Abstract
Foodborne illnesses remain a global challenge, requiring rapid and sensitive detection platforms. We developed a magnetosome-based electrochemical immunosensor for lipopolysaccharide (LPS) antigens from Escherichia coli and Salmonella typhimurium. Magnetosomes isolated from Magnetospirillum sp. RJS1 were characterized by HR-TEM and functionalized with antibodies (2 CFU mL−1), with FTIR confirming successful conjugation. The antibody–magnetosome complexes were immobilized on a chitosan/glutaraldehyde-modified glassy carbon electrode. AFM revealed globular (200–700 nm) and island-like (1–3 µm) features after antigen binding. Electrochemical impedance spectroscopy showed stepwise increases in charge-transfer resistance upon electrode modification and antigen interaction. The sensor exhibited high sensitivity toward E. coli (3–7 CFU mL−1) and Salmonella (3–8 CFU mL−1), achieving an immune sensitivity of 36.24 Ω/CFU mL−1 and a detection limit of 1 CFU mL−1. These results demonstrate the potential of magnetosome-based immunosensors as portable, efficient platforms for the rapid detection of foodborne pathogens in real samples.
1. Introduction
Foodborne pathogens such as Escherichia coli, Staphylococcus aureus, Campylobacter jejuni, Listeria monocytogenes, and Salmonella typhimurium are globally prevalent and responsible for a wide range of illnesses, including diarrhea, hemorrhagic colitis, meningitis, and kidney failure causative to the acute poisoning through chemical contaminants entering the body [1,2,3,4,5]. Among foodborne pathogens, E. coli and S. typhimurium are recognized as major contributors for prolonged illness globally affecting every 1 in 10 individuals annually resulting in 420,000 deaths approximately which is an associated economic burden of nearly US$ 110 billion [6,7,8,9]. In India, the Ministry of Health and Welfare identified non-O157 enterohemorrhagic E. coli (EHEC) from stool samples of patients with bloody diarrhea in Kolkata, and additional EHEC O157 cases with sorbitol-negative phenotypes were reported in water samples collected from the Ganges River in Varanasi [10,11,12]. An integrated report states that food contaminants are majorly attained from heavy metals, microbes, mycotoxins, microplastics, adulteration with common sources of E. coli, S. typhimurium infections, aflatoxins through poultry, beef, pork, oil, spices and turkey [13,14,15,16,17,18].
Bacterial pathogens such as Escherichia coli and Listeria monocytogenes are traditionally detected using conventional microbiological assays, including culture methods, the Complement Fixation Test (CFT), Rose Bengal Test (RBT), and Serum Agglutination Test (SAT). While these techniques are standardized and widely applied, they are often time-consuming (24–72 h), require skilled personnel, and achieve detection limits typically in the range of 103–105 CFU/mL. Molecular methods such as polymerase chain reaction (PCR) and quantitative PCR provide higher sensitivity (10–100 CFU/mL) with improved specificity but demand sophisticated laboratory infrastructure and complex sample preparation. Optical biosensors, such as surface plasmon resonance (SPR) and fluorescence-based assays, have also been employed for foodborne pathogen detection, achieving sensitivities down to 10–102 CFU/mL, yet remain limited by cost, bulky instrumentation, and susceptibility to matrix interference [19,20,21,22]. In comparison, electrochemical immunosensors offer a unique balance of sensitivity, rapidity, and portability. Recent studies have demonstrated detection limits as low as 1–10 CFU/mL for E. coli and Listeria using nanomaterial-modified electrodes. Magnetosome-based sensors combine the intrinsic biocompatibility and functional surface chemistry of biogenic nanoparticles with magnetic separation capability and enhanced electron transfer, thus providing a novel platform for ultra-sensitive and field-deployable pathogen detection [8,23,24,25]. The use of nanoparticles, particularly magnetic ones, in sensor design has proven to significantly enhance signal transduction and biomolecule immobilization. However, direct conjugation of chemically synthesized nanoparticles to biomolecules often necessitates complex linker chemistry [26,27,28,29]. Magnetosomes, biogenic magnetic nanoparticles synthesized through biomineralization in magnetotactic bacteria, over chemically synthesized counterparts. These naturally produced nanoparticles exhibit uniform cubo-octahedral shapes, narrow size distributions are enveloped in a lipid bilayer membrane containing approximately primary amine groups ideal for covalent coupling with biomolecules. Magnetosomes have previously been employed in a variety of immunochemical detection platforms due to their unique biogenic origin and surface properties. Their lipid bilayer provides reactive functional groups (–NH2, –OH, –COOH) that enable direct covalent conjugation of antibodies, enzymes, or nucleic acids without the need for extensive chemical modification. For example, magnetosome–antibody conjugates have been reported for the immunodetection of bacterial pathogens such as Salmonella and Listeria, as well as for the immobilization of enzymes like lipase for catalytic and environmental applications. In these studies, magnetosomes served not only as stable nanocarriers but also as magnetic separation tools, improving sensitivity by concentrating analytes from complex matrices. Compared to synthetic magnetic nanoparticles, magnetosomes offer superior uniformity, biocompatibility, and stability of functionalization. These advantages provide the basis for their integration into electrochemical immunosensors, where enhanced bioreceptor immobilization and electron-transfer mediation are critical for achieving high sensitivity and selectivity [30,31,32]. The use of biogenic magnetosomes not only improves immobilization efficiency but also enhances electrochemical sensing performance, exhibits a promising strategy for rapid and sensitive detection of foodborne pathogens. This magnetosome-based electrochemical immunosensor combines the unique biogenic properties of magnetosomes with CNT–chitosan hybrid electrodes, offering significant advantages in sensitivity, biocompatibility, and magnetic handling compared to conventional assays and synthetic nanomaterial-based sensors. However, challenges such as scalability of magnetosome production, batch variability, and long-term stability remain to be addressed before large-scale deployment. The lipopolysaccharide (LPS) layer in bacterial contaminants contains a highly conserved core region and pathogen-specific O-antigens that play a critical role in host–pathogen interactions and disease manifestation [33]. Hence, rapid and sensitive detection of LPS is pivotal for ensuring food safety. Furthermore, the reports on utilizing magnetosomes immobilized biological entity based electrochemical sensors were used to sense Salmonella, E. coli relying particularly in water and milk samples [34,35,36]. Recent advances in the field underscore the growing role of electrochemical biosensors for food safety, owing to their speed, sensitivity, and potential for in situ analysis [37,38]. Among these, magnetosome-based platforms have shown particularly promising performance: Sannigrahi et al. demonstrated magnetosome–antibody conjugates enabling rapid, specific detection of Salmonella, Listeria, and E. coli—achieving detection limits down to ~10 CFU/mL even in complex food matrices [35]. Reviews of magnetosomes highlight their uniform nanoscale morphology, lipid-bilayer surface chemistry, and magnetic and conductive properties as key advantages for biosensing [39]. Moreover, iron-based magnetic nanoparticles enhance biosensor sensitivity and enable integration with portable and microfluidic platforms [40]. Finally, chitosan–glutaraldehyde interfaces remain a foundational immobilization strategy, providing robust film formation and reactive anchoring for bioreceptors [41].
In this study, we report on the development of a magnetosome-based, label-free impedimetric biosensor coated on the surface of glassy carbon with biomaterials such as chitosan coated with carbon nanotubes and glutaraldehyde acting as a signal transducer for the detection and quantification of LPS antigens from Escherichia coli (O55:B5) and Salmonella typhimurium (ATCC 7823).
2. Experimental Section
2.1. Materials and Reagents
Potassium ferrocyanide (K4Fe(CN)6), potassium ferricyanide (K3Fe(CN)6) and potassium nitrate (KNO3) were purchased from Merck, Gangnam-gu, Seoul, Republic of Korea. Chitosam, Glutaraldehyde, Carbon Nanotubes (CNTs) were procured from Sigma Aldrich, Bangalore, Karnataka India. The neodymium magnets were purchased from Dura Magnets Pvt. Ltd. Maharashtra, India. The buffers and solutions used in this study were prepared as follows: PBS buffer (10 mM, pH 7.4), blocking buffer (1% BSA in PBS buffer), and electrolyte solution (1 mM K4Fe(CN)6, 1 mM K3Fe(CN)6 and 0.1 M KNO3). All solutions were prepared with deionized water in Merck Millipore system.
The primary antibody (Anti-Salmonella antibody, ab35156) was purchased from Abcam, Nagpur, Maharashtra India. Lipopolysaccharide from Escherichia coli (O55: B5) was purchased from Santa Cruz Biotechnology, Texas, USA. Lipopolysaccharide from Salmonella typhimurium (ATCC-7823) was purchased from Sigma Aldrich, Bangalore, Karnataka, India.
2.2. Culturing of Magnetosomes Extraction and Characterization
The Magnetotactic bacteria Magnetospirillum sp. RJS1 was cultured in Magnetospirillum specific medium (MSGM) by following Hungate anaerobic technique [42]. Introduced the Hungate technique, a strict anaerobic culture method that allows growth and study of oxygen-sensitive microorganisms. This method became foundational in microbiology, widely used for cultivating anaerobes, including magnetotactic bacteria (Magnetospirillum) in modern research. Proper monitoring of magnetosomes production inside the magnetotactic bacteria was regularly performed [43,44]. Sediment samples collected → enrichment using magnetic racetrack method → isolation of magnetotactic bacteria. Characterized via HR-TEM, and magnetosome morphology. Isolates belonged to Magnetospirillum spp., producing uniform magnetosomes of 1 mg/mL (nano-sized magnetite crystals). Magnetosomes extracted from Magnetospirillum were conjugated with lipase enzyme; characterization performed by FTIR and enzyme activity assays. Highlighted magnetosomes as eco-friendly, reusable, and efficient nanomaterials for enzyme immobilization, opening applications in biocatalysis and biosensors. Demonstrated the potential of saline lagoons as novel sources of magnetotactic bacteria for biotechnological and biosensing applications. The extracted magnetosomes were then characterized through HR-TEM (JEOL JEM2100, Tokyo, Japan) operating at 200 kv. For TEM analysis, the dispersed magnetosome suspension was dispersed in water and directly drop-cast onto copper grids and observed under the microscope. The FT-IR measurements were performed with Bruker INVENIO model, and the liquid samples were directly measured.
2.3. Magnetosomes Immobilization with Antibody
Magnetosomes (2 mg) were added in phosphate-buffer saline and sonicated for 10 min to disperse it. 100 μL of Anti-Salmonella antibody (0.8 µg/mL) was added to 900 μL of magnetosomes solution and incubated at 4 °C for 24 h. A neodymium magnet was used to accumulate the magnetosomes- anti-Salmonella antibody complex and further washing process were followed with phosphate-buffer saline containing 1% bovine serum albumin (BSA) 10–12 times. In this study, lipopolysaccharides of E. coli O55:B5, and S. typhimurium, a clinically relevant foodborne pathogen, were used as the target organism to evaluate the performance of the electrochemical biosensor. This strain was selected due to its pathogenic significance and well-characterized antigenic profile, which makes it a widely accepted model for validating sensitivity, specificity, and limit of detection in food safety applications. The binding of the antibody with magnetosomes was characterized using FT-IR analysis.
2.4. Preparation of Sample for AFM Imaging
To study the surface morphology of the electrode surface, AFM imaging was conducted. The glass slide was sliced into 1 × 1 cm area, and the materials were coated on it. Single walled carbon nanotubes were grafted with chitosan dispersed in weak acid, so that it enhances the conductive nature of the electrode. Furthermore, glutaraldehyde was coated on the surface to enhance the linkage with biomolecules. The topology of the modified glass side, after each modification was observed by atomic force microscopy (AFM) (Agilent Model No. 5500, Santa Clara, CA, USA).
2.5. Sensor Fabrication
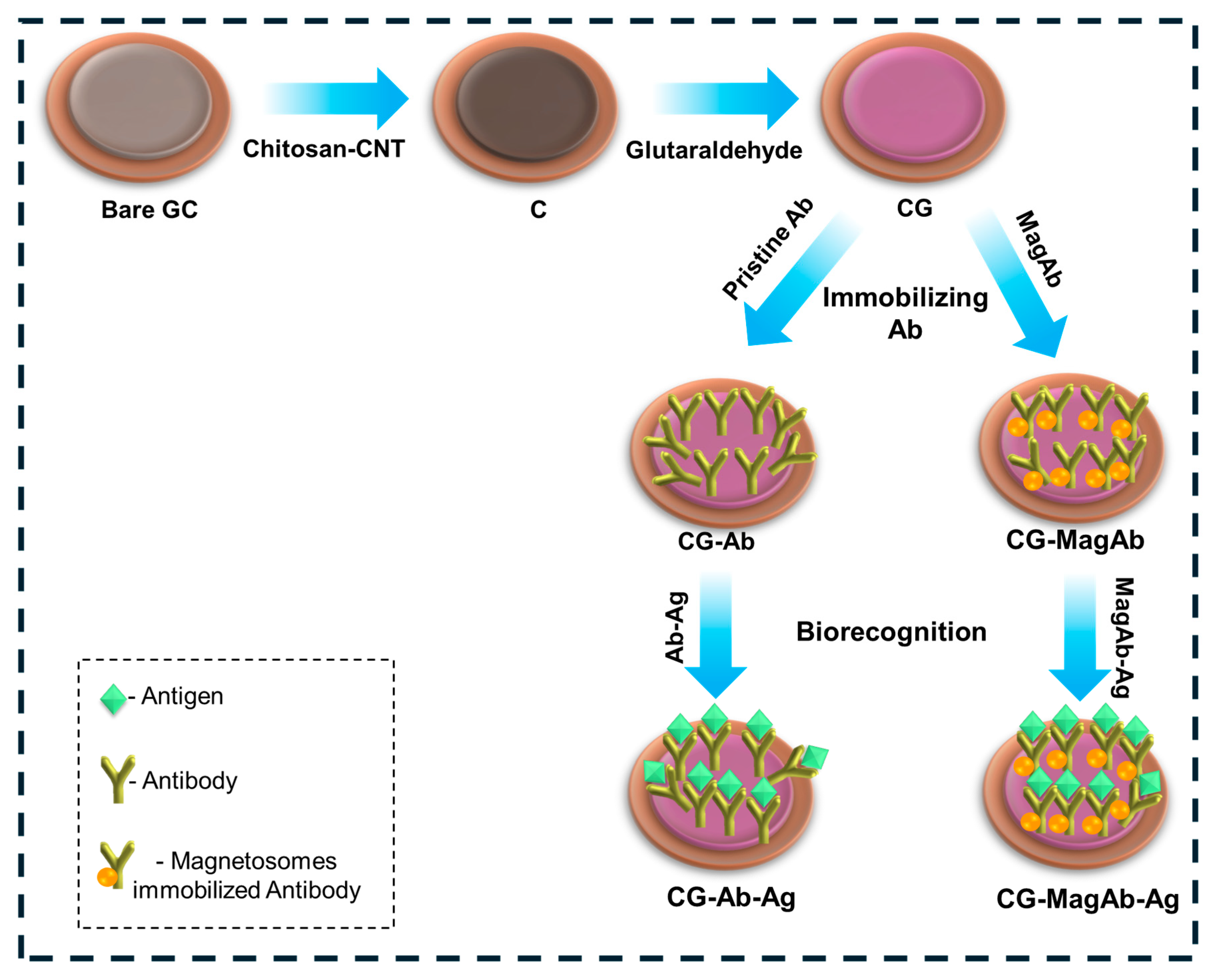
The electrochemical immunosensor was assembled layer-by-layer via direct physical adsorption; the fabrication process demonstrated in Figure 1. The reactions scheme shows (i) the covalent Schiff base formation between the amine groups of chitosan and the aldehyde groups of glutaraldehyde, (ii) subsequent availability of free aldehyde groups on the linker, and (iii) covalent attachment of antibody amine groups to the immobilized glutaraldehyde [45,46]. The conditions chosen for electrode functionalization and assay implementation were established from optimization studies and prior reports. A chitosan layer (0.5% w/v) provided sufficient amino and hydroxyl groups for crosslinking while maintaining film homogeneity. Glutaraldehyde (25% v/v) was used as a bifunctional linker, as higher concentrations led to excessive crosslinking and reduced antibody activity. Magnetosomes were employed at 2 mg mL−1, a concentration found to yield uniform dispersion and stable immobilization. Anti-salmonella and anti-E. coli antibodies were used at 2 CFU mL−1, which provided maximal electrochemical response without nonspecific background increase. Incubation for 20 min at 4 °C ensured efficient coupling while preserving antibody activity. These optimized conditions ensured reproducible electrode modification and sensitive antigen detection.
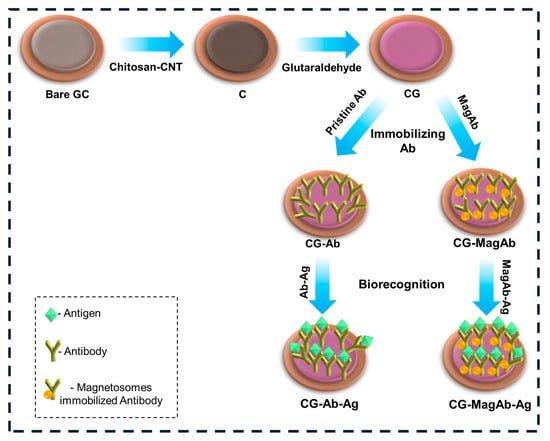
Figure 1.
Schematic diagram represents the modification of Glassy Carbon Electrode for electrochemical measurements in the perspective of food sensors.
Briefly, the glassy carbon electrode was coated with a solution of 0.5% Chitosan and single walled carbon nanotubes (CNT). CNTs are being used such that they increase the binding affinity. After an incubation of 60 min, the electrode was immersed in 25% glutaraldehyde solution to enhance the binding nature of the biological entity. Then, 10 µL of antibody (0.8 µg/mL) with or without magnetosomes was deposited onto the surface of a glassy carbon electrode and incubated at 4 °C for 20 min. In this work, the high concentration of (25%) glutaraldehyde was used to cause the over-cross linking, minimizing the flexibility of chitosan/CNT as well as the potential loss of activity of antibodies if directly exposed. The 25% treatment was only applied to the chitosan-CNT layer (before antibody immobilization), this may have been performed to create a very stable anchoring network and not directly interact with the antibody layer at the same time, antibody immobilization was performed at low temperature (4 °C, 20 min), which may minimize activity loss. Finally, the glassy carbon electrode surface was coated with lipopolysaccharide (Salmonella 0.01–0.5 µg/mL and E. coli 0.01–1 µg/mL) and the electrochemical response was measured. Hereafter the electrode modification of chitosan with CNTs are denoted as C, further modification with glutaraldehyde is denoted as CG, on immobilization with antibody and magnetosomes modified antibody are denoted as CG-Ab, CG-MagAb, further biogenic interactions with corresponding antigens are denoted as CG-Ab-Ag and CG-MagAb-Ag.
2.6. Electrochemical Characterization
Cyclic Voltammogram (CV) and electrochemical impedance spectroscopy (EIS) were performed in PARSTAT Multichannel Potentiostat (Ametek Scientific Instruments, Mahwah, NJ, USA) equipped with a conventional three electrode system and all measurements were carried out at room temperature in Fe(CN)63−/4−electrolyte. Glassy carbon electrode (GCE) was used as a working electrode, Ag/AgCl and a platinum wire was used as reference and counter electrode, respectively. Before the modification of GCE, it was subsequently polished with 0.05 µm Alumina slurries and washed with ultrapure water. After the addition of antigens (Salmonella 3–7 CFU ml−1 and E. coli 3–8 CFU ml−1) onto the electrode, electrochemical measurements were carried out by using PARSTAT electrochemical workstation. CV was performed at a range of −0.1 V to 0.7 V with a scan rate of 0.05 V/s. EIS was measured in the frequency of 0.1 Hz to 100 kHz in open circuit potential and a voltage amplitude of 5 mV. All experiments were performed in Fe(CN)63−/4− working solution; and the impedance (Z) was expressed in terms of real (Z’) and an imaginary (-Z”) component. The difference in charge transfer resistance (∆Rct) across the surface of the sensor was used to construct calibration curves. EIS data were used for simulation of the immunosensor by ZVIEW software(V.40h). Data points from each measured spectrum were automatically selected by the software for inputting into an equivalent circuit to generate a fitting spectrum. The difference in charge-transfer resistance before and after antigen binding to the sensor surface was taken as the signal produced by the immune reaction between immobilized antibodies and the cells. In addition, each experiment was repeated three times using different GCs to test the reproducibility of the immunosensor. All data were expressed with mean signals and ±standard deviations (S.D).
3. Results and Discussion
Magnetosomes are biogenic nanoparticles surrounded by a lipid bilayer, which provides abundant functional groups for biomolecule conjugation and biosensing applications. HR-TEM analysis confirmed their uniform cubo-octahedral morphology in the nanometer range, consistent with the equilibrium growth form of magnetite. A comparative table depicting the difference between the synthesize magnetosomes with previously reported studies are presented in Table S1. The successful conjugation of magnetosomes with antibodies was verified by FT-IR spectroscopy, where characteristic vibrational bands corresponding to secondary amide, hydroxyl, and carbonyl groups were observed in the antibody, magnetosomes, and antibody–magnetosome complexes.
For sensor fabrication, the glassy carbon electrode surface was activated with a thin chitosan film, a polysaccharide biopolymer providing amino and hydroxyl groups suitable for crosslinking [47,48]. Glutaraldehyde was then introduced as a bifunctional crosslinker, forming covalent bonds with the amino groups of chitosan [49,50]. Magnetosomes, carrying hydroxyl and amine functionalities, were immobilized via Schiff-base linkage to glutaraldehyde, thereby anchoring the antibody–magnetosome conjugates firmly onto the electrode surface [51]. The antibodies immobilized with magnetosomes on the surface of electrode are then open for antigen interaction. The immobilization of magnetosomes coated antibody provides a good platform for anchoring the antigens and measuring their sensitivity in an appropriate manner [52].
The sensing principle is based on the specific interaction between immobilized antibodies and their target antigens. Upon antigen binding, the insulating antigen–antibody complex perturbs the interfacial charge-transfer process at the electrode/electrolyte interface. This manifests as an increase in charge-transfer resistance (Rct) and a change in interfacial capacitance, which are readily detected by EIS. Thus, the antibody-functionalized magnetosomes act not only as a robust biorecognition element but also as a signal-amplifying nanointerface, enabling sensitive and selective electrochemical detection of foodborne pathogens.
3.1. Morphological and Spectral Characterization
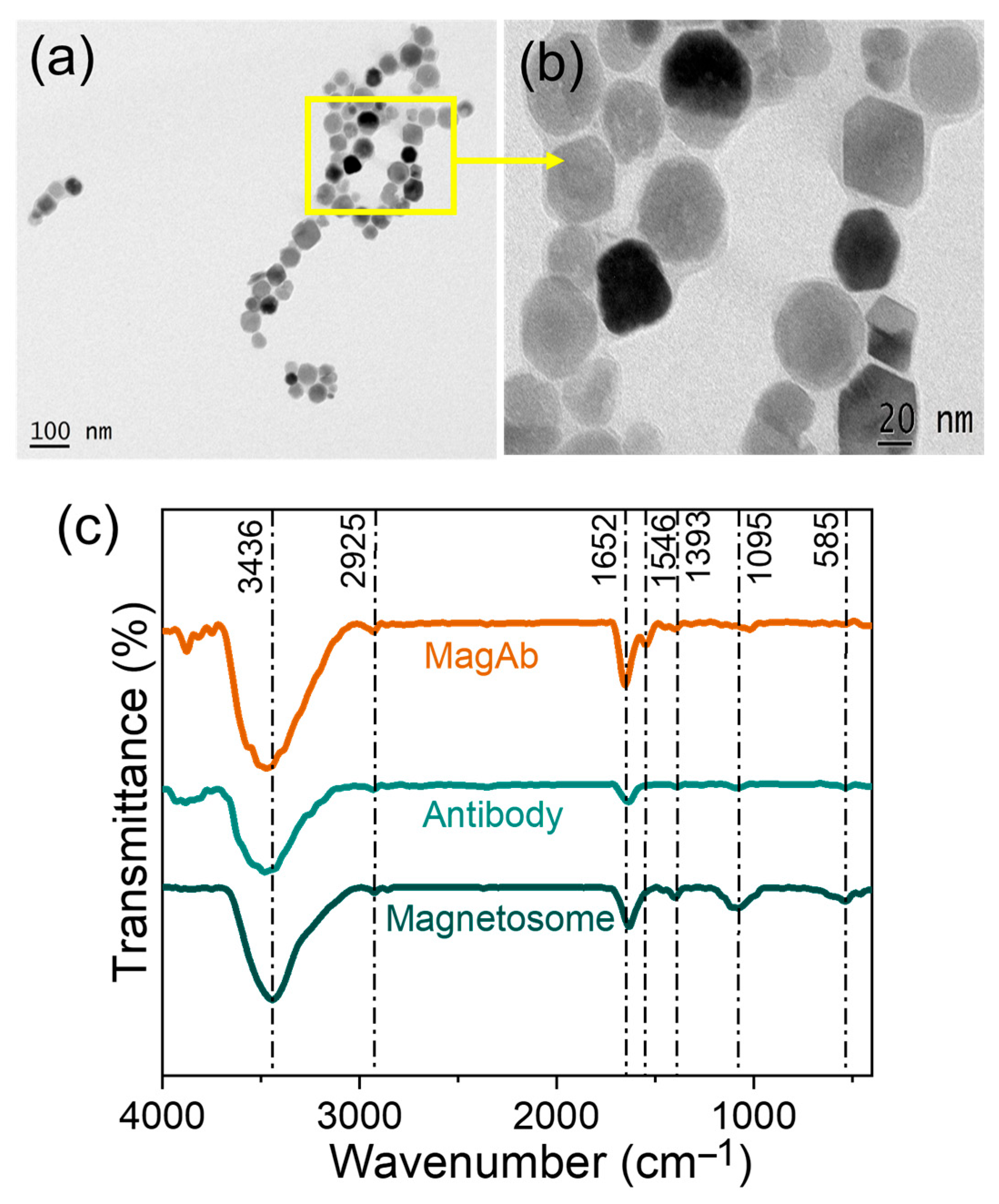
The extraction of magnetosomes was performed from Magnetospirillum sp. RJS1. Characterization of magnetosomes by HR-TEM confirmed uniform cubo-octahedral morphology and narrow size range (Figure 2a,b). Figure 2c shows the spectrum obtained for magnetosomes, antibody and magnetosome immobilized antibody. Based on these results, it was concluded that the observed IR vibrational signals of secondary amide bands are due to the IgG in the antibody. The spectrum also shows the presence of -OH group at 3436 cm−1 in stretching vibration, C-H at 2925 cm−1, C=N stretching at 1652 cm−1, N-O stretching at 1546 cm−1 present only in Magnetosome immobilized Ab, C-H moiety at 1393 cm−1, C-O moiety at 1095 cm−1, and Fe-O stretching being present at 585 cm−1. Magnetosomes showed Fe–O vibration at ~570 cm−1, and antibody immobilization was verified by the appearance of protein amide bands (Amide I ~1650 cm−1, Amide II ~1545 cm−1), together confirming the stable conjugation of antibodies to the magnetosome–chitosan–glutaraldehyde matrix [53,54,55].
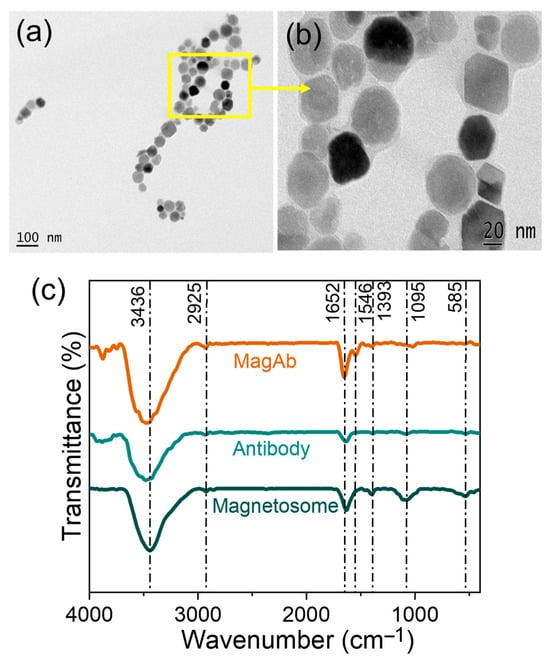
Figure 2.
(a,b) HR-TEM images of magnetosomes extracted from Magnetospirillum sp. RJS1 and (c) FT-IR spectrum analysis showing the peak for Magnetosomes (Mag), Antibody (Ab) and Magnetosome immobilized antibody (MagAb).
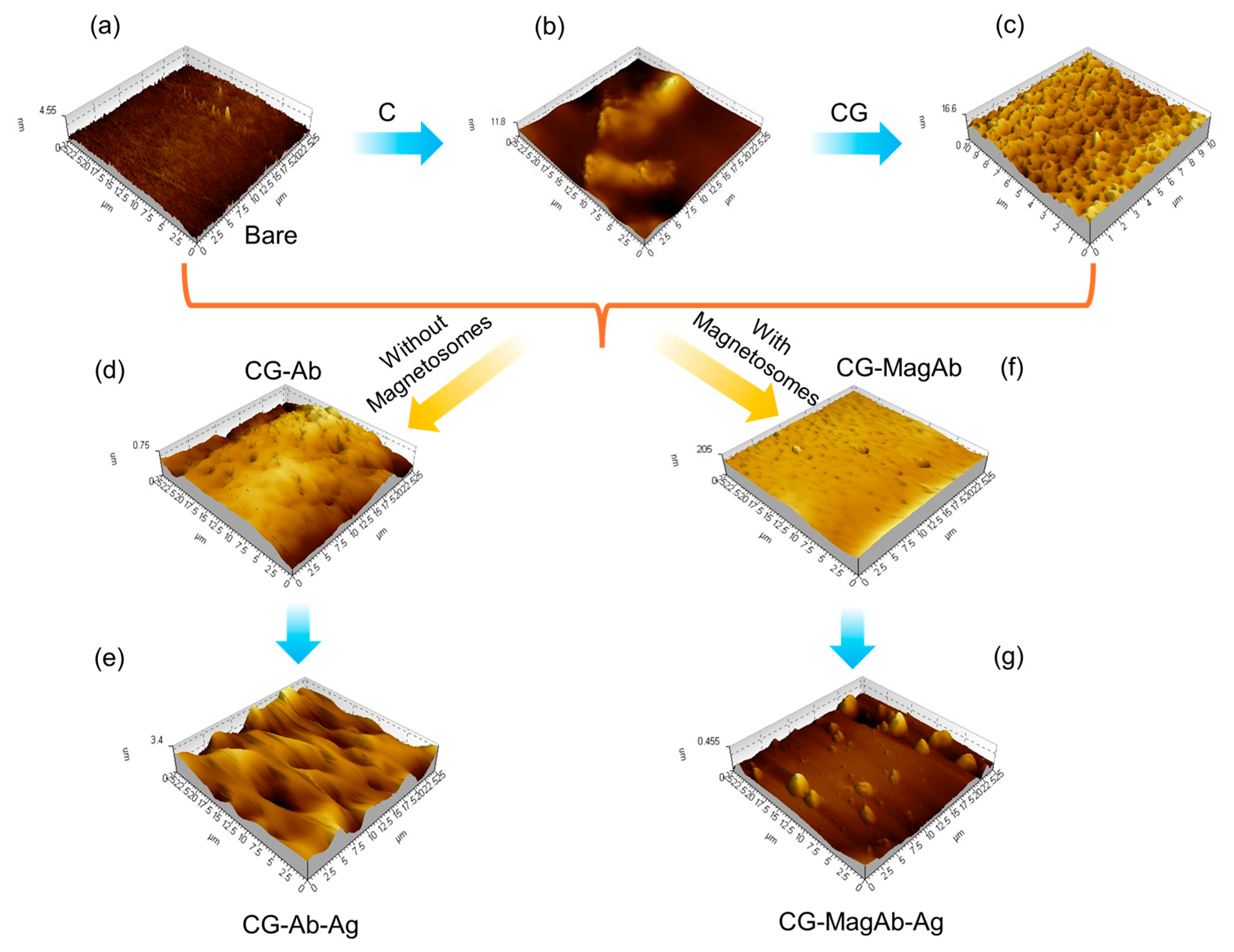
To investigate the nano-topology of the modified glass plate surface AFM technique was performed. Tapping mode atomic force microscope images each modifying layer of the immunosensor assembly were acquired on 1 × 1 cm sections of a glass slide (Figure 3). Figure S1 shows the AFM topography of the modified glass surfaces. The profile thickness of bare glass slide was observed to be around 4 nm. As the addition of C increases the surface conductivity nature of the electrode (Figure 3b); its profile thickness increases to 11 nm. Upon CG immobilization over the modified glass side enhances the biomolecules linkage over the modified surface, such as it makes the surface to be spiky for binding with the antibody (0.8 µg/mL) (Figure 3c); thickness of the surface enhances to around 17 nm. The spiky nature of the surface disappeared after immobilizing with antibody as they are covalently linked to yield a globular coating features of humps (Figure 3d); the roughness factor of the surface changes to be around 200–700 nm. A similar response was seen for magnetosomes immobilized antibody (0.8 µg/mL). The humps that are formed are modified into island upon the addition of Salmonella antigen (0.05 µg/mL) over the glass surface (Figure 3e); the profile extraction of the surface seems to be increasing to a range of 1–3 µm. Statistical analysis of the AFM images (roughness, particle size and distribution) was also measured using Agilent’s Pico View 1.20.3 software, those data are provided in Figure S2. Through this study, we conclude that homogenous sub monolayers of the proteins are being formed.
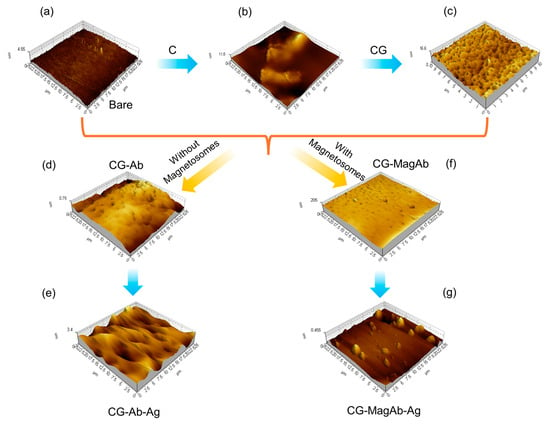
Figure 3.
AFM analysis using Tapping mode to image layers in sensor fabrication and use: (a) Bare glass plate; (b) Modification with chitosan and single walled carbon nanotubes (C); (c) Coating the modified glass side with Glutaraldehyde (CG); (d) Immobilizing the surface with antibody (CG-Ab); (e) Antigen–antibody interaction (CG-Ab-Ag); (f) Immobilizing the surface with magnetosomes coated antibody (CG-MagAb); (g) Antigen-magnetosome-antibody interaction (CG-MagAb-Ag).
3.2. Electrochemical Result and Discussion
3.2.1. Cyclic Voltammetry
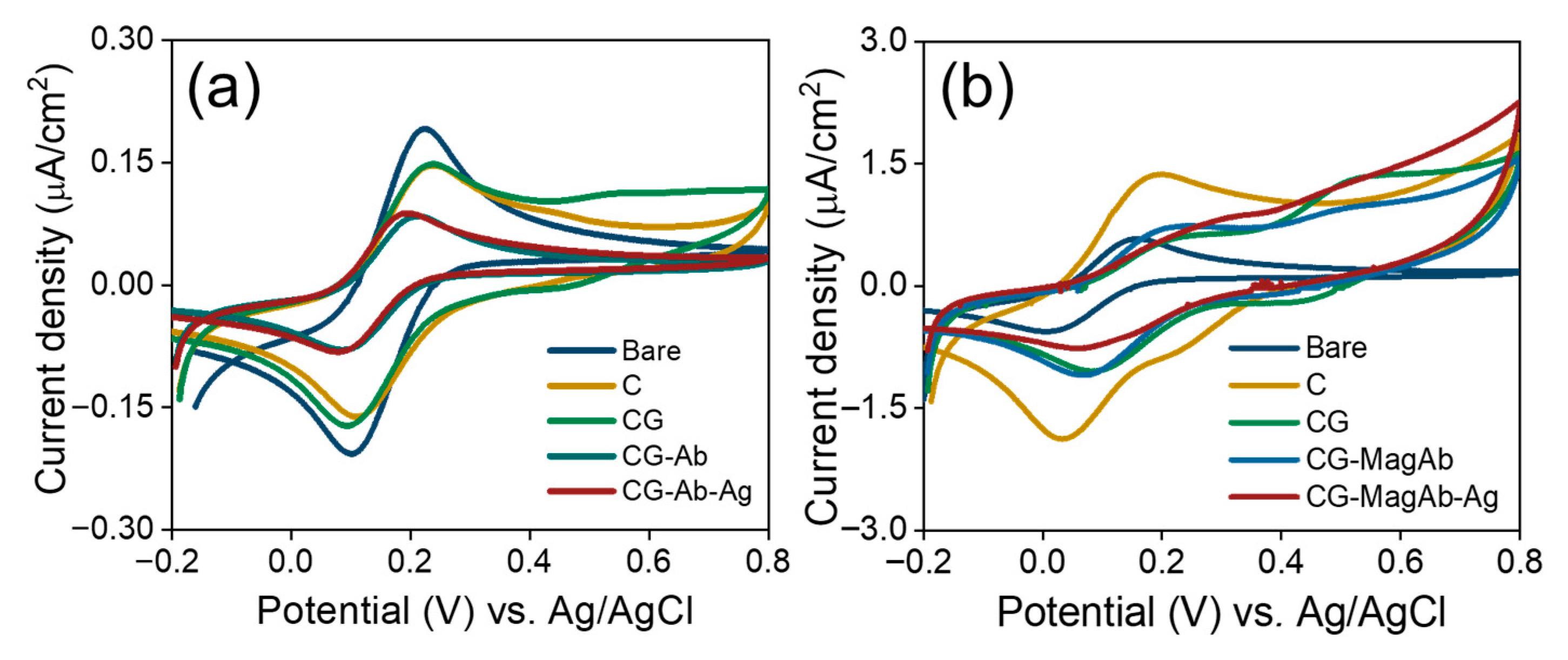
CV experiments were used to further confirm the surface changes for the attachment of antibody and antigens (Salmonella and E. coli) in the fabricated immunosensor. Theoretically, the changes in peak current and the separation between peak potentials in voltammograms obtained for modified electrode surfaces are related to the electron-transfer rate constant [56]. Figure 4a,b shows the cyclic voltammograms of modified GCs obtained under the following conditions: (i) bare; (ii) modification with C; (iii) CG; (iv) immobilization with antibody/magnetosome immobilized with antibody; (v) linking with concentration of antigen (Salmonella 0.01 µg/mL and E.coli 0.01 µg/mL) in the solution of 1 mM [Fe(CN)6]3−/4− and 0.1 M KNO3 prepared in 10 mM PBS (pH = 7.4). It is evident that the increase in peak potential and peak current is noted as the electrode is modified continuously. It also suggests that the surface is confined, and reversible diffusion occurs. The layer-specific controls indicate that this feature primarily arises from surface-confined oxidative processes within the chitosan/glutaraldehyde film, with a minor contribution from magnetosome Fe(II)/Fe(III) surface chemistry on GC/CGMagAb. The peak is therefore a film/electrostatic charging and redox signature of the modified interface rather than a mediator-driven faradaic process in solution.
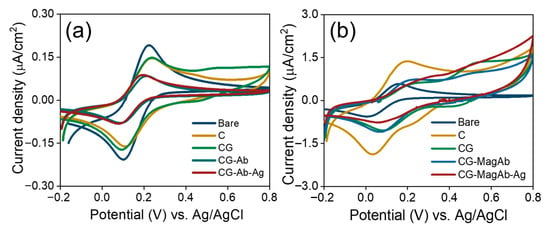
Figure 4.
(a,b) Cyclic Voltammograms of modified GC electrodes in the solution of 1 mM Fe(CN)63−/4− and 0.1 M KNO3 prepared in 10 mM PBS (pH = 7.4). Scan rate 0.5 V/s.
When the electrode was immobilized with magnetosome linked antibodies (Ab) and pristine Ab, an increase in potential (from 119 mV to 169 mV) and a decrease in peak current (from 23 µA to 19 µA) were observed. After the addition of antigen, an increase in peak potential (from 169 mV to 194 mV) and a decrease in peak current (from 19 µA to 11 µA) is noted. Especially, a decrease of 42.1% in peak current was clearly observed after the antigen–antibody interaction [57]. The increase in peak potential and a decrease in peak current suggests that the binding of cells perturb the electron transfer rate which is in good agreement with the increase in electron transfer resistance observed in the impedance spectra.
3.2.2. Impedimetric Spectroscopy
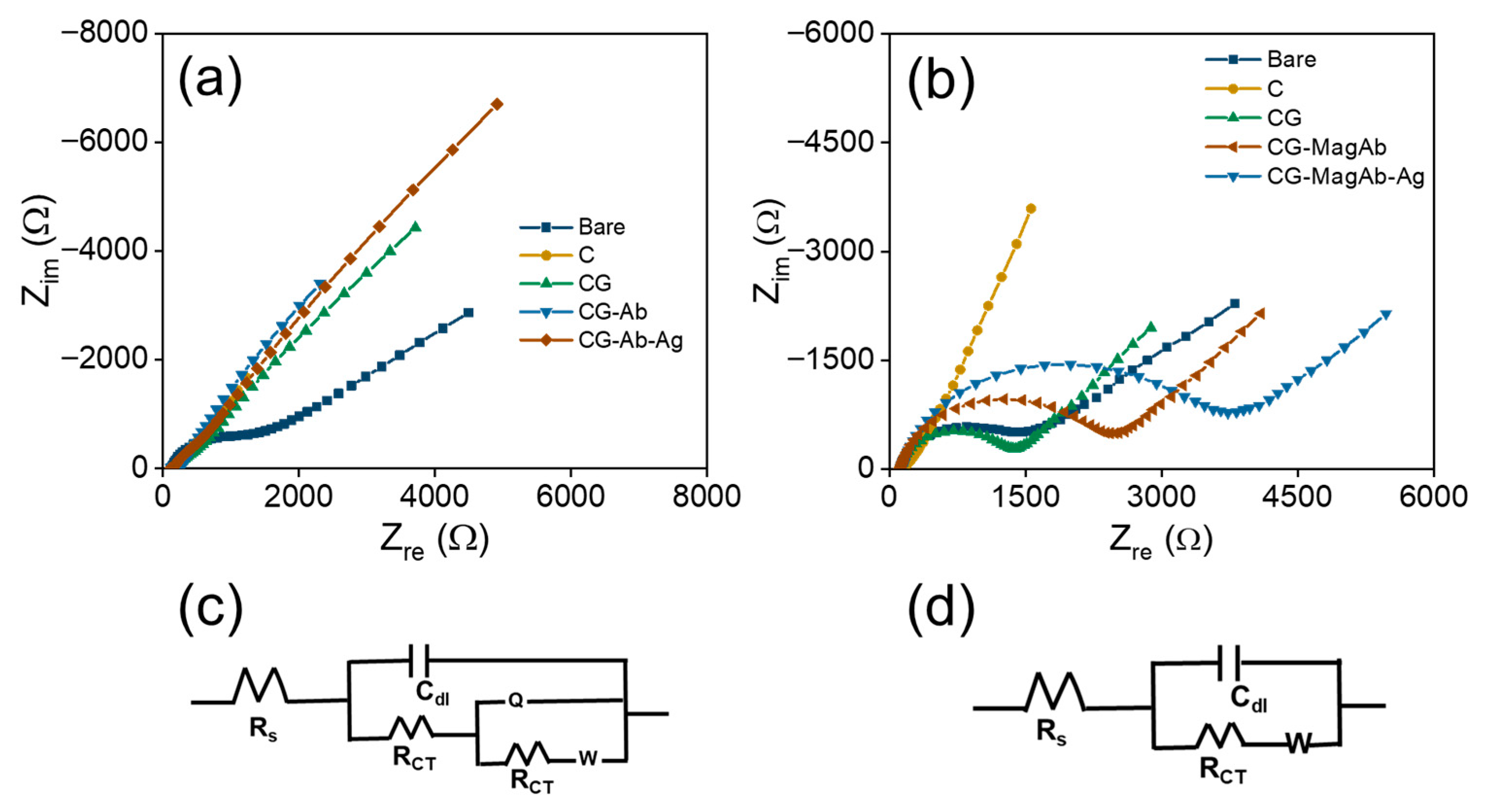
EIS is a powerful technique to analyze the interfacial electron transfer characteristics and to determine kinetic parameters like charge transfer resistance (Rct) and rate constant associated with that of electron transfer reaction. Particularly in this work, EIS is used to confirm each step of the modification process reflected as changes in Rct values. Basically, Rct is defined as the resistance offered by the modified electrode towards the electron transfer reaction. Each step of the glassy carbon electrode modification is studied using EIS and the corresponding spectra are shown as Nyquist plots in Figure 5a. These spectra were recorded using 1 mM Fe(CN)63−/4− and 0.1 M KNO3 prepared in 10 mM PBS (pH = 7.4) as a redox probe [58,59]. It can be seen from this figure that bare glassy carbon electrode exhibited a small semicircle at high frequency region and a straight line at low frequency regime denotes the Warburg resistance to mass transfer process at the interface. This result clearly indicates a diffusion-controlled process for the electron transfer reaction on bare glassy carbon electrode. On modifying glassy carbon electrode with C it exhibits an increase in Rct value but still retains the diffusion-controlled process [60,61]. Further on modification with CG that acts as an arm linker, Rct value is found to increase by more than 8 times due to the adsorption of glutaraldehyde on the C modified electrode. Furthermore, Fab’ fragments from antibody were covalently linked to Glutaraldehyde through chemical bond formation and this caused almost two-fold increase in Rct value, suggesting the electron transfer process is controlled by charge transfer rather than diffusion [62,63]. Finally, the addition of antigen to antibody modified electrode results in further enhancement of Rct value indicating a complete charge transfer controlled process as evidenced by the formation of semicircle almost in entire range of frequency used for the study. The corresponding Rct values are determined using an equivalent circuit fitting procedure by employing modified Randle’s equivalent circuits as shown in Figure 5c. A model shown in Figure 5c is used for fitting the impedance data obtained for pristine Ab-Ag interactions on electrode surface and similarly an equivalent circuit model shown in Figure 5d is used for fitting the impedance data recorded using magnetosome linked Ab-Ag interactions on the electrode surface. Various fitting parameters determined from equivalent circuit models including Rct values are shown in Table 1. It is clear from this table that Rct value changes systematically with respect to each modification step and these results confirm the electrode modification at each step and they are in good agreement with CV results discussed earlier.
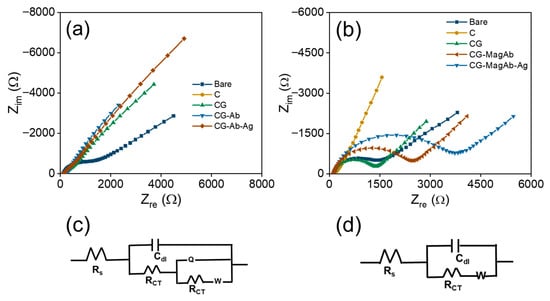
Figure 5.
Nyquist plots: (a) CG-Ab-Ag, (b) CG-MagAb-Ag modified GC electrodes within the frequency range from 0.1 Hz to 100 kHz. A sinusoidal potential modulation of ±10 mV amplitude was superimposed on the open circuit potential. Electrolyte: 1 mM Fe(CN)63−/4− and 0.1 M KNO3 as a supporting electrolyte in 10 mM PBS (pH = 7.4). (c,d) The corresponding equivalent circuit of the fabricated electrodes.

Table 1.
Determination of various parameters associated with the modified GC electrode obtained using equivalent circuit fitting of the measured impedance data.
Similarly, impedance spectroscopic studies are also used to investigate the electron transfer characteristics of antibodies immobilized with magnetosomes on the modified GC electrode. It is presumed that the usage of magnetosomes along with the antibody leads to enhancement in the sensitivity of pathogens detection in the food contaminants. Since the magnetosomes can also affect the electron transfer reaction, a varying range of Rct values is noted in the corresponding impedance plots shown in Figure 5b. These results clearly show the process is controlled by mass transfer, and this predominantly affects the diffusion as evidenced from the changes in Rct values shown in Table 2. These fitting parameters are determined from the controlled circuit fitting using the models shown in Figure 5d. It is noticed that the Rct value is increased from 814 Ω to 1403 Ω on adding antigen to antibody immobilized magnetosomes modified GC electrode and this tremendous enhancement confirms the modification process. The equivalent circuit in Figure 5c reflects a more resistive interface because antibody/antigen immobilization partially blocks electron transfer. The equivalent fit circuit in Figure 5d reflects an enhanced bio interface where magnetosomes improve antibody orientation, conductivity, and diffusion, resulting in lower baseline Rct, higher capacitance, and better biosensor performance. These results obtained from EIS measurement confirm the modification steps and further are in good agreement with the CV results discussed earlier. Furthermore, Table 3 compares the foodborne pathogens detection using different catalysts developed for sensor applicability.

Table 2.
Determination of various parameters associated with the magnetosomes modified GC electrode obtained using equivalent circuit fitting of the measured impedance data.

Table 3.
Comparison study of the foodborne pathogen detection using different catalysts.
3.3. Species Specificity
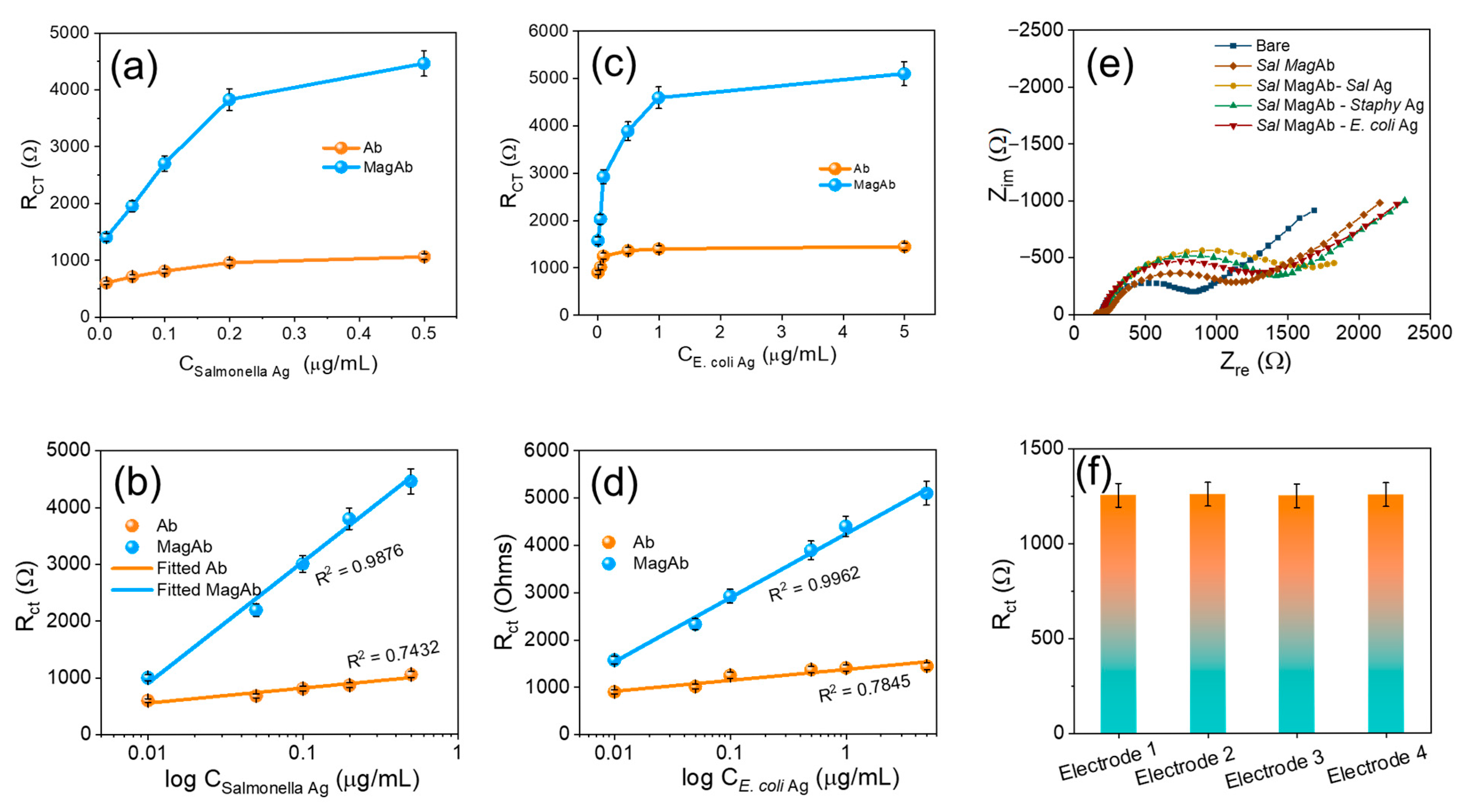
The sensor exhibited a linear correlation to the concentration of antigen vs. charge-transfer resistance, with a regression coefficient of 0.9876 and 0.9962, respectively. With an antigen–antibody modified electrode as a sensor, the calibration graph for the determination of antigen was obtained under optimal conditions (Figure 6a,b). Following the standard concentration of antigen and the charge-transfer resistance, an increase in antigen concentration increases the charge-transfer resistance in two linear ranges from (0.01–5 µg/mL) for E. coli antigen and from (0.01–0.5 µg/mL) for Salmonella antigen (Figure 6c,d). This indicates that the combination of magnetosome linked Ab-Ag increases resistance and decreases conductivity. The main advantages in the C-G-Ab-Ag composite preparation, electrode modification and finally immobilizing the antibody with magnetosomes employed in the present study are very simple when compared to the other detection methods. To assess the cross-reactivity the magnetosome linked Salmonella antibody was coupled with varying antigens of E. coli, Staphylococcus but the Rct response remains consistent intimidating the binding affinity towards the sensor (Figure 6e). Compared to conventional nanomaterial-based immunosensors, the proposed system leverages the natural lipid bilayer of magnetosomes for direct antibody conjugation, their magnetic responsiveness for efficient analyte handling, and their conductive Fe3O4 core for signal amplification. These features collectively yield superior sensitivity (LOD = 1 CFU mL−1), reproducibility, and field applicability, positioning the sensor as a more practical and effective alternative for foodborne pathogen detection. The reproducibility of the sensing remains consistent with different sets of EIS charge-transfer resistance as displayed in Figure 6f.
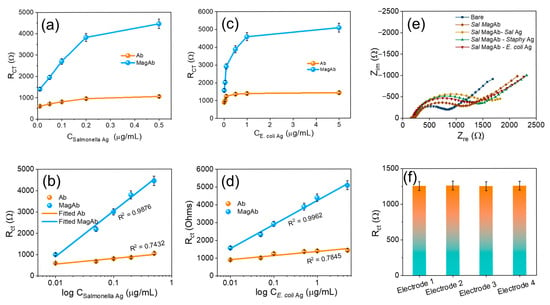
Figure 6.
(a) The Rct due to the varying concentration of Salmonella antigen. (b) Corresponding linear fit of (a). (c) show the Rct due to the varying concentration of E. coli antigen. (d) Corresponding linear fit of (b). (e) Visualizes the cross-reactivity while varying the Ag with Sal MagAb and (f) reproducibility in different sets of electrodes. The Rct was calculated by Rct (Ab-Ag) − Rct (Ab), where Rct (Ab-Ag) is the value of the electron transfer resistance after antigen binding to antibody, Rct (Ab) is the value of the electron transfer resistance after the addition of antibody to the modified GC electrode. Each value of Rct was derived from three independent measurements.
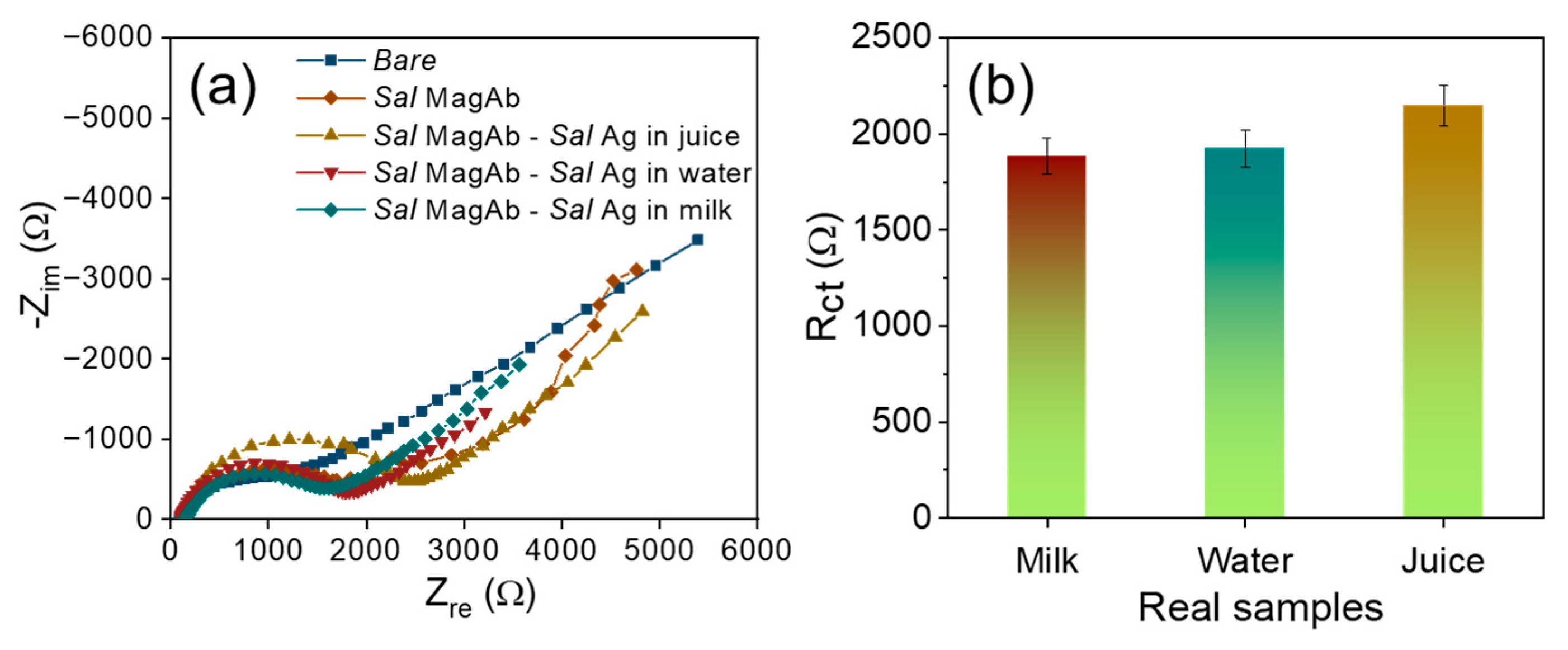
For real sample, assessment the magnetosomes immobilized Salmonella antibody was used to detect the LPS extracted from Salmonella contaminated food samples such as milk, water and apple juice. The samples are procured from the local vendors and are purified using Whatman filter paper to isolate the solid residues. The overnight Salmonella culture (0.01 µg/mL) was inoculated consecutively in each tested samples and the analysis was repeated three times. From Figure 7a,b, it could be observed that LPS protein extracted from the Salmonella cultured real samples exhibits a higher resistance of 1893 Ω (milk), 1957 Ω (water) and 2134 Ω (juice) depicting efficient detection of food contaminants in real samples.
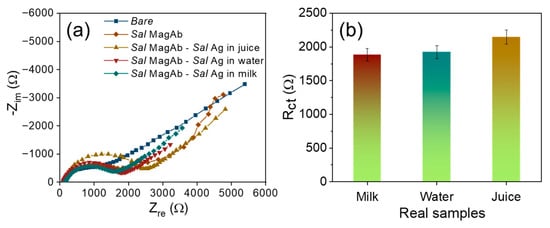
Figure 7.
(a) Nyquist diagram and (b) the corresponding bar plot for the interaction of magnetosome antibody complex with various concentrations of S. aureus in real samples.
4. Conclusions
In this study, we developed a magnetosome-based electrochemical immunosensor for the detection of LPS antigens from E. coli and Salmonella typhimurium, achieving high sensitivity of 36.24 Ω/CFU mL−1 and LOD = 1 CFU mL−1, reproducibility, and rapid response through the integration of biogenic magnetosomes with a chitosan/CNT-modified electrode. While morphological and electrochemical analyses confirmed successful stepwise modification and effective antigen binding, some limitations remain, including restricted reusability due to antibody degradation, lack of a standardized regeneration protocol, high production costs and limited scalability of magnetosomes, and the requirement for low-temperature storage to preserve stability. Addressing these challenges—particularly improving cycle stability, reducing cost through scalable bioprocessing, and enhancing robustness under field conditions—will be essential for translating this platform into a commercially viable and practical tool for food safety monitoring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13090355/s1, Figure S1: AFM images showing Topography details of the various modifications on the Glassy carbon electrode. (a) Bare glass plate; (b) Modification with Chitosan & single walled carbon Nanotubes; (c) Coating the modified electrode with Glutaraldehyde; (d) Immobilizing the surface with antibody; (e) Antigen -antibody interaction; (f) Immobilizing the surface with magnetosomes coated antibody; (g) Antigen-antibody interaction. Figure S2: AFM images showing Profile extraction details of the various modifications on the Glassy carbon electrode. (a) Bare glass plate; (b) Modification with Chitosan & single walled carbon Nanotubes; (c) Coating the modified electrode with Glutaraldehyde; (d) Immobilizing the surface with antibody; (e) Antigen-antibody interaction; (f) Immobilizing the surface with magnetosomes coated antibody; (g) Antigen-antibody interaction. Table S1: The table visualizes the features of magnetosomes compared with previous literature reports. References [43,44,52,68,69,70].
Author Contributions
CRediT authorship contribution statement: S.S. (Sankar Sekar): conceptualization, formal analysis, investigation, writing—original draft. S.K.A.: methodology, formal analysis, investigation, writing—original draft. S.L.: conceptualization, investigation, formal analysis, supervision. S.S. (Saravanan Sekar): data curation, formal analysis. S.S. (Sutha Sadhasivam): conceptualization, formal analysis. S.V.: data curation, validation. N.S.: conceptualization, formal analysis. E.K.: data curation, validation. S.-C.C.: data curation, conceptualization, validation, supervision. R.M.: methodology, conceptualization, formal analysis, investigation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation (NRF) of Korea through the basic science research programs (2021R1I1A1A01049638; RS-2023-NR076644, RS-2022-NR075805) funded by the Korean Government. This study was supported by the Engineering and Academic Research (R&D) Program through the NRF funded by the Ministry of Education (RS202300249778). This study was also supported by the NRF grant funded by the Korean government (MSIT) (RS-2021-NR060086).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef]
- Gourama, H. Foodborne pathogens. In Food Safety Engineering; Springer: Cham, Switzerland, 2020; pp. 25–49. [Google Scholar]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Sekar, S.; Manikandan, R.; Arumugasamy, S.K.; Sekar, S.; Lee, Y.; Chang, S.-C.; Lee, S. Rapid Microwave-Assisted Synthesis of CuSe Nanoparticles for High-Sensitivity Serotonin Biosensing in Serum. Chemosensors 2025, 13, 264. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, Y.S.; Lee, S.H.; Kim, S.H.; Park, K.H.; Bahk, G.J. Estimating the burden of foodborne disease, South Korea, 2008–2012. Foodborne Pathog. Dis. 2015, 12, 207–213. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Vital signs: Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne diseases active surveillance network, 10 U.S. sites, 1996–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 749–755. [Google Scholar]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.C.; Bhowmik, S.; Ali, A. Conventional and advanced detection techniques of foodborne pathogens: A comprehensive review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Khaki, P.; Jahanban-Esfahlan, A.; de la Guardia, M.; Mokhtarzadeh, A. State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1868–1912. [Google Scholar] [CrossRef] [PubMed]
- Albert, V.; Ramamurthy, T.; Das, S.; Dolma, K.G.; Majumdar, T.; Baruah, P.J.; Hazarika, S.C.; Apum, B.; Das, M. Comprehending the risk of foodborne and waterborne disease outbreaks: Current situation and control measures with special reference to the Indian scenario. Heliyon 2024, 10, e36344. [Google Scholar] [CrossRef]
- Bisht, A.; Kamble, M.P.; Choudhary, P.; Chaturvedi, K.; Kohli, G.; Juneja, V.K.; Sehgal, S.; Taneja, N.K. A surveillance of food borne disease outbreaks in India: 2009–2018. Food Control 2021, 121, 107630. [Google Scholar] [CrossRef]
- Sekar, S.; Yun, J.-S.; Lee, S. Metal-free electrocatalytic nanocomposites of poly azovan blue-decorated graphitic carbon nitride for simultaneously sensing paracetamol and 4-aminophenol. Environ. Res. 2023, 239, 117293. [Google Scholar] [CrossRef]
- Interagency Food Safety Analytics Collaboration. Foodborne Illness Source Attribution Estimates for 2017 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter Using Multi-Year Outbreak Surveillance Data, United States; U.S. Department of Health and Human Services, CDC, FDA, USDA/FSIS: Washington, DC, USA, 2019.
- Singh, P.; Balaraman, A.K.; Mehta, R.; Sah, S. Urgent need for enhanced food safety protocols in fast-food supply chains: Lessons from the recent multi-state E. coli outbreak. Infect. Dis. 2025, 57, 109–111. [Google Scholar] [CrossRef]
- Aladhadh, M. A review of modern methods for the detection of foodborne pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Su, W.; Liang, D.; Tan, M. Nucleic acid-based detection for foodborne virus utilizing microfluidic systems. Trends Food Sci. Technol. 2021, 113, 97–109. [Google Scholar] [CrossRef]
- Manikandan, R.; Rajarathinam, T.; Jayaraman, S.; Jang, H.-G.; Yoon, J.-H.; Lee, J.; Paik, H.-j.; Chang, S.-C. Recent advances in miniaturized electrochemical analyzers for hazardous heavy metal sensing in environmental samples. Coord. Chem. Rev. 2024, 499, 215487. [Google Scholar] [CrossRef]
- Manikandan, R.; Jang, H.-G.; Kim, C.-S.; Yoon, J.-H.; Lee, J.; Paik, H.-J.; Chang, S.-C. Nano-engineered paper-based electrochemical biosensors: Versatile diagnostic tools for biomarker detection. Coord. Chem. Rev. 2025, 523, 216261. [Google Scholar] [CrossRef]
- Priyanka, B.; Patil, R.K.; Dwarakanath, S. A review on detection methods used for foodborne pathogens. Indian J. Med. Res. 2016, 144, 327–338. [Google Scholar] [CrossRef]
- Xu, X.; Liu, G.; Huang, X.; Li, L.; Lin, H.; Xu, D. MALDI-TOF MS-based identification of bacteria and a survey of fresh vegetables with pathogenic bacteria in Beijing, China. Food Biosci. 2021, 41, 100746. [Google Scholar] [CrossRef]
- Zhang, R.; Belwal, T.; Li, L.; Lin, X.; Xu, Y.; Luo, Z. Nanomaterial-based biosensors for sensing key foodborne pathogens: Advances from recent decades. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1465–1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, H.; Lu, X.; Zheng, X.; Yang, Z. Recent advances in electrochemical biosensors for the detection of foodborne pathogens: Current perspective and challenges. Foods 2023, 12, 2795. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Suthindhiran, K. Development of magnetosomes-based biosensor for the detection of Listeria monocytogenes from food sample. IET Nanobiotechnol. 2020, 14, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Sudhakaran, R.; Suthindhiran, K. Detection of white spot syndrome virus in seafood samples using a magnetosome-based impedimetric biosensor. Arch. Virol. 2021, 166, 2763–2778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, X.; Ma, A.; Jiang, F.; Chen, Y. Multiplexed food-borne pathogen detection using an argonaute-mediated digital sensor based on a magnetic-bead-assisted imaging transcoding system. Nat. Food 2025, 6, 170–181. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, K.; Huang, M.; Zeng, M.; Deng, Y.; Li, S.; Chen, H.; Li, W.; Chen, Z. Research progress on detection techniques for point-of-care testing of foodborne pathogens. Front. Bioeng. Biotechnol. 2022, 10, 958134. [Google Scholar] [CrossRef]
- Techakasikornpanich, M.; Jangpatarapongsa, K.; Polpanich, D.; Zine, N.; Errachid, A.; Elaissari, A. Biosensor technologies: DNA-based approaches for foodborne pathogen detection. TrAC—Trends Anal. Chem. 2024, 180, 117925. [Google Scholar] [CrossRef]
- Sekar, S.; Huijun, J.; Liuzhu, Z.; Jin, C.; Lee, S.; Kim, D.Y.; Manikandan, R. Copper phthalocyanine conjugated graphitic carbon nitride nanosheets as an efficient electrocatalyst for simultaneous detection of natural antioxidants. Electrochim. Acta 2022, 413, 140150. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Manikandan, R.; Sekar, S.; Jin Lee, D.; Chang Jeon, H.; Lee, S.; Chang, S.-C.; Young Kim, D. Two dimensional FeVO4 nanoflakes decorated on Ti3C2 MXene hybrid nanocomposites as a novel effective electrochemical biosensor for ultrasensitive and selective detection of serotonin (5-HT). Appl. Surf. Sci. 2025, 680, 161411. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kosterov, A.; Koziaeva, V.V.; Sergienko, E.S.; Shevtsov, M.A. Magnetotactic bacteria and magnetosomes: Basic properties and applications. Magnetochemistry 2021, 7, 86. [Google Scholar] [CrossRef]
- Muñoz, D.; Marcano, L.; Martín-Rodríguez, R.; Simonelli, L.; Serrano, A.; García-Prieto, A.; Fdez-Gubieda, M.; Muela, A. Magnetosomes could be protective shields against metal stress in magnetotactic bacteria. Sci. Rep. 2020, 10, 11430. [Google Scholar] [CrossRef] [PubMed]
- Mickoleit, F.; Altintoprak, K.; Wenz, N.L.; Richter, R.; Wege, C.; Schüler, D. Precise Assembly of Genetically Functionalized Magnetosomes and Tobacco Mosaic Virus Particles Generates a Magnetic Biocomposite. ACS Appl. Mater. Interfaces 2018, 10, 37898–37910. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Kim, M.; Kang, R.; Lim, I.; Jang, Y. Rapid and simple on-site salmonella detection in food via direct sample loading using a lipopolysaccharide-imprinted polymer. J. Nanobiotechnol. 2025, 23, 279. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Kumar, A.S.; Mathiyarasu, J.; Suthindhiran, K. Detection of Escherichia coli in food samples by magnetosome-based biosensor. Biotechnol. Bioprocess Eng. 2023, 28, 152–161. [Google Scholar] [CrossRef]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J. Magnetosome-anti-Salmonella antibody complex based biosensor for the detection of Salmonella typhimurium. Mater. Sci. Eng. C 2020, 114, 111071. [Google Scholar] [CrossRef]
- Manikandan, R.; Mani, S.P.; Selvan, K.S.; Yoon, J.-H.; Chang, S.-C. Fabrication of S and O-incorporated graphitic carbon nitride linked poly (1,3,4-thiadiazole-2,5-dithiol) film for selective sensing of Hg2+ ions in water, fish, and crab samples. Food Chem. 2023, 425, 136483. [Google Scholar] [CrossRef] [PubMed]
- Hosseinikebria, S.; Khazaei, M.; Dervisevic, M.; Judicpa, M.A.; Tian, J.; Razal, J.M.; Voelcker, N.H.; Nilghaz, A. Electrochemical biosensors: The beacon for food safety and quality. Food Chem. 2025, 475, 143284. [Google Scholar] [CrossRef]
- Feroci, M.; Grasso, G.; Dragone, R.; Curulli, A. Electrochemical (Bio) Sensors for Toxins, Foodborne Pathogens, Pesticides, and Antibiotics Detection: Recent Advances and Challenges in Food Analysis. Biosensors 2025, 15, 468. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhou, X.; Long, R.; Xie, M.; Kankala, R.K.; Wang, S.; Zhang, Y.S.; Liu, Y. Biomedical applications of magnetosomes: State of the art and perspectives. Bioact. Mater. 2023, 28, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Adampourezare, M.; Hasanzadeh, M.; Hoseinpourefeizi, M.-A.; Seidi, F. Iron/iron oxide-based magneto-electrochemical sensors/biosensors for ensuring food safety: Recent progress and challenges in environmental protection. RSC Adv. 2023, 13, 12760–12780. [Google Scholar] [CrossRef]
- Curulli, A. Electrochemical biosensors in food safety: Challenges and perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef]
- Hungate, R. The anaerobic mesophilic cellulolytic bacteria. Bacteriol. Rev. 1950, 14, 1–49. [Google Scholar] [CrossRef]
- Revathy, T.; Jacob, J.J.; Jayasri, M.; Suthindhiran, K. Isolation and characterization of Magnetospirillum from saline lagoon. World J. Microbiol. Biotechnol. 2016, 32, 109. [Google Scholar] [CrossRef]
- Jacob, J.J.; Suthindhiran, K. Immobilisation of lipase enzyme onto bacterial magnetosomes for stain removal. Biotechnol. Rep. 2020, 25, e00422. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, H.; Cui, J.; Duan, L. A simple electrochemical immunosensor based on a chitosan/reduced graphene oxide nanocomposite for sensitive detection of biomarkers of malignant melanoma. RSC Adv. 2022, 12, 25844–25851. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, C.; Xi, F. Disposable amperometric label-free immunosensor on chitosan–graphene-modified patterned ITO electrodes for prostate specific antigen. Molecules 2022, 27, 5895. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.N.; Dasagrandhi, C.; Truong, V.G.; Kim, Y.-M.; Kang, H.W. Antibacterial activity of Staphylococcus aureus biofilm under combined exposure of glutaraldehyde, near-infrared light, and 405-nm laser. PLoS ONE 2018, 13, e0202821. [Google Scholar] [CrossRef]
- Liuzhu, Z.; Sekar, S.; Chen, J.; Lee, S.; Kim, D.Y.; Manikandan, R. A polyrutin/AgNPs coated GCE for simultaneous anodic stripping voltammetric determination of Pb(II) and Cd(II)ions in environmental samples. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129082. [Google Scholar] [CrossRef]
- Nga, N.T.A.; Raghavendra, V.B.; Sindhu, R.; Alshiekheid, M.; Sabour, A.; Krishnan, R.; Chi, N.T.L.; Pugazhendhi, A. Green fabrication of silver nanoparticles using Chloroxylon swietenia leaves and their application towards dye degradation and food borne pathogens. Food Chem. Toxicol. 2022, 165, 113192. [Google Scholar] [CrossRef]
- Capobianco, J.A.; Armstrong, C.M.; Lee, J.; Gehring, A.G. Detection of pathogenic bacteria in large volume food samples using an enzyme-linked immunoelectrochemical biosensor. Food Control 2021, 119, 107456. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.-K.; Zhang, T.-T.; Bi, Y.; Shu, M.; Zhong, C.; Tang, K.-J.; Wu, G.-P. A novel real-time loop-mediated isothermal amplification combined with immunomagnetic beads separation and ethidium bromide monoazide treatment for rapid and ultrasensitive detection of viable Escherichia coli O157: H7 in milk. Food Anal. Methods 2021, 14, 944–956. [Google Scholar] [CrossRef]
- Jacob, J.J.; Suthindhiran, K. Magnetotactic bacteria and magnetosomes–Scope and challenges. Mater. Sci. Eng. C 2016, 68, 919–928. [Google Scholar] [CrossRef]
- Giannetto, M.; Costantini, M.; Mattarozzi, M.; Careri, M. Innovative gold-free carbon nanotube/chitosan-based competitive immunosensor for determination of HIV-related p24 capsid protein in serum. RSC Adv. 2017, 7, 39970–39976. [Google Scholar] [CrossRef]
- Chahri, I.; Karrat, A.; Mohammadi, H.; Amine, A. Development of a new route for the immobilization of unmodified single-stranded DNA on chitosan beads and detection of released guanine after hydrolysis. Molecules 2023, 28, 2088. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Electrochemical immunosensor based on chitosan/conductive carbon black composite modified disposable ITO electrode: An analytical platform for p53 detection. Biosens. Bioelectron. 2018, 121, 80–89. [Google Scholar] [CrossRef] [PubMed]
- El-Moghazy, A.Y.; Wisuthiphaet, N.; Yang, X.; Sun, G.; Nitin, N. Electrochemical biosensor based on genetically engineered bacteriophage T7 for rapid detection of Escherichia coli on fresh produce. Food Control 2022, 135, 108811. [Google Scholar] [CrossRef]
- Lian, F.; Wang, D.; Yao, S.; Ge, L.; Wang, Y.; Zhao, Y.; Zhao, J.; Song, X.; Zhao, C.; Li, J. A detection method of Escherichia coli O157: H7 based on immunomagnetic separation and aptamers-gold nanoparticle probe quenching Rhodamine B’s fluorescence: Escherichia coli O157: H7 detection method based on IMS and Apt-AuNPs probe quenching Rho B’s fluorescence. Food Sci. Biotechnol. 2021, 30, 1129–1138. [Google Scholar] [PubMed]
- Yang, L.; Bashir, R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol. Adv. 2008, 26, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Guo, R.; Huang, F.; Qi, W.; Liu, Y.; Cai, G.; Lin, J. An impedance biosensor based on magnetic nanobead net and MnO2 nanoflowers for rapid and sensitive detection of foodborne bacteria. Biosens. Bioelectron. 2021, 173, 112800. [Google Scholar] [CrossRef]
- Liu, J.; Jasim, I.; Abdullah, A.; Shen, Z.; Zhao, L.; El-Dweik, M.; Zhang, S.; Almasri, M. An integrated impedance biosensor platform for detection of pathogens in poultry products. Sci. Rep. 2018, 8, 16109. [Google Scholar] [CrossRef]
- Chiriacò, M.S.; Parlangeli, I.; Sirsi, F.; Poltronieri, P.; Primiceri, E. Impedance sensing platform for detection of the food pathogen listeria monocytogenes. Electronics 2018, 7, 347. [Google Scholar] [CrossRef]
- Wang, L.; Xue, L.; Guo, R.; Zheng, L.; Wang, S.; Yao, L.; Huo, X.; Liu, N.; Liao, M.; Li, Y. Combining impedance biosensor with immunomagnetic separation for rapid screening of Salmonella in poultry supply chains. Poult. Sci. 2020, 99, 1606–1614. [Google Scholar] [CrossRef]
- Poltronieri, P.; Primiceri, E.; Radhakrishnan, R. EIS-Based Biosensors in Foodborne Pathogen Detection with a Special Focus on Listeria monocytogenes. Methods Mol. Biol. 2019, 1918, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.D.; Thang, C.X. Label-free electrochemical immunosensor based on cerium oxide nanowires for Vibrio cholerae O1 detection. Mater. Sci. Eng. C 2016, 58, 953–959. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Wang, X.; Zhang, W.; Yang, D.-P.; Cui, L.; Wang, X. Label-free 3D Ag nanoflower-based electrochemical immunosensor for the detection of Escherichia coli O157: H7 pathogens. Nanoscale Res. Lett. 2016, 11, 507. [Google Scholar] [CrossRef]
- Zhong, M.; Yang, L.; Yang, H.; Cheng, C.; Deng, W.; Tan, Y.; Xie, Q.; Yao, S. An electrochemical immunobiosensor for ultrasensitive detection of Escherichia coli O157: H7 using CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags. Biosens. Bioelectron. 2019, 126, 493–500. [Google Scholar] [CrossRef]
- Pandey, C.M.; Tiwari, I.; Singh, V.N.; Sood, K.; Sumana, G.; Malhotra, B.D. Highly sensitive electrochemical immunosensor based on graphene-wrapped copper oxide-cysteine hierarchical structure for detection of pathogenic bacteria. Sens. Actuators B. Chem. 2017, 238, 1060–1069. [Google Scholar] [CrossRef]
- Faivre, D.; Schuler, D. Magnetotactic bacteria and magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef] [PubMed]
- Staniland, S.; Ward, B.; Harrison, A.; van der Laan, G.; Telling, N. Rapid magnetosome formation shown by real-time x-ray magnetic circular dichroism. Proc. Natl. Acad. Sci. USA 2007, 104, 19524–19528. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Applications of magnetosomes synthesized by magnetotactic bacteria in medicine. Front. Bioeng. Biotechnol. 2014, 2, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).