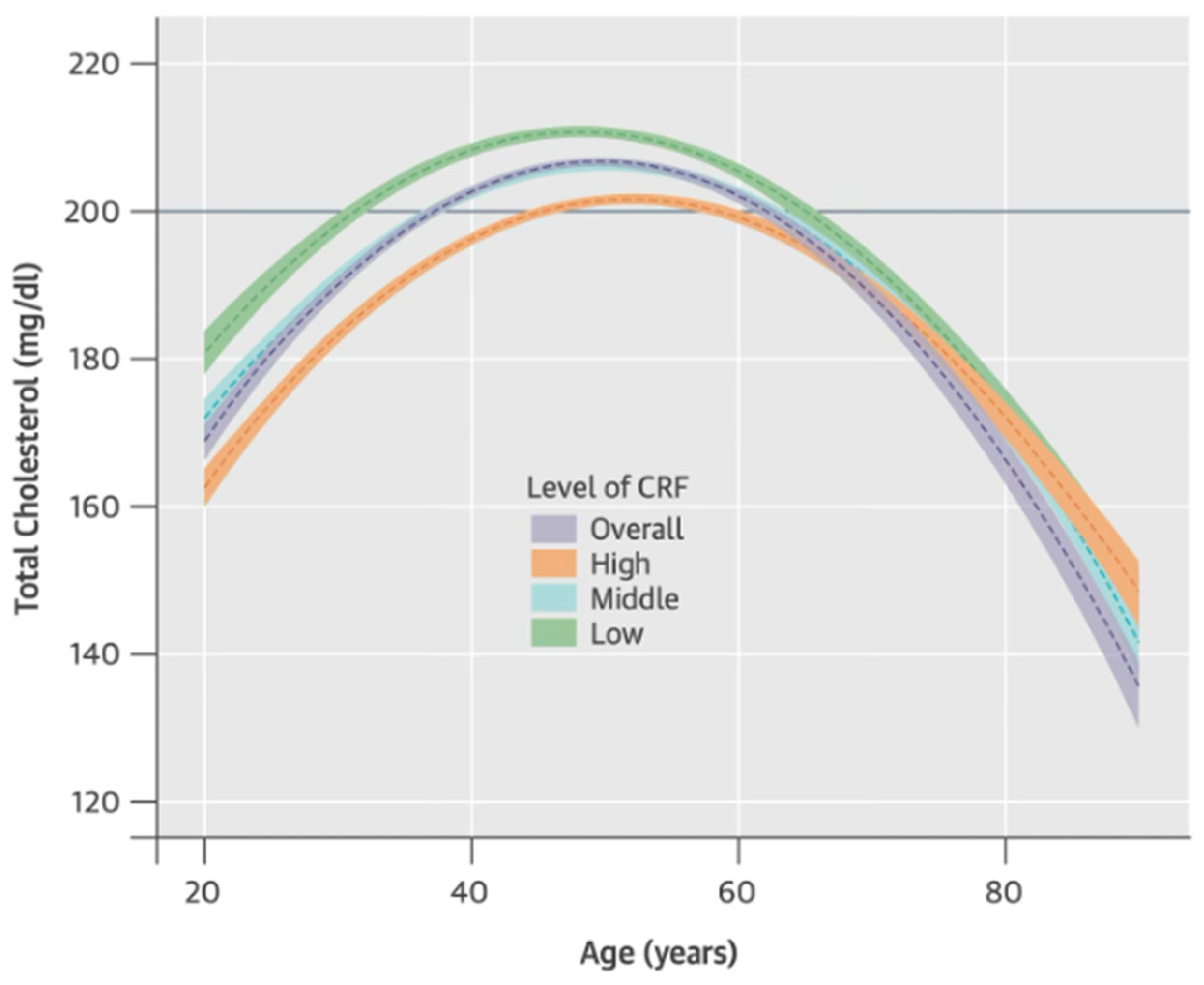
- Park, Y.-M.M.; Sui, X.; Liu, J.; Zhou, H.; Kokkinos, P.F.; Lavie, C.J.; Hardin, J.W.; Blair, S.N. The effect of cardiorespiratory fitness on age-related lipids and lipoproteins. J. Am. Coll. Cardiol. 2015, 65, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef]
- Lavie, C.J.; Kachur, S.; Sui, X. Impact of Fitness and Changes in Fitness on Lipids and Survival. Prog. Cardiovasc. Dis. 2019, 62, 431–435. [Google Scholar] [CrossRef]
- Breneman, C.B.; Polinski, K.; Sarzynski, M.A.; Lavie, C.J.; Kokkinos, P.F.; Ahmed, A.; Sui, X. The Impact of Cardiorespiratory Fitness Levels on the Risk of Developing Atherogenic Dyslipidemia. Am. J. Med. 2016, 129, 1060–1066. [Google Scholar] [CrossRef]
- Hung, R.K.; Al-Mallah, M.H.; Qadi, M.A.; Shaya, G.E.; Blumenthal, R.S.; Nasir, K.; Brawner, C.A.; Keteyian, S.J.; Blaha, M.J. Cardiorespiratory fitness attenuates risk for major adverse cardiac events in hyperlipidemic men and women independent of statin therapy: The Henry Ford ExercIse Testing Project. Am. Heart J. 2015, 170, 390–399.e6. [Google Scholar] [CrossRef]
- Jiménez-Pavón, D.; Artero, E.G.; Lee, D.C.; España-Romero, V.; Sui, X.; Pate, R.R.; Church, T.S.; Moreno, L.A.; Lavie, C.J.; Blair, S.N. Cardiorespiratory Fitness and Risk of Sudden Cardiac Death in Men and Women in the United States: A Prospective Evaluation From the Aerobics Center Longitudinal Study. Mayo Clin. Proc. 2016, 91, 849–857. [Google Scholar] [CrossRef]
- Mendoza, M.V.F.; Kachur, S.M.; Lavie, C.J. The Effects of ExerciseExerciseson Lipid BiomarkersBiomarkers. In Physical Exercise and Natural and Synthetic Products in Health and Disease [Internet]; Guest, P.C., Ed.; Springer: New York, NY, USA, 2022; pp. 93–117. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Dibben, G.; Faulkner, J.; Oldridge, N.; Rees, K.; Thompson, D.R.; Zwisler, A.D.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021, 11, CD001800. [Google Scholar] [PubMed]
- Franklin, B.A.; Thompson, P.D.; Al-Zaiti, S.S.; Albert, C.M.; Hivert, M.-F.; Levine, B.D.; Lobelo, F.; Madan, K.; Sharrief, A.Z.; Eijsvogels, T.M.; et al. Exercise-Related Acute Cardiovascular Events and Potential Deleterious Adaptations Following Long-Term Exercise Training: Placing the Risks Into Perspective–An Update: A Scientific Statement From the American Heart Association. Circulation 2020, 141, E705–E736. [Google Scholar] [CrossRef] [PubMed]
- Sulague, R.M.; Suan, N.N.M.; Mendoza, M.F.; Lavie, C.J. The associations between exercise and lipid biomarkers. Prog. Cardiovasc. Dis. 2022, 75, 59–68. [Google Scholar] [CrossRef]
- Mendoza, M.F.; Lavie, C.J. Clinical associations between exercise and lipoproteins. Curr. Opin. Lipidol. 2022, 33, 364–373. [Google Scholar] [CrossRef]
- Kokkinos, P.; Faselis, C.; Samuel, I.B.H.; Pittaras, A.; Doumas, M.; Murphy, R.; Heimall, M.S.; Sui, X.; Zhang, J.; Myers, J. Cardiorespiratory Fitness and Mortality Risk Across the Spectra of Age, Race, and Sex. J. Am. Coll. Cardiol. 2022, 80, 598–609. [Google Scholar] [CrossRef]
| Bacterial Translocation in PD | Inflammation in PD and CVD |
|---|---|
| In periodontal disease, periodontal vasculature is dilated and facilitates bacteremia | Periodontitis produces elevated levels of inflammatory mediators (CRP, haptoglobin, fibrinogen, TNF-α, IL-6, fibrinogen) |
| When the sulcular epithelium is disturbed, bacteria translocate into the bloodstream | Inflammatory mediators may modify serum lipid, inflammatory cell, and endothelial cell receptors, contributing to plaque development |
| Beginning with PGE2, an inflammatory cascade creates a reservoir of gram-negative bacteria and pro-inflammatory products (LPS) in the periodontium | Elevated CRP correlates with an increase in CIMT in PD patients, ultimately conferring increased stroke risk |
| Common dental plaque anaerobic bacteria have been found particularly in locations where atherosclerotic plaques are found (coronary arteries) | Patient with periodontitis has higher levels of LPS-LDL and oxLDL, which are modified lipid forms that promote atheroma development |
| Pathogens induce cross-reactive antibodies, which then produce inflammation systemically and within atheromas | Salivary assays for 8-hydroxyguanosine, malondialdehyde, protein carbonyl assay, and total antioxidant capacity are elevated in both chronic PD and ACS patients |
| Category | Exercise Type | Description |
|---|---|---|
| Mode | Aerobic ET | Brisk walking, running/jogging, swimming, bicycling, stair climbing, rowing, aerobic dancing, skiing |
| Resistance ET | Lifting weights, resistance/elastic bands, bodyweight exercises, heavy gardening, calisthenics | |
| Duration | Aerobic ET | 150–300 min/week (moderate intensity); 75–150 min/week (vigorous intensity); or an equivalent combination of moderate to vigorous intensity |
| Resistance ET | 8 to 12 repetitions to fatigue; at least 1 set for all muscle groups; 60 to 80% of single maximum repetition (70% in elderly) | |
| Frequency | Aerobic ET | Most days of the week (preferably 6–7 days/week) |
| Resistance ET | 2–3 non-consecutive days/week | |
| Intensity | Aerobic ET | Moderate to vigorous intensity |
| Resistance ET | Moderate to vigorous intensity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza, M.F.; Anzelmo, M.A.; Suan, N.M.; Cuccia, C.S.; Lavie, C.J. More than Just a Toothache: Inflammatory Mechanisms Linking Periodontal Disease to Cardiovascular Disease and the Protective Impact of Cardiorespiratory Fitness. Biomedicines 2025, 13, 1512. https://doi.org/10.3390/biomedicines13071512
Mendoza MF, Anzelmo MA, Suan NM, Cuccia CS, Lavie CJ. More than Just a Toothache: Inflammatory Mechanisms Linking Periodontal Disease to Cardiovascular Disease and the Protective Impact of Cardiorespiratory Fitness. Biomedicines. 2025; 13(7):1512. https://doi.org/10.3390/biomedicines13071512
Chicago/Turabian StyleMendoza, Michael F., Michael A. Anzelmo, Nina M. Suan, Chloe S. Cuccia, and Carl J. Lavie. 2025. "More than Just a Toothache: Inflammatory Mechanisms Linking Periodontal Disease to Cardiovascular Disease and the Protective Impact of Cardiorespiratory Fitness" Biomedicines 13, no. 7: 1512. https://doi.org/10.3390/biomedicines13071512
APA StyleMendoza, M. F., Anzelmo, M. A., Suan, N. M., Cuccia, C. S., & Lavie, C. J. (2025). More than Just a Toothache: Inflammatory Mechanisms Linking Periodontal Disease to Cardiovascular Disease and the Protective Impact of Cardiorespiratory Fitness. Biomedicines, 13(7), 1512. https://doi.org/10.3390/biomedicines13071512





