Connexins in Acquired Hearing Loss: Expanding Research Perspectives
Abstract
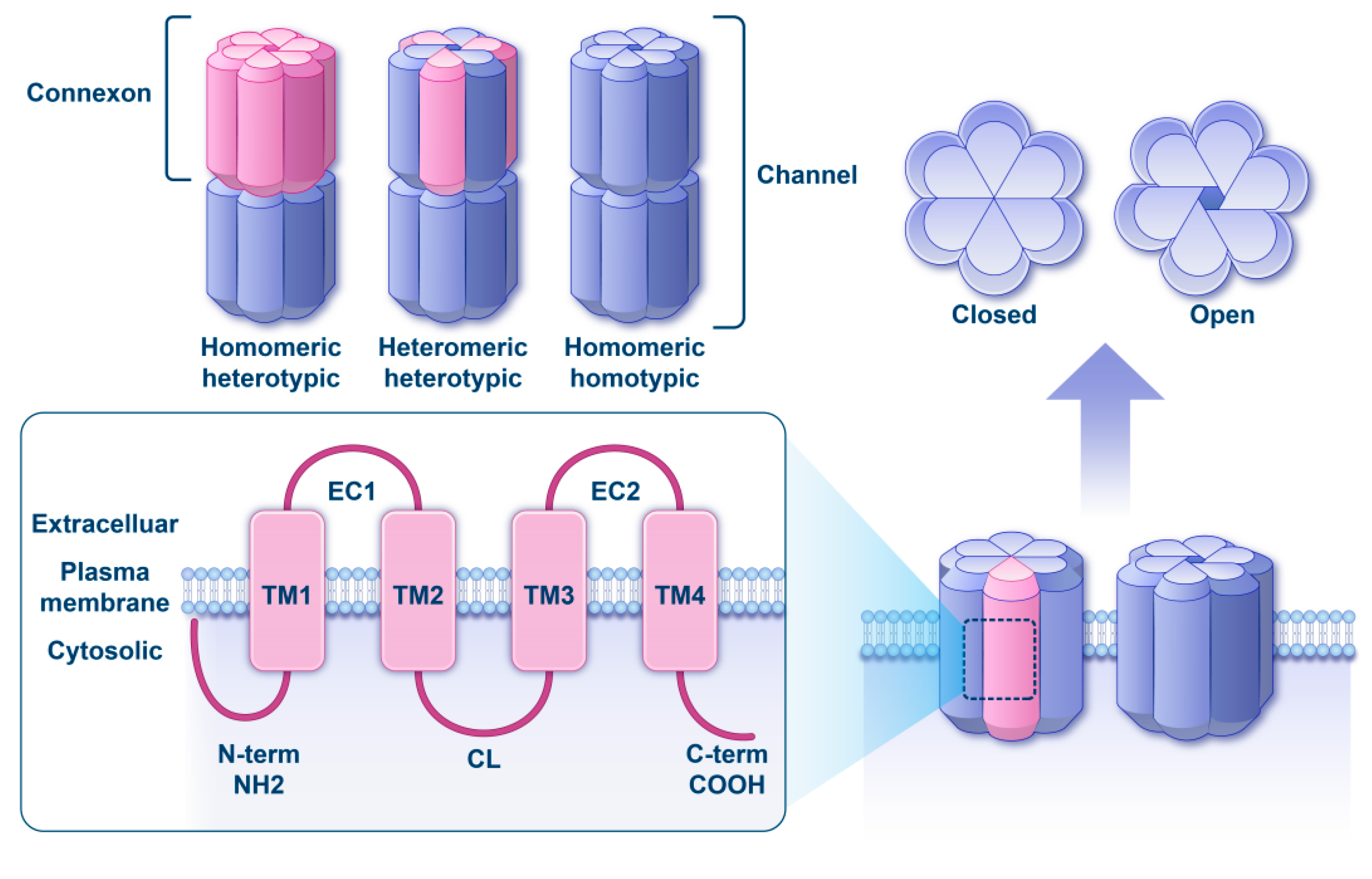
1. Introduction
2. Age-Related Hearing Loss (ARHL)
2.1. Cx26 Partial Loss and Cx30 Depletion Accelerated ARHL
2.2. A88V Mutation in Cx30 Stops Early Onset ARHL
2.3. Mechanism of Connexins in ARHL
3. Noise-Induced Hearing Loss (NIHL)
3.1. Mechanisms of NIHL
3.2. Effects of Noise Exposure on Connexins and Their Role in NIHL
4. The Relationship Between GJs and Cisplatin-Induced Ototoxicity
5. Cx31.9 and Ménière’s Disease
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| ABR | Auditory Brainstem Response |
| AMPK | AMP-activated protein kinase |
| ARHL | Age-Related Hearing Loss |
| ATP | Adenosine Triphosphate |
| BM | Basilar Membrane |
| CCL2 | Chemokine (C-C motif) Ligand 2 |
| CCR2 | C-C Chemokine Receptor type 2 |
| CD-1 | a mouse strain (originally “Carworth Farms”) |
| CM | Cochlear Microphonic |
| CREB | cAMP Response Element-Binding protein |
| Cx26 | Connexin-26 |
| Cx30 | Connexin-30 |
| Cx30.2 | Connexin-30.2 (mouse ortholog of human Cx31.9) |
| Cx31.9 | Connexin-31.9 |
| cKO | conditional Knockout |
| EC | extracellular loops |
| EP | Endocochlear Potential |
| FMD | familial Ménière’s Disease |
| GJ | Gap Junction |
| GJB2 | Gap Junction β-2 gene (encodes Cx26) |
| GJB6 | Gap Junction β-6 gene (encodes Cx30) |
| GJD3 | Gap Junction δ-3 gene (encodes Cx31.9 in humans) |
| HED | Hidrotic Ectodermal Dysplasia (Clouston syndrome) |
| HMGB1 | High-Mobility Group Box 1 protein |
| HO-1 | Heme Oxygenase-1 |
| IHC | Inner Hair Cell |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IP3 | Inositol 1,4,5-trisphosphate |
| JNK | c-Jun N-terminal kinase |
| K+ | Potassium ion |
| MAPK | Mitogen-Activated Protein Kinase |
| MIM | Mendelian Inheritance in Man (database identifier) |
| Na+ | Sodium ion |
| NAD+ | Nicotinamide Adenine Dinucleotide (oxidized form) |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate (reduced form) |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NIHL | Noise-Induced Hearing Loss |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OHC | Outer Hair Cell |
| OTOF | Otoferlin gene |
| P2 receptors | Purinergic receptors activated by ATP and related nucleotides |
| P5 | Postnatal day 5 |
| PD150606 | a selective calpain inhibitor (N-(4-fluorophenyl)sulfonyl-L-valyl-L-leucyl-4-aminobutyric acid) |
| PKA | Protein Kinase A |
| ROS | Reactive Oxygen Species |
| SGN | Spiral Ganglion Neuron |
| SL | Spiral Ligament |
| SLFs | Spiral Ligament Fibrocytes |
| SV | Stria Vascularis |
| TNF-α | Tumor Necrosis Factor-alpha |
| TLR4 | Toll-like receptor 4 |
| TM | transmembrane segment |
References
- Kelly, J.J.; Abitbol, J.M.; Hulme, S.; Press, E.R.; Laird, D.W.; Allman, B.L. The connexin 30 A88V mutant reduces cochlear gap junction expression and confers long-term protection against hearing loss. J. Cell Sci. 2019, 132, jcs224097. [Google Scholar] [CrossRef]
- Lefebvre, P.P.; Van De Water, T.R. Connexins, hearing and deafness: Clinical aspects of mutations in the connexin 26 gene. Brain Res. Brain Res. Rev. 2000, 32, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Xia, X.J.; Adams, J.; Chen, Z.Y.; Welch, K.O.; Tekin, M.; Ouyang, X.M.; Kristiansen, A.; Pandya, A.; Balkany, T.; et al. Mutations in GJA1 (connexin 43) are associated with non-syndromic autosomal recessive deafness. Hum. Mol. Genet. 2001, 10, 2945–2951. [Google Scholar] [CrossRef]
- Liu, X.Z.; Xia, X.J.; Xu, L.R.; Pandya, A.; Liang, C.Y.; Blanton, S.H.; Brown, S.D.; Steel, K.P.; Nance, W.E. Mutations in connexin31 underlie recessive as well as dominant non-syndromic hearing loss. Hum. Mol. Genet. 2000, 9, 63–67. [Google Scholar] [CrossRef]
- Forge, A.; Becker, D.; Casalotti, S.; Edwards, J.; Marziano, N.; Nevill, G. Gap junctions in the inner ear: Comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J. Comp. Neurol. 2003, 467, 207–231. [Google Scholar] [CrossRef]
- Escalera-Balsera, A.; Robles-Bolivar, P.; Parra-Perez, A.M.; Murillo-Cuesta, S.; Chua, H.C.; Rodríguez-de la Rosa, L.; Contreras, J.; Domarecka, E.; Amor-Dorado, J.C.; Soto-Varela, A.; et al. A rare haplotype of the GJD3 gene segregating in familial Meniere’s disease interferes with connexin assembly. Genome Med. 2025, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Hoang Dinh, E.; Ahmad, S.; Chang, Q.; Tang, W.; Stong, B.; Lin, X. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res. 2009, 1277, 52–69. [Google Scholar] [CrossRef]
- Verselis, V.K. Connexin hemichannels and cochlear function. Neurosci. Lett. 2019, 695, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef]
- Martínez, A.D.; Acuña, R.; Figueroa, V.; Maripillan, J.; Nicholson, B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid. Redox Signal. 2009, 11, 309–322. [Google Scholar] [CrossRef]
- Chan, D.K.; Chang, K.W. GJB2-associated hearing loss: Systematic review of worldwide prevalence, genotype, and auditory phenotype. Laryngoscope 2014, 124, E34–E53. [Google Scholar] [CrossRef]
- Santos-Sacchi, J.; Dallos, P. Intercellular communication in the supporting cells of the organ of Corti. Hear. Res. 1983, 9, 317–326. [Google Scholar] [CrossRef]
- Spiess, A.C.; Lang, H.; Schulte, B.A.; Spicer, S.S.; Schmiedt, R.A. Effects of gap junction uncoupling in the gerbil cochlea. Laryngoscope 2002, 112, 1635–1641. [Google Scholar] [CrossRef]
- Ceriani, F.; Pozzan, T.; Mammano, F. Critical role of ATP-induced ATP release for Ca2+ signaling in nonsensory cell networks of the developing cochlea. Proc. Natl. Acad. Sci. USA 2016, 113, E7194–E7201. [Google Scholar] [CrossRef]
- Johnson, S.L.; Ceriani, F.; Houston, O.; Polishchuk, R.; Polishchuk, E.; Crispino, G.; Zorzi, V.; Mammano, F.; Marcotti, W. Connexin-Mediated Signaling in Nonsensory Cells Is Crucial for the Development of Sensory Inner Hair Cells in the Mouse Cochlea. J. Neurosci. 2017, 37, 258–268. [Google Scholar] [CrossRef]
- Xie, L.; Chen, S.; Xu, K.; Cao, H.Y.; Du, A.N.; Bai, X.; Sun, Y.; Kong, W.J. Reduced postnatal expression of cochlear Connexin26 induces hearing loss and affects the developmental status of pillar cells in a dose-dependent manner. Neurochem. Int. 2019, 128, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Zhu, Y.; Liang, C.; Zhao, H.B. Deafness induced by Connexin 26 (GJB2) deficiency is not determined by endocochlear potential (EP) reduction but is associated with cochlear developmental disorders. Biochem. Biophys. Res. Commun. 2014, 448, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, W.; Zhang, J.; Chen, J.; Li, Y.; Sun, L.; Hou, S.; Yang, J. Pathological mechanisms of connexin26-related hearing loss: Potassium recycling, ATP-calcium signaling, or energy supply? Front. Mol. Neurosci. 2022, 15, 976388. [Google Scholar] [CrossRef]
- Pineros, J.; Zhu, X.; Ding, B.; Frisina, R.D. Connexins 30 and 43 expression changes in relation to age-related hearing loss. Hear. Res. 2024, 444, 108971. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Danzaki, K.; Ikeda, K.; Kamiya, K. Degradation and modification of cochlear gap junction proteins in the early development of age-related hearing loss. Exp. Mol. Med. 2020, 52, 166–175. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Zorzi, V.; Paciello, F.; Ziraldo, G.; Peres, C.; Raspa, M.; Scavizzi, F.; Salvatore, A.M.; Crispino, G.; Tognola, G.; et al. Cx26 partial loss causes accelerated presbycusis by redox imbalance and dysregulation of Nfr2 pathway. Redox Biol. 2018, 19, 301–317. [Google Scholar] [CrossRef]
- Jayakody, D.M.P.; Friedland, P.L.; Martins, R.N.; Sohrabi, H.R. Impact of Aging on the Auditory System and Related Cognitive Functions: A Narrative Review. Front. Neurosci. 2018, 12, 125. [Google Scholar] [CrossRef]
- Hou, S.; Chen, P.; He, J.; Chen, J.; Zhang, J.; Mammano, F.; Yang, J. Dietary intake of deuterium oxide decreases cochlear metabolism and oxidative stress levels in a mouse model of age-related hearing loss. Redox Biol. 2022, 57, 102472. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.A.; Mills, J.H. Presbycusis. Lancet 2005, 366, 1111–1120. [Google Scholar] [CrossRef]
- Wang, J.; Puel, J.L. Presbycusis: An Update on Cochlear Mechanisms and Therapies. J. Clin. Med. 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Picciotti, P.M.; Paludetti, G.; Troiani, D. Pathogenesis of presbycusis in animal models: A review. Exp. Gerontol. 2011, 46, 413–425. [Google Scholar] [CrossRef]
- Tavanai, E.; Mohammadkhani, G. Role of antioxidants in prevention of age-related hearing loss: A review of literature. Eur. Arch. Otorhinolaryngol. 2017, 274, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K. Age-related hearing loss: The status of Schuknecht’s typology. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 439–443. [Google Scholar] [CrossRef]
- Mills, D.M.; Schmiedt, R.A. Metabolic presbycusis: Differential changes in auditory brainstem and otoacoustic emission responses with chronic furosemide application in the gerbil. J. Assoc. Res. Otolaryngol. 2004, 5, 1–10. [Google Scholar] [CrossRef]
- Ichimiya, I.; Suzuki, M.; Mogi, G. Age-related changes in the murine cochlear lateral wall. Hear. Res. 2000, 139, 116–122. [Google Scholar] [CrossRef]
- Xu, K.; Chen, S.; Bai, X.; Xie, L.; Qiu, Y.; Liu, X.Z.; Wang, X.H.; Kong, W.J.; Sun, Y. Degradation of cochlear Connexin26 accelerate the development of age-related hearing loss. Aging Cell 2023, 22, e13973. [Google Scholar] [CrossRef]
- Gabriel, H.D.; Jung, D.; Bützler, C.; Temme, A.; Traub, O.; Winterhager, E.; Willecke, K. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J. Cell Biol. 1998, 140, 1453–1461. [Google Scholar] [CrossRef]
- Gridley, T.; Murray, S.A. Mouse mutagenesis and phenotyping to generate models of development and disease. Curr. Top. Dev. Biol. 2022, 148, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Liang, C.; Zong, L.; Chen, J.; Jones, R.O.; Zhao, H.B. Connexin26 (GJB2) deficiency reduces active cochlear amplification leading to late-onset hearing loss. Neuroscience 2015, 284, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, L.; Wang, X.; Sun, C.; Lin, X.; Li, L.; Mei, L.; Huang, Z.; Yang, T.; Wu, H. Characterization of a knock-in mouse model of the homozygous p.V37I variant in Gjb2. Sci. Rep. 2016, 6, 33279. [Google Scholar] [CrossRef]
- Lin, X.; Li, G.; Zhang, Y.; Zhao, J.; Lu, J.; Gao, Y.; Liu, H.; Li, G.L.; Yang, T.; Song, L.; et al. Hearing consequences in Gjb2 knock-in mice: Implications for human p.V37I mutation. Aging 2019, 11, 7416–7441. [Google Scholar] [CrossRef]
- Boulay, A.C.; del Castillo, F.J.; Giraudet, F.; Hamard, G.; Giaume, C.; Petit, C.; Avan, P.; Cohen-Salmon, M. Hearing is normal without connexin30. J. Neurosci. 2013, 33, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Paciello, F.; Zorzi, V.; Raspa, M.; Scavizzi, F.; Grassi, C.; Mammano, F.; Fetoni, A.R. Connexin 30 deletion exacerbates cochlear senescence and age-related hearing loss. Front. Cell Dev. Biol. 2022, 10, 950837. [Google Scholar] [CrossRef] [PubMed]
- Lamartine, J.; Munhoz Essenfelder, G.; Kibar, Z.; Lanneluc, I.; Callouet, E.; Laoudj, D.; Lemaître, G.; Hand, C.; Hayflick, S.J.; Zonana, J.; et al. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat. Genet. 2000, 26, 142–144. [Google Scholar] [CrossRef]
- Sugiura, K.; Teranishi, M.; Matsumoto, Y.; Akiyama, M. Clouston syndrome with heterozygous GJB6 mutation p.Ala88Val and GJB2 variant p.Val27Ile revealing mild sensorineural hearing loss and photophobia. JAMA Dermatol. 2013, 149, 1350–1351. [Google Scholar] [CrossRef]
- Jan, A.Y.; Amin, S.; Ratajczak, P.; Richard, G.; Sybert, V.P. Genetic heterogeneity of KID syndrome: Identification of a Cx30 gene (GJB6) mutation in a patient with KID syndrome and congenital atrichia. J. Investig. Dermatol. 2004, 122, 1108–1113. [Google Scholar] [CrossRef]
- Bosen, F.; Schütz, M.; Beinhauer, A.; Strenzke, N.; Franz, T.; Willecke, K. The Clouston syndrome mutation connexin30 A88V leads to hyperproliferation of sebaceous glands and hearing impairments in mice. FEBS Lett. 2014, 588, 1795–1801. [Google Scholar] [CrossRef]
- Lukashkina, V.A.; Levic, S.; Lukashkin, A.N.; Strenzke, N.; Russell, I.J. A connexin30 mutation rescues hearing and reveals roles for gap junctions in cochlear amplification and micromechanics. Nat. Commun. 2017, 8, 14530. [Google Scholar] [CrossRef] [PubMed]
- Essenfelder, G.M.; Bruzzone, R.; Lamartine, J.; Charollais, A.; Blanchet-Bardon, C.; Barbe, M.T.; Meda, P.; Waksman, G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004, 13, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Levic, S.; Lukashkina, V.A.; Simões, P.; Lukashkin, A.N.; Russell, I.J. A Gap-Junction Mutation Reveals That Outer Hair Cell Extracellular Receptor Potentials Drive High-Frequency Cochlear Amplification. J. Neurosci. 2022, 42, 7875–7884. [Google Scholar] [CrossRef]
- Kuang, Y.; Zorzi, V.; Buratto, D.; Ziraldo, G.; Mazzarda, F.; Peres, C.; Nardin, C.; Salvatore, A.M.; Chiani, F.; Scavizzi, F.; et al. A potent antagonist antibody targeting connexin hemichannels alleviates Clouston syndrome symptoms in mutant mice. EBioMedicine 2020, 57, 102825. [Google Scholar] [CrossRef]
- Yamasoba, T.; Lin, F.R.; Someya, S.; Kashio, A.; Sakamoto, T.; Kondo, K. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear. Res. 2013, 303, 30–38. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Keithley, E.M.; Canto, C.; Zheng, Q.Y.; Wang, X.; Fischel-Ghodsian, N.; Johnson, K.R. Cu/Zn superoxide dismutase and age-related hearing loss. Hear. Res. 2005, 209, 76–85. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chen, J.C.; Hsu, C.J.; Yang, W.S.; Liu, T.C. Plasma reactive oxygen species levels are correlated with severity of age-related hearing impairment in humans. Neurobiol. Aging 2012, 33, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Lopez, W.; Ramachandran, J.; Ayad, W.A.; Liu, Y.; Lopez-Rodriguez, A.; Harris, A.L.; Contreras, J.E. Glutathione release through connexin hemichannels: Implications for chemical modification of pores permeable to large molecules. J. Gen. Physiol. 2015, 146, 245–254. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, H.B. ATP-mediated potassium recycling in the cochlear supporting cells. Purinergic Signal. 2010, 6, 221–229. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yu, N.; Fleming, C.R. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. USA 2005, 102, 18724–18729. [Google Scholar] [CrossRef]
- Mammano, F. ATP-dependent intercellular Ca2+ signaling in the developing cochlea: Facts, fantasies and perspectives. Semin. Cell Dev. Biol. 2013, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, R.M.; Roest, G.; Bultynck, G.; Parys, J.B. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 2016, 60, 74–87. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Forge, A.; Jagger, D.J.; Kelly, J.J.; Taylor, R.R. Connexin30-mediated intercellular communication plays an essential role in epithelial repair in the cochlea. J. Cell Sci. 2013, 126, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Wingard, J.C.; Zhao, H.B. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss—A Common Hereditary Deafness. Front. Cell Neurosci. 2015, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Chen, J.; Zhu, Y.; Zhao, H.B. Progressive age-dependence and frequency difference in the effect of gap junctions on active cochlear amplification and hearing. Biochem. Biophys. Res. Commun. 2017, 489, 223–227. [Google Scholar] [CrossRef]
- Cohen-Salmon, M.; Regnault, B.; Cayet, N.; Caille, D.; Demuth, K.; Hardelin, J.P.; Janel, N.; Meda, P.; Petit, C. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc. Natl. Acad. Sci. USA 2007, 104, 6229–6234. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, P.; He, B.; Gong, T.; Li, Y.; Zhang, J.; Lv, J.; Mammano, F.; Hou, S.; Yang, J. Connexin30-Deficiency Causes Mild Hearing Loss With the Reduction of Endocochlear Potential and ATP Release. Front. Cell Neurosci. 2021, 15, 819194. [Google Scholar] [CrossRef]
- Lyu, A.R.; Kim, T.H.; Park, S.J.; Shin, S.A.; Jeong, S.H.; Yu, Y.; Huh, Y.H.; Je, A.R.; Park, M.J.; Park, Y.H. Mitochondrial Damage and Necroptosis in Aging Cochlea. Int. J. Mol. Sci. 2020, 21, 2505. [Google Scholar] [CrossRef] [PubMed]
- Peixoto Pinheiro, B.; Vona, B.; Löwenheim, H.; Rüttiger, L.; Knipper, M.; Adel, Y. Age-related hearing loss pertaining to potassium ion channels in the cochlea and auditory pathway. Pflug. Arch. 2021, 473, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Batts, S.; Stankovic, K.M. Noise-Induced Hearing Loss. J. Clin. Med. 2023, 12, 2347. [Google Scholar] [CrossRef]
- Shrestha, S.; Baral, B.; Dawadi, A.; Sherpa, P.; Regmi, D. Noise-induced Hearing Loss among Patients Requiring Pure Tone Audiometry Evaluation in a Tertiary Care Centre: A Descriptive Cross-sectional Study. JNMA J. Nepal. Med. Assoc. 2023, 61, 98–101. [Google Scholar] [CrossRef]
- Coyat, C.; Cazevieille, C.; Baudoux, V.; Larroze-Chicot, P.; Caumes, B.; Gonzalez-Gonzalez, S. Morphological consequences of acoustic trauma on cochlear hair cells and the auditory nerve. Int. J. Neurosci. 2019, 129, 580–587. [Google Scholar] [CrossRef]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 2002, 3, 248–268. [Google Scholar] [CrossRef]
- Yamashita, D.; Jiang, H.Y.; Schacht, J.; Miller, J.M. Delayed production of free radicals following noise exposure. Brain Res. 2004, 1019, 201–209. [Google Scholar] [CrossRef]
- Wang, J.; Ruel, J.; Ladrech, S.; Bonny, C.; van de Water, T.R.; Puel, J.L. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol. Pharmacol. 2007, 71, 654–666. [Google Scholar] [CrossRef]
- Sebe, J.Y.; Cho, S.; Sheets, L.; Rutherford, M.A.; von Gersdorff, H.; Raible, D.W. Ca2+-Permeable AMPARs Mediate Glutamatergic Transmission and Excitotoxic Damage at the Hair Cell Ribbon Synapse. J. Neurosci. 2017, 37, 6162–6175. [Google Scholar] [CrossRef]
- Bharadwaj, H.M.; Verhulst, S.; Shaheen, L.; Liberman, M.C.; Shinn-Cunningham, B.G. Cochlear neuropathy and the coding of supra-threshold sound. Front. Syst. Neurosci. 2014, 8, 26. [Google Scholar] [CrossRef]
- Shin, S.H.; Jung, J.; Park, H.R.; Sim, N.S.; Choi, J.Y.; Bae, S.H. The Time Course of Monocytes Infiltration After Acoustic Overstimulation. Front. Cell. Neurosci. 2022, 16, 844480. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Z.; Liu, J.; Zheng, Z.; Xiong, Y.; Li, C.; Feng, Y.; Yin, S. HMGB1 accumulation in cytoplasm mediates noise-induced cochlear damage. Cell Tissue Res. 2023, 391, 43–54. [Google Scholar] [CrossRef]
- Hill, K.; Yuan, H.; Wang, X.; Sha, S.H. Noise-Induced Loss of Hair Cells and Cochlear Synaptopathy Are Mediated by the Activation of AMPK. J. Neurosci. 2016, 36, 7497–7510. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, X.; Hill, K.; Chen, J.; Lemasters, J.; Yang, S.M.; Sha, S.H. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid. Redox Signal. 2015, 22, 1308–1324. [Google Scholar] [CrossRef] [PubMed]
- Varela-Nieto, I.; Murillo-Cuesta, S.; Calvino, M.; Cediel, R.; Lassaletta, L. Drug development for noise-induced hearing loss. Expert Opin. Drug Discov. 2020, 15, 1457–1471. [Google Scholar] [CrossRef]
- Zhou, X.X.; Chen, S.; Xie, L.; Ji, Y.Z.; Wu, X.; Wang, W.W.; Yang, Q.; Yu, J.T.; Sun, Y.; Lin, X.; et al. Reduced Connexin26 in the Mature Cochlea Increases Susceptibility to Noise-Induced Hearing Lossin Mice. Int. J. Mol. Sci. 2016, 17, 301. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, F.; Zhou, L.; Wang, S.; Li, J.; He, L. Drug-Therapeutic Strategies for Noise-Induced Hearing Loss: A Narrative Review. Noise Health 2025, 27, 203–209. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nagashima, R.; Yoneyama, M.; Shiba, T.; Ogita, K. Disruption of ion-trafficking system in the cochlear spiral ligament prior to permanent hearing loss induced by exposure to intense noise: Possible involvement of 4-hydroxy-2-nonenal as a mediator of oxidative stress. PLoS ONE 2014, 9, e102133. [Google Scholar] [CrossRef]
- Sun, T.; Li, W.; Shi, K.; Zhao, Y.; Guo, D.; Wang, D. The Role of Connexin26 and Connexin30 in the Mouse Cochlea of Noise-Induced Hearing Loss. Otolaryngol. Head Neck Surg. 2025, 172, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yoneyama, M.; Ogita, K. Calpain inhibitor alleviates permanent hearing loss induced by intense noise by preventing disruption of gap junction-mediated intercellular communication in the cochlear spiral ligament. Eur. J. Pharmacol. 2017, 803, 187–194. [Google Scholar] [CrossRef]
- Hsu, W.C.; Wang, J.D.; Hsu, C.J.; Lee, S.Y.; Yeh, T.H. Expression of connexin 26 in the lateral wall of the rat cochlea after acoustic trauma. Acta Otolaryngol. 2004, 124, 459–463. [Google Scholar] [CrossRef]
- Nagashima, R.; Yamaguchi, T.; Tanaka, H.; Ogita, K. Mechanism underlying the protective effect of tempol and Nω-nitro-L-arginine methyl ester on acoustic injury: Possible involvement of c-Jun N-terminal kinase pathway and connexin26 in the cochlear spiral ligament. J. Pharmacol. Sci. 2010, 114, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Y.; Chen, X.; Zhang, P.; Su, D.; Wang, L.; Yang, F.; Yang, J. Effects of D-methionine in mice with noise-induced hearing loss mice. J. Int. Med. Res. 2019, 47, 3874–3885. [Google Scholar] [CrossRef]
- Xiong, M.; Zhu, Y.; Lai, H.; Fu, X.; Deng, W.; Yang, C.; He, Q.; Zheng, G. Radix astragali inhibits the down-regulation of connexin 26 in the stria vascularis of the guinea pig cochlea after acoustic trauma. Eur. Arch. Otorhinolaryngol. 2015, 272, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gong, T.; Chen, P.; Zhu, J.; Huang, S.; Li, Y.; Li, G.; Zhang, Q.; Duan, M.; Song, Q.; et al. Connexin30-deficient mice increase susceptibility to noise via redox and lactate imbalances. Free Radic. Biol. Med. 2024, 225, 641–653. [Google Scholar] [CrossRef]
- Alamgir, H.; Turner, C.A.; Wong, N.J.; Cooper, S.P.; Betancourt, J.A.; Henry, J.; Senchak, A.J.; Hammill, T.L.; Packer, M.D. The impact of hearing impairment and noise-induced hearing injury on quality of life in the active-duty military population: Challenges to the study of this issue. Mil. Med. Res. 2016, 3, 11. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.; Tian, C.; Lim, H.J.; Kim, Y.S.; Chung, J.H.; Choung, Y.H. Prevention of cisplatin-induced ototoxicity by the inhibition of gap junctional intercellular communication in auditory cells. Cell. Mol. Life Sci. 2014, 71, 3859–3871. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.S.; Kim, H.; Jang, J.H.; Choung, Y.H. Gap Junction-Mediated Intercellular Communication of cAMP Prevents CDDP-Induced Ototoxicity via cAMP/PKA/CREB Pathway. Int. J. Mol. Sci. 2021, 22, 6327. [Google Scholar] [CrossRef]
- Abitbol, J.; Beach, R.; Barr, K.; Esseltine, J.; Allman, B.; Laird, D. Cisplatin-induced ototoxicity in organotypic cochlear cultures occurs independent of gap junctional intercellular communication. Cell Death Dis. 2020, 11, 342. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.Y. The HDOCK server for integrated protein-protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.J.; Liu, X.Z.; Tu, L.; Sun, Y. Cytomembrane Trafficking Pathways of Connexin 26, 30, and 43. Int. J. Mol. Sci. 2023, 24, 10349. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Liu, X.; Sun, Y. Regulatory mechanisms of connexin26. Neuroscience 2025, 570, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, H.; Mi, Y.; Jiang, W.; Guo, D.; Zhang, J.; Zhao, Y.; Tang, W. Mechanisms of hearing loss and cell death in the cochlea of connexin mutant mice. Am. J. Physiol. Cell Physiol. 2020, 319, C569–C578. [Google Scholar] [CrossRef]
- Mao, L.; Wang, Y.; An, L.; Zeng, B.; Wang, Y.; Frishman, D.; Liu, M.; Chen, Y.; Tang, W.; Xu, H. Molecular Mechanisms and Clinical Phenotypes of GJB2 Missense Variants. Biology 2023, 12, 505. [Google Scholar] [CrossRef]
| Noise | Region | Cx26 | Cx30 | Animal | Ref. | ||
|---|---|---|---|---|---|---|---|
| dB SPL | Type | Duration | |||||
| 115 | White noise | 48 h | SL | ↑ | — | Wistar rats | [84] |
| 110 | Octave band, 8 kHz | 1 h | SL | ↓ | — | Std-ddY mice | [85] |
| 100 | Narrow band, 4 kHz | 8 h/d × 3 d | BM, SV, SL | ↓ | ↓ | Kunming mice | [86] |
| 176 | Impulse noise (rifle shots) | 15 × 1 s | SV | ↓ | — | Albino guinea pigs | [87] |
| 120 | Broadband | 2–4 h | Whole cochlea | ↑ at 4 h → ↓ by day 7 | Mirrors Cx26 | C57BL/6 mice | [82] |
| 110 | 1 h | ↓ at 4 h → recovery by day 2 | Mirrors Cx26 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Zhu, J.; Zhang, J.; Gong, T.; Fei, Z.; Chen, P.; Hou, S.; Yang, J. Connexins in Acquired Hearing Loss: Expanding Research Perspectives. Biomedicines 2025, 13, 3109. https://doi.org/10.3390/biomedicines13123109
Huang S, Zhu J, Zhang J, Gong T, Fei Z, Chen P, Hou S, Yang J. Connexins in Acquired Hearing Loss: Expanding Research Perspectives. Biomedicines. 2025; 13(12):3109. https://doi.org/10.3390/biomedicines13123109
Chicago/Turabian StyleHuang, Sihan, Jingyi Zhu, Jifang Zhang, Tianyu Gong, Zhongyuan Fei, Penghui Chen, Shule Hou, and Jun Yang. 2025. "Connexins in Acquired Hearing Loss: Expanding Research Perspectives" Biomedicines 13, no. 12: 3109. https://doi.org/10.3390/biomedicines13123109
APA StyleHuang, S., Zhu, J., Zhang, J., Gong, T., Fei, Z., Chen, P., Hou, S., & Yang, J. (2025). Connexins in Acquired Hearing Loss: Expanding Research Perspectives. Biomedicines, 13(12), 3109. https://doi.org/10.3390/biomedicines13123109





