Real-World Efficacy of Minimally Invasive Revascularization in Diabetic Foot Ischemia: Impact of Device Selection and Lesion-Specific Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Procedure Details
2.3. Outcomes and Follow-Up Evaluation
2.4. Follow-Up Protocol
2.5. Complication Tracking
2.6. Statistical Analysis
3. Results
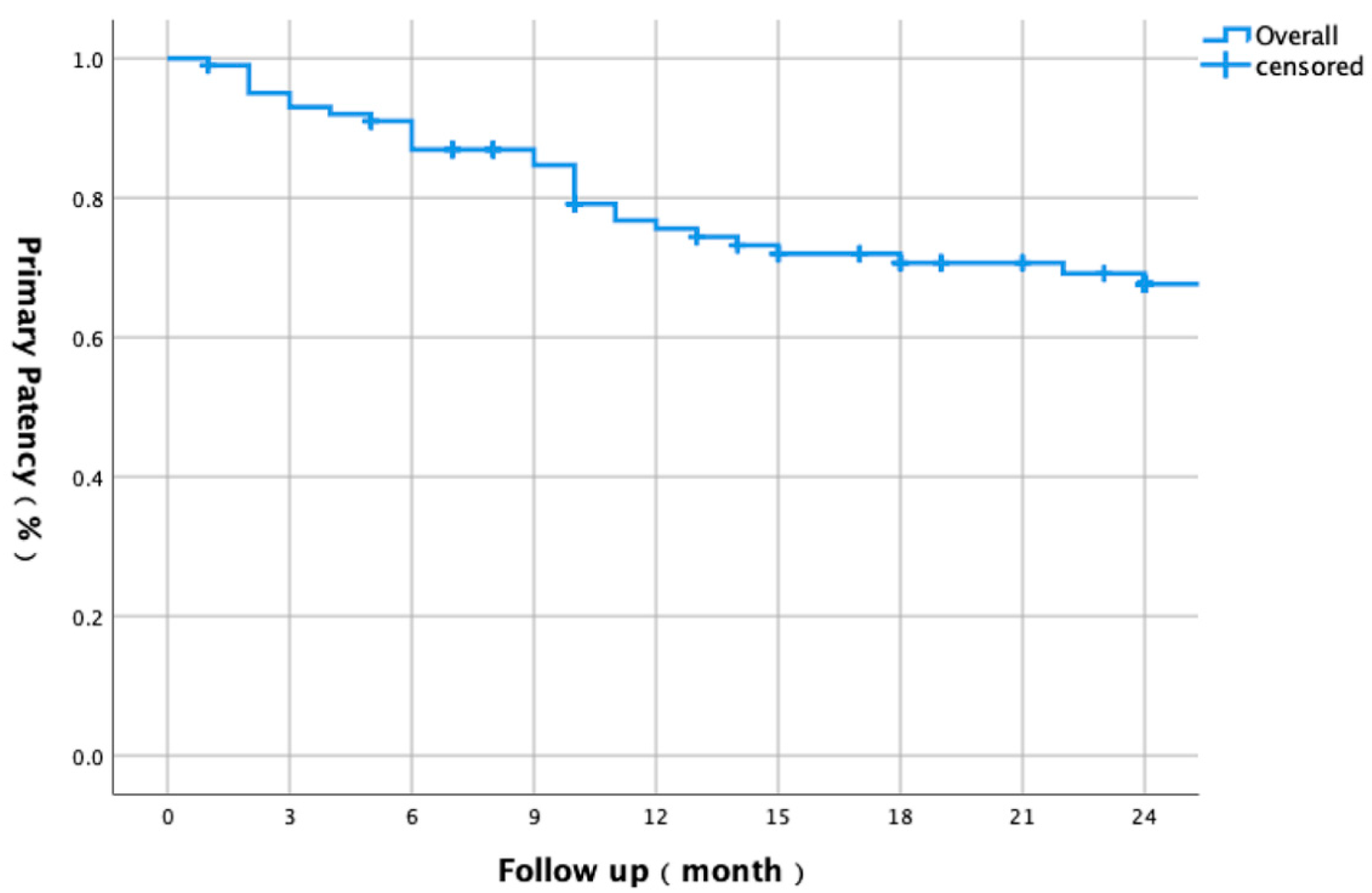
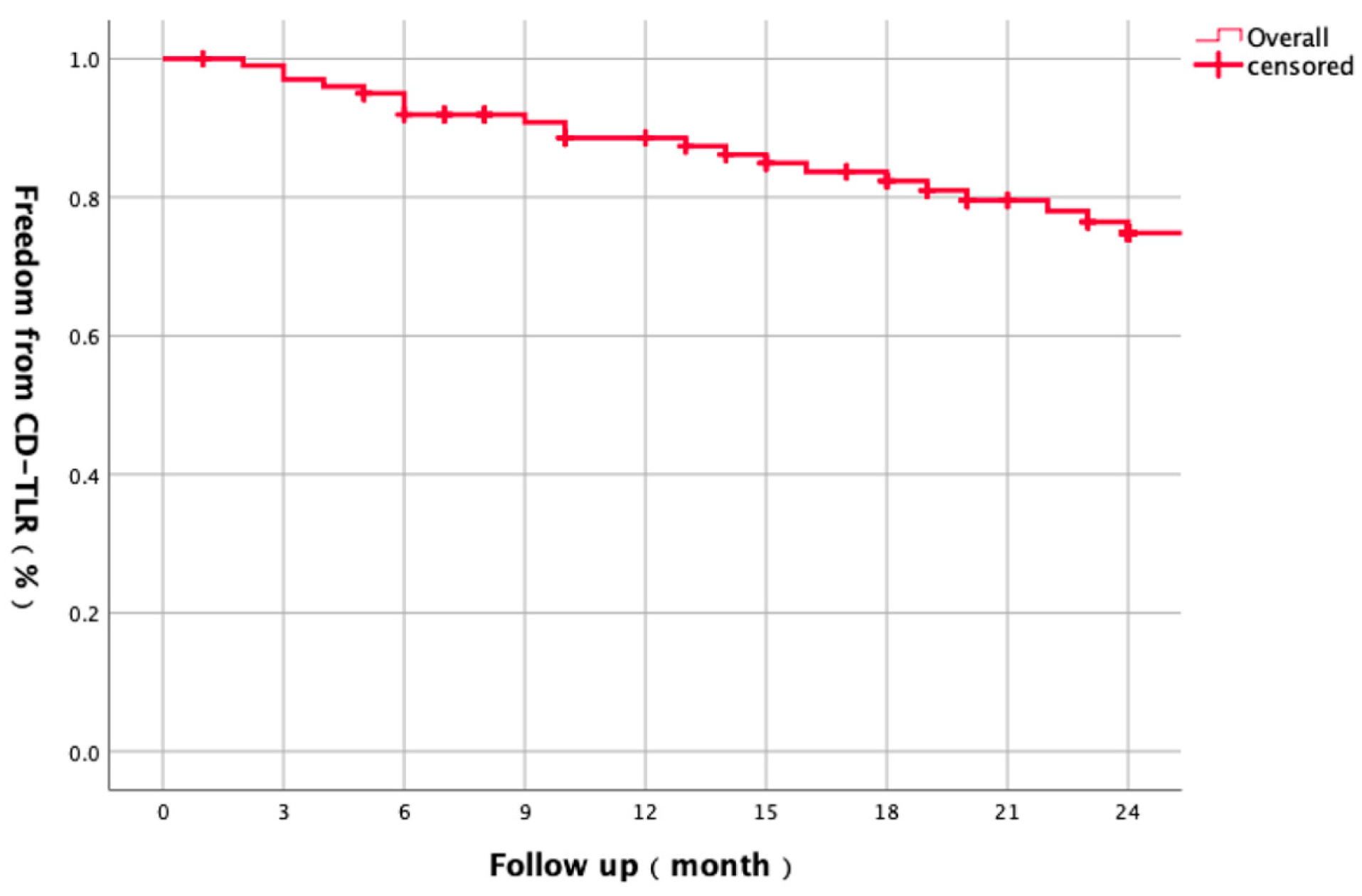
3.1. Baseline Characteristics and Overall Outcomes
3.2. Multivariate Cox Regression
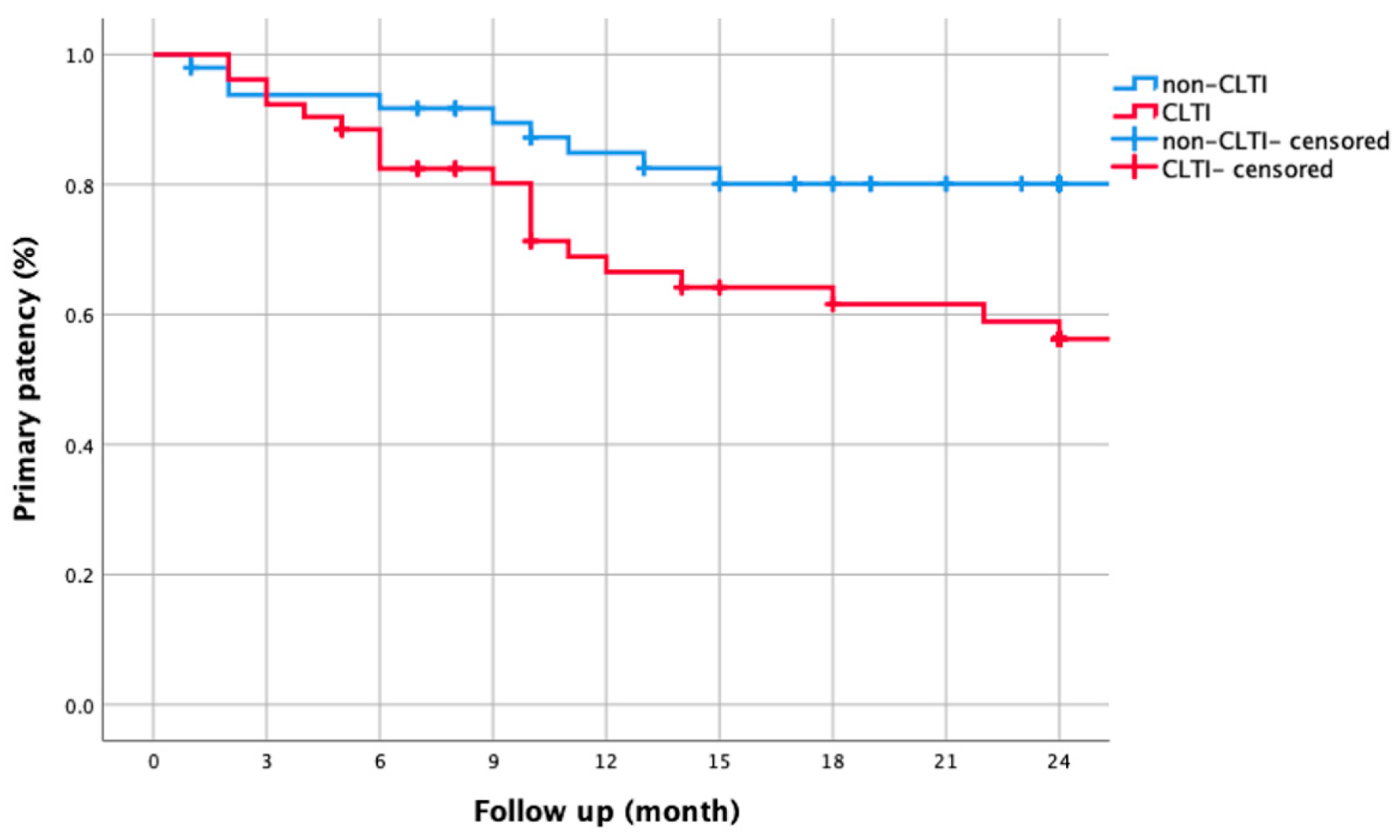
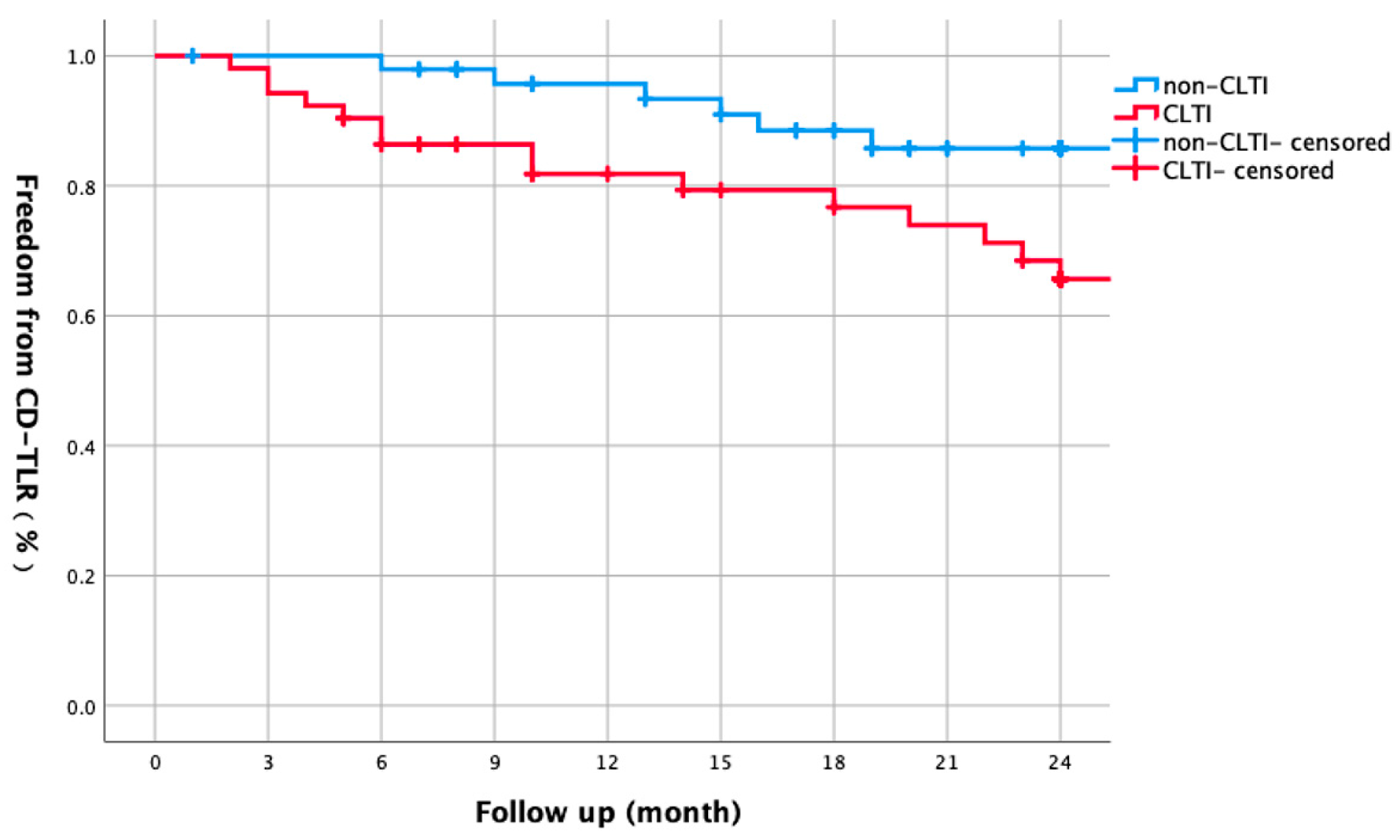
3.3. CLTI vs. Non-CLTI Comparisons
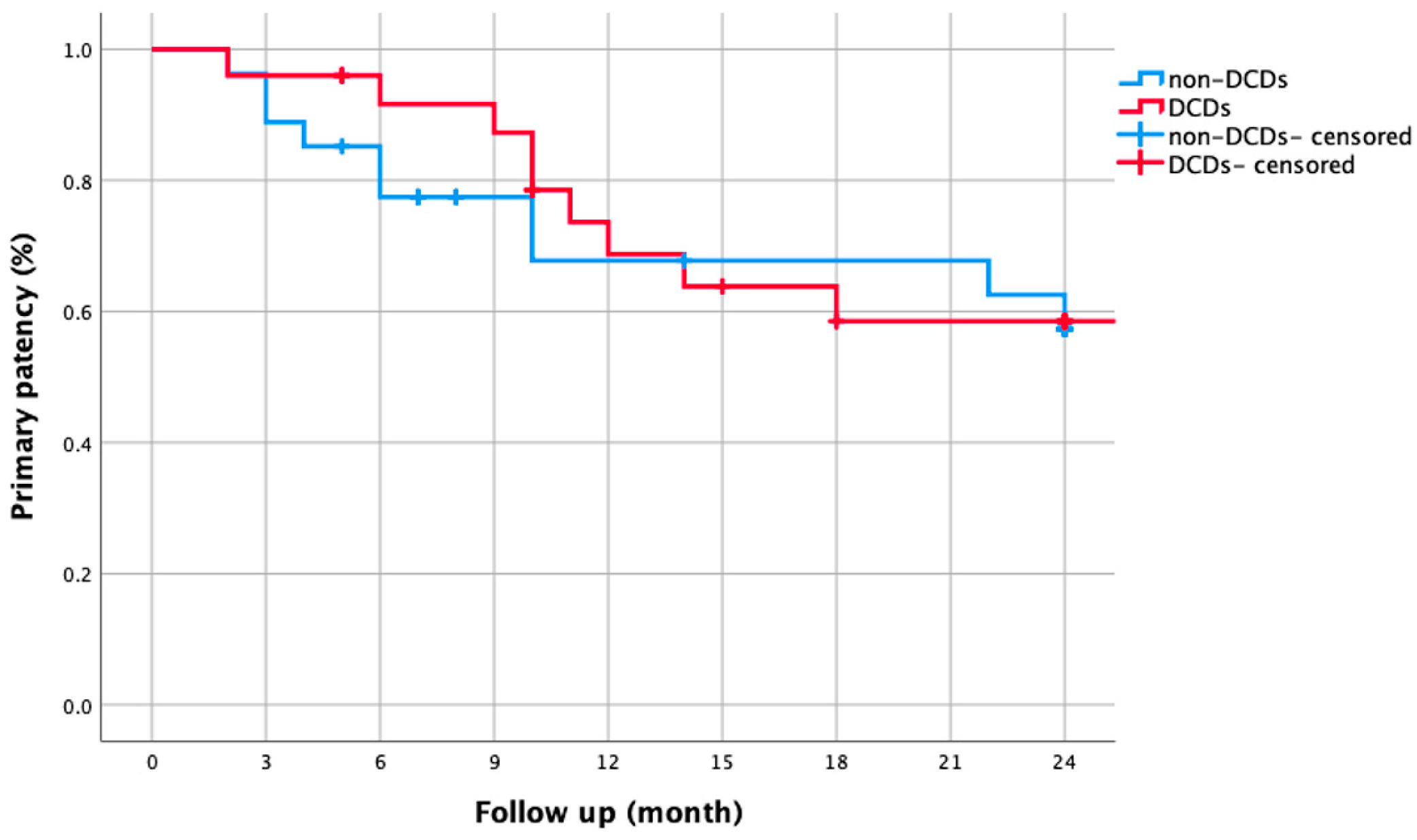
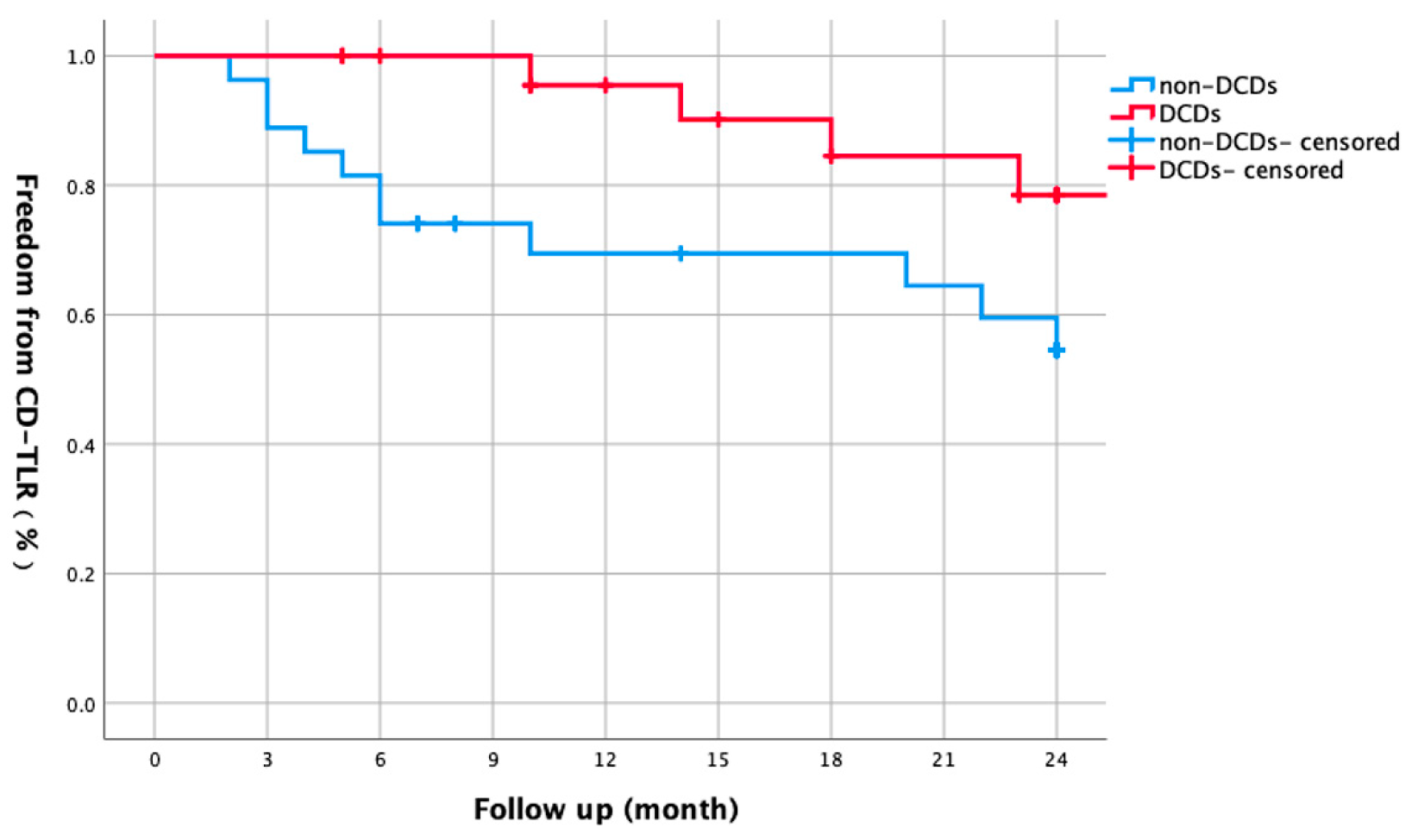
3.4. DCD Subgroup Analysis
3.5. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aiello, A.; Anichini, R.; Brocco, E.; Caravaggi, C.; Chiavetta, A.; Cioni, R.; Da Ros, R.; De Feo, M.; Ferraresi, R.; Florio, F.; et al. Treatment of peripheral arterial disease in diabetes: A consensus of the Italian Societies of Diabetes (SID, AMD), Radiology (SIRM) and Vascular Endovascular Surgery (SICVE). Nutr. Metab. Cardiovasc. Dis. 2014, 24, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Perera, G.B.; Lyden, S.P. Current trends in lower extremity revascularization. Surg. Clin. N. Am. 2007, 87, 1135–1147. [Google Scholar] [CrossRef]
- Morbach, S.; Furchert, H.; Gröblinghoff, U.; Hoffmeier, H.; Kersten, K.; Klauke, G.-T.; Klemp, U.; Roden, T.; Icks, A.; Haastert, B.; et al. Long-term prognosis of diabetic foot patients and their limbs: Amputation and death over the course of a decade. Diabetes Care 2012, 35, 2021–2027. [Google Scholar] [CrossRef]
- Choke, E.; Tang, T.Y.; Cheng, S.C.; Tay, J.S. Treatment of lower limb ischaemia in patients with diabetes. Diabetes/Metabolism Res. Rev. 2019, 36, e3262. [Google Scholar] [CrossRef]
- Liistro, F.; Porto, I.; Angioli, P.; Grotti, S.; Ricci, L.; Ducci, K.; Falsini, G.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): A randomized trial in diabetic patients with critical limb ischemia. Circulation 2013, 128, 615–621. [Google Scholar] [CrossRef]
- Micari, A.; Brodmann, M.; Keirse, K.; Peeters, P.; Tepe, G.; Frost, M.; Wang, H.; Zeller, T.; Torsello, G.; Scheinert, D.; et al. Drug-Coated Balloon Treatment of Femoropopliteal Lesions for Patients with Intermittent Claudication and Ischemic Rest Pain: 2-Year Results From the IN.PACT Global Study. JACC Cardiovasc. Interv. 2018, 11, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Reijnen, M.M.P.J.; van Wijck, I.; Zeller, T.; Micari, A.; Veroux, P.; Keirse, K.; Lee, S.-W.; Li, P.; Voulgaraki, D.; Holewijn, S. Outcomes After Drug-Coated Balloon Treatment of Femoropopliteal Lesions in Patients With Critical Limb Ischemia: A Post Hoc Analysis From the IN.PACT Global Study. J. Endovasc. Ther. 2019, 26, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kum, S.; Ipema, J.; Huizing, E.; Tan, Y.K.; Lim, D.; Lok, I.Y.; Hazenberg, C.E.; Ünlü, Ç. Outcomes of the paclitaxel-eluting Eluvia stent for long femoropopliteal lesions in Asian patients with predominantly chronic limb-threatening ischemia. Vasc. Med. 2021, 26, 267–272. [Google Scholar] [CrossRef]
- Rizzo, L.; D’Andrea, A.; Stella, N.; Orlando, P.; Taurino, M. The Influence of Diabetes Mellitus on the Outcome of Superficial Femoral Artery Recanalization is Debatable. Transl. Med. UniSa 2020, 21, 10–18. [Google Scholar]
- Pearce, B.J.; Toursarkissian, B. The current role of endovascular intervention in the management of diabetic peripheral arterial disease. Diabet. Foot Ankle 2012, 3, 18977. [Google Scholar] [CrossRef]
- Bracale, U.M.; Ammollo, R.P.; Hussein, E.A.; Hoballah, J.J.; Goeau-Brissonniere, O.; Taurino, M.; Setacci, C.; Pecoraro, F.; Bracale, G.; Giribono, A.M.; et al. Managing Peripheral Artery Disease in Diabetic Patients: A Questionnaire Survey from Vascular Centers of the Mediterranean Federation for the Advancing of Vascular Surgery (MeFAVS). Ann. Vasc. Surg. 2020, 64, 239–245. [Google Scholar] [CrossRef] [PubMed]
- DeRubertis, B.G.; Pierce, M.; Ryer, E.J.; Trocciola, S.; Kent, K.C.; Faries, P.L. Reduced primary patency rate in diabetic patients after percutaneous intervention results from more frequent presentation with limb-threatening ischemia. J. Vasc. Surg. 2008, 47, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.; Giri, J. Management of peripheral arterial disease patients: Comparing the ACC/AHA and TASC-II guidelines. Curr. Med. Res. Opin. 2008, 24, 2509–2522. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Suárez de Lezo, J.; Pan, M. Una clasificación simple de las lesiones coronarias en bifurcación (A new classification of coronary bifurcation lesions). Rev. Esp. Cardiol. 2006, 59, 183. [Google Scholar] [CrossRef]
- Iida, O.; Takahara, M.; Soga, Y.; Yamaoka, T.; Fujihara, M.; Kawasaki, D.; Ichihashi, S.; Kozuki, A.; Nanto, S.; Sakata, Y.; et al. 1-Year Outcomes of Fluoropolymer-Based Drug-Eluting Stent in Femoropopliteal Practice: Predictors of Restenosis and Aneurysmal Degeneration. JACC Cardiovasc. Interv. 2022, 15, 630–638. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, D.-S.; Tong, J.-J.; Shen, J. Efficacy of endoluminal interventional therapy in diabetic peripheral arterial occlusive disease: A retrospective trial. Cardiovasc. Diabetol. 2012, 11, 17. [Google Scholar] [CrossRef]
- Golzar, J.; Soga, Y.; Babaev, A.; Iida, O.; Kawasaki, D.; Bachinsky, W.; Park, J.; Prem, J.T.; Vermassen, F.; Diaz-Cartelle, J.; et al. Effectiveness and Safety of a Paclitaxel-Eluting Stent for Superficial Femoral Artery Lesions up to 190 mm: One-Year Outcomes of the Single-Arm IMPERIAL Long Lesion Substudy of the Eluvia Drug-Eluting Stent. J. Endovasc. Ther. 2020, 27, 296–303. [Google Scholar] [CrossRef]
- Soga, Y.; Fujihara, M.; Iida, O.; Kawasaki, D.; Hirano, K.; Yokoi, H.; Miyamoto, A.; Kichikawa, K.; Nakamura, M.; Ohki, T.; et al. Japanese Patients Treated in the IMPERIAL Randomized Trial Comparing Eluvia and Zilver PTX Stents. Cardiovasc. Interv. Radiol. 2019, 43, 215–222. [Google Scholar] [CrossRef]
- Müller-Hülsbeck, S.; Keirse, K.; Zeller, T.; Schroë, H.; Diaz-Cartelle, J. Long-Term Results from the MAJESTIC Trial of the Eluvia Paclitaxel-Eluting Stent for Femoropopliteal Treatment: 3-Year Follow-up. Cardiovasc. Interv. Radiol. 2017, 40, 1832–1838. [Google Scholar] [CrossRef]
- Müller-Hülsbeck, S.; Keirse, K.; Zeller, T.; Schroë, H.; Diaz-Cartelle, J. Twelve-Month Results from the MAJESTIC Trial of the Eluvia Paclitaxel-Eluting Stent for Treatment of Obstructive Femoropopliteal Disease. J. Endovasc. Ther. 2016, 23, 701–707. [Google Scholar] [CrossRef]
- Lyden, S.P.; Faries, P.L.; Niazi, K.A.K.; Sachar, R.; Jain, A.; Brodmann, M.; Werner, M.; Sood, A.; Krishnan, P. No Mortality Signal with Stellarex Low-Dose Paclitaxel DCB: ILLUMENATE Pivotal 4-Year Outcomes. J. Endovasc. Ther. 2022, 29, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Faries, P.; Niazi, K.; Sachar, R.; Jain, A.; Brodmann, M.; Werner, M.; Holden, A.; Tarricone, A.; Tarra, T.; et al. Stellarex Drug-Coated Balloon for the Treatment of Peripheral Artery Disease: Five-Year Results from the ILLUMENATE Pivotal Randomized Controlled Trial. Am. J. Cardiol. 2024, 227, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gouëffic, Y.; Torsello, G.; Zeller, T.; Esposito, G.; Vermassen, F.; Hausegger, K.A.; Tepe, G.; Thieme, M.; Gschwandtner, M.; Kahlberg, A.; et al. Efficacy of a Drug-Eluting Stent Versus Bare Metal Stents for Symptomatic Femoropopliteal Peripheral Artery Disease: Primary Results of the EMINENT Randomized Trial. Circulation 2022, 146, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Schroë, H.; Sachar, R.; Keirse, K.; Soga, Y.; Brodmann, M.; Rao, V.; Werner, M.; Holden, A.; Lopez, L.; Krishnan, P.; et al. The RANGER II superficial femoral artery trial: 1-year results of the long lesion cohort. Vasc. Med. 2022, 27, 457–465. [Google Scholar] [CrossRef]
- Sachar, R.; Soga, Y.; Ansari, M.M.; Kozuki, A.; Lopez, L.; Brodmann, M.; Schroë, H.; Ramanath, V.S.; Diaz-Cartelle, J.; Zeller, T. 1-Year Results from the RANGER II SFA Randomized Trial of the Ranger Drug-Coated Balloon. JACC Cardiovasc. Interv. 2021, 14, 1123–1133. [Google Scholar] [CrossRef]
- Ouriel, K.; Adelman, M.A.; Rosenfield, K.; Scheinert, D.; Brodmann, M.; Peña, C.; Geraghty, P.; Lee, A.; White, R.; Clair, D.G. Safety of Paclitaxel-Coated Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease. JACC Cardiovasc. Interv. 2019, 12, 2515–2524. [Google Scholar] [CrossRef]
- Scheinert, D.; Schmidt, A.; Zeller, T.; Müller-Hülsbeck, S.; Sixt, S.; Schröder, H.; Weiss, N.; Ketelsen, D.; Ricke, J.; Steiner, S.; et al. German Center Subanalysis of the LEVANT 2 Global Randomized Study of the Lutonix Drug-Coated Balloon in the Treatment of Femoropopliteal Occlusive Disease. J. Endovasc. Ther. 2016, 23, 409–416. [Google Scholar] [CrossRef]
| Variables | N = 98, legs = 101 |
|---|---|
| Age (years) | 72.11 ± 8.90 |
| Male [n (%)] | 74 (75.5%) |
| Follow-up duration (months) | 16.57 ± 10.25 |
| Comorbidities | |
| Hypertension [n(%)] | 83 (84.7%) |
| Smoking [n(%)] | 49 (50.0%) |
| Hyperlipidemia [n(%)] | 72 (73.5%) |
| Chronic renal insufficiency [n(%)] | 10 (10.2%) |
| Coronary heart disease [n(%)] | 21 (21.4%) |
| Clinical Parameters | |
| ABI * pre-procedure | 0.43 ± 1.40 |
| ABI * post-procedure | 0.81 ± 0.16 |
| Lesion length (cm) | 26.11 ± 9.47 |
| Claudication [n(%)] | 49 (49%) |
| CLTI * [n(%)] | 52 (51%) |
| Rest pain | 23 (22.5%) |
| Tissue loss | 29 (28.5%) |
| TASC * Classification [n(%)] | |
| A + B | 10 (10.1%) |
| C + D | 91 (91.9%) |
| Lesion Characteristics | |
| CTO * lesions [n(%)] | 88 (87.1%) |
| Stenosis [n(%)] | 12 (11.9%) |
| Runoff Grade [n(%)] | |
| 1 | 43 (42.6%) |
| 2 | 29 (28.7%) |
| 3 | 28 (27.7%) |
| Procedural Details | |
| Endovascular intervention [n(%)] | 96 (95.0%) |
| Hybrid procedure * [n(%)] | 5 (5.0%) |
| Concurrent BTK * outflow treatment [n(%)] | 31 (30.7%) |
| Stent procedure [n(%)] | 101 (100%) |
| Debulking device use [n(%)] | 2 (2.0%) |
| DCDs * use [n(%)] | 40 (39.6%) |
| Complications | |
| Total complications [n(%)] | 6 (5.9%) |
| MALE * | 4 (4.0%) |
| Hematoma | 1 (1.0%) |
| Cerebrovascular accident | 1 (1.0%) |
| Variable | B | SE | Wald | df | p-Value | HR | 95% CI |
|---|---|---|---|---|---|---|---|
| Gender | 0.109 | 0.564 | 0.037 | 1 | 0.847 | 1.115 | 0.369–3.365 |
| Smoking history | 0.469 | 0.506 | 0.86 | 1 | 0.354 | 1.599 | 0.593–4.314 |
| Hypertension | −0.021 | 0.594 | 0.001 | 1 | 0.972 | 0.979 | 0.305–3.138 |
| Hyperlipidemia | 0.056 | 0.439 | 0.016 | 1 | 0.898 | 1.058 | 0.448–2.499 |
| Coronary heart disease | 0.65 | 0.506 | 1.646 | 1 | 0.199 | 1.915 | 0.710–5.164 |
| Renal insufficiency | 0.811 | 0.624 | 1.69 | 1 | 0.194 | 2.25 | 0.662–7.644 |
| CLTI | 1.217 | 0.5 | 5.924 | 1 | 0.015 * | 3.375 | 1.267–8.990 |
| Procedural details | −0.261 | 0.852 | 0.094 | 1 | 0.76 | 0.771 | 0.145–4.091 |
| DCD usage | −0.645 | 0.524 | 1.515 | 1 | 0.218 | 0.525 | 0.188–1.465 |
| Concurrent treatment of BTK outflow | 0.228 | 0.461 | 0.244 | 1 | 0.621 | 1.256 | 0.509–3.097 |
| Outflow status | 0.265 | 0.457 | 0.336 | 1 | 0.562 | 1.303 | 0.532–3.190 |
| Type of lesions (stenosis or occlusion) | 0.263 | 0.89 | 0.087 | 1 | 0.768 | 1.301 | 0.227–7.447 |
| Lesion length(mm) | 0.037 | 0.024 | 2.515 | 1 | 0.113 | 1.038 | 0.991–1.087 |
| Variables | Non-CLTI (n = 49) | CLTI (n = 52) | Statistic | p-Value |
|---|---|---|---|---|
| Age (years) | 72.08 ± 9.43 | 72.13 ± 8.48 | T = −0.222 | 0.825 |
| Male [n(%)] | 41 (83.7%) | 36 (69.2%) | χ² = 3.128 | 0.077 |
| Follow-up duration (months) | 16.57 ± 10.25 | 18.32 ± 9.74 | T = −0.873 | 0.384 |
| Comorbidities | ||||
| Hypertension [n(%)] | 36 (73.5%) | 47 (90.4%) | χ² = 5.375 | 0.020 * |
| Smoking [n(%)] | 23 (46.9%) | 26 (50.0%) | χ² = 0.105 | 0.746 |
| Hyperlipidemia [n(%)] | 35 (71.4%) | 37 (71.2%) | χ² = 0.001 | 0.976 |
| Chronic renal insufficiency [n(%)] | 7 (14.3%) | 3 (5.8%) | χ² = 1.943 | 0.163 |
| Coronary heart disease [n(%)] | 9 (18.4%) | 12 (23.1%) | χ² = 0.375 | 0.54 |
| Lesion length (cm) | 24.29 ± 9.17 | 27.82 ± 9.52 | T = −1.902 | 0.06 |
| Device usage | ||||
| Debulking device [n(%)] | 1 (2.0%) | 2 (3.8%) | Fisher’s exact | 1 |
| DCD use [n(%)] | 15 (30.6%) | 25 (48.1%) | χ² = 3.629 | 0.057 |
| Lesion types [n(%)] | χ² = 1.795 | 0.18 | ||
| CTO lesions | 19 (38.8%) | 6 (11.5%) | ||
| Stenosis | 8 (16.3%) | 4 (7.7%) | ||
| Concurrent BTK outflow treatment [n(%)] | 13 (26.5%) | 18 (34.6%) | χ² = 0.889 | 0.346 |
| Run-off [n (%)] | χ² = 4.223 | 0.121 | ||
| 1 | 18 (36.7%) | 25 (48.1%) | ||
| 2 | 12 (24.5%) | 17 (32.7%) | ||
| 3 | 19 (38.8%) | 10 (19.2%) | ||
| Total complications [n(%)] | 0 (0%) | 6 (11.5%) | Fisher’s exact | 0.057 |
| MALE | 0 | 4 | ||
| Hematoma | 0 | 1 | ||
| Cerebrovascular accident | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Lv, F.; Huang, Y.; Chen, G.; Hong, S.; Hong, X.; Xie, X.; Lu, W.; Fu, W. Real-World Efficacy of Minimally Invasive Revascularization in Diabetic Foot Ischemia: Impact of Device Selection and Lesion-Specific Factors. Biomedicines 2025, 13, 1384. https://doi.org/10.3390/biomedicines13061384
Lin Y, Lv F, Huang Y, Chen G, Hong S, Hong X, Xie X, Lu W, Fu W. Real-World Efficacy of Minimally Invasive Revascularization in Diabetic Foot Ischemia: Impact of Device Selection and Lesion-Specific Factors. Biomedicines. 2025; 13(6):1384. https://doi.org/10.3390/biomedicines13061384
Chicago/Turabian StyleLin, Yue, Fanzhen Lv, Yulong Huang, Gang Chen, Shichai Hong, Xiang Hong, Xinsheng Xie, Weifeng Lu, and Weiguo Fu. 2025. "Real-World Efficacy of Minimally Invasive Revascularization in Diabetic Foot Ischemia: Impact of Device Selection and Lesion-Specific Factors" Biomedicines 13, no. 6: 1384. https://doi.org/10.3390/biomedicines13061384
APA StyleLin, Y., Lv, F., Huang, Y., Chen, G., Hong, S., Hong, X., Xie, X., Lu, W., & Fu, W. (2025). Real-World Efficacy of Minimally Invasive Revascularization in Diabetic Foot Ischemia: Impact of Device Selection and Lesion-Specific Factors. Biomedicines, 13(6), 1384. https://doi.org/10.3390/biomedicines13061384






