Comparative Study of Elevated CA19-9 Levels in Non-Gastrointestinal Tumors Patients: Evaluation of Different Immunoassay Methods and Analysis of Potential Interfering Factors
Abstract
1. Introduction
2. Methods
2.1. Sample Collection
2.2. CA19-9 Detection Platform
2.3. Interference Exclusion Experiment
2.4. Statistical Analysis
3. Results
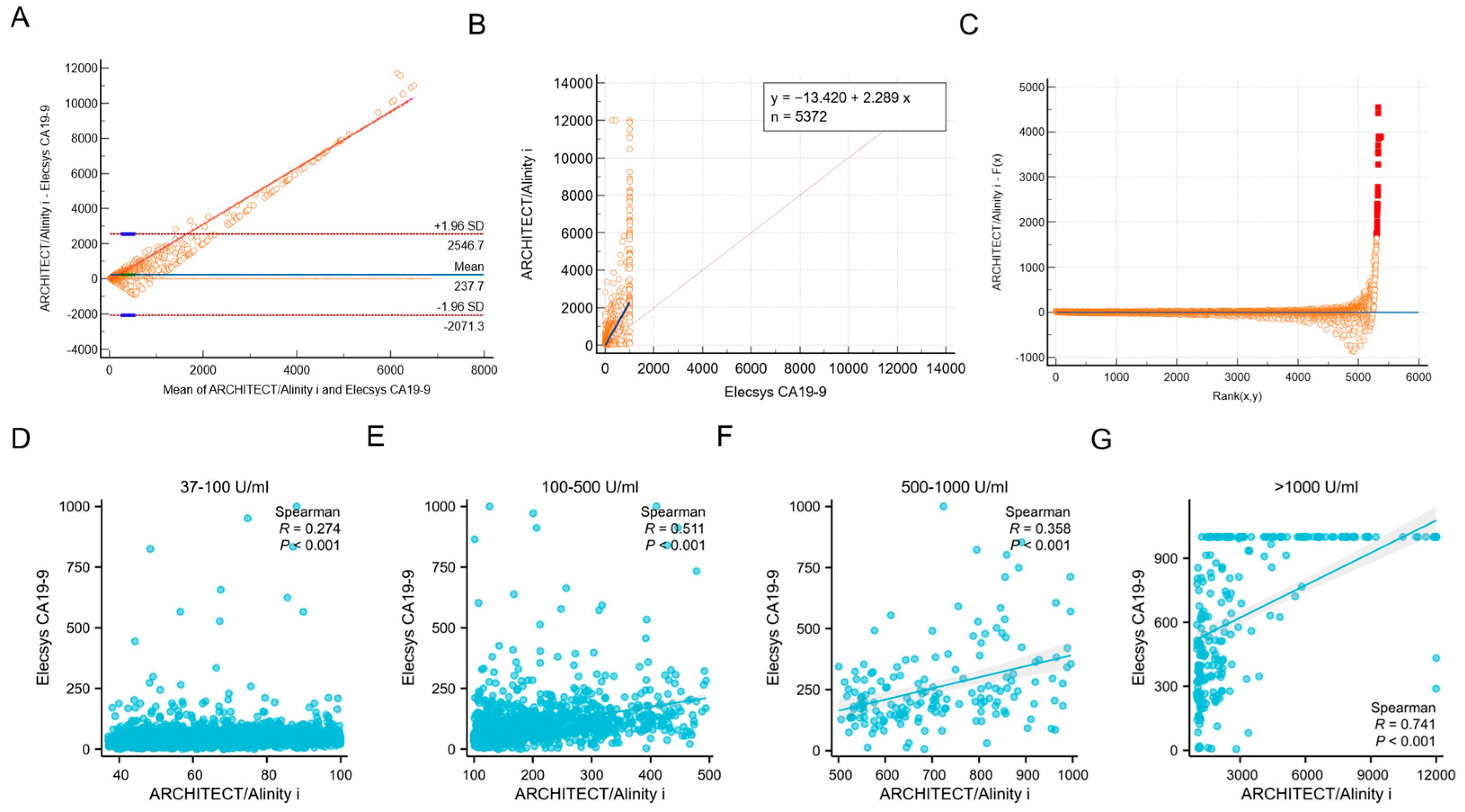
3.1. Consistency Analysis of ARCHITECT/Alinity I and Elecsys CA19-9 Assays
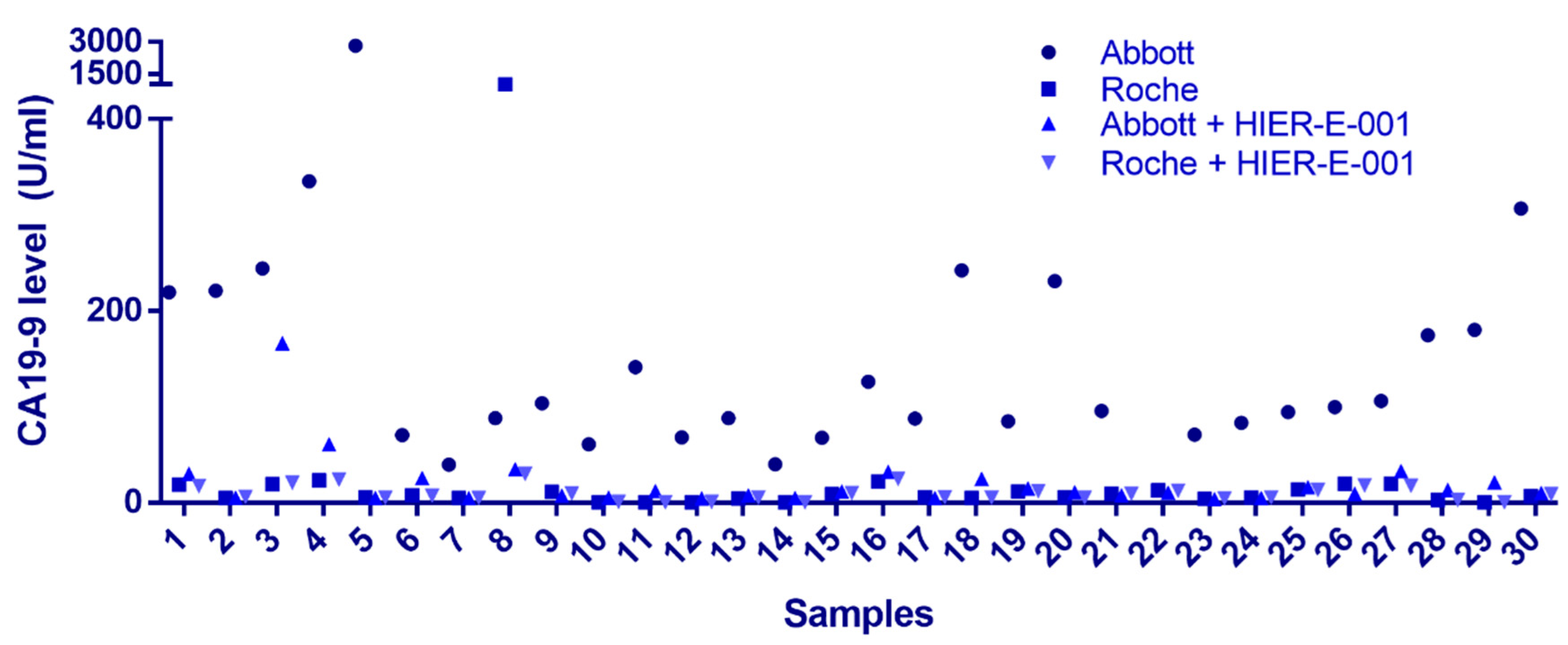
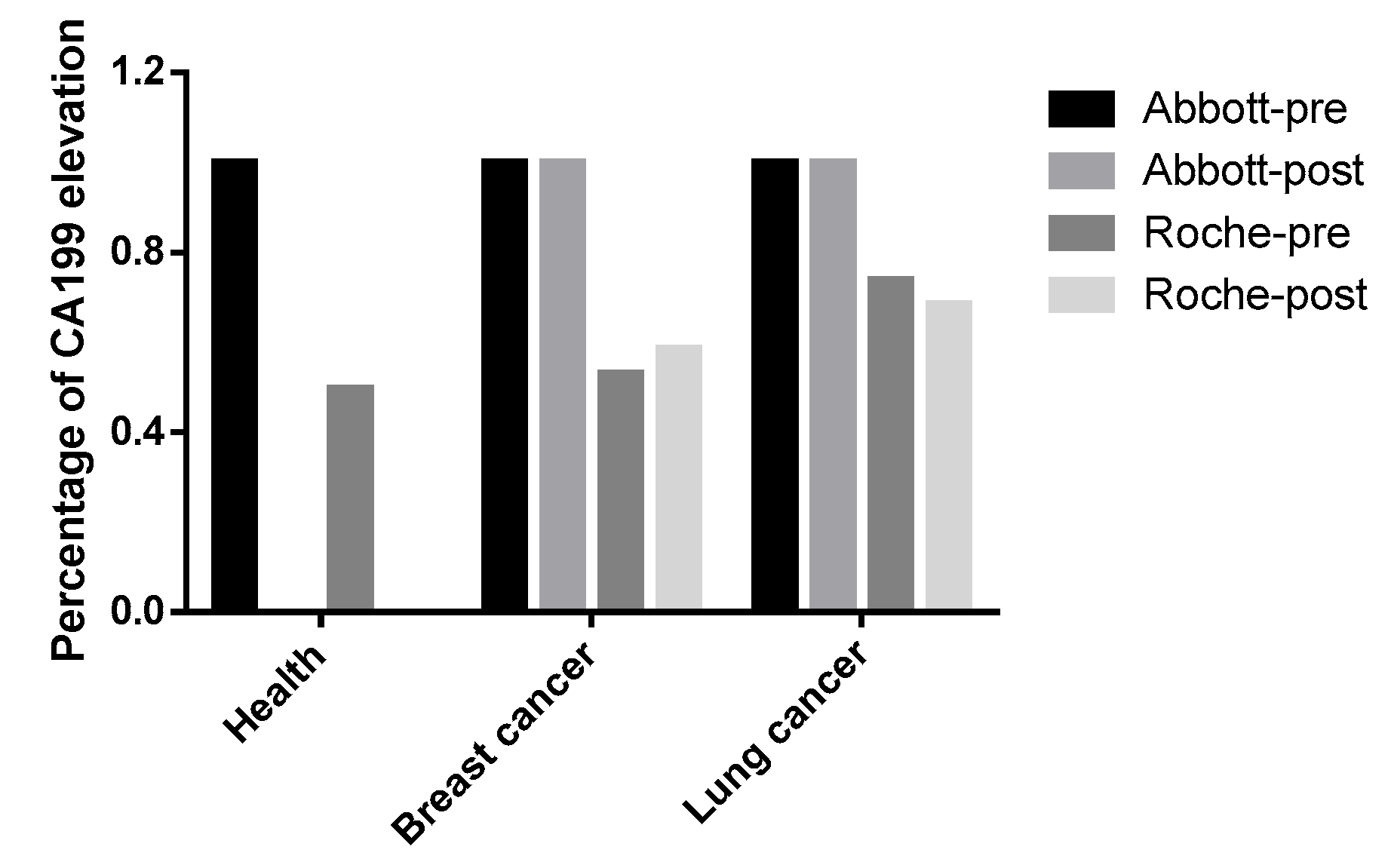
3.2. Identification of Endogenous Interfering Substances
3.3. Compliance Analysis of Results with Clinical Diagnosis
3.4. Assessment of Disease Risk Factors for Non-Specific Elevated CA19-9
3.5. Correlation Between Non-Specific Elevation of CA19-9 with Age
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, H.; Shen, K.; Li, B.; Li, R.; Wang, Z.; Xie, Z. Clinical significance and diagnostic value of serum NSE, CEA, CA19-9, CA125 and CA242 levels in colorectal cancer. Oncol. Lett. 2020, 20, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Lertkhachonsuk, A.A.; Buranawongtrakoon, S.; Lekskul, N.; Rermluk, N.; Wee-Stekly, W.W.; Charakorn, C. Serum CA19-9, CA-125 and CEA as tumor markers for mucinous ovarian tumors. J. Obstet. Gynaecol. Res. 2020, 46, 2287–2291. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Yao, C.; Wang, X.F.; Zhang, N.P.; Chen, Y.J.; Pan, D.; Zhao, G.P.; Shen, X.Z.; Wu, H.; Liu, T.T. Diagnostic and economic value of carcinoembryonic antigen, carbohydrate antigen 19-9, and carbohydrate antigen 72-4 in gastrointestinal cancers. World J. Gastroenterol. 2023, 29, 706–730. [Google Scholar] [CrossRef] [PubMed]
- Nakisa, A.; Sempere, L.F.; Chen, X.; Qu, L.T.; Woldring, D.; Crawford, H.C.; Huang, X. Tumor-Associated Carbohydrate Antigen 19-9 (CA 19-9), a Promising Target for Antibody-Based Detection, Diagnosis, and Immunotherapy of Cancer. ChemMedChem 2024, 19, e202400491. [Google Scholar] [CrossRef]
- Tang, J.; Ge, Q.M.; Huang, R.; Shu, H.Y.; Su, T.; Wu, J.L.; Pan, Y.C.; Liang, R.B.; Zhang, L.J.; Shao, Y.; et al. Clinical Significance of CYFRA21-1, AFP, CA-153, CEA, and CA-199 in the Diagnosis of Lung Cancer Ocular Metastasis in Hypertension Population. Front. Cardiovasc. Med. 2021, 8, 670594. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, Q.; Fei, X.; Li, C.; Li, B. Elevated tumor markers in a benign lung disease. J. Cardiothorac. Surg. 2021, 16, 308. [Google Scholar] [CrossRef]
- Wang, M.; Bu, H.; Luo, W.; Zeng, X.; Chen, G.; He, Y.; Cao, D. CA19-9, CEA and PIVKA-II as a novel panel of serum markers for diagnosis of pancreatic cancer. Clin. Biochem. 2025, 137, 110902. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Materon, E.M.; Furuta, R.H.M.; Wilson, D.; do Nascimento, G.F.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Oliveira, O.N., Jr.; Gonçalves, D. Screen-printed electrodes modified with carbon black and polyelectrolyte films for determination of cancer marker carbohydrate antigen 19-9. Mikrochim. Acta 2020, 187, 417. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Hu, B.; Guo, C.; Song, Y.; Jia, Q.; He, L.; Zhang, Z.; Fang, S. Bimetallic cerium and ferric oxides nanoparticles embedded within mesoporous carbon matrix: Electrochemical immunosensor for sensitive detection of carbohydrate antigen 19-9. Biosens. Bioelectron. 2019, 135, 22–29. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, G.C.; Abdel-Halim, E.S.; Zhang, J.R.; Zhu, J.J. Ultrasensitive photoelectrochemical immunoassay for CA19-9 detection based on CdSe@ZnS quantum dots sensitized TiO2NWs/Au hybrid structure amplified by quenching effect of Ab2@V2+ conjugates. Biosens. Bioelectron. 2016, 77, 339–346. [Google Scholar] [CrossRef]
- Sha, Y.; Guo, Z.; Chen, B.; Wang, S.; Ge, G.; Qiu, B.; Jiang, X. A one-step electrochemiluminescence immunosensor preparation for ultrasensitive detection of carbohydrate antigen 19-9 based on multi-functionalized graphene oxide. Biosens. Bioelectron. 2015, 66, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Passerini, R.; Cassatella, M.C.; Boveri, S.; Salvatici, M.; Radice, D.; Zorzino, L.; Galli, C.; Sandri, M.T. The pitfalls of CA19-9: Routine testing and comparison of two automated immunoassays in a reference oncology center. Am. J. Clin. Pathol. 2012, 138, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Berth, M.; Bosmans, E.; Everaert, J.; Dierick, J.; Schiettecatte, J.; Anckaert, E.; Delanghe, J. Rheumatoid factor interference in the determination of carbohydrate antigen 19-9 (CA 19-9). Clin. Chem. Lab. Med. 2006, 44, 1137–1139. [Google Scholar] [CrossRef]
- Bolstad, N.; Warren, D.J.; Nustad, K. Heterophilic antibody interference in immunometric assays. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 647–661. [Google Scholar] [CrossRef]
- Ward, G.; Simpson, A.; Boscato, L.; Hickman, P.E. The investigation of interferences in immunoassay. Clin. Biochem. 2017, 50, 1306–1311. [Google Scholar] [CrossRef]
- Weber, T.H.; Käpyaho, K.I.; Tanner, P. Endogenous interference in immunoassays in clinical chemistry. A review. Scand. J. Clin. Lab. Investig. Suppl. 1990, 201, 77–82. [Google Scholar] [CrossRef]
- Ghazal, K.; Brabant, S.; Prie, D.; Piketty, M.L. Hormone Immunoassay Interference: A 2021 Update. Ann. Lab. Med. 2022, 42, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Strum, S.; Vincent, M.; Gipson, M.; McArthur, E.; Breadner, D. Assessment of serum tumor markers CEA, CA-125, and CA19-9 as adjuncts in non-small cell lung cancer management. Oncotarget 2024, 15, 381–388. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Zhang, J.; Meng, Q.; Ma, L. Correlation of TBK1, AR, and other serum cancer-related biomarkers in breast cancer patients: An observational study. Medicine 2022, 101, e29996. [Google Scholar] [CrossRef]
- Ercan, Ş.; Kaymaz, Ö.; Yücel, N.; Orçun, A. Serum concentrations of CA 125, CA 15-3, CA 19-9 and CEA in normal pregnancy: A longitudinal study. Arch. Gynecol. Obstet. 2012, 285, 579–584. [Google Scholar] [CrossRef]
- La’ulu, S.L.; Roberts, W.L. Performance characteristics of five automated CA 19-9 assays. Am. J. Clin. Pathol. 2007, 127, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Deinzer, M.; Faissner, R.; Metzger, T.; Kaminski, W.E.; Löhr, M.; Neumaier, M.; Brinkmann, T. Comparison of two different methods for CA19-9 antigen determination. Clin. Lab. 2010, 56, 319–325. [Google Scholar] [PubMed]
- Serdarevic, N. The Comparison Between Different Immunoassays for Serum Carbohydrate Antigen (CA 19-9) Concentration Measurement. Acta Inform. Medica 2018, 26, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, G.D. Spurious Serum Hormone Immunoassay Results: Causes, Recognition, Management. touchREVIEWS Endocrinol. 2022, 18, 141–147. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Pan, Q.; Wang, Z.; Xing, R.; Li, W.; Zhang, J.; Ding, M.; Guo, J.; Wu, L.; et al. Identification and elimination of heterophilic antibody interference during antibody pair screening. Anal. Biochem. 2012, 430, 1–3. [Google Scholar] [CrossRef]
- Lavoie, S.; Caswell, D.; Gill, M.J.; Kadkhoda, K.; Charlton, C.L.; Levett, P.N.; Hatchette, T.; Garceau, R.; Maregmen, J.; Mazzulli, T.; et al. Heterophilic interference in specimens yielding false-reactive results on the Abbott 4th generation ARCHITECT HIV Ag/Ab Combo assay. J. Clin. Virol. 2018, 104, 23–28. [Google Scholar] [CrossRef]
- Choe, J.W. Approach to the Patients with Elevated CA 19-9. Korean J. Gastroenterol. 2023, 81, 185–188. [Google Scholar] [CrossRef]
- Ge, L.; Wang, S.; Liu, H.; Shi, X.; Shi, J.; Feng, R. Intralobar pulmonary sequestration with aspergillus infection and elevated serum CA19-9 and CA242: A case report. Transl. Cancer Res. 2021, 10, 1169–1176. [Google Scholar] [CrossRef]
- Hachiya, T.; Koyama, S.; Kubo, K.; Sekiguchi, M.; Honda, T. Elevated serum CA19-9 level and regional lymphadenopathy in a young man with allergic bronchopulmonary aspergillosis. Intern. Med. 1998, 37, 91–93. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Zhou, L.; Ye, A.; Zhu, Q. Changes of CA19-9 levels and related influencing factors in patients with type 2 diabetes mellitus after antidiabetic therapy. Sci. Rep. 2025, 15, 1264. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhou, Y.W.; Xie, X.Y.; Li, G.L.; Wang, X.H.; Li, F.Y.; Le, Z.A.; Zhong, Y.H.; Zhang, J. The levels of carbohydrate antigen 19-9 are associated with gender and age in Chinese population. Clin. Lab. 2013, 59, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Wu, L.W.; Hu, J.M.; Chang, P.K. Serum CA19-9 as a predictor of incident metabolic syndrome in obese middle-aged and older men: A 9-year cohort study. Cancer Biomark. 2025, 42, 18758592241296282. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, K.T.; Moon, T.G.; Kang, P.; Lee, J.K.; Kim, J.J.; Rhee, J.C. How do we interpret an elevated carbohydrate antigen 19-9 level in asymptomatic subjects? Dig. Liver Dis. 2009, 41, 364–369. [Google Scholar] [CrossRef]
- Partyka, K.; Maupin, K.A.; Brand, R.E.; Haab, B.B. Diverse monoclonal antibodies against the CA 19-9 antigen show variation in binding specificity with consequences for clinical interpretation. Proteomics 2012, 12, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Matsumoto, Y.; Dombek, G.E.; Stackhouse, K.A.; Ore, A.S.; Glickman, J.N.; Heimburg-Molinaro, J.; Cummings, R.D. Differential expression of CD175 and CA19-9 in pancreatic adenocarcinoma. Sci. Rep. 2025, 15, 4177. [Google Scholar] [CrossRef]
- Farley, A.M.; Braxton, D.R.; Li, J.; Trounson, K.; Sakar-Dey, S.; Nayer, B.; Ikeda, T.; Lau, K.X.; Hardikar, W.; Hasegawa, K.; et al. Antibodies to a CA 19-9 Related Antigen Complex Identify SOX9 Expressing Progenitor Cells In Human Foetal Pancreas and Pancreatic Adenocarcinoma. Sci. Rep. 2019, 9, 2876. [Google Scholar] [CrossRef] [PubMed]
- Lima-Oliveira, G.; Volanski, W.; Lippi, G.; Picheth, G.; Guidi, G.C. Pre-analytical phase management: A review of the procedures from patient preparation to laboratory analysis. Scand. J. Clin. Lab. Investig. 2017, 77, 153–163. [Google Scholar] [CrossRef]
- Plebani, M. Errors in clinical laboratories or errors in laboratory medicine? Clin. Chem. Lab. Med. 2006, 44, 750–759. [Google Scholar] [CrossRef]
- Ismail, A.A. Interference from endogenous antibodies in automated immunoassays: What laboratorians need to know. J. Clin. Pathol. 2009, 62, 673–678. [Google Scholar] [CrossRef]


| Disease | Number | χ2 Value | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Diabetes | 257 | 11.96 | 0.0005 | 1.671 (1.252–2.230) |
| Cerebral Infarction | 81 | 2.685 | 0.101 | 1.506 (0.920–2.465) |
| Chronic Gastritis | 122 | 0.130 | 0.719 | 1.072 (0.733–1.568) |
| Pulmonary Infection | 90 | 9.886 | 0.0017 | 2.354 (1.384–4.003) |
| Breast Nodules | 71 | 9.858 | 0.0017 | 0.466 (0.291–0.745) |
| Ovarian Cysts | 144 | 0.036 | 0.845 | 1.052 (0.742–1.492) |
| Uterine Leiomyoma | 113 | 9.883 | 0.0017 | 0.544 (0.375–0.790) |
| Gender | Positive Count | Total | Positive Rate |
|---|---|---|---|
| Male | 1419 | 1994 | 71.16% |
| Female | 2051 | 3378 | 61.72% |
| Total | 3470 | 5372 | |
| χ2 value | 59.38 | ||
| p-value | <0.0001 |
| Age | Male Positive Rate | Female Positive Rate | χ2 Value | p-Value |
|---|---|---|---|---|
| 0–20 | 58.82% (10/17) | 46.15% (36/78) | 0.190 | 0.663 |
| >20–30 | 37.97% (30/79) | 51.30% (198/386) | 4.656 | 0.031 |
| >30–40 | 52.87% (92/174) | 51.28% (321/626) | 0.139 | 0.710 |
| >40–50 | 65.04% (173/266) | 54.00% (466/863) | 10.090 | 0.002 |
| >50–60 | 69.11% (311/450) | 65.77% (442/672) | 1.360 | 0.244 |
| >60–70 | 78.97% (383/485) | 75.12% (305/406) | 1.858 | 0.173 |
| >70–80 | 79.51% (295/371) | 80.58% (195/242) | 0.103 | 0.748 |
| >80 | 82.23% (125/152) | 83.81% (88/105) | 0.108 | 0.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, W.; Tang, S.; Wu, R.; Wang, Y.; Shao, F. Comparative Study of Elevated CA19-9 Levels in Non-Gastrointestinal Tumors Patients: Evaluation of Different Immunoassay Methods and Analysis of Potential Interfering Factors. Biomedicines 2025, 13, 1386. https://doi.org/10.3390/biomedicines13061386
Liu Y, Li W, Tang S, Wu R, Wang Y, Shao F. Comparative Study of Elevated CA19-9 Levels in Non-Gastrointestinal Tumors Patients: Evaluation of Different Immunoassay Methods and Analysis of Potential Interfering Factors. Biomedicines. 2025; 13(6):1386. https://doi.org/10.3390/biomedicines13061386
Chicago/Turabian StyleLiu, Yangyang, Wenxuan Li, Shaoxi Tang, Ruihao Wu, Yumin Wang, and Fanggui Shao. 2025. "Comparative Study of Elevated CA19-9 Levels in Non-Gastrointestinal Tumors Patients: Evaluation of Different Immunoassay Methods and Analysis of Potential Interfering Factors" Biomedicines 13, no. 6: 1386. https://doi.org/10.3390/biomedicines13061386
APA StyleLiu, Y., Li, W., Tang, S., Wu, R., Wang, Y., & Shao, F. (2025). Comparative Study of Elevated CA19-9 Levels in Non-Gastrointestinal Tumors Patients: Evaluation of Different Immunoassay Methods and Analysis of Potential Interfering Factors. Biomedicines, 13(6), 1386. https://doi.org/10.3390/biomedicines13061386






