Abstract
Background/Objective: Cutaneous melanoma is a highly heterogeneous malignancy and life-threatening skin cancer with rising global incidence. Although various therapeutic options are available, their clinical efficacy remains limited, highlighting the urgent need for novel strategies that facilitate prevention, diagnosis, and treatment. The aim of this study was to explore the potential causal association between medication use and the risk of developing cutaneous melanomas. Methods: Using summary data from Genome-Wide Association Studies (GWASs), we performed Mendelian randomization (MR) to investigate the causal effect of medication use on cutaneous melanoma risk. Exposure data were based on self-reported medication uses from ~320,000 European participants in the UK Biobank. The outcomes included GWAS results from 2824 cutaneous melanoma cases. Single-nucleotide polymorphisms (SNPs) significantly associated with medication use were used as instruments and analyzed with IVW, weighted median, weighted mode, and MR-Egger methods. Sensitivity analyses were used to assess pleiotropy and heterogeneity. Results: The analysis revealed that genetically predicted high use of adrenergics, inhalers, glucocorticoids, and opioids was suggestively associated with a reduced risk of cutaneous melanoma. Sensitivity analyses supported the robustness of these findings, showing no evidence of horizontal pleiotropy or influence from outliers. Conclusions: The results presented herein suggest that certain medication uses may lower the risk of developing cutaneous melanomas, offering potential new avenues for future prevention and treatment strategies.
1. Introduction
Cutaneous melanoma, the most aggressive type of skin cancer, arises from melanocytes and has seen a steady increase in incidence over recent decades. At a global scale, approximately 325,000 new cases of melanoma are reported annually [1]. Although melanoma constitutes only roughly 4% of all skin cancers, it accounts for 75% of skin cancer-related deaths [2]. Based on a 2019 Global Burden of Disease assessment, cutaneous melanoma ranked 16th out of 38 cancers in terms of disability-adjusted life years (DALYs) in the United States [3]. Despite advances in treatment [4], the disease burden imposed by melanoma remains substantial, underscoring the need to identify novel biomarkers to improve diagnosis, treatment, and prognosis.
The development of cutaneous melanoma is influenced by a complex interplay of genetic and environmental factors [5]. Among potential risk modifiers, medication use has emerged as an area of growing concern. Clinical observations and the results of epidemiological studies have suggested that some medications may alter melanoma risk; however, findings across different substances have been inconsistent. For instance, while the results of studies on β-blockers have suggested reduced metastasis, recurrence, and improved survival rates among cancer patients [6], the authors of other studies have not reported significant differences [7]. These inconsistencies highlight the need for in-depth investigations, particularly into medication effects specific to cutaneous melanoma.
Several medication classes have been examined to determine their impact on melanoma risk. Glucocorticoids, due to their anti-inflammatory effects, may theoretically reduce pro-carcinogenic inflammation and have been shown to inhibit melanoma cell proliferation at high doses [8]. Yet, long-term oral glucocorticoid use may slightly increase the risk of melanoma [9], and definitive evidence remains lacking. Opioids, commonly used for chronic pain, have been linked to increased risk of various cancers [10]. Although the results of some studies suggest that they may inhibit tumor angiogenesis through the suppression of VEGF signaling [11], their net effect on melanoma remains unclear. Non-steroidal anti-inflammatory drugs (NSAIDs) have demonstrated protective effects in some large cohort studies, with regular use associated with decreased skin cancer risk, including melanoma [12], possibly due to long-term suppression of tumor growth [13]. Statins, widely prescribed to treat hypercholesterolemia, may modulate inflammation and immune responses; however, their relationship with melanoma is controversial: the results of some cohort studies have demonstrated no significant effect [14], whereas the results of others have indicated increased risk with lipophilic statins and decreased risk of basal cell carcinoma with hydrophilic statins [15]. From the results presented above, while these medication classes have proven efficacy in other health contexts, their specific roles as risk or protective factors for cutaneous melanoma remain uncertain.
Given these conflicting findings, Mendelian randomization (MR) offers a powerful, unbiased approach to investigate potential causal relationships between medication use and melanoma risk. MR uses genetic variants as instrumental variables to proxy for exposures, enabling inference about causality while minimizing confounding and reverse causation [16]. MR has been increasingly applied in melanoma research to clarify the causal impact of various exposures on disease risk (see [17,18]). Recent MR studies have provided valuable insights into the genetic determinants of melanoma susceptibility and the causal roles of modifiable factors, emphasizing the value of this methodology in melanoma epidemiology.
In this context, we performed a two-sample MR analysis using publicly available GWAS datasets to systematically examine the potential causal associations between the use of 23 medication classes and the risk of cutaneous melanoma. We also conducted comprehensive sensitivity analyses to assess robustness. Through our findings, we aim to provide new perspectives on the prevention and management of cutaneous melanoma and to inform future research directions.
2. Materials and Methods
2.1. Research Framework
Our study follows the STrengthening the Reporting of Observational studies in Epidemiology using Mendelian Randomization (STROBE-MR) guidelines and is based on three core assumptions [19]:
- (1)
- Relevance: The genetic instruments (IVs) are strongly associated with the exposure (medication use).
- (2)
- Independence from confounders: The IVs are not associated with confounders of the exposure–outcome relationship.
- (3)
- Exclusion restriction: The IVs affect the outcome (cutaneous melanoma) only through the exposure, not via alternative pathways.
Given the potential for confounding by indication in medication use traits, we further screened instruments for pleiotropy using LDtrait (see Section 2.6) and performed sensitivity analyses to evaluate the robustness of the exclusion restriction assumption.
No further ethical approval was required because the present study was based on publicly available GWAS data.
2.2. Data Acquisition
GWAS summary statistics for medication use were obtained from published sources [20] covering 23 medication categories in approximately 132,367 to 320,000 UK Biobank participants. To minimize sample overlap, the primary GWAS for cutaneous melanoma was sourced from FinnGen Release 12 (C3_MELANOMA_SKIN_EXALLC: 5753 cases and 378,749 controls), which does not include UK Biobank participants. Secondary analyses were performed using a UK Biobank-based melanoma GWAS (GCST90041829: 2824 cases and 453,524 controls) (Supplementary Table S1) [21]. Sample overlap between exposure and outcome GWASs was assessed and minimized; FinnGen results are reported as primary.
2.3. Instrumental Variable Selection
SNPs associated with the genome-wide significance of medication use and minor allele frequency (MAF) > 0.01 were screened. The threshold should satisfy p < 5 × 10−8 [22]. Based on R2 < 0.001 and a window size = 10,000 kb standard, we eliminate the linkage disequilibrium effect (LD) between SNPs [23]. When the selected IV does not exist in the summary data of the outcome, SNPS that have a high LD (R2 > 0.8) with this IV will be replaced as proxy SNPs. The F-value of each SNP in IV was calculated to evaluate the intensity of IV and to exclude the possible weak instrumental variable bias between IV and exposure factors. The calculation formula was as follows: F = R2 × (N − 2)/(1 − R2), where R2 is the proportion of variation in exposure that can be explained by the SNP in IV, and the requirement for the F-value is >10. Allele harmonization was performed to align effect alleles between the exposure and outcome datasets. Palindromic SNPs (A/T or C/G) with ambiguous strand orientation and an MAF close to 0.5 were excluded. Detailed harmonization procedures are described in the Supplementary Methods. For exposures with fewer than three valid IVs after harmonization and LD pruning, they were excluded from MR analysis to ensure the robustness of causal inference.
2.4. MR Analyses
We performed an MR analysis to investigate the causal association between medication use and cutaneous melanomas. Four commonly used MR methods are used for features containing multiple IVs: the random-effects inverse variance weighted method (IVW), weighted mode, weighted median estimation (WME), and MR-Egger regression. IVW is the most important and weighted mode method to interpret the results of MR; it takes the inverse variance of each SNP as the weight to calculate the weighted average of the effect size [24]. Therefore, IVW was used as the main analysis method to evaluate the causal association between medication use exposure and the risk of cutaneous melanoma outcomes by calculating the odds ratio (OR) and 95% confidence interval (CI). In addition, the MR-Egger, WME, and weighted mode methods were used to test the robustness of the analysis results [25]. The MR-Egger method can provide an accurate estimation of the causal effect in the case of pleiotropic bias because it considers the existence of an intercept term. The WME method assumes the validity of half of the IV to analyze the causal association between exposure and outcome. IVW was considered the primary method; results from the MR-Egger, weighted median, and weighted mode were used for sensitivity assessment. Results with FDR-adjusted p < 0.05 were considered statistically significant; p < 0.05 without FDR correction was considered suggestive evidence only. Where applicable, MR-RAPS (Robust Adjusted Profile Score) and Radial MR were also used to assess sensitivity to outlying instruments and horizontal pleiotropy. Given the multiple comparisons across 23 medications, we controlled the false discovery rate (FDR) using the Benjamini–Hochberg procedure. Associations were considered statistically significant at FDR < 0.05. Nominally significant results (p < 0.05, FDR ≥ 0.05) were described as “suggestive associations”.
2.5. Sensitivity Analysis
Sensitivity analysis is used to detect potential pleiotropy that may be present in MR studies. In this analysis, Cochran’s Q test, MR-Egger regression, MR-PRESSO, and leave-one-out methods were used to evaluate potential pleiotropy. Heterogeneity among IVs was assessed using Cochran’s Q test [26], and p > 0.05 was considered to be low heterogeneity, indicating that the estimates between IVs vary randomly, and the effect on IVW results is not significant. Secondly, considering that the pleiotropy of genetic variation may have an impact on the estimation of an association effect, we adopted the MR-Egger regression method to investigate the presence or absence of horizontal pleiotropy and determine the existence of pleiotropy by the intercept term of MR-Egger regression (when it approaches 0 or has no statistical significance, indicating that there is no pleiotropy) [27]. In addition, we also used the MR-PRESSO method to detect the possible presence of outliers (SNPs with p < 0.05) [28]. Radial MR was used for outlier and influential point detection, and MR-RAPS was used for robust effect estimates in the presence of weak instruments or outliers. Outliers detected using these methods were iteratively removed until all of the MR-PRESSO global test p > 0.05, MR-Egger Q-test p > 0.05, and MR-Egger intercept p > 0.05 were satisfied.
2.6. Instrument Pleiotropy and Confounder Screening
For exposures with nominally significant MR results, all instrument SNPs (and proxies) were cross-referenced in LDtrait (window ± 500 kb, R2 = 1) to identify associations with potential confounders, particularly pigmentation, sun exposure, and immune-related traits.
2.7. Additional Analyses and Statistical Corrections
To assess the validity of the causal direction from medication use to cutaneous melanoma, we performed the Steiger directionality test, which compares the variance explained in exposure and outcome by the selected instruments. We estimated the statistical power of our MR analyses using the mRnd online calculator (https://shiny.cnsgenomics.com/mRnd/, (accessed on 13 September 2025)). Minimum detectable odds ratios for various instrument strengths (R2) are presented in Supplementary Table S2 to contextualize the findings. All analyses were performed using R version 4.3.3. Forest plots, scatter plots, and funnel plots were produced with the TwoSampleMR and ggplot2 packages.
3. Results
3.1. Selection of Instrumental Variables (IVs)
A total of 910 independent SNPs were selected as instrumental variables (IVs) across 23 medication use categories (Figure 1 for flow chart of the study design; Supplementary Table S1 for exposure GWAS details). The mean F-statistics for all exposures ranged from 33.3 to 229.7, exceeding the conventional threshold of 10, indicating adequate instrument strength (Supplementary Table S2). Palindromic SNPs with ambiguous strand orientation were excluded during harmonization, and proxies (R2 > 0.8) were used where necessary, as described in the Methods section. The full list of IVs, F-statistic distributions, and details of removed outlier SNPs are provided in Supplementary Table S3.
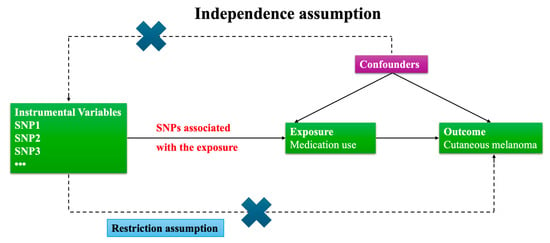
Figure 1.
Flow chart of the study design. This flow chart outlines the overall study design and analytical workflow of the Mendelian randomization analysis. The diagram summarizes the selection of medication use exposures and melanoma outcome GWAS datasets, the identification and quality control of instrumental variables (IVs), data harmonization, main MR analysis, sensitivity analyses, and interpretation of the results.
Given the relatively modest number of melanoma cases in the GWAS datasets (FinnGen: 5753 cases; UK Biobank: 2824 cases), and the generally low variance explained (R2) for medication use by the genetic instruments, statistical power to detect modest effect sizes was limited for most exposures. For example, for agents acting on the renin–angiotensin system (R2 ≈ 0.07), statistical power for an OR of approximately 1.09 did not exceed 20% (Supplementary Table S2 for minimum detectable ORs and power for each exposure–outcome pair). To improve robustness, primary analyses were conducted using FinnGen melanoma GWAS data, with additional results for UK Biobank cutaneous melanoma. Exposures with fewer than 5 SNPs as IVs (e.g., opioids) are interpreted as exploratory.
3.2. Mendelian Randomization Analysis
The MR analysis revealed several suggestive associations between genetically predicted medication use and melanoma outcomes (Table 1, Figure 2). In the FinnGen dataset, genetic liability to the use of agents acting on the renin–angiotensin system (C09) was suggestively associated with an increased risk of malignant melanoma. The IVW method estimated an OR of 1.10 (95% CI: 1.03–1.17, p = 0.0059, FDR = 0.0712, Supplementary Figure S1A–E), and this finding was consistent with the robust adjusted profile score (RAPS) method (OR = 1.10, 95% CI: 1.02–1.18, p = 0.0087). Diuretics (C03) also showed a suggestive association with higher malignant melanoma risk (IVW OR = 1.07, 95% CI: 1.00–1.15, p = 0.0449, FDR = 0.3138, Supplementary Figure S2A–E), though this did not reach the threshold for FDR significance. In contrast, thyroid preparations (H03A) were suggestively associated with a decreased risk of malignant melanoma (IVW OR = 0.96, 95% CI: 0.92–1.00, p = 0.0499, FDR = 0.3138), with other MR methods yielding consistent but non-significant results. Notably, the use of diabetes drugs (A10) was associated with a reduced risk of malignant melanoma (IVW OR = 0.91, 95% CI: 0.86–0.97, p = 0.0018, FDR = 0.0712, Supplementary Figure S3A–E), and this finding was supported by the RAPS method (OR = 0.91, 95% CI: 0.86–0.97, p = 0.0024).

Table 1.
Mendelian randomization results for all exposures and melanoma outcomes.
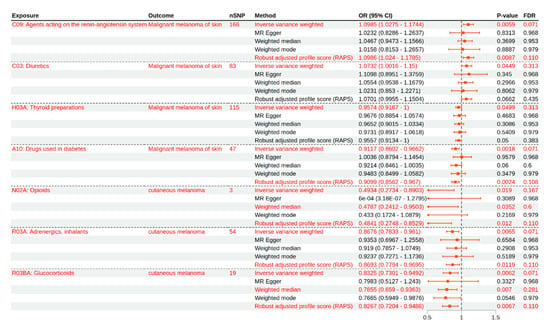
Figure 2.
Mendelian randomization estimates for the associations between medication use and melanoma risk. This forest plot presents the odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between genetically predicted medication use (by class) and melanoma outcomes, as estimated using different MR methods. The results are shown for the primary inverse variance weighted (IVW) analysis, in addition to the MR-Egger, weighted median, weighted mode, and robust adjusted profile score (RAPS) methods. Associations reaching suggestive significance after FDR correction are highlighted. Data are shown for both FinnGen (malignant melanoma of the skin) and UK Biobank (cutaneous melanoma) outcomes.
For cutaneous melanoma outcomes in the UK Biobank dataset, several exposures demonstrated suggestive inverse associations. Genetically predicted higher use of adrenergics, inhalants (R03A), was associated with a lower risk of cutaneous melanoma (IVW OR = 0.87, 95% CI: 0.78–0.96, p = 0.0065, FDR = 0.0712, Supplementary Figure S4A–D), and this finding was corroborated by the RAPS method (OR = 0.84, 95% CI: 0.72–0.99, p = 0.0384). Similarly, genetically predicted glucocorticoid use (R03BA) was suggestively associated with a decreased risk of cutaneous melanoma (IVW OR = 0.86, 95% CI: 0.73–0.95, p = 0.0062, FDR = 0.0712, Supplementary Figure S5A–D). This association remained in the weighted median (OR = 0.77, 95% CI: 0.60–0.98, p = 0.0454) and RAPS (OR = 0.83, 95% CI: 0.72–0.95, p = 0.0067) analyses. For opioid use (N02A), although only three SNPs were available as instruments, higher genetically predicted exposure was suggestively associated with a reduced risk of cutaneous melanoma (IVW OR = 0.49, 95% CI: 0.27–0.89, p = 0.019, FDR = 0.167, Supplementary Figure S6A–D), with similar results from the weighted median (OR = 0.48, 95% CI: 0.26–0.89, p = 0.0206). However, given the limited number of IVs, this finding should be interpreted as exploratory.
Overall, after correction for multiple testing, the most consistent suggestive evidence was observed for increased risk of malignant melanoma with genetic liability to renin–angiotensin system agents and decreased risk of cutaneous melanoma with genetic liability to adrenergics, inhalants, and glucocorticoids. The results obtained using the different MR methods were generally consistent in direction, and all of the main findings are detailed in Table 1 and visualized in Figure 2. For exposures with fewer SNPs, such as opioids, the results require cautious interpretation due to limited instrument strength.
3.3. Sensitivity and Pleiotropy Analysis
Sensitivity analyses were conducted to assess the heterogeneity and horizontal pleiotropy across all primary associations (Table 1, Table 2 and Table 3). Heterogeneity was evaluated using Cochran’s Q statistic, whereas horizontal pleiotropy was assessed by the MR-Egger intercept. After outlier removal, no significant heterogeneity (Cochran’s Q p > 0.05) or horizontal pleiotropy (MR-Egger intercept p > 0.05) was detected for any main association (Table 2 and Table 3). For exposures with fewer than five SNPs, such as opioids, these tests have limited power, and the results should be interpreted with caution.

Table 2.
MR-PRESSO results for main exposures and melanoma outcomes after outlier removal.

Table 3.
Sensitivity analyses after outlier removal.
The robustness of the main positive findings was further supported by a series of sensitivity plots, including scatter, forest, funnel, and leave-one-out analyses, as presented in Supplementary Figures S1–S6. The Steiger directionality test further confirmed that the inferred causal direction was from medication use to melanoma risk for all exposure–outcome pairs (all p < 1 × 10−5; Table 4). In addition, MR-RAPS and Radial MR analyses demonstrated effect estimates that were consistent in direction and magnitude with those from the IVW and weighted median/mode methods (Table 3). For exposures with significant outliers, the effect sizes remained stable after outlier removal, supporting the robustness of the primary results.

Table 4.
Steiger directionality test for each exposure–outcome pair.
4. Discussion
In this study, we used MR to explore the potential causal associations between genetic liability to specific medication use and the risk of cutaneous melanoma. Our findings provide suggestive evidence that genetic liability to the use of certain medications—particularly agents acting on the renin–angiotensin system, diuretics, thyroid preparations, and diabetes drugs in the FinnGen dataset and adrenergics, inhalants, glucocorticoids, and opioids in the UK Biobank dataset—may be associated with melanoma risk. However, these associations should be interpreted as exploratory and hypothesis-generating, rather than definitive evidence of causality, due to various limitations, including genetic instrument strength, potential confounding, and the modest effect sizes observed.
By leveraging both FinnGen and UK Biobank GWAS datasets, we sought to strengthen the robustness and generalizability of our results. Notably, the FinnGen-based associations involved medications typically prescribed for cardiovascular or metabolic conditions (e.g., agents acting on the renin–angiotensin system, diuretics, thyroid preparations, and diabetes drugs); in comparison, the UK Biobank-based associations were observed for medications more commonly used for pain or respiratory diseases (e.g., opioids, adrenergics, inhalants, and glucocorticoids). This lack of overlap between significant associations in the two datasets may reflect differences in phenotype definitions, comorbidity patterns, population genetic background, and environmental exposures, in addition to limitations in statistical power.
It is crucial to emphasize the relevance and strength of the genetic instruments used in MR. In our analysis, the average F-statistics for all exposures exceeded 10, suggesting adequate instrument strength and reducing the risk of weak instrument bias. However, for some exposures—particularly those with fewer than five SNPs (e.g., opioids)—the power to detect and correct for pleiotropy was limited, and such findings must be considered exploratory. Our power calculations, based on mRnd, highlight the limited ability to detect small effect sizes given the available sample sizes and instrument strength.
Our FinnGen-based results suggest that genetic liability to the use of agents acting on the renin–angiotensin system, diuretics, thyroid preparations, or diabetes drugs may be associated with melanoma risk. These medications are commonly prescribed for cardiovascular and metabolic diseases—conditions that themselves may be linked with skin cancer risk through shared risk factors (e.g., obesity, chronic inflammation, and metabolic dysregulation). The observed associations could be influenced by underlying disease liability or by pleiotropic genetic effects and thus require cautious interpretation and further validation.
For adrenergics, inhalants, and glucocorticoids—primarily used in asthma—our findings are consistent with some preclinical studies suggesting potential biological pathways linking these medications and melanoma biology [29,30,31,32,33,34]. For example, β2-adrenergic receptor agonists can modulate intracellular cAMP in melanoma cells [31], and glucocorticoids may affect cell survival and immune interactions [8,35]. However, these findings from cell lines or animal models should not be assumed to translate directly to human population effects; cultured cells behave differently than cells in vivo, and results at the population level are subject to complex confounding.
With opioids, the observed association (in the UK Biobank dataset) was based on only three SNPs and should be regarded as exploratory. While the results of previous studies have suggested that opioid use may be linked to cancer risk through immune modulation or angiogenesis inhibition [10,11,36], its clinical relevance for melanoma is uncertain; thus, further research is needed.
It is well established that the vast majority of melanomas occur in individuals with fair skin (low melanin), high nevus counts, or a genetic predisposition, particularly those with substantial ultraviolet (UV) exposure or severe sunburns [37]. Environmental and inherited factors remain the dominant determinants of melanoma risk [38]. The potential impact of medication use on melanoma risk is likely to be much smaller than these major risk factors [39]. With our findings, we aim to uncover new, potentially modifiable or targetable biological pathways beyond the primary environmental and genetic determinants, rather than to suggest that medication use is a dominant factor.
Interpretation of these associations must be cautious, as medication use often reflects underlying disease states (e.g., asthma, cardiovascular disease, diabetes, and chronic pain), which themselves may influence melanoma risk through shared mechanisms or confounding [40]. Rates of diseases such as respiratory disorders, cardiovascular disease, diabetes, obesity, and orthopedic or autoimmune conditions can affect both the likelihood of medication exposure and the baseline melanoma risk [41]. Social habits (e.g., outdoor activity, sun protection, and occupational exposures) and unmeasured environmental factors may also play crucial roles. Our MR approach partially addresses confounding but cannot fully disentangle these complex relationships, particularly with polygenic traits and pleiotropic loci.
It is critical to underscore that MR estimates reflect the effect of genetic liability to medication use, not the direct pharmacologic effect of actual drug exposure. The findings of this study must not be interpreted as a recommendation to use these medications for melanoma prevention. Both glucocorticoids and opioids have well-documented and potentially serious side effects, including immunosuppression, metabolic disturbances, addiction, and increased risk of infection or other cancers [42]. The goal of this research is to identify biological pathways that may be targeted in the future by safer interventions, not to advocate for increased use of these specific drugs.
Our study population consisted of individuals of broadly European ancestry. However, there is substantial heterogeneity within European populations regarding skin pigmentation, nevus density, sun sensitivity, environmental exposures, and social habits. Individuals of Northern European descent (e.g., English, Irish, Scottish, and Scandinavian) with lighter skin are at the highest melanoma risk due to both genetic and environmental factors [41]. In our analysis, we could not stratify by these finer subgroups, and the findings may not generalize to non-European or admixed populations. More diverse studies are needed to understand effect modification by ancestry, skin type, and environment.
The two-sample MR design used in this study leveraged data from large-scale GWASs, effectively excluding confounding factors and providing relatively unbiased causal evidence [16]. However, the study has its limitations. First, the number of IVs was limited for certain medication exposures (e.g., opioids, with only three SNPs), resulting in reduced statistical power and restricting the ability to robustly assess heterogeneity and horizontal pleiotropy using sensitivity analyses. For all exposures with fewer than five IVs, the findings should be interpreted as exploratory rather than confirmatory. Second, while we applied multiple robust MR methods (including random-effects IVW, MR-Egger, and MR-PRESSO), the accuracy of pleiotropy detection and correction is inherently limited when the number of instruments is low. Third, our analyses rely solely on bioinformatic approaches using publicly available GWAS summary statistics and are not accompanied by experimental or mechanistic validation. Future studies integrating laboratory-based functional assays or clinical data are needed to support and clarify the biological mechanisms underlying these genetic associations. Fourth, medication use was primarily self-reported in the original GWAS, which may introduce misclassification or recall bias and reduce the reliability of exposure data.
Fifth, although the GWAS data were described as being from individuals of European ancestry, there is considerable heterogeneity within European populations in terms of skin pigmentation, environmental exposures (e.g., UV radiation), and social habits, all of which may influence melanoma risk. In our study, we were unable to account for these internal population differences, and the findings may not generalize to non-European or more diverse populations. Lastly, while the random-effects IVW model can accommodate some degree of heterogeneity, results for exposures or outcomes with significant heterogeneity should still be interpreted with caution.
The authors of future studies should aim to validate these findings in larger, more diverse cohorts and ideally incorporate prospective or experimental approaches to provide mechanistic insights. Stratified analyses by finer ancestry groups, skin type, and environmental factors would improve our understanding of gene–environment interactions. Ultimately, a deeper understanding of these associations could help develop personalized medicine strategies to reduce melanoma risk without compromising treatment efficacy.
5. Conclusions
In conclusion, through this study, we provide new evidence of a potential causal association between medication use and the risk of cutaneous melanoma, particularly with adrenergics, inhalants, glucocorticoids, and opioids. These findings not only offer new biological insights into the association between medication use and cutaneous melanoma but also provide possible targets for developing new prevention and treatment strategies. The authors of future studies should explore in greater depth the mechanisms of action of these medications and validate their effectiveness and safety in clinical practice. Such efforts are critical for guiding clinical recommendations and improving patient outcomes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines13102477/s1. Table S1. Detailed information on the GWAS data; Table S2. Information on the instrument variable (IV). Table S3. Detailed SNPs and their statistical information. STROBE-MR checklist: Title: STROBE-MR checklist of recommended items to address in reports of Mendelian randomization studies. Supplementary Figure S1. (A–E) Sensitivity analyses for the association between genetically predicted use of agents acting on the renin–angiotensin system and malignant melanoma of skin (FinnGen). (A) Scatter plot of SNP effects; (B) forest plot of individual SNP estimates; (C) funnel plot for heterogeneity; (D) leave-one-out analysis; (E) radial plot for outlier estimation. These plots demonstrate the robustness of the causal estimate after outlier removal. Supplementary Figure S2. (A–E) Sensitivity analyses for the association between genetically predicted use of thyroid preparations and malignant melanoma of skin (FinnGen). (A) Scatter plot of SNP effects; (B) forest plot of individual SNP estimates; (C) funnel plot for heterogeneity; (D) leave-one-out analysis; (E) radial plot for outlier estimation. These plots demonstrate the consistency and robustness of the causal estimate after outlier removal. Supplementary Figure S3. (A–E) Sensitivity analyses for the association between genetically predicted use of drugs used in diabetes and malignant melanoma of skin (FinnGen). (A) Scatter plot of SNP effects; (B) forest plot of individual SNP estimates; (C) funnel plot for heterogeneity; (D) leave-one-out analysis; (E) radial plot for outlier estimation. These plots demonstrate the robustness of the causal estimate after outlier removal. Supplementary Figure S4. (A–D) Sensitivity analyses for the association between genetically predicted use of adrenergics, inhalants, and cutaneous melanoma (UK Biobank). (A) Scatter plot of SNP effects; (B) forest plot of individual SNP estimates; (C) funnel plot for heterogeneity; (D) leave-one-out analysis. These plots support the robustness of the results. Supplementary Figure S5. (A–D) Sensitivity analyses for the association between genetically predicted glucocorticoid use and cutaneous melanoma (UK Biobank). (A) Scatter plot of SNP effects; (B) forest plot of individual SNP estimates; (C) funnel plot for heterogeneity; (D) leave-one-out analysis. These plots support the robustness of the results. Supplementary Figure S6. (A–D) Sensitivity analyses for the association between genetically predicted opioid use and cutaneous melanoma (UK Biobank). (A) Scatter plot of SNP effects; (B) forest plot of individual SNP estimates; (C) funnel plot for heterogeneity; (D) leave-one-out analysis. Due to the limited number of SNPs, this result should be interpreted as exploratory.
Author Contributions
Conceptualization, H.W., L.Z., Q.Z., T.X., and D.Z.; methodology, H.W., L.Z., T.X., and D.Z.; investigation, H.W., L.Z., and J.S.; writing—original draft preparation, H.W., and L.Z.; writing—review and editing, Q.Z., M.T., K.T., H.M., T.X., and D.Z.; supervision, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from Chengdu Municipal Health Commission (2024025) and the Key Research and Development Project of Sichuan Provincial Science and Technology Department (2023YFS0311; 2022YFS0310).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files. GWAS summary statistics for medication use were obtained from Wu et al. [20], and those for melanoma were obtained from the GWAS Catalog (GCST90041829) and FinnGen Release 12 (https://r12.finngen.fi/, (accessed on 20 September 2025)). The harmonized datasets and analysis scripts (including the code for TwoSampleMR v4.0.5) are available at https://github.com/phoenixwhy/my-project?tab=readme-ov-file#my-project (accessed on 20 September 2025).
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
The following abbreviations are used in this manuscript:
| (GWASs) | Genome-Wide Association Studies |
| (MR) | Mendelian randomization |
| (DALYs) | Disability-adjusted life years |
| (NSAIDs) | Nonsteroidal anti-inflammatory medications |
| (COX) | Cyclooxygenase |
| (IVW) | Inverse variance weighted method |
| (WME) | Weighted median estimation |
| (GORD) | Gastro-esophageal reflux disease |
References
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Aggarwal, P.; Knabel, P.; Fleischer, A.B., Jr. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J. Am. Acad. Dermatol. 2021, 85, 388–395. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Zappalà, A.R.; Passarelli, F.; Ricci, F.; Abeni, D.; Michelozzi, P. Early inflammatory biomarkers and melanoma survival. Int. J. Dermatol. 2023, 62, 752–758. [Google Scholar] [CrossRef]
- Caini, S.; Gandini, S.; Sera, F.; Raimondi, S.; Fargnoli, M.C.; Boniol, M.; Armstrong, B.K. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinico-pathological variant. Eur. J. Cancer 2009, 45, 3054–3063. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Zänker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Musselman, R.P.; Bennett, S.; Li, W.; Mamdani, M.; Gomes, T.; van Walraven, C.; Boushey, R.; Al-Obeed, O.; Al-Omran, M.; Auer, R.C. Association between perioperative beta blocker use and cancer survival following surgical resection. Eur. J. Surg. Oncol. 2018, 44, 1164–1169. [Google Scholar] [CrossRef]
- Dobos, J.; Kenessey, I.; Tímár, J.; Ladányi, A. Glucocorticoid receptor expression and antiproliferative effect of dexamethasone on human melanoma cells. Pathol. Oncol. Res. 2011, 17, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Thomsen, H.F.; Engebjerg, M.C.; Olesen, A.B.; Friis, S.; Karagas, M.R.; Sørensen, H.T. Use of oral glucocorticoids and risk of skin cancer and non-Hodgkin’s lymphoma: A population-based case-control study. Br. J. Cancer 2009, 100, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lin, J.A.; Chang, C.L.; Wu, S.Y.; Zhang, J. Association between long-term opioid use and cancer risk in patients with chronic pain: A propensity score-matched cohort study. Br. J. Anaesth. 2022, 129, 84–91. [Google Scholar] [CrossRef]
- Yamamizu, K.; Furuta, S.; Hamada, Y.; Yamashita, A.; Kuzumaki, N.; Narita, M.; Doi, K.; Katayama, S.; Nagase, H.; Yamashita, J.K.; et al. κ Opioids inhibit tumor angiogenesis by suppressing VEGF signaling. Sci. Rep. 2013, 3, 3213. [Google Scholar] [CrossRef]
- Johannesdottir, S.A.; Chang, E.T.; Mehnert, F.; Schmidt, M.; Olesen, A.B.; Sørensen, H.T. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: A population-based case-control study. Cancer 2012, 118, 4768–4776. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Motilva, V.; Martorell-Calatayud, A.; Nagore, E. Non-steroidal anti-inflammatory drugs and melanoma. Curr. Pharm. Des. 2012, 18, 3966–3978. [Google Scholar] [CrossRef]
- Al Rahmoun, M.; Ghiasvand, R.; Cairat, M.; Mahamat-Saleh, Y.; Cervenka, I.; Severi, G.; Boutron-Ruault, M.C.; Robsahm, T.E.; Kvaskoff, M.; Fournier, A. Statin Use and Skin Cancer Risk: A Prospective Cohort Study. J. Investig. Dermatol. 2022, 142, 1318–1325.e5. [Google Scholar] [CrossRef]
- Wang, D.; Dai, S.; Lou, D.; Wang, T.; Wang, S.; Zheng, Z. Association between statins exposure and risk of skin cancer: An updated meta-analysis. Int. J. Dermatol. 2023, 62, 1332–1344. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Li, Y.; Li, Q.; Cao, Z.; Wu, J. Multicenter proteome-wide Mendelian randomization study identifies causal plasma proteins in melanoma and non-melanoma skin cancers. Commun. Biol. 2024, 7, 857. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Nakayama, T.; Asaba, K. Systematic proteome-wide Mendelian randomization to prioritize causal plasma proteins for skin cancers. Commun. Biol. 2024, 7, 1681. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Wu, Y.; Byrne, E.M.; Zheng, Z.; Kemper, K.E.; Yengo, L.; Mallett, A.J.; Yang, J.; Visscher, P.M.; Wray, N.R. Genome-wide association study of medication-use and associated disease in the UK Biobank. Nat. Commun. 2019, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zheng, Z.; Fang, H.; Yang, J. A generalized linear mixed model association tool for biobank-scale data. Nat. Genet. 2021, 53, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Zhao, Q.; Lawlor, D.A.; Sheehan, N.A.; Thompson, J.; Davey Smith, G. Improving the accuracy of two-sample summary-data Mendelian randomization: Moving beyond the NOME assumption. Int. J. Epidemiol. 2019, 48, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Ong, J.S.; MacGregor, S. Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner’s perspective. Genet. Epidemiol. 2019, 43, 609–616. [Google Scholar] [CrossRef]
- Demenais, F.; Margaritte-Jeannin, P.; Barnes, K.C.; Cookson, W.O.C.; Altmüller, J.; Ang, W.; Barr, R.G.; Beaty, T.H.; Becker, A.B.; Beilby, J.; et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat. Genet. 2018, 50, 42–53. [Google Scholar] [CrossRef]
- Yim, R.P.; Koumbourlis, A.C. Tolerance & resistance to β2-agonist bronchodilators. Paediatr. Respir. Rev. 2013, 14, 195–198. [Google Scholar] [CrossRef]
- Matarrese, P.; Maccari, S.; Ascione, B.; Vona, R.; Vezzi, V.; Stati, T.; Grò, M.C.; Marano, G.; Ambrosio, C.; Molinari, P. Crosstalk between β2- and α2-Adrenergic Receptors in the Regulation of B16F10 Melanoma Cell Proliferation. Int. J. Mol. Sci. 2022, 23, 4634. [Google Scholar] [CrossRef]
- Switzer, B.; Puzanov, I.; Gandhi, S.; Repasky, E.A. Targeting beta-adrenergic receptor pathways in melanoma: How stress modulates oncogenic immunity. Melanoma Res. 2024, 34, 89–95. [Google Scholar] [CrossRef]
- Yao, C.Y.; Chen, F. β2AR is a potential biomarker for prognosis of malignant melanoma. Genom. Appl. Biol. 2019, 38, 4800–4805. [Google Scholar]
- Barnes, P.J. Inhaled Corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Oriol-Caballo, M.; Moreno-Murciano, P.; Estrela, J.M. Survival Mechanisms of Metastatic Melanoma Cells: The Link between Glucocorticoids and the Nrf2-Dependent Antioxidant Defense System. Cells 2023, 12, 418. [Google Scholar] [CrossRef]
- Gupta, M.A.; Gupta, A.K.; Vujcic, B.; Piccinin, M. Use of opioid analgesics in skin disorders: Results from a nationally representative US sample. J. Dermatol. Treat. 2015, 26, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Tasdogan, A.; Sullivan, R.J.; Katalinic, A.; Lebbe, C.; Whitaker, D.; Puig, S.; van de Poll-Franse, L.V.; Massi, D.; Schadendorf, D. Cutaneous melanoma. Nat. Rev. Dis. Primers 2025, 11, 23. [Google Scholar] [CrossRef]
- Davey, M.G.; Miller, N.; McInerney, N.M. A Review of Epidemiology and Cancer Biology of Malignant Melanoma. Cureus 2021, 13, e15087. [Google Scholar] [CrossRef]
- Yang, B.; Wang, H.; Song, W.; Feng, J.; Hou, S. Lipid-lowering medications and risk of malignant melanoma: A Mendelian randomization study. Front. Oncol. 2024, 14, 1408972. [Google Scholar] [CrossRef]
- Li, S.; Du, H.; An, K.; He, L.; Li, J.; Li, S. Values and preferences of medication use in patients for primary and secondary prevention of cardiovascular diseases: A mixed-methods exploratory study. Chin. General. Pract. J. 2024, 1, 100022. [Google Scholar] [CrossRef]
- Garg, R.K. The alarming rise of lifestyle diseases and their impact on public health: A comprehensive overview and strategies for overcoming the epidemic. J. Res. Med. Sci. 2025, 30, 1. [Google Scholar] [CrossRef]
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Freedman, C.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018, 124, 3706–3714. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).