Dedicated Bifurcation Stents vs. Regular Drug-Eluting Stents in Coronary Bifurcation Treatment: A Systematic Review and Meta-Analysis of 1-Year and 4-Year Outcomes, Including Left Main and Non-Left Main Subgroup Comparisons
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Eligibility Criteria
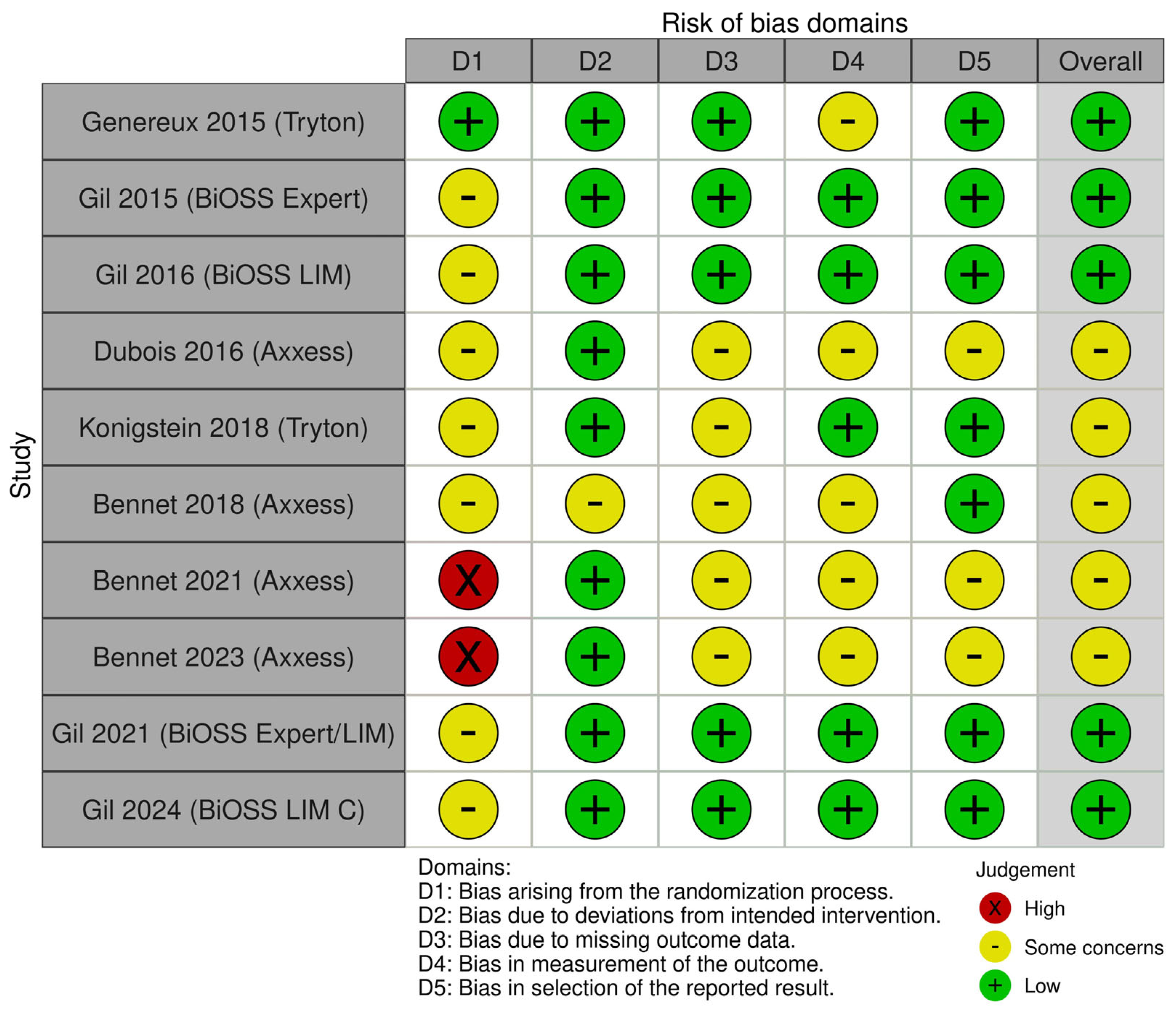
2.3. Data Extraction and Quality Appraisal
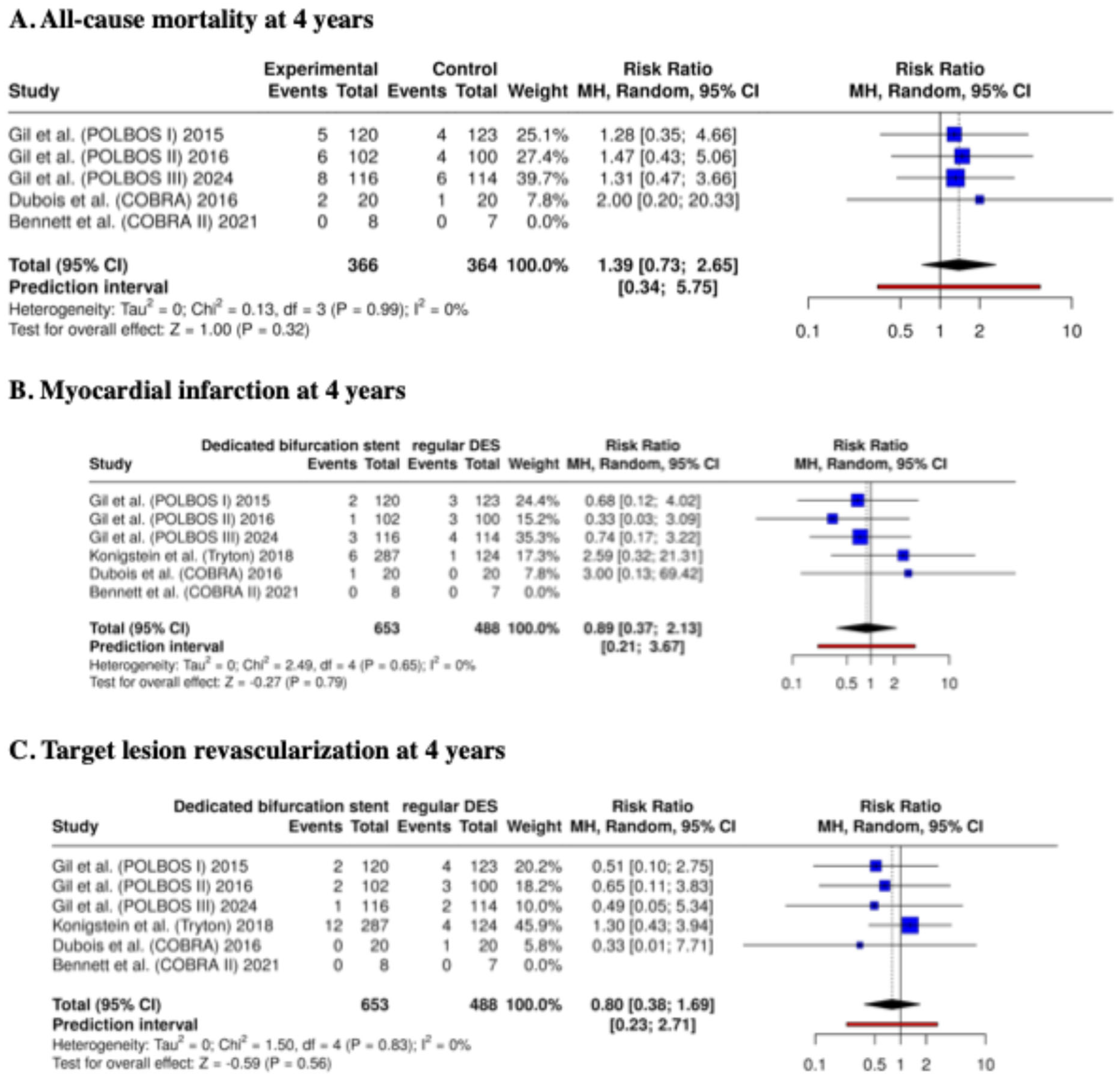
- All-cause mortality
- Target lesion revascularization (TLR),
- Myocardial infarction,
2.4. Data Synthesis and Statistical Analysis
3. Results
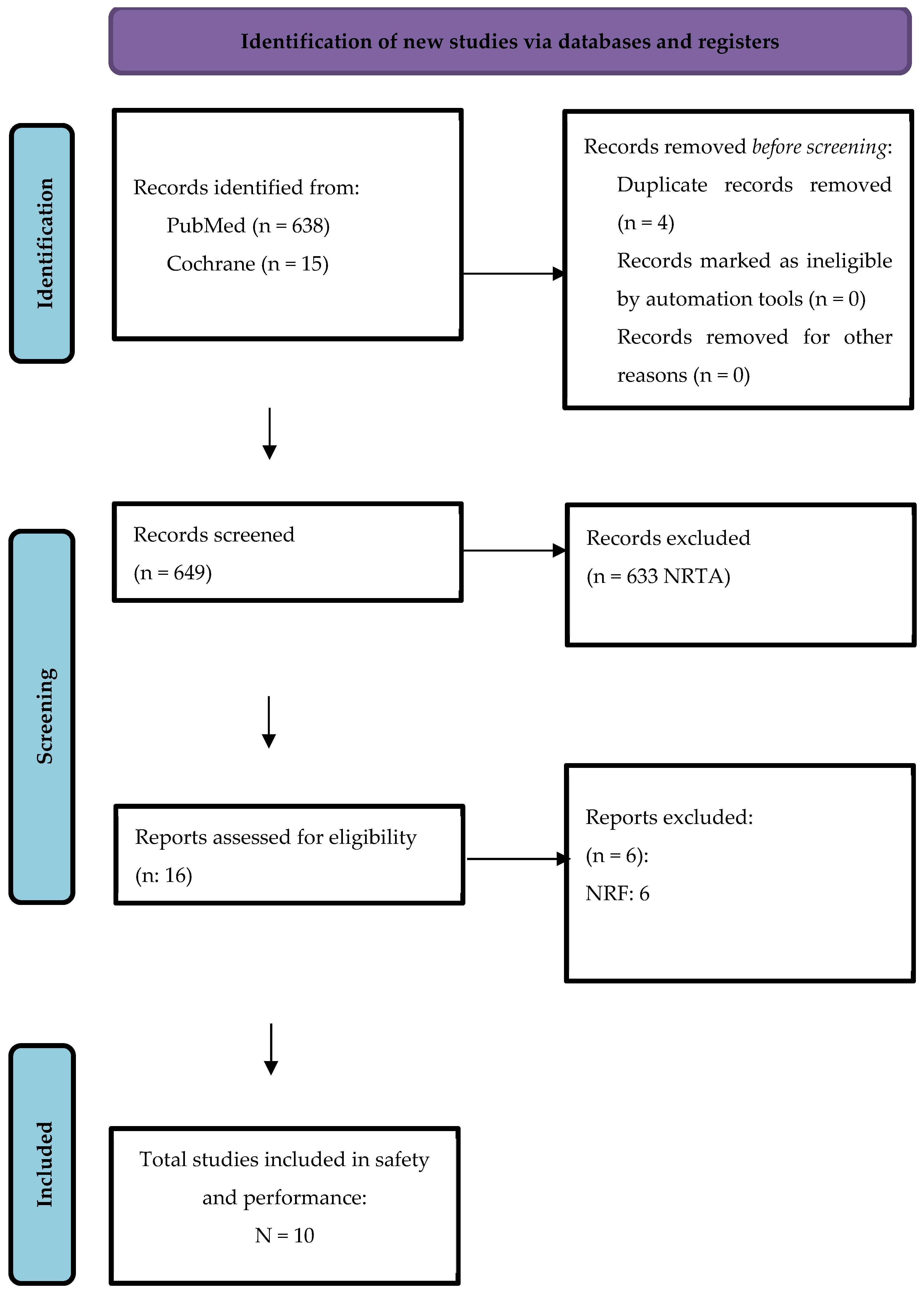
3.1. Study Selection
3.2. Study Characteristics
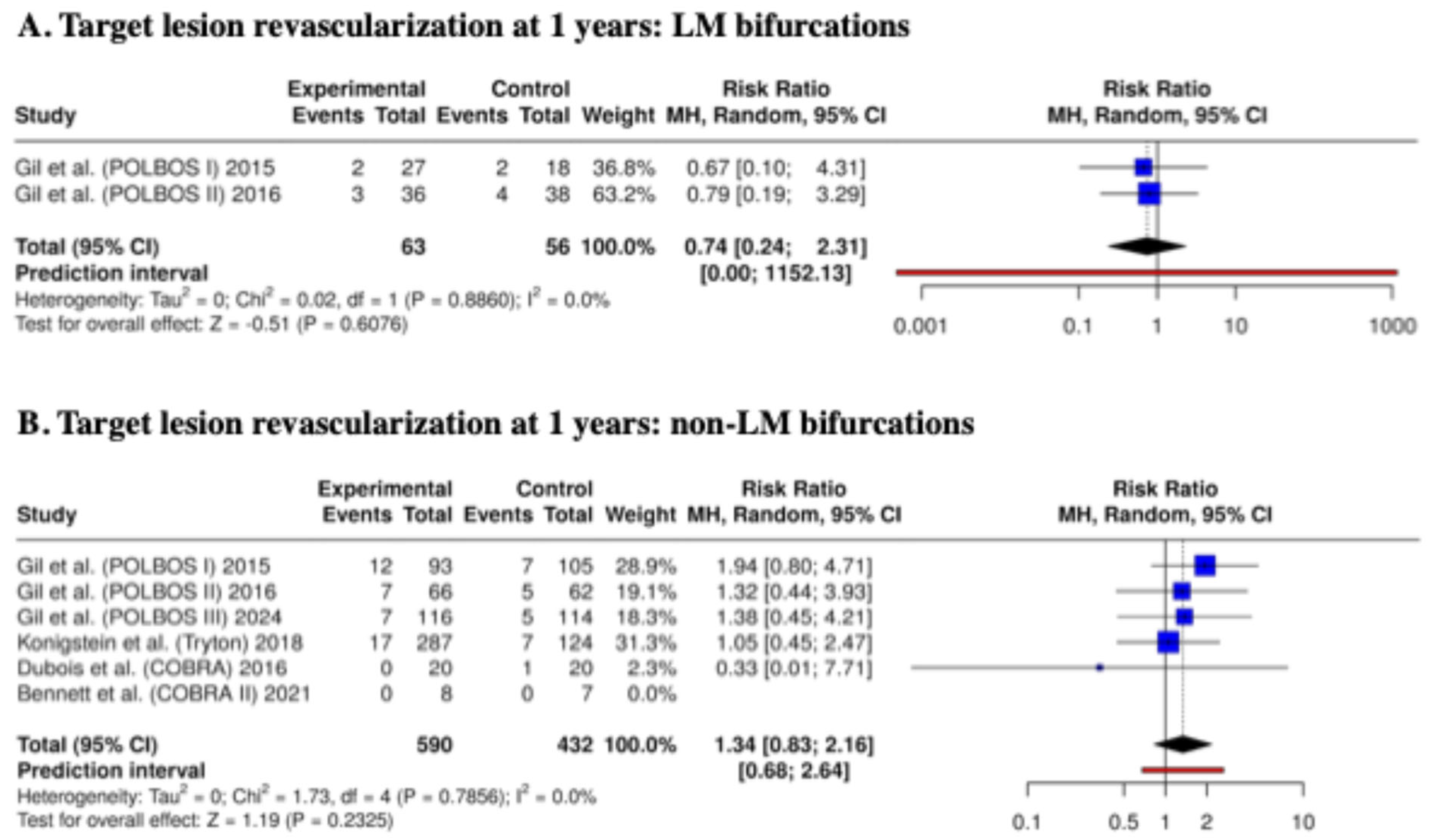
3.3. Clinical Outcomes at 1 Year
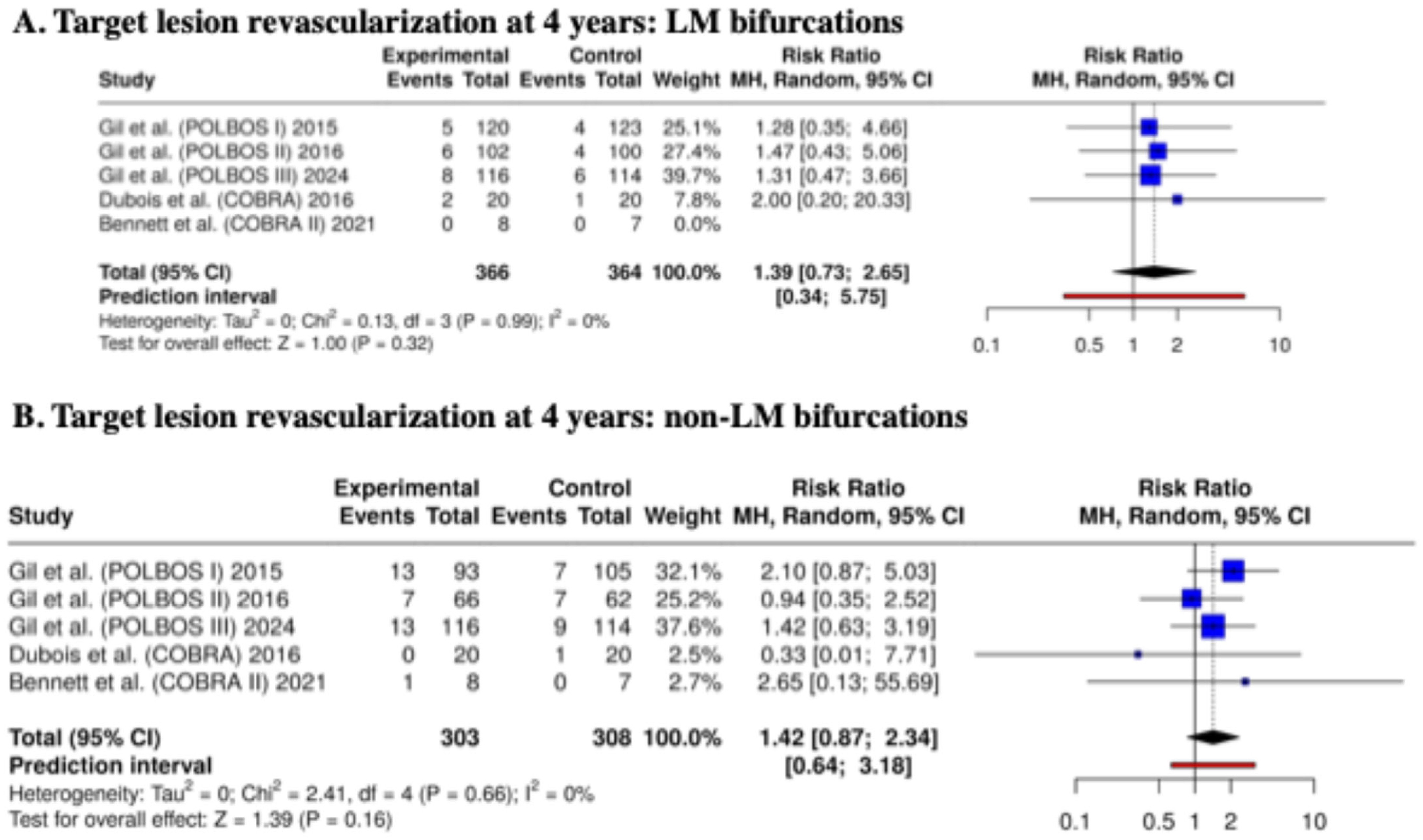
3.4. Clinical Outcomes at 4 Years
4. Discussion
- Defining patient and lesion subsets most likely to benefit from DBS, particularly in bifurcations with side branches ≥ 2.25 mm or marked vessel size mismatch.
- Integrating intravascular imaging (IVUS or OCT) and procedural standardization, such as systematic POT and side branch POT, into trial designs to improve precision in bifurcation PCI.
- Evaluating newer DBS iterations with thinner struts, improved deliverability, and optimized polymer coatings (e.g., BiOSS LIM C) to determine whether these refinements can reduce restenosis and enhance vessel healing.
- BIOSS LIM C (Balton): Features a stepped balloon and tapered design to match the anatomy of bifurcations better and facilitate main vessel (MV) and side branch (SB) coverage. This design allows for simplified crossover stenting and optimized proximal stent apposition. However, long-term clinical outcome data remain limited, and their adoption is still restricted mainly to specialized centers [10].
- Biomime Branch (Meril Life Sciences): Designed for a “main vessel–side branch” approach, this stent incorporates an open-cell configuration at the SB ostium to facilitate SB access while maintaining scaffold integrity in the proximal MV segment. Importantly, the Biomime Branch is used in combination with a standard DES for the main vessel, resembling the staged concept previously introduced with the Tryton stent. Although early reports on procedural feasibility are encouraging, robust long-term safety and efficacy data are still lacking [11].
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ABPM | Ambulatory blood pressure monitoring |
| BMS | Bare-metal stent |
| BVS | Bioresorbable vascular scaffold |
| CAD | Coronary artery disease |
| CBL | Coronary bifurcation lesion |
| CI | Confidence interval |
| CTO | Chronic total occlusion |
| DBS | Dedicated bifurcation stent |
| DES | Drug-eluting stent |
| DK | Double kissing (technique) |
| EBC | European Bifurcation Club |
| EES | Everolimus-eluting stent |
| FKB | Final kissing balloon (inflation) |
| IVUS | Intravascular ultrasound |
| LAD | Left anterior descending coronary artery |
| LCx | Left circumflex coronary artery |
| LM | Left main coronary artery |
| MACE | Major adverse cardiac events |
| MI | Myocardial infarction |
| MV | Main vessel |
| OCT | Optical coherence tomography |
| PCI | Percutaneous coronary intervention |
| PES | Paclitaxel-eluting stent |
| POT | Proximal optimization technique |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized controlled trial |
| rDES | Regular (conventional) drug-eluting stent |
| RR | Risk ratio |
| SB | Side branch |
| SES | Sirolimus-eluting stent |
| ST | Stent thrombosis |
| TSA | Trial sequential analysis |
| TLR | Target lesion revascularization |
| TVF | Target vessel failure |
| ZES | Zotarolimus-eluting stent |
References
- Gu, D.; Qu, J.; Zhang, H.; Zheng, Z. Revascularization for Coronary Artery Disease: Principle and Challenges. Adv. Exp. Med. Biol. 2020, 1177, 75–100. [Google Scholar]
- Dillen, D.M.M.; Otsuki, H.; Takahashi, K.; Kobayashi, Y.; Piroth, Z.; Noiseux, N.; El Nakadi, B.; Kalinauskas, G.; Szekely, L.; Davidavičius, G.; et al. Impact of Bifurcation Lesions on Outcomes After FFR-Guided PCI or CABG. Circ. Cardiovasc. Interv. 2024, 18, e014610. [Google Scholar] [CrossRef]
- Kumar, A.; Shariff, M.; Singal, A.; Bhat, V.; Stulak, J.; Reed, G.; Kalra, A. A Bayesian meta-analysis of double kissing (DK) crush or provisional stenting for coronary artery bifurcation lesions. Indian Heart J. 2024, 76, 113–117. [Google Scholar] [CrossRef]
- Burzotta, F.; Louvard, Y.; Lassen, J.F.; Lefèvre, T.; Finet, G.; Collet, C.; Legutko, J.; Lesiak, M.; Hikichi, Y.; Albiero, R.; et al. Percutaneous coronary intervention for bifurcation coronary lesions using optimised angiographic guidance: The 18th consensus document from the European Bifurcation Club. EuroIntervention 2024, 20, e915–e926. [Google Scholar] [CrossRef]
- Pan, M.; Lassen, J.F.; Burzotta, F.; Ojeda, S.; Albiero, R.; Lefèvre, T.; Hildick-Smith, D.; Johnson, T.W.; Chieffo, A.; Banning, A.P.; et al. The 17th expert consensus document of the European Bifurcation Club—Techniques to preserve access to the side branch during stepwise provisional stenting. EuroIntervention 2023, 19, 26–36. [Google Scholar] [CrossRef]
- Lassen, J.F.; Albiero, R.; Johnson, T.W.; Burzotta, F.; Lefèvre, T.; Iles, T.L.; Pan, M.; Banning, A.B.; Chatzizisis, Y.C.; Ferenc, M.; et al. Treatment of coronary bifurcation lesions, part II: Implanting two stents. The 16th expert consensus document of the European Bifurcation Club. EuroIntervention 2022, 18, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, A.; Guo, L.; Sakamoto, A.; Jinnouchi, H.; Sato, Y.; Kuntz, S.; Kawakami, R.; Mori, M.; Fernandez, R.; Fuller, D.; et al. Histopathologic and physiologic effect of bifurcation stenting: Current status and future prospects. Expert. Rev. Med. Devices 2020, 17, 189–200. [Google Scholar] [CrossRef]
- Rawasia, W.F.; Alkhouli, M. Dedicated Bifurcation Stents vs. Conventional Stenting Strategy for Coronary Bifurcation Lesions: Insights from Randomized Clinical Trials. Cardiovasc. Revasc. Med. 2020, 21, 556–558. [Google Scholar] [CrossRef]
- Briguori, C.; Capodanno, D.; Contarini, M.; Donahue, M.E.; Evola, S.; Garro, N.; Greco, F.; LA Manna, A.; Murè, P.; Nicosia, A.; et al. Acute and long-term results of percutaneous coronary intervention of bifurcation lesions with the dedicated Bioss Lim C stent: The Italian BIfurcation Observational Spontaneous Study (IBIOSS). Minerva Med. 2024, 115, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.J.; Bil, J.; Kaczynski, M.; Milewski, K.P. BiOSS LIM C: Thin-strut cobalt-chromium version of the dedicated bifurcation stent. Expert. Rev. Med. Devices 2017, 14, 279–284. [Google Scholar] [CrossRef]
- Bartorelli, A.L.; Monizzi, G.; Grancini, L.; Gallinoro, E.; Mastrangelo, A.; Mallia, V.; Fabbiocchi, F. Coronary bifurcation lesion treatment with the BioMime Branch sirolimus-eluting coronary side-branch stent system: A single-center experience. Cardiovasc. Revasc. Med. 2025, 75, 39–45. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shanseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Kumsars, I.; Lesiak, M.; Kini, A.; Fontos, G.; Slagboom, T.; Ungi, I.; Metzger, D.C.; Wykrzykowska, J.J.; Stella, P.R.; et al. A randomized trial of a dedicated bifurcation stent versus provisional stenting in the treatment of coronary bifurcation lesions. J. Am. Coll. Cardiol. 2015, 65, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.J.; Bil, J.; Dzavik, V.; Vassilev, D.; Kern, A.; Formuszewicz, R.; Zalewska-Adamiec, M.; Dobrzycki, S. Regular Drug-Eluting Stent vs Dedicated Coronary Bifurcation BiOSS Expert Stent: Multicenter Open-Label Randomized Controlled POLBOS I Trial. Can. J. Cardiol. 2015, 31, 671–678. [Google Scholar] [CrossRef]
- Gil, R.J.; Bil, J.; Grundeken, M.J.; Kern, A.; Garcia, L.I.; Vassilev, D.; Pawłowski, T.; Formuszewicz, R.; Dobrzycki, S.; Wykrzykowska, J.; et al. Regular drug-eluting stents versus the dedicated coronary bifurcation sirolimus-eluting BiOSS LIM(R) stent: The randomised, multicentre, open-label, controlled POLBOS II trial. EuroIntervention 2016, 12, e1404–e1412. [Google Scholar] [CrossRef]
- Dubois, C.; Bennett, J.; Dens, J.; De Cock, D.; Desmet, W.; Belmans, A.; Ughi, G.J.; Sinnaeve, P.; Vrolix, M.; D’hOoge, J.; et al. COmplex coronary Bifurcation lesions: RAndomized comparison of a strategy using a dedicated self-expanding biolimus-eluting stent versus a culotte strategy using everolimus-eluting stents: Primary results of the COBRA trial. EuroIntervention 2016, 11, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Konigstein, M.; Srdanovic, I.; Gore, A.K.; Rahim, H.M.; Généreux, P.; Ben-Yehuda, O.; Kumsars, I.; Lesiak, M.; Kini, A.; Fontos, G.; et al. Outcomes of the Tryton-dedicated bifurcation stent for the treatment of true coronary bifurcations: Individual-patient-data pooled analysis. Catheter. Cardiovasc. Interv. 2019, 93, 1255–1261. [Google Scholar] [CrossRef]
- Bennett, J.; Adriaenssens, T.; McCutcheon, K.; Dens, J.; Desmet, W.; Sinnaeve, P.; Vrolix, M.; Dubois, C. 5-Year clinical follow-up of the COBRA (complex coronary bifurcation lesions: Randomized comparison of a strategy using a dedicated self-expanding biolimus A9-eluting stent vs. a culotte strategy using everolimus-eluting stents) study. Catheter. Cardiovasc. Interv. 2018, 92, E375–E380. [Google Scholar] [CrossRef]
- Bennett, J.; Desmet, W.; Dubois, C. The COBRA II (COmplex Bifurcation lesions: RAndomized comparison of a fully bioresorbable modified-T stenting strategy versus bifurcation reconstruction with a dedicated self-expanding stent in combination with bioresorbable scaffolds) study: Final 5-year follow-up. Indian Heart J. 2023, 75, 473–476. [Google Scholar]
- Bennett, J.; McCutcheon, K.; Vanhaverbeke, M.; Pauwels, R.; Adriaenssens, T.; Sinnaeve, P.; Desmet, W.; Dubois, C. COmplex Bifurcation Lesions: RAndomized Comparison of Modified-T Stenting vs Reconstruction With Self-Expanding Stent and Bioresorbable Scaffold: COBRA II. J. Invasive Cardiol. 2021, 33, E281–E293. [Google Scholar] [CrossRef]
- Gil, R.J.; Kern, A.; Bojko, K.; Gziut-Rudkowska, A.; Vassilev, D.; Bil, J. The Randomized, Multicenter, Open-Label, Controlled POLBOS 3 Trial Comparing Regular Drug-Eluting Stents and the Sirolimus-Eluting BiOSS LIM C Dedicated Coronary Bifurcation Stent: Four-Year Results. Biomedicines 2024, 12, 938. [Google Scholar] [CrossRef]
- Gil, R.J.; Kern, A.; Formuszewicz, R.; Garcia, L.A.I.; Dobrzycki, S.; Vassilev, D.; Bil, J. 6-year results of BiOSS stents in coronary bifurcation treatment. Eur. J. Clin. Investig. 2021, 51, e13555. [Google Scholar] [CrossRef]
- Albiero, R.; Burzotta, F.; Lassen, J.F.; Lefèvre, T.; Banning, A.B.; Chatzizisis, Y.C.; Johnson, T.J.; Ferenc, M.; Pan, M.; Darremont, O.; et al. Treatment of coronary bifurcation lesions, part I: Implanting the first stent in the provisional pathway. The 16th expert consensus document of the European Bifurcation Club. EuroIntervention 2022, 18, e362–e376. [Google Scholar] [CrossRef]
- Di Gioia, G.; Sonck, J.; Ferenc, M.; Chen, S.L.; Colaiori, I.; Gallinoro, E.; Mizukami, T.; Kodeboina, M.; Nagumo, S.; Franco, D.; et al. Clinical Outcomes Following Coronary Bifurcation PCI Techniques: A Systematic Review and Network Meta-Analysis Comprising 5711 Patients. JACC Cardiovasc. Interv. 2020, 13, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.B.; Ahn, J.M.; Kang, D.Y.; Park, S.J.; Park, D.W. Contemporary State-of-the-Art PCI of Left Main Coronary Artery Disease. Circ. Cardiovasc. Interv. 2024, 17, e014026. [Google Scholar] [CrossRef]
- Genuardi, L.; Chatzizisis, Y.S.; Chiastra, C.; Sgueglia, G.; Samady, H.; Kassab, G.S.; Migliavacca, F.; Trani, C.; Burzotta, F. Local fluid dynamics in patients with bifurcated coronary lesions undergoing percutaneous coronary interventions. Cardiol. J. 2021, 28, 321–329. [Google Scholar] [CrossRef]
- Tiroch, K.; Mehilli, J.; Byrne, R.A.; Schulz, S.; Massberg, S.; Laugwitz, K.-L.; Vorpahl, M.; Seyfarth, M.; Kastrati, A. Impact of coronary anatomy and stenting technique on long-term outcome after drug-eluting stent implantation for unprotected left main coronary artery disease. JACC Cardiovasc. Interv. 2014, 7, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Lassen, J.F.; Lefevre, T.; Banning, A.P.; Chatzizisis, Y.S.; Johnson, T.W.; Ferenc, M.; Rathore, S.; Albiero, R.; Pan, M.; et al. Percutaneous coronary intervention for bifurcation coronary lesions: The 15(th) consensus document from the European Bifurcation Club. EuroIntervention 2021, 16, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Aedma, S.K.; Naik, A.; Kanmanthareddy, A. Coronary Bifurcation Stenting: Review of Current Techniques and Evidence. Curr. Cardiol. Rev. 2023, 19, e060422203185. [Google Scholar] [CrossRef]
- Digne, F.; Darmon, A.; Nejjari, M.; Stratiev, V.; Maxo, L.; Abdellaoui, M. A Modified POT-Side-POT Technique for Distal Stent Expansion in Bifurcation PCI: Introducing the OTUS Strategy (Optimization Technique Using a Single Balloon). JACC Cardiovasc. Interv. 2025, 18, 2548–2553. [Google Scholar] [CrossRef]
- Giustino, G.; Sharma, S.K.; Kini, A. Systematic Proximal Optimization Technique During Bifurcation Stenting: Where Is the Evidence? JACC Cardiovasc. Interv. 2024, 17, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Annone, U.; Paraggio, L.; D’aScenzo, F.; Biondi-Zoccai, G.; Aurigemma, C.; Romagnoli, E.; Verdirosi, D.; Trani, C.; Crea, F. Clinical outcome after percutaneous coronary intervention with drug-eluting stent in bifurcation and nonbifurcation lesions: A meta-analysis of 23,981 patients. Coron. Artery Dis. 2020, 31, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, I.G.R.; Saputra, P.B.T.; Rurus, M.; Saputra, M.E.; Widiarti, W.; Multazam, C.E.C.Z.; Alkaff, F.F. Comparison between provisional and dual systematic stenting approach for left main bifurcation disease: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102633. [Google Scholar] [CrossRef] [PubMed]
| Search Number | Query | Sort By | Filters | Search Details |
|---|---|---|---|---|
| 10 | (coronary) AND ((((“dedicated bifurcation stent”) OR (DBS)) OR ((coronary) AND (bifurcation))) AND (randomized)) | Publication Date | (“coronaries”[All Fields] OR “heart”[MeSH Terms] OR “heart”[All Fields] OR “coronary”[All Fields]) AND ((“dedicated bifurcation stent”[All Fields] OR “DBS”[All Fields] OR ((“coronaries”[All Fields] OR “heart”[MeSH Terms] OR “heart”[All Fields] OR “coronary”[All Fields]) AND (“bifurcate”[All Fields] OR “bifurcated”[All Fields] OR “bifurcates”[All Fields] OR “bifurcating”[All Fields] OR “bifurcation”[All Fields] OR “bifurcational”[All Fields] OR “bifurcations”[All Fields]))) AND (“random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “random”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields])) | |
| 7 | (((“dedicated bifurcation stent”) OR (DBS)) OR ((coronary) AND (bifurcation))) AND (randomized) | Publication Date | (“dedicated bifurcation stent”[All Fields] OR “DBS”[All Fields] OR ((“coronaries”[All Fields] OR “heart”[MeSH Terms] OR “heart”[All Fields] OR “coronary”[All Fields]) AND (“bifurcate”[All Fields] OR “bifurcated”[All Fields] OR “bifurcates”[All Fields] OR “bifurcating”[All Fields] OR “bifurcation”[All Fields] OR “bifurcational”[All Fields] OR “bifurcations”[All Fields]))) AND (“random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “random”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields]) | |
| 9 | “dedicated bifurcation stent” | Publication Date | “dedicated bifurcation stent”[All Fields] | |
| 8 | (((“dedicated bifurcation stent”) OR (DBS)) OR ((coronary) AND (bifurcation))) AND (randomized) | Publication Date | Randomized Controlled Trial | ((“dedicated bifurcation stent”[All Fields] OR “DBS”[All Fields] OR ((“coronaries”[All Fields] OR “heart”[MeSH Terms] OR “heart”[All Fields] OR “coronary”[All Fields]) AND (“bifurcate”[All Fields] OR “bifurcated”[All Fields] OR “bifurcates”[All Fields] OR “bifurcating”[All Fields] OR “bifurcation”[All Fields] OR “bifurcational”[All Fields] OR “bifurcations”[All Fields]))) AND (“random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “random”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields])) AND (randomizedcontrolledtrial[Filter]) |
| 6 | ((“dedicated bifurcation stent”) OR (DBS)) OR ((coronary) AND (bifurcation)) | Publication Date | “dedicated bifurcation stent”[All Fields] OR “DBS”[All Fields] OR ((“coronaries”[All Fields] OR “heart”[MeSH Terms] OR “heart”[All Fields] OR “coronary”[All Fields]) AND (“bifurcate”[All Fields] OR “bifurcated”[All Fields] OR “bifurcates”[All Fields] OR “bifurcating”[All Fields] OR “bifurcation”[All Fields] OR “bifurcational”[All Fields] OR “bifurcations”[All Fields])) | |
| 5 | (coronary) AND (bifurcation) | Publication Date | (“coronaries”[All Fields] OR “heart”[MeSH Terms] OR “heart”[All Fields] OR “coronary”[All Fields]) AND (“bifurcate”[All Fields] OR “bifurcated”[All Fields] OR “bifurcates”[All Fields] OR “bifurcating”[All Fields] OR “bifurcation”[All Fields] OR “bifurcational”[All Fields] OR “bifurcations”[All Fields]) | |
| 4 | randomized | Publication Date | “random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “randomization”[All Fields] OR “randomized”[All Fields] OR “random”[All Fields] OR “randomisation”[All Fields] OR “randomisations”[All Fields] OR “randomise”[All Fields] OR “randomised”[All Fields] OR “randomising”[All Fields] OR “randomizations”[All Fields] OR “randomize”[All Fields] OR “randomizes”[All Fields] OR “randomizing”[All Fields] OR “randomness”[All Fields] OR “randoms”[All Fields] | |
| 3 | bifurcation | Publication Date | “bifurcate”[All Fields] OR “bifurcated”[All Fields] OR “bifurcates”[All Fields] OR “bifurcating”[All Fields] OR “bifurcation”[All Fields] OR “bifurcational”[All Fields] OR “bifurcations”[All Fields] | |
| 2 | coronary | Publication Date | “coronaries”[All Fields] OR “heart”[MeSH Terms] OR “heart”[All Fields] OR “coronary”[All Fields] | |
| 1 | (“dedicated bifurcation stent”) OR (DBS) | Publication Date | “dedicated bifurcation stent”[All Fields] OR “DBS”[All Fields] |
| Study | N (Patients) | Age (yrs) | Female (%) | Hypertension (%) | Diabetes (%) | Prior MI (%) | LM Bifurcation (%) | Main Lesion Location | Devices Compared | Follow-up/Outcomes [DBS vs. rDES] |
|---|---|---|---|---|---|---|---|---|---|---|
| Gil et al., 2024 [21] | 230 | 64.3 ± 9.6 | 19.6 | 79.6 | 34.8 | 39.6 | 0 | LAD 56.5%, LCx 25.2%, RCA 18.3 | BiOSS LIM C vs. rDES (Xience, Resolute, Orsiro, Synergy) | 48 mo: MACE 18.1% vs. 14.9%; Cardiac Death 4.3% vs. 3.5%; MI 2.6% vs. 3.5%; TLR 11.2% vs. 7.9% |
| Gil et al., 2021 [22] | 445 | 66.4 ± 9.5 | 28.3 | 78.9 | 34.4 | 42.5 | 26.7 | LM 26.7%, non-LM 73.3% | BiOSS Expert/LIM vs. rDES (SES, PES, EES, ZES) | 72 mo: MACE 25.7% vs. 25.1%; Cardiac Death 3.1% vs. 4.0%; MI 3.6% vs. 4.9%; TLR 18.9% vs. 16.1% |
| Gil et al., 2016 [15] | 202 | 67 | 23.1 vs. 25 | 84.3 vs. 81 | 44.1 vs. 32 | 43.1 vs. 48 | 35.3 vs. 38 | LAD 44.1/43%, LCx 15.7/15%, RCA 4.9/4% | BiOSS LIM vs. rDES | 12 mo: MACE 11.8% vs. 15%; TLR 9.8% vs. 9% |
| Gil et al., 2015 [14] | 243 | 66 | 31.2 vs. 31.7 | 78.3 vs. 73.2 | 37.5 vs. 25.2 | 45.8 vs. 35 | 22.5 vs. 15 | LAD 52.5/70%, LCx 18/13%, RCA 8/2% | BiOSS Expert vs. rDES | 12 mo: MACE 13.3% vs. 12.2%; TLR 11.5% vs. 7.3% |
| Genereux et al., 2015 [13] | 704 | 64 | 28.2 vs. 26.6 | 73.2 vs. 73.6 | 23.9 vs. 28.1 | 30 vs. 37.8 | 0 | LAD 75.8%; LCx 18.2%, RCA 6% | Tryton vs. provisional rDES | 9 mo: TVF 17.4% vs. 12.8%; SB stenosis 31.6% vs. 38.6% |
| Konigstein et al., 2018 [17] | 411 | 64.8 | 25.1 vs. 16.9 | 76 vs. 77.2 | 26.9 vs. 29 | 31.1 vs. 41 | 1 case in Tryton | LAD 72.5/68.5%; LCx 19.9/22.6%; RCA 7.3%/8.9% | Tryton vs. provisional rDES | 12 mo: MACE 31% vs. 12%; TLR 17% vs. 7% |
| Dubois et al., 2016 [16] | 40 | 65 | 25–30 | 70–75 | 20–25 | 10–30 | 0 | LAD 97.5%; RCA 2.5% | Axxess + Biomatrix vs. culotte Xience | 12 mo: MACE 10% vs. 10%; TLR 0 vs. 5% |
| Bennet et al., 2021 [20] | 15 | 63 | 37–0 | 63–71 | 25–43 | 25–29 | 0 | LAD 88/71%; LCx 12/29% | Axxess + Biomatrix vs. modified-T Absorb BVS | 12 mo: MACE/TLR 12.5% vs. 0 |
| Bennett et al., 2023 [19] | 40 | 65 | 25–30 | 70–75 | 20–25 | 10–30 | 0 | LAD 97.5%; RCA 2.5% | Axxess + Biomatrix vs. culotte Xience | 5 yr: MACE 12% vs. 0 |
| Bennet et al., 2018 [18] | 40 | 65 | 25–30 | 70–75 | 20–25 | 10–30 | 0 | LAD 97.5%; RCA 2.5% | Axxess + Biomatrix vs. culotte Xience | 5 yr: MACE 0 vs. 10%; TLR 0 vs. 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bil, J.; Kern, A.; Gziut-Rudkowska, A.I.; Zalewski, J.; Bojko, K.; Gil, R.J. Dedicated Bifurcation Stents vs. Regular Drug-Eluting Stents in Coronary Bifurcation Treatment: A Systematic Review and Meta-Analysis of 1-Year and 4-Year Outcomes, Including Left Main and Non-Left Main Subgroup Comparisons. Biomedicines 2025, 13, 2763. https://doi.org/10.3390/biomedicines13112763
Bil J, Kern A, Gziut-Rudkowska AI, Zalewski J, Bojko K, Gil RJ. Dedicated Bifurcation Stents vs. Regular Drug-Eluting Stents in Coronary Bifurcation Treatment: A Systematic Review and Meta-Analysis of 1-Year and 4-Year Outcomes, Including Left Main and Non-Left Main Subgroup Comparisons. Biomedicines. 2025; 13(11):2763. https://doi.org/10.3390/biomedicines13112763
Chicago/Turabian StyleBil, Jacek, Adam Kern, Aneta I. Gziut-Rudkowska, Jarosław Zalewski, Krystian Bojko, and Robert J. Gil. 2025. "Dedicated Bifurcation Stents vs. Regular Drug-Eluting Stents in Coronary Bifurcation Treatment: A Systematic Review and Meta-Analysis of 1-Year and 4-Year Outcomes, Including Left Main and Non-Left Main Subgroup Comparisons" Biomedicines 13, no. 11: 2763. https://doi.org/10.3390/biomedicines13112763
APA StyleBil, J., Kern, A., Gziut-Rudkowska, A. I., Zalewski, J., Bojko, K., & Gil, R. J. (2025). Dedicated Bifurcation Stents vs. Regular Drug-Eluting Stents in Coronary Bifurcation Treatment: A Systematic Review and Meta-Analysis of 1-Year and 4-Year Outcomes, Including Left Main and Non-Left Main Subgroup Comparisons. Biomedicines, 13(11), 2763. https://doi.org/10.3390/biomedicines13112763