Electrochemical Assessment of Microbial Activity Using PEDOT:PSS-Immobilized Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Microorganisms
2.3. PEDOT:PSS Biosensor Fabrication
2.4. Biosensor Characterization
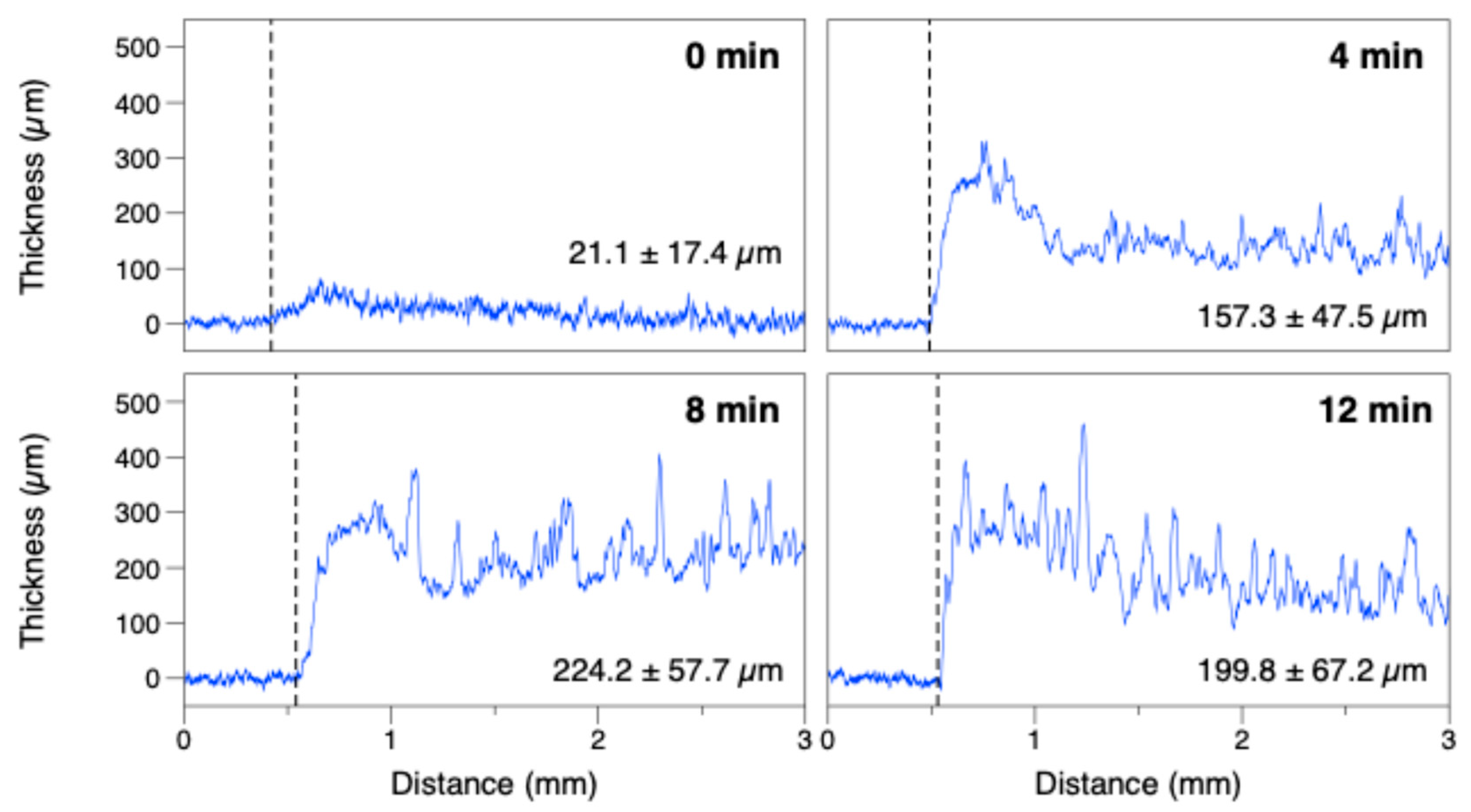
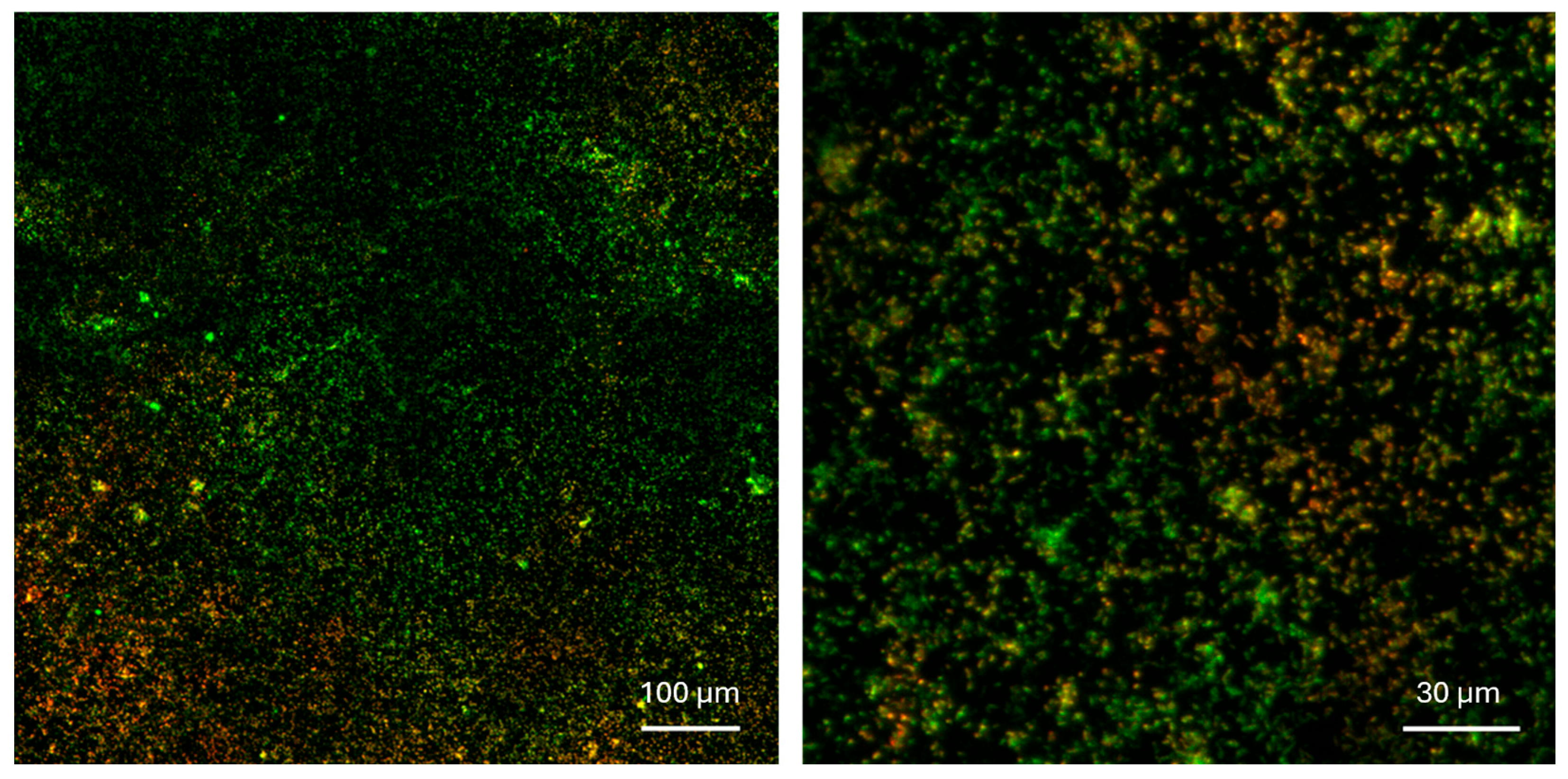
2.4.1. Confocal Imaging and Profilometry
2.4.2. Ferricyanide Respirometry
2.4.3. Toxicity Assay
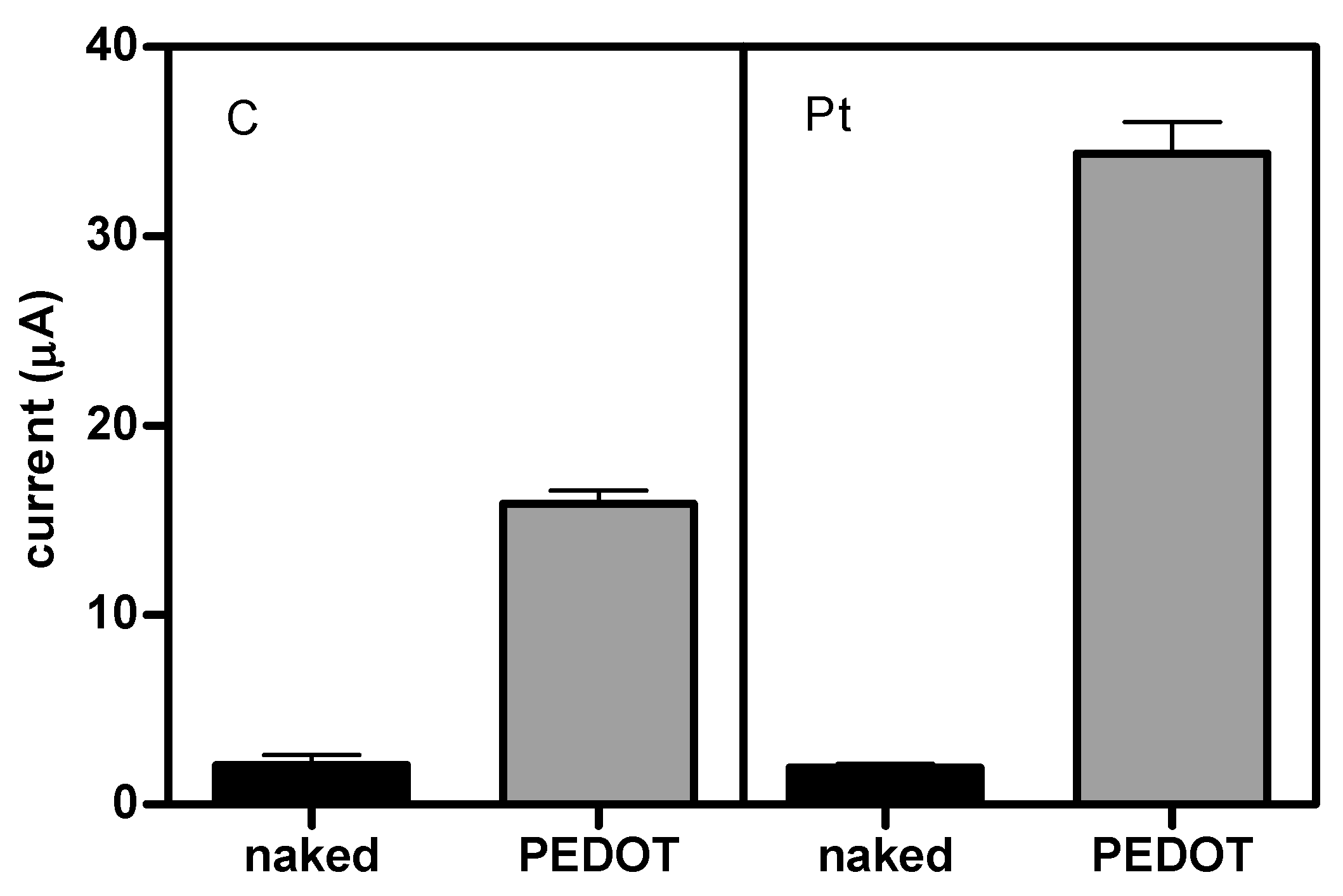
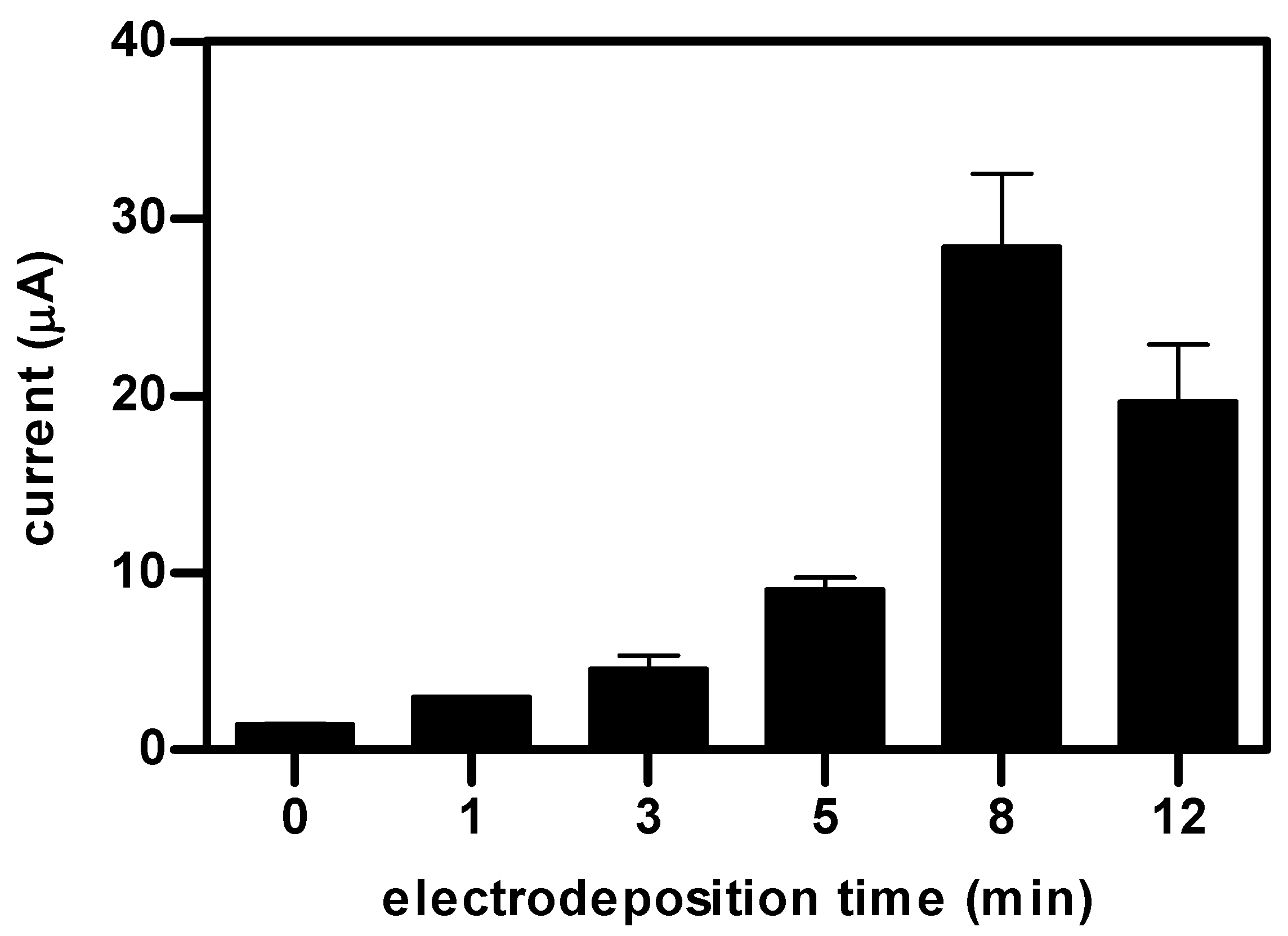
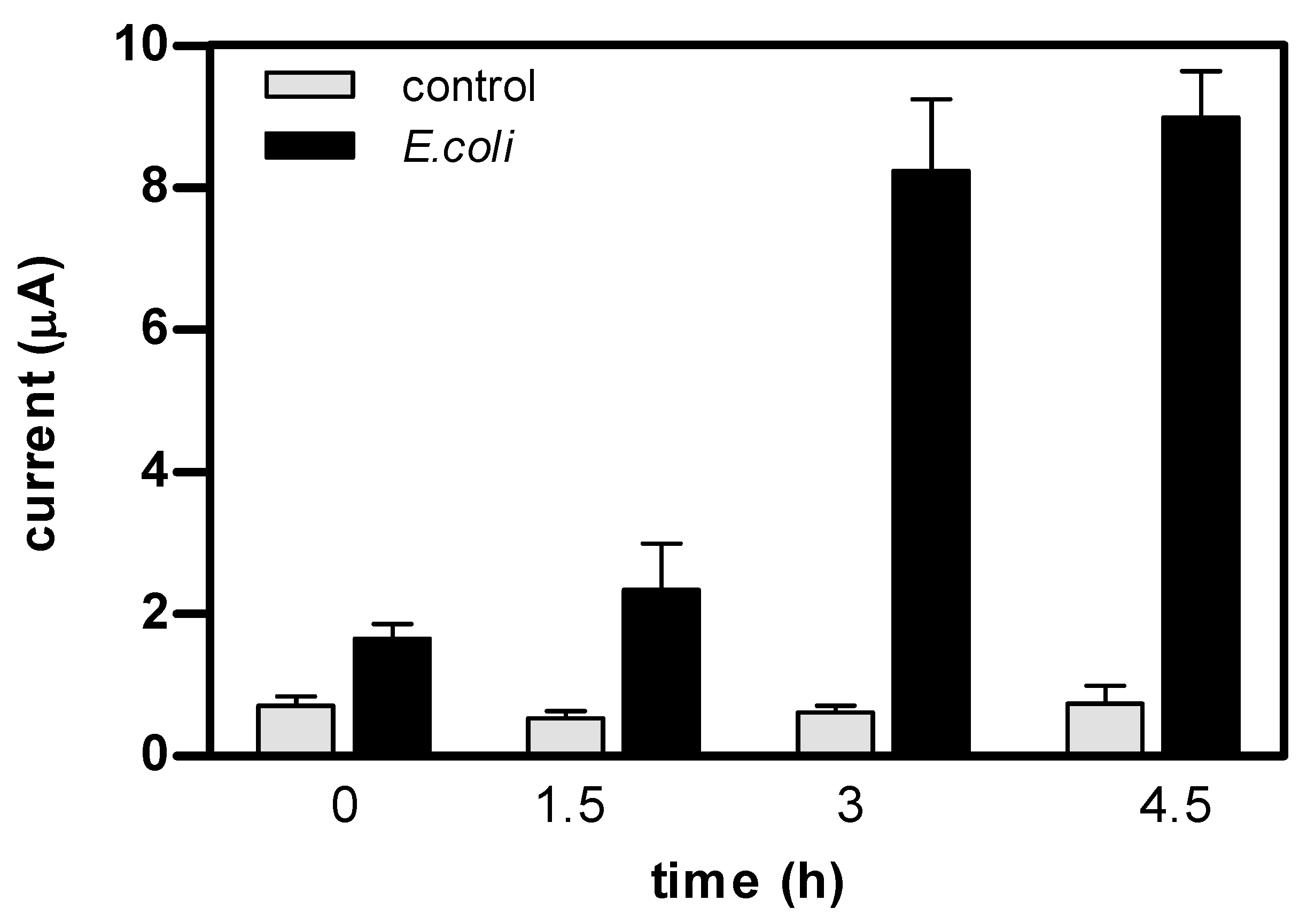
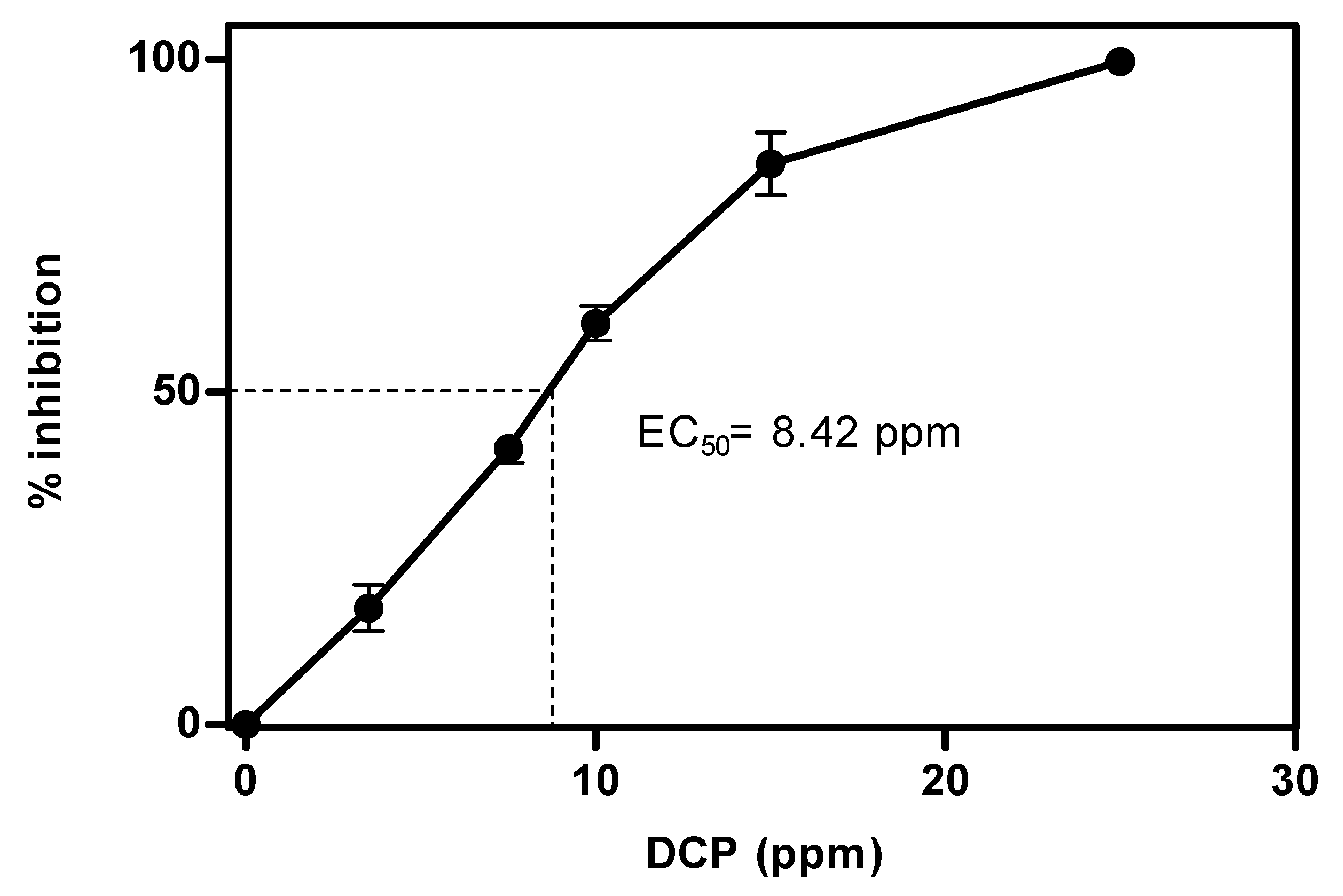
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PEDOT:PSS | poly(3,4-ethylenedioxythiophene):polystyrene |
| 3,5-DCP | 3,5-dichlorophenol |
| EC50 | half-maximum effective concentration |
| PVA | polyvinyl alcohol |
| PVP | polyvinylpyrrolidone |
| PPy | polypyrrole |
| Pth | polythiophene |
| PANI | polyaniline |
| EDOT | 3,4-ethylenedioxythiophene |
| PSS | sulfonated polystyrene |
| SPE | screen-printed electrodes |
| NaPSS | sodium poly(4-styrenesulfonate) |
| LB | Luria–Bertani |
| PBS | phosphate-buffered saline |
| I% | percentage of inhibition |
References
- Lei, Y.; Chen, W. Microbial biosensors. Anal. Chem. Acta 2006, 568, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar]
- Ding, L.; Du, D.; Zhang, X.; Ju, H. Trends in cell-based electrochemical biosensors. Curr. Med. Chem. 2008, 15, 3160–3170. [Google Scholar] [CrossRef]
- Vigués, N.; Pujol-Vila, F.; Marquez-Maqueda, A.; Muñoz-Berbel, X.; Mas, J. Electro-addressable conductive alginate hydrogel for bacterial trapping and general toxicity determination. Anal. Chim. Acta 2018, 1036, 115–120. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Manjulahumari, D. Microbial biosensors and bioelectronics. Res. J. Biotech. 2012, 7, 102–108. [Google Scholar]
- D’Souza, S.F. Microbial biosensors. Biosens. Bioelectron. 2001, 16, 337–353. [Google Scholar] [CrossRef]
- Trelles, J.A.; Rivero, C.W. Whole cell entrapment techniques. Methods Mol. Biol. 2013, 1051, 365–374. [Google Scholar] [PubMed]
- Efremenko, E.N.; Senko, O.V.; Aleskerova, L.E.; Alenina, K.A.; Mazhul, M.M.; Ismailov, A.D. Biosensors based on the luminous bacteria Photobaterium phosphoreum immobilized in polyvinyl alcohol cryogel for the monitoring of ecotoxicants. Appl. Biochem. Microbiol. 2014, 50, 477–482. [Google Scholar] [CrossRef]
- Ruan, B.; Wu, P.; Chen, M.; Lai, X.; Chen, L.; Yu, L.; Gong, B.; Kang, C.; Dang, Z.; Shi, Z.; et al. Immobilization of Shingomonas sp. GY2B in polyvinyl alcohol-alginate-kaolin beads for efficient degradation of phenol against unfavorable environmental factors. Ecotox. Environ. Saf. 2018, 162, 103–111. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Shamsabadipour, A.; Aslani, A.; Eshaghi, M.M.; Rahdar, M.A.; Pandey, S. Development of polyvinylpyrrolidone-based nanomaterials for biosensors applications: A review. Inorg. Chem. Commun. 2023, 152, 110714. [Google Scholar] [CrossRef]
- Shin, H.J. Agarose-gel-immobilized recombinant bacterial biosensors for simple and disposable on-site detection of phenolic compounds. Appl. Microbiol. Biotechnol. 2012, 93, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Vigués, N.; Pujol-Vila, F.; Macanás, J.; Muñoz, M.; Muñoz-Berbel, X.; Mas, J. Fast fabrication of reusable polyethersulfone microbial biosensors through biocompatible phase separation. Talanta 2020, 206, 120192. [Google Scholar] [CrossRef] [PubMed]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2020, 12, 481–494. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting polymers in the design of biosensors and biofuel cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Apetrei, R.M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Sensitivity enhancement for microbial biosensors through cell self-coating with polypyrrole. Int. J. Polym. Mater. 2019, 68, 1058–1067. [Google Scholar] [CrossRef]
- Canbay, E.; Habip, A.; Kara, G.; Eren, Z.; Akyilmaz, E. A microbial biosensor based on Lactobacillus delbruecki sp. bacterial cells for simultaneous determination of lactic and pyruvic acid. Food Chem. 2015, 169, 197–202. [Google Scholar] [CrossRef]
- Terán-Alcocer, A.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomater 2021, 11, 252. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Wang, P.; Qin, J. Application of polypyrrole-based electrochemical biosensor for the early diagnosis of colorectal cancer. Nanomater 2023, 13, 674. [Google Scholar] [CrossRef]
- Girtan, M.; Mallet, R.; Socol, M.; Stanculescu, A. On the physical properties PEDOT: PSS thin films. Mater. Today Commun. 2020, 22, 100735. [Google Scholar] [CrossRef]
- Hu, L.; Song, J.; Yin, X.; Su, Z.; Li, Z. Research progress on polymer solar cells based on PEDOT: PSS electrodes. Polymers 2020, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077–2088. [Google Scholar] [CrossRef]
- Hu, F.; Xue, Y.; Xu, J.; Lu, B. PEDOT-based conducting polymer actuators. Front. Robot. AI 2019, 6, 114. [Google Scholar] [CrossRef]
- Benoudjit, A.; Bader, M.M.; Wan Salim, W.W.A. Study of electropolymerized PEDOT: PSS transducers for application as electrochemical sensors in aqueous media. Sens. Bio-Sens. Res. 2018, 17, 18–24. [Google Scholar] [CrossRef]
- Pujol-Vila, F.; Vigués, N.; Díaz-González, M.; Muñoz-Berbel, X.; Mas, J. Fast and sensitive optical toxicity bioassay based on dual wavelength analysis of bacterial ferricyanide reduction kinetics. Biosens Bioelectron. 2015, 67, 272–279. [Google Scholar] [CrossRef]
- Zajdel, T.J.; Baruch, M.; Méhes, G.; Stavrinidou, E.; Berggren, M.; Maharbiz, M.M.; Simon, D.T.; Ajo-Franklin, C.M. PDOT:PSS-based Multilayer Bacterial-Composite Films for Bioelectronics. Sci. Rep. 2018, 8, 15293. [Google Scholar] [CrossRef] [PubMed]
- Tizzard, A.; Webber, J.; Goonertane, R.; John, R.; Hay, J.; Pasco, N. Micredox: Application for rapid biotoxicity assessment. Anal. Chim. Acta 2004, 522, 197–2005. [Google Scholar] [CrossRef]
- Zhai, J.; Yong, D.; Li, J.; Dong, A. A novel colorimetric biosensor for monitoring and detecting acute toxicity in water. Analyst 2012, 138, 702–707. [Google Scholar] [CrossRef]
- Qian, J.; Li, J.; Fang, D.; Yu, Y.; Zhi, J. A disposable biofilm-modified amperometric biosensor for the sensitive determination of pesticide biotoxicity in water. RSC Adv. 2014, 4, 55473–55482. [Google Scholar] [CrossRef]
- Gao, G.; Qian, J.; Fang, D.; Yu, Y.; Zhi, J. Development of mediated whole cell-based electrochemical biosensor for joint toxicity assessment of multi-pollutants using a mixed microbial consortium. Anal. Chim. Acta 2016, 924, 21–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigués, N.; Cantallops-Vilà, C.; Mas, J. Electrochemical Assessment of Microbial Activity Using PEDOT:PSS-Immobilized Cells. Chemosensors 2025, 13, 211. https://doi.org/10.3390/chemosensors13060211
Vigués N, Cantallops-Vilà C, Mas J. Electrochemical Assessment of Microbial Activity Using PEDOT:PSS-Immobilized Cells. Chemosensors. 2025; 13(6):211. https://doi.org/10.3390/chemosensors13060211
Chicago/Turabian StyleVigués, N., C. Cantallops-Vilà, and J. Mas. 2025. "Electrochemical Assessment of Microbial Activity Using PEDOT:PSS-Immobilized Cells" Chemosensors 13, no. 6: 211. https://doi.org/10.3390/chemosensors13060211
APA StyleVigués, N., Cantallops-Vilà, C., & Mas, J. (2025). Electrochemical Assessment of Microbial Activity Using PEDOT:PSS-Immobilized Cells. Chemosensors, 13(6), 211. https://doi.org/10.3390/chemosensors13060211




