The Potential of Matrine in the Treatment of Breast Cancer: A Review
Abstract
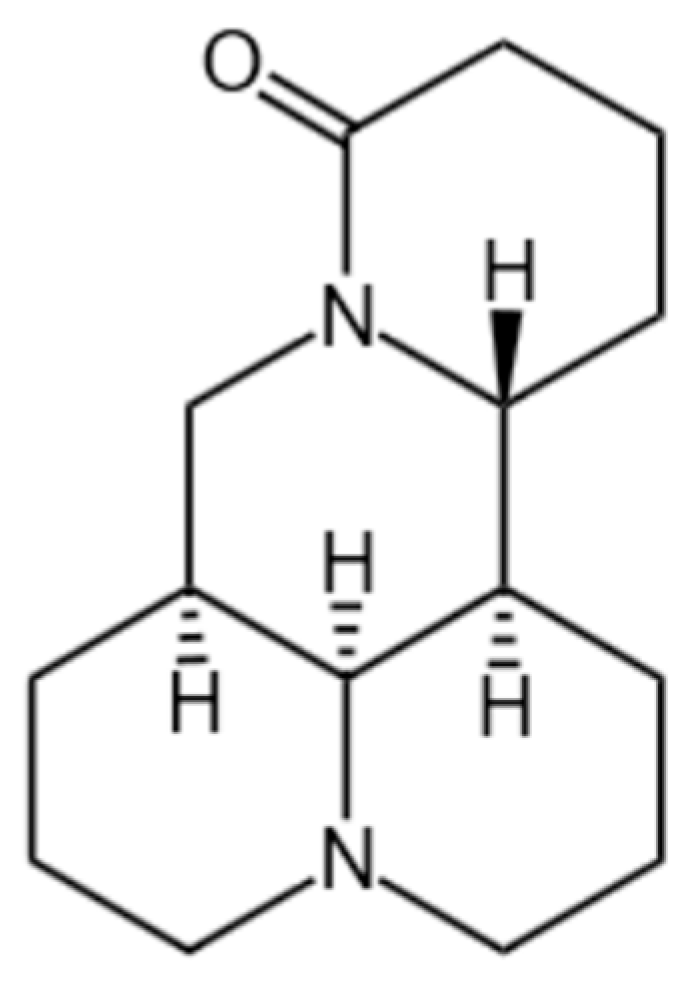
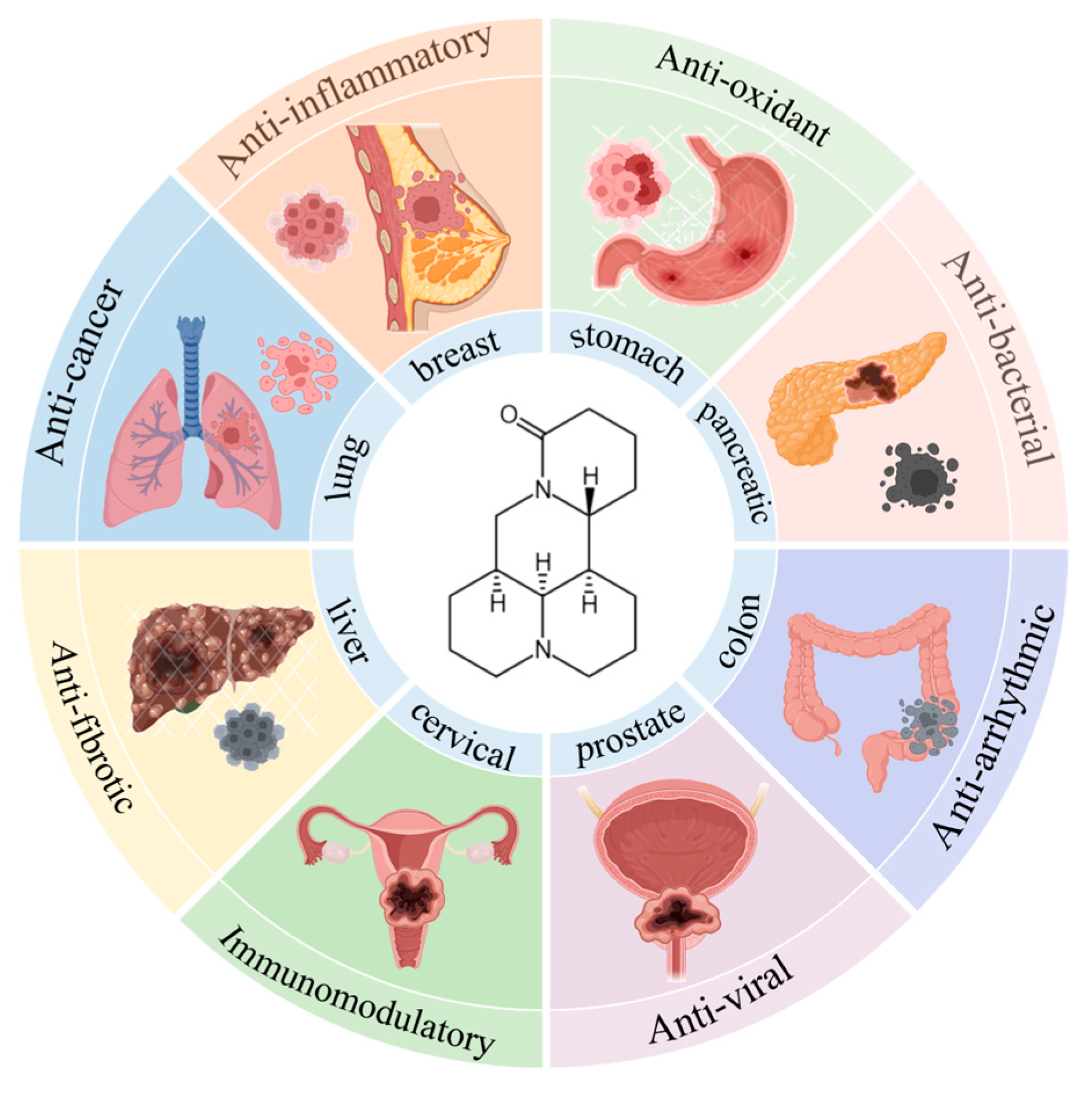
1. Introduction
2. Materials and Methods
3. Results
3.1. In Vitro Experimental Study of MT on Breast Cancer Cells
3.2. Analysis of In Vivo Experimental Studies of MT Against Breast Cancer Cell Lines
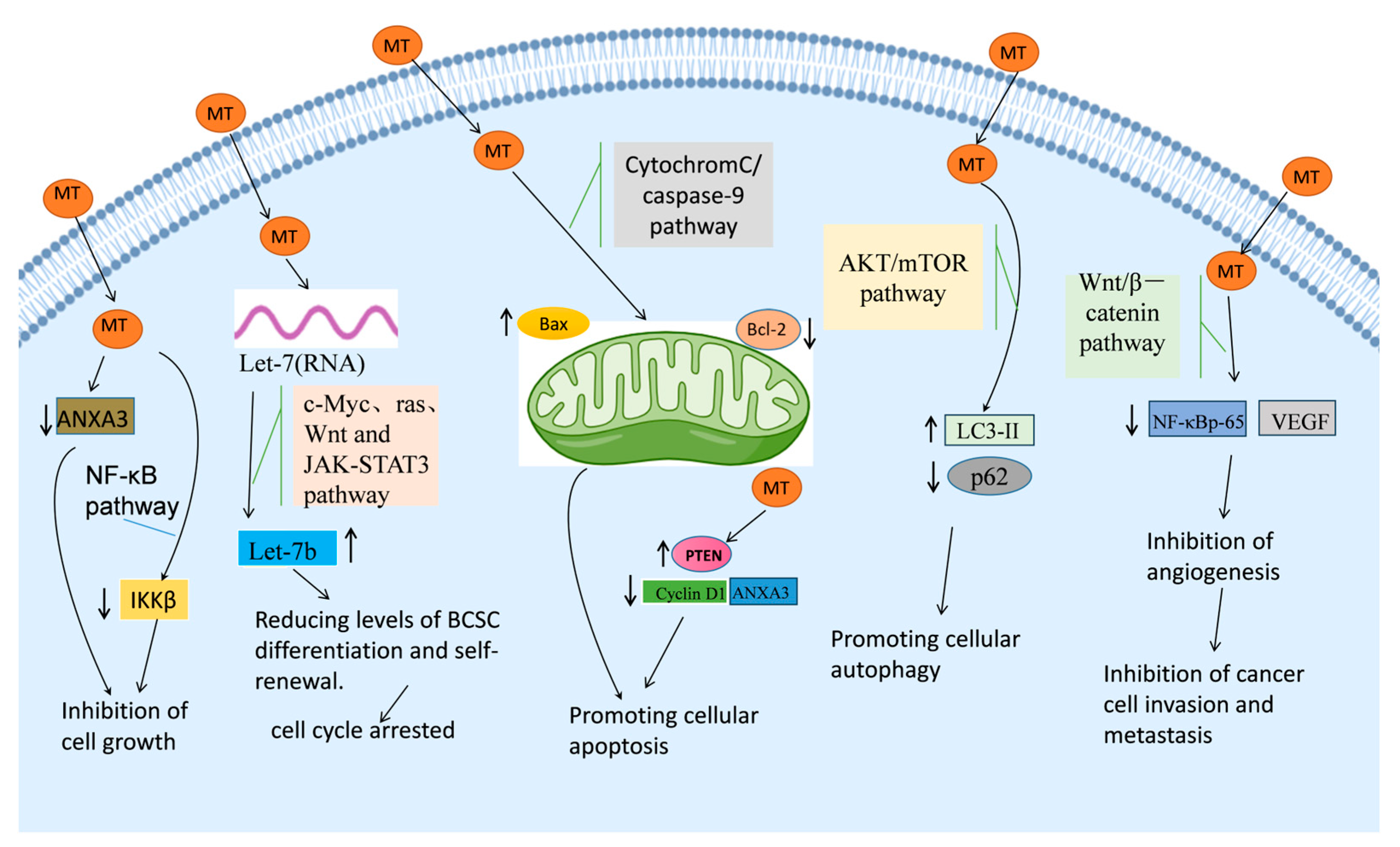
4. Molecular Mechanisms of MT in Breast Cancer
4.1. Cytotoxic Effects of MT in Breast Cancer Cells
4.2. Induction of Cancer Cell Cycle Arrest by MT
4.3. Induction of Apoptosis in Cancer Cells by MT
4.4. Induction of Autophagy in Cancer Cells by MT
4.5. Inhibition of Angiogenesis by MT
4.6. Inhibition of Cancer Cell Metastasis
4.7. Regulation of Immune Function by MT
4.8. Reversing Drug Resistance in Cancer Cells by MT
5. Medications and Combination Therapies of MT
6. Pharmacokinetics and Toxicological Profile of MT
6.1. Pharmacokinetics and Dose Optimization
6.2. Toxicological Profile and Reported Adverse Effects
7. Summary and Prospect
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Guo, J.H.; Cui, Y.L.; Liu, J.G.; Liu, T.J. Research progress on structural modifications of matrine andits anticancer activity. Drugs Clin. 2015, 30, 600–604. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research Advances on Clinical Pharmacological Action of Anti-inflammatory Agent and lmmunosuppressant of Matrine. Anti-Infect. Pharm. 2018, 15, 737–743. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research progress on antinociceptive effects of matrine-type alkaloids. Drug Eval. Res. 2018, 41, 904–911. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research advance on central suppression and neuroprotection of reduced matrine-type alkaloids. Drug Eval. Res. 2018, 41, 1541–1547. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research Advances of Pharmacological Action of Matrine Against Acute Liver Injury. Anti-Infect. Pharm. 2018, 15, 1657–1662. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research Advances in Pharmacological Effects of Matrine Against Chronic Liver Injuries. Anti-Infect. Pharm. 2018, 15, 2025–2029. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Progress in Efficacy Evaluation of Drug Synergistic Effects of Matrine for ViralHepatitis. Anti-Infect. Pharm. 2019, 16, 185–189. [Google Scholar]
- Zhang, M.-F.; Shen, Y.-Q. Research advances on effects of matrine-type alkaloids against lymphocytic leukemia, lymphoma and myeloma. Drug Eval. Res. 2019, 42, 799–804. [Google Scholar]
- Liu, J.; Guo, S.R. Research Progress on the Effect of Matrine on Cardiovascular and its Mechanism. J. Jishou Univ. (Nat. Sci. Ed.) 2011, 32, 103–106. [Google Scholar]
- Hen, F.; Pan, Y.; Xu, J.; Liu, B.; Song, H. Research progress of matrine’s anticancer activity and its molecular mechanism. J. Ethnopharmacol. 2022, 286, 114914. [Google Scholar]
- Huang, W.-C.; Chan, C.-C.; Wu, S.-J.; Chen, L.-C.; Shen, J.-J.; Kuo, M.-L.; Chen, M.-C.; Liou, C.-J. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J. Ethnopharmacol. 2014, 151, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.-C.; Zhu, L.; Liu, N.; Zhang, G.-X. Matrine suppresses expression of adhesion molecules and chemokines as a mechanism underlying its therapeutic effect in CNS autoimmunity. Immunol. Res. 2013, 56, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-P.; He, X.-W.; Jiang, Y.; Chen, F. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 2003, 375, 264–269. [Google Scholar] [CrossRef]
- Yang, S.Y.; Liu, Y.Q. Research Progress on Chemical Constituents, Pharmacological Effects and Clinical Application of Lightyellow Sophora Root. Chin. J. Drug Abus. Prev. Treat. 2024, 30, 80–83. [Google Scholar]
- Chen, J.; Liu, Y.; Zhi, W.B.; An, Y.Y.; Li, S.S.; Liu, Q.Q.; Li, Y.; Zhang, H. Research Progress of Sophora alopecuroides Chemical Constituents and Pharmacological Activities of Sophora alopecuroides. Spec. Res. 2023, 45, 162–173+179. [Google Scholar]
- Chi, Z.L. Dietary Safety and Nutritional Evaluation of Cassiasurattensis Burm, f. eaf and lts Protein Products. Master’s Dissertation, Southwestern University, Chongqi, China, 2009. [Google Scholar]
- Xu, Z.R.; Liu, Y.; Liu, S.; Zhi, W.B.; Jiang, S.N.; Li, Y.; Zhang, H. Research Progress on the Chemical Constituents, Quality Standard and Pharmacological Action of Sophora davidii. Chin. Wild Plant Resour. 2023, 42, 70–78. [Google Scholar]
- Peng, S.S. Chinese Pharmaceutical Yearbook; Shanghai Second Military Medical University Press: Shanghai, China, 2010. [Google Scholar]
- Zheng, P.R.; Zhou, S.N. Handbook of Food Hygiene; Red Flag Press: Hangzhou, China, 1996. [Google Scholar]
- Ling, M.; Li, Z.Q.; Luo, L.; Huang, R.; Zhou, P. Study on Chemical Constituents from Roots of Sophora Dunnii Prain. J. Yunnan Univ. (Nat. Sci. Ed.) 2000, 22, 446–448. [Google Scholar]
- Zhao, N.S.; Ji, P.; Wei, Y.M.; Wu, F.L. Research progress in the determination, extraction process and biologicaactivity of alkaloids from Sophora moorcroftiana. Nat. Prod. Res. Dev. 2020, 32, 1614–1620. [Google Scholar]
- Zou, J.B. Studies on Chemical Constituents of the Seeds of Sophora tonkinensis Gagnep. and Their Bioactivities. Master’s Thesis, Guiyang Medical University, Guizhou, China, 2022. [Google Scholar]
- Wang, X.; Wang, Y.; Zhang, B.; Lin, Z.J. Research progress on herbaceous, chemical constituents andpharmacological effects of different medicinal parts of Sophora japonica. Chin. Tradit. Herb. Drugs 2018, 49, 4461–4467. [Google Scholar]
- Yuan, C.J.; Linghu, N.K.; Wang, H.D.; Wang, S.; Dai, X.Y.; Ding, F.J.; Wu, H.L. Research Progress on Active Components and Their Influencing Factors of Sophora tonkinensis. Chin. Wild Plant Resour. 2023, 42, 84–89. [Google Scholar]
- Li, H.C.; Yuan, D.P.; Liu, Y. Research progress on chemical constituents in plants of Euchresta J. Benn andtheir biological activities. Chin. Tradit. Herb. Drugs 2014, 45, 3486–3493. [Google Scholar]
- Zhang, C.; Zhao, K.X.; Pan, H.R.; Yu, Y.J.; Mi, J.B.; Su, M.Y.; Yang, Y.C. Determination of Picrasidine and Picrasidine Oxide Residues in Lycium barbarum by Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Res. Dev. 2024, 45, 189–195. [Google Scholar]
- Song, W.R. Studies on the Chemical Constituents of Incarvillea Sinensis Andtheir Activity for Tumor Cell Migration. Master’s Thesis, Qinghai University, Xining, China, 2023. [Google Scholar]
- Sun, X.J.; Zhou, Z.; Wang, Q.; Zhang, B.Q. A Preliminary Discussion on the Application of Sophora flavescens in Various Dynasties. Hunan J. Tradit. Chin. Med. 2018, 34, 139–140. [Google Scholar]
- Liu, W.; Tiang, J.H.; Wang, Y.D. Research progress on Sophora flavescens Ait. Lishizhen Med. Mater. Medica Res. 2006, 05, 829–830. [Google Scholar]
- Sun, X.Y.; Jia, L.Y.; Rong, Z. Research advances on matrine. Front. Chem. 2022, 10, 867318. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Sun, X. Matrine: A promising natural product with various pharmacological activities. Front. Pharmacol. 2020, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, F.; Wu, L. Matrine exerts pharmacological effects through multiple signaling pathways: A comprehensive review. Drug Des. Dev. Ther. 2022, 533–569. [Google Scholar] [CrossRef]
- Li, Y.F.; Gao, X.J.; Wang, X.X. Wang Xixing’s Experience in Treating Tumors with Danggui Beimu Kushen Pills. Shanxi J. Tradit. Chin. Med. 2011, 27, 4–5+7. [Google Scholar]
- Jin, Y.; Yang, Q.; Liang, L.; Ding, L.; Liang, Y.; Zhang, D.; Wu, B.; Yang, T.; Liu, H.; Huang, T. Compound kushen injection suppresses human acute myeloid leukaemia by regulating the Prdxs/ROS/Trx1 signalling pathway. J. Exp. Clin. Cancer Res. CR 2018, 37, 277. [Google Scholar] [CrossRef]
- Gao, L.; Wang, K.X.; Zhou, Y.Z.; Fang, J.S.; Qin, X.M.; Du, G.H. Uncovering the anticancer mechanism of Compound Kushen Injection against HCC by integrating quantitative analysis, network analysis and experimental validation. Sci. Rep. 2018, 8, 624. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xin, W. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sci. 2018, 192, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, D.; Wang, X.; Hu, Z.; Yan, Y.; Huang, J. Matrine suppresses proliferation and invasion of SGC7901 cells through inactivation of PI3K/Akt/uPA pathway. Ann. Clin. Lab. Sci. 2016, 46, 457–462. [Google Scholar]
- Chang, C.; Liu, S.P.; Fang, C.H.; He, R.S.; Wang, Z.; Zhu, Y.Q.; Jiang, S.W. Effects of matrine on the proliferation of HT29 human colon cancer cells and its antitumor mechanism. Oncol. Lett. 2013, 6, 699–704. [Google Scholar] [CrossRef]
- Huang, H.; Du, T.; Xu, G.; Lai, Y.; Fan, X.; Chen, X.; Li, W.; Yue, F.; Li, Q.; Liu, L. Matrine suppresses invasion of castration-resistant prostate cancer cells by downregulating MMP-2/9 via NF-κB signaling pathway. Int. J. Oncol. 2017, 50, 640–648. [Google Scholar] [CrossRef]
- Vardarlı, A.T.; Düzgün, Z.; Erdem, C.; Kaymaz, B.T.; Eroglu, Z.; Çetintas, V.B. Matrine induced G0/G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia (T-ALL) cells. Bosn. J. Basic Med. Sci. 2018, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xu, M. Matrine inhibits the migratory and invasive properties of nasopharyngeal carcinoma cells. Mol. Med. Rep. 2015, 11, 4158–4164. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Cai, D.; Li, M. Matrine inhibits the metastatic properties of human cervical cancer cells via downregulating the p38 signaling pathway. Oncol. Rep. 2017, 38, 1312–1320. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Feng, J.Y.; You, L.S.; Meng, H.T.; Qian, W.B. Matrine and CYC116 synergistically inhibit growth and induce apoptosis in multiple myeloma cells. Chin. J. Integr. Med. 2015, 21, 635–639. [Google Scholar] [CrossRef]
- Jin, H.; Sun, Y.; Wang, S.; Cheng, X. Matrine activates PTEN to induce growth inhibition and apoptosis in V600EBRAF harboring melanoma cells. Int. J. Mol. Sci. 2013, 14, 16040–16057. [Google Scholar] [CrossRef]
- Ur Rashid, H.; Xu, Y.; Muhammad, Y.; Wang, L.; Jiang, J. Research advances on anticancer activities of matrine and its derivatives: An updated overview. Eur. J. Med. Chem. 2019, 161, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Y.; Ji, W.; Li, X.; Sun, B.; Gao, Q.; Su, C. Anti-tumor activities of matrine and oxymatrine: Literature review. Tumor Biol. 2014, 35, 5111–5119. [Google Scholar] [CrossRef] [PubMed]
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and activity of pentacyclic triterpenes codrugs. A review. Mini Rev. Med. Chem. 2021, 21, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Betulinic acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef]
- Liu, L.M.; Liu, H.G.; Mao, L.; Chen, Z.F.; Wang, H.S. Effects of matrine and oxymatrine on tumor cell proliferation in vitro. Chin. J. Exp. Tradit. Med. Formulae 2008, 11, 35–36. [Google Scholar]
- Mao, L. Effect of Au (III) complex with oxymatrine and matrine on proliferation of tumor cells in vitro and its mechanisms. Chin. Tradit. Herb. Drugs 2019, 50, 639–646. [Google Scholar]
- Wang, X.L.; Li, H.X.; Wang, L.H.; Liu, X.M. Killing effect of matrine combined with DC—CIK cells on breast cancer cells. Chin. J. Clin. Pharmacol. 2023, 39, 2311–2315. [Google Scholar]
- Jia, S.H.; Sun, M.Y.; Ding, H.X.; Jin, S.P. Study on the Effect of Matrine on Autophagy and Apoptosis of Human Breast Cancer MCF-7 Cells. J. Chin. Med. Mater. 2023, 46, 724–729. [Google Scholar]
- Sun, L.; Qian, S.Y.; Mao, X.P.; Gu, X. Biotransformation preparation and anti-tumor activity of matrine derivatives. Pharm. Clin. Res. 2017, 25, 24–26. [Google Scholar]
- Ou, X.Y. Study on the Mechanism and Related Pharmacological Effects of Matrine Induced Endoplasmic Reticulum Stress. Ph.D. Thesis, Peking Union Medical College Hospital, Beijing, China, 2014. [Google Scholar]
- Zhao, W.G.; Sun, Y.; Zhuang, J.; Feng, F.B.; Li, J.; Sun, C.G. Evaluation of the synergistic effect of Picrasidine combined with Adriamycin on human breast cancer cells by the Kim Jung Mean Q-value method. Lishizhen Med. Mater. Medica Res. 2018, 29, 33–36. [Google Scholar]
- Sui, H. Effects of matrine on proliferation of human breast cancer cells. China J. Mod. Med. 2013, 23, 41–43. [Google Scholar]
- Wang, S.Q.; Li, H.J.; Zhang, L.X.; Li, Y.Z.; Hao, X.Q. Matrine induces apoptosis of breast cancer MCF-7 cells and its effect on Bax expression. Chin. J. Gerontol. 2012, 32, 3489–3491. [Google Scholar]
- Li, H.J.; Zhao, X.X.; Bai, M.L.; Zhang, L.X.; Li, Y.Z.; Liu, H. Effects of matrine on apoptosis and mitochondrial transmembrane potential of breast cancer MCF-7 cells. Lishizhen Med. Mater. Medica Res. 2011, 22, 2042–2043. [Google Scholar]
- Gu, M.R.; Li, J.B.; Zhang, X.Y.; Yin, Y.M. Analysis of induced apoptosis effect of matrine on human breast cancer MCF-7 cell and its effect on mitochondrial transmembrane potential. Chin. J. Biochem. Pharm. 2015, 35, 39–41. [Google Scholar]
- Li, H.J.; Wang, J.M.; Tian, Y.T.; Bai, M.L.; Zhang, L.X.; Zhao, X.X. Effect of Matrine on Fas, VEGF, and Activities of Telomerase of MCF-7 Cells. Chin. J. Integr. Tradit. West. Med. 2013, 33, 1247–1251. [Google Scholar]
- Wei, C.S. Marine Cooperates with Chemotherapy Drug and Reverses Multidrug Resistance in Breast Cancer Drug-Resistant Cell Line MCF-7/ADR Through Inhibiting PI3K/AKT Signal Pathway. Master’s Thesis, Ningxia Medical University, Yinchuan, China, 2015. [Google Scholar]
- Zhou, B.G.; Sun, J.Z.; Su, G.; Ma, D.Q. Apoptosis of human breast cancer MCF-7/ADR cells induced by matrine. Chin. J. Exp. Surg. 2003, 06, 36–37. [Google Scholar]
- Ren, L.Q. Effects and Mechanisms of Matrine on Human Breast Cancer Cells. Master’s Thesis, Liaoning University of Traditional Chinese Medicine, Shenyang, China, 2023. [Google Scholar]
- Guo, Q.S. Study on the Mechanism of Matrine Combined with Docetaxel in Promoting Apoptosis of Triple Negative Human Breast Cancer MDA-MB-231 Cells. Ph.D. Thesis, ZheJiang University of TCM, Hangzhou, China, 2022. [Google Scholar]
- Xiao, X.; Ao, M.; Shi, W.D.; Pu, Z.F.; Li, D.X.; Hu, J.L. Effects of matrine on proliferation and apoptosis of Bcap-37 cells in breast cancer. Chin. Tradit. Pat. Med. 2018, 40, 2750–2754. [Google Scholar]
- Shi, W.D.; Li, D.X.; Hu, J.L.; Wang, Y.; Yang, N.; Xiao, X. Matrine inhibits proliferation of breast cancer 4T1 cells by regulating expression of annexin A3. Ningxia Med. J. 2018, 40, 967–969. [Google Scholar]
- Xiao, X.; Ao, M.; Shi, W.D.; Pu, Z.F.; Li, D.X.; Hu, J.L. Effects of matrine on proliferation and apoptosis of breast cancer SK-BR-3 cells. ShanDong Med. J. 2018, 58, 24–27. [Google Scholar]
- Zhou, B.G.; Wei, C.S.; Zhang, S.; Zhang, Z.; Wang, J. The matrine reverse multidrug resistance on breast cancer MCF-7 /ADR cell line and have an influence on downstream factors of PI3K/AKT signal pathway. J. Mod. Oncol. 2017, 25, 9–13. [Google Scholar]
- Ren, L.L.; Lan, T.; Wang, X.J. Antitumor Effect of Matrine in Human Breast Cancer Bcap-37 Cells by Apoptosis and Autophagy. Chin. Arch. Tradit. Chin. Med. 2014, 32, 2756–2759. [Google Scholar]
- Yu, M.; Cao, F.; Zhu, R.; Ding, H.Z. Effect of ZIC1 associated with matrine on proliferation, migration and apoptosis in MDA-MB-231 human breast cancer cell line. J. Mod. Oncol. 2016, 24, 1528–1533. [Google Scholar]
- Wei, C.S.; Shen, Y.J.; Zhang, Z.; Wang, J.; Zhou, B.G. Matrine reverses multidrug resistance in breast cancer drug-resistant cell line MCF-7 /ADR through inhibiting PI3K/AKT signal pathway. J. Army Med. Univ. 2014, 36, 2254–2258. [Google Scholar]
- Zhen, R.Z.; Zhang, J.Y.; Shao, X.Y.; Wang, X.J. Inhibition of the Growth of Human Breast Cancer Cell Line Bcap-37 with Matrine. Zhejiang J. Integr. Tradit. Chin. West. Med. 2012, 22, 945–947. [Google Scholar]
- Zhen, R.Z.; Zhang, J.Y.; Shao, X.Y.; Wang, X.J. Mechanism of Matrine Promoting Apoptosis Induced by Tamoxifen in Breast Cancer Cell Bcap-37. J. Chin. Oncol. 2012, 18, 840–843. [Google Scholar]
- Li, H.J.; Zhao, X.; Bai, M.L.; Zhang, L.X.; Liu, H.; Ge, L.P. Effects of matrine on proliferation and apoptosis in human breast cancer MCF-7 cells. Jiangsu Med. J. 2011, 37, 396–398+368. [Google Scholar]
- Zhen, R.Z.; Zhang, J.Y.; Shao, X.Y.; Wang, X.J. In Mechanism of Matrine Promoting Apoptosis in Breast Cancer Cell Bcap-37, The Third Conference of Traditional Chinese and Western Medicine Physicians of the Chinese Medical Association in 2012. 2012; 134. [Google Scholar]
- Zhou, B.G.; Ge, L.J.; Jin, L.Y.; Wu, Y.Z.; Fan, Y.Z.; Su, G. Effect of matrine on the expression of bcl-2 protein, bax protein and Fas antigen in breast cancer cells. Chin. J. Exp. Surg. 2007, 24, 1261. [Google Scholar]
- Jia, S.H.; Ding HSun, M.Y.; Jin, S.P. Research on Matrine Induced Autophagy of Human Breast Cancer MCF-7 Cells Based on PI3K/Akt/mTOR Signal Pathway and Its Mechanism. Food Drug 2023, 25, 159–163. [Google Scholar]
- Li, H.Y.; Chen, Y.; He, Q.Y. Mechanism of Combination of Matrine and Tetrandrine for Reversal of Multidrug Resistance of Breast Carcinoma MCF-7 Cells. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 275–278. [Google Scholar]
- Wang, X.K.; Li, J.; Wei, L.X.; Yan, Y.N.; Wang, R.Q. The Effects of Inhibiting Tumor in vitro of Alkaloidsfrom the Seed of Sophora viciifolia. J. Beijing Univ. Tradit. Chin. Med. 1996, 02, 59–60+73. [Google Scholar]
- Du, J.; Li, J.; Song, D.; Li, Q.; Li, L.; Li, B.; Li, L. Matrine exerts anti-breast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF-7 cells. Mol. Med. Rep. 2020, 22, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Yang, B.; Hu, R.; Wang, Y. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-κB signaling pathway. Oncol. Lett. 2013, 6, 517–520. [Google Scholar] [CrossRef]
- Xiao, X.; Ao, M.; Xu, F.; Li, X.; Hu, J.; Wang, Y.; Li, D.; Zhu, X.; Xin, C.; Shi, W. Effect of matrine against breast cancer by downregulating the vascular endothelial growth factor via the Wnt/β-catenin pathway. Oncol. Lett. 2018, 15, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, T.; Chen, S.S.; Wang, M.; Wang, R.; Li, K.; Wang, J.C.; Xu, C.W.; Du, N.; Qin, S. Matrine suppression of self-renewal was dependent on regulation of LIN28A/Let-7 pathway in breast cancer stem cells. J. Cell. Biochem. 2020, 121, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Q.; Li, X.-L.; Wang, L.; Du, W.-J.; Guo, R.; Liang, H.-H.; Liu, X.; Liang, D.-S.; Lu, Y.-J.; Shan, H.-L. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell. Physiol. Biochem. 2012, 30, 631–641. [Google Scholar] [CrossRef]
- Ren, L.; Mo, W.; Wang, L.; Wang, X. Matrine suppresses breast cancer metastasis by targeting ITGB1 and inhibiting epithelial-to-mesenchymal transition. Exp. Ther. Med. 2020, 19, 367–374. [Google Scholar] [CrossRef]
- Shi, Y.; Su, G.; Fang, H. Method for quantitative determination of matrine in Sophora alopecuroides L. and its inhibitory effect on breast cancer MCF-7 cell proliferation. Biomed. Res. 2015, 26, 461–466. [Google Scholar]
- Guo, Q.; Yu, Y.; Tang, W.; Zhou, S.; Lv, X. Matrine exerts an anti-tumor effect via regulating HN1 in triple breast cancer both in vitro and in vivo. Chem. Biol. Drug Des. 2023, 102, 1469–1477. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, D.; Wang, H.; Wu, D.; Chen, Y.; Ji, K.; Qin, T.; Wu, L. Matrine suppresses the ER-positive MCF cells by regulating energy metabolism and endoplasmic reticulum stress signaling pathway. Phytother. Res. 2017, 31, 671–679. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Bai, M.; Suo, Y.; Zhang, G.; Cao, X. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int. J. Clin. Exp. Pathol. 2015, 8, 14793. [Google Scholar]
- Jiang, L.; Wu, L.; Yang, F.; Almosnid, N.; Liu, X.; Jiang, J.; Altman, E.; Wang, L.; Gao, Y. Synthesis, biological evaluation and mechanism studies of matrine derivatives as anticancer agents. Oncol. Lett. 2017, 14, 3057–3064. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.; Hu, S.; Chen, X.; Bai, X. Screening and research of anti-cancer matrine components based on hollow fiber cell fishing with high-performance liquid chromatography. Chromatographia 2016, 79, 125–136. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Ma, X.; Yao, Y.; Zhou, Q.; Zhang, W.; Zhou, C.; Zhuang, J. MAT as a promising therapeutic strategy against triple-negative breast cancer via inhibiting PI3K/AKT pathway. Sci. Rep. 2023, 13, 12351. [Google Scholar] [CrossRef]
- Li, H.; Tan, G.; Jiang, X.; Qiao, H.; Pan, S.; Jiang, H.; Kanwar, J.R.; Sun, X. Therapeutic effects of matrine on primary and metastatic breast cancer. Am. J. Chin. Med. 2010, 38, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Sarem, M.; Xiang, S.; Hu, H.; Xu, W.; Shastri, V.P. Autophagy inhibition enhances Matrine derivative MASM induced apoptosis in cancer cells via a mechanism involving reactive oxygen species-mediated PI3K/Akt/mTOR and Erk/p38 signaling. BMC Cancer 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Thang, P.N.T.; Tran, V.-H.; Vu, T.A.; Vinh, N.N.; Huynh, D.T.M.; Pham, D.T. Determination of antioxidant, cytotoxicity, and acetylcholinesterase inhibitory activities of alkaloids isolated from Sophora flavescens Ait. Grown in Dak Nong, Vietnam. Pharmaceuticals 2022, 15, 1384. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.H.; Motaez, M.; Zamiri-Akhlaghi, A.; Emami, S.A.; Tayarani-Najaran, Z. In-vitro evaluation of cytotoxic and apoptogenic properties of Sophora pachycarpa. Iran. J. Pharm. Res. IJPR 2014, 13, 665. [Google Scholar] [PubMed]
- Yu, P.; Liu, Q.; Liu, K.; Yagasaki, K.; Wu, E.; Zhang, G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κ B signaling. Cytotechnology 2009, 59, 219–229. [Google Scholar] [CrossRef]
- Chui, C.H.; Lau, F.Y.; Tang, J.C.O.; Kan, K.L.; Cheng, G.Y.M.; Wong, R.S.M.; Kok, S.H.L.; Lai, P.B.S.; Ho, R.; Gambari, R. Activities of fresh juice of Scutellaria barbata and warmed water extract of Radix Sophorae Tonkinensis on anti-proliferation and apoptosis of human cancer cell lines. Int. J. Mol. Med. 2005, 16, 337–341. [Google Scholar] [CrossRef]
- Dong, H.D. Effect of Matrine on JNK1/AP-1 Signaling Pathway in Mice Model with 4T1Breast Cancer. J. Liaoning Univ. Tradit. Chin. Med. 2019, 21, 64–68. [Google Scholar]
- Zhang, R.K.; Wang, C. Effect of matrine on tumor growth and inflammatory factors and immune function in Wistar rat with breast cancer. Chin. J. Appl. Physiol. 2018, 34, 375–378. [Google Scholar]
- Mao, H.S.; Liu, H.; Li, C.; Song, Y.S.; Sun, H.; Feng, Y.M. Modulation of the Malignant Phenotype and theImmunological Response of Tumor Cellsby Sophora Flavescens Ait(AF-7). Chin. J. Clin. Oncol. 1996, 11, 44–48. [Google Scholar]
- Bai, Y.L.; Li, X.J.; Liang, L.; Li, X.Q. Effect of matrine injection on sensitization of TM40D human breast cancer bearing mice and its mechanism. Propr. Chin. Med. 2016, 38, 1608–1610. [Google Scholar]
- Zhou, B.G.; Wei, C.S.; Zhang, S.; Su, Z.M.; Zhang, Z.; Wang, J. Effects of matrine on the growth of human breast cancer MCF-7/ADR cells transplanted in nude mice and apoptosis factors of phosphoinositol 3 kinase/protein kinase B signaling pathway. Chin. J. Exp. Surg. 2017, 34, 4. [Google Scholar]
- Zhang, X. Study on the Molecular Mechanisms of the Antitumor Effect of Matrine. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2020. [Google Scholar]
- Zhang, X.J.; Zhang, X.; Ma, H.G.; Hu, X.Q. Mechanism of Chinese medicine Fuzheng in reversing paclitaxel resistance in TLR4 over-expressed breast cancer. J. Wenzhou Med. Univ. 2019, 49, 826–831. [Google Scholar]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Wen, F.; Lu, Z.M. Progression of the roles of apoptosis in breast cancer therapy. Chin. Clin. Oncol. 2017, 22, 175–179. [Google Scholar]
- Pierce, B.L.; Ballard-Barbash, R.; Bernstein, L.; Baumgartner, R.N.; Neuhouser, M.L.; Wener, M.H.; Baumgartner, K.B.; Gilliland, F.D.; Sorensen, B.E.; McTiernan, A. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 2009, 27, 3437–3444. [Google Scholar] [CrossRef]
- Foo, J.B.; Yazan, L.S.; Tor, Y.S.; Wibowo, A.; Ismail, N.; Armania, N.; Cheah, Y.K.; Abdullah, R. Dillenia suffruticosa dichloromethane root extract induced apoptosis towards MDA-MB-231 triple-negative breast cancer cells. J. Ethnopharmacol. 2016, 187, 195–204. [Google Scholar] [CrossRef]
- Ren, L.L.; Wang, L.; Wang, X.J. Relationship Between Matrine Induced Autophagy and mTOR in Human Breast Cancer Bcap-37 Cells. Zhejiang JITCWM 2016, 26, 783–786. [Google Scholar]
- Wang, S.; Wang, K.; Wang, H.; Han, J.; Sun, H. Autophagy is essential for flavopiridol-induced cytotoxicity against MCF-7 breast cancer cells. Mol. Med. Rep. 2017, 16, 9715–9720. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Udristioiu, A.; Nica-Badea, D. Autophagy dysfunctions associated with cancer cells and their therapeutic implications. Biomed. Pharmacother. 2019, 115, 108892. [Google Scholar] [CrossRef]
- Xie, Z.; Xie, Y.; Xu, Y.; Zhou, H.; Xu, W.; Dong, Q. Bafilomycin A1 inhibits autophagy and induces apoptosis in MG63 osteosarcoma cells. Mol. Med. Rep. 2014, 10, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z. Regulation of neuronal autophagy in axon: Implication of autophagy in axonal function and dysfunction/degeneration. Autophagy 2007, 3, 139–141. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor family genes: When did the three genes phylogenetically segregate? 2002, 383, 1573–1579. Biol. Chem. 2002, 383, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Fedewa, S.A.; Goding Sauer, A.; Kramer, J.L.; Smith, R.A.; Jemal, A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA A Cancer J. Clin. 2016, 66, 31–42. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Zhao, X.X.; Tian, T.; Yan, W.J.; Ma, X.C.; Zhang, H.; Wang, W. Regulating Effects and Molecular Mechanisms of Traditional Chinese Medicine in the Tumor Immunity. J. Mod. Oncol. 2016, 24, 306–309. [Google Scholar]
- Peng, R.Q.; Zhang, X. New perspectives on tumor immunotherapy and its clinical practice. J. Pract. Oncol. 2013, 28, 339–342. [Google Scholar]
- Yaguchi, T.; Sumimoto, H.; Kudo-Saito, C.; Tsukamoto, N.; Ueda, R.; Iwata-Kajihara, T.; Nishio, H.; Kawamura, N.; Kawakami, Y. The mechanisms of cancer immunoescape and development of overcoming strategies. Int. J. Hematol. 2011, 93, 294–300. [Google Scholar] [CrossRef]
- Tian, T.D.; Yue, L.Y.; Tian, T.L.; Fan, Y.X.; Zhang, X.F. Relationship between tumor inflammatory microenvironment and immunity and intervention strategy of traditional Chinese medicine. J. Tradit. Chin. Med. 2017, 58, 209–213. [Google Scholar]
- He, S.; Liu, F.; Xie, Z.; Zu, X.; Xu, W.; Jiang, Y. P-Glycoprotein/MDR1 regulates pokemon gene transcription through p53 expression in human breast cancer cells. Int. J. Mol. Sci. 2010, 11, 3039–3051. [Google Scholar] [CrossRef]
- Kim, D.; Cheng, G.Z.; Lindsley, C.W.; Yang, H.; Cheng, J.Q. Targeting the phosphatidylinositol-3 kinase/Akt pathway for the treatment of cancer. Curr. Opin. Investig. Drugs 2005, 6, 1250–1258. [Google Scholar] [PubMed]
- Tian, T.; Nan, K.J.; Guo, H.; Wang, W.J.; Ruan, Z.P.; Wang, S.H.; Liang, X.; Lu, C.X. PTEN inhibits the migration and invasion of HepG2 cells by coordinately decreasing MMP expression via the PI3K/Akt pathway. Oncol. Rep. 2010, 23, 1593–1600. [Google Scholar] [PubMed]
- Lee, C.S.; Kim, Y.J.; Jang, E.R.; Myung, S.C.; Kim, W. Akt inhibitor enhances apoptotic effect of carboplatin on human epithelial ovarian carcinoma cell lines. Eur. J. Pharmacol. 2010, 632, 7–13. [Google Scholar] [CrossRef]
- Burris, H.A. Overcoming acquired resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR pathway. Cancer Chemother. Pharmacol. 2013, 71, 829–842. [Google Scholar] [CrossRef]
- Calderaro, J.; Rebouissou, S.; de Koning, L.; Masmoudi, A.; Hérault, A.; Dubois, T.; Maille, P.; Soyeux, P.; Sibony, M.; de la Taille, A. PI3K/AKT pathway activation in bladder carcinogenesis. Int. J. Cancer 2014, 134, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Tapia, O.; Riquelme, I.; Leal, P.; Sandoval, A.; Aedo, S.; Weber, H.; Letelier, P.; Bellolio, E.; Villaseca, M.; Garcia, P. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014, 465, 25–33. [Google Scholar] [CrossRef]
- Guo, C.Y.; Ke WFSong, K.Y.; Wang, J.F.; Zhou, L.; Li, K. PI3K/AKT signalling pathway involves in the modulation of multidrug resistance and metastasis in breast cancer. Prog. Mod. Biomed. 2012, 12, 4809–4812. [Google Scholar]
- Liu, F.; Liu, S.; He, S.; Xie, Z.; Zu, X.; Jiang, Y. Survivin transcription is associated with P-glycoprotein/MDR1 overexpression in the multidrug resistance of MCF-7 breast cancer cells. Oncol. Rep. 2010, 23, 1469–1475. [Google Scholar] [PubMed]
- Ren, D.; Jia, L.; Li, Y.; Gong, Y.; Liu, C.; Zhang, X.; Wang, N.; Zhao, Y. ST6GalNAcII mediates the invasive properties of breast carcinoma through PI3K/Akt/NF-κB signaling pathway. IUBMB Life 2014, 66, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Choi, Y.; Shin, J.M.; Cho, H.J.; Kang, J.H.; Park, K.K.; Choe, J.Y.; Bae, Y.S.; Han, S.M.; Kim, C.H. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem. Toxicol. 2014, 68, 218–225. [Google Scholar] [CrossRef]
- Huemer, F.; Bartsch, R.; Gnant, M. The PI3K/AKT/MTOR signaling pathway: The role of PI3K and AKT inhibitors in breast cancer. Curr. Breast Cancer Rep. 2014, 6, 59–70. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, Z.; Yao, H.; Sun, L.; Lin, L. An effective drug sensitizing agent increases gefitinib treatment by down regulating PI3K/Akt/mTOR pathway and up regulating autophagy in non-small cell lung cancer. Biomed. Pharmacother. 2019, 118, 109169. [Google Scholar] [CrossRef]
- Liu, N. Clinical Effect of Compound Sophora Flavescens Lotion Combined with Clotrimazole Vaginal Tablet in the Treatment of Candida Vaginitis during Pregnancy. J. Med. Inf. 2018, 31, 145–146. [Google Scholar]
- Lv, P.G.; Jin, Y.N.; Xu, H.Y. The clinical observation of 40 cases of infantile acute eczema treated with Fufang Kushen Lotion. J. Pediatr. Tradit. Chin. Med. 2017, 13, 50–52. [Google Scholar]
- Wu, X.; Yamashita, F.; Hashida, M.; Chen, X.; Hu, Z. Determination of matrine in rat plasma by high-performance liquid chromatography and its application to pharmacokinetic studies. Talanta 2003, 59, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Z.; Wen, L.; Jiang, C.; Feng, Q. Matrine: A review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches. J. Ethnopharmacol. 2021, 269, 113682. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Q.; He, Z.; Yin, L.; Zhang, Y.; Wang, S. Liver, blood microdialysate and plasma pharmacokinetics of matrine following transdermal or intravenous administration. Die Pharm.-Int. J. Pharm. Sci. 2017, 72, 167–170. [Google Scholar]
- Yang, L.Y. Analysis on Matrine Toxicity and HPLC Determination of Matrine in Sheep Plasma. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2015. [Google Scholar]
- Gong, X.; Gao, Y.; Guo, G.; Vondran, F.W.; Schwartlander, R.; Efimova, E.; Pless, G.; Sauera, I.M.; Neuhaus, P. Effect of matrine on primary human hepatocytes in vitro. Cytotechnology 2015, 67, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yuan, T.J.; Gu, L.L.; Lu, H. Study of Hepatotoxicity and Neural Behavioral Changes of Sophora Flavescens and Matrine in Mice. Chin. J. Mod. Appl. Pharm. 2015, 32, 1444–1448. [Google Scholar]
- Gu, Y.M.; Lu, J.Y.; Sun, W.; Jin, R.M.; Ohira, T.; Zhang, Z. Oxymatrine and its metabolite matrine contribute to the heparotoxicity induced by radix Sophorae tonkinensis in mice. Exp. Ther. Med. 2019, 17, 2519–2528. [Google Scholar] [PubMed]
- Guo, Q.P.; Chen, G.Y.; Zhou, Q.; Jin, R.M. Comparison of hepatotoxicity and toxic mechanisms of matrine and oxymatrine using in vivo and in vitro models. Chin. J. Compart. Med. 2018, 28, 44–50. [Google Scholar]
- Li, P.P. Study on the rule of residue elimination and hepatotoxicity of dichroine and matrine in broyers. Master’s Thesis, Agricultural University Of Hebei, Baoding, China, 2022. [Google Scholar]
- You, L.T.; Yang, C.J.; Du, Y.Y.; Liu, Y.; Chen, G.S. Matrine exerts hepatotoxic effects via the ROS-dependent mitochondrial apoptosis pathway and inhibition ofNrf2-mediated antioxidant response. Oxidative Med. Cell. Longev. 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Luo, T.; Zou, Q.; He, Y.; Wang, H.; Li, N.; Zeng, X. Matrine Inhibits Mouse Sperm Function by Reducing Sperm [Ca2+]iand Phospho-ERK1/2. Cell. Physiol. Biochem. 2015, 35, 374–385. [Google Scholar] [CrossRef]

| Familia | Genus | Name | Part Used | Reference |
|---|---|---|---|---|
| Leguminosae | Sophora L. | Sophora flavescens Aiton | Roots | Yang et al., 2024 [15] |
| Leguminosae | Sophora L. | Sophora alopecuroides L. | Fruits | Chen et al., 2023 [16] |
| Leguminosae | Sophora L. | Sophora xanthoantha C. Y. Ma | Flowers, buds | Chi ZL, 2011 [17] |
| Leguminosae | Sophora L. | Sophora davidii Kom. ex Pavol. | Stem, leaf, flower, seed, roots | Xu et al., 2023 [18] |
| Leguminosae | Sophora L. | Sophora mollis Graham. | Roots | Peng et al., 2010 [19] |
| Leguminosae | Sophora L. | Sophora pachycarpa Schrenk ex C. A. Mey | Roots | Zheng et al., 1996 [20] |
| Leguminosae | Sophora L. | Sophora dunnii Prain | Roots | Ling et al., 2000 [21] |
| Leguminosae | Sophora L. | Sophora moorcroftiana (Benth.) Baker | Seeds | Zhao et al., 2020 [22] |
| Leguminosae | Sophora L. | Sophora tonkinensis Gagnep. | Seeds | Zou et al., 2023 [23] |
| Leguminosae | Styphnolobium Schott | Styphnolobium japonicum (L.) Schott | Flowers, leaves, branches, roots, fruits | Wang et al., 2018 [24] |
| Leguminosae | Euchresta J. Benn. | Euchresta japonica Benth. ex Oliv. | Roots | Yuan et al., 2023 [25] |
| Leguminosae | Euchresta J. Benn. | Euchresta formosana (Hayata) Ohwi | Roots | Li et al., 2014 [26] |
| Leguminosae | Euchresta J. Benn. | Euchresta horsfieldii (Lesch.) J. Benn.) | Roots | Li et al., 2014 [26] |
| Leguminosae | Euchresta J. Benn. | Euchresta tubulosa Dunn | Roots | Li et al., 2014 [26] |
| Solanaceae Juss. | Lycium L. | Lycium chinense Miller | Fruits | Zhang et al., 2024 [27] |
| Bignoniaceae Juss. | Incarvillea Juss. | Incarvillea sinensis Lam. | Grass | Song et al., 2023 [28] |
| Cells Type | Composition (mg·mL −1) | Time of Administration (h) | Cell Proliferation Inhibition Rate/ Cell Apoptosis | Influenced Gene/ Protein | Other | Ref. |
|---|---|---|---|---|---|---|
| MCF-7 | MT | 48 | Cell proliferation was detected by MTT assay, and the IC50 value was 0.68 ± 2.00 mg·mL−1. | NA | NA | Liu et al., 2008 [50] |
| MCF-7 | MT | 24, 48, 72 | Cell proliferation was detected by MTT assay, and the IC50 value was 0.93 ± 9.03 mg·mL−1. | NA | NA | Mao et al., 2019 [51] |
| MCF-7 | MT | 48 | The cell survival rate detected by the MTT method with 4.0 mg·mL−1 MT was 43.13 ± 5.37%. | Significant decrease in p-JAK2, p-STAT3 protein levels. | NA | Wang et al., 2023 [52] |
| MCF-7 | MT | 48 | The cell survival rate detected by MTT assay, and the IC50 value was 0.85 mg·mL−1. | LC3-I/LC3-II levels were significantly increased, and Beclin-1 protein expression was increased. | NA | Jia et al., 2023 [53] |
| MCF-7 | MT | NA | Cell proliferation was detected by MTT assay, and the IC50 value was 15.8 mg·mL−1. | NA | NA | Sun et al., 2017 [54] |
| MCF-7 | MT | 72 | Cell proliferation was detected by MTT assay, and the IC50 value was 4.83 mg·mL−1. Cycle retention in the G1 phase, S phase, and G2/M phase cell numbers decreased. | The expression of p21 and p27 was upregulated, p53 and cyclin-D1 were downregulated, and the accumulation of LC3-II was increased. | NA | Ou, 2014 [55] |
| MCF-7 | MT | 48 | Cell proliferation was detected by MTT assay, and the IC50 value was 2.00 ± 0.17 mg·mL−1, and it showed concentration-dependent inhibition. | Bax protein expression was upregulated. | NA | Zhao et al., 2018 [56] |
| MCF-7 | MT | 24, 48, 72 | The inhibition rate at 72 h of action time with 4.0 mg·mL−1 MT was 73.29 ± 4.07%, and it showed concentration-dependent inhibition. Cell proliferation was stagnant in the G0/G1 phase, and the number of cells in S phase was reduced. | Bax protein expression was upregulated and downregulated Bcl-2 protein expression. | NA | Sui, 2013 [57] |
| MCF-7 | MT | 24, 48, 72 | The inhibition rate at 72 h of action time with 2.0 mg·mL−1 MT was 68. 31 ± 2.13% and it showed concentration-dependent inhibition and promoted apoptosis. | Decreased mitochondrial transmembrane potential and increased Bax expression. | NA | Wang et al., 2012 [58] |
| MCF-7 | MT | 24 | The inhibition rate at 24 h of action time with 2.0 mg·mL−1 MT was 19.63 ± 0.17%. | Decreased mitochondrial transmembrane potential. | Cells show shortened protrusions, reduced cytoplasm, intracytoplasmic vacuoles, and nuclear consolidation and fragmentation. | Li et al., 2011 [59] |
| MCF-7 | MT | 24, 48, 72 | The inhibition rate at 72 h of action time with 2.0 mg·mL−1 MT was 67.16 ± 2.14%, and it showed concentration-dependent inhibition. The apoptosis rate of 2.0 mg·mL−1 MT for 24 h was 20.16 ± 0.16%. | Decreased mitochondrial transmembrane potential | NA | Gu et al., 2015 [60] |
| MCF-7 | MT | 24 | Significantly inhibited growth and promoted apoptosis. | Upregulation of Fas protein expression and downregulation of VEGF protein and telomerase activity gradually decreased with increasing concentrations of MT. | Cells show shortened protrusions, reduced cytoplasm, and nuclear consolidation and fragmentation with a budding of the cell membrane. | Li et al., 2013 [61] |
| MCF-7 | MT | 24 | The MTT assay showed a concentration- and time-dependent inhibition rate of 22.35 ± 0.84% for mg·mL−1 MT at 24 h. | Decreased p-pg, MRP1 protein, and p-AKT protein expression in the PI3K/AKT-signaling pathway and increased PTEN protein expression. | NA | Wei et al., 2016 [62] |
| MCF-7 | MT | 72 | The inhibition rate of MCF-7/ADR cells at 1.25 mg·mL−1 MT was 66.2% with an IC50 of 0.92 mg·mL−1. The proportion of S-phase cells increased, and the proportion of G2/M cells decreased. | NA | Cytosol shrinkage and cytoplasmic condensation. | Zhou et al., 2003 [63] |
| MCF-7 | MT | 24 | After 24 h, the concentrations of 2.0 mg·mL−1 and 2.4 mg·mL−1 of MT induced the late apoptosis rate to be 23.5% ± 0.024 and 56.82% ± 0.042. The IC50 at 24 h was 2.729 mg·mL−1. | Inhibits the expression of IL-6, JAK1, P-JAK1, STAT3, and P-STAT3 proteins by a molecular mechanism that may be related to its effective regulation of the IL-6/STAT3 pathway. | NA | Ren, 2023 [64] |
| MDA-MB-231 | MT | 24, 48, 72 | The inhibition rate at 72 h of action time with 5 mg·mL−1 MT was 67.41%. | Downregulation of HN1 protein expression and decreased expression of VEGF and CD31. | NA | Guo, 2022 [65] |
| Bcap-37 | MT | 48 | The inhibition rate at 48 h of action time with 2.0 mg·mL−1 MT was 18.25 ± 1.12%. | Decreased Cyclin D1 and c-Myc protein expression. | NA | Xiao et al., 2018 [66] |
| 4T1 | MT | 48 | The inhibition rate at 48 h of action time with 0.4 mg·mL−1 MT was 17.32 ± 3.09%. | Significantly decreased ANXA3 protein expression. | NA | Shi et al., 2018 [67] |
| SK-BR-3 | MT | 48 | The inhibition rate at 48 h of action time with 3.0 mg·mL−1 MT was 46.6 ± 3.2% and showed concentration-dependent inhibition. | Inhibition of miR-21 expression and upregulation of PTEN protein expression. | NA | Xiao et al., 2018 [68] |
| MCF-7 | MT | 72 | Cell proliferation was detected by MTT assay, and the IC50 value was 0.92 mg·mL−1. | The relative expression of Bcl-2 and p65 was decreased, and the relative expression of Bax, PARP, and GSK-3β genes was increased. | NA | Zhou et al., 2017 [69] |
| Bcap-37 | MT | 24, 48, 72 | Cell proliferation was detected by MTT assay, proliferation inhibition existed in a concentration and time-dependent manner, and the apoptosis rate was 19.58% at 24 h of the action time of 2 mg·mL−1. | LC3b-II expression was upregulated. | NA | Ren et al., 2014 [70] |
| MDA-MB-231 | MT | 48 | MTT assay was used to detect cell proliferation, and the inhibition rate was 49.21 ± 1.12% at 0.2 mg·mL−1 action time 48 h. | It may be related to PI3K/Akt and MAPK/ERK-signaling pathway. | NA | Yu et al., 2016 [71] |
| MCF-7/ADR cell | MT | 24 | The MTT assay showed a concentration- and time-dependent inhibition rate of 11.82 ± 0.66% for 0.6 mg·mL−1 at 24 h. | Decreased expression of MDR1, MRP1, and AKT gene and increased expression of PTEN gene. | NA | Wei et al., 2014 [72] |
| Bcap-37 | MT | 24, 48, 72 | Cell proliferation was detected by MTT assay, and the proliferation inhibition was concentration- and time-dependent with IC50s of 0.63, 0.42, and 0.29 mg·mL−1 at 24, 48, and 72 h. | The expression of caspase-3 and Bax proteins was increased, and the expression of Bcl-2 protein was decreased. | NA | Zheng et al., 2012 [73] |
| Bcap-37 | MT | 24, 48, 72 | The highest apoptosis rate of 6.90 ± 0.19% was observed when the action time of 0.08 mg·mL−1 was 48 h. | The expression of p53 and Bax proteins was increased, and Bcl-2 protein was decreased. | NA | Zheng et al., 2012 [74] |
| MCF-7 | MT | 24, 48, 72 | Cell proliferation was detected by MTT assay, and proliferation inhibition was concentration- and time-dependent, with inhibition rates ranging from 10.86% to 70.23% at 72 h. | Bax protein expression was increased, and Bc-l2 protein was decreased. | NA | Li et al., 2011 [75] |
| Bcap-37 | MT | 24, 48, 72 | Cell proliferation was detected by MTT assay, and proliferation inhibition was concentration- and time-dependent. | Increased expression of p53 and Bax proteins and decreased expression of Bcl-2 proteins. | Decreased cytoplasm, rounding, and vacuolization of cells. | Zheng et al., 2012 [76] |
| MCF-7/ADR cell | MT | 48 | Cell proliferation was detected by MTT assay, and proliferation inhibition was concentration- and time-dependent. | Increased expression of Fas and bax proteins and decreased Bcl-2 proteins. | NA | Zhou et al., 2007 [77] |
| MCF-7 | MT | 24 | Cellular autophagy phenomenon was obvious and concentration-dependent. | The expression of Beclin-1 was increased, and PI3K, Akt, and TOR were decreased. | NA | Jia et al., 2023 [78] |
| MCF-7 | MT | 24 | MTT results showed that the IC50 of MT for MCF-7 was 1.38 ± 0.09 mg·mL−1. | The expression of P-gp was decreased. | NA | Li et al., 2013 [79] |
| Bcap | MT | 72 | The MTT assay showed that the inhibition rate of 0.1 mg·mL−1 was less than 40% for 72 h. | NA | NA | Wang et al., 1996 [80] |
| MCF-7 | MT | 24, 48, 72 | CCK-8 test showed MT inhibited cancer cell proliferation in a time-dose-dependent manner, and the apoptosis rate was 72.81±3.83% at 8 mg·mL−1 of MT. | Reduced p62 expression and p-AKT/AKT ratio. | Cells showed contraction, membrane blistering, balloon-like protrusions, and partial detachment. | Du et al., 2020 [81] |
| MCF-7 BT-474 MDA-MB-231 | MT | 48 | 3 mg·mL−1 MT resulted in a reduction of cell number to 15.5–23.6% by MTT assay, and MDA-MB-231 was the most sensitive cell. 3 mg·mL−1 MT acted for 48 h, and the apoptosis rate was about 90%. | Reduced IKKβ expression, which may be related to the NF-κB-signaling pathway. | NA | Shao et al., 2018 [82] |
| MCF-7 | MT | 24, 48, 72 | MTT assay showed that it inhibited cell proliferation in a concentration- and time-dependent manner and induced apoptosis with an IC50 value of 0.86 mg·mL−1 at 48 h. | Inhibited the expression of vascular endothelial growth factor and down-regulated the Wnt/β-catenin pathway. | NA | Xiao et al., 2018 [83] |
| MCF-7 | MT | 24, 48, 72 | MTT assay showed that MT inhibited cell growth in a concentration- and time-dependent manner with an IC50 (48 h) of approximately 0.8 mg·mL−1. After the action of MT with 0.8 mg·mL−1, the apoptosis rate was 25.6 ± 4.8%, inducing cell cycle arrest in G1/S phase. | Increased expression of PTEN, p21/WAF1/CIP1, p27/KIP1 and decreased expression of pAkt, pBad | NA | Li et al., 2019 [84] |
| MCF-7 T47-D | MT | 24, 48, 72 | MTT assay showed that MT inhibited cell growth in a concentration- and time-dependent manner with an IC50 (48 h) of approximately 0.8 mg·mL−1. | Increased expression of Let-7b and decreased expression of CD133, KLF4. | NA | Liang et al., 2019 [85] |
| MDA-MB-231 MCF-7 | MT | 24, 48 | MTT assay showed that MT inhibited cell growth in a concentration- and time-dependent manner and induced apoptosis. | Increased expression of E-calmodulin and decreased expression of N-calmodulin and waveform protein. | MT(2 mg/mL) significantly inhibited cell migration. | Ren et al., 2020 [86] |
| MCF-7 | MT | 48 | MTT assay showed MT significantly inhibited cell proliferation and induced cell apoptosis. S-phase cells decreased and G0/G1-phase cells increased. | Bax protein expression increased and Bcl-2 protein expression decreased. | NA | Shi et al., 2015 [87] |
| MDA-MB-453 HCC-1806 | MT | NA | CCK-8 tests indicate that MT inhibits cell proliferation and promotes apoptosis. | HN1 protein expression is inhibited and Cleared-Caspase-3 protein expression is increased. | NA | Guo et al., 2023 [88] |
| MCF-7 | MT | 24, 48, 72 | Cell viability was detected by MTT assay with an IC50 value of 0.53 ± 34.6 mg·mL−1.MT promoted apoptosis. | Promoted the release of cytochrome C and enhances caspase-3 activity, while p-eIF2a was elevated. | NA | Xiao et al., 2017 [89] |
| MCF-7 | MT | 24, 48, 72 | MTT assay showed that the inhibition of cell proliferation after 72 h was 10.86–70.23% in a time- and concentration-dependent manner, and the apoptosis rate was 4.17–19.63% in the 0.25–2.0 mg·mL−1 MT group. | Increased expression of Bax and decreased expression of Bcl-2. | NA | Li et al., 2015 [90] |
| BT-20 MCF-7 | MT | 48 | The IC50 values of cell viability after 48 h were 0.31 ± 23.45 mg·mL−1 and 0.22 ± 16.89 mg·mL−1 as detected by the MTT assay. | NA | NA | Jiang et al., 2017 [91] |
| MCF-7 | MT | 24, 48, 72 | Cell survival was 77.5%, and apoptosis was 32.6% as detected by flow cytometry. | NA | NA | Zhang et al., 2016 [92] |
| MDA-MB-231 MDA-MB-468 | MT | 24, 48, 72 | MT inhibited cell proliferation, and flow cytometry results showed that MT blocked the cell cycle and induced apoptosis. | Inhibition of BCL-2 expression and up-regulation of caspase-3, LC3-II expression. | NA | Wei et al., 2023 [93] |
| MCF-7 | MT | 24, 48, 72 | MTT assay showed that MT inhibited cell growth in a concentration- and time-dependent manner and induced apoptosis. | Decreased Bcl-2/Bax ratio, downregulated VEGF and VEGFR-2 expression, and increased caspase-3 and caspase-9. | NA | Li et al., 2010 [94] |
| MCF-7 MDA-MB-231 | MT | 8, 16, 24 | MTT assay showed that MT inhibited cell growth in a concentration- and time-dependent manner. | Increased expression of Akt, Erk1/2, and p38. | NA | Zou et al., 2019 [95] |
| MDA-MB-231 | MT | NA | The inhibition rate of cell proliferation after 0.1 mg·mL−1 of MT was 20.29 ± 0.84% as determined by MTT assay. | NA | NA | Thang PNT et al., 2022 [96] |
| MCF-7 | MT | 48 | The MTT assay showed MT inhibited cell viability in a concentration-dependent manner, and the IC50 value for cell viability after 48 h was 0.53 mg·mL−1. | NA | NA | Mousavi SH et al., 2014 [97] |
| MDA-MB-231 | MT | 24, 48, 72 | MTT assay showed that MT inhibited cell growth in a concentration- and time-dependent manner. | Decreased Bcl-2/Bax protein and decreased mRNA levels of MMP-9, MMP-2, EGF, and VEGFR1. | NA | Yu et al., 2009 [98] |
| MDA-MB-231 | MT | 24 | MT inhibited cell growth and induced apoptosis. | Increased caspase-3 activation. | NA | Chui CH et al., 2005 [99] |
| Cells Type | Composition/Concentration | Dosage | Time Administer Drug | Methods | Results | Ref. |
|---|---|---|---|---|---|---|
| 4T1 | MT, 3.5–7.5 mg·mL−1 | 10 mL·kg−1 | 10 | Mice in the modeling group were inoculated with 0.2 mL of 1 × 106 cells-mL−1 of single-cell suspension of mouse 4T1 breast cancer cells in the right axilla, and after successful modeling, mice were injected with MT once/d for 10 d. | The tumor weight of mice in groups of MT was reduced, and the number of apoptotic cells was higher. The effect may be related to the activation of the JNK1/AP-1-signaling pathway and the regulation of p53, Bax, and other apoptosis-related proteins. | Dong, 2019 [100] |
| MDA-MB-231 | MT, 20 mg·mL−1 | 50 mL·kg−1 | 15 | The nude mice were inoculated with 1 × 107 cells-mL−1 of MDA-MB-231 cell suspension under the right mammary gland, and MT 50 mg/kg was injected intraperitoneally five times/week for a total of 15 times. | Tumor growth was inhibited in nude mice after the administration of MT. MT can induce apoptosis. At day 22, the inhibition of tumor volume by ascorbic acid alone was 28.72%. | Guo et al., 2022 [65] |
| Walker 256 | MT, 0.5–20 mg·mL−1 | 10 mL·kg−1 | 28 | Rats were subcutaneously inoculated with 0.4 mL of Walker 256 breast cancer cell suspension (5 × 107 cells-mL−1) in the right axilla. The drug was administered intraperitoneally at a volume of 10 mL·kg−1 twice/day for 14 d. | The tumor inhibition rates of the low-, medium-, and high-dose groups of MT were 24.6%, 31.7%, and 36.3%, respectively, showing a dose-dependent inhibition of tumor growth. | Zhang et al., 2018 [101] |
| MA737 | MT, NA | 20 mL·kg−1 | 28 | MA737 cells 1 × 105 cells/mouse were inoculated subcutaneously in the right inguinal area, randomly grouped, and sacrificed 14 days after administration, and thymus, spleen, and tumor weights, as well as body weights of the mice, were weighed. | MT can increase the weight of the thymus and spleen, and the weight of the tumor is smaller. After the treatment of MT, the TH/TS ratio of mice is significantly increased, which can improve the immunity of mice. | Mao et al., 1996 [102] |
| MDA-MB-453 | MT, NA | NA | NA | NA | MT inhibited tumor growth and decreased the expression of HN1 protein but promoted the protein expression of Cleared-Caspase-3. | Guo Q et al., 2023 [88] |
| 4T1 | MT, NA | NA | 14 | Injected 4 × 104 4T1 cells into the left inguinal mammary fat pads of mice, after 15 days, received daily i.p. injection of MT for 14 days, and then euthanized, and the tumors, lungs, and livers were removed. | The volume of tumors of mice treated with MT was significantly smaller, and the numbers of tumor on the lung and livers surface of MT-treated mice were lowered. | Li H et al., 2010 [94] |
| 4T1 | MT, NA | NA | 21 | Injected 4 × 104 4T1 cells (100 µL, 5 × 106 cells-mL−1) into the right side of the fourth mammary gland of mice, mice received i.p. injection of MT, the mice were sacrificed on day 21, and the tumors were removed rapidly and weighed. | MT can inhibit the growth of tumors and induce apoptosis while decreasing the level of VEGF. | Xiao X et al., 2018 [83] |
| TM40D | MT, 5–20 mg·mL−1 | 10 mL·kg−1 | 20 | TM40D cells with cell density adjusted to 2.0 × 107 cells-mL−1, subcutaneous injection of 0.1 mL per nude mouse’s back, and intraperitoneal injection of the corresponding dose of drug. On the 20th day, the mice were euthanized, and tumor blocks were removed and weighed. | MT can reduce tumor weight and promote cancer cell apoptosis. | Bai et al., 2016 [103] |
| MCF-7/ADR | MT, 50, 100 mg·mL−1 | NA | 21 | Nude mice were subcutaneously inoculated with MCF-7/ADR cells. MT was injected for 21 days, and the tumor inhibition rate was measured using in vivo tumor inhibition experiments. | The tumor inhibition rates of the high-dose and low-dose groups of MT were 60.7% and 42.1%. MT can promote cancer cell apoptosis. | Zhou et al., 2017 [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, Y.; Liao, S.; Gao, P.; Tian, J.; Fu, C.; Qin, X.; Jin, S. The Potential of Matrine in the Treatment of Breast Cancer: A Review. Biomedicines 2025, 13, 1355. https://doi.org/10.3390/biomedicines13061355
Yang Y, Li Y, Liao S, Gao P, Tian J, Fu C, Qin X, Jin S. The Potential of Matrine in the Treatment of Breast Cancer: A Review. Biomedicines. 2025; 13(6):1355. https://doi.org/10.3390/biomedicines13061355
Chicago/Turabian StyleYang, Yumin, Yufeng Li, Shanshan Liao, Pan Gao, Jie Tian, Cheng Fu, Xuhua Qin, and Shenrui Jin. 2025. "The Potential of Matrine in the Treatment of Breast Cancer: A Review" Biomedicines 13, no. 6: 1355. https://doi.org/10.3390/biomedicines13061355
APA StyleYang, Y., Li, Y., Liao, S., Gao, P., Tian, J., Fu, C., Qin, X., & Jin, S. (2025). The Potential of Matrine in the Treatment of Breast Cancer: A Review. Biomedicines, 13(6), 1355. https://doi.org/10.3390/biomedicines13061355







