Abstract
A 3D-printed device was designed and printed by a stereolithographic technique (SLA) and coated with a highly selective solid phase extraction resin for on-site diclofenac extraction from wastewater, avoiding the transport and treatment of large volumes of samples in the laboratory. The best results in terms of chemical and mechanical resistance were obtained with Rigid 10K resin. The “stick-and-cure” impregnation technique was used to coat the 3D-printed device with Oasis® HLB resin. The coated 3D-printed device can be reused up to eight times without losing extraction efficiency. The eluent and derivatization reagent volumes were optimized by a multivariate design. The proposed method allowed for the extraction and determination of diclofenac by PTV-GC-MS, achieving methodological detection and quantification limits of 0.019 and 0.055 μg L−1, respectively, with a preconcentration factor of 46. The analysis time was 23 min per sample. To validate the proposed methodology, addition/recovery tests were carried out in different wastewater samples, obtaining recoveries above 90%. The methodology was applied at the wastewater treatment plant (WWTP) of Calvià (Mallorca, Spain), finding diclofenac in concentrations of 15.39 ± 0.07 μg L−1 at the input of the primary decantation process, 4.48 ± 0.03 μg L−1 at the output of the secondary decantation, and 0.099 ± 0.001 μg L−1 at the output of the tertiary treatment, demonstrating the feasibility of the on-site extraction method in monitoring diclofenac over a wide concentration range. Finally, a greenness index of 0.58 for the proposed on-site sample preparation was achieved according to the AGREEprep metrics, making it an eco-friendly alternative for diclofenac monitoring.
1. Introduction
Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID), which is widely used in human and veterinary medicine. In general, NSAIDs are non-prescription medicines that, due to their anti-inflammatory and antipyretic properties, are highly consumed. Among these, the most used ones are ibuprofen, diclofenac, and naproxen, whose constant use causes the generation and release of waste into the environment []. The biological action of these drugs is the inhibition of cyclooxygenase enzymes (COX-1 and COX-2), which are responsible for regulating the production of arachidonic acid and its conversion into prostaglandins and thromboxanes, present in inflammatory, febrile, and painful processes []. The generation of waste derived from the use of drugs is currently considered an emerging environmental pollution issue [].
Emerging pollutants, such as diclofenac, are not included in any regulations that control their concentration in the environment or their scope in environmental risk assessments, including both drugs and their metabolites. Each year, more than 100,000 t of NSAIDs are consumed worldwide both in human and veterinary medicine, resulting in significant waste generation and its environmental release, especially into aquatic ecosystems [,], causing severe effects due to chronic exposure [].
Wastewater treatment plants (WWTPs) receive a large proportion of NSAIDs and their metabolites through human urine. They are usually concentrated in sludge, which is subsequently deposited in soil or used as fertilizer []. In conventional WWTPs, a removal rate of diclofenac between 30 and 70% is generally obtained []. In this context, NSAID monitoring in WWTPs is gaining increasing interest. Recent studies have revealed the presence of low concentrations of diclofenac in freshwater bodies, which can affect aquatic organisms since their metabolites are biologically active, especially affecting fish at concentrations of 5 to 50 μg L−1 [,].
Due to the low concentrations (µg L−1 or below) of diclofenac in environmental water samples, preconcentration techniques are required for its determination. In addition, the complexity of environmental sample matrices requires the use of extraction techniques to clean the samples. In this sense, solid phase extraction (SPE) is one of the most widely used extraction techniques due to its high selectivity and preconcentration factor [,]. Among the most used SPE resins for diclofenac determination, it is worth highlighting Oasis® HLB, Oasis® MCX, and Sep-Pak tC18, which were satisfactorily used for biological and environmental sample analysis [,,].
For diclofenac detection, chromatographic techniques are the most used, e.g., high-performance liquid chromatography with a diode array detector (HPLC-DAD) [], liquid chromatography coupled with mass spectrometry (LC-MS-MS) [], liquid chromatography coupled with electro spray ionization (ESI-LC-MS-MS) [], and gas chromatography coupled with mass spectrometry (GC-MS) [].
The 3D printing technique has become an essential technology for the evolution of multiple scientific fields, including analytical chemistry []. The recent evolution in 3D design has allowed a trend toward the miniaturization and portability of analytical systems with exponential growth in environmental field analyses [,]. Stereolithography (SLA), or layer-by-layer printing, is one of the most widely used 3D printing techniques due to its speed and simplicity in prototype production []. This technique is based on photosensitive resin photopolymerization, or curing by ultraviolet light, solidifying the soft material through a polymer cross-linking process, resulting in non-porous structures or units that are functional for the handling of fluids.
Sample preparation is a crucial step in analytical method development, as it significantly impacts the quality of measurement outcomes. However, from the perspective of green chemistry, it poses significant environmental risks due to solvent consumption and other factors, such as analyst–sample interactions, the degree of automation, and energy consumption, among others. In response to this concern, tools like AGREE and AGREEPrep have been developed to assess the environmental impact of sample preparation methods. These tools provide comprehensive evaluations, facilitating the development of greener, more environmentally friendly methods [,].
In this context, the main objective of this work was to develop a greener sample preparation protocol for the on-site extraction of diclofenac from wastewater matrices based on a 3D-printed device coated with a selective SPE resin, avoiding the transport of large volumes of samples to be treated in the laboratory. Given the high complexity of wastewater matrices, both the design and material of the 3D-printed device, together with the selective extraction resin and the impregnation technique, were optimized. This resulted in good sensitivity while transporting only a few milliliters of eluate to the laboratory for GC-MS analysis.
2. Experimental
2.1. Reagents, Materials, and Instrumentation
All solutions were of analytical–reagent grade, and ultrapure Milli-Q water was used to prepare solutions. All glassware was carefully cleaned three times with acetone, and then heated in a muffle furnace at a temperature of 500 °C for an hour to eliminate any possible contamination.
The standard solutions of diclofenac (0.5 to 2000 μg L−1) were prepared from a high-purity solid reagent (Sigma Aldrich, Madrid, Spain, 99%). N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA, >99.9%) from Scharlab (Barcelona, Spain) was used for diclofenac derivatization. Other reagents used, i.e., ethyl acetate, hexane, methanol, ammonium hydroxide, 2-propanol, and acetone, were also provided by Scharlab.
Resins for 3D printing, i.e., Clear, Durable, and Rigid 10K, from Formlabs (Formlabs Inc, Somerville, MA, USA) were tested to fabricate the 3D-printed devices using a Form 3+ SLA printer (Formlabs).
Oasis® HLB, Oasis® MCX, and Sep-Pak tC18 resins were tested as SPE adsorbents for coating the 3D-printed devices.
Polyvinylidene fluoride (PVDF) and dimethylformamide (DMF) (99.5%), used for coating the 3D-printed device, were purchased from Scharlab.
2.2. Three-Dimensional-Printed Device
The device was designed using Rhinoceros 6 software, with a focus on the on-site extraction procedure employing a variable-flow pump (1–4.5 L min−1). Therefore, the 3D-printed device had to have channels to provide a large surface/volume ratio to be coated with the SPE resin. In addition, a 3D-printed container was designed to allow for easy connection to flexible tubes, facilitating sample pumping, and to include screw caps for easy opening and replacement of the 3D-printed device coated with the extraction resin.
Once the 3D printing of each part was complete, they were rinsed with 2-propanol (90% v/v) to remove residual non-polymerized resin. Posteriorly, to complete the polymerization of printing resins, the parts of the 3D-printed container (caps and cylinders) were immediately exposed to ultraviolet light (curing step) in a UV reactor (CL-1000 Ultraviolet Crosslinker, λ = 365 nm) for approximately 6 h. By contrast, 3D-printed devices were first coated with SPE resin and then cured in the UV reactor (see Section 2.4).
2.3. Resin Selection for 3D Printing
Preliminary assays with Clear, Durable, and Rigid 10K printing resins were performed to obtain the highest chemical and mechanical resistance. Depending on their composition, 3D printing resins provide elasticity and resistance to organic solvents, allowing for device reuse.
The solvents studied were selected based on the SPE steps (conditioning, washing, and elution) used for the Oasis® HLB, Oasis® MCX, and tC18 resins, i.e., methanol (100%), methanol and ethyl acetate (50% v/v); methanol and water (50% v/v), methanol and ammonium hydroxide (98% v/v methanol), and ethyl acetate and acetone (50% v/v).
For this, cube-shaped pieces (1 cm3) were printed with each type of 3D printing resin, and exposed to solvents (24 h) commonly used in SPE with the extraction resins tested.
2.4. SPE Resin Selection and Impregnation Technique
The affinity of the SPE resins for diclofenac was evaluated by analyzing the extraction capacity of 50 mg L−1 standard solutions. The tests were performed using commercial cartridges according to the manufacturer’s recommendations. The eluates were analyzed by GC–MS.
Once the SPE resin was selected, three coating techniques were tested, i.e., stick-and-cure, PVDF, and a combination of both []. Briefly, the stick-and-cure procedure involved rinsing the 3D-printed device with 2-propanol just after printing and letting it dry. The device was placed in a 50 mL plastic tube containing SPE resin, which was shaken manually (~2 min). After that, the device coated with the resin beads (40 ± 4 mg, n = 10) was cured in a UV reactor. Finally, it was washed with Milli-Q water to remove any non-impregnated SPE resin. The PVDF technique was performed on cured devices by coating them with a resin dispersion prepared in a PVDF/DMF solution. First, 150 mg of SPE resin was added to 5 mL of acetone and sonicated during 30 min. Then, this dispersion was mixed with 1 g of the PVDF/DMF solution (7.5% w/w) and sonicated for a further 30 min. Finally, the obtained solution (SPE/PVDF/DMF) was added drop by drop onto the cured 3D-printed device until it was completely covered by the solution. The excess was removed with nitrogen gas, and it was left to dry at 60 °C for 30 min. Using this technique, each 3D-printed device was coated with resin beads (37 ± 6 mg, n = 10). In addition, a combination of both techniques was assayed by first performing the stick-and-cure technique and then performing the PVDF technique on the same device.
2.5. Variable Optimization
The variables of elution volume (45–75 mL) and derivatization reagent volume (30–70 µL) were optimized by multivariate analysis using a composite central design (Minitab Statistical Software 19). Ranges for both variables were selected based on previous GC-MS analysis in order to ensure the extraction and determination of 200 μg L−1 diclofenac using the developed coated 3D-printed devices.
2.6. Analytical Procedure
For the field application of the developed 3D-printed device coated with SPE resin for the on-site extraction and preconcentration of diclofenac from wastewater, the analytical protocol was as follows:
- A 3D-printed device coated with Oasis® HLB resin was submerged in conditioning solution for 15 min (a plastic tube containing 20 mL of 50% v/v methanol). It is recommended that the time does not exceed 20 min to prolong the life of the device.
- Then, the device was placed in the 3D-printed container, which was connected on one end to the tube of the pump and on the other end to the sampling tube.
- The pump was set to a flow rate of 2.0 L min−1 for the required time (in this case, 120 s allowed for a 4 L sample of wastewater).
- The sampling tube was placed in the wastewater stream of the input or output of the WWTP treatments, and the pump was turned on.
- Once the sampling was finished, the 3D-printed device was rinsed with ultrapure water and placed in a hermetic bottle containing the eluent solution (85 mL of methanol), which was transported to the laboratory in an insulated plastic tube.
- Once in the laboratory, the 3D-printed device was immediately removed, and the eluate was treated in order to derivatize the diclofenac and subsequently analyze it by PTV-GC-MS.
2.7. Conditions of GC-MS
The analysis was performed using a HP 7890 series GC (Agilent Technologies, Palo Alto, CA, USA), equipped with a programmable temperature vaporizing (PTV) and a HP 5973C mass selective detector system.
The PTV was set to solvent vent mode, and to control the initial temperature, liquid nitrogen cooling was required at the end of each run. The initial PTV temperature was set at 50 °C and held for 1 min. The vent flow was adjusted to 30 mL min−1, with a vent pressure of 5.0 psi, and a total vent time of 2 min. After this period, the split valve was closed, and the injector was flash-heated at 12 °C/s to a final temperature of 280 °C.
The oven temperature program started at 100 °C (held for 1 min), increased at 20 °C min−1 to 200 °C, and then at 5 °C min−1 to 280 °C, where it was held for 8 min. The PTV was operated in solvent vent mode at a flow of 30 mL min−1, maintaining a constant pressure in the column of 10.52 psi, resulting in approximately 1 mL min−1 of carrier (helium 99.999%). The diclofenac was separated using an Agilent HP-5MS column, 30 m in length, with a 0.25 µm film thickness of 5% methyl phenyl polysiloxane. A solvent delay time of 10 min was applied to avoid overworking the excitation filament, since diclofenac has a retention time of 12.3 min. Therefore, the resulting analysis time for diclofenac was 23 min to ensure flushing of residual sample, avoiding cross-contamination.
The detector was configured in SIM mode, with m/z values of 179, 214, and 242, since in previous analysis, it was observed that the most characteristic fragments of the derivatized diclofenac were found at these values.
Chromatographic data was recorded using HP Chemstation F.01.03.2365 software (Agilent Technologies, Palo Alto, CA, USA).
2.8. On-Site Extraction
The on-site extraction of diclofenac was performed by placing a preconditioned 3D-printed device coated with Oasis® HLB resin in a specially designed 3D-printed container, which was connected to an aspiration pump. To investigate the on-site sampling under real conditions, the input stream of the primary decantation process and the output streams of the secondary decantation and the tertiary treatment were sampled at a WWTP (Calvià-Mallorca, Spain). Once the extraction step was finished, the 3D-printed devices were placed in plastic tubes containing the elution solution and transported to the laboratory in insulated plastic tubes.
2.9. Green Metrics Assessment
A comprehensive analysis of the developed method was conducted using AgreePrep 0.91 software, objectively following the guidelines outlined in the methodology proposed by the authors []. The AGREE prep tool estimates the degree of sustainability of a sample treatment methodology by quantifying the weighted values of ten impact categories, from 0 to 1, related to the ten principles of green sample preparation. Therefore, this analysis method allowed for the identification of the strengths and weaknesses of the current procedure for the determination of diclofenac in wastewater matrices.
3. Results and Discussion
3.1. SPE Resin Selection
Three SPE resins packed in cartridges were tested in order to find the best extraction efficiency, i.e., Sep-pack tC18, Oasis® HLB, and Oasis® MCX, following the procedures recommended by each manufacturer (Table S1). All of them have previously been used for the extraction of β-blockers and β2-agonists and other pharmaceuticals from wastewater [,].
These resins are effective due to their retention mechanisms. Sep-pack tC18 is hydrophobic, while HLB (hydrophilic lipophilic balance) is a reversed-phase dimer-type sorbent (co-polymer: polypropylene, 80 Å pore size, 30 μm particle size). It is a macro-porous copolymer made with a balanced ratio of two monomers, namely lipophilic divinylbenzene and hydrophilic N-vinylpyrrolidone. The retention mechanisms of HLB are hydrophilicity and lipophilicity. In addition, a mixed-mode cation-exchange sorbent (Oasis MCX) was used.
Table 1 presents the results obtained. The highest extraction efficiency (97%) of diclofenac was achieved with the Oasis HLB extraction resin. Therefore, HLB was the SPE resin used in further experiments.

Table 1.
Extraction efficiency of diclofenac for the packaged solid-phase extraction resins tested. The results are expressed as the mean ± SD (n = 3).
3.2. Coated 3D-Printed Device and 3D-Printed Container
To perform the diclofenac on-site extraction, a 3D-printed device was successfully designed and manufactured using Rigid 10K printing resin due to its higher mechanical resistance to all the solvents evaluated (Figure S1, Supplementary Material). The 3D-printed device was coated with SPE resin and placed in a 3D-printed container.
To meet the sampling requirements, the 3D-printed device consists of the following parts (Figure 1):
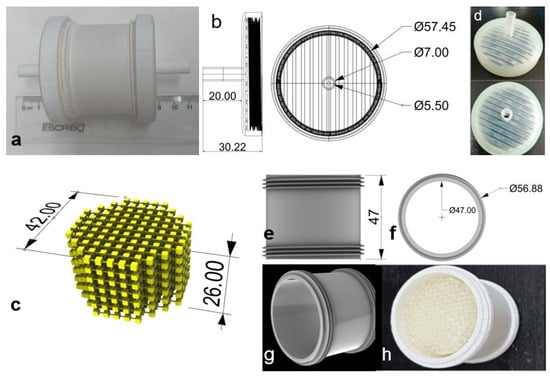
Figure 1.
Three-dimensional-printed device designed for on-site diclofenac extraction. (a) Image of external 3D-printed container; (b) two threaded caps on ends allow for connection with suction pump and sampling tube; (c) internal cube coated with SPE resin; (d) image of 3D-printed caps; (e) design of 3D-printed container with external thread; (f) 3D-printed container diameter (top view); (g) isometric view of 3D-printed container; (h) 3D-printed container with 3D-printed extraction device. All dimensions are expressed in mm.
- Caps: Two pieces that have a socket on one side to attach to a hose of 0.7 cm i.d., and on the other, an internal thread of 3.5 turns.
- A cube composed of a network of interconnected cubes: An internal device coated with the SPE resin, providing a greater area of contact with the analyte of interest.
- External 3D-printed container: This protects the internal coated 3D-printed device, with external threads to connect to the caps.
3.3. Coating Technique Selection
With the aim of selecting the most suitable impregnation technique for applying the selected SPE resin onto the 3D-printed extraction device, the stick-and-cure technique was compared with the PVDF coating technique and with a combination of both. As can be seen in Table 2, better results in terms of the extraction capacity (88%) were obtained using the stick-and-cure technique. As expected, the extraction percentage of the supported resin decreased compared to the packaged resin, due to the loss of useful extraction surface that occurred when applied to the 3D-printed device. The PVDF and combination techniques did not allow for diclofenac extraction, probably due to the mechanical obstruction of the functional sites of the resins.

Table 2.
Extraction efficiency of diclofenac using different coating techniques on 3D-printed devices. The results are expressed as the mean ± SD (n = 3). --, not calculated.
3.4. Optimized Variables
Two variables that affect the methodology were optimized by experimental design, including the elution volume of the SPE protocol and the derivatization volume for diclofenac determination by GC-MS. The ANOVA results (Table S2) indicate that both variables were significant within the tested experimental domains. Finally, using the desirability function of Minitab 19 software, a summary graph was obtained that reflects the arrangement of the variable values to achieve the maximum response within the experimental domain studied (Figure 2). An optimal value for the derivatization volume of 58.68 μL was estimated. For practical purposes, this was set at 60 μL. However, the optimal eluent volume was not determined, as it only showed an upward trend, even when it reached the highest level analyzed; therefore, it underwent a univariate study. For this, a 60 μL derivatization volume was employed, varying the eluent volume (75, 85, 95, and 105 mL). Each trial was performed in triplicate. The maximum peak area was reached with a volume of 85 mL (Figure 3). Therefore, the elution volume to perform the SPE was set at 85 mL. To complete the analytical protocol, 60 μL of MSTFA as a derivatization reagent was added to the eluate, followed by thermal treatment at 80 °C for 30 min in an oven.
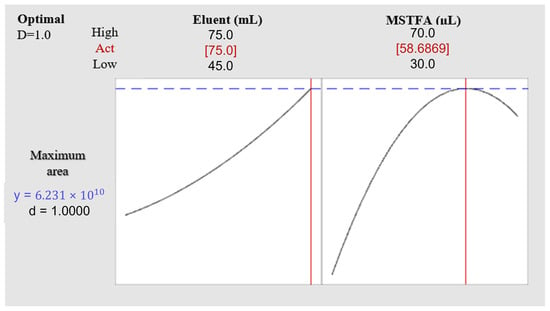
Figure 2.
Desirability function from the multivariate optimization of derivatization and eluent volumes. MSTFA: N-methyl-N-trimethylsilyl trifluoroacetamide (derivatization reagent). The red line indicates the optimized value of each variable; the dashed blue line indicates the desired optimal response, and the black line represents the variable responses within the experimental domain.
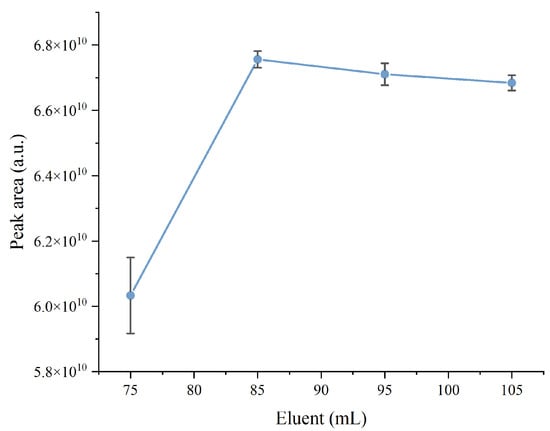
Figure 3.
Univariate study of eluent volume. Error bars represent SD (n = 3).
3.5. Analytical Parameters
The retention time (tR) of diclofenac was 12.3 min, with a 10 min solvent delay added to avoid overworking the excitation filament. Therefore, 2.6 samples per hour were analyzed by GC-MS.
The linearity of the instrumental response was evaluated over the diclofenac concentration range of 0.5–2000 µg L−1 (y = 8.64 × 105 x −1.42 × 107, R2 = 0.9984, n = 8). Additionally, the linearity was also evaluated by applying the proposed methodology. In this case, the methodological calibration was also satisfactory (y = 2.15 × 109 x 7.52 × 108, R2 = 0.9984, n = 8), presenting a much narrower diclofenac concentration range, i.e., 0.01–43.3 µg L−1, and a higher sensitivity (>2000 times) than the instrumental curve (Figure 4).
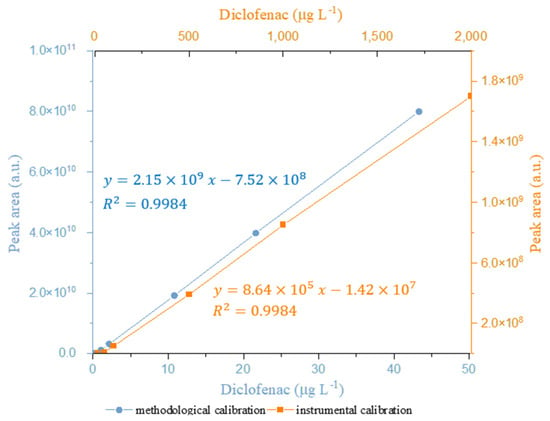
Figure 4.
Calibration curves of diclofenac. Orange represents instrumental calibration using GC-MS. Blue represents methodological calibration using the 3D-printed device for the extraction/preconcentration of diclofenac before GC-MS analysis.
According to IUPAC [], the LOD and LOQ were calculated as three and ten times, respectively, the standard deviation of the reagent blank analytical signal (n = 10) divided by the slope of the calibration curve. Thus, the instrumental LOD and LOQ achieved for diclofenac were 0.86 and 2.86 μg L−1, while the methodological LOD and LOQ were 0.019 and 0.055 μg L−1, respectively.
An interday precision of 0.4% relative standard deviation (RSD, n = 3) was calculated using different coated 3D-printed devices on different working days, based on the analytical signal from a 5 µg L−1 standard solution (Table S3). In addition, 0.85% RSD (n = 3) was calculated from real sample concentrations using the same coated 3D-printed device, demonstrating the good precision of the proposed methodology.
The preconcentration factor (PF) was calculated considering a sample volume of 4 L and elution volume of 85 mL, to which were added 0.9 mL of ethyl acetate and 0.6 mL of derivative agent. Thus, a PF of 46.2 was reached.
In addition, assays were conducted to evaluate the reusability of the 3D-printed device. Aliquots of the same sample were analyzed employing a unique device performing 10 consecutives cycles of the analytical protocol. The analytical signals, i.e., the peak areas, were compared. The results obtained with only one device are promising, showing it can be used up to 10 times. Among the first eight cycles, the analytical signal remained practically constant, while the analytical signal decreased by 6% in the last cycle (Figure S2).
A summary of all analytical parameters is shown in Table 3.

Table 3.
Figures of merit of developed on-site extraction by 3D-printed device and subsequent GC-MS analysis.
3.6. Method Validation and Application to Environmental Samples
Calibration curves (0; 0.01; 0.1; 1; 10; 25; and 50 μg L−1) were obtained by applying the proposed methodology and GC-MS for diclofenac quantification. The satisfactory results obtained by GC-MS were possible thanks to the derivatization technique and the use of a PTV injector, which enhanced the analytical signal of the instrument. This combination, together with the PF, allowed for improvement of the detection and quantification limits [].
Firstly, addition/recovery assays were conducted at the laboratory by adding 4.1 μg L−1 of diclofenac to deionized water and tap water to estimate its recovery using the proposed method. Satisfactory recoveries greater than 89% were obtained, as shown in Table 4.

Table 4.
Recovery of diclofenac using the developed on-site extraction 3D-printed device and subsequent GC-MS analysis. The results are expressed as the mean ± SD (n = 3).
Then, the on-site extraction of diclofenac using the developed coated 3D-printed device was performed at three different sites (primary decantation input, secondary decantation output, and tertiary treatment output) at the WWTP of Calvià (Mallorca, Spain). A typical chromatogram of a real wastewater sample is shown in Figure S3.
In addition, samples were also collected at the same sites and transported to the laboratory, where diclofenac (20 μg L−1) was added to calculate the percentage of recovery. The WWTP samples contained diclofenac in concentrations of 15.39 ± 0.07 μg L−1 at the input of the primary decantation process, 4.48 ± 0.03 μg L−1 at the output of secondary decantation, and 0.099 ± 0.001 μg L−1 at the output of tertiary treatment. As wastewater treatment increases, the concentration of diclofenac decreases, up to trace concentrations in the regenerated water that will then be used for different purposes. It is important to note that despite the significant decrease (approximately 170-fold), the proposed method allows for diclofenac to be monitored throughout the entire wastewater treatment process. The results of real sample analysis are summarized in Table 5.

Table 5.
Diclofenac concentrations found through on-site extraction using the 3D-printed device and subsequent GC-MS analysis in three stages at the Calvià WWTP (Mallorca). In addition, the samples were spiked at the laboratory and analyzed by the proposed methodology. The results are expressed as the mean ± SD (n = 3). --, not calculated.
3.7. Green Metrics
The environmental impact of the proposed methodology has been assessed using the analytical sustainability metric tool AGREEprep. A summary of the aspects considered in each category is presented in Table S4, together with the calculated score values. The results of the evaluation (0.58 score) using the pre-determined weighting factors for each principle are shown in Figure 5.
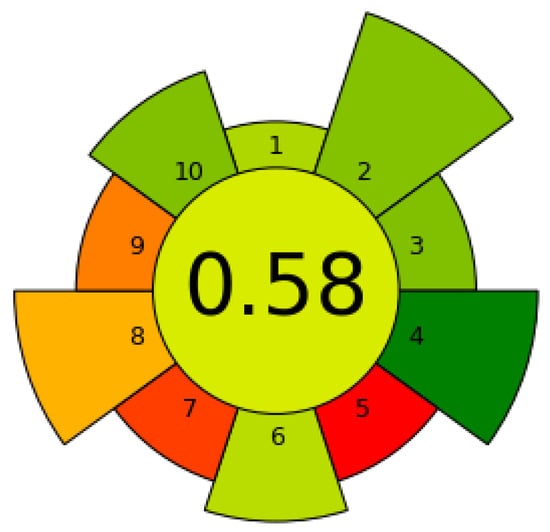
Figure 5.
Analytical greenness metric for sample preparation (AGREEprep) for on-site diclofenac extraction using the developed coated 3D-printed device. Red (low), yellow (middle), green (high): Color scale for the ten principles of green sample preparation, with the resulting score in the center.
The developed method has several positive aspects, such as the use of more than 75% sustainable and reusable materials, which significantly reduces the environmental impact compared to traditional single-use methods. In addition, the use of hazardous materials was optimized through experimental design, minimizing the amount required without compromising process efficiency. This improvement increases operator safety by limiting exposure to hazardous materials only to MSTFA.
The method also demonstrated the ability to prepare at least 15 samples per hour, which is efficient in terms of throughput. However, there are associated limitations, i.e., the large sample volumes required to quantify the low concentration of diclofenac in the environmental matrix, as well as the low automation and moderate energy consumption due to manual processes and equipment with high energy requirements. These aspects should be considered for future improvements.
However, the overall approach strikes a positive balance by reducing environmental impact using sustainable materials and optimizing hazardous substances, contributing to operator safety and process success.
In comparison with previous methods for extracting diclofenac and other NSAIDs from wastewater based on SPE, which obtained AGREEprep scores between 0.32 and 0.64 [,,,], the score of the proposed methodology (0.58) could be considered a green alternative with a good competitive environmental performance.
4. Conclusions
A 3D-printed device was designed and built with Rigid 10K printing resin through SLA, then coated with Oasis HLB extraction resin by the stick-and-cure technique. In this way, it achieved an inert support based on complex geometries that help to improve extraction conditions, with the lowest degree of lixiviation of non-polymerized resin and the highest mechanical resistance to all the solvents evaluated.
The coated 3D-printed device was incorporated into a pumping system, allowing for diclofenac on-site extraction, and subsequent analysis by PTV-GC-MS using derivatization, yielding satisfactory results. The proposed method was optimized and validated. A preconcentration factor of 46, methodological detection and quantification limits of 0.019 and 0.055 μg L−1, respectively, and recoveries above 90% were achieved.
The coated 3D-printed device was satisfactorily applied to the on-site extraction of diclofenac from wastewater at the Calvià WWTP (Mallorca, Spain), demonstrating the capacity to monitor diclofenac at different steps of wastewater treatment, in a wide range of concentrations.
Finally, an AGREEprep score of 0.58 was obtained, with limitations due to the larger sample volumes required to quantify low concentrations. However, the overall approach strikes a positive balance by reducing environmental impact using sustainable materials and optimizing hazardous substances, contributing to operator safety and process success.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors13060212/s1: Figure S1: Three-dimensional printing resins. Clear resin (a), Durable resin (b) and Rigid 10K resin (c), exposed to solvents (24 h) commonly used in SPE (methanol, ammonium hydroxide, and ethyl acetate); Figure S2: Reuse tests of diclofenac extraction by coated 3D-printed device and GC-MS quantification for 10 cycles.; Figure S3: Wastewater sample chromatogram showing diclofenac peak at tR = 12.3 min; Table S1: Solid-phase extraction procedures based on manufacturer’s recommendations for the evaluation of the extraction capacity of SPE resins (50 mg L−1 diclofenac standard solutions); Table S2: ANOVA of optimization variables, i.e., eluent and derivatizing volumes; Table S3: Peak areas corresponding to a diclofenac pattern of 5 μg L−1 to calculate the interday precision of the method; Table S4: Assessment of analytical sample preparation greenness of the proposed method using the AGREEprep tool.
Author Contributions
Conceptualization, L.F. and L.O.L.; methodology, C.C.-G.; software, C.C.-G.; validation, E.P.; formal analysis, C.C.-G.; investigation, C.C.-G.; resources, E.P.; data curation, L.F.; writing—original draft preparation, C.C.-G.; writing—review and editing, L.F.; visualization, L.F.; supervision, R.R.-M. and L.F.; project administration, L.F.; funding acquisition, L.O.L. and L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from project PID2019-107604RB-I00 funded by MCIN/AEI/10.13039/501100011033, and from project FECTI/2024/CV-CDF/015, funded by Fondo Estatal de Ciencia, Tecnología e Innovación of Gobierno del Estado de Chihuahua (Mexico).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
César Castro acknowledges the support from the SECIHTI and CIMAV for the PhD fellowship. The authors thank Calvià2000 for the access to WWTP to analyze the wastewater samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Świacka, K.; Maculewicz, J.; Smolarz, K.; Caban, M. Long-term stability of diclofenac and 4-hydroxydiclofenac in the seawater and sediment microenvironments: Evaluation of biotic and abiotic factors. Environ. Pollut. 2022, 304, 119243. [Google Scholar] [CrossRef]
- Voilley, N.; De Weille, J.; Mamet, J.; Lazdunski, M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 2001, 21, 8026–8033. [Google Scholar] [CrossRef]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Klaper, R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 2013, 93, 2116–2123. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, E.; Yoon, S.H.; Chang, H.R.; Kim, K.; Kwon, J.H. Enzymatic and microbial transformation assays for the evaluation of the environmental fate of diclofenac and its metabolites. Chemosphere 2012, 87, 969–974. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Bian, Y.; Zhang, Y.; Du, R.; Li, M.; Tan, Y.; Feng, X. Non-steroidal anti-inflammatory drugs (NSAIDs) in the environment: Recent updates on the occurrence, fate, hazards and removal technologies. Sci. Total Environ. 2023, 904, 166897. [Google Scholar] [CrossRef]
- Plaza, P.I.; Wiemeyer, G.M.; Lambertucci, S.A. Veterinary pharmaceuticals as a threat to endangered taxa: Mitigation action for vulture conservation. Sci. Total Environ. 2022, 817, 152884. [Google Scholar] [CrossRef]
- Dey, S.; Bano, F.; Malik, A. 1—Pharmaceuticals and personal care product (PPCP) contamination—a global discharge inventory. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Butterworth-Heinemann: Oxford, UK, 2019; pp. 1–26. ISBN 9780128161890. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Intern. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Hoeger, B.; Köllner, B.; Dietrich, D.R.; Hitzfeld, B. Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquat. Toxicol. 2005, 75, 53–64. [Google Scholar] [CrossRef]
- Lolić, A.; Paíga, P.; Santos, L.H.; Ramos, S.; Correia, M.; Delerue-Matos, C. Assessment of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawaters of North of Portugal: Occurrence and environmental risk. Sci. Total Environ. 2015, 508, 240–250. [Google Scholar] [CrossRef]
- Reinholds, I.; Pugajeva, I.; Zacs, D.; Lundanes, E.; Rusko, J.; Perkons, I.; Bartkevics, V. Determination of acidic non-steroidal anti-inflammatory drugs in aquatic samples by liquid chromatography-triple quadrupole mass spectrometry combined with carbon nanotubes-based solid-phase extraction. Environ. Monit. Assess. 2017, 189, 568. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Qawariq, R.F.; Abdelghani, J.I. Adsorption and magnetic solid-phase extraction of NSAIDs from pharmaceutical wastewater using magnetic carbon nanotubes: Effect of sorbent dimensions, magnetite loading and competitive adsorption study. Environ. Technol. Innov. 2019, 16, 100496. [Google Scholar] [CrossRef]
- Millership, J.; Hare, L.; Farry, M.; Collier, P.; McElnay, J.; Shields, M.; Carson, D. The use of hydrophilic lipophilic balanced (HLB) copolymer SPE cartridges for the extraction of diclofenac from small volume paediatric plasma samples. J. Pharm. Biomed. Anal. 2001, 25, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Migowska, N.; Caban, M.; Łukaszewicz, P.; Stepnowski, P. Simultaneous determination of non-steroidal anti-inflammatory drugs and oestrogenic hormones in environmental solid samples. Sci. Total Environ. 2015, 508, 498–505. [Google Scholar] [CrossRef]
- Zhou, J.; Broodbank, N. Sediment-water interactions of pharmaceutical residues in the river environment. Water Res. 2014, 48, 61–70. [Google Scholar] [CrossRef]
- Tartaglia, A.; Kabir, A.; D’Ambrosio, F.; Ramundo, P.; Ulusoy, S.; Ulusoy, H.; Merone, G.; Savini, F.; D’Ovidio, C.; Grazia, U.D.; et al. Fast off-line FPSE-HPLC-PDA determination of six NSAIDs in saliva samples. J. Chromatogr. B 2020, 1144, 122082. [Google Scholar] [CrossRef]
- Sandrin, V.S.S.; Oliveira, G.M.; Weckwerth, G.M.; Polanco, N.L.D.H.; Faria, F.A.C.; Santos, C.F.; Calvo, A.M. Analysis of different methods of extracting NSAIDs in biological fluid samples for LC-MS/MS assays: Scoping review. Metabolites 2022, 12, 751. [Google Scholar] [CrossRef]
- Kretschmer, A.; Giera, M.; Wijtmans, M.; de Vries, L.; Lingeman, H.; Irth, H.; Niessen, W. Derivatization of carboxylic acids with 4-APEBA for detection by positive-ion LC-ESI–MS(/MS) applied for the analysis of prostanoids and NSAID in urine. J. Chromatogr. B 2011, 879, 1393–1401. [Google Scholar] [CrossRef]
- Krokos, A.; Tsakelidou, E.; Michopoulou, E.; Raikos, N.; Theodoridis, G.; Gika, H. NSAIDs determination in human serum by GC-MS. Separations 2018, 5, 37. [Google Scholar] [CrossRef]
- Gross, B.; Lockwood, S.Y.; Spence, D.M. Recent advances in analytical chemistry by 3D printing. Anal. Chem. 2017, 89, 57–70. [Google Scholar] [CrossRef]
- Agrawaal, H.; Thompson, J.E. Additive manufacturing (3D printing) for analytical chemistry. Talanta Open 2021, 3, 100036. [Google Scholar] [CrossRef]
- Belka, M.; Baczek, T. Additive manufacturing and related technologies—The source of chemically active materials in separation science. Trends Anal. Chem. 2021, 142, 116322. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical greenness metric for sample preparation. Trends Anal. Chem—TRAC 2022, 149, 116553. [Google Scholar] [CrossRef]
- Barzallo, D.; Palacio, E.; March, J.; Ferrer, L. 3D printed device coated with solid-phase extraction resin for the on-site extraction of seven sulfonamides from environmental water simples preceding HPLC-DAD analysis. Microchem. J. 2023, 190, 108609. [Google Scholar] [CrossRef]
- Hanafiah, Z.M.; Mohtar, W.H.; Manan, T.S.; Bachi, N.A.; Abdullah, N.A.; Hamid, H.H.; Beddu, S.; Kamal, N.L.; Ahmad, A.; Rasdi, N.W. The occurrence of non-steroidal anti-inflammatory drugs (NSAIDs) in Malaysian urban domestic wastewater. Chemosphere 2022, 287, 132134. [Google Scholar] [CrossRef]
- Lavén, M.; Alsberg, T.; Yu, Y.; Adolfsson-Erici, M.; Sun, H. Serial mixed-mode cation- and anion-exchange solid-phase extraction for separation of basic, neutral and acidic pharmaceuticals in wastewater and analysis by high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2009, 216, 49–62. [Google Scholar] [CrossRef]
- Gold, V. Limit of detection in analysis. In The IUPAC Compendium of Chemical Terminology; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, USA, 2014. [Google Scholar] [CrossRef]
- Pavón, J.L.P.; Ferreira, A.M.C.; Laespada, M.E.F.; Cordero, B.M. In situ derivatization reaction and determination of ibuprofen in water samples using headspace generation-programmed temperature vaporization-gas chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 6728–6734. [Google Scholar] [CrossRef]
- Godlewska, K.; Lis, H.; Caban, M.; Paszkiewicz, M. Advances in monitoring pharmaceuticals in an aquatic environment: Greenness assessment of analytical procedures. TrAC Trends Anal. Chem. 2024, 180, 117921. [Google Scholar] [CrossRef]
- Peña-Velasco, G.; Hinojosa-Reyes, L.; Escamilla-Coronado, M.; Turnes-Palomino, G.; Palomino-Cabello, C.; Guzmán-Mar, J.L. Iron metal-organic framework supported in a polymeric membrane for solid-phase extraction of anti-inflammatory drugs. Anal. Chim. Acta 2020, 1136, 157–167. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; Lu, N.; Wu, X.; Zhang, Y.; Hou, X. Development and application of metal-organic framework@GA based on solid-phase extraction coupling with UPLC-MS/MS for the determination of five NSAIDs in water. Talanta 2021, 225, 121846. [Google Scholar] [CrossRef]
- Mejías, C.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Automatised on-line SPE-chiral LC-MS/MS method for the enantiomeric determination of main fluoroquinolones and their metabolites in environmental water samples. Microchem. J. 2023, 185, 108217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).