Combined Single-Session Stereotactic Biopsy and Microwave Ablation of Primary and Secondary Liver Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
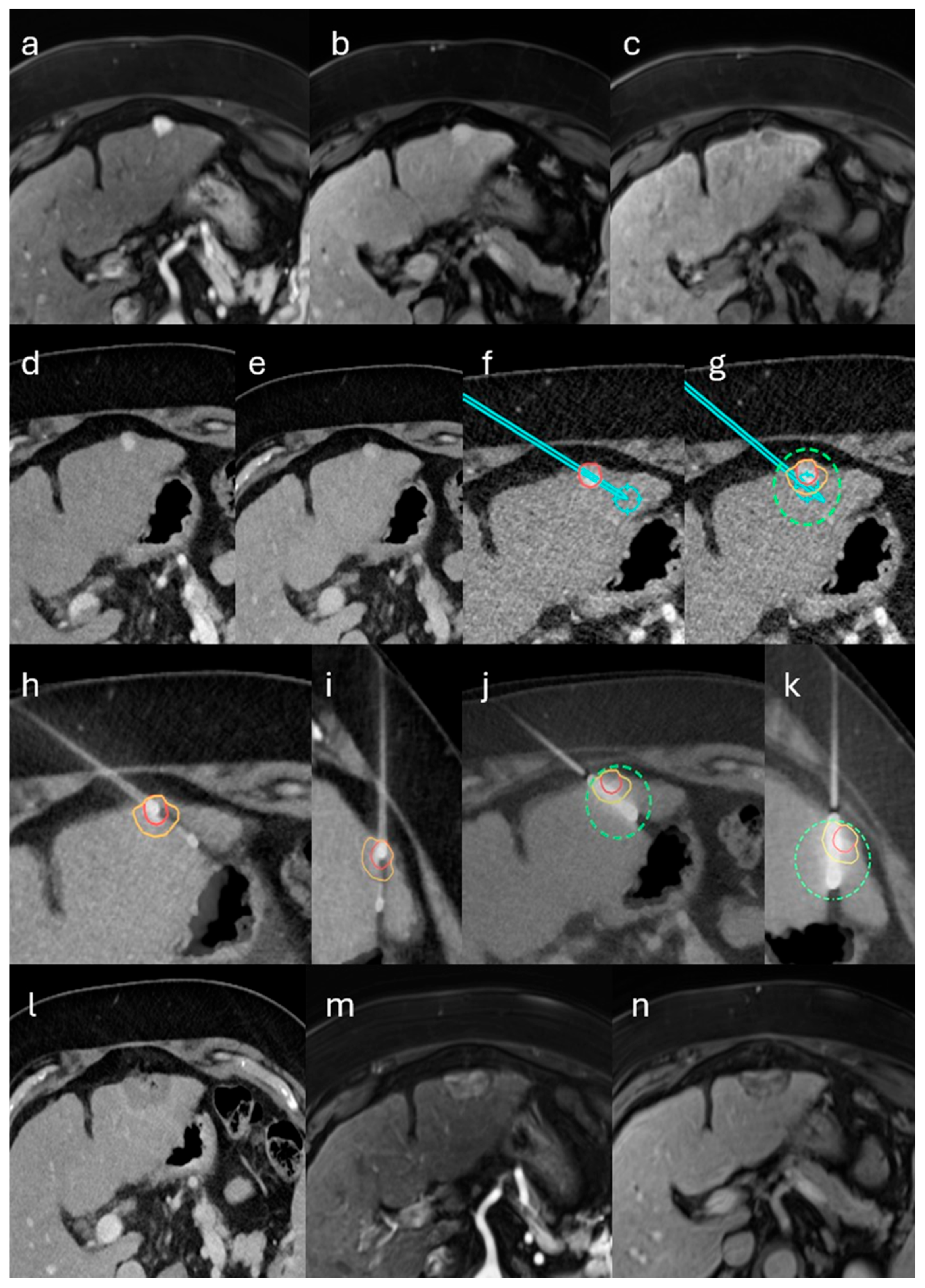
2.2. Stereotactic Biopsy and MWA
2.3. Endpoints and Statistical Analysis
3. Results
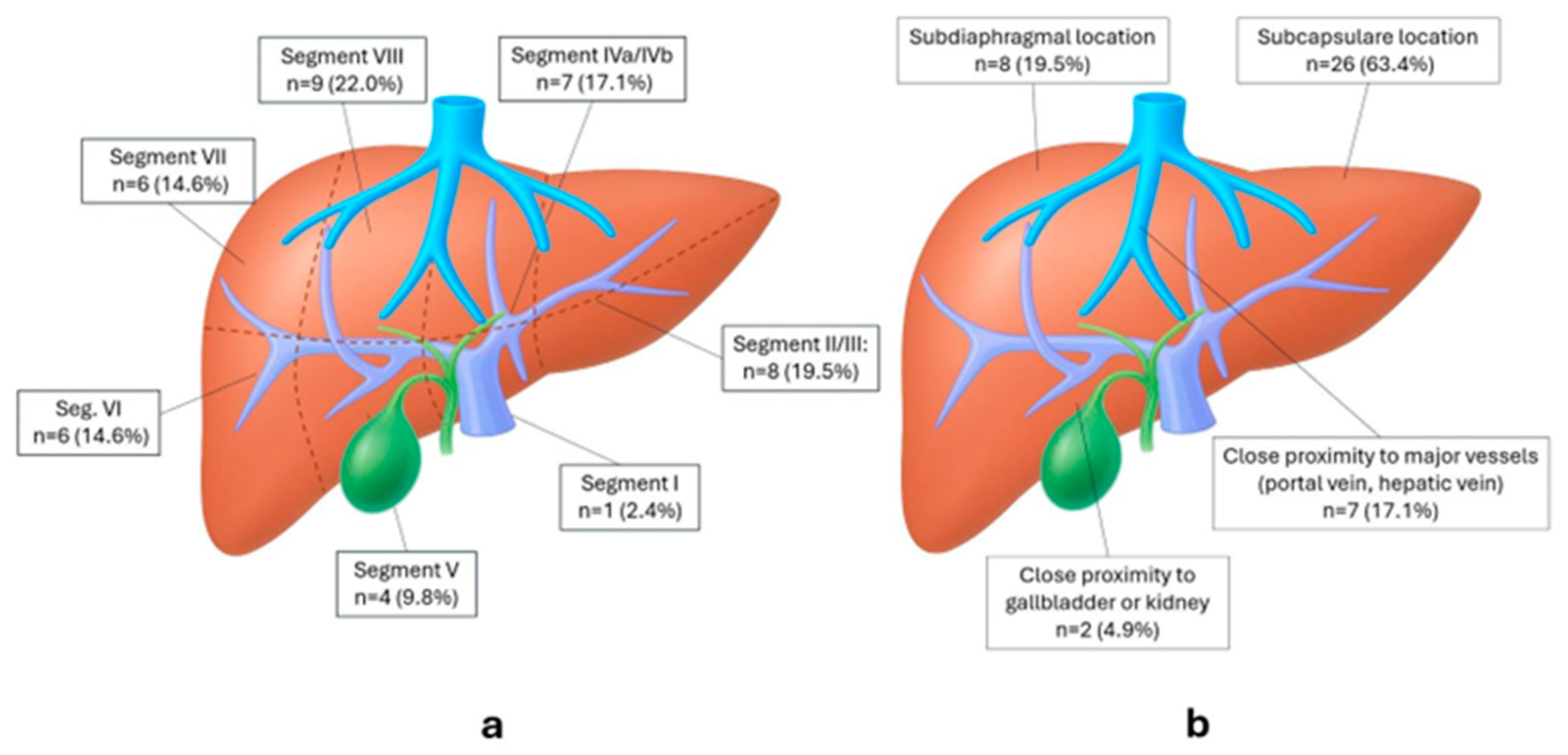
3.1. Patient, Tumor and Treatment Characteristics
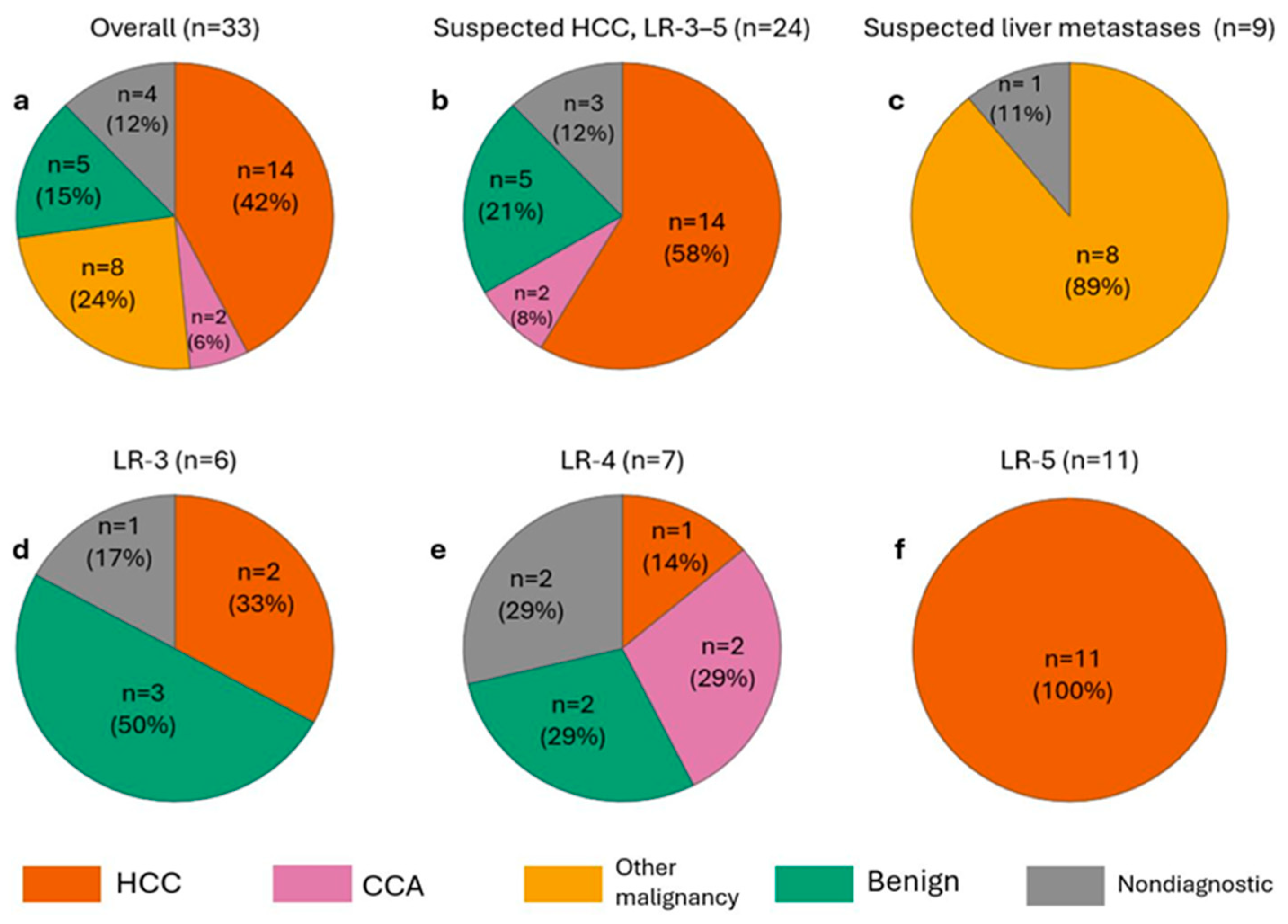
3.2. Primary Technique Efficacy (PTE)
3.3. 6-Month Local Tumor Progression (LTP)
3.4. Complications
3.5. Histopathological Yield and Correlation with Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ceppa, E.P.; Collings, A.T.; Abdalla, M.; Onkendi, E.; Nelson, D.W.; Ozair, A.; Miraflor, E.; Rahman, F.; Whiteside, J.; Shah, M.M.; et al. SAGES/AHPBA guidelines for the use of microwave and radiofrequency liver ablation for the surgical treatment of hepatocellular carcinoma or colorectal liver metastases less than 5 cm. Surg. Endosc. 2023, 37, 8991–9000. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; Baere, T.d.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Crocetti, L.; Baére, T.d.; Pereira, P.L.; Tarantino, F.P. CIRSE Standards of Practice on Thermal Ablation of Liver Tumours. Cardiovasc. Intervent. Radiol. 2020, 43, 951–962. [Google Scholar] [CrossRef]
- Vogel, A.; Chan, S.L.; Dawson, L.A.; Kelley, R.K.; Llovet, J.M.; Meyer, T.; Ricke, J.; Rimassa, L.; Sapisochin, G.; Vilgrain, V.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025, 36, 491–506. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Poulou, L.S.; Botsa, E.; Thanou, I.; Ziakas, P.D.; Thanos, L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1054–1063. [Google Scholar] [CrossRef]
- Radosevic, A.; Quesada, R.; Serlavos, C.; Sánchez, J.; Zugazaga, A.; Sierra, A.; Coll, S.; Busto, M.; Aguilar, G.; Flores, D.; et al. Microwave versus radiofrequency ablation for the treatment of liver malignancies: A randomized controlled phase 2 trial. Sci. Rep. 2022, 12, 316. [Google Scholar] [CrossRef]
- Wright, A.S.; Sampson, L.A.; Warner, T.F.; Mahvi, D.M.; Lee, F.T. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005, 236, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Cannella, R.; Berman, Z.T.; Tirukkovalur, N.V.; Tohme, S.T.; Minervini, M.I.; Furlan, A. Role of imaging-guided biopsy for hepatocellular carcinoma. Eur. J. Radiol. 2025, 191, 112271. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Yang, W.; Wang, C.; Guo, T.; Yang, J.; Shao, Z.; Cai, G.; Cai, S.; Zhang, L.; et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology 2023, 165, 414–428.e7. [Google Scholar] [CrossRef]
- Parra, N.S.; Ross, H.M.; Khan, A.; Wu, M.; Goldberg, R.; Shah, L.; Mukhtar, S.; Beiriger, J.; Gerber, A.; Halegoua-DeMarzio, D. Advancements in the Diagnosis of Hepatocellular Carcinoma. IJTM 2023, 3, 51–65. [Google Scholar] [CrossRef]
- Wang, M.-D.; Diao, Y.-K.; Yao, L.-Q.; Fan, Z.-Q.; Wang, K.-C.; Wu, H.; Gu, L.-H.; Xu, J.-H.; Li, C.; Lv, G.-Y.; et al. Emerging role of molecular diagnosis and personalized therapy for hepatocellular carcinoma. ILIVER 2024, 3, 100083. [Google Scholar] [CrossRef]
- Yang, X.; Yang, C.; Zhang, S.; Geng, H.; Zhu, A.X.; Bernards, R.; Qin, W.; Fan, J.; Wang, C.; Gao, Q. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell 2024, 42, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Kiran, N.S.; Yashaswini, C.; Maheshwari, R.; Bhattacharya, S.; Prajapati, B.G. Advances in Precision Medicine Approaches for Colorectal Cancer: From Molecular Profiling to Targeted Therapies. ACS Pharmacol. Transl. Sci. 2024, 7, 967–990. [Google Scholar] [CrossRef] [PubMed]
- Veltri, A.; Bargellini, I.; Giorgi, L.; Almeida, P.A.M.S.; Akhan, O. CIRSE Guidelines on Percutaneous Needle Biopsy (PNB). Cardiovasc. Intervent. Radiol. 2017, 40, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Sheth, R.A.; Baerlocher, M.O.; Connolly, B.L.; Dariushnia, S.R.; Shyn, P.B.; Vatsky, S.; Tam, A.L.; Gupta, S. Society of Interventional Radiology Quality Improvement Standards on Percutaneous Needle Biopsy in Adult and Pediatric Patients. J. Vasc. Interv. Radiol. 2020, 31, 1840–1848. [Google Scholar] [CrossRef]
- Sabir, S.H.; Krishnamurthy, S.; Gupta, S.; Mills, G.B.; Wei, W.; Cortes, A.C.; Mills Shaw, K.R.; Luthra, R.; Wallace, M.J. Characteristics of percutaneous core biopsies adequate for next generation genomic sequencing. PLoS ONE 2017, 12, e0189651. [Google Scholar] [CrossRef]
- Huang, S.-C.; Liang, J.-D.; Hsu, S.-J.; Hong, T.-C.; Yang, H.-C.; Kao, J.-H. Direct comparison of biopsy techniques for hepatic malignancies. Clin. Mol. Hepatol. 2021, 27, 305–312. [Google Scholar] [CrossRef]
- Tinguely, P.; Frehner, L.; Lachenmayer, A.; Banz, V.; Weber, S.; Candinas, D.; Maurer, M.H. Stereotactic Image-Guided Microwave Ablation for Malignant Liver Tumors-A Multivariable Accuracy and Efficacy Analysis. Front. Oncol. 2020, 10, 842. [Google Scholar] [CrossRef]
- Schaible, J.; Lürken, L.; Wiggermann, P.; Verloh, N.; Einspieler, I.; Zeman, F.; Schreyer, A.G.; Bale, R.; Stroszczynski, C.; Beyer, L. Primary efficacy of percutaneous microwave ablation of malignant liver tumors: Comparison of stereotactic and conventional manual guidance. Sci. Rep. 2020, 10, 18835. [Google Scholar] [CrossRef]
- Durand, P.; Moreau-Gaudry, A.; Silvent, A.-S.; Frandon, J.; Chipon, E.; Médici, M.; Bricault, I. Computer assisted electromagnetic navigation improves accuracy in computed tomography guided interventions: A prospective randomized clinical trial. PLoS ONE 2017, 12, e0173751. [Google Scholar] [CrossRef]
- Laimer, G.; Schullian, P.; Bale, R. Stereotactic Thermal Ablation of Liver Tumors: 3D Planning, Multiple Needle Approach, and Intraprocedural Image Fusion Are the Key to Success-A Narrative Review. Biology 2021, 10, 644. [Google Scholar] [CrossRef]
- Perrodin, S.; Lachenmayer, A.; Maurer, M.; Kim-Fuchs, C.; Candinas, D.; Banz, V. Percutaneous stereotactic image-guided microwave ablation for malignant liver lesions. Sci. Rep. 2019, 9, 13836. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Chan, G.; Huang, I.K.H.; Kwan, J.; Lim, G.H.T.; Quek, L.H.H.; Pua, U. Combined Biopsy and Imaging-Guided Microwave Ablation by Using a Coaxial Guiding Needle. J. Belg. Soc. Radiol. 2021, 105, 25. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Intervent. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Kimura, S.; Sone, M.; Sugawara, S.; Itou, C.; Oshima, T.; Ozawa, M.; Nakama, R.; Murakami, S.; Matsui, Y.; Arai, Y.; et al. Risk factors of non-diagnostic percutaneous liver tumor biopsy: A single-center retrospective analysis of 938 biopsies based on cause of error. Jpn. J. Radiol. 2025, 43, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Tinguely, P.; Paolucci, I.; Ruiter, S.J.S.; Weber, S.; Jong, K.P.d.; Candinas, D.; Freedman, J.; Engstrand, J. Stereotactic and Robotic Minimally Invasive Thermal Ablation of Malignant Liver Tumors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 713685. [Google Scholar] [CrossRef] [PubMed]
- Schaible, J.; Pregler, B.; Verloh, N.; Einspieler, I.; Bäumler, W.; Zeman, F.; Schreyer, A.; Stroszczynski, C.; Beyer, L. Improvement of the primary efficacy of microwave ablation of malignant liver tumors by using a robotic navigation system. Radiol. Oncol. 2020, 54, 295–300. [Google Scholar] [CrossRef]
- Zhang, L.; Luerken, L.; Mayr, V.; Goetz, A.; Schlitt, A.; Stroszczynski, C.; Einspieler, I. The Efficacy and Safety of a Microwave Ablation System with a Dipole Antenna Design Featuring Floating Sleeves and Anti-Phase Technology in Stereotactic Percutaneous Liver Tumor Ablation: Results from a Prospective Study. Cancers 2024, 16, 4211. [Google Scholar] [CrossRef]
- Beyer, L.P.; Lürken, L.; Verloh, N.; Haimerl, M.; Michalik, K.; Schaible, J.; Stroszczynski, C.; Wiggermann, P. Stereotactically navigated percutaneous microwave ablation (MWA) compared to conventional MWA: A matched pair analysis. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1991–1997. [Google Scholar] [CrossRef]
- Tinguely, P.; Ruiter, S.J.S.; Engstrand, J.; Haas, R.J.d.; Nilsson, H.; Candinas, D.; Jong, K.P.d.; Freedman, J. A prospective multicentre trial on survival after Microwave Ablation VErsus Resection for Resectable Colorectal liver metastases (MAVERRIC). Eur. J. Cancer 2023, 187, 65–76. [Google Scholar] [CrossRef]
- Lachenmayer, A.; Tinguely, P.; Maurer, M.H.; Frehner, L.; Knöpfli, M.; Peterhans, M.; Weber, S.; Dufour, J.-F.; Candinas, D.; Banz, V. Stereotactic image-guided microwave ablation of hepatocellular carcinoma using a computer-assisted navigation system. Liver Int. 2019, 39, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Pausch, A.-M.; Ghali, T.; Wertheimer, T.; Zeman, F.; Mueller, K.; Doppler, M.C.; Einspieler, I.; Beyer, L.P.; Schleder, S.; Stroszczynski, C.; et al. Stereotactic Microwave Ablation of Hepatocellular Carcinoma: The Impact of Tumor Size and Minimal Ablative Margin on Therapeutic Success. Tomography 2022, 9, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Odisio, B.C.; Wah, T.M.; Fotiadis, N.; Anton, K.; Yoon, C.J.; Stanziola, J.; Mamun, A.; Meyers, E.E.; Leon, H.d. Safety and Effectiveness of Microwave Ablation of Liver Tumors: Initial Real-World Results from the Multinational NeuWave Observational Liver Ablation (NOLA) Registry. J. Vasc. Interv. Radiol. 2025, 36, 813–822.e5. [Google Scholar] [CrossRef]
- Schullian, P.; Widmann, G.; Lang, T.B.; Knoflach, M.; Bale, R. Accuracy and diagnostic yield of CT-guided stereotactic liver biopsy of primary and secondary liver tumors. Comput. Aided Surg. 2011, 16, 181–187. [Google Scholar] [CrossRef]
- Fotiadis, N.; Paepe, K.N.d.; Bonne, L.; Khan, N.; Riddell, A.; Turner, N.; Starling, N.; Gerlinger, M.; Rao, S.; Chau, I.; et al. Comparison of a coaxial versus non-coaxial liver biopsy technique in an oncological setting: Diagnostic yield, complications and seeding risk. Eur. Radiol. 2020, 30, 6702–6708. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Cyrany, J.; Kopecky, J.; Hoffmannova, M.; Ryska, P.; Hulek, M.; Dvorak, P. Percutaneous CT-guided Biopsy of Focal Liver Lesions—Long- term Experience with more than 300 Procedures. J. Gastrointestin. Liver Dis. 2023, 32, 197–205. [Google Scholar] [CrossRef]
- Elsayes, K.M.; Ellis, J.H.; Elkhouly, T.; Ream, J.M.; Bowerson, M.; Khan, A.; Caoili, E.M. Diagnostic yield of percutaneous image-guided tissue biopsy of focal hepatic lesions in cancer patients: Ten percent are not metastases from the primary malignancy. Cancer 2011, 117, 4041–4048. [Google Scholar] [CrossRef]
- Vernuccio, F.; Rosenberg, M.D.; Meyer, M.; Choudhury, K.R.; Nelson, R.C.; Marin, D. Negative Biopsy of Focal Hepatic Lesions: Decision Tree Model for Patient Management. AJR Am. J. Roentgenol. 2019, 212, 677–685. [Google Scholar] [CrossRef]
- Langenbach, M.C.; Vogl, T.J.; Buchinger, A.; Eichler, K.; Scholtz, J.-E.; Hammerstingl, R.; Gruber-Rouh, T. CT-guided biopsies of unspecified suspect intrahepatic lesions: Pre-procedure Lipiodol-marking improves the biopsy success rate. Radiol. Oncol. 2023, 57, 158–167. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.-Y.; Shin, J.; Son, W.J.; Roh, Y.H.; Choi, J.-Y.; Sirlin, C.B.; Chernyak, V. Percentages of Hepatocellular Carcinoma in LI-RADS Categories with CT and MRI: A Systematic Review and Meta-Analysis. Radiology 2023, 307, e220646. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.S.; Park, S.H.; Kim, K.M.; Yu, E.; Park, Y.; Shin, Y.M.; Lee, M.-G. LI-RADS Classification and Prognosis of Primary Liver Cancers at Gadoxetic Acid-enhanced MRI. Radiology 2019, 290, 388–397. [Google Scholar] [CrossRef]
- Di Tommaso, L.; Spadaccini, M.; Donadon, M.; Personeni, N.; Elamin, A.; Aghemo, A.; Lleo, A. Role of liver biopsy in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 6041–6052. [Google Scholar] [CrossRef] [PubMed]
- Galun, D.; Mijac, D.; Filipovic, A.; Bogdanovic, A.; Zivanovic, M.; Masulovic, D. Precision Medicine for Hepatocellular Carcinoma: Clinical Perspective. J. Pers. Med. 2022, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2024, 22, e240029. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Value |
|---|---|
| Demographics | |
| Number of patients | 33 |
| Age, years (mean ± SD) | 65.1 ± 8.3 |
| Sex—Male | 19 (58%) |
| Sex—Female | 14 (42%) |
| Liver status | |
| Cirrhotic | 19 (58%) |
| — Child-Pugh A | 13 |
| — Child-Pugh B | 6 |
| Non-cirrhotic | 14 (42%) |
| Indication | |
| Suspected HCC | 24 (73%) |
| — LI-RADS 5 | 11 (46% of HCC) |
| — LI-RADS 4 | 7 (29% of HCC) |
| — LI-RADS 3 | 6 (25% of HCC) |
| Suspected metastatic liver tumors | 9 (27%) |
| — Colorectal cancer | 5 (15%) |
| — Pancreatic cancer | 2 (6%) |
| — Breast cancer | 1 (3%) |
| — Thyroid cancer | 1 (3%) |
| Tumor counts and size | |
| Number of biopsied tumors (1 per case) | 33 |
| Number of ablated tumors | 41 |
| Tumor diameter, mm (median [IQR]) | 14 [10–20] |
| Tumor size categories | |
| — Tumor diameter < 10 mm | 16 (39%) |
| — Tumor diameter 10–20 mm | 16 (39%) |
| — Tumor diameter > 20 mm | 9 (22%) |
| Interventional metrics | |
| Total anesthesia time, minutes (median [IQR]) | 118 (104–155) |
| Intervention time, minutes (median [IQR]) | 79 (60–97) |
| — Biopsy time, minutes (median [IQR]) | 10 (8–13) |
| — Ablation time, minutes (median [IQR]) | 36 (28–46) |
| — Other intraprocedural time, minutes (median [IQR]) | 28 (22–42) |
| Number of control scans per case (median [IQR]) | 4 (2–6) |
| CT DLP per case, mGy·cm (median [IQR]) | 2110 (1928–2570) |
| MWA antenna (per ablation) | |
| — NEUWAVE™ PRXT | 15 (37%) |
| — Dophi™ M150E | 23 (56%) |
| — Emprint™ HP | 3 (7%) |
| Postinterventional hospital stay, days (median [IQR]) | 2 (2–2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ngu, A.; Kupke, L.S.; Mayr, V.; Strotzer, Q.; Brandenstein, M.; Stroszczynski, C.; Einspieler, I. Combined Single-Session Stereotactic Biopsy and Microwave Ablation of Primary and Secondary Liver Tumors. Biomedicines 2025, 13, 2865. https://doi.org/10.3390/biomedicines13122865
Zhang L, Ngu A, Kupke LS, Mayr V, Strotzer Q, Brandenstein M, Stroszczynski C, Einspieler I. Combined Single-Session Stereotactic Biopsy and Microwave Ablation of Primary and Secondary Liver Tumors. Biomedicines. 2025; 13(12):2865. https://doi.org/10.3390/biomedicines13122865
Chicago/Turabian StyleZhang, Liang, Anthony Ngu, Laura Sophia Kupke, Vinzenz Mayr, Quirin Strotzer, Moritz Brandenstein, Christian Stroszczynski, and Ingo Einspieler. 2025. "Combined Single-Session Stereotactic Biopsy and Microwave Ablation of Primary and Secondary Liver Tumors" Biomedicines 13, no. 12: 2865. https://doi.org/10.3390/biomedicines13122865
APA StyleZhang, L., Ngu, A., Kupke, L. S., Mayr, V., Strotzer, Q., Brandenstein, M., Stroszczynski, C., & Einspieler, I. (2025). Combined Single-Session Stereotactic Biopsy and Microwave Ablation of Primary and Secondary Liver Tumors. Biomedicines, 13(12), 2865. https://doi.org/10.3390/biomedicines13122865






