Abstract
Breast cancer ranks as the fifth-most-prevalent malignancy worldwide, characterized by high heterogeneity and multifactorial etiology across molecular subtypes. Despite advancements in conventional therapies, including surgery and chemotherapy, persistent challenges such as treatment-related adverse effects and acquired drug resistance necessitate alternative therapeutic strategies. Matrine, a naturally occurring alkaloid derived from Sophora flavescens, has demonstrated significant anticancer potential through multiple mechanisms. Experimental evidence indicates that matrine exerts inhibitory effects on tumor cell proliferation, promotes apoptosis, and attenuates metastatic progression via modulation of critical signaling pathways, particularly PI3K/Akt, JAK/STAT, NF-κB, MAPK/ERK, and Wnt/β-catenin. This review systematically examines subtype-specific responses to matrine treatment, highlighting its potential utility in precision oncology for distinct breast cancer classifications. Furthermore, we evaluate matrine’s capacity to synergize with standard chemotherapeutic regimens, potentially overcoming drug resistance while reducing required dosages. By integrating current preclinical and clinical findings, this analysis provides new perspectives on matrine’s therapeutic applications and underscores the imperative for translational studies to establish optimized treatment protocols for clinical implementation.
1. Introduction
Breast cancer has emerged as a leading global health challenge, representing one of the most prevalent and aggressive malignancies. According to the International Agency for Research on Cancer (IARC) 2020 report, it is the fifth-leading cause of cancer-related mortality worldwide, and its incidence continues to rise [1]. This epidemiological trend has intensified the search for safer and more effective therapeutic agents, positioning natural compounds as promising candidates for oncological research. Among these, matrine (MT), a tetracyclic quinolizidine alkaloid, has garnered significant scientific interest due to its multifaceted pharmacological profile. Isolated primarily from Sophora flavescens Aiton (S. flavescens), with additional sources including Sophora alopecuroides L. (S. alopecuroides), and Euchresta japonica Benth. ex Oliv. (E. japonica) [2]. This compound exhibits a remarkable spectrum of bioactivities spanning anti-neoplastic, anti-inflammatory, antimicrobial, antiviral, and neuroprotective properties [3,4,5,6,7,8,9,10,11,12,13]. Among these diverse pharmacological activities, its anti-cancer efficacy, particularly in breast cancer, has been widely explored, with promising results observed in both preclinical and clinical settings. However, despite these advances, a comprehensive analysis of its mechanisms of action and therapeutic potential, specifically in breast cancer, remains scarce.
MT, a tetracyclic quinolizidine alkaloid first isolated from S. flavescens in 1958, has been identified as a key bioactive component in traditional Chinese medicine (TCM) [3,14]. The chemical formula is C15H24N2O with a molecular weight of 248.36, as depicted in Figure 1. It is found in various natural plants. Detailed information on its botanical sources is provided in Table 1 and Figure 2 [15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Historical records from Shen Nong Ben Cao Jing (circa 200 CE) described S. flavescens in treating tumor-like masses and toxin-related disorders, laying the empirical foundation for modern pharmacological investigations [29]. Subsequent experimental studies validated its antitumor properties, particularly through MT-mediated inhibition of tumor cell proliferation [30]. Several literature studies have shown that MT can exert anti-cancer activity through multiple signal pathways, and it has pharmacological activity against breast cancer, lung cancer, liver cancer, blood cancer, gastric cancer, pancreatic cancer, and so on [31,32,33]. Clinical applications were advanced by Professor Wang Xixing’s successful use of S. flavescens in gynecological tumor management [34]. Additionally, the Compound Kushen Injection (CKI) demonstrated efficacy in alleviating cancer-related pain and hemorrhage [35,36]. These historical and clinical observations collectively support MT’s potential in suppressing tumor growth, inducing apoptosis, and inhibiting metastasis [31,32,33].
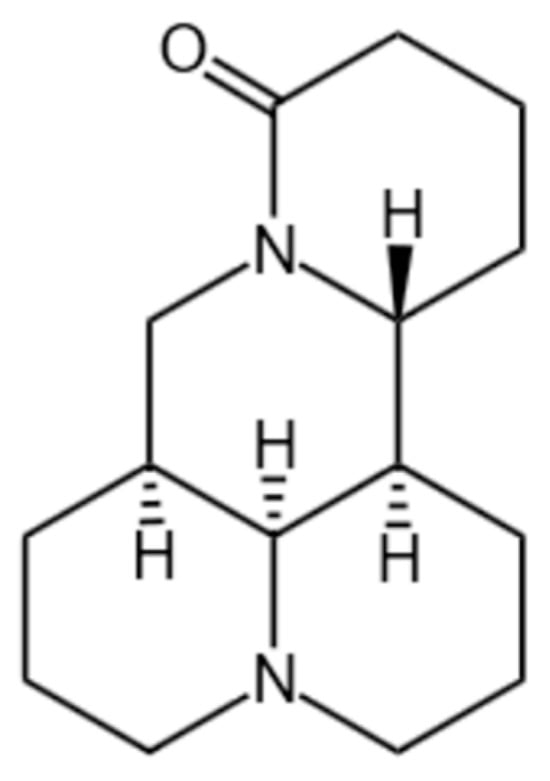
Figure 1.
Structural formula of MT.

Table 1.
The natural sources of matrine.

Figure 2.
Pharmacological activity diagram of MT.
In breast cancer, MT exhibits multifaceted antitumor effects via modulation of critical signaling pathways. Mechanistic studies reveal its dual regulatory action on the PI3K/Akt/mTOR and MAPK/ERK axes, coupled with suppression of JAK/STAT signaling. Furthermore, MT activates apoptosis through upregulation of Bax and caspase-3 while simultaneously promoting autophagy via LC3-II conversion and Beclin-1 activation. Its antimetastatic properties are mediated through the downregulation of MMP-9 and VEGF, effectively inhibiting angiogenesis and epithelial–mesenchymal transition (EMT). Preclinical models consistently demonstrate MT’s capacity to impede breast cancer progression across multiple molecular subtypes.
Beyond breast cancer, MT displays broad-spectrum anticancer activity against lung, liver, and pancreatic malignancies through tissue-specific mechanisms [37,38,39,40,41,42,43,44,45,46,47,48,49] (as shown in Figure 3). The ability of MT to modulate oxidative stress and reduce inflammation further contributes to its potential as a multi-target therapy for breast cancer. Its anti-inflammatory effects are particularly noteworthy, as inflammation is a known contributor to cancer progression and metastasis. Additionally, MT has demonstrated neuroprotective effects, making it a promising candidate for patients experiencing cancer-related pain, or those undergoing chemotherapy.
Figure 3.
Graph of activity of MT.
While substantial evidence supports the therapeutic potential of MT in breast cancer treatment, further research is needed to fully elucidate its molecular mechanisms of action, optimize dosing strategies, and assess its safety profile in clinical settings. This review aims to provide a thorough overview of MT’s anti-breast cancer properties. This review fills the gap in the existing literature on MT treatment of breast cancer.
2. Materials and Methods
A comprehensive and systematic literature search was conducted across eight major biomedical databases, including ScienceDirect, PubMed, CNKI, and VIP, covering studies published from January 1996 to May 2025. The search strategy utilized Medical Subject Headings (MeSH) terms in combination with Boolean operators, as follows: (“matrine” OR “Sophora flavescens”) AND (“breast neoplasms” [MeSH] OR “breast cancer” OR “mammary carcinoma”) AND (“in vivo” OR “in vitro”).
Inclusion criteria required original experimental studies evaluating the antitumor effects of matrine using in vitro and/or in vivo models. Review articles, commentaries, and studies lacking mechanistic data were excluded. In total, 2137 records were initially retrieved. After independent screening by two reviewers, a final set of 59 eligible studies was selected for analysis.
The following parameters were extracted and analyzed: Cellular-level outcomes, including IC50 values, apoptosis rates (via Annexin V/PI assays), and migration inhibition (via scratch/wound healing assays), as well as in vivo pharmacodynamic indicators, such as tumor volume reduction (%) and tumor weight changes in xenograft mouse models, and molecular mechanisms.
Studies that lacked both in vitro and in vivo experimental data on matrine in the context of breast cancer were excluded from the final synthesis. Importantly, no registered clinical trials evaluating the efficacy of matrine in breast cancer patients were identified during the search. Data synthesis and quantitative analyses were performed using STATA version 17, where applicable, to conduct meta-analyses and evaluate pooled effect sizes.
3. Results
MT exhibits dose-dependent antiproliferative effects across diverse breast cancer subtypes, as evidenced by in vitro and in vivo studies summarized in Table 2 [50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99] and Table 3 [65,83,88,94,100,101,102,103,104]. This study aims to characterize the pharmacological profile of MT against breast cancer and to explore potential subtype-specific differences in its mechanisms of action. The ultimate objective is to support the development of personalized, subtype-targeted therapeutic strategies for breast cancer treatment.

Table 2.
Effect of matrine on breast cancer cells.

Table 3.
Anti-breast cancer effect of matrine in vivo.
3.1. In Vitro Experimental Study of MT on Breast Cancer Cells
A systematic review of the literature was performed to assess the in vitro anticancer properties of MT in breast cancer. The included studies investigated MT’s effects in a wide array of breast cancer cell lines, including triple-negative breast cancer (TNBC) cells such as MDA-MB-231, BT474, MDA-MB-468, HCC-1806, and BT20; ER+/PR+ breast cancer cell lines such as MCF-7, T47-D, and Bcap-37; HER2+ cell lines such as SK-BR-3; and the murine breast cancer cell line 4T1. Assays used to evaluate cell viability and proliferation included MTT, CCK-8, crystal violet (CV), propidium iodide (PI), and SRB. Apoptosis and cell cycle arrest were assessed using methods such as flow cytometry, TUNEL immunofluorescence, and quantitative image analysis, as shown in Table 2.
Experimental results consistently demonstrated that MT exerts significant cytotoxic effects on multiple breast cancer cell types by inhibiting proliferation, inducing cell cycle arrest, and promoting apoptosis and autophagy. Furthermore, MT was found to suppress cancer cell migration and invasion, highlighting its strong potential as an anti-breast cancer agent.
In TNBC cell lines (e.g., MDA-MB-231, MDA-MB-468, BT474, and BT20), MT inhibited cell proliferation and induced apoptosis in a concentration- and time-dependent manner. For instance, Shao et al. [82] reported that treatment with 3 mg/mL MT for 48 h resulted in an approximately 90% apoptosis rate in MDA-MB-231 cells as measured by the MTT assay, indicating potent cytotoxic effects at relatively low concentrations. Mechanistically, the anti-TNBC effects of MT were mediated via modulation of the PI3K/Akt, MAPK/ERK, and NF-κB-signaling pathways. MT downregulated the expression of N-cadherin, vimentin, Bcl-2, IκB, HN1, VEGF, and CD31 while decreasing the Bcl-2/Bax ratio. Additionally, mRNA levels of MMP-9, MMP-2, EGF, and VEGFR1 were reduced, whereas the expression of E-cadherin, AKT, ERK1/2, and p38 was elevated. Caspase-3 activation was increased, and LC3-II expression was enhanced, further supporting MT’s pro-apoptotic and pro-autophagic actions in TNBC cells.
In ER+/PR+ breast cancer cell lines (MCF-7, T47-D, and Bcap-37), MT exhibited strong inhibitory effects, with reported IC50 values ranging from 0.3 to 15.8 mg/mL. MCF-7 cells demonstrated the highest sensitivity, with IC50 values between 0.7–0.9 mg/mL. The underlying mechanism involved mitochondrial membrane potential disruption and regulation of apoptosis-related gene expression. MT treatment led to the downregulation of Bcl-2 and NF-κB p65 while upregulating Bax, p53, caspase-3, PARP, Beclin-1, LC3b-II, and GSK-3β. Additionally, MT inhibited the IL-6/JAK/STAT3-signaling pathway, as evidenced by decreased expression of IL-6, JAK1, p-JAK1, STAT3, and p-STAT3. It also downregulated Wnt/β-catenin signaling and VEGF expression. Specific molecular alterations included decreased levels of IKKβ, cyclin D1, c-Myc, PI3K, Akt, P-gp, p62, CD133, and KLF4, while the expression of p27/Kip1 and let-7b was increased. These changes promoted cytochrome c release and caspase-3 activation, culminating in apoptosis. Furthermore, MT induced cell cycle arrest in MCF-7 cells, as shown by increased accumulation in the G0/G1 or G1 phases, with corresponding reductions in S and G2/M phase populations. MT also inhibited telomerase activity, contributing to reduced cell proliferation and enhanced apoptotic activity.
In HER2+ breast cancer cell lines, the anticancer effects of MT were associated with inhibition of miR-21 expression and upregulation of PTEN, resulting in suppressed cell proliferation. In the murine 4T1 cell line, treatment with 0.4 mg/mL MT for 48 h resulted in an apoptosis rate of 17.32 ± 3.09% [84], indicating moderate cytotoxic and pro-apoptotic effects. Further investigations revealed that MT may exert its antitumor effect in this model through the regulation of ANXA3 protein expression.
3.2. Analysis of In Vivo Experimental Studies of MT Against Breast Cancer Cell Lines
A systematic review of in vivo studies evaluating the anti-tumor efficacy of MT in breast cancer models was conducted. These studies employed tumor-bearing animal models established using various breast cancer cell lines, including MCF-7, TM40D, 4T1, MDA-MB-231, MDA-MB-451, Walker 256, and MA737. The therapeutic effects of MT on tumor progression were assessed primarily by measuring tumor volume and weight, as detailed in Table 3. Results from these in vivo studies demonstrated that MT significantly inhibited tumor growth and delayed tumor formation in treated animals. Notably, MT also exhibited preventive effects against early-stage mammary tumor development, suggesting its potential role in both therapeutic and prophylactic settings. Histopathological analyses of tumor tissues from MT-treated groups revealed several notable changes: a reduction in mitotic index, decreased invasiveness of tumor cells, and a marked suppression of angiogenesis and apoptosis dysregulation. Following the establishment of tumors, MT administration led to a significant reduction in tumor growth rate, final tumor weight, and the number of pulmonary metastatic nodules, indicating its potential to inhibit both primary tumor expansion and metastatic spread.
Collectively, these in vivo findings corroborate the potent anti-tumor properties of MT observed in in vitro settings and underscore its therapeutic promise for breast cancer. However, despite these encouraging outcomes, the current body of in vivo research remains relatively limited in scope and depth. Therefore, future studies should prioritize comprehensive in vivo investigations, including dose-response evaluations, long-term toxicity assessments, and comparative studies across different breast cancer subtypes, to further clarify MT’s therapeutic potential and facilitate its clinical translation.
4. Molecular Mechanisms of MT in Breast Cancer
In order to further study the mechanism of the effects of MT on breast cancer, this paper summarizes and analyzes the results of the above experimental studies and describes the anti-breast cancer effects of MT through multi-targets and multi-signaling pathways, which are described as follows.
4.1. Cytotoxic Effects of MT in Breast Cancer Cells
The cytotoxic impact of MT on various breast cancer cell lines has been extensively studied, showcasing a robust reduction in cell viability. In a study conducted by Shao et al., it was observed that MT treatment at a concentration of 3 mg/mL for 48 h led to a significant reduction in cancer cell viability, with reductions ranging from 76.4% to 84.5% across MCF-7, BT-474, and MDA-MB-231 cells [82]. This suggests a potent cytotoxic effect of MT against these breast cancer cells. Zhang’s research also reinforced this finding, revealing concentration- and time-dependent inhibitory effects on MCF-7 cell proliferation, with the optimal concentration identified as 2.5 mg/mL [105]. Additionally, Xiao et al. demonstrated that MT suppressed the proliferation of SK-BR-3 cells, with molecular investigations indicating a reduction in miR-21 expression and upregulation of PTEN, which may contribute to the anti-proliferative action of MT [68]. Furthermore, MT was shown to downregulate ANXA3 protein expression in 4T1 cells, leading to a reduction in cell proliferation [67]. In a related study by Zhang et al., the inhibition of AKT phosphorylation and the enhancement of PTEN expression were observed following MT treatment, providing additional evidence for its cytotoxic potential against cancer cells [106]. Moreover, Du et al. observed notable morphological alterations such as cell contraction, membrane blistering, and partial detachment in MCF-7 cells after 24 h of MT exposure, indicative of its cytotoxicity in breast cancer cells [81].
4.2. Induction of Cancer Cell Cycle Arrest by MT
The cell cycle, a fundamental process regulating cell division and growth, consists of distinct phases: G0 (quiescent), G1 (early DNA synthesis), S (DNA synthesis), G2 (late DNA synthesis), and M (mitosis). The progression through key checkpoints, particularly G0/S and G2/M, is essential for successful cell division and homeostasis [57]. In vitro experiments conducted by Sui Hui et al. revealed that MT significantly altered the dynamics of the cell cycle in breast cancer cells. MT induced a marked increase in the proportion of cells arrested in the G0/G1 phase, accompanied by a decrease in the S phase population, thereby halting the cell cycle and triggering apoptosis in cancer cells [57]. This cell cycle arrest was likely mediated through the modulation of cell cycle-related proteins, which play a pivotal role in regulating tumor progression [107]. Li et al. found that MT treatment increased the expression of Let-7, a miRNA known for its tumor suppressive properties, particularly Let-7b, which is involved in the regulation of c-Myc, ras, and JAK-STAT3 pathways [79]. The upregulation of Let-7b led to a blockade of the Wnt-signaling pathway and inhibited the differentiation and self-renewal of breast cancer stem cells (BCSCs), further contributing to the overall anticancer effects of MT. Additionally, MT also inhibited key signaling pathways such as c-Myc, ras, Wnt, and JAK-STAT3 through Let-7 modulation, which likely played a central role in disrupting the cancer cell cycle and mediating its anticancer effects, as summarized in Figure 4.
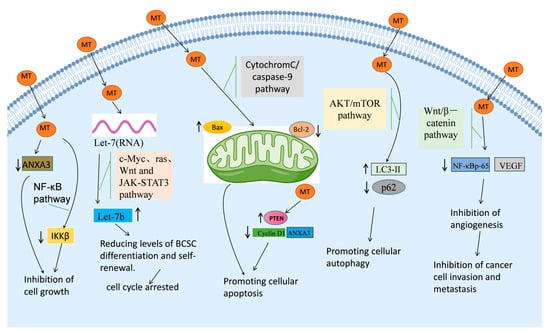
Figure 4.
Anti-cancer mechanism of MT against breast cancer.
4.3. Induction of Apoptosis in Cancer Cells by MT
Apoptosis, a genetically programmed form of cell death, is essential for maintaining cellular homeostasis and eliminating damaged cells. Dysregulated apoptosis is a hallmark of cancer, allowing for the evasion of cell death and tumor progression [108,109,110]. In breast cancer, apoptosis evasion is largely mediated by an imbalance between pro-apoptotic and anti-apoptotic proteins, notably Bax and Bcl-2. Bax induces mitochondrial outer membrane permeabilization (MOMP), leading to the release of cytochrome c and subsequent activation of caspases, which drive apoptosis [42]. MT has been shown to trigger apoptotic cell death in MCF-7 cells by altering the mitochondrial membrane potential (MMP), resulting in structural changes that facilitate the release of apoptotic factors. This cascade of events includes the regulation of Bcl-2 and Bax proteins, which ultimately culminates in the promotion of apoptosis [90,111]. Additionally, MT has been demonstrated to inhibit the PI3K/Akt pathway by suppressing mTOR activity, thus sensitizing cells to apoptosis. In Bcap-37 cells, treatment with 2 mg/mL MT led to a 19.58% apoptosis rate after 24 h of treatment, highlighting the dose-dependent pro-apoptotic effect of MT [66]. Furthermore, MT upregulated PTEN expression and inhibited miR-21, further confirming its ability to promote apoptosis in SK-BR-3 cells through the suppression of cell-proliferation pathways [68]. The induction of apoptosis by MT in MCF-7 cells was also evidenced by an increase in apoptotic vesicle formation after 48 h of treatment, with a significant increase in the apoptosis rate to 49.12 ± 3.79%. This suggests that apoptosis is a key mechanism by which MT exerts its anticancer effects in vivo.
4.4. Induction of Autophagy in Cancer Cells by MT
Cell death encompasses three distinct pathways: apoptosis, autophagy, and necrosis [112,113]. Autophagy, a process by which cells degrade and recycle their damaged components, is another form of programmed cell death and has gained attention as a therapeutic target in cancer treatment [114,115]. MT has been found to induce autophagy in MCF-7 cells through the modulation of key signaling pathways. Specifically, Du et al. observed an increase in LC3-II, an autophagy marker, alongside a reduction in p62, a protein involved in the autophagic degradation process [81]. Furthermore, MT treatment led to a decrease in the phosphorylation levels of AKT and mTOR in MCF-7 cells, signaling the activation of autophagy. Ren Lili et al. demonstrated that MT inhibited the phosphorylation of mTOR, suppressing the downstream activation of p70S6k and eIF4E, key regulators of protein synthesis and cell growth, thereby promoting autophagy in Bcap-37 cells [111]. These findings suggest that MT can modulate the AKT/mTOR pathway to enhance autophagic flux and promote autophagy in cancer cells, contributing to its anti-cancer effects, as depicted in Figure 4.
4.5. Inhibition of Angiogenesis by MT
Angiogenesis, the formation of new blood vessels, is crucial for tumor growth and metastasis [116]. In breast cancer, tumor angiogenesis is primarily regulated by the VEGF-signaling pathway, which is activated by the hypoxic conditions in tumors. MT has been shown to suppress angiogenesis by inhibiting the Wnt/β-catenin-signaling pathway and reducing VEGF expression, thereby limiting tumor blood supply and growth [83]. VEGF is a potent pro-angiogenic factor that promotes the formation of new blood vessels by binding to VEGFR1 and VEGFR2 on endothelial cells [117]. Li et al. demonstrated that MT treatment effectively downregulated VEGF protein expression, consequently inhibiting breast cancer cell proliferation and tumor angiogenesis [56]. Additionally, the experiments by Yu et al. [98] demonstrated that MT was found to reduce NF-κB p65 protein levels and lower the expression of MMP-9, MMP-2, EGF, and VEGFR1 mRNA in MDA-MB-231 cells, highlighting its anti-angiogenic potential in breast cancer treatment.
4.6. Inhibition of Cancer Cell Metastasis
Cancer metastasis, the spread of tumor cells to distant organs, is a significant cause of breast cancer mortality [118,119]. One of the key mechanisms involved in metastasis is epithelial-to-mesenchymal transition (EMT), characterized by the downregulation of e-cadherin and upregulation of N-cadherin, leading to enhanced cell motility and invasiveness. In a study by Ren et al., MT treatment led to an upregulation of e-cadherin and downregulation of N-cadherin in MDA-MB-231 and MCF-7 cells, effectively inhibiting EMT and reducing the metastatic potential of these cells [86]. By modulating EMT-related markers, MT may disrupt the metastatic cascade, offering a promising therapeutic strategy to prevent the spread of breast cancer.
4.7. Regulation of Immune Function by MT
Breast cancer progression is closely associated with immune dysfunction, particularly in the modulation of cytokine levels [120,121,122,123]. Significant changes in serum cytokine levels were observed in experiments with breast cancer cells in rats. The levels of IL-2 and IFN-γ exhibited a significant reduction, while the levels of IL-6, IL-10, and TGF-β showed an increase in the model group as compared to the control group. MT showed a remarkable ability to reverse these changes, restoring the balance of inflammatory factors and effectively preventing the growth of mammary tumors [101]. MT also modulated T-lymphocyte subpopulations, increasing CD8+ levels while decreasing CD3+ and CD4+ levels, indicating a shift towards a more effective anti-tumor immune response. Additionally, MT treatment resulted in reduced serum immunoglobulin levels (IgG, IgM, and IgA), suggesting its ability to restore normal immune function and prevent breast tumor growth.
4.8. Reversing Drug Resistance in Cancer Cells by MT
Multidrug resistance (MDR) is a major challenge in cancer treatment, often associated with the overexpression of drug-resistant genes such as MDR1, which encodes P-glycoprotein (P-gp). This protein pumps chemotherapeutic agents out of cancer cells, thereby reducing their efficacy [106,124]. MT has been shown to reverse drug resistance in MCF-7 cells by regulating the expression of drug-resistant genes and inhibiting the function of drug-resistant proteins such as P-gp and MRP1. The phenomenon of multidrug resistance may also be intricately linked to the PI3K/AKT-signaling pathway. In their 2005 study, Kim et al. [125] discovered that activation of the PI3K/AKT-signaling pathway not only stimulated the proliferation and differentiation of breast cancer cells but also inhibited cell apoptosis, consequently bolstering their resistance to chemotherapeutic agents [126,127,128,129,130]. Activation of the PI3K/AKT pathway rendered breast tumor cells resistant to cisplatin, paclitaxel, and pirarubicin chemotherapy drugs [131,132,133,134,135]. Upon exposure of breast cancer cells to chemotherapeutic agents, AKT phosphorylation levels increase, activating the PI3K/AKT-signal pathway. Activated AKT subsequently triggers downstream factors that make breast cancer cells resistant to chemotherapeutic agents, ultimately leading to treatment failure. In a comparative experiment involving the AKT channel inhibitor MK2206, the potent impact of MT on PTEN within the PI3K/AKT pathway, as compared to the MK2206 positive control, suggests that MT may function as a natural AKT channel inhibitor. Furthermore, MT demonstrated a dose-dependent reduction in the expression of MDR1 gene products P-gp and MRP1 protein. Western blot analysis revealed that increasing concentrations of MT were associated with elevated levels of PTEN protein expression, leading to inhibition of AKT activation. This finding is consistent with the observed gradual decrease in p-AKT expression levels with escalating concentrations of MT. MT was found to inhibit this pathway, reduce P-gp and MRP1 expression, and enhance the expression of PTEN, a tumor suppressor that counteracts AKT activation. These findings suggest that MT may be a promising natural compound to overcome drug resistance in breast cancer, offering a new therapeutic avenue for resistant tumors [62].
5. Medications and Combination Therapies of MT
MT, a major bioactive alkaloid extracted from S. flavescens, has been employed in clinical practice in China since 1995. Formulations such as CKI, which contains both MT and oxymatrine, are approved by the National Medical Products Administration (NMPA) and widely used as adjuvant therapies for managing symptoms associated with various malignancies, including breast cancer, hepatocellular carcinoma, and lung cancer [35,36,136]. These formulations are primarily utilized for palliative purposes, such as alleviating cancer-related pain and controlling hemorrhage. Topical MT-based products, such as Compound Kushen Lotion and MT Lotion, have also been introduced in dermatological and gynecological indications, including eczema and vaginitis, where they exert anti-inflammatory and antimicrobial effects [137,138].
In contrast, preclinical studies have consistently demonstrated that MT may enhance the therapeutic efficacy of conventional anticancer agents. For example, in Bcap-37 cells, the combination of MT with tamoxifen increased apoptosis by 62% compared to tamoxifen alone (p < 0.01), mediated by ERα/BCL-2-signaling modulation [74]. In MCF-7 cells, co-treatment with MT and doxorubicin resulted in a 78% reduction in cell viability versus 45% with monotherapies, along with a substantial shift in IC50 values (from 1.2 μM to 0.4 μM) [56]. In triple-negative MDA-MB-231 cells, MT enhanced docetaxel’s anti-metastatic effect through downregulation of HN1 (↓82%) and VEGF/CD31 expression (↓67%) [65]. Pirarubicin–cisplatin combination therapy showed 42% higher tumor inhibition when administered with MT (p < 0.001), attributed to reduced ABC transporter activity [62]. Despite their clinical availability, it is important to note that rigorous randomized clinical trials evaluating MT or CKI in combination with standard chemotherapeutics in breast cancer are currently lacking. Their application in oncology remains largely empirical and based on traditional use or observational data. The efficacy and safety of these combination strategies have not been systematically validated in human populations.
These findings support the potential use of MT as a chemosensitizing and adjuvant compound, particularly in drug-resistant or metastatic phenotypes. Currently, no clinical studies have directly compared MT monotherapy with combination therapy (e.g., MT + tamoxifen) in breast cancer patients. The promising synergy observed in preclinical studies underscores the need for well-controlled, multi-arm clinical trials to assess treatment efficacy, safety, and optimal combinations.
Due to its multi-targeted pharmacological actions, including modulation of apoptosis, angiogenesis inhibition, and reversal of chemoresistance, MT presents a promising adjuvant candidate in breast cancer therapy. However, its clinical application should be restricted to adjuvant use until high-quality human data become available.
6. Pharmacokinetics and Toxicological Profile of MT
6.1. Pharmacokinetics and Dose Optimization
Understanding the pharmacokinetics (PK) of MT is essential for guiding rational dose selection and optimizing its therapeutic application in oncology. Preclinical studies in rats have shown that MT is rapidly absorbed following oral administration, with a peak plasma concentration typically reached within 0.5 to 1.5 h and an elimination half-life ranging from 1.9 to 3.2 h [139,140]. MT distributes broadly to the liver, kidneys, and lungs and is primarily eliminated via hepatic metabolism and renal excretion. However, the oral bioavailability of MT is limited due to first-pass hepatic metabolism, and maintaining therapeutic concentrations may require frequent dosing or alternative delivery routes. Research has found that the plasma concentration of MT after transdermal administration is higher than that after intravenous administration [141]. Due to the short half-life and modest bioavailability, dose optimization for anticancer purposes will require PK–PD modeling, ideally supported by clinical data from cancer patients, to ensure adequate tumor exposure without inducing toxicity.
6.2. Toxicological Profile and Reported Adverse Effects
Although MT is generally considered safe at clinical doses, multiple studies have reported dose-dependent toxicological effects, particularly when administered at high doses or over extended periods.
In an acute toxicity assay, Yang et al. [142] determined the median lethal dose (LD50) of MT in mice to be 570.26 mg/kg, indicating moderate acute toxicity. Gong et al. [143] found that MT concentrations above 140 mg/L induced hepatotoxicity in hepatocytes after 72 h, evidenced by reduced cell viability and total protein content. Chronic exposure to MT at 40 mg/kg/day for 60 days also resulted in signs of neurotoxicity in mice [144].
Biochemical analysis by Gu et al. [145] revealed elevated serum ALT and AST levels after MT administration, suggesting hepatic stress. Zebrafish studies further confirmed MT-induced liver injury, potentially mediated by oxidative stress, as shown by altered levels of malondialdehyde (MDA) and glutathione (GSH) [146]. Similarly, Li et al. [147] and others reported that inflammation and redox imbalance contribute to MT-induced hepatotoxicity. In HL-7702 cells, MT treatment resulted in a time- and concentration-dependent reduction of superoxide dismutase (SOD) and GSH levels, supporting this mechanism [148].
In terms of reproductive safety, Luo et al. [149] found that MT at concentrations ≥ 100 µM impaired sperm function in mice following only 2 h of exposure in the cauda epididymis, raising concerns about potential reproductive toxicity.
Taken together, these findings suggest that MT’s adverse effects are closely related to both dose and duration of exposure, with the liver, nervous system, and reproductive organs being particularly vulnerable. While clinical use remains relatively safe within approved indications and doses, rigorous toxicity profiling and close monitoring will be necessary in the context of long-term or high-dose anticancer applications.
7. Summary and Prospect
MT is a bioactive alkaloid that has exhibited robust anti-breast cancer activity in both in vitro and in vivo preclinical models. This review provides a comprehensive evaluation of MT’s effects on key cellular processes, including proliferation, viability, apoptosis, autophagy, and migration, across various breast cancer subtypes. We have summarized how MT modulates the expression of multiple genes and proteins, emphasizing the importance of concentration, dosage, and treatment duration across different cell lines. The current literature indicates that MT exerts strong dose- and time-dependent cytotoxicity, with MCF-7 cells being particularly sensitive (IC50 = 0.7–0.9 mg/mL), suggesting potential subtype-specific responsiveness.
The molecular mechanisms underlying MT’s anti-tumor effects vary with the breast cancer subtype. In ER+/PR+ cell lines, MT has been shown to reduce mitochondrial membrane potential, arrest the cell cycle, and inhibit telomerase activity. In TNBC models, MT primarily acts through the PI3K/Akt, MAPK/ERK, and NF-κB pathways, leading to autophagy activation, enhanced apoptosis, and reduced migration. In HER2+ cells, MT decreases miR-21 levels and concurrently upregulates PTEN and ANXA3, highlighting its pleiotropic and subtype-dependent activity. Beyond direct effects on proliferation and survival, MT regulates multiple oncogenic and tumor-suppressor pathways, including Let-7, c-Myc, Ras, Wnt/β-catenin, and JAK/STAT3, thereby halting tumor growth and cell cycle progression. MT also modulates apoptosis-related proteins (e.g., Bax, Bcl-2), inhibits telomerase activity, and promotes ANXA3 expression, all of which contribute to tumor suppression. It activates autophagy via the AKT/mTOR axis (↑LC3-II, ↓p62), inhibits EMT-related proteins, and impedes metastasis. Furthermore, MT downregulates VEGF and Wnt/β-catenin signaling, thereby impairing angiogenesis. Importantly, MT enhances immune modulation and has been reported to reverse multidrug resistance by inhibiting P-gp and MRP1, increasing PTEN levels, and reducing p-AKT activation.
Despite these encouraging findings, several gaps remain. First, the majority of studies focus solely on tumor cell lines, with limited data available on non-tumor breast epithelial cells, which hinders assessment of MT’s selectivity and safety profile. Future investigations should include comparisons between cancerous and non-cancerous cells to better understand therapeutic windows. Second, although multiple subtypes of breast cancer cells (e.g., MCF-7, T47-D, MDA-MB-231, SK-BR-3, and 4T1) have been studied, comparative analyses between subtypes are largely lacking, and the findings have not been extended to clinical trials. Third, we emphasize that all such findings are limited to in vitro and in vivo animal models. The translational gap between these controlled laboratory conditions and complex clinical scenarios, such as interpatient variability, pharmacokinetics, immune modulation, and tumor heterogeneity, must be carefully considered. As such, the anticancer activity observed in cell lines should not be assumed to reflect clinical efficacy without further clinical validation. Moreover, MT’s utility as an adjuvant to standard chemotherapy warrants further exploration. Some preclinical studies suggest synergistic effects when MT is combined with agents like tamoxifen, doxorubicin, and docetaxel, but no clinical trials have been conducted to evaluate these combinations in patients with breast cancer. Finally, MT exhibits poor water solubility and limited oral bioavailability, restricting its clinical applicability. Future studies should prioritize the development of enhanced drug-delivery systems (e.g., nanoparticles, liposomes) to improve MT pharmacokinetics and the therapeutic index.
In summary, MT exhibits significant preclinical promise for the treatment of breast cancer. However, its full therapeutic potential will depend on resolving key challenges, including mechanistic elucidation across subtypes, in vivo validation, pharmacokinetic optimization, and rigorous clinical evaluation. This review underscores the need for further comparative and translational research, particularly in ER+, PR+, HER2+, and TNBC subtypes, to support MT’s integration into future breast cancer therapeutic strategies.
Author Contributions
Conceptualization, Y.Y.; methodology, Y.Y.; software, Y.Y.; validation, Y.Y., Y.L., S.L., and P.G.; formal analysis, Y.Y. and Y.L.; investigation, Y.Y. and Y.L.; resources, S.J.; data curation, Y.Y., Y.L., and X.Q.; writing—original draft preparation, Y.Y. and Y.L.; writing—review and editing, Y.Y.; visualization, J.T., C.F.; supervision, X.Q. and S.J.; project administration, X.Q. and S.J.; funding acquisition, X.Q. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by State Administration of Traditional Chinese Medicine Famous Elderly Chinese Medicine Experts Inheritance Program (003112011013).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
ANXA3, annexin A3; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; BCSC, breast cancer stem cells; BP, biological process; CC, cellular component; CKI, the compound Kushen injection, E. japonica, Euchresta japonica Benth. ex Oliv. EMT epithelial-mesenchymal transition; ER+, Estrogen receptor (+); GO, gene ontology; HER2+, Human Epidermal Growth Factor Receptor 2 (+); IARC, International Agency for Research on Cancer; JAK-STAT, Janus kinase-signal transducer and activator of transcription; KEGG, kyoto encyclopedia of genes and genomes; IL-17, Interleukin 17; MAPK/ERK, mitogen-activated protein kinase/extracellular regulated protein kinases; MT, matrine; MMP, mitochondrial membrane potential; MRP1, Multidrug Resistance-Associated Protein 1; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-B; PI3K-Akt, Phosphoinositide 3-kinase-Akt; PK, pharmacokinetics; PR+, progesterone receptor (+); PTEN, Phosphatase and Tensin Homolog; P-gp, P-glycoprotein; S. flavescens, Sophora flavescens Aiton; S. alopecuroides, Sophora alopecuroides L.; TCM, Traditional Chinese Medicine; TNBC, triple-negative breast cancer; TNF, Tumor Necrosis Factor; VEGF, vascular endothelial growth factor.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Guo, J.H.; Cui, Y.L.; Liu, J.G.; Liu, T.J. Research progress on structural modifications of matrine andits anticancer activity. Drugs Clin. 2015, 30, 600–604. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research Advances on Clinical Pharmacological Action of Anti-inflammatory Agent and lmmunosuppressant of Matrine. Anti-Infect. Pharm. 2018, 15, 737–743. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research progress on antinociceptive effects of matrine-type alkaloids. Drug Eval. Res. 2018, 41, 904–911. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research advance on central suppression and neuroprotection of reduced matrine-type alkaloids. Drug Eval. Res. 2018, 41, 1541–1547. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research Advances of Pharmacological Action of Matrine Against Acute Liver Injury. Anti-Infect. Pharm. 2018, 15, 1657–1662. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research Advances in Pharmacological Effects of Matrine Against Chronic Liver Injuries. Anti-Infect. Pharm. 2018, 15, 2025–2029. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Progress in Efficacy Evaluation of Drug Synergistic Effects of Matrine for ViralHepatitis. Anti-Infect. Pharm. 2019, 16, 185–189. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research advances on effects of matrine-type alkaloids against lymphocytic leukemia, lymphoma and myeloma. Drug Eval. Res. 2019, 42, 799–804. [Google Scholar]
- Liu, J.; Guo, S.R. Research Progress on the Effect of Matrine on Cardiovascular and its Mechanism. J. Jishou Univ. (Nat. Sci. Ed.) 2011, 32, 103–106. [Google Scholar]
- Hen, F.; Pan, Y.; Xu, J.; Liu, B.; Song, H. Research progress of matrine’s anticancer activity and its molecular mechanism. J. Ethnopharmacol. 2022, 286, 114914. [Google Scholar]
- Huang, W.-C.; Chan, C.-C.; Wu, S.-J.; Chen, L.-C.; Shen, J.-J.; Kuo, M.-L.; Chen, M.-C.; Liou, C.-J. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J. Ethnopharmacol. 2014, 151, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.-C.; Zhu, L.; Liu, N.; Zhang, G.-X. Matrine suppresses expression of adhesion molecules and chemokines as a mechanism underlying its therapeutic effect in CNS autoimmunity. Immunol. Res. 2013, 56, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-P.; He, X.-W.; Jiang, Y.; Chen, F. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 2003, 375, 264–269. [Google Scholar] [CrossRef]
- Yang, S.Y.; Liu, Y.Q. Research Progress on Chemical Constituents, Pharmacological Effects and Clinical Application of Lightyellow Sophora Root. Chin. J. Drug Abus. Prev. Treat. 2024, 30, 80–83. [Google Scholar]
- Chen, J.; Liu, Y.; Zhi, W.B.; An, Y.Y.; Li, S.S.; Liu, Q.Q.; Li, Y.; Zhang, H. Research Progress of Sophora alopecuroides Chemical Constituents and Pharmacological Activities of Sophora alopecuroides. Spec. Res. 2023, 45, 162–173+179. [Google Scholar]
- Chi, Z.L. Dietary Safety and Nutritional Evaluation of Cassiasurattensis Burm, f. eaf and lts Protein Products. Master’s Dissertation, Southwestern University, Chongqi, China, 2009. [Google Scholar]
- Xu, Z.R.; Liu, Y.; Liu, S.; Zhi, W.B.; Jiang, S.N.; Li, Y.; Zhang, H. Research Progress on the Chemical Constituents, Quality Standard and Pharmacological Action of Sophora davidii. Chin. Wild Plant Resour. 2023, 42, 70–78. [Google Scholar]
- Peng, S.S. Chinese Pharmaceutical Yearbook; Shanghai Second Military Medical University Press: Shanghai, China, 2010. [Google Scholar]
- Zheng, P.R.; Zhou, S.N. Handbook of Food Hygiene; Red Flag Press: Hangzhou, China, 1996. [Google Scholar]
- Ling, M.; Li, Z.Q.; Luo, L.; Huang, R.; Zhou, P. Study on Chemical Constituents from Roots of Sophora Dunnii Prain. J. Yunnan Univ. (Nat. Sci. Ed.) 2000, 22, 446–448. [Google Scholar]
- Zhao, N.S.; Ji, P.; Wei, Y.M.; Wu, F.L. Research progress in the determination, extraction process and biologicaactivity of alkaloids from Sophora moorcroftiana. Nat. Prod. Res. Dev. 2020, 32, 1614–1620. [Google Scholar]
- Zou, J.B. Studies on Chemical Constituents of the Seeds of Sophora tonkinensis Gagnep. and Their Bioactivities. Master’s Thesis, Guiyang Medical University, Guizhou, China, 2022. [Google Scholar]
- Wang, X.; Wang, Y.; Zhang, B.; Lin, Z.J. Research progress on herbaceous, chemical constituents andpharmacological effects of different medicinal parts of Sophora japonica. Chin. Tradit. Herb. Drugs 2018, 49, 4461–4467. [Google Scholar]
- Yuan, C.J.; Linghu, N.K.; Wang, H.D.; Wang, S.; Dai, X.Y.; Ding, F.J.; Wu, H.L. Research Progress on Active Components and Their Influencing Factors of Sophora tonkinensis. Chin. Wild Plant Resour. 2023, 42, 84–89. [Google Scholar]
- Li, H.C.; Yuan, D.P.; Liu, Y. Research progress on chemical constituents in plants of Euchresta J. Benn andtheir biological activities. Chin. Tradit. Herb. Drugs 2014, 45, 3486–3493. [Google Scholar]
- Zhang, C.; Zhao, K.X.; Pan, H.R.; Yu, Y.J.; Mi, J.B.; Su, M.Y.; Yang, Y.C. Determination of Picrasidine and Picrasidine Oxide Residues in Lycium barbarum by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Res. Dev. 2024, 45, 189–195. [Google Scholar]
- Song, W.R. Studies on the Chemical Constituents of Incarvillea Sinensis Andtheir Activity for Tumor Cell Migration. Master’s Thesis, Qinghai University, Xining, China, 2023. [Google Scholar]
- Sun, X.J.; Zhou, Z.; Wang, Q.; Zhang, B.Q. A Preliminary Discussion on the Application of Sophora flavescens in Various Dynasties. Hunan J. Tradit. Chin. Med. 2018, 34, 139–140. [Google Scholar]
- Liu, W.; Tiang, J.H.; Wang, Y.D. Research progress on Sophora flavescens Ait. Lishizhen Med. Mater. Medica Res. 2006, 05, 829–830. [Google Scholar]
- Sun, X.Y.; Jia, L.Y.; Rong, Z. Research advances on matrine. Front. Chem. 2022, 10, 867318. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Sun, X. Matrine: A promising natural product with various pharmacological activities. Front. Pharmacol. 2020, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, F.; Wu, L. Matrine exerts pharmacological effects through multiple signaling pathways: A comprehensive review. Drug Des. Dev. Ther. 2022, 533–569. [Google Scholar] [CrossRef]
- Li, Y.F.; Gao, X.J.; Wang, X.X. Wang Xixing’s Experience in Treating Tumors with Danggui Beimu Kushen Pills. Shanxi J. Tradit. Chin. Med. 2011, 27, 4–5+7. [Google Scholar]
- Jin, Y.; Yang, Q.; Liang, L.; Ding, L.; Liang, Y.; Zhang, D.; Wu, B.; Yang, T.; Liu, H.; Huang, T. Compound kushen injection suppresses human acute myeloid leukaemia by regulating the Prdxs/ROS/Trx1 signalling pathway. J. Exp. Clin. Cancer Res. CR 2018, 37, 277. [Google Scholar] [CrossRef]
- Gao, L.; Wang, K.X.; Zhou, Y.Z.; Fang, J.S.; Qin, X.M.; Du, G.H. Uncovering the anticancer mechanism of Compound Kushen Injection against HCC by integrating quantitative analysis, network analysis and experimental validation. Sci. Rep. 2018, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xin, W. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sci. 2018, 192, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, D.; Wang, X.; Hu, Z.; Yan, Y.; Huang, J. Matrine suppresses proliferation and invasion of SGC7901 cells through inactivation of PI3K/Akt/uPA pathway. Ann. Clin. Lab. Sci. 2016, 46, 457–462. [Google Scholar]
- Chang, C.; Liu, S.P.; Fang, C.H.; He, R.S.; Wang, Z.; Zhu, Y.Q.; Jiang, S.W. Effects of matrine on the proliferation of HT29 human colon cancer cells and its antitumor mechanism. Oncol. Lett. 2013, 6, 699–704. [Google Scholar] [CrossRef]
- Huang, H.; Du, T.; Xu, G.; Lai, Y.; Fan, X.; Chen, X.; Li, W.; Yue, F.; Li, Q.; Liu, L. Matrine suppresses invasion of castration-resistant prostate cancer cells by downregulating MMP-2/9 via NF-κB signaling pathway. Int. J. Oncol. 2017, 50, 640–648. [Google Scholar] [CrossRef]
- Vardarlı, A.T.; Düzgün, Z.; Erdem, C.; Kaymaz, B.T.; Eroglu, Z.; Çetintas, V.B. Matrine induced G0/G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia (T-ALL) cells. Bosn. J. Basic Med. Sci. 2018, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xu, M. Matrine inhibits the migratory and invasive properties of nasopharyngeal carcinoma cells. Mol. Med. Rep. 2015, 11, 4158–4164. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Cai, D.; Li, M. Matrine inhibits the metastatic properties of human cervical cancer cells via downregulating the p38 signaling pathway. Oncol. Rep. 2017, 38, 1312–1320. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Feng, J.Y.; You, L.S.; Meng, H.T.; Qian, W.B. Matrine and CYC116 synergistically inhibit growth and induce apoptosis in multiple myeloma cells. Chin. J. Integr. Med. 2015, 21, 635–639. [Google Scholar] [CrossRef]
- Jin, H.; Sun, Y.; Wang, S.; Cheng, X. Matrine activates PTEN to induce growth inhibition and apoptosis in V600EBRAF harboring melanoma cells. Int. J. Mol. Sci. 2013, 14, 16040–16057. [Google Scholar] [CrossRef]
- Ur Rashid, H.; Xu, Y.; Muhammad, Y.; Wang, L.; Jiang, J. Research advances on anticancer activities of matrine and its derivatives: An updated overview. Eur. J. Med. Chem. 2019, 161, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumor Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and activity of pentacyclic triterpenes codrugs. A review. Mini Rev. Med. Chem. 2021, 21, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Betulinic acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef]
- Liu, L.M.; Liu, H.G.; Mao, L.; Chen, Z.F.; Wang, H.S. Effects of matrine and oxymatrine on tumor cell proliferation in vitro. Chin. J. Exp. Tradit. Med. Formulae 2008, 11, 35–36. [Google Scholar]
- Mao, L. Effect of Au (III) complex with oxymatrine and matrine on proliferation of tumor cells in vitro and its mechanisms. Chin. Tradit. Herb. Drugs 2019, 50, 639–646. [Google Scholar]
- Wang, X.L.; Li, H.X.; Wang, L.H.; Liu, X.M. Killing effect of matrine combined with DC—CIK cells on breast cancer cells. Chin. J. Clin. Pharmacol. 2023, 39, 2311–2315. [Google Scholar]
- Jia, S.H.; Sun, M.Y.; Ding, H.X.; Jin, S.P. Study on the Effect of Matrine on Autophagy and Apoptosis of Human Breast Cancer MCF-7 Cells. J. Chin. Med. Mater. 2023, 46, 724–729. [Google Scholar]
- Sun, L.; Qian, S.Y.; Mao, X.P.; Gu, X. Biotransformation preparation and anti-tumor activity of matrine derivatives. Pharm. Clin. Res. 2017, 25, 24–26. [Google Scholar]
- Ou, X.Y. Study on the Mechanism and Related Pharmacological Effects of Matrine Induced Endoplasmic Reticulum Stress. Ph.D. Thesis, Peking Union Medical College Hospital, Beijing, China, 2014. [Google Scholar]
- Zhao, W.G.; Sun, Y.; Zhuang, J.; Feng, F.B.; Li, J.; Sun, C.G. Evaluation of the synergistic effect of Picrasidine combined with Adriamycin on human breast cancer cells by the Kim Jung Mean Q-value method. Lishizhen Med. Mater. Medica Res. 2018, 29, 33–36. [Google Scholar]
- Sui, H. Effects of matrine on proliferation of human breast cancer cells. China J. Mod. Med. 2013, 23, 41–43. [Google Scholar]
- Wang, S.Q.; Li, H.J.; Zhang, L.X.; Li, Y.Z.; Hao, X.Q. Matrine induces apoptosis of breast cancer MCF-7 cells and its effect on Bax expression. Chin. J. Gerontol. 2012, 32, 3489–3491. [Google Scholar]
- Li, H.J.; Zhao, X.X.; Bai, M.L.; Zhang, L.X.; Li, Y.Z.; Liu, H. Effects of matrine on apoptosis and mitochondrial transmembrane potential of breast cancer MCF-7 cells. Lishizhen Med. Mater. Medica Res. 2011, 22, 2042–2043. [Google Scholar]
- Gu, M.R.; Li, J.B.; Zhang, X.Y.; Yin, Y.M. Analysis of induced apoptosis effect of matrine on human breast cancer MCF-7 cell and its effect on mitochondrial transmembrane potential. Chin. J. Biochem. Pharm. 2015, 35, 39–41. [Google Scholar]
- Li, H.J.; Wang, J.M.; Tian, Y.T.; Bai, M.L.; Zhang, L.X.; Zhao, X.X. Effect of Matrine on Fas, VEGF, and Activities of Telomerase of MCF-7 Cells. Chin. J. Integr. Tradit. West. Med. 2013, 33, 1247–1251. [Google Scholar]
- Wei, C.S. Marine Cooperates with Chemotherapy Drug and Reverses Multidrug Resistance in Breast Cancer Drug-Resistant Cell Line MCF-7/ADR Through Inhibiting PI3K/AKT Signal Pathway. Master’s Thesis, Ningxia Medical University, Yinchuan, China, 2015. [Google Scholar]
- Zhou, B.G.; Sun, J.Z.; Su, G.; Ma, D.Q. Apoptosis of human breast cancer MCF-7/ADR cells induced by matrine. Chin. J. Exp. Surg. 2003, 06, 36–37. [Google Scholar]
- Ren, L.Q. Effects and Mechanisms of Matrine on Human Breast Cancer Cells. Master’s Thesis, Liaoning University of Traditional Chinese Medicine, Shenyang, China, 2023. [Google Scholar]
- Guo, Q.S. Study on the Mechanism of Matrine Combined with Docetaxel in Promoting Apoptosis of Triple Negative Human Breast Cancer MDA-MB-231 Cells. Ph.D. Thesis, ZheJiang University of TCM, Hangzhou, China, 2022. [Google Scholar]
- Xiao, X.; Ao, M.; Shi, W.D.; Pu, Z.F.; Li, D.X.; Hu, J.L. Effects of matrine on proliferation and apoptosis of Bcap-37 cells in breast cancer. Chin. Tradit. Pat. Med. 2018, 40, 2750–2754. [Google Scholar]
- Shi, W.D.; Li, D.X.; Hu, J.L.; Wang, Y.; Yang, N.; Xiao, X. Matrine inhibits proliferation of breast cancer 4T1 cells by regulating expression of annexin A3. Ningxia Med. J. 2018, 40, 967–969. [Google Scholar]
- Xiao, X.; Ao, M.; Shi, W.D.; Pu, Z.F.; Li, D.X.; Hu, J.L. Effects of matrine on proliferation and apoptosis of breast cancer SK-BR-3 cells. ShanDong Med. J. 2018, 58, 24–27. [Google Scholar]
- Zhou, B.G.; Wei, C.S.; Zhang, S.; Zhang, Z.; Wang, J. The matrine reverse multidrug resistance on breast cancer MCF-7 /ADR cell line and have an influence on downstream factors of PI3K/AKT signal pathway. J. Mod. Oncol. 2017, 25, 9–13. [Google Scholar]
- Ren, L.L.; Lan, T.; Wang, X.J. Antitumor Effect of Matrine in Human Breast Cancer Bcap-37 Cells by Apoptosis and Autophagy. Chin. Arch. Tradit. Chin. Med. 2014, 32, 2756–2759. [Google Scholar]
- Yu, M.; Cao, F.; Zhu, R.; Ding, H.Z. Effect of ZIC1 associated with matrine on proliferation, migration and apoptosis in MDA-MB-231 human breast cancer cell line. J. Mod. Oncol. 2016, 24, 1528–1533. [Google Scholar]
- Wei, C.S.; Shen, Y.J.; Zhang, Z.; Wang, J.; Zhou, B.G. Matrine reverses multidrug resistance in breast cancer drug-resistant cell line MCF-7 /ADR through inhibiting PI3K/AKT signal pathway. J. Army Med. Univ. 2014, 36, 2254–2258. [Google Scholar]
- Zhen, R.Z.; Zhang, J.Y.; Shao, X.Y.; Wang, X.J. Inhibition of the Growth of Human Breast Cancer Cell Line Bcap-37 with Matrine. Zhejiang J. Integr. Tradit. Chin. West. Med. 2012, 22, 945–947. [Google Scholar]
- Zhen, R.Z.; Zhang, J.Y.; Shao, X.Y.; Wang, X.J. Mechanism of Matrine Promoting Apoptosis Induced by Tamoxifen in Breast Cancer Cell Bcap-37. J. Chin. Oncol. 2012, 18, 840–843. [Google Scholar]
- Li, H.J.; Zhao, X.; Bai, M.L.; Zhang, L.X.; Liu, H.; Ge, L.P. Effects of matrine on proliferation and apoptosis in human breast cancer MCF-7 cells. Jiangsu Med. J. 2011, 37, 396–398+368. [Google Scholar]
- Zhen, R.Z.; Zhang, J.Y.; Shao, X.Y.; Wang, X.J. In Mechanism of Matrine Promoting Apoptosis in Breast Cancer Cell Bcap-37, The Third Conference of Traditional Chinese and Western Medicine Physicians of the Chinese Medical Association in 2012. 2012; 134. [Google Scholar]
- Zhou, B.G.; Ge, L.J.; Jin, L.Y.; Wu, Y.Z.; Fan, Y.Z.; Su, G. Effect of matrine on the expression of bcl-2 protein, bax protein and Fas antigen in breast cancer cells. Chin. J. Exp. Surg. 2007, 24, 1261. [Google Scholar]
- Jia, S.H.; Ding HSun, M.Y.; Jin, S.P. Research on Matrine Induced Autophagy of Human Breast Cancer MCF-7 Cells Based on PI3K/Akt/mTOR Signal Pathway and Its Mechanism. Food Drug 2023, 25, 159–163. [Google Scholar]
- Li, H.Y.; Chen, Y.; He, Q.Y. Mechanism of Combination of Matrine and Tetrandrine for Reversal of Multidrug Resistance of Breast Carcinoma MCF-7 Cells. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 275–278. [Google Scholar]
- Wang, X.K.; Li, J.; Wei, L.X.; Yan, Y.N.; Wang, R.Q. The Effects of Inhibiting Tumor in vitro of Alkaloidsfrom the Seed of Sophora viciifolia. J. Beijing Univ. Tradit. Chin. Med. 1996, 02, 59–60+73. [Google Scholar]
- Du, J.; Li, J.; Song, D.; Li, Q.; Li, L.; Li, B.; Li, L. Matrine exerts anti-breast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF-7 cells. Mol. Med. Rep. 2020, 22, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Yang, B.; Hu, R.; Wang, Y. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-κB signaling pathway. Oncol. Lett. 2013, 6, 517–520. [Google Scholar] [CrossRef]
- Xiao, X.; Ao, M.; Xu, F.; Li, X.; Hu, J.; Wang, Y.; Li, D.; Zhu, X.; Xin, C.; Shi, W. Effect of matrine against breast cancer by downregulating the vascular endothelial growth factor via the Wnt/β-catenin pathway. Oncol. Lett. 2018, 15, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, T.; Chen, S.S.; Wang, M.; Wang, R.; Li, K.; Wang, J.C.; Xu, C.W.; Du, N.; Qin, S. Matrine suppression of self-renewal was dependent on regulation of LIN28A/Let-7 pathway in breast cancer stem cells. J. Cell. Biochem. 2020, 121, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Q.; Li, X.-L.; Wang, L.; Du, W.-J.; Guo, R.; Liang, H.-H.; Liu, X.; Liang, D.-S.; Lu, Y.-J.; Shan, H.-L. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell. Physiol. Biochem. 2012, 30, 631–641. [Google Scholar] [CrossRef]
- Ren, L.; Mo, W.; Wang, L.; Wang, X. Matrine suppresses breast cancer metastasis by targeting ITGB1 and inhibiting epithelial-to-mesenchymal transition. Exp. Ther. Med. 2020, 19, 367–374. [Google Scholar] [CrossRef]
- Shi, Y.; Su, G.; Fang, H. Method for quantitative determination of matrine in Sophora alopecuroides L. and its inhibitory effect on breast cancer MCF-7 cell proliferation. Biomed. Res. 2015, 26, 461–466. [Google Scholar]
- Guo, Q.; Yu, Y.; Tang, W.; Zhou, S.; Lv, X. Matrine exerts an anti-tumor effect via regulating HN1 in triple breast cancer both in vitro and in vivo. Chem. Biol. Drug Des. 2023, 102, 1469–1477. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, D.; Wang, H.; Wu, D.; Chen, Y.; Ji, K.; Qin, T.; Wu, L. Matrine suppresses the ER-positive MCF cells by regulating energy metabolism and endoplasmic reticulum stress signaling pathway. Phytother. Res. 2017, 31, 671–679. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Bai, M.; Suo, Y.; Zhang, G.; Cao, X. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int. J. Clin. Exp. Pathol. 2015, 8, 14793. [Google Scholar]
- Jiang, L.; Wu, L.; Yang, F.; Almosnid, N.; Liu, X.; Jiang, J.; Altman, E.; Wang, L.; Gao, Y. Synthesis, biological evaluation and mechanism studies of matrine derivatives as anticancer agents. Oncol. Lett. 2017, 14, 3057–3064. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.; Hu, S.; Chen, X.; Bai, X. Screening and research of anti-cancer matrine components based on hollow fiber cell fishing with high-performance liquid chromatography. Chromatographia 2016, 79, 125–136. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Ma, X.; Yao, Y.; Zhou, Q.; Zhang, W.; Zhou, C.; Zhuang, J. MAT as a promising therapeutic strategy against triple-negative breast cancer via inhibiting PI3K/AKT pathway. Sci. Rep. 2023, 13, 12351. [Google Scholar] [CrossRef]
- Li, H.; Tan, G.; Jiang, X.; Qiao, H.; Pan, S.; Jiang, H.; Kanwar, J.R.; Sun, X. Therapeutic effects of matrine on primary and metastatic breast cancer. Am. J. Chin. Med. 2010, 38, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sarem, M.; Xiang, S.; Hu, H.; Xu, W.; Shastri, V.P. Autophagy inhibition enhances Matrine derivative MASM induced apoptosis in cancer cells via a mechanism involving reactive oxygen species-mediated PI3K/Akt/mTOR and Erk/p38 signaling. BMC Cancer 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Thang, P.N.T.; Tran, V.-H.; Vu, T.A.; Vinh, N.N.; Huynh, D.T.M.; Pham, D.T. Determination of antioxidant, cytotoxicity, and acetylcholinesterase inhibitory activities of alkaloids isolated from Sophora flavescens Ait. Grown in Dak Nong, Vietnam. Pharmaceuticals 2022, 15, 1384. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.H.; Motaez, M.; Zamiri-Akhlaghi, A.; Emami, S.A.; Tayarani-Najaran, Z. In-vitro evaluation of cytotoxic and apoptogenic properties of Sophora pachycarpa. Iran. J. Pharm. Res. IJPR 2014, 13, 665. [Google Scholar] [PubMed]
- Yu, P.; Liu, Q.; Liu, K.; Yagasaki, K.; Wu, E.; Zhang, G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κ B signaling. Cytotechnology 2009, 59, 219–229. [Google Scholar] [CrossRef]
- Chui, C.H.; Lau, F.Y.; Tang, J.C.O.; Kan, K.L.; Cheng, G.Y.M.; Wong, R.S.M.; Kok, S.H.L.; Lai, P.B.S.; Ho, R.; Gambari, R. Activities of fresh juice of Scutellaria barbata and warmed water extract of Radix Sophorae Tonkinensis on anti-proliferation and apoptosis of human cancer cell lines. Int. J. Mol. Med. 2005, 16, 337–341. [Google Scholar] [CrossRef]
- Dong, H.D. Effect of Matrine on JNK1/AP-1 Signaling Pathway in Mice Model with 4T1Breast Cancer. J. Liaoning Univ. Tradit. Chin. Med. 2019, 21, 64–68. [Google Scholar]
- Zhang, R.K.; Wang, C. Effect of matrine on tumor growth and inflammatory factors and immune function in Wistar rat with breast cancer. Chin. J. Appl. Physiol. 2018, 34, 375–378. [Google Scholar]
- Mao, H.S.; Liu, H.; Li, C.; Song, Y.S.; Sun, H.; Feng, Y.M. Modulation of the Malignant Phenotype and theImmunological Response of Tumor Cellsby Sophora Flavescens Ait(AF-7). Chin. J. Clin. Oncol. 1996, 11, 44–48. [Google Scholar]
- Bai, Y.L.; Li, X.J.; Liang, L.; Li, X.Q. Effect of matrine injection on sensitization of TM40D human breast cancer bearing mice and its mechanism. Propr. Chin. Med. 2016, 38, 1608–1610. [Google Scholar]
- Zhou, B.G.; Wei, C.S.; Zhang, S.; Su, Z.M.; Zhang, Z.; Wang, J. Effects of matrine on the growth of human breast cancer MCF-7/ADR cells transplanted in nude mice and apoptosis factors of phosphoinositol 3 kinase/protein kinase B signaling pathway. Chin. J. Exp. Surg. 2017, 34, 4. [Google Scholar]
- Zhang, X. Study on the Molecular Mechanisms of the Antitumor Effect of Matrine. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2020. [Google Scholar]
- Zhang, X.J.; Zhang, X.; Ma, H.G.; Hu, X.Q. Mechanism of Chinese medicine Fuzheng in reversing paclitaxel resistance in TLR4 over-expressed breast cancer. J. Wenzhou Med. Univ. 2019, 49, 826–831. [Google Scholar]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Wen, F.; Lu, Z.M. Progression of the roles of apoptosis in breast cancer therapy. Chin. Clin. Oncol. 2017, 22, 175–179. [Google Scholar]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef]
- Foo, J.B.; Yazan, L.S.; Tor, Y.S.; Wibowo, A.; Ismail, N.; Armania, N.; Cheah, Y.K.; Abdullah, R. Dillenia suffruticosa dichloromethane root extract induced apoptosis towards MDA-MB-231 triple-negative breast cancer cells. J. Ethnopharmacol. 2016, 187, 195–204. [Google Scholar] [CrossRef]
- Ren, L.L.; Wang, L.; Wang, X.J. Relationship Between Matrine Induced Autophagy and mTOR in Human Breast Cancer Bcap-37 Cells. Zhejiang JITCWM 2016, 26, 783–786. [Google Scholar]
- Wang, S.; Wang, K.; Wang, H.; Han, J.; Sun, H. Autophagy is essential for flavopiridol-induced cytotoxicity against MCF-7 breast cancer cells. Mol. Med. Rep. 2017, 16, 9715–9720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Udristioiu, A.; Nica-Badea, D. Autophagy dysfunctions associated with cancer cells and their therapeutic implications. Biomed. Pharmacother. 2019, 115, 108892. [Google Scholar] [CrossRef]
- Xie, Z.; Xie, Y.; Xu, Y.; Zhou, H.; Xu, W.; Dong, Q. Bafilomycin A1 inhibits autophagy and induces apoptosis in MG63 osteosarcoma cells. Mol. Med. Rep. 2014, 10, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z. Regulation of neuronal autophagy in axon: Implication of autophagy in axonal function and dysfunction/degeneration. Autophagy 2007, 3, 139–141. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor family genes: When did the three genes phylogenetically segregate? 2002, 383, 1573–1579. Biol. Chem. 2002, 383, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Fedewa, S.A.; Goding Sauer, A.; Kramer, J.L.; Smith, R.A.; Jemal, A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA A Cancer J. Clin. 2016, 66, 31–42. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Zhao, X.X.; Tian, T.; Yan, W.J.; Ma, X.C.; Zhang, H.; Wang, W. Regulating Effects and Molecular Mechanisms of Traditional Chinese Medicine in the Tumor Immunity. J. Mod. Oncol. 2016, 24, 306–309. [Google Scholar]
- Peng, R.Q.; Zhang, X. New perspectives on tumor immunotherapy and its clinical practice. J. Pract. Oncol. 2013, 28, 339–342. [Google Scholar]
- Yaguchi, T.; Sumimoto, H.; Kudo-Saito, C.; Tsukamoto, N.; Ueda, R.; Iwata-Kajihara, T.; Nishio, H.; Kawamura, N.; Kawakami, Y. The mechanisms of cancer immunoescape and development of overcoming strategies. Int. J. Hematol. 2011, 93, 294–300. [Google Scholar] [CrossRef]
- Tian, T.D.; Yue, L.Y.; Tian, T.L.; Fan, Y.X.; Zhang, X.F. Relationship between tumor inflammatory microenvironment and immunity and intervention strategy of traditional Chinese medicine. J. Tradit. Chin. Med. 2017, 58, 209–213. [Google Scholar]
- He, S.; Liu, F.; Xie, Z.; Zu, X.; Xu, W.; Jiang, Y. P-Glycoprotein/MDR1 regulates pokemon gene transcription through p53 expression in human breast cancer cells. Int. J. Mol. Sci. 2010, 11, 3039–3051. [Google Scholar] [CrossRef]
- Kim, D.; Cheng, G.Z.; Lindsley, C.W.; Yang, H.; Cheng, J.Q. Targeting the phosphatidylinositol-3 kinase/Akt pathway for the treatment of cancer. Curr. Opin. Investig. Drugs 2005, 6, 1250–1258. [Google Scholar] [PubMed]
- Tian, T.; Nan, K.J.; Guo, H.; Wang, W.J.; Ruan, Z.P.; Wang, S.H.; Liang, X.; Lu, C.X. PTEN inhibits the migration and invasion of HepG2 cells by coordinately decreasing MMP expression via the PI3K/Akt pathway. Oncol. Rep. 2010, 23, 1593–1600. [Google Scholar] [PubMed]
- Lee, C.S.; Kim, Y.J.; Jang, E.R.; Myung, S.C.; Kim, W. Akt inhibitor enhances apoptotic effect of carboplatin on human epithelial ovarian carcinoma cell lines. Eur. J. Pharmacol. 2010, 632, 7–13. [Google Scholar] [CrossRef]
- Burris, H.A. Overcoming acquired resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR pathway. Cancer Chemother. Pharmacol. 2013, 71, 829–842. [Google Scholar] [CrossRef]
- Calderaro, J.; Rebouissou, S.; de Koning, L.; Masmoudi, A.; Hérault, A.; Dubois, T.; Maille, P.; Soyeux, P.; Sibony, M.; de la Taille, A. PI3K/AKT pathway activation in bladder carcinogenesis. Int. J. Cancer 2014, 134, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Tapia, O.; Riquelme, I.; Leal, P.; Sandoval, A.; Aedo, S.; Weber, H.; Letelier, P.; Bellolio, E.; Villaseca, M.; Garcia, P. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014, 465, 25–33. [Google Scholar] [CrossRef]
- Guo, C.Y.; Ke WFSong, K.Y.; Wang, J.F.; Zhou, L.; Li, K. PI3K/AKT signalling pathway involves in the modulation of multidrug resistance and metastasis in breast cancer. Prog. Mod. Biomed. 2012, 12, 4809–4812. [Google Scholar]
- Liu, F.; Liu, S.; He, S.; Xie, Z.; Zu, X.; Jiang, Y. Survivin transcription is associated with P-glycoprotein/MDR1 overexpression in the multidrug resistance of MCF-7 breast cancer cells. Oncol. Rep. 2010, 23, 1469–1475. [Google Scholar] [PubMed]
- Ren, D.; Jia, L.; Li, Y.; Gong, Y.; Liu, C.; Zhang, X.; Wang, N.; Zhao, Y. ST6GalNAcII mediates the invasive properties of breast carcinoma through PI3K/Akt/NF-κB signaling pathway. IUBMB Life 2014, 66, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Choi, Y.; Shin, J.M.; Cho, H.J.; Kang, J.H.; Park, K.K.; Choe, J.Y.; Bae, Y.S.; Han, S.M.; Kim, C.H. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem. Toxicol. 2014, 68, 218–225. [Google Scholar] [CrossRef]
- Huemer, F.; Bartsch, R.; Gnant, M. The PI3K/AKT/MTOR signaling pathway: The role of PI3K and AKT inhibitors in breast cancer. Curr. Breast Cancer Rep. 2014, 6, 59–70. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, Z.; Yao, H.; Sun, L.; Lin, L. An effective drug sensitizing agent increases gefitinib treatment by down regulating PI3K/Akt/mTOR pathway and up regulating autophagy in non-small cell lung cancer. Biomed. Pharmacother. 2019, 118, 109169. [Google Scholar] [CrossRef]
- Liu, N. Clinical Effect of Compound Sophora Flavescens Lotion Combined with Clotrimazole Vaginal Tablet in the Treatment of Candida Vaginitis during Pregnancy. J. Med. Inf. 2018, 31, 145–146. [Google Scholar]
- Lv, P.G.; Jin, Y.N.; Xu, H.Y. The clinical observation of 40 cases of infantile acute eczema treated with Fufang Kushen Lotion. J. Pediatr. Tradit. Chin. Med. 2017, 13, 50–52. [Google Scholar]
- Wu, X.; Yamashita, F.; Hashida, M.; Chen, X.; Hu, Z. Determination of matrine in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies. Talanta 2003, 59, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Z.; Wen, L.; Jiang, C.; Feng, Q. Matrine: A review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches. J. Ethnopharmacol. 2021, 269, 113682. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Q.; He, Z.; Yin, L.; Zhang, Y.; Wang, S. Liver, blood microdialysate and plasma pharmacokinetics of matrine following transdermal or intravenous administration. Die Pharm.-Int. J. Pharm. Sci. 2017, 72, 167–170. [Google Scholar]
- Yang, L.Y. Analysis on Matrine Toxicity and HPLC Determination of Matrine in Sheep Plasma. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2015. [Google Scholar]
- Gong, X.; Gao, Y.; Guo, G.; Vondran, F.W.; Schwartlander, R.; Efimova, E.; Pless, G.; Sauera, I.M.; Neuhaus, P. Effect of matrine on primary human hepatocytes in vitro. Cytotechnology 2015, 67, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yuan, T.J.; Gu, L.L.; Lu, H. Study of Hepatotoxicity and Neural Behavioral Changes of Sophora Flavescens and Matrine in Mice. Chin. J. Mod. Appl. Pharm. 2015, 32, 1444–1448. [Google Scholar]
- Gu, Y.M.; Lu, J.Y.; Sun, W.; Jin, R.M.; Ohira, T.; Zhang, Z. Oxymatrine and its metabolite matrine contribute to the heparotoxicity induced by radix Sophorae tonkinensis in mice. Exp. Ther. Med. 2019, 17, 2519–2528. [Google Scholar] [PubMed]
- Guo, Q.P.; Chen, G.Y.; Zhou, Q.; Jin, R.M. Comparison of hepatotoxicity and toxic mechanisms of matrine and oxymatrine using in vivo and in vitro models. Chin. J. Compart. Med. 2018, 28, 44–50. [Google Scholar]
- Li, P.P. Study on the rule of residue elimination and hepatotoxicity of dichroine and matrine in broyers. Master’s Thesis, Agricultural University Of Hebei, Baoding, China, 2022. [Google Scholar]
- You, L.T.; Yang, C.J.; Du, Y.Y.; Liu, Y.; Chen, G.S. Matrine exerts hepatotoxic effects via the ROS-dependent mitochondrial apoptosis pathway and inhibition ofNrf2-mediated antioxidant response. Oxidative Med. Cell. Longev. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Luo, T.; Zou, Q.; He, Y.; Wang, H.; Li, N.; Zeng, X. Matrine Inhibits Mouse Sperm Function by Reducing Sperm [Ca2+]iand Phospho-ERK1/2. Cell. Physiol. Biochem. 2015, 35, 374–385. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).