Abstract
Background/Objectives: Cardiometabolic risk clustering (CMRC), the coexistence of multiple risk factors, markedly increases the risk of cardiovascular disease and diabetes. While obesity is central, the independent roles of vitamin D status and systemic inflammation remain unclear. This study examined determinants of CMRC in middle-aged Korean adults, focusing on vitamin D and C-reactive protein (CRP). Methods: Cross-sectional data were analyzed from 2062 adults aged 40–64 years in the 2023 Korea National Health and Nutrition Examination Survey. CMRC was defined as ≥3 of abdominal obesity, hypertension, diabetes, hypertriglyceridemia, and low high-density lipoprotein cholesterol. Serum 25-hydroxyvitamin D [25(OH)D], CRP, lifestyle behaviors, and covariates were assessed. Complex-sample logistic regression identified factors associated with CMRC. Results: CMRC prevalence was 16.5%. Older age (OR = 1.04, 95% CI: 1.02–1.06), current smoking (OR = 1.76, 95% CI: 1.26–2.45), elevated CRP (1–3 mg/L: OR = 1.40, 95% CI: 1.04–1.87; ≥3 mg/L: OR = 1.63, 95% CI: 1.00–2.66), and obesity (OR = 8.29, 95% CI: 6.12–11.21) increased CMRC risk. Protective factors included male sex (OR = 0.60, 95% CI: 0.45–0.81), sufficient vitamin D (≥20 ng/mL: OR = 0.76, 95% CI: 0.58–0.99), and meeting World Health Organization physical activity guidelines (OR = 0.71, 95% CI: 0.55–0.92). Conclusions: These survey-weighted associations may help identify at-risk mid-life adults at the population level and motivate longitudinal evaluation of vitamin D deficiency and inflammation in risk assessment and targeted prevention.
Keywords:
metabolic syndrome; vitamin D; C-reactive protein; obesity; epidemiology; smoking; physical activity 1. Introduction
Cardiometabolic risk factors—including obesity, hypertension, dyslipidemia, impaired fasting glucose, and systemic inflammation—are recognized as major drivers of the global burden of chronic disease [1]. These risk factors frequently cluster within individuals, a phenomenon referred to as cardiometabolic risk clustering (CMRC) [2]. The simultaneous presence of multiple risk factors exerts additive and possibly synergistic effects, leading to substantially higher risks of cardiovascular disease, type 2 diabetes, and premature mortality compared with single risk exposures [3]. With population aging, CMRC has become an important focus for clinical management and public health policy [2].
In Korea, the prevalence of cardiometabolic conditions has risen markedly in recent decades, reflecting demographic shifts and lifestyle transitions [4]. Middle-aged adults (40–64 years) represent a critical stage in which cardiometabolic risks accumulate, yet preventive actions remain possible [5]. Identifying determinants of CMRC in this group is therefore essential for strategies that may delay or prevent disease progression.
Obesity is a well-established contributor to CMRC, primarily through links with insulin resistance, abnormal lipid metabolism, and hypertension [6]. However, exclusive attention to adiposity may overlook other modifiable influences. Increasing research has focused on vitamin D status as a potential correlate beyond adiposity. Vitamin D, mainly obtained through sunlight and diet, has traditionally been linked to bone and mineral metabolism [7], with emerging evidence for broader roles in insulin sensitivity, lipid regulation, and immune modulation [8,9]. Low serum 25-hydroxyvitamin D [25(OH)D] concentrations have been associated with insulin resistance, metabolic syndrome, and hypertension [10]; yet, evidence for clustering per se is heterogeneous once central adiposity and lipids are accounted for [11].
Systemic inflammation has likewise been implicated in CMRC. CRP, a marker of low-grade inflammation, has been linked to obesity, insulin resistance, vascular dysfunction, and atherosclerosis [12]. Elevated CRP levels are also associated with increased risks of metabolic syndrome and cardiovascular events independent of traditional risk factors [13]. However, the role of inflammation in CMRC among middle-aged adults, and its interaction with vitamin D or lifestyle factors, remains insufficiently examined.
Lifestyle behaviors—including smoking, alcohol use, physical activity, and sleep—further influence cardiometabolic health [14]. For example, adherence to the World Health Organization (WHO) physical activity guideline is protective, whereas smoking contributes to vascular dysfunction and insulin resistance [15]. Sleep duration, though less studied, has been linked to obesity and metabolic dysregulation [16]. Yet, the interplay between these behaviors, vitamin D status, and systemic inflammation in relation to CMRC is not fully clarified.
Taken together, current evidence highlights the multifactorial nature of CMRC while revealing knowledge gaps, particularly regarding the independent and combined roles of vitamin D [25(OH)D] and systemic inflammation (hs-CRP). Although Korea is a relevant setting—given rapid population aging and nationally representative health data—most prior studies examined these biomarkers in isolation, focused on older adults, or did not apply survey-weighted models to CMRC as an integrated outcome. Leveraging the most recent Korea National Health and Nutrition Examination Survey (KNHANES), our study addresses this gap by jointly evaluating 25(OH)D and hs-CRP in relation to CMRC in mid-life (40–64 years) using survey-weighted multivariable analyses.
We anticipated that lower serum 25(OH)D and higher hs-CRP would be associated with greater odds of CMRC in mid-life, independent of sociodemographic characteristics, lifestyle behaviors, and adiposity, and we explored their joint associations and potential sex differences. Using KNHANES 2023 and survey-weighted multivariable models, we aimed to (i) estimate the prevalence of CMRC in adults aged 40–64 years; (ii) quantify the independent and joint associations of 25(OH)D and hs-CRP with CMRC; and (iii) conduct prespecified sensitivity analyses (alternative 25(OH)D cut-offs; exclusion of extreme CRP values) and exploratory interaction checks to assess robustness.
2. Materials and Methods
2.1. Study Design and Data Source
This study employed a cross-sectional design using data from the 2023 KNHANES, which was released in December 2024. KNHANES is a nationally representative survey conducted annually by the Korea Disease Control and Prevention Agency (KDCA) to monitor the health and nutritional status of the Korean population. The survey consists of three components: a health interview, a health examination, and a nutrition survey. Participants are selected through a two-stage stratified cluster sampling design based on age, sex, and geographic region, ensuring representativeness of the civilian, non-institutionalized Korean population [17]. Detailed information regarding the survey design, sampling methods, and quality assurance procedures is available in the official KNHANES reports.
2.2. Study Population
A total of 6929 individuals participated in the 2023 KNHANES. Of these, 1836 adults aged 65 years or older and 2312 individuals younger than 40 years were excluded to restrict the analysis to middle-aged adults. Among the remaining 2781 participants, 719 were further excluded due to missing data on study variables required for analysis. The final analytic sample, therefore, comprised 2062 adults aged 40–64 years with complete information on all variables of interest (Figure 1).
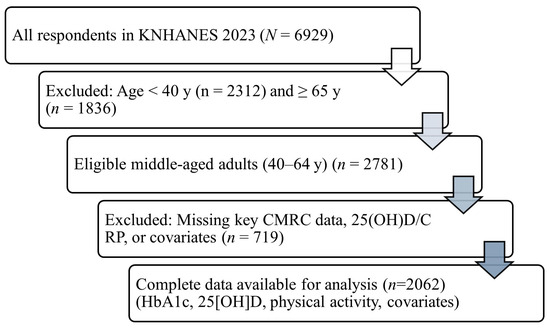
Figure 1.
Flow of participant selection for the analytic sample, KNHANES 2023. From all respondents in 2023 (n = 6929), participants aged ≥65 years (n = 1836) and <40 years (n = 2312) were excluded to restrict analyses to middle-aged adults. Among the remaining 2781 participants, those with missing data for CMRC components, serum 25(OH)D, CRP, or prespecified covariates were excluded (n = 719), yielding a final analytic sample of n = 2062. Abbreviations: CMRC, cardiometabolic risk clustering; 25(OH)D, 25-hydroxyvitamin D; CRP, C-reactive protein; KNHANES, Korea National Health and Nutrition Examination Survey. Notes: CMRC was defined as the coexistence of ≥3 major metabolic risk factors (central adiposity, blood pressure/hypertension, dyslipidemia, impaired fasting glucose). Survey-weighted procedures (weights, strata, primary sampling units) were used in all analyses.
2.3. Measures
2.3.1. Outcome Variable
CMRC was defined as the presence of three or more cardiometabolic risk factors, consistent with the criteria applied in a previous study [18]. The risk factors included abdominal obesity, hypertension, diabetes, hypertriglyceridemia, and low high-density lipoprotein (HDL) cholesterol. Abdominal obesity was defined as a waist circumference of ≥90 cm for men and ≥85 cm for women. Hypertension was defined as systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg, or current use of antihypertensive medication. Diabetes was defined as fasting glucose ≥ 126 mg/dL, a physician diagnosis, or use of glucose-lowering medication. Hypertriglyceridemia was defined as triglycerides ≥ 150 mg/dL or current treatment for dyslipidemia, and low HDL cholesterol as <40 mg/dL for men and <50 mg/dL for women.
2.3.2. Independent Variables
The primary independent variables of interest were serum 25(OH)D and high-sensitivity CRP. Serum 25(OH)D concentration was measured using liquid chromatography–tandem mass spectrometry, and vitamin D deficiency was defined as <20 ng/mL. Serum CRP concentration was measured by immunoturbidimetry and categorized into <1.0 mg/L, 1–3 mg/L, and ≥3 mg/L.
2.3.3. Covariates
Sociodemographic covariates included age (continuous), sex (male/female), education (≤high school, ≥college), and household income (low–middle, middle–high). Lifestyle factors included alcohol use in the past year (yes/no), current smoking (yes/no), physical activity, and sleep duration. Physical activity was assessed according to the WHO guideline, classifying participants as meeting or not meeting the aerobic activity recommendation. Average sleep duration was dichotomized as <7 h/day vs. ≥7 h/day. Anthropometric and clinical measures included body mass index (BMI, kg/m2), waist circumference (cm), systolic and diastolic blood pressure (mmHg), fasting glucose (mg/dL), triglycerides (mg/dL), and HDL cholesterol (mg/dL).
Detailed diet quality, vitamin D intake/supplement use, season of blood draw, menopausal status, and medication use were unavailable/limited and could not be fully adjusted for.
2.4. Statistical Analysis
All models were estimated using IBM SPSS Complex Samples (v29) with sampling weights, strata, and primary sampling units (PSUs) to obtain design-based inferences. We prespecified covariates (age, sex, education, smoking, alcohol use, physical activity, adiposity, blood pressure/hypertension, lipids, and glycaemic markers/diabetes) while avoiding over-adjustment for components defining CMRC. Variance inflation factors (VIFs) were <2.5 in all multivariable models. Model performance included Nagelkerke R2; influence diagnostics were inspected. Sensitivity analyses varied 25(OH)D cut-offs (<10/<20/<30 ng/mL) and excluded extreme CRP values; vitamin D supplement use was not available and could not be adjusted for. We estimated survey-weighted logistic models (weights, strata, PSUs) and reported ORs (95% CIs). Multicollinearity was low (VIF < 2.5); model performance was summarized by Nagelkerke R2. Sensitivity varied 25(OH)D cut-offs and excluded extreme CRP values; exploratory interactions (sex × 25(OH)D, sex × CRP) were screened.
2.5. Ethical Considerations
The KNHANES is conducted by the KDCA under the National Health Promotion Act. All survey protocols were approved by the KDCA Institutional Review Board (IRB approval numbers: 2022-11-16-R-A). Written informed consent was obtained from all participants prior to data collection. De-identified datasets are publicly available for research purposes through the official KNHANES website. The present study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
3. Results
3.1. Baseline Characteristics of the Study Population
A total of 2062 participants aged 40–64 years were included in the analysis. The mean age was 53.0 years (SD = 7.3), and 43.5% were male. Nearly half of the participants had attained at least a college education (46.4%), and 68.0% reported middle-to-high household income. The prevalence of current smoking and alcohol use in the past year was 19.1% and 77.0%, respectively. Approximately 46.4% of participants met the WHO physical activity guideline, and 55.7% reported sleeping ≥ 7 h per day. The mean body mass index (BMI) was 24.5 kg/m2 (SD = 3.6), and 40.0% were classified as obese (BMI ≥ 25 kg/m2). The mean serum 25(OH)D concentration was 24.4 ng/mL (SD = 10.8), while the mean CRP level was 1.29 mg/L (SD = 3.09). The average CMRC score was 1.25 (SD = 1.22) (Table 1).

Table 1.
Descriptive statistics of participant characteristics (N = 2062).
3.2. Sociodemographic, Lifestyle, and Clinical Differences Between CMRC Groups
When stratified by CMRC status, significant differences were observed across multiple sociodemographic, lifestyle, and clinical characteristics (Table 2). Participants with CMRC were older (54.2 vs. 52.8 years, p = 0.001) and more likely to be male (64.5% vs. 39.1%, p < 0.001). Higher educational attainment (≥college) was less common in the CMRC group compared with the non-CMRC group (39.3% vs. 47.8%, p = 0.004), whereas household income did not differ significantly (p = 0.211). Regarding lifestyle behaviors, the CMRC group had higher rates of current smoking (29.0% vs. 17.1%, p < 0.001) and lower adherence to the WHO physical activity guideline (38.7% vs. 48.0%, p = 0.002). Average sleep duration was slightly shorter in the CMRC group (6.71 vs. 6.86 h/day, p = 0.018), although the proportion reporting ≥7 h of sleep per day did not differ significantly (p = 0.240). In terms of anthropometric and clinical markers, obesity was substantially more prevalent among participants with CMRC (80.1% vs. 32.0%, p < 0.001). The CMRC group also had higher BMI, waist circumference, systolic and diastolic blood pressure, fasting glucose, and triglyceride levels, alongside lower HDL-cholesterol and vitamin D levels (all p < 0.001). CRP levels were also significantly elevated in the CMRC group compared to the non-CMRC group (1.62 vs. 1.22 mg/L, p = 0.030).

Table 2.
Comparison of participant characteristics according to CMRC (N = 2062).
3.3. Factors Independently Associated with CMRC
The results of multivariable logistic regression are presented in Table 3. Older age was independently associated with increased odds of CMRC (OR = 1.04, 95% CI [1.02–1.06], p < 0.001). Current smoking (OR = 1.76, 95% CI [1.26–2.45], p = 0.001), elevated CRP (1–3 mg/L: OR = 1.40, 95% CI [1.04–1.87], p = 0.026; ≥3 mg/L: OR = 1.63, 95% CI [1.00–2.66], p = 0.033), and obesity (OR = 8.29, 95% CI [6.12–11.21], p < 0.001) significantly increased the likelihood of CMRC. In contrast, male sex (OR = 0.60, 95% CI [0.45–0.81], p < 0.001), meeting the WHO physical activity guideline (OR = 0.71, 95% CI [0.55–0.92], p = 0.010), and sufficient vitamin D levels (≥20 ng/mL: OR = 0.76, 95% CI [0.58–0.99], p = 0.039) were protective factors. Education, household income, alcohol use, and sleep duration were not significantly associated with CMRC after adjustment for covariates.

Table 3.
Survey-weighted multivariable logistic regression: associations of 25(OH)D, hs-CRP, and covariates with CMRC (N = 2062).
4. Discussion
In this nationally representative sample of Korean adults aged 40–64 years, the prevalence of CMRC was 16.5%. In survey-weighted multivariable models, sufficient vitamin D (≥20 ng/mL) was associated with lower odds of CMRC, whereas elevated CRP (≥1.0 mg/L), obesity, and current smoking were associated with higher odds; meeting the WHO physical activity guideline was protective, while education, income, alcohol use, and sleep duration were not significant after adjustment. Sensitivity analyses varying vitamin D cut-offs and excluding extreme CRP values yielded directionally similar estimates (Supplement Tables S1 and S2). Taken together, these findings reinforce the multifactorial nature of CMRC, spanning biologic markers and modifiable behaviors, while we interpret them as associations given the cross-sectional design. Given the cross-sectional design, these patterns are associational and should be interpreted with caution.
4.1. Comparison with Previous Studies
The prevalence we observed is somewhat lower than in older populations where clustering is more common [19], consistent with evidence that cardiometabolic risk accumulates with age. Even so, a substantial proportion of mid-life adults already exhibit multiple risk factors, underscoring the need for targeted identification in this age group [20]. Our vitamin D finding aligns with reports of inverse associations between lower 25(OH)D and adverse metabolic profiles (insulin resistance, dyslipidemia, hypertension) [21], but evidence for clustering per se is heterogeneous once central adiposity and lipids are rigorously accounted for [13]. Such inconsistencies likely reflect differences in age structure, covariate adjustment (especially adiposity), and analytic approaches across studies [22].
The protective association of meeting the WHO physical activity guideline and the adverse association of current smoking are consistent with prior studies: physical activity improves insulin sensitivity, lipid metabolism, and blood pressure [23], whereas smoking contributes to inflammation and endothelial dysfunction [24]. Evidence linking sleep duration to metabolic dysregulation is less consistent [16].
Lifestyle patterns also played an important role. The protective effect of meeting the WHO physical activity guideline and the harmful effect of current smoking are consistent with prior studies. Physical activity improves insulin sensitivity, lipid metabolism, and blood pressure [23], while smoking contributes to inflammation and endothelial dysfunction [24]. The inverse association for male sex contrasts with reports of higher risk in men [25]. Differences in fat distribution, hormonal milieu, or behaviors may contribute, but these explanations are speculative. Where feasible, sex-stratified models and sex × 25(OH)D/sex × CRP interactions should be examined to clarify whether associations differ by sex. The inverse association for male sex contrasts with several reports; our exploratory interaction screens did not yield consistent evidence of effect modification. Formal sex-stratified analyses in longitudinal data are warranted.
4.2. Possible Mechanisms
Several biological and behavioral pathways may explain the observed associations. Vitamin D has been implicated in insulin secretion, lipid regulation, and blood pressure control through effects on pancreatic β-cell function, insulin receptor activity, the renin–angiotensin–aldosterone system, and vascular endothelial health [26]. Deficiency may therefore be compatible with a pro-atherogenic and pro-inflammatory milieu that co-occurs with CMRC, although causal pathways remain to be established [10].
Systemic inflammation, as indicated by elevated CRP, may also play a central role. CRP, produced in response to cytokines such as interleukin-6 and tumor necrosis factor-α, reflects chronic low-grade inflammation that promotes insulin resistance, endothelial dysfunction, and dyslipidemia [27]. Elevated CRP may thus serve as both a marker and mediator of cardiometabolic dysregulation [28]. Part of the CRP–CMRC association may be mediated or confounded by adiposity-related pathways, consistent with reports of attenuation after adiposity adjustment [29].
Lifestyle behaviors further modulate these pathways. Physical activity reduces inflammation and improves insulin sensitivity and body composition, while smoking induces oxidative stress and vascular injury, exacerbating metabolic dysfunction [23,24]. These mechanisms align with our findings that adherence to the WHO physical activity guideline was protective, whereas current smoking increased the risk of CMRC.
Finally, the observed sex differences may reflect hormonal and physiological distinctions. Estrogen exerts protective effects on lipid metabolism and vascular function, which decline after menopause, while differences in fat distribution between men and women may also contribute [30]. However, the precise mechanisms underlying these sex-specific associations remain to be clarified.
4.3. Public Health and Clinical Implications
The findings of this study have important implications for public health and clinical practice. Vitamin D sufficiency was associated with lower odds of CMRC, suggesting that improving vitamin D status may help reduce cardiometabolic risk. Although causality cannot be inferred, intervention studies indicate that supplementation can improve insulin sensitivity, glucose metabolism, and lipid profiles in deficient individuals [31]. Public health measures such as fortification, supplementation, and safe sun exposure could therefore be beneficial in Korea.
The independent association between elevated CRP and CMRC highlights the role of inflammation in cardiometabolic health. Routine CRP measurement, a simple and widely available test, may provide added value in identifying individuals at increased risk of clustering [32]. Incorporating inflammatory markers into preventive strategies could be especially useful for middle-aged adults not yet meeting clinical thresholds for disease. The protective effects of physical activity and the risks of smoking reaffirm the importance of lifestyle interventions. Promoting exercise and supporting smoking cessation remain priorities for chronic disease prevention, and their benefits appear to extend beyond single outcomes to CMRC more broadly [23]. These benefits, well established for single outcomes, appear to extend to CMRC at the population level, consistent with our associational findings.
Finally, the higher risk observed among women suggests that middle-aged Korean women may represent a particularly vulnerable subgroup. This underscores the need for sex-specific approaches to risk assessment and prevention, and for further research into the biological and social determinants of these patterns.
4.4. Strengths and Limitations
This study has several strengths. We used data from the 2023 KNHANES, a nationally representative survey with standardized measurements and complex sampling. Analyses were conducted with survey-weighted models to obtain design-based inferences. The sample of middle-aged adults afforded adequate precision to examine associations across sociodemographic, lifestyle, and clinical domains. In addition, we concurrently evaluated biochemical markers (vitamin D and CRP) and behavioral factors (smoking, physical activity, and sleep), enabling a comprehensive assessment of CMRC. Model diagnostics indicated low multicollinearity (VIF < 2.5) and modest explanatory power (Nagelkerke R2 ≈ 0.27), typical of multifactorial population models. Prespecified sensitivity analyses (alternative vitamin D cut-offs; exclusion of extreme CRP values) yielded directionally similar results, supporting robustness.
Several limitations warrant emphasis. First, the cross-sectional design precludes causal inference because temporality cannot be established. Second, vitamin D supplement use and detailed dietary information were unavailable, and information on the season of blood draw was limited, which may introduce residual confounding. Third, lifestyle variables (alcohol use, smoking, sleep duration) were self-reported and thus susceptible to recall or misclassification. Fourth, additional unmeasured factors—such as menopausal status and medication use (e.g., statins, antihypertensives)—could not be fully addressed. Fifth, single-time-point measurements of 25(OH)D and CRP may not capture long-term status or variability; however, our findings were robust to the exclusion of extreme CRP values in sensitivity analyses.
Although estimates are derived from a Korean national sample, the biological links among vitamin D, adiposity, lipids, and glycemic regulation are broadly relevant across populations. Even so, external validity beyond East Asia requires confirmation. We therefore interpret our results as associational and hypothesis-generating, and encourage multicountry replication using harmonized CMRC definitions and longitudinal designs.
5. Conclusions
In this nationally representative sample of Korean adults aged 40–64 years, approximately one in six exhibited CMRC. In survey-weighted models, sufficient vitamin D and meeting World Health Organization physical activity guidelines were associated with lower odds of CMRC, whereas elevated hs-CRP, obesity, and current smoking were associated with higher odds. These findings may inform risk identification at the population level but should be interpreted as associations rather than causal effects given the cross-sectional design. Longitudinal and interventional studies—incorporating detailed diet, seasonality, medication, and sex-specific analyses—are warranted to confirm these associations and to evaluate their utility in risk assessment and targeted prevention for mid-life populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13112762/s1, Table S1: Survey-weighted logistic regression for cardiometabolic risk clustering using alternative 25(OH)D cut-offs; Table S2: Survey-weighted logistic regression for cardiometabolic risk clustering after excluding participants with extreme CRP values.
Author Contributions
Conceptualization, C.L. and K.J.; methodology, K.J.; formal analysis, K.J.; data curation, K.J.; writing—original draft preparation, C.L.; writing—review and editing, C.L. and K.J.; visualization, K.J.; supervision, K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved on 16 November 2022 by the Institutional Review Board of the Korea Disease Control and Prevention Agency (IRB No. 2022-11-16-R-A).
Informed Consent Statement
I confirm that all participants in the KNHANES provided informed consent through the KDCA, and that this secondary analysis was conducted entirely with de-identified data, without access to any personal identifiers. No additional informed consent was required for this study.
Data Availability Statement
The data that support the findings of this study are openly available from the KNHANES website (https://knhanes.kdca.go.kr, accessed on 12 July 2025), managed by the Korea Disease Control and Prevention Agency.
Acknowledgments
The author thanks the Korea Disease Control and Prevention Agency (KDCA) for providing access to the KNHANES data.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CMRC | Cardiometabolic Risk Clustering |
| CRP | C-Reactive Protein |
| WHO | World Health Organization |
| KNHANES | Korea National Health and Nutrition Examination Survey |
| KDCA | Korea Disease Control and Prevention Agency |
| IRB | Institutional Review Board |
| BMI | Body Mass Index |
| HDL | High-Density Lipoprotein |
| SD | Standard Deviation |
| OR | Odds Ratio |
| CI | Confidence Interval |
| 25(OH)D | 25-Hydroxy Vitamin D |
References
- Brauer, M.; Roth, G.A.; Aravkin, A.Y.; Zheng, P.; Abate, K.H.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasi, M.A.; Abbasian, M.; et al. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203, Erratum in Lancet 2024, 404, 244. [Google Scholar] [CrossRef]
- Stefan, N.; Schulze, M.B. Metabolic health and cardiometabolic risk clusters: Implications for prediction, prevention, and treatment. Lancet Diabetes Endocrinol. 2023, 11, 426–440. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, L.; Wang, X.; Chen, Z.; Zheng, C.; Chen, L.; Zhou, H.; Cai, J.; Hu, Z.; Tian, Y.; et al. Cardiovascular disease and all-cause mortality associated with individual and combined cardiometabolic risk factors. BMC Public Health 2023, 23, 1725. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, D.R.; Kim, J.Y.; Kim, W.; Jeong, Y.W.; Chun, K.-H.; Han, S.H.; Koh, K.K. Metabolic syndrome fact sheet 2024: Executive report. CardioMetabolic Syndr. J. 2024, 4, 70. [Google Scholar] [CrossRef]
- Kwok, Z.C.-M.; Tao, A.; Chan, H.Y.-L. Effects of health coaching on cardiometabolic health in middle-aged adults: A systematic review and meta-analysis. Am. J. Health Promot. 2023, 37, 555–565. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500, Erratum in Circ. Res. 2020, 127, 107. [Google Scholar] [CrossRef]
- Chen, X.; Shen, L.; Gao, C.; Weng, R.; Fan, Y.; Xu, S.; Zhang, Z.; Hu, W. Vitamin D status and its associations with bone mineral density, bone turnover markers, and parathyroid hormone in Chinese postmenopausal women with osteopenia and osteoporosis. Front. Nutr. 2023, 10, 1307896. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Shin, S.; Han, S.N. Multifaceted roles of vitamin D for diabetes: From immunomodulatory functions to metabolic regulations. Nutrients 2024, 16, 3185. [Google Scholar] [CrossRef]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The role of vitamin D and its molecular bases in insulin resistance, diabetes, metabolic syndrome, and cardiovascular disease: State of the art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Shahdadian, F.; Moslemi, E.; Mirenayat, F.S.; Saneei, P. Serum vitamin D levels in relation to metabolic syndrome: A systematic review and dose–response meta-analysis of epidemiologic studies. Obes. Rev. 2021, 22, e13223. [Google Scholar] [CrossRef]
- Mohammed, J.A.; Basil, B.; Mba, I.N.; Abubakar, N.D.; Lawal, A.O.; Momoh, J.A.; Yahaya, I.A. Elevated high-sensitivity C-reactive protein and dyslipidaemia in type 2 diabetes mellitus: Implications for cardiovascular risk prediction in Nigerian patients. BMC Endocr. Disord. 2025, 25, 100. [Google Scholar] [CrossRef]
- Amezcua-Castillo, E.; González-Pacheco, H.; Sáenz-San Martín, A.; Méndez-Ocampo, P.; Gutierrez-Moctezuma, I.; Massó, F.; Sierra-Lara, D.; Springall, R.; Rodríguez, E.; Arias-Mendoza, A.; et al. C-reactive protein: The quintessential marker of systemic inflammation in coronary artery disease—Advancing toward precision medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef]
- Verswijveren, S.J.; Dingle, S.; Donnelly, A.E.; Dowd, K.P.; Ridgers, N.D.; Carson, B.P.; Kearney, P.M.; Harrington, J.M.; Chappel, S.E.; Powell, C. How are different clusters of physical activity, sedentary, sleep, smoking, alcohol, and dietary behaviors associated with cardiometabolic health in older adults? A cross-sectional latent class analysis. J. Act. Sedentary Sleep Behav. 2023, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Saldana, G.; Liu, L.; German, C.A. Physical activity, steps, and cardiovascular disease: A literature review. Heart Mind 2025, 9, 94. [Google Scholar] [CrossRef]
- Pienaar, P.R.; Roden, L.C.; Boot, C.R.; van Mechelen, W.; Suter, J.A.; Lambert, E.V.; Rae, D.E. Associations between habitual sleep characteristics and cardiometabolic disease risk in corporate executives. Sleep Health 2024, 10, 550–557. [Google Scholar] [CrossRef]
- Oh, K.; Kim, Y.; Kweon, S.; Kim, S.; Yun, S.; Park, S.; Lee, Y.-K.; Kim, Y.-T.; Park, O.; Jeong, E.K. Korea National Health and Nutrition Examination Survey, 20th anniversary: Accomplishments and future directions. Epidemiol. Health 2021, 43, e2021025. [Google Scholar] [CrossRef]
- Mezhal, F.; Oulhaj, A.; Abdulle, A.; AlJunaibi, A.; Alnaeemi, A.; Ahmad, A.; Leinberger-Jabari, A.; Al Dhaheri, A.S.; Tuzcu, E.M.; AlZaabi, E.; et al. The interrelationship and accumulation of cardiometabolic risk factors amongst young adults in the United Arab Emirates: The UAE Healthy Future Study. Diabetol. Metab. Syndr. 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, C.; Song, B.; Chen, D.; Teng, D.; Shan, Z.; Teng, W. The prevalence and clustering of metabolic syndrome risk components in Chinese population: A cross-sectional study. Front. Endocrinol. 2023, 14, 1290855. [Google Scholar] [CrossRef]
- Shin, S. Association between nut consumption and metabolic syndrome in Korean adults. J. Lipid Atheroscler. 2025, 14, 219–228. [Google Scholar] [CrossRef]
- Nejabat, A.; Emamat, H.; Afrashteh, S.; Jamshidi, A.; Jamali, Z.; Farhadi, A.; Talkhabi, Z.; Nabipour, I.; Larijani, B.; Spitz, J. Association of serum 25-hydroxy vitamin D status with cardiometabolic risk factors and total and regional obesity in southern Iran: Evidence from the PoCOsteo study. Sci. Rep. 2024, 14, 17983. [Google Scholar] [CrossRef]
- Strange, R.C.; E Shipman, K.; Ramachandran, S. Metabolic syndrome: A review of the role of vitamin D in mediating susceptibility and outcome. World J. Diabetes 2015, 6, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Małkowska, P. Positive effects of physical activity on insulin signaling. Curr. Issues Mol. Biol. 2024, 46, 5467–5487. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y. Smoking cessation and vascular endothelial function. Hypertens. Res. 2023, 46, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Strack, C.; Behrens, G.; Sag, S.; Mohr, M.; Zeller, J.; Lahmann, C.; Hubauer, U.; Loew, T.; Maier, L.; Fischer, M.; et al. Gender differences in cardiometabolic health and disease in a cross-sectional observational obesity study. Biol. Sex Differ. 2022, 13, 8. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Masouri, M.M.; Khozani, A.A.S.A.; Soltani, S.M.; Nejadghaderi, S.A. Effects of vitamin D supplementation on blood pressure in patients With type 1 diabetes mellitus: A systematic review of clinical trials. Health Sci. Rep. 2025, 8, e70524. [Google Scholar] [CrossRef]
- Cecerska-Heryć, E.; Engwert, W.; Michałów, J.; Marciniak, J.; Birger, R.; Serwin, N.; Heryć, R.; Polikowska, A.; Goszka, M.; Wojciuk, B.; et al. Oxidative stress markers and inflammation in type 1 and 2 diabetes are affected by BMI, treatment type, and complications. Sci. Rep. 2025, 15, 23605. [Google Scholar] [CrossRef]
- Xue, Q.; Yang, X.; Huang, Y.; Zhu, D.; Wang, Y.; Wen, Y.; Zhao, J.; Liu, Y.; Yang, C.-X.; Pan, J.; et al. Association between baseline and changes in high-sensitive C-reactive protein and metabolic syndrome: A nationwide cohort study and meta-analysis. Nutr. Metab. 2022, 19, 2. [Google Scholar] [CrossRef]
- Anderson, J.J.; Deo, S.V.; Welsh, P.; MacKay, D.F.; Ho, F.K.; Ferguson, L.D.; Celis-Morales, C.; Gill, J.M.R.; Pell, J.P.; Sattar, N. In which common chronic conditions can (or cannot) obesity and lifestyle factors explain higher concentrations of C-reactive protein? Diabetes Obes. Metab. 2024, 26, 5786–5794. [Google Scholar] [CrossRef]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and women’s cardiovascular health: Is it really an obvious relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef]
- Chen, W.; Liu, L.; Hu, F. Efficacy of vitamin D supplementation on glycaemic control in type 2 diabetes: An updated systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2024, 26, 5713–5726. [Google Scholar] [CrossRef] [PubMed]
- Gowdaiah, P.; R, M.; Nirgude, D.; Hosamani, P. High sensitivity C-reactive protein in metabolic syndrome. Int. J. Adv. Med. 2016, 3, 607–610. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).