Abstract
Telomeres, which serve as protective ends on chromosomes, and telomerase, the enzyme that preserves telomere length, play crucial roles in ensuring genomic stability and delaying cellular aging. Dysregulation of these proteins is a key characteristic of cancer development. This review aimed to explore the complex processes involved in telomere and telomerase dysregulation in cancer and evaluate the therapeutic potential of curcumin. Curcumin has attracted significant interest due to its anticancer, antioxidant, and anti-inflammatory properties. Curcumin modulates telomere dynamics and inhibits telomerase activity, leading to cancer cell senescence and telomere shortening. Curcumin downregulates human telomerase reverse transcriptase expression and reduces telomerase activity in various cancer cell lines. Despite its potential, its clinical use is restricted by its poor water solubility and limited bioavailability. This review underscores the critical role of telomere/telomerase dysregulation in cancer and highlights curcumin as a promising modulator of these pathways, thereby offering potential novel strategies for cancer treatment. This review integrates the literature published up to September 2025 to ensure the inclusion of the most recent advances in curcumin-related telomerase modulation.
1. Introduction
Telomeres are repetitive DNA-protein that protect the ends of chromosomes and help preserve their genomic stability and integrity. Telomerase is a ribonucleoprotein enzyme complex that prevents telomere shortening by adding repeated DNA sequences to the ends of telomeres during cell division. Dysregulation of telomeres and telomerases has implications in cancer initiation and progression [1]. Telomeres become shorter with each round of cell division in normal cells until they reach a point where the cell either stops dividing or undergoes apoptosis, thereby serving as a safeguard against uncontrolled cell proliferation. Telomerases play an important role in maintaining telomere length, which is essential for cellular longevity and stability. Under normal physiological conditions, telomeres become shorter with each round of cell division, leading to cellular aging and eventual senescence [2]. However, telomerase counteracts this shortening by adding repetitive nucleotide sequences to telomeres, thereby extending their length and allowing cells to divide beyond the typical limit. This activity is particularly important in germ cells (sperm and eggs) and undifferentiated stem cells, such as the skin, intestine, and hematopoietic system, where maintaining telomere length is vital for tissue regeneration and reproductive capacity.
In cancer cells, the mechanisms that maintain telomere length can overcome the replicative limit, allowing them to proliferate indefinitely and achieve an immortal status. Cancer cells with dysfunctional telomeres contribute to chromosomal instability and are involved in oncogenic transformation, metastasis, and resistance to therapy [3,4]. Therefore, the complex dysregulation of telomeres in cancer and telomerase activity highlights the necessity of developing therapeutic interventions based on an understanding of these mechanisms. The telomer elongation pathway regulates cancer growth, and targeting this pathway has significant therapeutic potential for preventing the growth and progression of most cancers. Curcumin, a polyphenol derived from the rhizome of Curcuma longa, has garnered significant research interest due to its diverse pharmacological characteristics, including anticancer, antioxidant, and anti-inflammatory effects [5].
Curcumin modulates telomere and telomerase dynamics, offering potential therapeutic benefits in cancer treatment. Curcumin blocks telomerase activity by downregulating various telomerase components, including human telomerase reverse transcriptase (hTERT), in various malignant cell lines [6]. Curcumin also shortens telomere length and triggers senescence-like growth arrest in several cancer cells, thus reducing their potential to proliferate. It also exhibits powerful antioxidant activity, which may reduce the telomere damage caused by oxidative stress, a characteristic feature of many cancer cells. The multidimensional effects of curcumin on the telomere/telomerase pathways hold promise for the development of new therapeutic strategies in cancer management [5].
Considering the crucial role of telomeres and telomerase dynamics in cancer development, along with new evidence that curcumin can influence these pathways, this review provides a foundation for future studies. This highlights the promise of curcumin as a new therapeutic agent and suggests new directions for creating targeted cancer treatment that takes advantage of its diverse benefits. Although the anticancer properties of curcumin are well documented, its modulatory effects on telomere and telomerase dynamics in cancer remain unclear. This literature review aimed to explore the complex interactions between telomeres, telomerases, and cancer development, focusing on understanding the mechanisms underlying the deregulation of these processes, together with the potential therapeutic role of curcumin in this context. We also summarized the molecular pathways involved in telomere maintenance, examined the influence of curcumin on these pathways, and proposed the potential therapeutic benefits of curcumin as a modulator of these molecular pathways.
The overall anticancer properties of curcumin are well documented. This review exclusively consolidates the evidence of its precise telomerase-inhibitory mechanisms, including hTERT transcriptional suppression, G-quadruplex stabilization, and chaperone disruption, in comparison with synthetic telomerase inhibitors.
We performed a comprehensive literature search across Google Scholar, PubMed, Web of Science, and Scopus databases. The search utilized keywords including: “telomeres,” “telomerase,” “curcumin,” “curcumin analogues,” “nanocurcumin,” and “cancer.” Of the more than 100 articles initially retrieved, 120 were included in this review based on their relevance and scientific quality. The final literature search was conducted on 30 September 2025.
2. Telomeres
Telomeres are sequences of repetitive non-coding nucleotides that are rich in guanine nucleotides (GGTTAG in humans). This sequence is attached to a protein complex known as shelterin, which involves six protein subunits: repressor activator protein (RAP1), telomere repeat binding factors 1 and 2 (TRF1 and TRF2), TRF1-interacting nuclear factor 2 (TIN2), protection of telomeres-1 (POT1), and TINT1/PTOP/PIP1 (TPP1) (Figure 1) [7]. This specialized structure is found at the terminus of linear chromosomes, where it forms a protective cap that regulates telomere metabolism, prevents chromatids from being recognized as double-stranded breaks, and is inappropriately repaired by fusion or degradation [8]. Telomeres participate in various cellular activities, including protecting chromosome ends, facilitating DNA replication, and regulating the cell cycle, thus helping to determine the lifespan of somatic cells by acting as a biological clock [9].
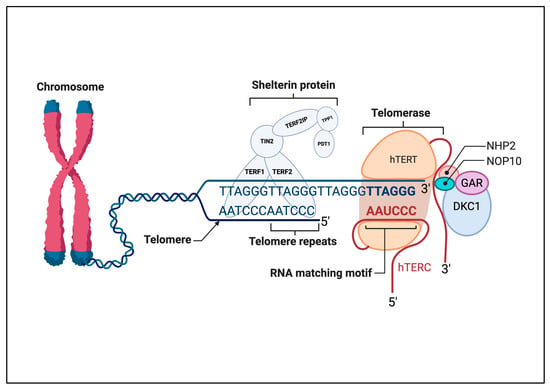
Figure 1.
Schematic illustration of the interaction between telomerase and the telomere region at the very end of chromosomes. Telomerase is a ribonucleoprotein enzyme complex, with one molecule of telomerase reverse transcriptase (hTERT) and one molecule of telomerase RNA component (hTERC) serving as a template for the synthesis of the telomeric DNA repeat GGTTAG. Shelterin proteins TRF1, TRF2, TIN2, POT1, TPP1, and RAP1 bind to the telomeric DNA to cap the telomeres and maintain telomere length. The RNA templating motif of hTERC is aligned with the telomere repeats to allow base pairing for the extension of the telomere by hTERT. Other associated proteins, such as NHP2, NOP10, GAR, and DKC1, contribute to the stability and activity of the telomerase complex. This figure outlines the key components and interactions crucial for telomere maintenance and stability. Abbreviations: DKC1, dyskerin; hTERC, human telomerase RNA component; hTERT, human telomerase reverse transcriptase; POT1, protection of telomeres 1; RAP1, repressor activator protein 1; TCAB1, telomerase Cajal body protein 1; TIN2, TRF1-interacting nuclear factor 2; TPP1, TINT1/PTOP/PIP1; TRF1/2, telomere repeat-binding factor 1/2. Created in BioRender. ALBADRANI, h. (2025) https://BioRender.com/k16a761 (accessed on 17 September 2025).
Telomeres play a crucial role in preserving genetic information during DNA replication by acting as buffer zones that ensure the completion of DNA synthesis without compromising chromosomal integrity. Moreover, telomeres maintain the correct separation of chromosomes during cell division and protect chromosomes from degradation, thus preventing the formation of aneuploid cells [10]. A typical cycle involves shortening of the telomere, with approximately 50–200 bases being lost from the sequence at the end of the chromosomes after each division until the telomere reaches a certain length, at which point the replication ability of the chromosome is lost, triggering cellular suicide [11]. The delicate balance between telomere shortening and telomerase activity is crucial for cellular health, as it ensures proper cell function and longevity. Disruption of this balance can lead to age-related diseases or carcinogenesis, in which telomerase is often reactivated to sustain uncontrolled cell proliferation. When the telomere becomes short, the response to DNA damage may be activated, which leads the cells to enter senescence or undergo apoptosis, preventing tumor formation [12]. In most cases, this barrier is overcome by upregulating telomerase activity or activating alternative telomere-lengthening mechanisms, allowing limitless proliferation and malignant transformation [13]. Incomplete or absent telomere function in cancer cells leads to genomic instability, which has significant consequences for tumor heterogeneity, metastasis, and therapeutic resistance, underscoring the importance of telomere regulation in cancer biology [14].
3. Telomerase and Cancer Progression
Telomerase is a specialized reverse transcriptase that increases telomere length by the addition of repetitive sequences (GGTTAG) to the ends of the telomeric region, counteracting the gradual shortening of telomeres that occurs with each round of cell division [15]. This enzyme complex is made up of two essential subunits and several complementary proteins: the integral human telomerase RNA component (hTERC), which acts as an RNA template, and the catalytic subunit of the hTERT (Figure 1). The protein complex comprises dyskerin (DKC1), telomerase Cajal body protein 1 (TCAP 1), NHP2, NOP10, and GAR, and has an important role in the modification and stabilization of specific uridine sequences in newly synthesized ribosomal RNA [15]. The main role of hTERT is to catalyze the addition of repetitive sequences to the RNA template. During telomeric elongation, hTERT binds to hTERC, which acts as a template, and the other complementary protein complex helps to localize and stabilize telomerase on the telomere (Figure 1). TCAP 1 is involved in recruiting hTERT to the telomere, whereas DKC1, NHP2, NOP10, and GAR1 stabilize binding during this process [16,17,18]. The G-quadruplex is a non-canonical, four-stranded secondary structure formed by nucleic acids, particularly in guanine-rich regions. Often located in telomeres, these structures play a protective role by capping chromosome ends and regulating telomerase activity [19].
In somatic cells, the activity of telomerase is tightly regulated and is inactive in most normal cells. However, it remains active in some tissues that require preservation of the telomere length and ensures ongoing regeneration and reproductive capacity [20,21,22]. However, telomerase dysregulation contributes to cancer development and enables cancer cells to maintain telomere length, allowing for indefinite proliferation and bypassing cellular senescence. This dysregulation can involve mutations in the TERT promoter region, epigenetic modifications, or alterations in signaling pathway activity (e.g., in the c-Myc and Wnt/β-catenin pathways), upregulating telomerase activity in cancer cells. Telomerase activity is upregulated in 90% of cancers and immortalized cells [23,24]. Elevated telomerase activity is linked to more aggressive tumor phenotypes, greater metastatic potential, and poor resistance to conventional cancer therapies. In esophageal cancer, the administration of low-dose chemotherapy or radiotherapy can accelerate the malignant phenotypic transformation in normal fibroblasts, leading to the activation of cancer-associated fibroblasts and increased tumor malignancy [25]. Mutations in telomerase and ATRX are independent predictors of poor prognosis in pheochromocytomas and paragangliomas, resulting in worse overall and metastasis-free survival [26]. Telomerase activity is significantly higher in breast cancer tissues than in normal tissues. Its activity is positively correlated with histological grade, tumor size, axillary nodal status, and the expression of both Ki-67 and HER-2/neu protein, and negatively correlated with estrogen receptor expression [27]. Studies have indicated that telomerase activation is linked to aggressive tumor behavior, heightened potential for metastasis, and limited efficacy of conventional cancer treatments.
4. Molecular Mechanisms of Telomer Elongation Contribute to Tumor Progression
4.1. Telomerase Activation Mechanism
Cancer cells stimulate various mechanisms to upregulate telomerase activity, thereby preserving telomere length and sustaining proliferation. One key mechanism involves mutations in the non-coding regions of the hTERT promoter, which create ETS factor-binding sites that lead to transcriptional hyperactivation of telomerase [28]. Additionally, viral oncoproteins can interact with the regulatory elements of host cells, further enhancing TERT gene activation [29]. hTERT expression is elevated in cancer cells, resulting in higher telomerase activity, whereas in normal cells, hTERT expression remains suppressed [30]. Epigenetic modifications, such as histone modifications, DNA methylation, and non-coding RNAs, also regulate telomerase by influencing TERT gene expression [31]. Most tumor cells exhibit increased hTERT promoter hypermethylation, which is associated with tumorigenesis. In contrast, telomerase-negative normal tissues exhibit hypomethylation [32,33]. The histone methyltransferase SMYD3 adds another layer of regulation by binding to the TERT promoter and activating its transcription through the methylation of histone H3K4 in both fibroblasts and cancer cells [34]. The TERT promoter commonly exhibits histone modifications; for example, the active histone marks H3K27ac and H3K4me3 and epigenetic silencing marks H3K9me3 and H3K27me3. H3K4me3, which is linked to active transcription, is significantly enriched in induced pluripotent stem cells and cancer cells. In contrast, HP-1α and H3K27me3, which form heterochromatin, are highly enriched in somatic cells. Additionally, numerous non-coding RNAs bind to TERT, including various microRNAs (miRNAs) that target the 3′-untranslated regions (3′-UTRs) and open reading frames of TERT, thereby modulating its expression in various cancer cell types [35]. miRNAs can modulate transcription by inhibiting transcription factors associated with TERT.
4.2. Curcumin Suppresses Telomerase Activity by Modulating Specific microRNAs
This miRNA-mediated mechanism represents a promising pathway for telomerase inhibition. Additionally, the antitumor activity of curcumin in osteosarcoma involves miR-138, which has been shown to inhibit cell proliferation and invasion [36], highlighting the broader role of miRNA regulation in its mechanism of action. A review of the literature has suggested that miR-135-5p may mediate curcumin’s anti-telomerase effects in ovarian cancer [37]. Furthermore, other microRNAs, such as miR-21, also regulate TERT by targeting different pathways, including STAT3 signaling in glioblastoma and the PTEN/ERK1/2 pathway in colorectal cancer (Figure 2) [38,39].
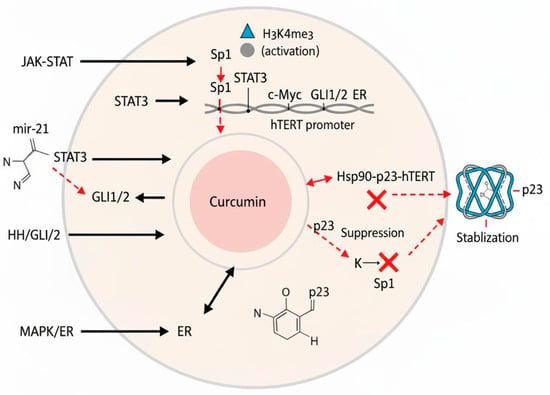
Figure 2.
Diagram illustrating the regulation of hTERT (human telomerase reverse transcriptase) and curcumin’s multifaceted inhibitory mechanisms. Transcription factors (Sp1, STAT3, c-Myc, GLI1/2, and ER), epigenetic modifications (H3K4me3 activation and H3K27me3 repression), and miRNAs (e.g., miR-21) modulate hTERT promoter activity. Curcumin inhibits telomerase by disrupting the Hsp90–p23–hTERT complex, suppressing Sp1/STAT3 activity and expression, and stabilizing the telomeric G-quadruplex DNA structure. Abbreviations: ER, estrogen receptor; G4, G-quadruplex DNA; hTERT, human telomerase reverse transcriptase; Hsp90, heat shock protein 90; miRNA, microRNA; Sp1, specificity protein 1; STAT3, signal transducer and activator of transcription 3.
Some studies have explored the role of TERT in both breast and ovarian cancers, highlighting its promise as a therapeutic target. Wang et al. assessed hTERT function in ovarian cancer using the SKOV3 cell line. Their findings showed that reducing hTERT led to a higher rate of apoptosis and a decline in cell proliferation [40]. Similarly, Su et al. [41] employed a mouse transplantation tumor model and found that lowering hTERT levels significantly suppressed ovarian cancer growth by enhancing apoptosis and upregulating senescence-associated genes, such as p21 and p53, which are essential for controlling cell cycle and apoptosis [41]. Additionally, notable progress has been made in developing breast cancer treatments that focus on targeting hTERT G-quadruplex (G4) structures. Researchers have focused on designing small-molecule ligands that specifically bind to the G4 structures, aiming to downregulate hTERT expression [42].
In cancer cells, telomerase activity is stimulated by certain signaling pathways. The JAK-STAT pathway, which plays a significant role in activating telomerase in cancer cells [43]. In hematological malignancies, the JAK-STAT pathway is frequently upregulated, contributing to telomerase activation [43]. The JAK-STAT pathway mediates signaling through growth factors and cytokine receptors, and its activation leads to the phosphorylation of JAK and STAT proteins [44]. Phosphorylated STAT proteins bind directly to the hTERT promoter, resulting in telomerase activation [43]. Additionally, the JAK-STAT pathway is linked to the mTORC1/PI3K/HSP90/AKT pathway, which regulates telomerase activity [45].
Furthermore, the Hedgehog (HH)/GLI axis influences telomerase activity by directly targeting the hTERT gene in cancers [46,47]. Activation of the HH/GLI signaling pathway increases the production of the GLI1 and GLI2 transcription factors, which attach to the hTERT promoter and stimulate its transcription [48]. Inhibiting the function of GLI1/GLI2 using a pharmacological inhibitor or a truncated GLI3 repressor mutant led to decreased protein levels of hTERT and reduced telomerase activity in various cancer cell lines [47,49]. Conversely, introducing a constantly active GLI2 mutant resulted in elevated mRNA and protein levels, as well as increased hTERT promoter activity (Figure 2) [50].
Moreover, androgen receptor (AR)-mediated signaling upregulates telomerase expression in cancer cells. In androgen-dependent prostate cancer cells, ARs positively regulate hTERT expression [51]. In contrast, in castration-resistant prostate cancer cells, AR-mediated signaling negatively regulates hTERT expression [52]. This negative regulation involves the transcription factor EGR1, which positively regulates hTERT expression [53].
In endometrial cancer cells, the MAPK pathway also contributes to the stimulation of estrogen-mediated induction of telomerase activity [54]. When cells are exposed to estrogen, the MAPK pathway, specifically the p44/42 MAPK, ERK1/2, and P38 members, is activated, which in turn regulates telomerase activity [54,55,56,57,58]. Estrogen-driven MAPK activation results in the phosphorylation of hTERT, the enzyme’s catalytic subunit, and promotes the association of 14-3-3 protein and NF-κB with hTERT. Additionally, the MAPK pathway is involved in the transcriptional regulation of hTERT through activation of the hTERT promoter, which contains estrogen-responsive elements. The MAPK family members JNK, ERK1/2, and P38 play distinct roles in regulating hTERT levels and telomerase activity. Ultimately, radiation stimulates telomerase activity in cancer cells through the Ras/phosphatidylinositol 3-kinase/Akt pathway [59].
4.3. Mechanism of Alternative Lengthening of Telomeres
Telomeres can also elongate through telomerase-independent mechanisms, such as alternative lengthening of telomeres (ALT). In this process, telomere length is maintained by homologous recombination between telomeric DNA sequences. This process takes place when the telomere is absent and is often triggered by defects in the telomere maintenance machinery, including mutations in the chromatin remodeling complex (DAXX/ATRX), which are commonly found in certain cancer types [31]. This alternative pathway is often observed in cancers that manifest telomerase activity or in tumors with mutations that increase telomerase function. Specific genomic alterations and histological features are associated with ALT activation in various cancers. ALT activation is characterized by heterogeneous telomere lengths, extrachromosomal telomeric DNA circles, and specialized nuclear structures known as ALT-associated PML bodies. However, it is unclear how ALT is activated in cancer cells at a molecular level. However, several key factors that trigger the ATL mechanism in cancer cells have been identified [60]. One of the primary triggers is loss of function or mutations in the ATRX/DAXX chromatin remodeling complex, which is common in tumors with ALT. Homologous recombination proteins, such as RAD52, are involved in one of the break-induced replications (BIR) that are the basis of ALT activation. Other key BIR events involve the fork remodeler SMARCAL1 and nuclease MUS81, which maintain replication fork stability and encourage DNA repair [61]. Another key contributor to this alternative lengthening method is the activation of the DNA damage response, which promotes telomere elongation via homologous recombination. In addition, somatic mutations or dysregulation of telomere maintenance mechanisms can trigger bypass of the ALT pathway, allowing cancer cells to preserve telomere length through normal telomerase activity [62]. This constitutes an unequivocal avoidance of cellular senescence, which allows continuous proliferation.
The ALT pathway is also associated with two distinct BIR processes: one dependent on RAD52 and the other dependent on the fork remodelers SMARCAL1 and MUS81 nuclease [61]. Furthermore, the ALT pathway involves telomerase activation or activation of TMMs through reactivation of telomerase activity [62]. These molecular mechanisms provide insights into the processes involving ALT and potential therapeutic targets for the ALT pathway in cancer cells [63].
5. Telomerase and Curcumin
Curcumin, widely recognized as turmeric, is a natural yellow polyphenolic compound isolated from Curcuma longa. Curcumin has been used in alternative medicine for many years as a compound with medicinal properties [64]. Curcumin is chemically composed of diferuloyl methane, which consists of two ferulic acid residues joined by a methylene bridge to yield a bright yellow pigment. This bioactive compound exists in different forms, including free curcumin, curcumin complexes, and derivatives such as tetrahydrocurcumin, all of which have different bioavailabilities and therapeutic potentials [65].
Turmeric has been used for its putative healing properties in skin, pulmonary, and gastrointestinal ailments, and for the treatment of wounds and sprains. Curiously, many of these traditional uses have been verified in recent studies that have investigated the cytoprotective mechanisms of curcumin in several pro-inflammatory diseases. The therapeutic benefits of curcumin are due to its anti-inflammatory and antioxidant properties [66].
One of the most promising areas of research is the potential use of curcumin as an anticancer agent. Curcumin can induce programmed cell death and apoptosis in cancer cells, inhibit the proliferation of cancer cells, and inhibit the diffuse growth of tumor cells. Several studies have demonstrated that curcumin effectively inhibits the growth of colorectal cancer cells and has also shown promising results in breast and pancreatic cancers [67,68,69]. These results underscore the notable promise of curcumin in cancer therapy, making it a focal point of ongoing medical research.
The mechanism by which curcumin inhibits telomerase is fundamentally different from that of synthetic agents. Unlike imetelstat, which directly blocks the hTERC RNA template, curcumin epigenetically silenced hTERT. This hypothesis is supported by two lines of evidence: first, the hTERT subunit is known to be regulated by epigenetic mechanisms, including DNA methylation [70], and second, curcumin has been demonstrated to function as a DNA-demethylating agent, as shown by its ability to reactivate silenced tumor suppressor genes in lung cancer models [71]. This epigenetic activity acts in concert with curcumin’s ability to disrupt key transcription factor networks, such as Sp1, STAT3, and c-Myc, to achieve multitargeted inhibition of telomerase.
6. Mechanisms of Curcumin-Mediated Telomerase Regulation
Curcumin suppresses the activity of telomerase, leading to apoptosis and shortening of telomeres in various cancer cell types, including brain tumor [9], human leukemia [72,73], and MCF-7 breast cancer [6].
Telomerase activity is reactivated in immortal cells and in most human malignancies. Curcumin suppresses telomerase activity and reduces hTERT mRNA levels, which leads to telomere shortening of telomere [9]. Telomerase activity is extremely high in cancer cells. Curcumin has been shown to reduce telomerase levels in human leukemia cells by 55% and 78% after 24 h of treatment with 10 and 50 mM curcumin, respectively [73], suggesting that curcumin inhibits the translocation of hTERT from the nucleus to the cytosol. Nuclear localization of hTERT involves its interaction with the Hsp90-p23 complex. Curcumin disrupts this interaction by dissociating p23 from hTERT, leading to its cytoplasmic accumulation, subsequent proteasomal degradation, and ubiquitination, while leaving the binding of Hsp90 to hTERT unaffected [74].
Khaw and colleagues investigated the effects of curcumin on telomere shortening and telomerase activity in human glioblastoma cell lines. Curcumin was administered at concentrations ranging from 0 to 100 µM, demonstrating a dose-dependent effect on cell viability. Curcumin downregulates hTERT mRNA expression and inhibits telomerase activity, resulting in telomere shortening in these cancer cells. This inhibition resulted in growth arrest at the G2/M phase and induced apoptosis via increased caspase-3/7 activity and overexpression of pro-apoptotic proteins, such as Bax, while decreasing the expression of anti-apoptotic proteins, such as Bcl-2 [9]. Nanoencapsulation of curcumin increases its delivery to cancerous cells, leading to a significant decrease in hTERT gene expression [75,76]. This approach is further supported by recent in vivo and clinical evidence. For instance, reviews on nutraceutical telomerase inhibitors and nanocurcumin formulations have shown that PLGA–curcumin nanoparticles can downregulate hTERT expression and shorten telomeres in colorectal tumors [77,78,79]. However, direct validation in well-controlled primary studies is essential.
The downregulation of hTERT mRNA expression occurs via different mechanisms. First, it generates ROS, which eventually inhibits Sp1 binding activity, thereby downregulating hTERT expression [80]. Furthermore, curcumin reduces the expression of Sp1, a major transcription factor that regulates hTERT, via a proteasomal mechanism, ultimately leading to the inhibition of hTERT mRNA levels [80]. Moreover, curcumin’s ability to downregulate DNMT3b mRNA levels contributes to the suppression of hTERT levels, as demonstrated in lung cancer cells, revealing a novel molecular pathway by which curcumin may act as a chemopreventive agent in lung cancer involving the reactivation of tumor suppressor genes such as RARβ, which ultimately impacts hTERT expression [71]. Collectively, these findings suggest that curcumin exerts its anticancer effects, in part, by downregulating hTERT mRNA expression through multiple pathways.
Curcumin interacts with telomeric G-quadruplexes and inhibits telomerase activity [19]. This interaction involves groove binding to G-quadruplex DNA, and curcumin has been shown to binds strongly to these structures. Curcumin also affects other telomere-associated proteins. Curcumin reduces the expression of telomerase-associated protein 1, which is essential for the stability and activity of the telomerase complex. The downregulation of TEP1 expression is due to the reduced expression of protein arginine methyltransferase 5, a key enzyme that participates in histone methylation and modulates the expression of TEP1 and other proteins involved in telomerase regulation [81].
Curcumin indirectly inhibits telomerase activity by modifying several cellular signaling pathways, including its selective targets on proteins associated with the regulation of cell cycle control. For example, curcumin induces cell cycle arrest in the G1 phase by targeting cyclin-dependent kinase 2 (CDK2) activity, thereby suppressing cancer cell proliferation [82]. Additionally, curcumin analogs, such as chemoprevention curcumin analog-1.1 (CCA-1.1) and pentagamavunone-1 (PGV-1), induce mitotic arrest and cellular senescence in hepatocellular carcinoma cells, further emphasizing the significance of curcumin derivatives in cell cycle regulation [83,84]. Chemically modified curcumin (mCur) induces multiphase cell cycle arrest, including G2/M phase arrest, by downregulating cyclin-dependent kinases and polo-like kinase 1 (PLK1), CCNE1, E2F1, and CDK2, while enhancing PTEN gene expression. Xu et al. used colorectal cancer cell lines to evaluate the impact of curcumin nanoprodrugs on cell cycle progression. The suppression of CDKs and downregulation of PLK1 may contribute to cellular stress and growth arrest, which can indirectly affect telomere maintenance [85]. In addition, curcumin triggers senescence by inducing mitotic slippage, DNA damage, and p21waf1/cip1-dependent heterochromatin loss in cancer cells [86]. Curcumin enhances DLC1, a tumor suppressor gene that inhibits the DYRK1A-EGFR axis, triggers DNA damage, and ultimately leads to cancer cell senescence [87]. These findings collectively highlight the multifaceted molecular pathways through which curcumin exerts its senescence-inducing effects on cancer cells while affecting the cell cycle and telomerase activity.
7. Mechanistic Pathways
Curcumin exerts multiple anticancer effects by modulating key oncogenic signaling pathways, including JAK/STAT, MAPK/ERK, the androgen receptor axis, and PI3K/AKT [37,88]. It also regulates microRNAs involved in telomerase control [37,89].
Curcumin directly inhibits the JAK2/STAT signaling cascade, inducing apoptosis in JAK2-mutated cells through suppression of this pathway [90]. This finding is further supported by evidence from gastric cancer models, where curcumin suppresses JAK2/STAT signaling to overcome chemoresistance [91].
It suppresses the PI3K/AKT pathway by restoring PTEN expression, leading to a 60% decrease in p-AKT (Ser473) levels and downstream mTOR signaling [36,37,92]. Curcumin decreases p-ERK1/2 levels, resulting in the inhibition of ELK1-driven hTERT transcription and reduced GLI1 and PTCH1 transcripts within the hedgehog/GLI pathway [71,75,92]. It also inhibits AKT-dependent AR phosphorylation, reducing AR nuclear localization and PSA expression in prostate cancer cells by approximately 60% [51,93,94].
Curcumin inhibits telomerase by reducing oncogenic microRNA-21 levels by 50%, thereby relieving the suppression of PTEN and SOCS3, which in turn decreases AKT and STAT3 signaling [38,95]. Conversely, it increases miR-138, a tumor-suppressive microRNA that directly targets the 3′ UTR of hTERT mRNA, leading to an approximately 50% reduction in telomerase activity, an effect confirmed in osteosarcoma, glioblastoma, and ovarian cancer models, where blocking miR-138 attenuates curcumin’s anti-telomerase action [89,95]. Additionally, miR-21 influences hTERT regulation via the STAT3 and PTEN/ERK1/2 pathways, linking curcumin-mediated modulation of miRNAs to reduced telomerase expression and telomere shortening [37,38,95]. Together, these results demonstrate that curcumin impedes cancer cell immortality by inhibiting oncogenic signaling and telomerase via a microRNA-dependent mechanism [37,71,92].
8. Comparative Perspective
The broad, multitargeted modulatory effects of curcumin on telomerase differ from those of synthetic inhibitors, such as imetelstat, the most extensively studied direct telomerase antagonist [96]. Imetelstat prevents telomeric DNA extension by binding to the telomerase RNA template. However, due to dose-related hematologic toxicity and the emergence of resistance mechanisms, its clinical use remains limited despite its efficacy against hematologic malignancies [97,98].
In contrast, curcumin employs a pleiotropic strategy: inhibiting telomerase through both transcriptional and post-translational mechanisms, stabilizing telomeric G-quadruplexes, suppressing pro-inflammatory signaling, and exerting antioxidant activity [77,99,100]. This polypharmacological profile may lower the likelihood of resistance and enhance the potential for synergistic therapy, although it simultaneously poses challenges for standardization and clinical application [93,99].
9. Challenges and Future Directions
9.1. Overcoming Pharmacokinetic Limitations
The clinical application of curcumin is hindered primarily by its poor pharmacokinetic properties, including low water solubility, rapid metabolism, and rapid systemic elimination [93,99,101]. When administered orally, curcumin undergoes extensive first-pass metabolism in the liver, producing metabolites that are swiftly cleared from the bloodstream [93,99]. These pharmacokinetic barriers limit the achievement of therapeutically effective drug concentrations in the target tissues. Consequently, as highlighted in recent reviews of clinical trials, substantial research has focused on overcoming these limitations by using advanced formulation strategies. Karaboga Arslan et al. (2022) reported the use of innovative delivery systems, such as phytosomal complexes (e.g., Meriva®) and nanoparticle-based formulations (e.g., Theracurmin®), which enhance curcumin’s bioavailability and therapeutic efficacy [102].
9.2. Promising Strategies: Analogs and Delivery System
To address these limitations, research has focused on synthesizing curcumin analogs and developing advanced delivery systems (Figure 3). Analogs such as CCA-1.1, PGV-1, and EF24 have demonstrated improved potency, stability, and bioavailability [103,104]. Notably, the difluorinated curcumin analog (CDF) exhibits 10–30-fold greater hTERT inhibition than native curcumin, primarily through the suppression of STAT3 phosphorylation [105]. Similarly, the synthetic analog EF24 shows potent telomerase inhibition in glioblastoma xenografts by suppressing Sp1-mediated hTERT transcription [106].
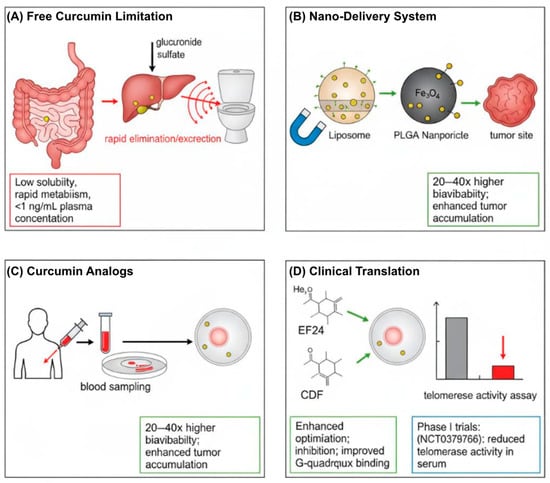
Figure 3.
Strategies to overcome the bioavailability limitations of curcumin for telomerase-targeted cancer therapy. (A) Free curcumin exhibits poor solubility, rapid hepatic metabolism, and rapid systemic clearance, resulting in subtherapeutic plasma concentrations. (B) Nanoformulations (e.g., liposomes, PLGA nanoparticles, and metal–curcumin complexes) enhance solubility, prolong circulation time, and enable tumor-targeted delivery. (C) Structurally optimized analogs (e.g., EF24, CDF) demonstrate superior hTERT inhibition and G-quadruplex stabilization. (D) Potential clinical outcome: nano-curcumin-mediated suppression of telomerase activity in patient serum. Arrows in the diagram generally indicate processes, pathways, or transitions between states. Abbreviations: CDF, difluorinated curcumin; G4, G-quadruplex DNA; hTERT, human telomerase reverse transcriptase; PLGA, poly(lactic-co-glycolic acid).
Beyond organic derivatives, metal–curcumin complexes represent another promising avenue. For instance, in colorectal cancer models, an aluminum–curcumin complex demonstrated superior potency compared to native curcumin [107]. Collectively, the development of such enhanced analogs and metal–curcumin complexes constitutes an actively pursued strategy to overcome the bioavailability and efficacy limitations of native curcumin [103,108].
To circumvent these challenges, a great deal of research has been dedicated to the synthesis of curcumin analogs and their delivery systems. Analogs and Derivatives: Compounds such as CCA-1.1, PGV-1 and EF24 show increased stability and efficiency [83,104]. For example, the curcumin analog CDF (difluorinated curcumin) displays 10–30× more potent hTERT suppression than the precursor compound curcumin through the suppression of STAT3 phosphorylation and Sp1 nuclear translocation [71] grafts by suppressing Sp1-mediated hTERT transcription [105,106].
Nanotechnological approaches—including polymeric nanoparticles [101,108,109], liposomal or micellar systems [110,111,112], and other advanced nanocarriers—have markedly improved curcumin’s bioavailability, metabolic stability, and tumor-targeting efficiency [113,114]. Recent preclinical and systematic reviews have confirmed these benefits [78], and several early-phase clinical trials are currently evaluating these formulations in patients [115].
A Phase I clinical trial demonstrated the safety and feasibility of administering liposomal curcumin (LipoCurc) to patients with malignant pleural effusion [115]. Preclinical studies have indicated that dendrosomal and solid lipid nanocurcumin formulations enhance bioavailability and anticancer activity in vitro. For example, dendrosomal nanocurcumin decreased telomerase activity via the TGF-β1 pathway in hepatocellular carcinoma cells [92], whereas curcumin-loaded OA400 nanoparticles suppressed HPV oncogene expression in cervical cancer cells [116]. Furthermore, PLGA-based nanoparticles have been investigated for the treatment of malignant pleural effusion [115] and several other cancers [102]; however, direct evidence of telomerase inhibition in human trials remains limited.
Encouraging findings from advanced formulations have also emerged from clinical studies. For instance, a Phase II trial reported improved disease control rates and median overall survival using a phytosomal curcumin formulation combined with gemcitabine in pancreatic cancer [84]. Preclinical PLGA-based curcumin nanoparticles showed increased tumor accumulation associated with hTERT downregulation in experimental models [117]. In a pilot clinical study, oral curcumin improved oxidative status in prostate cancer patients [20]; however, robust clinical evidence demonstrating significant effects on leukocyte telomere length and telomerase activity is still lacking.
9.3. Comparative Analysis of Telomerase Inhibitors
Comparative evaluation indicated that the multitargeted mode of action of curcumin differs from that of synthetic agents such as BIBR1532, a direct telomerase catalytic inhibitor, or G-quadruplex stabilizers. Although these agents exhibit higher potency in specific contexts, curcumin offers a broader safety margin, pleiotropic efficacy across diverse tumor types, and a favorable potential for combination therapy [93,99,118]. Nevertheless, its naturally low bioavailability remains a major challenge compared to that of certain synthetic inhibitors [39,78,108] (Figure 3). Table 1 summarizes the key characteristics of representative telomerase inhibitors to contextualize these differences.

Table 1.
Comparative profile of telomerase inhibitors in oncology.
9.4. Future Research
Future investigations should prioritize well-designed in vivo studies that directly assess telomere length and telomerase activity as primary endpoints. Developing a standardized bioavailable curcumin formulation is essential for successful clinical translation (Figure 3) [39,78,108]. Clinical trials should incorporate telomere-related biomarkers to accurately evaluate the potential of curcumin as a telomerase-targeted therapy [77,92]. Additionally, exploring combination strategies with standard chemotherapeutics or other telomerase inhibitors may yield synergistic anticancer effects [93,96]. This synergism may extend to natural compounds such as piperine, which enhances curcumin’s bioavailability and efficacy [119]. Furthermore, curcumin’s potential role as a geroprotective agent against age-related diseases, including cancer, through telomere maintenance warrants further investigation [120].
10. Conclusions
This review highlights the pivotal role of telomeres and telomerase in tumor progression, emphasizing the complex regulatory mechanisms that enable tumor cells to evade senescence, either through telomerase reactivation or alternative lengthening of telomeres (ALT). The unlimited replication potential, genomic instability, and resistance to conventional therapy underscore telomere maintenance as a compelling therapeutic target.
Curcumin has emerged as a promising natural modulator of this axis. It downregulates hTERT expression, disrupts telomerase assembly (e.g., interference with the Hsp90–hTERT complex), stabilizes telomeric G-quadruplex structures, and modulates key oncogenic signaling pathways, including JAK/STAT, NF-κB, and Wnt/β-catenin. Collectively, these effects induce cell-cycle arrest, senescence, and apoptosis in multiple cancer types.
However, translating these robust preclinical outcomes remains constrained by pharmacokinetic limitations, including rapid hepatic and intestinal degradation, hydrophobicity, and low systemic bioavailability. To address these challenges, several measures are critical: (1) application of nanotechnology to enhance solubility, slow metabolic degradation, and increase serum concentration; (2) design of optimized derivatives with high selectivity and affinity for telomerase-associated molecular targets; and (3) rigorous, biomarker-driven clinical trials using standardized protocols to evaluate efficacy, safety, and patient stratification based on telomerase activity or ALT pathway activation.
Through the integration of these strategies, curcumin may evolve from a promising phytochemical into a clinically viable telomerase-modulating agent. Its mechanistic multi-targeted activity across the telomere–telomerase axis offers distinct advantages over synthetic inhibitors that typically act at a single molecular site. Ultimately, curcumin-based interventions present an opportunity to develop innovative therapeutic approaches to improve outcomes across diverse malignancies.
Author Contributions
Conceptualization: H.M.A. and A.F.Z.; Data curation: H.M.A. and A.F.Z.; Formal Analysis; Funding acquisition: H.M.A. and A.F.Z.; Investigation: H.M.A. and A.F.Z.; Methodology: H.M.A. and A.F.Z.; Project administration; Resources; Software; Supervision: H.M.A. and A.F.Z.; Validation; Visualization; Writing—original draft: H.M.A. and A.F.Z.; Writing—review and editing: H.M.A. and A.F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Conflicts of Interest
The authors declare no conflicts of interest related to this work.
Abbreviations
| ALT | alternative lengthening of telomeres |
| AR | androgen receptor |
| CCA-1.1 | chemoprevention curcumin analog-1.1 |
| CDF | difluorinated curcumin |
| c-Myc | cellular myelocytomatosis oncogene |
| DKC1 | dyskerin |
| EF24 | curcumin analog EF24 |
| ER | estrogen receptor |
| G4 | G-quadruplex DNA |
| GLI1/GLI2 | GLI family zinc-finger 1/2 |
| H3K4me3 | histone 3 lysine 4 trimethylation |
| H3K27me3 | histone 3 lysine 27 trimethylation |
| Hsp90 | heat shock protein 90 |
| hTERC | human telomerase RNA component |
| hTERT | human telomerase reverse transcriptase |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| MAPK | mitogen-activated protein kinase |
| mCur | chemically modified curcumin |
| miRNAs | microRNAs |
| PGV-1 | pentagamavunone-1 |
| PI3K/AKT | phosphoinositide 3-kinase/protein kinase B pathway |
| PLGA | poly(lactic-co-glycolic acid) |
| PLK1 | polo-like kinase 1 |
| POT1 | protection of telomeres 1 |
| RAP1 | repressor activator protein 1 |
| SMYD3 | set and mynd domain-containing protein 3 |
| Sp1 | specificity protein 1 |
| STAT3 | signal transducer and activator of transcription 3 |
| TCAB1 | telomerase Cajal body protein 1 |
| TEP1 | telomerase-associated protein 1 |
| TIN2 | TRF1-interacting nuclear factor 2 |
| TPP1 | TINT1/PTOP/PIP1 (protection of telomeres 1-interacting protein) |
| TRF1/2 | telomere repeat-binding factors 1 and 2 |
References
- Gruber, H.-J.; Semeraro, M.D.; Renner, W.; Herrmann, M. Telomeres and Age-Related Diseases. Biomedicines 2021, 9, 1335. [Google Scholar] [CrossRef]
- Chakravarti, D.; LaBella, K.A.; DePinho, R.A. Telomeres: History, Health, and Hallmarks of Aging. Cell 2021, 184, 306–322. [Google Scholar] [CrossRef]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of Telomeres and Telomerase in Cancer, and Advances in Telomerase-Targeted Therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Soleti, R.; Panaro, M.A.; Torre, M.E.; Monda, V.; Messina, G.; Porro, C. Curcumin as Prospective Anti-Aging Natural Compound: Focus on Brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin Inhibits Telomerase Activity through Human Telomerase Reverse Transcritpase in MCF-7 Breast Cancer Cell Line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef]
- Hu, H.; Yan, H.L.; Nguyen, T.H.D. Structural Biology of Shelterin and Telomeric Chromatin: The Pieces and an Unfinished Puzzle. Biochem. Soc. Trans. 2024, 52, 1551–1564. [Google Scholar] [CrossRef]
- Lange, T. Shelterin-Mediated Telomere Protection. Annu. Rev. Genet. 2018, 52, 223–247. [Google Scholar] [CrossRef]
- Khaw, A.K.; Hande, M.P.; Kalthur, G.; Hande, M.P. Curcumin Inhibits Telomerase and Induces Telomere Shortening and Apoptosis in Brain Tumour Cells. J. Cell Biochem. 2013, 114, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Londoño-vallejo, A. Telomeres and Cancer. In Telomeres: Chromosome Sentinels; Saintome, C., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 263–290. [Google Scholar]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The Impact of Oxidative DNA Damage and Stress on Telomere Homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of Cellular Senescence. Telomere Shortening as a Marker of Cellular Senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- Rosso, I.; Jones-Weinert, C.; Rossiello, F.; Cabrini, M.; Brambillasca, S.; Munoz-Sagredo, L.; Lavagnino, Z.; Martini, E.; Tedone, E.; Garre’, M. Alternative Lengthening of Telomeres (ALT) Cells Viability Is Dependent on C-Rich Telomeric RNAs. Nat. Commun. 2023, 14, 7086. [Google Scholar] [CrossRef]
- Maciejowski, J.; Lange, T. Telomeres in Cancer: Tumour Suppression and Genome Instability. Nat. Rev. Mol. Cell Biol. 2017, 18, 175–186, Erratum in Nat. Rev. Mol. Cell Biol. 2019, 20, 259. https://doi.org/10.1038/nrm.2016.171. [Google Scholar] [CrossRef] [PubMed]
- Patrick, E.M.; Slivka, J.D.; Payne, B.; Comstock, M.J.; Schmidt, J.C. Observation of Processive Telomerase Catalysis Using High-Resolution Optical Tweezers. Nat. Chem. Biol. 2020, 16, 801–809. [Google Scholar] [CrossRef]
- Egan, E.D.; Collins, K. Specificity and Stoichiometry of Subunit Interactions in the Human Telomerase Holoenzyme Assembled in Vivo. Mol. Cell Biol. 2010, 30, 2775–2786. [Google Scholar] [CrossRef]
- Machado-Pinilla, R.; Carrillo, J.; Manguan-Garcia, C.; Sastre, L.; Mentzer, A.; Gu, B.W.; Mason, P.J.; Perona, R. Defects in mTR Stability and Telomerase Activity Produced by the Dkc1 A353V Mutation in Dyskeratosis Congenita Are Rescued by a Peptide from the Dyskerin TruB Domain. Clin. Transl. Oncol. 2012, 14, 755–763. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibáñez-Cabellos, J.S.; Seco-Cervera, M.; Picher-Latorre, C.; Pérez-Machado, G.; García-Giménez, J.L.; Pallardó, F.V. Acute Depletion of Telomerase Components DKC1 and NOP10 Induces Oxidative Stress and Disrupts Ribosomal Biogenesis via NPM1 and Activation of the P53 Pathway. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118845. [Google Scholar] [CrossRef]
- Jha, N.S.; Mishra, S.; Mamidi, A.S.; Mishra, A.; Jha, S.K.; Surolia, A. Targeting Human Telomeric G-Quadruplex DNA with Curcumin and Its Synthesized Analogues under Molecular Crowding Conditions. RSC Adv. 2016, 6, 7474–7487. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, G.; Wu, R.; Mack, D.L.; Sun, X.S.; Maxwell, J.; Guan, X.; Atala, A.; Zhang, Y. Differentiation Capacity of Human Urine-Derived Stem Cells to Retain Telomerase Activity. Front. Cell Dev. Biol. 2022, 10, 890574. [Google Scholar] [CrossRef]
- Kuru, G.; Küçüksolak, M.; Pulat, G.; Karaman, O.; Bedir, E. The Effects of Novel Telomerase Activators on Human Adipose-Derived Mesenchymal Stem Cell (hAD-MSC) Proliferation and Osteogenic Differentiation. Planta Med. 2022, 88, 1500. [Google Scholar] [CrossRef]
- Ma, B.; Martínez, P.; Sánchez-Vázquez, R.; Blasco, M.A. Telomere Dynamics in Human Pluripotent Stem Cells. Cell Cycle 2023, 22, 2505–2521. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Shay, J.W.; Bacchetti, S. A Survey of Telomerase Activity in Human Cancer. Eur. J. Cancer 1997, 33, 787–791. [Google Scholar] [CrossRef]
- Komoto, S.; Noma, K.; Kato, T.; Kobayashi, T.; Nishiwaki, N.; Narusaka, T.; Sato, H.; Katsura, Y.; Kashima, H.; Kikuchi, S. Conventional Cancer Therapies Can Accelerate Malignant Potential of Cancer Cells by Activating Cancer-Associated Fibroblasts in Esophageal Cancer Models. Cancers 2023, 15, 2971. [Google Scholar] [CrossRef]
- Job, S.; Draskovic, I.; Burnichon, N.; Buffet, A.; Cros, J.; Lépine, C.; Venisse, A.; Robidel, E.; Verkarre, V.; Meatchi, T. Telomerase Activation and ATRX Mutations Are Independent Risk Factors for Metastatic Pheochromocytoma and Paraganglioma. Clin. Cancer Res. 2019, 25, 760–770. [Google Scholar] [CrossRef]
- Kulić, A.; Plavetić, N.D.; Gamulin, S.; Jakić-Razumović, J.; Vrbanec, D.; Sirotković-Skerlev, M. Telomerase Activity in Breast Cancer Patients: Association with Poor Prognosis and More Aggressive Phenotype. Med. Oncol. 2016, 33, 23. [Google Scholar] [CrossRef]
- Sharma, S.; Chowdhury, S. Emerging Mechanisms of Telomerase Reactivation in Cancer. Trends Cancer 2022, 8, 632–641. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Cerasuolo, A.; Starita, N.; Tornesello, A.L.; Bonelli, P.; Tuccillo, F.M.; Buonaguro, L.; Isaguliants, M.G.; Buonaguro, F.M. The Molecular Interplay between Human Oncoviruses and Telomerase in Cancer Development. Cancers 2022, 14, 5257. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Jian, Y.; Gu, L.; Zhang, D.; Zhou, H.; Wang, Y.; Xu, Z.X. The Regulations of Telomerase Reverse Transcriptase (TERT) in Cancer. Cell Death Dis. 2024, 15, 90. [Google Scholar] [CrossRef]
- Dogan, F.; Forsyth, N.R. Telomerase regulation: A role for epigenetics. Cancers 2021, 13, 1213. [Google Scholar] [CrossRef] [PubMed]
- Dessain, S.K.; Yu, H.; Reddel, R.R.; Beijersbergen, R.L.; Weinberg, R.A. Methylation of the Human Telomerase Gene CpG Island. Cancer Res. 2000, 60, 537–541. [Google Scholar]
- Guilleret, I.; Yan, P.; Grange, F.; Braunschweig, R.; Bosman, F.T.; Benhattar, J. Hypermethylation of the Human Telomerase Catalytic Subunit (hTERT) Gene Correlates with Telomerase Activity. Int. J. Cancer 2002, 101, 335–341. [Google Scholar] [CrossRef]
- Liu, C.; Fang, X.; Ge, Z.; Jalink, M.; Kyo, S.; Björkholm, M.; Gruber, A.; Sjöberg, J.; Xu, D. The Telomerase Reverse Transcriptase (hTERT) Gene Is a Direct Target of the Histone Methyltransferase SMYD3. Cancer Res. 2007, 67, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, S.; Nunes-Souza, E.; Marchi, R.; Ruthes, M.O.; Okano, L.M.; Tofolo, M.V.; Centa, A.; Fonseca, A.S.; Rosolen, D.; Cavalli, L.R. MicroRNAs Role in Telomere Length Maintenance and Telomerase Activity in Tumor Cells. J. Mol. Med. 2024, 102, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; An, F.; He, X.; Cao, X. Curcumin Inhibits the Proliferation and Invasion of Human Osteosarcoma Cell Line MG-63 by Regulating miR-138. Int. J. Clin. Exp. Pathol. 2015, 8, 14946. [Google Scholar] [PubMed]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Sun, G.; Luo, H.; Wang, X.F.; Lan, F.M.; Yue, X.; Fu, L.S.; Pu, P.Y.; Kang, C.S.; Liu, N. MiR-21 Modulates hTERT through a STAT3-Dependent Manner on Glioblastoma Cell Growth. CNS Neurosci. Ther. 2012, 18, 722–728. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Activity. ACS Omega 2022, 7, 35875–35884. [Google Scholar] [CrossRef]
- Wang, Q.-A.-Z.; Liang, X.; Yang, Y. Downregulation of hTERT Contributes to Ovarian Cancer Apoptosis and Inhibits Proliferation of Ovarian Cancer Cells. Transl. Cancer Res. 2020, 9, 1448–1454. [Google Scholar] [CrossRef]
- Su, C.; Luo, Y.; Yang, Y.; Yi, Y. ShRNA-Mediated Silencing of hTERT Promote Apoptosis and Senescence in Human Ovarian Cancer Cells. Transl. Cancer Res. 2019, 8, 567–573. [Google Scholar] [CrossRef]
- Long, W.; Zeng, Y.-X.; Zheng, B.-X.; Li, Y.-B.; Wang, Y.-K.; Chan, K.-H.; She, M.-T.; Lu, Y.-J.; Cao, C.; Wong, W.-L. Targeting hTERT Promoter G-Quadruplex DNA Structures with Small-Molecule Ligand to Downregulate hTERT Expression for Triple-Negative Breast Cancer Therapy. J. Med. Chem. 2024, 67, 13363–13382. [Google Scholar] [CrossRef]
- Yamada, O.; Kawauchi, K. The Role of the JAK-STAT Pathway and Related Signal Cascades in Telomerase Activation during the Development of Hematologic Malignancies. JAK-STAT 2013, 2, 25256. [Google Scholar] [CrossRef]
- Brooks, A.J.; Putoczki, T. JAK-STAT Signalling Pathway in Cancer. Cancers 2020, 12, 1971. [Google Scholar] [CrossRef]
- Dutta, P.; Li, W.X. Role of the JAK-STAT Signalling Pathway in Cancer. In eLS; John Wiley & Sons, Limited: Chichester, UK, 2013. [Google Scholar]
- Chai, J.Y.; Sugumar, V.; Alshawsh, M.A.; Wong, W.F.; Arya, A.; Chong, P.P.; Looi, C.Y. The Role of Smoothened-Dependent and -Independent Hedgehog Signaling Pathway in Tumorigenesis. Biomedicines 2021, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, T.; Sandhu, R.; Qadan, M.; DeVecchio, J.; Magloire, V.; Agyeman, A.; Li, B.; Houghton, J.A. Hedgehog signaling regulates telomerase reverse transcriptase in human cancer cells. PLoS ONE 2013, 8, 75253. [Google Scholar] [CrossRef]
- Tusa, I.; Gagliardi, S.; Tubita, A.; Pandolfi, S.; Menconi, A.; Lulli, M.; Dello Sbarba, P.; Stecca, B.; Rovida, E. The Hedgehog-GLI pathway regulates MEK5-ERK5 expression and activation in melanoma cells. Int. J. Mol. Sci. 2021, 22, 11259. [Google Scholar] [CrossRef] [PubMed]
- Doheny, D.; Manore, S.G.; Wong, G.L.; Lo, H.-W. Hedgehog Signaling and Truncated GLI1 in Cancer. Cells 2020, 9, 2114. [Google Scholar] [CrossRef]
- Sigafoos, A.N.; Paradise, B.D.; Fernandez-Zapico, M.E. Hedgehog/GLI Signaling Pathway: Transduction, Regulation, and Implications for Disease. Cancers 2021, 13, 3410. [Google Scholar] [CrossRef]
- Bartsch, S.; Mirzakhani, K.; Neubert, L.; Stenzel, A.; Ehsani, M.; Esmaeili, M.; Pungsrinont, T.; Kacal, M.; Rasa, S.M.M.; Kallenbach, J. Antithetic hTERT Regulation by Androgens in Prostate Cancer Cells: hTERT Inhibition Is Mediated by the ING1 and ING2 Tumor Suppressors. Cancers 2021, 13, 4025. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nayak, S.; Kakar, R.; Chaudhari, U.K.; Joshi, D.; Vundinti, B.R.; Fernandes, G.; Barai, R.S.; Kholkute, S.D.; Sachdeva, G. A Triad of Telomerase, Androgen Receptor and Early Growth Response 1 in Prostate Cancer Cells. Cancer Biol. Ther. 2016, 17, 439–448. [Google Scholar] [CrossRef]
- Liu, S.; Qi, Y.; Ge, Y.; Duplessis, T.; Rowan, B.G.; Ip, C.; Cheng, H.; Rennie, P.S.; Horikawa, I.; Lustig, A.J. Telomerase as an Important Target of Androgen Signaling Blockade for Prostate Cancer Treatment. Mol. Cancer Ther. 2010, 9, 2016–2025. [Google Scholar] [CrossRef]
- Zhou, C.; Steplowski, T.A.; Dickens, H.K.; Malloy, K.M.; Gehrig, P.A.; Boggess, J.F.; Bae-Jump, V.L. Estrogen Induction of Telomerase Activity through Regulation of the Mitogen-Activated Protein Kinase (MAPK) Dependent Pathway in Human Endometrial Cancer Cells. PLoS ONE 2013, 8, 55730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kyo, S.; Takakura, M.; Tanaka, M.; Yatabe, N.; Maida, Y.; Fujiwara, M.; Hayakawa, J.; Ohmichi, M.; Koike, K. Progesterone Regulates Human Telomerase Reverse Transcriptase Gene Expression via Activation of Mitogen-Activated Protein Kinase Signaling Pathway. Cancer Res. 2000, 60, 5376–5381. [Google Scholar]
- Kyo, S.; Takakura, M.; Kanaya, T.; Zhuo, W.; Fujimoto, K.; Nishio, Y.; Orimo, A.; Inoue, M. Estrogen activates telomerase. Cancer Res. 1999, 59, 5917–5921. [Google Scholar]
- Kimura, A.; Ohmichi, M.; Kawagoe, J.; Kyo, S.; Mabuchi, S.; Takahashi, T.; Ohshima, C.; Arimoto-Ishida, E.; Nishio, Y.; Inoue, M. Induction of hTERT Expression and Phosphorylation by Estrogen via Akt Cascade in Human Ovarian Cancer Cell Lines. Oncogene 2004, 23, 4505–4515. [Google Scholar] [CrossRef]
- Lamy, E.; Herz, C.; Lutz-Bonengel, S.; Hertrampf, A.; Márton, M.-R.; Mersch-Sundermann, V. The MAPK Pathway Signals Telomerase Modulation in Response to Isothiocyanate-Induced DNA Damage of Human Liver Cancer Cells. PLoS ONE 2013, 8, 53240. [Google Scholar] [CrossRef]
- Ram, R.; Uziel, O.; Eldan, O.; Fenig, E.; Beery, E.; Lichtenberg, S.; Nordenberg, Y.; Lahav, M. Ionizing Radiation Up-Regulates Telomerase Activity in Cancer Cell Lines by Post-Translational Mechanism via Ras/Phosphatidylinositol 3-Kinase/Akt Pathway. Clin. Cancer Res. 2009, 15, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Goralsky, J.A.; Shay, J.W.; Min, J. The Molecular Mechanisms and Therapeutic Prospects of Alternative Lengthening of Telomeres (ALT). Cancers 2023, 15, 1945. [Google Scholar] [CrossRef] [PubMed]
- Broderick, R.; Cherdyntseva, V.; Nieminuszczy, J.; Dragona, E.; Kyriakaki, M.; Evmorfopoulou, T.; Gagos, S.; Niedzwiedz, W. Pathway Choice in the Alternative Telomere Lengthening in Neoplasia Is Dictated by Replication Fork Processing Mediated by EXD2’s Nuclease Activity. Nat. Commun. 2023, 14, 2428. [Google Scholar] [CrossRef]
- Stundon, J.L.; Ijaz, H.; Gaonkar, K.S.; Kaufman, R.S.; Jin, R.; Karras, A.; Vaksman, Z.; Kim, J.; Corbett, R.J.; Lueder, M.R. Alternative Lengthening of Telomeres (ALT) in Pediatric High-Grade Gliomas Can Occur without ATRX Mutation and Is Enriched in Patients with Pathogenic Germline Mismatch Repair (MMR) Variants. Neuro-Oncology 2023, 25, 1331–1342. [Google Scholar] [CrossRef]
- Sun, H.; Chen, G.; Guo, B.; Lv, S.; Yuan, G. Potential Clinical Treatment Prospects behind the Molecular Mechanism of Alternative Lengthening of Telomeres (ALT). J. Cancer 2023, 14, 417–433. [Google Scholar] [CrossRef]
- Ayub, H.; Islam, M.; Saeed, M.; Ahmad, H.; Al-Asmari, F.; Ramadan, M.F.; Alissa, M.; Arif, M.A.; Rana, M.U.J.; Subtain, M. On the Health Effects of Curcumin and Its Derivatives. Food Sci. Nutr. 2024, 12, 8623–8650. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Kanai, M. Therapeutic Applications of Curcumin for Patients with Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 9384–9391. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. Curcumin as an Enhancer of Therapeutic Efficiency of Chemotherapy Drugs in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2144. [Google Scholar] [CrossRef]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.-S.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G. Anticancer Properties of Curcumin against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tollefsbol, T.O. Regulation of the Telomerase Reverse Transcriptase Subunit through Epigenetic Mechanisms. Front. Genet. 2016, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Wang, X.; Shan, X.; Li, Y.; Wang, P.; Jiang, P.; Feng, Q. Curcumin Reactivates Silenced Tumor Suppressor Gene RARβ by Reducing DNA Methylation. Phytother. Res. 2015, 29, 1237–1245. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Roy, M. Inhibition of Telomerase Activity and Induction of Apoptosis by Curcumin in K-562 Cells. Mutat. Res. 2006, 596, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Dey, S.; Roy, M. Curcumin-Induced Apoptosis in Human Leukemia Cell HL-60 Is Associated with Inhibition of Telomerase Activity. Mol. Cell Biochem. 2007, 297, 31–39. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, I.K. Curcumin Inhibits Nuclear Localization of Telomerase by Dissociating the Hsp90 Co-Chaperone P23 from hTERT. Cancer Lett. 2010, 290, 76–86. [Google Scholar] [CrossRef]
- Bagheri, R.; Sanaat, Z.; Zarghami, N. Synergistic Effect of Free and Nano-Encapsulated Chrysin-Curcumin on Inhibition of hTERT Gene Expression in SW480 Colorectal Cancer Cell Line. Drug Res. 2018, 68, 335–343, Erratum in Drug Res. 2018, 68, e2. https://doi.org/10.1055/s-0043-121338. [Google Scholar] [CrossRef]
- Sadeghzadeh, H.; Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Dariushnejad, H.; Sanjarian, F.; Zarghami, N. The Effects of Nanoencapsulated Curcumin-Fe3O4 on Proliferation and hTERT Gene Expression in Lung Cancer Cells. Anti Cancer Agents Med. Chem. 2017, 17, 1363–1373. [Google Scholar] [CrossRef]
- Mustafa, Y.F. Nutraceutical-Based Telomerase Inhibitors: Renewed Hope for Cancer Therapy. Phytomed. Plus 2024, 4, 100537. [Google Scholar] [CrossRef]
- Boroughani, M.; Moaveni, A.K.; Hatami, P.; Mansoob Abasi, N.; Seyedoshohadaei, S.A.; Pooladi, A.; Moradi, Y.; Rahimi Darehbagh, R. Nanocurcumin in Cancer Treatment: A Comprehensive Systematic Review. Discov. Oncol. 2024, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, R.; Kondapavuluri, B.K.; Thangavelu, L. Nanoparticle-Based Strategies with Bioactive Compounds for Targeted Cancer Therapy. Ind. Crops Prod. 2025, 227, 120804. [Google Scholar] [CrossRef]
- Hsin, I.-L.; Sheu, G.-T.; Chen, H.-H.; Chiu, L.-Y.; Wang, H.-D.; Chan, H.-W.; Hsu, C.-P.; Ko, J.-L. N-Acetyl Cysteine Mitigates Curcumin-Mediated Telomerase Inhibition through Rescuing of Sp1 Reduction in A549 Cells. Mutat. Res. 2010, 688, 72–77. [Google Scholar] [CrossRef]
- Chatterjee, B.; Ghosh, K.; Suresh, L.; Kanade, S.R. Curcumin Ameliorates PRMT5-MEP50 Arginine Methyltransferase Expression by Decreasing the Sp1 and NF-YA Transcription Factors in the A549 and MCF-7 Cells. Mol. Cell Biochem. 2019, 455, 73–90. [Google Scholar] [CrossRef]
- Lim, T.-G.; Lee, S.-Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin Suppresses Proliferation of Colon Cancer Cells by Targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef]
- Moordiani, M.; Novitasari, D.; Susidarti, R.A.; Ikawati, M.; Kato, J.; Meiyanto, E. Curcumin Analogs PGV-1 and CCA-1.1 Induce Cell Cycle Arrest in Human Hepatocellular Carcinoma Cells with Overexpressed MYCN. Indones. Biomed. J. 2023, 15, 141–149. [Google Scholar] [CrossRef]
- Pastorelli, D.; Fabricio, A.S.C.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Dalt, G.; et al. Phytosome Complex of Curcumin as Complementary Therapy of Advanced Pancreatic Cancer Improves Safety and Efficacy of Gemcitabine: Results of a Prospective Phase II Trial. Pharmacol. Res. 2018, 132, 72–79. [Google Scholar] [CrossRef]
- Xu, D.; Feng, X.; Wan, Y.; Yang, L.; Gao, Q.; Yang, Z.; Du, C. Curcumin Nano-Prodrug Induces Multi-Phase Cell Cycle Arrest in Colorectal Cancer through Suppression of CDKs and Specific down-Regulation of PLK1. Smart Mater. Med. 2023, 4, 648–660, Erratum in Smart Mater. Med. 2024, 5, 181. https://doi.org/10.1016/j.smaim.2023.06.001. [Google Scholar] [CrossRef]
- Barra, V.; Chiavetta, R.F.; Titoli, S.; Provenzano, I.M.; Carollo, P.S.; Leonardo, A. Specific Irreversible Cell-Cycle Arrest and Depletion of Cancer Cells Obtained by Combining Curcumin and the Flavonoids Quercetin and Fisetin. Genes 2022, 13, 1125. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Ma, Y.; Liu, K.; Gao, J.; Li, S.; Sun, X.; Li, G. Resveratrol Induces DNA Damage-Mediated Cancer Cell Senescence through the DLC1–DYRK1A–EGFR Axis. Food Funct. 2023, 14, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Bolourinezhad, M.; Teng, Y.; Sahebkar, A. The Multifaceted Therapeutic Mechanisms of Curcumin in Osteosarcoma: State-of-the-Art. J. Oncol. 2021, 2021, 3006853. [Google Scholar] [CrossRef]
- Tarazón, E.; Unamuno Bustos, B.; Murria Estal, R.; Pérez Simó, G.; Sahuquillo Torralba, A.; Simarro, J. MiR-138-5p Suppresses Cell Growth and Migration in Melanoma by Targeting Telomerase Reverse Transcriptase. Genes 2021, 12, 1931. [Google Scholar] [CrossRef] [PubMed]
- Petiti, J.; Rosso, V.; Lo Iacono, M.; Panuzzo, C.; Calabrese, C.; Signorino, E.; Pironi, L.; Cartellà, A.; Bracco, E.; Pergolizzi, B.; et al. Curcumin Induces Apoptosis in JAK2-Mutated Cells by the Inhibition of JAK2/STAT and mTORC1 Pathways. J. Cell Mol. Med. 2019, 23, 4349–4357. [Google Scholar] [CrossRef]
- Ham, I.H.; Wang, L.; Lee, D.; Woo, J.; Kim, T.H.; Jeong, H.Y.; Oh, H.J.; Choi, K.S.; Kim, T.M.; Hur, H. Curcumin Inhibits the Cancer-associated Fibroblast-derived Chemoresistance of Gastric Cancer through the Suppression of the JAK/STAT3 Signaling Pathway. Int. J. Oncol. 2022, 61, 85. [Google Scholar] [CrossRef]
- Shariati, M.; Hajigholami, S.; Veisi Malekshahi, Z.; Entezari, M.; Bodaghabadi, N.; Sadeghizadeh, M. Nanocurcumin-Mediated Down-Regulation of Telomerase Via Stimulating TGFβ1 Signaling Pathway in Hepatocellular Carcinoma Cells. Iran. Biomed. J. 2017, 2018, 171. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.C. Curcumin in Cancer Therapy: Exploring Molecularmechanisms and Overcoming Clinical Challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef]
- Hashemi, M.; Mirzaei, S.; Barati, M.; Hejazi, E.S.; Kakavand, A.; Entezari, M.; Salimimoghadam, S.; Kalbasi, A.; Rashidi, M.; Taheriazam, A.; et al. Curcumin in the Treatment of Urological Cancers: Therapeutic Targets, Challenges and Prospects. Life Sci. 2022, 309, 120984. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, Y.; Liu, J.; Long, S.; Liu, H.; Zhang, J. The Role of miR-21/RECK in the Inhibition of Osteosarcoma by Curcumin. Mol. Cell Probes. 2020, 51, 101534. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.L.; Huang, F.; Behrs, M.K.; González-Sales, M.; Bhise, N.; Wan, Y.; Sun, L.; Berry, T.; Feller, F.; Morcos, P.N. Imetelstat, a Novel, First-in-Class Telomerase Inhibitor: Mechanism of Action, Clinical, and Translational Science. Clin. Transl. Sci. 2024, 17, e70076. [Google Scholar] [CrossRef]
- Thomas, X. Examining the Safety and Efficacy of Imetelstat in Low-Risk Myelodysplastic Syndrome. Expert Opin. Pharmacother. 2025, 26, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Santini, V.; Fenaux, P.; Sekeres, M.A.; Savona, M.R.; Madanat, Y.F.; Díez-Campelo, M.; Valcárcel, D.; Illmer, T.; Jonášová, A.; et al. Imetelstat in Patients with Lower-Risk Myelodysplastic Syndromes Who Have Relapsed or Are Refractory to Erythropoiesis-Stimulating Agents (IMerge): A Multinational, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2024, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of Curcumin: An Emerging Paradigm for Improved Remedial Application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef]
- ClinicalTrialsgov Trial of Curcumin to Prevent Progression of Low-Risk Prostate Cancer Under Active Surveillance. Available online: https://www.clinicaltrials.gov/study/NCT03769766 (accessed on 15 October 2025).
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory, and Anticancer Activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar] [CrossRef]
- Nowacka, A.; Ziółkowska, E.; Smuczyński, W.; Bożiłow, D.; Śniegocki, M. Potential of Curcumin and Its Analogs in Glioblastoma Therapy. Antioxidants 2025, 14, 351. [Google Scholar] [CrossRef]
- Simsek, E.; Sunguroglu, A.; Kilic, A.; Özgültekin, N.; Ozensoy Guler, O. Effects of Thymoquinone and the Curcumin Analog EF-24 on the Activity of the Enzyme Paraoxonase-1 in Human Glioblastoma Cells U87MG. J. Enzyme Inhib. Med. Chem. 2024, 39, 2339901. [Google Scholar] [CrossRef]
- Jabbari, N.; Sabokrouh, A. Enhanced Anticancer Efficacy of Aluminum-Curcumin Complex Compared to Curcumin in Colorectal Cancer Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 9109–9124. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef] [PubMed]
- Iranpoor, N.; Jafari-Gharabaghlou, D.; Abdulzehra, S.; Dashti, M.R.; Gorbanzadeh, F.; Zarghami, N. Development and Characterization of Curcumin Loaded PEGylated Niosomal Nanoparticles: Potential Anti-Cancer Effect on Breast Cancer Cells through RFC Gene Expression. Asian Pac. J. Cancer Prev. 2025, 26, 1017–1026. [Google Scholar] [CrossRef]
- Mostajeran, N. Liposome-based curcumin delivery systems as cancer therapeutics. In Curcumin-Based Nanomedicines as Cancer Therapeutics; Smith, J., Doe, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 209–240. [Google Scholar]
- Kumar, S.; Astalakshmi, N.; Dhivya, K.; Sarathi, M.; Lokesh, D.; Nivethitha, S.; Praveen Kumar, M. Recent Advances in Phytosomes for the Safe Management of Cancer. Phytomed. Plus 2024, 4, 100540. [Google Scholar] [CrossRef]
- Yakub, G.; Manolova, N.E.; Rashkov, I.B.; Markova, N.; Toshkova, R. Pegylated Curcumin Derivative: Water-Soluble Conjugates with Antitumor and Antibacterial Activity. Colloid. Polym. Sci. 2022, 300, 123–134. [Google Scholar] [CrossRef]
- Yan, Y.; Kulsoom; Sun, Y.; Li, Y.; Wang, Z.; Xue, L.; Wang, F. Advancing Cancer Therapy: Nanomaterial-Based Encapsulation Strategies for Enhanced Delivery and Efficacy of Curcumin. Mater. Today Bio 2025, 33, 101963. [Google Scholar] [CrossRef]
- Rathod, N.V.; Mishra, S. Nano-Curcumin for Cancer Therapy: A Strategic Approach to Improve Bioavailability and Modulate Tumor Resistance. Chem. Biodivers. 2025, 22, 2025012. [Google Scholar] [CrossRef]
- Hocking, A.J.; Farrall, A.L.; Newhouse, S.; Sordillo, P.; Greco, K.; Karapetis, C.S.; Dougherty, B.; Klebe, S. Study Protocol of a Phase 1 Clinical Trial Establishing the Safety of Intrapleural Administration of Liposomal Curcumin: Curcumin as a Palliative Treatment for Malignant Pleural Effusion (IPAL-MPE). BMJ Open 2021, 11, 047075. [Google Scholar] [CrossRef]
- Sadeghi, R.V.; Parsania, M.; Sadeghizadeh, M.; Haghighat, S. Investigation of Curcumin-Loaded OA400 Nanoparticle’s Effect on the Expression of E6 and E7 Human Papilloma-Virus Oncogenes and P53 and Rb Factors in HeLa Cell Line. Iran. J. Pharm. Res. 2022, 21, e130762. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Javidfar, S.; Lotfi-Attari, J.; Sadeghzadeh, H.; Shafiei-Irannejad, V.; Zarghami, N. Nano-Encapsulated Metformin-Curcumin in PLGA/PEG Inhibits Synergistically Growth and hTERT Gene Expression in Human Breast Cancer Cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Imran, M.; Hussain, M.; Alsagaby, S.A.; Momal, U.; Naeem, H.; Al Jbawi, E. Curcumin: A Bioactive Compound with Molecular Targets for Human Malignancies. Food Agric. Immunol. 2023, 34, 2280524. [Google Scholar] [CrossRef]
- Parvin, N.; Aslam, M.; Joo, S.W.; Mandal, T.K. Nano-Phytomedicine: Harnessing Plant-Derived Phytochemicals in Nanocarriers for Targeted Human Health Applications. Molecules 2025, 30, 3177. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Targeting Aging Pathways with Natural Compounds: A Review of Curcumin, Epigallocatechin Gallate, Thymoquinone, and Resveratrol. Immun. Ageing 2025, 22, 28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).