Normal Blood Flow in Rat Abdominal Aorta: An Ultrasound Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ultrasound Diagnostics
2.3. The Studied Ultrasound Parameters
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future perspectives in small-diameter vascular graft engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Légaré, J.F.; Nanton, M.A.; Bryan, P.; Lee, T.D.; Ross, D.B. Aortic valve graft implantation in rats: A new functional model. J. Thorac. Cardiovasc. Surg. 2000, 120, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Dokuchaeva, A.A.; Mochalova, A.B.; Timchenko, T.P.; Podolskaya, K.S.; Pashkovskaya, O.A.; Karpova, E.V.; Ivanov, I.A.; Filatova, N.A.; Zhuravleva, I.Y. In Vivo Evaluation of PCL Vascular Grafts Implanted in Rat Abdominal Aorta. Polymers 2022, 14, 3313. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.M.; Yu, L.; Song, S.H.; Woo, H.M.; Kwak, H.H. Vascular reconstruction: A major challenge in developing a functional whole solid organ graft from decellularized organs. Acta Biomater. 2020, 103, 68–80. [Google Scholar] [CrossRef]
- Cuenca, J.P.; Kang, H.J.; Fahad, M.A.A.; Park, M.; Choi, M.; Lee, H.Y.; Lee, B.T. Physico-mechanical and biological evaluation of heparin/VEGF-loaded electrospun polycaprolactone/decellularized rat aorta extracellular matrix for small-diameter vascular grafts. J. Biomater. Sci. Polym. Ed. 2022, 33, 1664–1684. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Kayumov, M.; Kwak, Y.; Cho, H.J.; Park, C.H.; Park, J.K.; Jeong, Y.J.; Lee, D.W.; Kim, D.W.; Jeong, I.S. Rapid remodeling observed at mid-term in-vivo study of a smart reinforced acellular vascular graft implanted on a rat model. J. Biol. Eng. 2023, 17, 1. [Google Scholar] [CrossRef]
- Liu, J.X.; Fang, C.L.; Zhang, K.; Ma, R.F.; Zhou, H.S.; Chen, L.; Wang, Q.L.; Lu, Y.X.; Wang, T.H.; Xiong, L.L. Transcranial Doppler Ultrasonography detection on cerebrovascular flow for evaluating neonatal hypoxic-ischemic encephalopathy modeling. Front. Neurosci. 2023, 17, 962001. [Google Scholar] [CrossRef]
- Scholz, A.M.; Bünger, L.; Kongsro, J.; Baulain, U.; Mitchell, A.D. Non-invasive methods for the determination of body and carcass composition in livestock: Dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: Invited review. Animal 2015, 9, 1250–1264. [Google Scholar] [CrossRef]
- Corvino, A.; Catalano, O.; de Magistris, G.; Corvino, F.; Giurazza, F.; Raffaella, N.; Vallone, G. Usefulness of doppler techniques in the diagnosis of peripheral iatrogenic pseudoaneurysms secondary to minimally invasive interventional and surgical procedures: Imaging findings and diagnostic performance study. J. Ultrasound 2020, 23, 563–573. [Google Scholar] [CrossRef]
- McCall, J.R.; Santibanez, F.; Belgharbi, H.; Pinton, G.F.; Dayton, P.A. Non-invasive transcranial volumetric ultrasound localization microscopy of the rat brain with continuous, high volume-rate acquisition. Theranostics 2023, 13, 1235. [Google Scholar] [CrossRef]
- Mynard, J.P.; Kondiboyina, A.; Kowalski, R.; Cheung, M.M.; Smolich, J.J. Measurement, analysis and interpretation of pressure/flow waves in blood vessels. Front. Physiol. 2020, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Hopper, S.E.; Cuomo, F.; Ferruzzi, J.; Burris, N.S.; Roccabianca, S.; Humphrey, J.D.; Figueroa, C.A. Comparative study of human and murine aortic biomechanics and hemodynamics in vascular aging. Front. Physiol. 2021, 12, 746796. [Google Scholar] [CrossRef] [PubMed]
- Andreollo, N.A.; Santos, E.F.D.; Araújo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? ABCD Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef]
- Koch, S.E.; de Kort, B.J.; Holshuijsen, N.; Brouwer, H.F.M.; van der Valk, D.C.; Dankers, P.Y.W.; van Luijk, J.A.K.R.; Hooijmans, C.R.; de Vries, R.B.M.; Bouten, C.V.C.; et al. Animal studies for the evaluation of in situ tissue-engineered vascular grafts—A systematic review, evidence map, and meta-analysis. NPJ Regen. Med. 2022, 7, 17. [Google Scholar] [CrossRef]
- Boon, J.A. Two-Dimensional and M-Mode Echocardiography for the Small Animal Practitioner, 2nd ed.; Willey Blackwell: Philadelphia, PA, USA, 2017; pp. 1–125. [Google Scholar]
- Van Daele, M.; Cooper, S.L.; Pannucci, P.; Wragg, E.S.; March, J.; de Jong, I.; Woolard, J. Monitoring haemodynamic changes in rodent models to better inform safety pharmacology: Novel insights from in vivo studies and waveform analysis. JRSM Cardiovasc. Dis. 2022, 11, 20480040221092893. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xie, M.; Qiu, H. The progress of advanced ultrasonography in assessing aortic stiffness and the application discrepancy between humans and rodents. Diagnostics 2021, 11, 454. [Google Scholar] [CrossRef]
- Dawson, J.A.; Morley, C.J. Monitoring oxygen saturation and heart rate in the early neonatal period. Semin. Fetal Neonatal Med. 2010, 15, 203–207. [Google Scholar] [CrossRef]
- Disatian, S.; Bright, J.M.; Boon, J. Association of age and heart rate with pulsed-wave Doppler measurements in healthy, nonsedated cats. J. Vet. Intern. Med. 2008, 22, 351–356. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Jadidi, M.; Habibnezhad, M.; Anttila, E.; Maleckis, K.; Desyatova, A.; MacTaggart, J.; Kamenskiy, A. Mechanical and structural changes in human thoracic aortas with age. Acta Biomater 2020, 103, 172–188. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Harrison, D.G.; Figueroa, C.A.; Lacolley, P.; Laurent, S. Central artery stiffness in hypertension and aging: A problem with cause and consequence. Circ. Res. 2016, 118, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Rizzoni, M.; Nardin, M.; Chiarini, G.; Agabiti-Rosei, C.; Aggiusti, C.; Paini, A.; Salvetti, M.; Muiesan, M.L. Vascular aging and disease of the small vessels. High. Blood Press. Cardiovasc. Prev. 2019, 26, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial dysfunction in obesity-induced inflammation: Molecular mechanisms and clinical implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Giudici, A.; Wilkinson, I.B.; Khir, A.W. Review of the techniques used for investigating the role elastin and collagen play in arterial wall mechanics. IEEE Rev. Biomed. Eng. 2020, 14, 256–269. [Google Scholar] [CrossRef]
- Halsey, G.; Sinha, D.; Dhital, S.; Wang, X.; Vyavahare, N. Role of elastic fiber degradation in disease pathogenesis. BBA-Mol. Basis Dis. 2023, 1869, 166706. [Google Scholar] [CrossRef]
- Trębacz, H.; Barzycka, A. Mechanical properties and functions of elastin: An overview. Biomolecules 2023, 13, 574. [Google Scholar] [CrossRef]
- Cai, Z.; Gong, Z.; Li, Z.; Li, L.; Kong, W. Vascular extracellular matrix remodeling and hypertension. Antioxid. Redox Signal. 2021, 34, 765–783. [Google Scholar] [CrossRef]
- Atkinson, J. Elastin, calcium and age-related stiffening of the arterial wall. In Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases, 1st ed.; Safar, M., O’Rourke, M., Frohlich, E., Eds.; Springer: London, UK, 2014; pp. 75–81. [Google Scholar] [CrossRef]
- Sutton, N.R.; Malhotra, R.; St Hilaire, C.; Aikawa, E.; Blumenthal, R.S.; Gackenbach, G.; Goyal, P.; Johnson, A.; Nigwekar, S.; Shanahan, C.; et al. Molecular mechanisms of vascular health: Insights from vascular aging and calcification. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 15–29. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Castelli, R.; Gidaro, A.; Casu, G.; Merella, P.; Profili, N.I.; Donadoni, M.; Maioli, M.; Delitala, A.P. Aging of the arterial system. Int. J. Mol. Sci. 2023, 24, 6910. [Google Scholar] [CrossRef]
- Roosens, B.; Bala, G.; Droogmans, S.; Hostens, J.; Somja, J.; Delvenne, E.; Lahoutte, T.; Van Camp, G.; Cosyns, B. Occurrence of cardiovascular calcifications in normal, aging rats. Exp. Gerontol. 2012, 47, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Fabricio, M.F.; Jordão, M.T.; Miotto, D.S.; Ruiz, T.F.; Vicentini, C.A.; Lacchini, S.; Santos, C.F.; Michelini, L.C.; Amaral, S.L. Standardization of a new non-invasive device for assessment of arterial stiffness in rats: Correlation with age-related arteries’ structure. MethodsX 2020, 7, 100901. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular stiffness in aging and disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, A.; Matus, K.; Mammoto, T. Extracellular matrix in aging aorta. Front. Cell Dev. Biol. 2022, 10, 822561. [Google Scholar] [CrossRef]

| Age | Body Weight, g ± m |
|---|---|
| 2 months (8 weeks) | 126 ± 3.47 |
| 3 months (12 weeks) | 214 ± 4.48 |
| 4 months (16 weeks) | 304 ± 4.6 |
| 5 months (20 weeks) | 430 ± 13.43 |
| 6 months (24 weeks) | 530 ± 21 |
| 24 months (104.29 weeks) | 742 ± 8.09 |
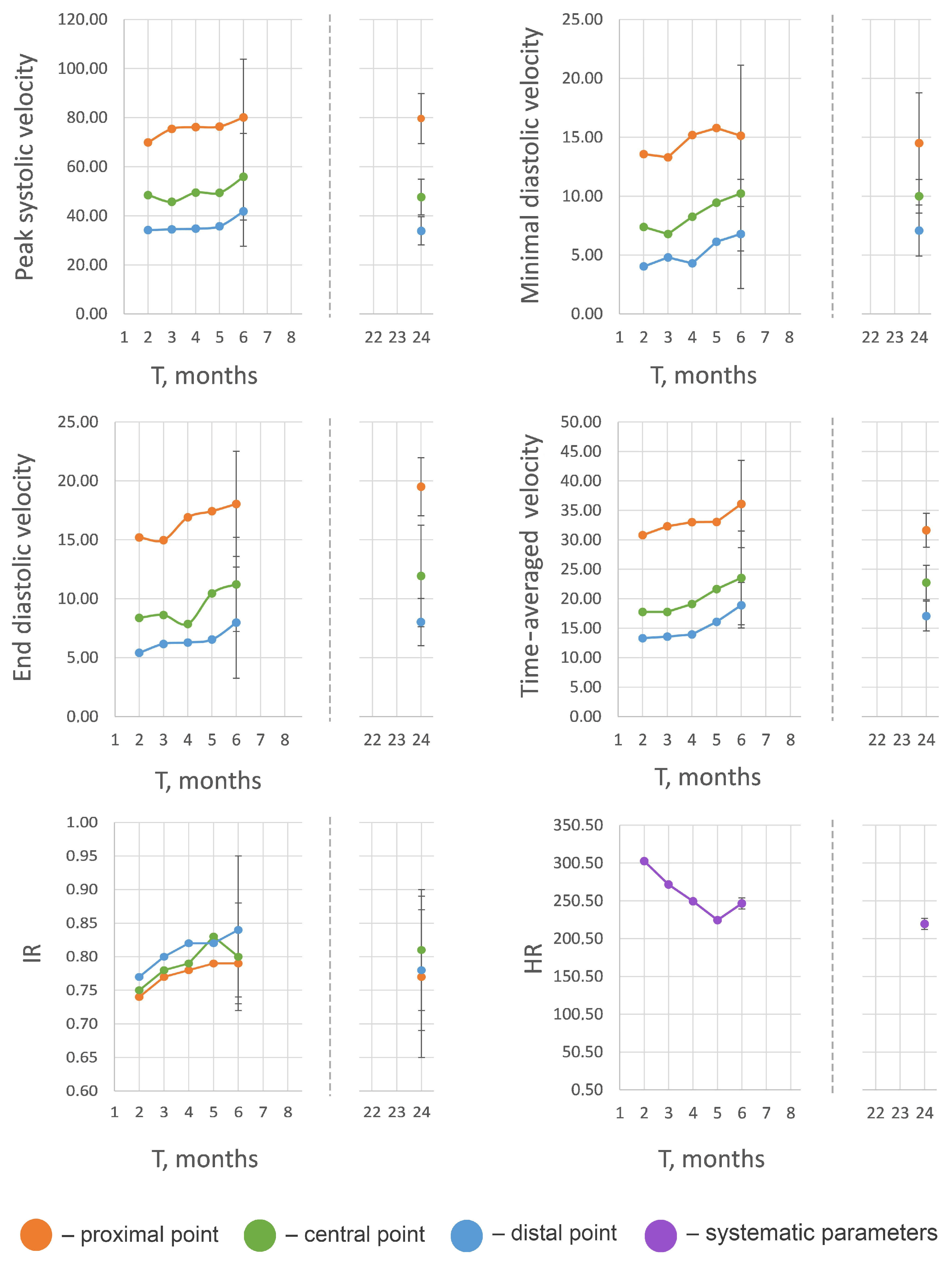
| Parameter * | Investigation Area | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | 2 Years |
|---|---|---|---|---|---|---|---|
| PSV | Proximal | 69.88 ± 13.45 | 75.41 ± 17.33 | 76.14 ± 15.75 | 76.37 ± 18.87 | 80.11 ± 23.66 | 79.60 ± 10.20 |
| Central | 48.45 ± 10.12 | 45.71 ± 10.94 | 49.46 ± 12.48 | 49.38 ± 11.94 | 55.89 ± 17.63 | 47.62 ± 7.24 | |
| Distal | 34.16 ± 7.38 | 34.51 ± 6.58 | 34.76 ± 8.27 | 35.72 ± 7.71 | 41.81 ± 14.24 | 33.85 ± 5.74 | |
| EDV | Proximal | 15.21 ± 2.90 | 14.98 ± 4.78 | 16.91 ± 3.25 | 17.43 ± 3.50 | 18.05 ± 4.46 | 19.51 ± 2.46 |
| Central | 8.38 ± 3.73 | 8.62 ± 4.49 | 7.86 ± 4.71 | 10.46 ± 4.86 | 11.22 ± 4.92 | 11.94 ± 4.31 | |
| Distal | 5.41 ± 3.27 | 6.17 ± 3.50 | 6.29 ± 6.20 | 6.54 ± 4.61 | 7.98 ± 4.72 | 8.02 ± 2.01 | |
| MDV | Proximal | 13.57 ± 2.65 | 13.30 ± 6.09 | 15.19 ± 4.17 | 15.78 ± 4.16 | 15.12 ± 6.57 | 14.97 ± 4.28 |
| Central | 7.38 ± 3.44 | 6.78 ± 4.28 | 8.26 ± 3.86 | 9.45 ± 4.42 | 10.23 ± 4.88 | 9.99 ± 1.42 | |
| Distal | 4.03 ± 3.34 | 4.80 ± 3.48 | 4.29 ± 3.04 | 6.13 ± 4.44 | 6.79 ± 4.63 | 7.08 ± 2.17 | |
| TAV | Proximal | 30.80 ± 3.66 | 32.32 ± 5.66 | 33.02 ± 5.08 | 33.05 ± 5.62 | 36.08 ± 7.41 | 31.64 ± 2.88 |
| Central | 17.78 ± 4.77 | 17.78 ± 4.28 | 19.10 ± 4.01 | 21.66 ± 5.58 | 23.54 ± 7.94 | 22.75 ± 2.93 | |
| Distal | 13.31 ± 2.25 | 13.59 ± 3.88 | 13.95 ± 2.86 | 16.07 ± 5.07 | 18.91 ± 3.86 | 17.07 ± 2.53 | |
| RI | Proximal | 0.74 ± 0.05 | 0.77 ± 0.05 | 0.78 ± 0.07 | 0.79 ± 0.08 | 0.79 ± 0.05 | 0.77 ± 0.12 |
| Central | 0.75 ± 0.10 | 0.78 ± 0.13 | 0.79 ± 0.05 | 0.83 ± 0.11 | 0.80 ± 0.08 | 0.81 ± 0.09 | |
| Distal | 0.77 ± 0.12 | 0.80 ± 0.15 | 0.82 ± 0.08 | 0.82 ± 0.11 | 0.84 ± 0.11 | 0.78 ± 0.09 | |
| HR, bpm | 303 ± 7.81 | 272 ± 5.13 | 250 ± 23.43 | 225 ± 5.69 | 247 ± 5.20 | 220 ± 5.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dokuchaeva, A.A.; Podolskaya, K.S.; Zhuravleva, I.Y. Normal Blood Flow in Rat Abdominal Aorta: An Ultrasound Study. Biomedicines 2025, 13, 1385. https://doi.org/10.3390/biomedicines13061385
Dokuchaeva AA, Podolskaya KS, Zhuravleva IY. Normal Blood Flow in Rat Abdominal Aorta: An Ultrasound Study. Biomedicines. 2025; 13(6):1385. https://doi.org/10.3390/biomedicines13061385
Chicago/Turabian StyleDokuchaeva, Anna A., Kseniya S. Podolskaya, and Irina Yu. Zhuravleva. 2025. "Normal Blood Flow in Rat Abdominal Aorta: An Ultrasound Study" Biomedicines 13, no. 6: 1385. https://doi.org/10.3390/biomedicines13061385
APA StyleDokuchaeva, A. A., Podolskaya, K. S., & Zhuravleva, I. Y. (2025). Normal Blood Flow in Rat Abdominal Aorta: An Ultrasound Study. Biomedicines, 13(6), 1385. https://doi.org/10.3390/biomedicines13061385






