Abstract
This review aims to summarize possible methods for the detection of limonene in the gas phase at low to very low concentrations. Limonene has historically been of interest as a fragrance in cosmetics, the food industry, pharmaceutics, and the production of solvents. The development of analytical methods for limonene was initially driven by its use in relevant industries such as chemical, pharmaceutics, cosmetics, food, agriculture, and forestry. More recently, it has been recognized as a potent biomarker for human metabolic conditions, such as liver disease and certain cancers. The interest in improved limonene detection in exhaled human breath has increased, particularly from the medical field, which demands high reliability, very low detection limits in the parts per billion (ppb) and even parts per trillion (ppt) range, and excellent selectivity against other exhaled volatile organic compounds (VOC). In addition, the detection methods should be portable and affordable to facilitate potential mass screening. This review paper aims to explore all possible detection methods by evaluating their proven analytical capabilities for limonene or discussing their potential usefulness, benefits, and applicability for limonene detection.
1. Importance and Occurrence of Limonene
1.1. What Is Limonene and Its Natural Origins
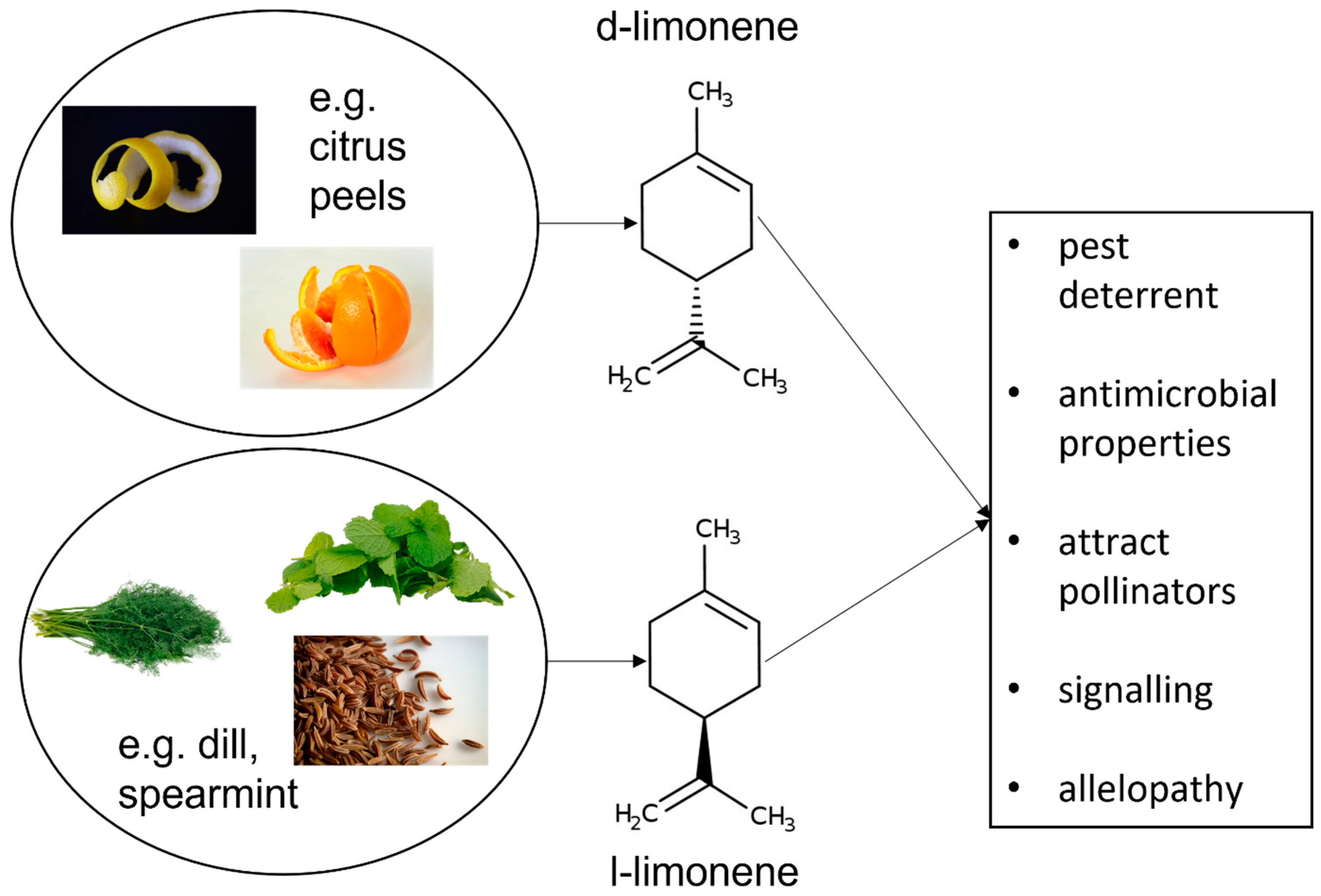
Limonene is a naturally occurring hydrocarbon compound, classified as a cyclic terpene, which takes the form of a colourless liquid (Figure 1). There are two main types of limonene, which are also enantiomers: D-limonene and L-limonene. D-limonene (also known as R-limonene, thanks to the absolute configuration of the chiral centre in the molecule) predominantly exhibits a dextrorotatory (clockwise) rotation of plane-polarized light, while L-limonene (or S-limonene) exhibits a laevorotatory (counter-clockwise) rotation. This difference in optical activity is due to the spatial arrangement of atoms around the chiral centre in the molecule [1].
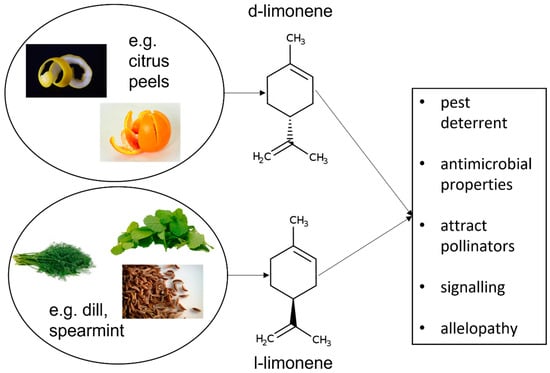
Figure 1.
Chemical structure of limonene, C10 H16, 1-Methyl-4-(prop-1-en-2-yl) cyclohex-1-en (IUPAC) and its role in plants.
Erasto and Viljoen have described in a review several important roles of limonene in plants. The compound constitutes a defence mechanism for pest deterrent, where the strong citrus scent of limonene acts as a repellent to many herbivorous insects and pests, reducing the likelihood of plant damage. Moreover, limonene has antimicrobial properties that can help protect plants from various pathogens, including fungi and bacteria. Another evolutionary purpose of limonene is the attraction of pollinators. The aroma of limonene can attract pollinators such as bees and other insects, aiding in the plant’s reproduction for facilitating pollination. Additionally, some plants release limonene into the surrounding soil, which can inhibit the growth of competing plants. This phenomenon, known as allelopathy, helps the limonene-producing plant reduce competition for resources such as nutrients, water, and light (Figure 1) [2].
Limonene, like many other volatile organic compounds (VOCs), can serve as a signalling molecule in plant-to-plant communication. For example, limonene released by a damaged plant can signal neighbouring plants to bolster their own defensive mechanisms. These functions make limonene a multifaceted compound that contributes to the survival and reproductive success of the plants that produce it.
Limonene is an exogenous substance for humans and its potential availability in the human metabolism only depends on external sources via uptake by food or by uptake via the skin.
For the majority of investigations cited in the following review, D-limonene enantiomer has been used and analysed. Only in exceptional cases, both enantiomers have been tested. For such cases, this fact is explicitly mentioned.
1.2. Application of Limonene in Everyday Use
Limonene is used in food and cosmetic products as a flavour and fragrance component. The substance also has a variety of industrial applications, including its use as a solvent in cleaning products and as a precursor in the production of other chemicals. Limonene is widely used in cleaning products due to its pleasant citrus scent and effective grease-cutting properties, generally without causing harm when used as directed [3,4]. It is generally regarded as safe for humans in common concentrations found in food, beverages, and consumer products such as air fresheners, under normal use conditions [5]. However, like many substances, its safety depends on the amount and form of exposure.
As a cosmetic ingredient limonene is considered safe as used in current practices and concentrations [6]. However, there are some potential risks and toxicity concerns. In its concentrated form (3.0% w/w), limonene can cause skin irritation and allergic reactions in some individuals, especially with prolonged or repeated contact, which can manifest as dermatitis [7]. High concentrations of limonene vapour can cause respiratory irritation. People with respiratory conditions or sensitivities may experience symptoms such as coughing or difficulty breathing when exposed to high levels of limonene in the air. According to Sunil et al., the threshold for such symptoms is 5.2 ppm, whereas Anderson et al. establish a sensitivity range of 500 ppb to 20 ppm [8,9,10]. While ingestion of limonene in typical dietary amounts is safe, consuming it in large quantities (20 g) can lead to gastrointestinal disturbances, such as nausea, vomiting, and diarrhoea [11,12].
Limonene has shown even potential health benefits including antioxidant, anti-inflammatory, and anticancer properties [13,14,15,16]. Some research suggests it may have therapeutic effects in various conditions, such as stress, anxiety, and gastrointestinal issues [17,18].
In conclusion, limonene is in general not considered toxic to humans at the levels typically encountered in foods, beverages, and household products.
2. Detection of Limonene in the Exhaled Human Breath Enabling Early Diagnosis of Liver Diseases
The detection of limonene in breath has recently seen increased attention in the research and development field due to its potential as a biomarker for various health conditions. The substance is normally not found in the exhaled air of healthy individuals/populations [19]. Externally ingested or inhaled limonene is metabolized in the liver by the enzymes from superfamily P450 (CYP2C9 and CYP2C19) producing trans-carveol and perillyl alcohol [20]. Xie et al. described thoroughly on the basis of several articles and research findings the process of metabolization of limonene [21]. What is more, it was found that limonene undergoes metabolization at a rapid pace, leaving the organism free from the metabolites within 24 h of ingestion of the substance [22].
A direct correlation between liver disease (i.e., cirrhosis) and limonene is already proven and described by several authors [23,24,25]. Exhaled limonene is elevated in individuals with liver cirrhosis and cirrhosis-induced hepatocellular carcinoma (HCC) compared to controls, but there’s no significant difference between the two diseased groups [26]. Analysis shows that limonene levels correlate with serum bilirubin, albumin, and the international normalized ratio, reflecting impaired liver function, but not with alanine aminotransferase. Therefore, limonene in breath can be considered a valuable biomarker for early diagnosis of liver diseases [26,27,28]. This suggests that exhaled limonene may serve as a non-invasive marker for liver metabolic capacity, primarily due to reduced liver functional capability in cirrhosis rather than the presence of HCC. Further, in patients with breast cancer [29] and head and neck cancer [30], limonene in exhaled air was investigated as a potential biomarker, however, the authors refrain from directly linking it with the disease in both cases due to insufficient data.
2.1. Types and Occurrence of Liver Diseases
Liver diseases cause an increasing burden on the global population, especially in the male population. Among more than 2 million liver-related deaths in the 2017 year, almost two-thirds were due to liver cirrhosis, with the rest associated with liver cancer [31,32]. In general, the highest rates can be found in Africa, Central America, Central Asia and Indochina, while Australia, Canada, China, Japan and Western Europe have the lowest rates [33].
Estimated age-standardized death rates due to cirrhosis in 2019 varied greatly between countries as well as regions. For instance, when accounting for regions, the lowest absolute number of deaths associated with cirrhosis was recorded in the Western Pacific (including Singapore with the lowest international rate) and highest in the Eastern Mediterranean region (including Egypt with the highest international rate).
Non-contagious liver diseases encompass a variety of conditions that are not caused by infections, but by other factors such as lifestyle, genetics, and autoimmune processes. Common types are Non-alcoholic fatty liver disease (NAFLD) and alcohol-related liver disease (ALD).
NAFLD is one of the most common liver diseases worldwide [34], affecting about 25% of the global population. Risk factors encompass obesity, type 2 diabetes, metabolic syndrome, dyslipidaemia, and poor diet [35]. There are several subtypes of this disease, including hepatitis steatosis, where fat accumulates in the liver without significant inflammation or damage, or non-alcoholic steatohepatitis (NASH), where fat accumulation causes inflammation and liver cell damage, which then in turn can progress to fibrosis, cirrhosis, and liver cancer [36].
ALD is a major cause of liver disease in many countries, especially where alcohol consumption is high, but varies widely from country to country [37]. The main risk factor is chronic, heavy alcohol use. There are several stages, with the initial stage of alcoholic fatty liver disease occurring as fat accumulation in the liver. Alcoholic hepatitis is the second stage encompassing inflammation and liver cell damage due to alcohol. The final stage is cirrhosis which consists of severe scarring and loss of liver function.
In 2019, cirrhosis was related to 2.4% of global deaths. However, this figure includes also cases of cirrhosis caused by infections like hepatitis. Despite viral hepatitis remaining the leading cause of cirrhosis worldwide, the prevalence of non-alcoholic fatty liver disease (NAFLD) and alcohol-associated cirrhosis (ALD) is rising in several regions of the world and the number of deaths by cirrhosis is expected to increase in the next decade. A total of 23% of decompensated cirrhosis in 2017 was estimated to be associated with alcohol. Concerning mortality, in 2019 an estimated 25% of deaths due to cirrhosis globally were associated with alcohol [38]. Recently, the National Institute on Alcohol Abuse and Alcoholism released data indicating that more than 50% of cirrhosis deaths in the USA in 2023 were alcohol-related [39]. The highest percentage of cirrhosis deaths associated with alcohol grouped by region in 2019 was observed in Europe (42%) [37].
Further, there are several discernible types of autoimmune liver diseases (autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), Wilson’s disease, haemochromatosis or alpha-1 antitrypsin deficiency) [40,41,42,43].
Drug-induced liver injury (DILI) tends to be more prevalent in hospitalized patients in comparison to the general population. The studies report an incidence of 23.8 in 100,000 persons/year in hospitalized patients in mainland China, lower numbers in general population analysis of 13.9 and 19.1 in 100,000 persons/year in France and Iceland, respectively, and as low as 2.3 in 100,000 persons/year in out-patients in Sweden. In this case, severe liver damage is caused by medications, supplements, or herbal products [44].
2.2. State-of-the-Art Early Diagnosis of Liver Diseases
Early diagnosis of cirrhosis is essential for effective management and to prevent progression to end-stage liver disease. Currently, there are several examinations that can indicate the presence of liver disease in the patient, including liver function tests (LFT) [45,46,47,48,49,50,51,52], ultrasound [53,54,55,56], CT and MRI [53,55,57,58].
Liver biopsy has long been considered the gold standard for diagnosing cirrhosis and staging liver fibrosis. However, due to its invasive nature and associated risks, there has been a growing interest in non-invasive alternatives. It provides detailed histological information, including the extent of fibrosis and other cellular markers, which are crucial for staging liver diseases [59,60].
However, liver biopsy has several limitations:
- The procedure is invasive and carries risks such as pain, bleeding, and other complications [61,62].
- Biopsies only sample a small portion of the liver, which may not be representative of the entire organ due to the heterogeneous distribution of fibrosis [59,60].
2.3. Importance of Early Diagnosis
Early diagnosis of cirrhosis is critical for [63,64,65]:
- Preventing complications: such as variceal bleeding, ascites, hepatic encephalopathy (HE), and HCC.
- Limiting the severity of disease with the help of lifestyle modifications: including alcohol cessation, weight management, and dietary changes.
- Managing the disease with medications and treatments: antiviral therapies for hepatitis, medications to manage complications, and potentially antifibrotic agents.
- Monitoring patient’s status and follow-up appointments: regular monitoring to manage disease progression and early detection of complications.
Early diagnosis is a critical factor in managing non-infectious liver diseases effectively. It enables prompt interventions that can prevent disease progression, improve patient outcomes, and reduce healthcare costs. Regular screening, patient education, and awareness of risk factors are essential strategies for achieving early diagnosis and better management of these liver conditions. Quantifying the benefits of early diagnosis in non-infectious liver diseases shows significant improvements in patient outcomes, reduced disease progression, and lower healthcare costs. Regular screening, patient education, and early interventions are crucial in achieving these positive outcomes. Early interventions can prevent complications such as ascites, HE, and variceal bleeding, improving the overall quality of life for patients. Regular health check-ups are important, especially for individuals with risk factors such as obesity, diabetes, chronic hepatitis, alcohol use disorder, metabolic syndrome, or a family history of liver disease. The development and availability of an easy screening procedure by detecting and measuring the limonene metabolism in the breath will contribute greatly to these measures and contribute to effective prevention. Hence, mitigating the abovementioned risk factors by effective measures for early detection, widespread screening, and prevention.
Early identification of hepatomegaly, splenomegaly, and jaundice through physical examination and symptom assessment is essential for diagnosing systemic conditions. While physical examination can reveal these signs, confirmation through imaging and laboratory tests is often necessary. Symptoms such as fatigue, abdominal discomfort, and jaundice are critical indicators that warrant further investigation to determine the underlying aetiology [66,67].
Early diagnosis and management of liver diseases can improve 5-year survival rates in many cases of liver disease [68]. Screening high-risk populations with serum AFP tests and real-time ultrasound can detect primary liver cancer (PLC) at early stages, increasing resection rates and prolonging survival. In a study, 76.8% of patients in the screening group were diagnosed at a subclinical stage, with 1- and 2-year survival rates of 88.1% and 77.5%, respectively, after 70.6% of them underwent surgical intervention. In contrast, none of the patients in the control group survived over 1 year [69].
Early intervention in NAFLD and NASH can reduce healthcare costs by preventing the progression of advanced liver disease, which is significantly more expensive to manage [70]. Liver transplantation costs in the U.S. can exceed $500,000 per patient, influenced by factors such as disease severity, complications, and healthcare system characteristics. Effective cost management strategies are essential to mitigate the financial burden on both patients and the healthcare system [71].
2.4. Correlation of Limonene Content in Breath with Liver Disease
Limonene detection in exhaled breath has been studied as a potential non-invasive biomarker for liver disease, including cirrhosis and hepatocellular carcinoma (HCC). Elevated levels of limonene in breath are associated with liver dysfunction due to impaired metabolic capacity. Patients with liver cirrhosis and HCC show significantly higher levels of exhaled limonene compared to healthy controls. This elevation is linked to reduced liver function, as the liver’s ability to metabolize limonene is compromised [26,27,28]. Limonene levels in breath correlate with serum bilirubin, albumin, and prothrombin time, indicating its potential as a marker for liver metabolic capacity. However, it does not correlate with alanine aminotransferase levels [26]. The utilization of dynamic breath testing for limonene has demonstrated considerable potential in the diagnosis of cirrhosis, exhibiting both high sensitivity and specificity. It also correlates with disease severity indicators like MELD scores and signs of portal hypertension [24,72]. Limonene breath testing is a non-invasive method that could be used for early diagnosis and monitoring of liver disease progression and response to treatment [73,74]. Limonene levels are particularly elevated in patients with hepatic encephalopathy and non-cholestatic liver disease, suggesting its specificity for certain liver conditions [25,27].
Studies have shown that limonene levels decrease significantly after liver transplantation, suggesting its utility in assessing graft liver function [28]. Dynamic breath testing, which measures limonene levels before and after administration, enhances diagnostic performance and correlates with disease severity. Limonene detection in exhaled breath is a promising non-invasive biomarker for liver disease, particularly cirrhosis and HCC. It correlates with liver function metrics and disease severity, offering potential for early diagnosis and monitoring. The establishment of the detection of limonene at high sensitivity (up to ppb levels or even lower) in the exhaled breath as an additional early diagnosis test is therefore important, particularly in monitoring and thereby preventing liver cirrhosis via breath analysis.
3. Detection Methods of Limonene in the Exhaled Human Breath
3.1. Breath Analysis as a Diagnostic Tool
Breath diagnostics have roots in ancient medicine and have evolved into a promising field for non-invasive disease diagnosis, including recent studies on SARS-CoV-2. Exhaled breath contains endogenous and exogenous volatiles, offering potential biomarkers for health monitoring [75,76]. Moreover, the diagnosis of a disease can be conducted with the help of identifying specific compounds. Volatolomics is a branch of chemistry where volatolome—a collection of volatile compounds emitted by the body—is analysed to identify potential disease markers. Such biomarkers (i.e., VOCs) can be detected in a promising non-invasive method—breath analysis. Such techniques have an advantage over traditional spectrometric techniques, because they are cheaper, portable, and do not require specialized personnel. Thus, this field holds promise for enhancing the accuracy and reliability of disease diagnostics [77]. Several types of sensors have been utilized for this purpose, each offering different advantages in terms of sensitivity, specificity, and practicality for breath analysis. However, a major challenge remains in establishing and verifying a solid relationship between specific volatolomes and particular diseases. Another challenge is developing effective and precise methods for detecting these volatile compounds. The advances in nanotechnology have spurred the development of innovative chemosensors that enhance diagnostic performance. Hu et al. review major chemosensor categories used in detecting exhaled gas analytes for disease diagnosis, highlighting the role of nanomaterials [78].
For general health monitoring, the detection of various VOCs in exhaled breath can provide insights into a patient’s metabolic status and disease diagnosis. This includes monitoring for diseases like diabetes, liver disease, and infections [79,80]. The review paper of Bruderer et al. highlights the potential and current state of on-line breath analysis in providing insights into an individual’s metabolism non-invasively and continuously. The review emphasizes the need for standardization to facilitate broader application and it calls for continuous improvements in existing methodologies. In summary, on-line breath analysis presents promising, non-invasive diagnostic and monitoring possibilities with several advanced technologies available. However, establishing standard protocols and overcoming data interpretation challenges are critical for its widespread use in clinical settings [81].
In a recent review, the detection of VOCs (among them also limonene) by nanomaterial-based chemosensors in human exhaled breath is described comprehensively [77]. The article reviews the methodologies used in volatolomics research, providing a critical analysis of the approaches and techniques employed to study volatile biomarkers. It systematically categorizes volatolomes based on different diseases and sources within the body, offering a detailed summary of the available data and findings. The review highlights the various analytical instruments used to identify volatolomes, showcasing the range of technologies available for these analyses. Versatile, portable technologies such as e-nose or photonic nose, which mimic the olfactory system to detect and analyse VOCs with high precision, serve as an important tool in volatolomics. The presented wide range of possible applications of volatolomics (including medicine, chemistry, materials science, and electronics engineering), emphasizes the interdisciplinary nature of the field and provides guidelines for choosing target VOCs and detection technologies.
3.2. Comparison of Different Sampling Methods of VOCs Like Limonene
As highlighted, the detection of limonene in breath is a promising diagnostic tool, but the effectiveness of different sampling methods varies. Hereby, we present a quick overview of some of the available methods of sampling the limonene.
Tedlar bags are commonly used for collecting whole-breath samples. They are effective for capturing limonene and are suitable for biomarker discovery and untargeted searches [82,83]. The limonene-containing sample can then be transferred to a headspace vial for analysis with the help of the cryotransfer method. This method is highly sensitive and allows for long-term storage at low temperatures, making it optimal for large-scale studies [84].
Direct-breath solid-phase microextraction (DB-SPME) uses fibre to directly extract VOCs like limonene from breath. It is rapid and has a low background signal, but it is less sensitive compared to other methods [84].
The Mistral sampler collects the end-tidal breath, which is less affected by ambient air contaminants compared to whole-breath sampling [82].
The Respiration Collector for In Vitro Analysis (ReCIVA) device collects simultaneously both, whole and alveolar breath. It shows lower levels due to fluctuating flow rates, but the alveolar fraction is less contaminated by ambient air [82].
Alveolar Breath Sampling captures alveolar breath and is less effective in detecting a wide range of VOCs compared to polymeric bags. It tends to dilute volatiles and introduce ambient air, reducing the levels of endogenous breath volatiles [83].
In case of the mechanically ventilated patients, a closed suction catheter can be used. This method retrieves higher yields, but may have compromised detection limits due to background signals from plastic and rubber components [85].
Different breath sampling methods offer various advantages and limitations. The cryotransfer method demonstrates high sensitivity and specificity for detecting VOCs like limonene in breath. Tedlar bags are effective, while Bio-VOC™ and DB-SPME are less sensitive but offer rapid and immediate analysis [83,84,86]. When it comes to the contamination of the alveolar breath by the ambient air, sampling methods like Mistral and ReCIVA, are less affected by ambient air contaminants compared to whole-breath sampling [82].
Considering different mechanisms and characteristics, those methods can be implemented with different goals in mind. Tedlar bags and cryotransfer methods are highly sensitive and suitable for large-scale studies. Devices like Mistral and ReCIVA are effective in reducing ambient air contamination. Therefore, the choice of method depends on the specific requirements of the study, such as sensitivity, specificity, and the need for rapid analysis.
Various techniques for detecting Limonene in the exhaled breath such as gas chromatography-mass spectrometry (GC-MS), proton transfer reaction-mass spectrometry (PTR-MS), and selected ion flow tube-mass spectrometry (SIFT-MS), have been used to detect and quantify limonene. These three techniques are considered the gold standards due to their high sensitivity and specificity; however, they are more suited to laboratory settings. Hereby, different techniques and methods for VOCs/limonene detection are presented.
3.3. Mass Spectrometry Techniques for Breath Analysis
3.3.1. Gas Chromatography-Mass Spectrometry (GC-MS)
Advanced analytical techniques like mass spectrometry (MS), gas chromatography (GC), and others are essential for breath analysis due to the complexity and low concentration of volatiles. These methods enable the detection and characterization of metabolites, helping in diagnosing diseases beyond respiratory conditions. Despite advancements, breath analysis faces challenges in clinical practice [75].
Gas chromatography-mass spectrometry (GC-MS) is widely used for its high sensitivity, specificity, and capability to separate complex mixtures. It combines the separation capabilities of gas chromatography with the high sensitivity and specificity of mass spectrometry. The technique provides detailed qualitative and quantitative data, making it ideal for comprehensive profiling of VOCs. Thus, it can provide detailed analysis and quantification of limonene in complex mixtures, including breath samples, even at very low concentrations of limonene, often in the parts per billion (ppb) range. However, the method requires substantial sample preparation and typically longer analysis times compared to other techniques, like PTR-MS and SIFT-MS, due to the chromatographic separation process. GC-MS was used in various studies to detect limonene in the breath of patients with liver cirrhosis, suggesting it could serve as a biomarker for the disease [25,87].
GC-MS was used in a study conducted by Dadamio et al., as an alternative to the traditional liver biopsy, which is invasive and impractical for frequent monitoring of patients. The authors investigated alveolar breath samples from cirrhosis patients and healthy volunteers in order to verify whether specific breath biomarkers can detect asymptomatic cirrhosis in patients with chronic liver disease [87]. D-limonene was investigated as one of the compounds identifying patients suffering from cirrhosis, however, despite observed differences in the results between healthy and sick participants, the compound was not identified by authors as one fulfilling set criteria of high sensitivity and specificity. Instead, a specific pattern involving 24 models of 8 non-limonene compounds, identified via linear discriminant analysis, was analysed. The model could distinguish cirrhosis with sensitivity and specificity between 82–88% and 96–100%, respectively, suggesting a new non-invasive diagnostic method.
Friedman et al. examine the presence of D-limonene, in the lung air of patients with liver disease compared to healthy controls [25]. Using GC-MS, researchers analysed expired lung air samples from 24 liver disease patients and 24 healthy individuals. Findings revealed that patients with liver disease had significantly higher levels of limonene (7.0 µg/20 L) compared to healthy controls (0.1 µg/20 L). However, elevated limonene levels were only observed in half of the patients. Among these patients, those with non-cholestatic liver disease had significantly higher limonene levels (13.8 µg/20 L) compared to those with cholestatic liver disease (0.2 µg/20 L). Dietary patterns and preferences reported through questionnaires suggested that the elevated limonene levels in those patients likely had a dietary origin, possibly due to higher consumption of foods containing limonene, highlighting the issue of specificity of limonene measurements.
Ferrandino et al. explore a novel method for detecting cirrhosis through dynamic breath testing, as current biomarkers used in primary care often fail to diagnose the condition before it progresses [72]. Researchers enrolled 29 subjects with cirrhosis and 29 healthy controls, using a non-invasive breath biopsy technique to collect breath samples before and after administering 100 mg of D-limonene. Next, GC-MS was used to measure limonene levels in collected samples. The results showed that all participants experienced a significant increase in breath limonene levels post-administration, with higher bioavailability in those with cirrhosis. The dynamic test improved classification performance and limonene bioavailability correlated with cirrhosis severity. The dynamic breath test presents a promising non-invasive tool for early cirrhosis detection and monitoring, potentially allowing for timely intervention and management within primary care settings.
Schulz et al. developed and compared methods for sampling VOCs using solid-phase microextraction (SPME) for analysis by GC-MS [84]. An in-house method, DB–SPME, was optimized for directly extracting VOCs from breath. It was compared to two methods using Tedlar bags: Tedlar–SPME and cryotransfer. The cryotransfer method was most sensitive overall, particularly for storing VOCs long-term, while Tedlar–SPME excelled in detecting low molecular weight compounds like acetone and isoprene. Although less sensitive, DB–SPME was fast and produced low background signals, making it efficient for immediate analysis. Each method effectively detects a wide range of VOCs. However, cryotransfer showed the highest sensitivity to the VOCs with relatively high molecular weights, including limonene.
The publication of Han et al. discusses the development and effectiveness of a micro-GC column for analysing VOCs in human breath, particularly for the diagnosis of NAFLD. The micro-GC column is designed to separate VOCs from breath samples. These VOCs can serve as biomarkers for early detection and diagnosis of NAFLD. The column utilizes micro-electromechanical systems (MEMS) technology, leading to minimal band broadening. A serpentine column structure was selected for this application. The column was integrated into a conventional gas chromatography setup. Separation tests were conducted with a nonpolar alkane mixture, which included pentane, an NAFLD biomarker. The column separated alkanes ranging from C5 to C12 within 5 min [88]. Quick and effective separation of VOCs suggests that the column is suitable for breath analysis in diagnosing NAFLD and potentially other liver-related metabolic disorders. There is potential for this technology to be developed into a portable breath analysis device, incorporating a microdetector and preconcentrator.
Both, Dadamio et al. and Friedman et al., bring into question the relevance of limonene in the diagnosis of liver disease, at least with GC-MS as a detection and analysis method. However, more recent studies conclude on more promising use of limonene detection. Hence, the older studies and their results should be assumed as indicators of a rather complex status of patients suffering from liver diseases and a need for taking into consideration additional variables, i.e., dietary patterns, during breath analyses, in developing sensors for the detection of liver diseases.
3.3.2. Proton Transfer Reaction-Mass Spectrometry (PTR-MS)
Proton transfer reaction-mass spectrometry (PTR-MS) allows real-time detection and quantification of VOCs in the breath (Figure 2). It involves the chemical ionization of the sample gas by proton transfer from H3O+ ions (hydronium ions), where the ions react with VOCs but not with common air constituents. This technique uses a soft ionization method (proton transfer reaction) which minimally fragments the ions, preserving the molecular structure of the analytes.
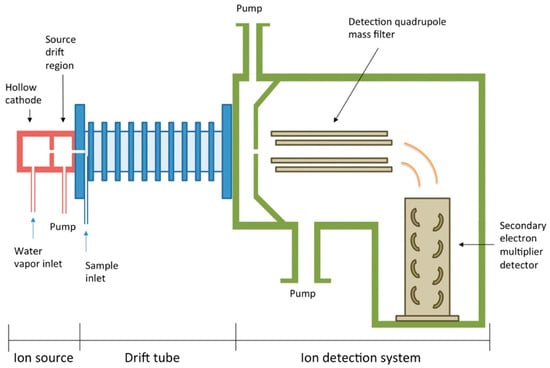
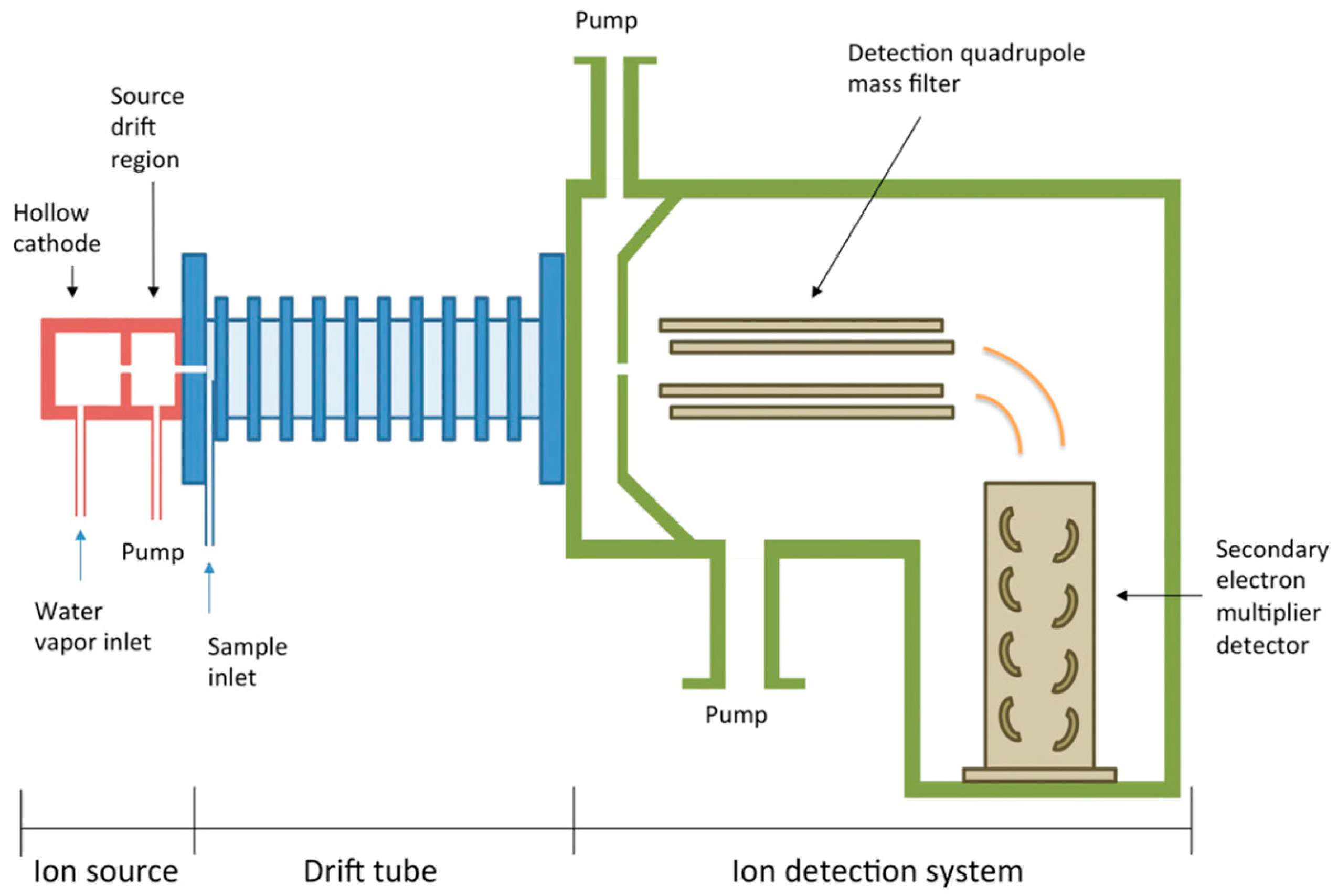
Figure 2.
Schematic diagram of PTR-MS used for the detection of VOCs (reprinted with permission from Ref. [89]).
This method is suitable for real-time analysis of VOCs in breath with high sensitivity and low detection limits [90]. It is ideal for dynamic and continuous monitoring of breath VOCs due to its fast response time. This approach facilitates non-invasive diagnostics and real-time tracking of physiological changes and requires little to no sample preparation. With the implementation of a PTR-MS, it was found that individuals with cirrhosis, especially those with HE, had higher levels of limonene in their breath [28,91].
Zhan et al. review recent advancements in PTR-MS technology, drawing attention to its applications in medical research, and methods for distinguishing isobaric VOCs. Additionally, it discusses specialized sample inlet designs that enhance PTR-MS for detecting aqueous and non-volatile samples, potentially broadening the field of application [90].
As mentioned, PTR-MS technology has also shown potential for using breath analysis to diagnose liver disease, with limonene emerging as a potential marker in patients with encephalopathy [91]. The authors concluded that limonene, unlike methanol or 2-pentanone, is able to discriminate between patients with no current symptoms and those suffering from encephalopathy [27].
In a study using a two-stage biomarker discovery process, alveolar breath samples from 31 cirrhosis patients and 30 healthy controls were mass-spectrometrically analysed with PTR-MS, revealing seven elevated volatiles in patients. Five of these, including limonene, decreased significantly post-transplant. Limonene alone had the best diagnostic capability when analysing the AUROC (Area Under the Receiver Operating Characteristic Curve), which gives results of plotting true positive rates against the false positive rates at different thresholds, with AUROC = 1 representing the perfect model. Fernández del Río et al. have obtained AUROC = 0.91, which could be further improved when combined with methanol and 2-pentanone (AUROC = 0.95, sensitivity = 97% and specificity = 70%). Furthermore, monitoring the wash-out of limonene after transplantation could non-invasively assess liver graft function, indicating potential as early-stage liver disease markers [28].
3.3.3. Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS)
The selected ion flow tube (SIFT) technique analyses in real time trace gases in air and breath by using soft chemical ionization, targeting trace gases while excluding major ones [92]. The sample is introduced into a flow tube where it reacts with selected precursor ions, and the resulting product ions are analysed to identify and quantify the VOCs (Figure 3).
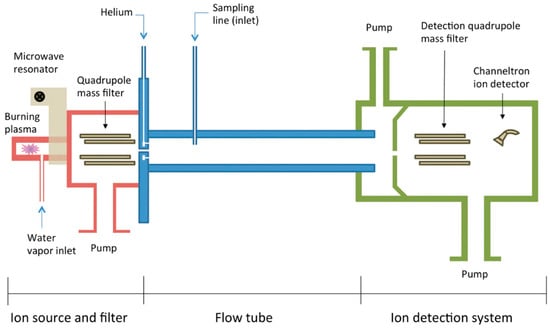
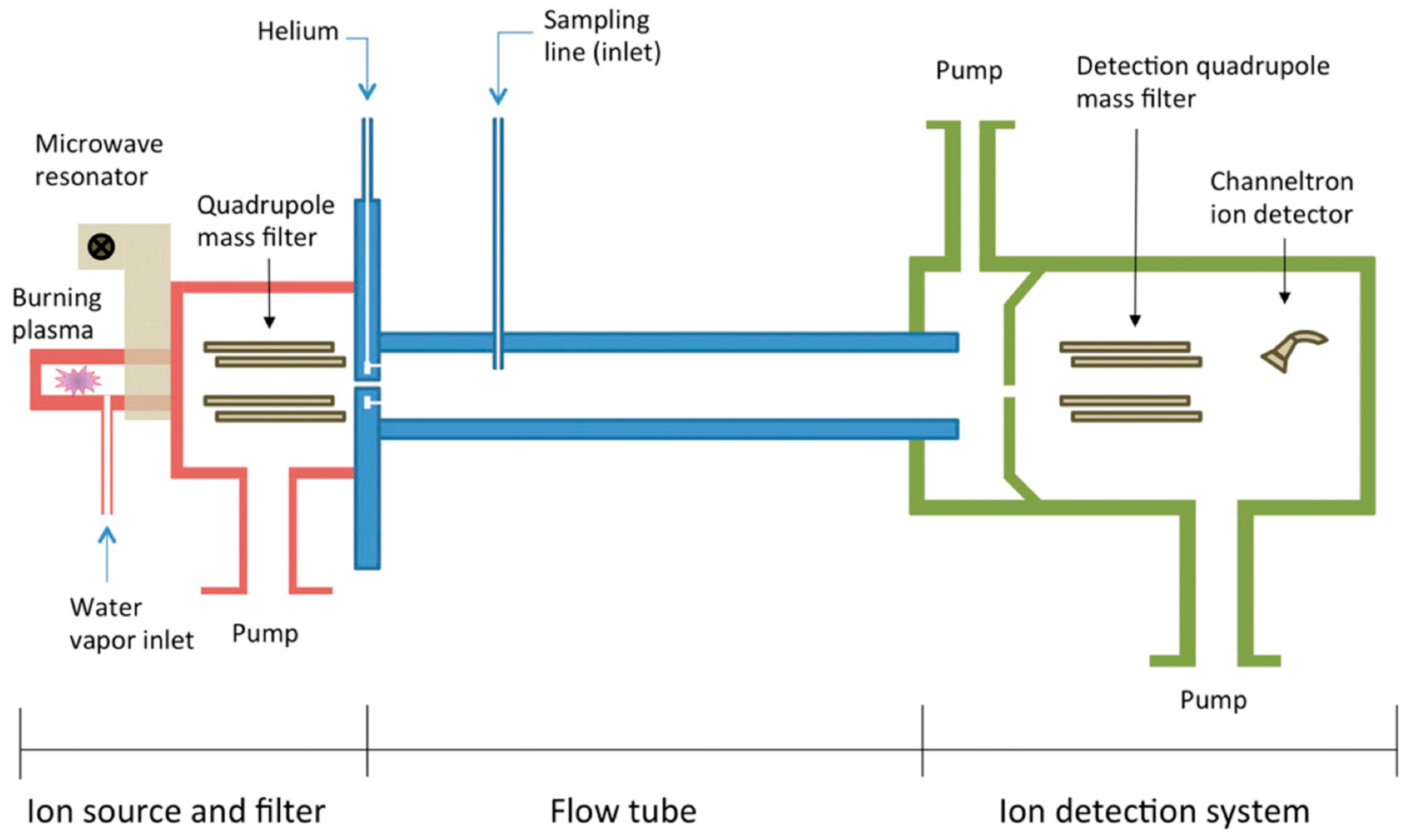
Figure 3.
Schematic diagram of SIFT-MS used for the detection of VOCs (reprinted with permission from Ref. [89]).
Common precursor ions used in selected ion flow tube-mass spectrometry (SIFT-MS) are H3O+, NO+ and O2+. H3O+ is the same ion as in PTR-MS, creating a parallelism between the processes, however, both methods differ in the way the reagent ions are generated and the place in which the reaction with the analyte takes place [93]. The ion reacts with many VOCs through proton transfer reactions, producing protonated molecular ions. There are two more ions used in SIFT-MS, but not in PTR-MS. First, NO+ (nitric oxide ion) is primarily used to react with VOCs via charge transfer or association reactions. It provides an alternative ionization method for compounds that may not react efficiently with H3O+. Second, O2+ (dioxygen ion) can interact with sample molecules via charge transfer or oxygen addition reactions and is useful for ionizing compounds that have a high ionization potential. These ions are chosen due to their well-understood reaction mechanisms and their ability to provide selective and efficient ionization for a broad range of analytes. Using these ions, SIFT-MS achieves versatile and selective ionization, making it a powerful tool for real-time and sensitive analysis of trace gases and VOCs in various applications, including medical diagnostics and environmental monitoring [92,94]. SIFT can measure trace gas partial pressures as low as 10 ppb, with a rapid response time of 20 milliseconds, allowing for detailed concentration profiles during breathing cycles. However, in general, SIFT-MS has higher limits of detection, compared to PTR-MS, due to the lower efficiency of creating reagent ions in the SIFT-MS technique.
Belluomo et al. offer a detailed workflow for breath analysis using SIFT-MS, allowing for the analysis of 50 samples in under 3 h [94]. Exploiting SIFT-MS’s ability for real-time results and direct quantification, the protocol includes methods for analysing disease-specific VOCs like fatty acids and aldehydes.
SIFT-MS has been shown to have potential in non-invasive diagnosis of esophagogastric cancer. On a group of 335 patients, Markar et al. reported 80% sensitivity and 81% specificity in diagnosing esophagogastric cancer with SIFT-MS [95].
The detection of monoterpenes like limonene has been successfully undertaken [96]. Lacko et al. highlight a method to improve the differentiation and quantification of VOC isomers using soft chemical ionization mass spectrometry (SCI-MS), particularly focusing on monoterpenes, which are challenging to differentiate due to their similar structures [97]. The method employs a fast GC system with a specially designed electrically heated metallic capillary column coupled to a SIFT-MS instrument. In this setup, the SIFT-MS uses H3O+ and NO+ reagent ions to generate specific analyte ions, allowing for the effective identification of monoterpene structures based on their characteristic fragment ion profiles. Two types of GC columns were tested: MXT-1 and MXT-Volatiles, each offering unique advantages. The MXT-1 column allows for very rapid quantification (less than 45 s), whereas the MXT-Volatiles column provides better temporal separation in less than 180 s. However, testing took place with compounds extracted from plants, not human expired air.
Wang et al. in a laboratory study have proven that both D- and L-limonene can be detected by SIFT-MS, but with only limited capability to distinguish between paired terpene isomers, bringing light to the possible limitation in the implementation of SIFT-MS in diagnostic setting [98]. Nonetheless, the approach offers a promising solution for real-time, accurate VOC analysis, particularly in environmental monitoring and quality control in various industries. This technique could allow for real-time analysis and direct measurement of limonene concentrations without the need for complex sample preparation or calibration curves. Thus, due to its high sensitivity and fast response time, SIFT-MS could be particularly effective for non-invasive monitoring and detection of limonene. However, as of now, it has not been clinically tested in the patient setup.
3.3.4. Summary of Mass Spectrometry Methods for the Use of Limonene Detection
These advanced MS techniques are instrumental in breath analysis for the detection of limonene and other VOCs. They provide crucial non-invasive diagnostic and monitoring tools in medical science. Each technique has unique advantages that make it suitable for specific applications, particularly in the analysis of VOCs in human breath.
GC-MS is widely used for its accuracy and reliability in detecting specific compounds like limonene and offers detailed compound identification and quantification. It is ideal for detailed and comprehensive profiling with high sensitivity and specificity.
PTR-MS provides real-time, on-line analysis of exhaled breath and is useful for monitoring dynamic changes in breath VOCs. This technique would be best suited for real-time, continuous monitoring with minimal sample preparation and soft ionization to preserve molecular structure.
Finally, the SIFT-MS allows real-time breath analysis with minimal sample preparation and is capable of detecting low-concentration VOCs including limonene. This method provides direct, real-time quantification with selective ion chemistry for enhanced specificity and versatility in VOC analysis. SIFT-MS uses a variety of precursor ions for the selective ionization of analytes. The choice of precursor ions that are to interact with the sample molecules is crucial for the specificity and sensitivity of the method.
3.4. Devices Emulating Olfactory System—Electronic Nose (e-Nose)
Electronic noses (e-Noses) are devices designed to detect and identify VOCs such as limonene by mimicking the human olfactory system. They can be used for various applications, such as pattern recognition of complex breath mixtures and non-invasive and rapid breath analysis. This would allow for the detection of specific compounds, like limonene, in human breath. These devices often incorporate sensor arrays (metal oxide, conducting polymer, etc.) that respond to different chemical compounds (various VOCs, including limonene), and their pattern recognition capabilities can be used for medical diagnostics, environmental monitoring, and food quality control. Those devices offer a non-invasive method for monitoring health conditions, particularly liver diseases. The method is appreciated for being non-invasive, portable, convenient, safe, simple, and for eliminating discomfort. Studies confirm the technology’s effectiveness and potential for medical diagnostics by identifying specific chemical patterns associated with various diseases.
Li et al. provide an in-depth review of the progress and potential applications of exhaled breath analysis, particularly focusing on the utilization of e-Nose technology [99]. The review meticulously identifies and discusses the challenges and limitations faced by current breath analysis technologies, particularly e-Nose. The authors mention an electrochemical (EC) sensor developed by Nazir et al., which detects D-limonene (Figure 4) [100].
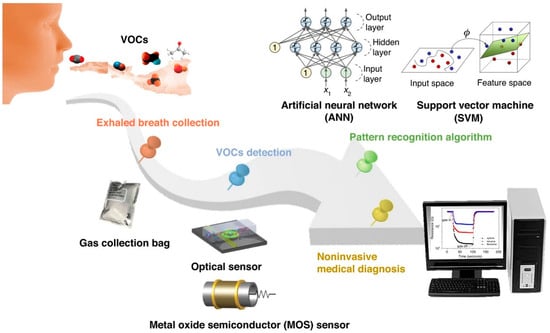
Figure 4.
Schematic diagram of non-invasive breath detection via the e-nose system (reprinted with permission from Ref. [99]).
A clinical exploratory study with 30 patients in three different groups was conducted by Voss et al., in order to prove that the status of cirrhotic patients can be detected with high accuracy by using an electronic nose for the analysis of the VOC composition in breath analysis based on MOx gas sensors [101]. The wearable, non-invasive system provides a promising tool to detect liver dysfunctions on a functional basis. A complex data analysis of the obtained response of nine different MOx sensor layers was performed. A distinction between the characteristic VOC composition of each patient group was successful, with specificity, sensitivity and accuracy of separating healthy and unhealthy cases equal to 1.00.
3.4.1. Electrochemical Sensors (ECs)
Electrochemical (EC) sensors are widely used for their sensitivity, selectivity, and rapid response in detecting various compounds. These sensors operate by measuring changes in electrical properties when target molecules interact with their surface. Hence, ECs are suitable for specific and selective detection of limonene with relatively low power consumption.
EC sensors present a promising approach for the detection of limonene in breath, offering high sensitivity, selectivity, and real-time monitoring capabilities. Their customizable nature and operational convenience make them valuable tools for non-invasive VOC detection in medical diagnostics and environmental monitoring.
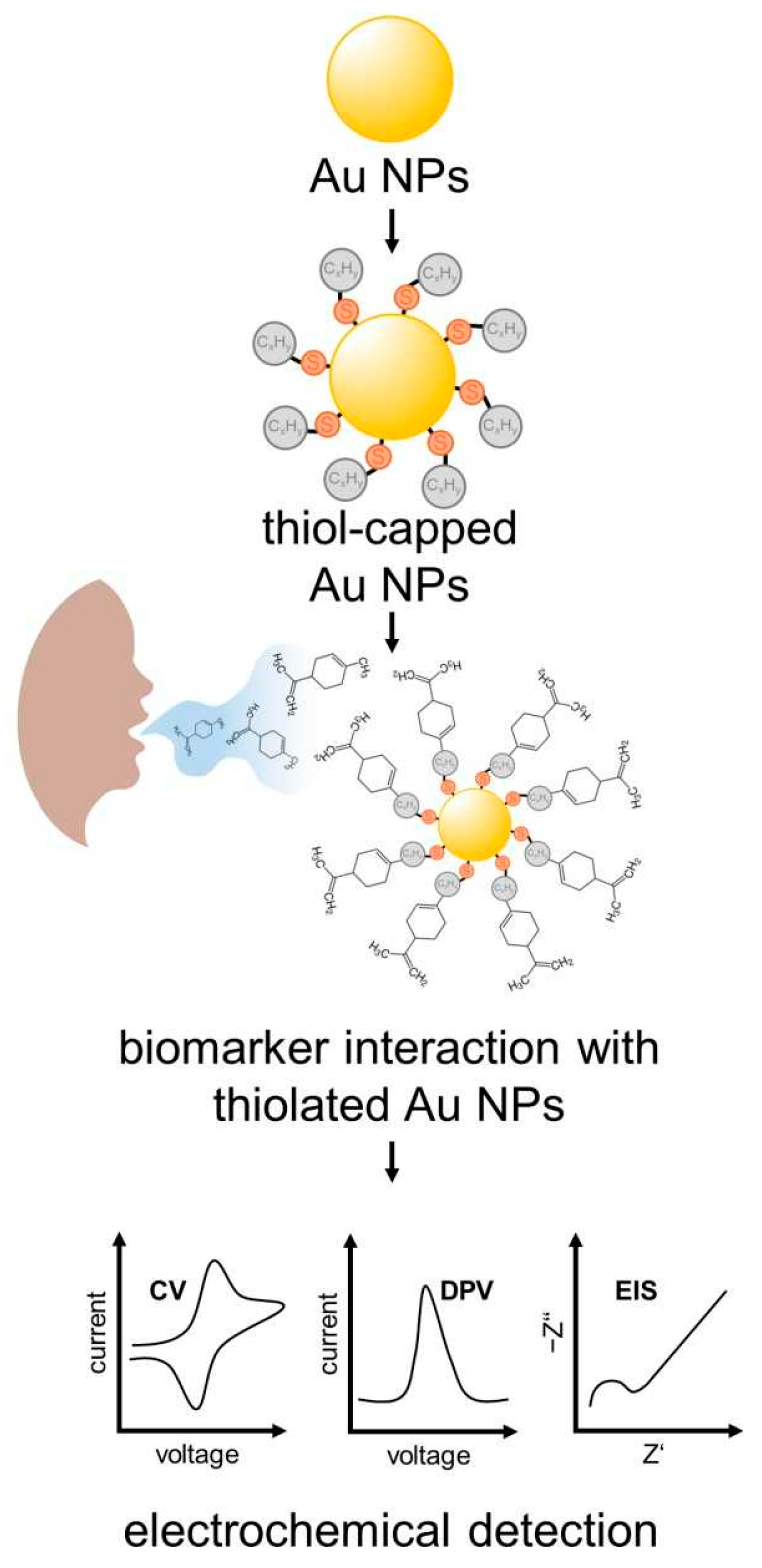
Hexanethiol, Decanethiol and Dodecanethiol AuNPs were tested as an EC sensor for the detection of limonene (Figure 5). Hexanethiol-capped AuNPs showed the best performance, with high sensitivity for limonene achieving a limit of detection (LoD) of 0.2 mmol L−1. The EC detection of D-limonene in the breath of cirrhotic patients was determined to be 800 ppm. This value was confirmed by GC-MS measurements [100].
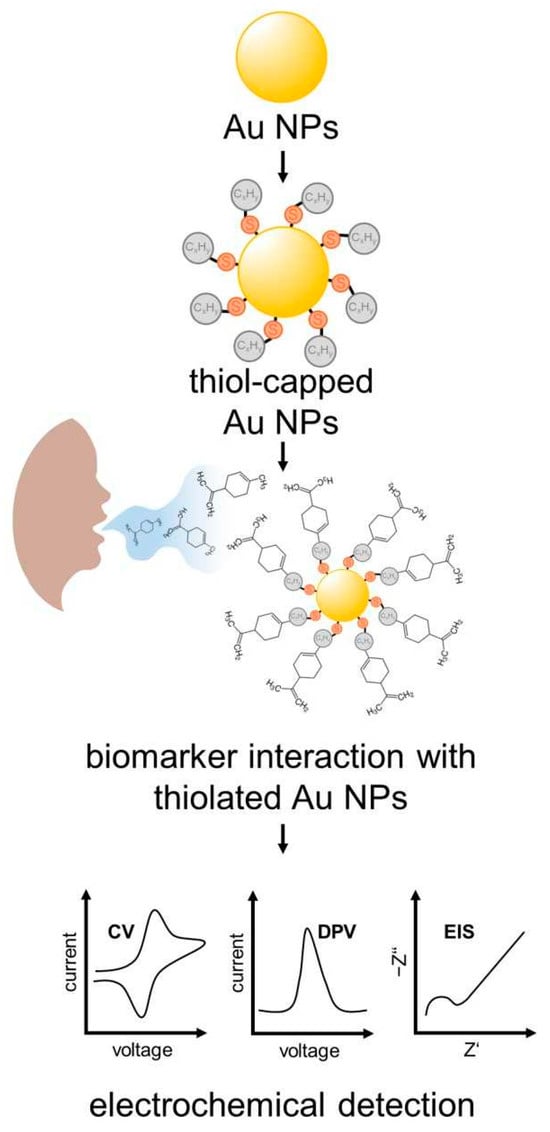
Figure 5.
Scheme of surface functionalization and biomarkers used in electrochemical detection of EC sensors on basis of the monolayer thiol-capped AuNPs sensors.
3.4.2. Surface Acoustic Wave Sensors (SAW)
Surface acoustic wave (SAW) sensors are a type of piezoelectric device used for detecting various gases, including VOCs, by measuring changes in acoustic wave properties as they interact with the target molecules. SAW sensors are highly sensitive to VOCs due to their ability to detect minute changes in acoustic wave properties, when target molecules adsorb onto the sensor’s surface. SAW sensors could provide fast and real-time detection, making them suitable for applications requiring immediate feedback, such as breath analysis. The sensors can operate efficiently at room temperature, providing practical advantages for non-invasive medical diagnostics. The ability of SAW sensors to be functionalized with different coatings allows for the selective detection of a wide range of VOCs, including limonene. Thus, SAW sensors are in principle highly effective tools for detecting limonene in exhaled breath, offering a combination of sensitivity, specificity, and rapid response, but will need further investigations and development. However, their unique properties could make them suitable for non-invasive diagnostics and continuous monitoring applications.
Chang et al. investigate the use of SAW sensors for detecting various organic vapours. The sensors showed high sensitivity and rapid response to changes in VOC concentration, indicating their potential for real-time breath analysis applications [102]. Those sensors have the potential for use in a portable e-Nose system for the discrimination of VOCs and the selection of gas-sensitive materials.
Yang et al. in a small and experimental study measured SAW velocities to identify five odorant molecules. The SAW device was functionalized with Cynops pyrrhogaster lipocalin (Cp-Lip1) protein. The findings of the study demonstrated a decline in the propagation velocity with an increase in concentration. These preliminary findings are important for future research in SAW sensing of limonene [103].
Grate et al. highlight the design considerations of chemically selective coatings for SAW sensors aimed at detecting VOCs. The findings apply to developing sensors for breath analysis [104].
A fast gas chromatography with surface acoustic wave detection (FGC-SAW) was developed and used to identify the major volatile components in fruit juice. The recorded analysis time in testing orange juice for headspace sampling, GC analysis and instrumental recycling, was 46 s. The major volatiles in orange juice were identified as terpenes (limonene) and esters. In total, 15 components could be detected by SAW. These research findings point to the general development potential of using SAW for the detection of terpenes like limonene, but will need further development before it could be used as a fast detection method in breath analysis [105].
3.4.3. Quartz Crystal Microbalance Sensors (QCM)
Quartz crystal microbalance (QCM) sensors are another effective technology used for detecting VOCs in breath. QCM sensors operate by measuring changes in the frequency of a quartz crystal resonator, which occurs when mass is deposited onto its surface. This mass change can be correlated to the concentration of the detected substance, thus, making them suitable for detecting low concentrations of VOCs such as limonene. They provide direct detection of VOCs without the need for complex sample preparation, which is ideal for rapid and real-time breath analysis. The sensor surface can be functionalized with various coatings to enhance selectivity towards specific compounds (like limonene). QCM sensors are versatile and can be used in medical diagnostics, environmental monitoring, and other fields where VOC detection is crucial. QCM sensors offer a promising approach for the detection of limonene in exhaled breath, combining high sensitivity and the possibility for real-time analysis with minimal sample preparation. They are particularly advantageous for non-invasive diagnostic applications. Moreover, they can be implemented as part of the e-Nose sensor.
Organic salts were proven to be promising recognition elements in QCM-based sensor arrays [106]. Phthalocyanine-based recognition elements have been used for VOC sensing utilizing a QCM-based sensor array. The vapour sensing properties of these materials for VOCs were examined, resulting in a discrimination of analytes into different functional group classes with 98.6% accuracy.
Cao et al. emphasize the drawback of using metal-organic frameworks (MOFs) as a sensing element of VOC in QCM-based sensing, namely its hydrophilicity [107]. This has been solved by depositing an additional layer of polydimethylsiloxane (PDMS). This hydrophobic PDMS layer improves the moisture resistance of the sensor in terms of shifts in resonant frequency by a factor of 4, and by a factor of 14 in scattering parameters. The performance of the sensor is maintained over 2 days despite water treatment, highlighting the high water resistance of the setup. The VOC ethanol, 2-propanol, acetone, and acetonitrile have been investigated in this study, but limonene has not been considered. The findings provide, however, a promising solution for QCM detection of VOC in high-humidity environments, a condition prominent in breath analysis.
Additional functionalization of the sensor by carbon nanotubes (CNTs) has been investigated by several studies. QCM acoustic and silica optical fibre (SOF) optical sensors with a film consisting of single-walled carbon nanotubes (SWCNTs) have been found to be promising in the recognition of VOC at room temperature [108]. Torad et al. reported on highly selective and excellent sensing activity in chemical-vapour discrimination QCM sensors with hairy-like layers of graphitic-like CNTs. The authors focused on the discrimination of toxic volatile aromatic hydrocarbons (e.g., benzene, c-hexane), due to the favoured strong π-π interactions, of aromatic compounds, thus unfortunately not useful in the detection of aliphatic compounds like limonene [109]. Therefore, despite the improvement of the sensitivity of QCM sensors with the implementation of CNTs, such functionalization has not yet been proven to be suitable in the detection of limonene.
The QCM sensors are proven to work in detecting limonene in air as a way for checking the freshness of the fruit, and therefore can be a promising tool in determining the limonene in exhaled breath and consequently diagnosing patients [110,111]. A very sensitive QCM method is presented for the detection of limonene by using a molecularly imprinted polymer (MIP), polystyrene-co-divinylbenzene based, by Völkle et al. A concentration as low as 50 ppm of D-limonene could be detected by the sensor [112]. The research proves the validity of using selective, low-cost polymeric materials as a base for organic semiconductors to enhance the sensitivity of gas sensors.
The functionalization of QCM sensors by using specific coatings of ethyl cellulose enhances the selectivity for detecting different VOCs, including limonene. The modified sensors showed significant improvements in selectivity and sensitivity. The LoD for D-limonene was determined to be 300 mg m−3 in pure gas. The lifetime of the sensor was more than one month with an acceptable drift of only 3.40% in their sensing characteristics [110].
Sasaki et al. reported on a QCM device tested for the detection of odorant molecules, including limonene. The sensor is based on cyclodextrin film combined with QCM. The vapourized limonene (0.66 mmol/L) was successfully detected by three types of cyclodextrin, with the best results for β-cyclodextrin [113].
3.4.4. Metal-Oxide Semiconductor Sensors (MOS)
Metal-oxide semiconductor (MOS) sensors are widely used for detecting VOCs due to their high sensitivity, fast response time, and relatively low cost. These sensors work by measuring changes in the electrical resistance of a metal-oxide material when it interacts with gas molecules. MOS sensors can detect low concentrations of VOCs and they provide rapid detection of gases and quick return to baseline levels.
They are effective for sensing various VOCs, including alkanes, alkenes, and aromatics and are generally less expensive than other types of sensors such as mass spectrometry instruments.
MOS sensors are highly sensitive to limonene, capable of detecting low ppm levels and they offer fast response and recovery times, which is crucial for real-time breath analysis. Functionalization with MIPs or other selective coatings can improve the specific detection of limonene. MOS sensors are cost-effective and straightforward to implement in practical devices like electronic noses.
Rossi et al. highlighted crucial advancements in the field of portable gas sensing technology, particularly focusing on D-(+)-limonene detection using a chemoresistive gas sensor array. The study investigated the capabilities of miniaturized and affordable sensors, that can be integrated into Internet of Things (IoT) systems for detecting D-(+)-limonene. Among the tested array of seven metal-oxide sensors, the WO3 (tungsten trioxide)-based sensor was identified as the most promising for D-(+)-limonene detection. The sensor exhibited high sensitivity at sub-ppm concentrations with a notable response of 2.5 at 100 ppb. The WO3 sensor operated effectively at a relatively low temperature (200 °C) compared to other sensors and functioned consistently well under various humidity conditions, maintaining its performance with relative humidity levels above 20%. The sensor demonstrated a high degree of selectivity towards D-(+)-limonene over other gases, making it suitable for real-world applications. Therefore, it has the potential to enhance the detection and monitoring of D-(+)-limonene in various industries, including cosmetics, food, and industrial solvents. This research contributes significantly to the field by providing evidence that WO3-based sensors can meet the demands of modern detection systems, combining sensitivity, selectivity, and robustness in varying environmental conditions [114].
Weber et al. present the results of developing a compact and low-cost device for dynamic and selective detection of breath D-limonene, which serves as a biomarker for liver disease screening. The design was focused on providing a non-invasive, simple-to-use, and effective diagnostic tool to aid in early diagnosis. The device integrates a chemoresistive sensor made of Si/WO3 nanoparticles, and utilises a packed bed Tenax separation column, which was used to pre-screen the breath samples at room temperature. The device was tested on healthy volunteers who ingested limonene capsules (either by swallowing or chewing). It monitored the dynamics of breath limonene release and metabolism in real time. The detector was found to be able to selectively detect limonene down to 20 ppb even when other substances such as acetone, ethanol, hydrogen, methanol, and 2-propanol were present at much higher concentrations (up to three orders of magnitude higher). The device demonstrated consistent performance across a wide range of humidity levels (10–90% relative humidity). This attribute ensures its reliability in a variety of environmental conditions. The results showed an excellent correlation (R2 = 0.98), with high-resolution PTR-MS validating the accuracy and effectiveness of the device. This study underscores the potential of the developed detector as a non-invasive, cost-effective tool for routine monitoring of limonene levels in exhaled breath. It can facilitate early diagnosis of liver dysfunction, improving clinical outcomes through timely intervention, due to the combination of sensitivity, selectivity, robustness to humidity, and real-time monitoring capabilities. This device holds potential for widespread use in routine health screenings, thereby enabling early diagnosis and better management of liver diseases [73].
3.5. Infrared Spectroscopy Sensors (IR)
Infrared spectroscopy (IR) has emerged as a powerful tool for non-invasive breath analysis, offering significant potential for diagnosing and monitoring various diseases through the detection of VOCs and other biomarkers in exhaled breath [115]. Commonly used alone or in combination with other methods, excels in detecting VOCs across diverse concentration ranges.
Near-infrared photoacoustic spectroscopy (NIR-PAS) is highlighted for its high sensitivity and selectivity in identifying VOCs in exhaled breath. This technique is fast, non-invasive, and simple to implement, making it suitable for real-world health applications [116].
Mid-infrared (MIR) spectroscopy, particularly laser-based techniques, has shown promise for point-of-care diagnostics. It offers high sensitivity and molecular selectivity, essential for detecting trace levels of biomarkers in breath [79,117,118]. Techniques such as tuneable diode laser absorption spectroscopy (TDLAS), cavity ring-down spectroscopy (CRDS), and photoacoustic spectroscopy (PAS) are commonly used in MIR spectroscopy for breath analysis [79,118]. Laser absorption spectroscopy (LAS) in the mid-infrared region is effective for the quantitative analysis of trace gases in human breath, enabling real-time monitoring of biomarkers like nitric oxide, ammonia, and carbon monoxide [80].
IR spectroscopy sensors have been investigated for the detection of several VOCs, and thus, their possible implementation in diagnosing diseases. However, the successful detection of limonene in breath analysis by IR methods has not yet been described more specifically in any of the review papers or in thematically focused research papers. Nonetheless, IR techniques provide future potential for the detection of limonene in breath samples and should be further investigated for their applicability in limonene detection.
3.6. Ion Mobility Spectrometry (IMS)
Ion mobility spectrometry (IMS) is a technique that separates ionized molecules based on their mobility under an electric field as they travel through a gas. The sample is first ionized, typically using one of several ionization methods, such as atmospheric pressure chemical ionization (APCI), radioactive sources (e.g., Ni-63), and corona discharge. The generated ions are injected into a drift tube where they will be subjected to an electric field. Inside the drift tube, ions move through a neutral buffer gas (often nitrogen or air) under the influence of an electric field. At the end of the drift tube, ions are detected, usually by a Faraday plate or an electron multiplier. The arrival time of ions at the detector, known as the drift time, is recorded. The drift times are analysed and compared to known standards to identify the ions.
The mobility of each ion, which depends on its size, shape, and charge, determines how quickly it drifts through the tube. This drift time is highly characteristic of the ion and provides a basis for separation. Drift time libraries are used to identify ions by comparing the drift times with those in a library of known substances. The measured ion mobility can be related to the ion’s collision cross-section, which can provide structural information about the molecule. The IMS technique is often coupled with mass spectrometry (IMS-MS), where ions are analysed based on their mass-to-charge ratio. This tandem technique provides two layers of separation and identification, enhancing the specificity and sensitivity of the analysis.
IMS is a highly effective technique for the non-invasive detection of limonene in breath. It offers the ability to detect low concentrations of VOC with high specificity, making it suitable for medical diagnostics. The IMS has been demonstrated to be capable of detecting compounds at levels as low as ppb or, indeed, at even lower levels of parts per trillion (ppt). The fast response time of IMS allows for real-time monitoring of breath samples, providing immediate feedback. Operating at ambient conditions simplifies the process and reduces costs, making IMS practical for widespread use. Thus, it is a valuable tool for real-time breath analysis in medical diagnostics and environmental monitoring. When combined with other methods like gas chromatography (GC-IMS), the detection and identification capabilities of IMS are significantly enhanced.
A specific type of IMS that offers an additional separation of ions based on their differential mobility in high electric fields is a field asymmetric ion mobility spectrometry (FAIMS). The technique relies on the fact that ions will have different mobilities in high-field and low-field conditions, and by applying an asymmetric waveform to the ions, it is possible to separate them based on their mobility differences [119].
The monograph of Eiceman et al. provides a detailed explanation of IMS technology and its applications, including the GC-IMS. The combination of two techniques (GC and IMS) enhances the detection and identification capabilities for VOCs such as limonene in complex samples like breath [120].
3.7. Colorimetric Sensors (CS) and Luminescence Sensors
Colourimetric sensors (CS) are optical devices that change colour in response to specific analytes, making them a valuable tool for the detection of various compounds, including VOCs. CS provide a direct and visual indication of the presence of target compounds through observable colour changes. These sensors offer a simple, rapid, and cost-effective method for detecting target molecules based on visible colour changes. Hence, they could be implemented for portable or on-site analysis in medical diagnostics and environmental monitoring.
The study of Janzen et al. presents the development of a colourimetric sensor array for detecting VOCs, demonstrating its potential for analysis of 100 common VOC, including the detection of some hydrocarbons, but not of limonene directly. Selectivity and sensitivity of the sensors have been found to be satisfactory in principle for the detection of VOC as biomarkers. The arrays were essentially nonresponsive to changes in humidity, avoiding interference from changes in humidity [121].
In the preprint study, Gurung et al. colourimetric sensor arrays based on different porphyrins derivates with Cu, Fe, Mn, Co, and Zn as their core atoms were developed and tested with the liver biomarker D- limonene. A distinct, visually discernible response when exposed to limonene vapour for 5 min could be detected and categorized, proving that the proposed scheme works in principle and could be further developed to be useful as a cost-effective and simple analysis method for limonene. However, questions of sensitivity and selectivity have not been investigated so far in the described study [122].
A colourimetric biosensor was developed that employs immobilized acetylcholinesterase (AChE) on a zinc-based metal–organic framework (AChE@Zn-MOF). D-limonene inhibits AChE activity, disrupting acetylcholine hydrolysis and inducing a detectable pH shift, resulting in a visual colour change [123].
MOFs have garnered significant attention in the development of luminescent sensors for detecting VOCs, including limonene. Their unique structural properties—such as high porosity, tuneable functionality, and the ability to incorporate luminescent centres—make them ideal candidates for sensitive and selective sensing applications.
MOFs’ porous structures facilitate the concentration of analytes near luminescent centres, enhancing detection sensitivity. Functionalization of MOFs with specific ligands or enzymes can tailor their selectivity toward target analytes like limonene. The modular nature of MOFs allows for the customization of their structural and photophysical properties to suit specific sensing requirements. Recent research has demonstrated the efficacy of MOF-based luminescent sensors in detecting various VOCs. For instance, a study highlighted the development of a MOF-stabilized enzyme-based colourimetric biosensor integrated with smartphone-assisted image processing, enabling the detection of d-limonene at concentrations as low as 1 ppm. This approach combines the selectivity of enzymatic reactions with the sensitivity of MOF structures, offering a promising avenue for limonene detection. Although not yet specifically detailed for limonene, LMOFs are highlighted for their potential in detecting various VOCs due to their tuneable pore sizes and luminescence properties. They offer a promising avenue for developing luminescent sensors for limonene detection [124].
3.8. Sensors Based on Electrospun Nanofibres (ESNs)
Electrospun nanofibres (ESNs) have been utilized in the detection of various compounds, particularly due to their advantageous properties like high surface area, porosity, and ease of functionalization. The ESNs have shown potential for use in biosensors, including those designed for breath analysis. They can detect trace amounts of VOCs due to their enhanced surface interaction with target molecules, as they offer a large surface-to-volume ratio, enhancing interactions with target molecules like limonene. The composition and structure of nanofibres can be modified to optimize sensing characteristics. They can be functionalized with specific receptors or materials for selective VOC detection. These properties make them highly sensitive and selective for detecting various analytes. ESNs provide several benefits over traditional sensing materials, such as improved sensitivity, rapid response times, and the ability to be incorporated into portable and wearable devices.
ESN sensors present a promising avenue for the sensitive and selective detection of limonene in breath analysis. Their tuneable properties, high surface area, and enhanced sensing capabilities make them valuable tools for VOC detection in various applications.
The review of Ding et al. explores in general the use of electrospun nanomaterials for fabricating ultrasensitive sensors. Customized nanofibres can be tailored to specific VOCs, for advanced sensor applications. Some predominant sensing approaches such as acoustic wave, resistive, photoelectric, optical, amperometric, etc. are presented. The detection of limonene is, however, not specifically described, only the detection of some hydrocarbons is elucidated further [125]. There are a larger number of similar reviews on the use of nanofibres for sensing applications [126,127,128,129,130].
In a recent study by Macagnano et al., a conductive composite electrospun nanofibrous fabric of polyvinylpyrrolidone (PVP), polyacrylic acid (PAA) and carbon nanotubes (MWCNTs) deposited onto microelectrodes was used as a stereoselective L-limonene sensor. The sensor was exposed to monoterpenes ((±)-α-pinene and (±)-linalool) and the D-enantiomer of limonene in environmental air, in order to prove its sensitivity, selectivity and stereoselectivity [131]. Therefore, the design is proven to be a promising option for sensing limonene and its application in the detection of the compound in the environment, including exhaled breath from patients suffering from liver diseases.
3.9. Aptamer-Based Sensors (APT)
The recognition elements of aptamers are short, single-stranded DNA or RNA molecules that can fold into specific three-dimensional structures capable of binding to target molecules with high affinity and specificity. They are often referred to as “chemical antibodies”.
Aptamers are typically selected from a large pool of random sequences using a process called SELEX (Systematic Evolution of Ligands by EXponential enrichment). This iterative process involves binding, separation, and amplification steps to isolate aptamers that bind strongly to the target molecule. The binding between aptamers and their targets is based on non-covalent interactions, including hydrogen bonding, electrostatic interactions, and van der Waals forces. The high specificity is due to the unique three-dimensional structure of the aptamer that complements the target molecule.
APT sensors are used in a variety of fields, including medical diagnostics, environmental monitoring, and food safety. They are valued for their high specificity, ability to be regenerated and reused, and the ability to bind to a wide range of targets, including small molecules, proteins, and even cells. They are in general biocompatible and non-immunogenic.
Appropriate transducers to convert the aptamer-limonene interaction into a measurable signal may be: electrochemical transducers (EC) (measure changes in current, voltage, or impedance), optical transducers (measure changes in light absorption, fluorescence, or surface plasmon resonance—SPR), or mass-sensitive transducers (measure changes in mass, such as in QCM).
Aptamers have a high binding affinity for the target compound under diverse conditions, ensuring efficient and consistent detection. They are known for their stability under various environmental conditions, making them suitable for sensor applications in real-time monitoring.
Aptamers can be tailored to various target compounds, enhancing their versatility for sensor applications. APT sensors enable quick and real-time monitoring of limonene concentrations in breath samples, due to high specificity and selectivity. Their molecular recognition capabilities make them valuable tools for non-invasive diagnostics in medical and environmental contexts.
In the review of Gaggiotti et al., the latest developments in gas sensors and gas sensor arrays are described. The authors summarized the use of peptides, MIP and DNA elements in the development of GS for sensing. The first, peptides, are described as an extension of the olfactory receptors, with great variability of the response to various VOCs, while the latter, DNA, is mentioned to have interesting results, and can lead to a combined, synergistic peptide-DNA array. QCM, FET (field-effect transistor) and surface plasmon resonance imaging are described as transduction principles for sensing [132].
Limonene was found to be captured at specific binding sites of acetylcholinesterase (AChE), both, in molecular dynamics simulations first and experimental proof. Using a similar principle as in a previously described colourimetric biosensor [123], a sensing signal was provided by using an ion-sensitive FET, as a result of limonene inhibiting the catalytic activity of AChE towards Ach hydrolysis, thus creating a dependence between limonene concentration and catalytic inhibition. A detection limit of 5.7 μM was determined, with the selectivity of the D-limonene detection being proven against pinene and perillic acid [133].
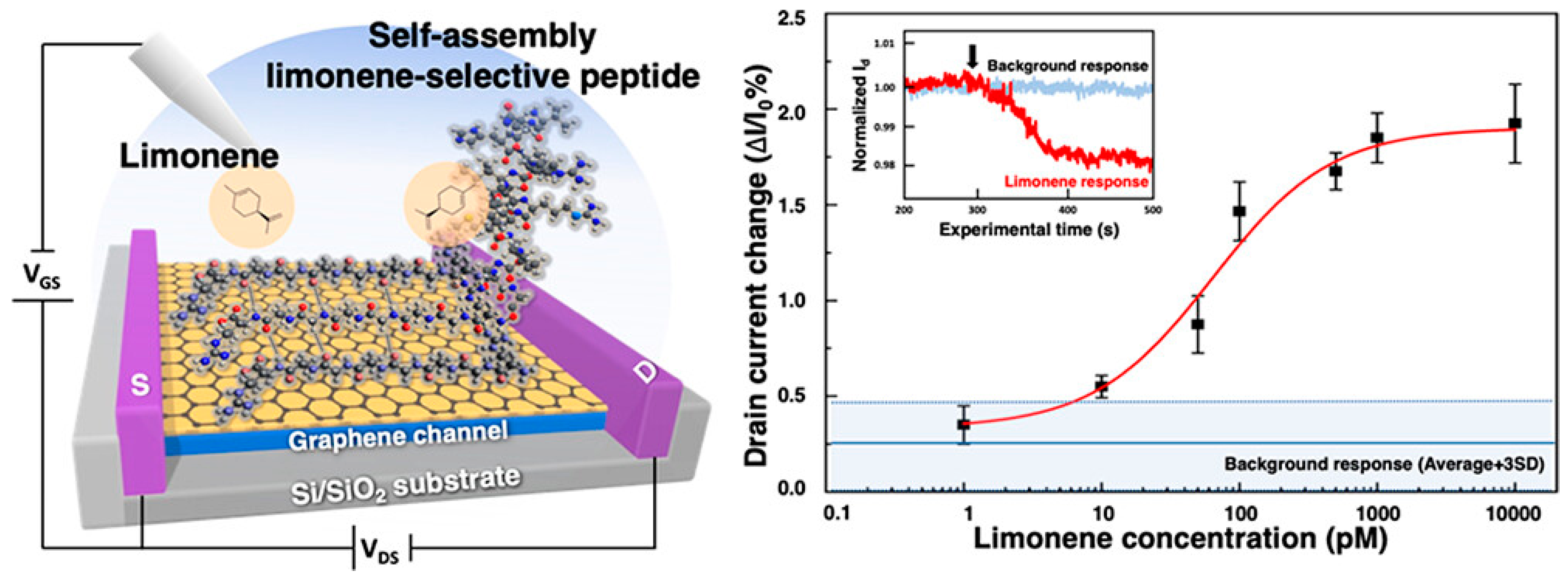
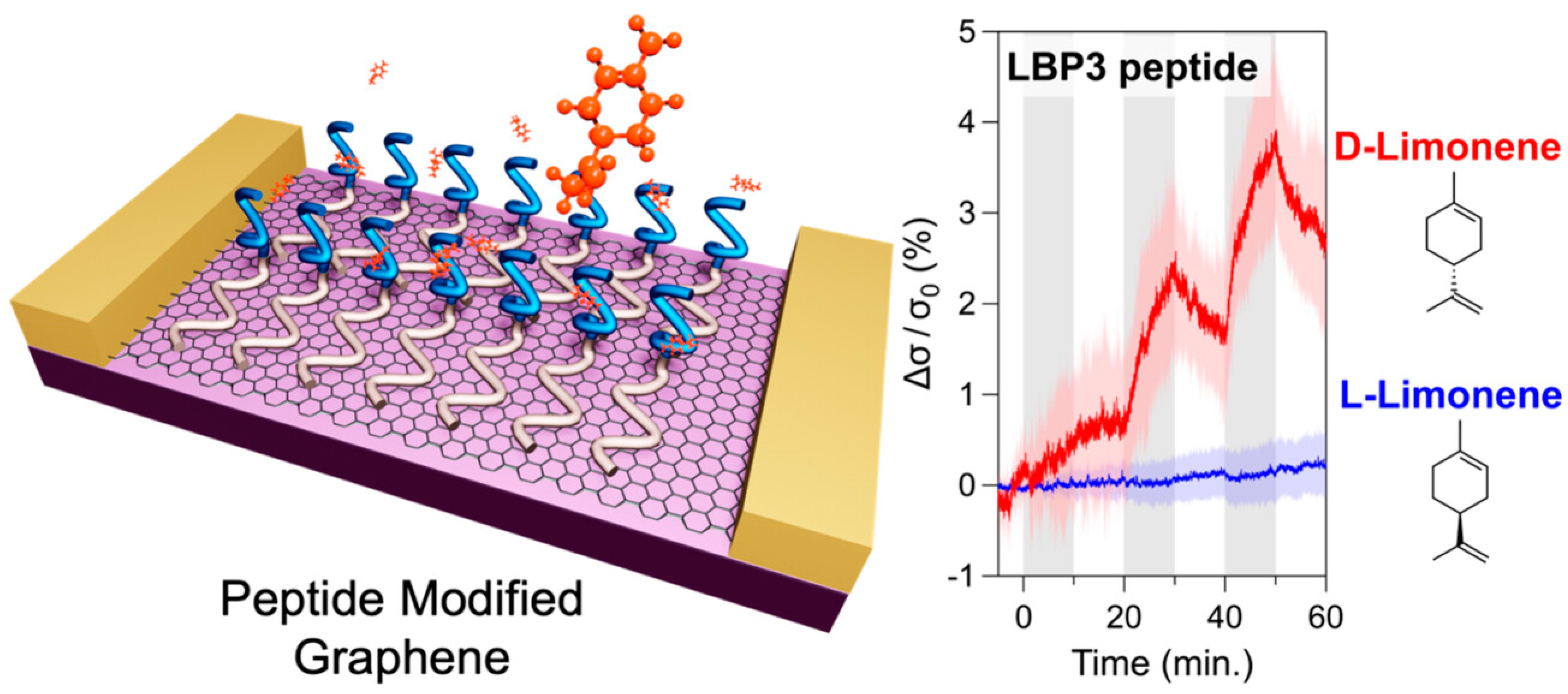
The pursuit of replicating the sense of smell in biomimetic devices has faced significant challenges, particularly in detecting nonpolar odour molecules in humid environments. Traditional graphene sensors are highly sensitive to water vapour, which has hindered their practical application. A graphene FET (gFET) based olfactory receptor was designed and analysed by Rungreungthanapol et al., in order to assess the viability of the use of combined peptide array and gas chromatography for the sensitive and selective gFET detection of limonene. The sensor was created with peptides designed to mimic the olfactory receptor of fruit flies. The sensor probe achieved a highly sensitive and selective detection of limonene within a detection range of 8–1000 pM (Figure 6) [134]. Yamazaki et al. expanded on this research, reporting on the effectiveness of peptide-functionalized graphene sensors in reducing unwanted humidity responses and enhancing selective detection of odorant molecules (Figure 7) [135]. The sensors achieved precise and specific odorant detection, even demonstrating a 35-fold signal contrast between D- and L-limonene. These novel sensors are versatile and offer significant potential for practical applications in various fields due to their robust, selective detection capabilities under normal atmospheric conditions.
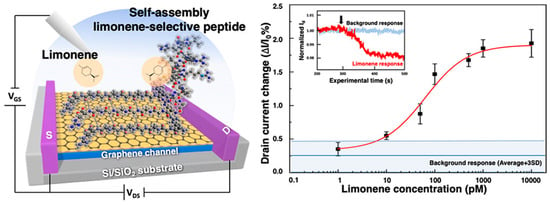
Figure 6.
Limonene detection on a mimetic peptide-modified graphene field-effect transistor (gFET), representing drain current change over the initial current as a response after exposure to limonene (reprinted with permission from Ref. [134], Copyright 2023 American Chemical Society).
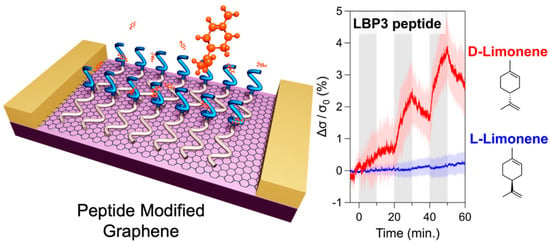
Figure 7.
Signal contrast between D- and L-Limonene from a peptide-modified graphene sensor, representing conductivity changes responding to enantiomers of limonene (reprinted with permission from Ref. [135]).
3.10. Molecularly Imprinted Polymer-Based Sensors (MIP)
The recognition element for molecularly imprinted polymers is synthetic polymers with a predetermined selectivity for a given analyte. They are created by polymerizing functional and cross-linking monomers in the presence of the target molecule (template). The synthesis process involves mixing the target molecule with monomers that can interact with the target. These monomers polymerize and form a network around the target molecule. After polymerization, the target molecule is removed, leaving behind a cavity that is complementary in shape and functional groups to the target molecule.
The binding mechanism in MIPs is based on the physical and chemical complementarity between the imprinted cavities and the target molecules. Interactions can include hydrogen bonds, ionic interactions, and hydrophobic effects. MIP-based sensors are widely used in environmental monitoring, food analysis, and pharmaceutical applications. They are particularly useful for detecting small molecules and have been used in solid-phase extraction and chromatographic separation.
The advantages of MIP-based sensors are as follows:
- High stability (thermal, chemical, and physical);
- Cost-effective and easy to produce;
- Robust and can be used in harsh conditions.
Aptamer-based sensors and sensors based on MIPs are both used for the selective detection of target molecules, but they differ in their principles of recognition, synthesis, and applications (Table 1).

Table 1.
Summary of differences between APT and MIP sensors.
In a mini-review Cuypers and Lieberzeit describe the use of the MIP strategy for enhancing the sensitivity of electronic nose approaches [136]. The sensing of limonene and other terpenes is described as an example of the high selectivity of such MIP devices.
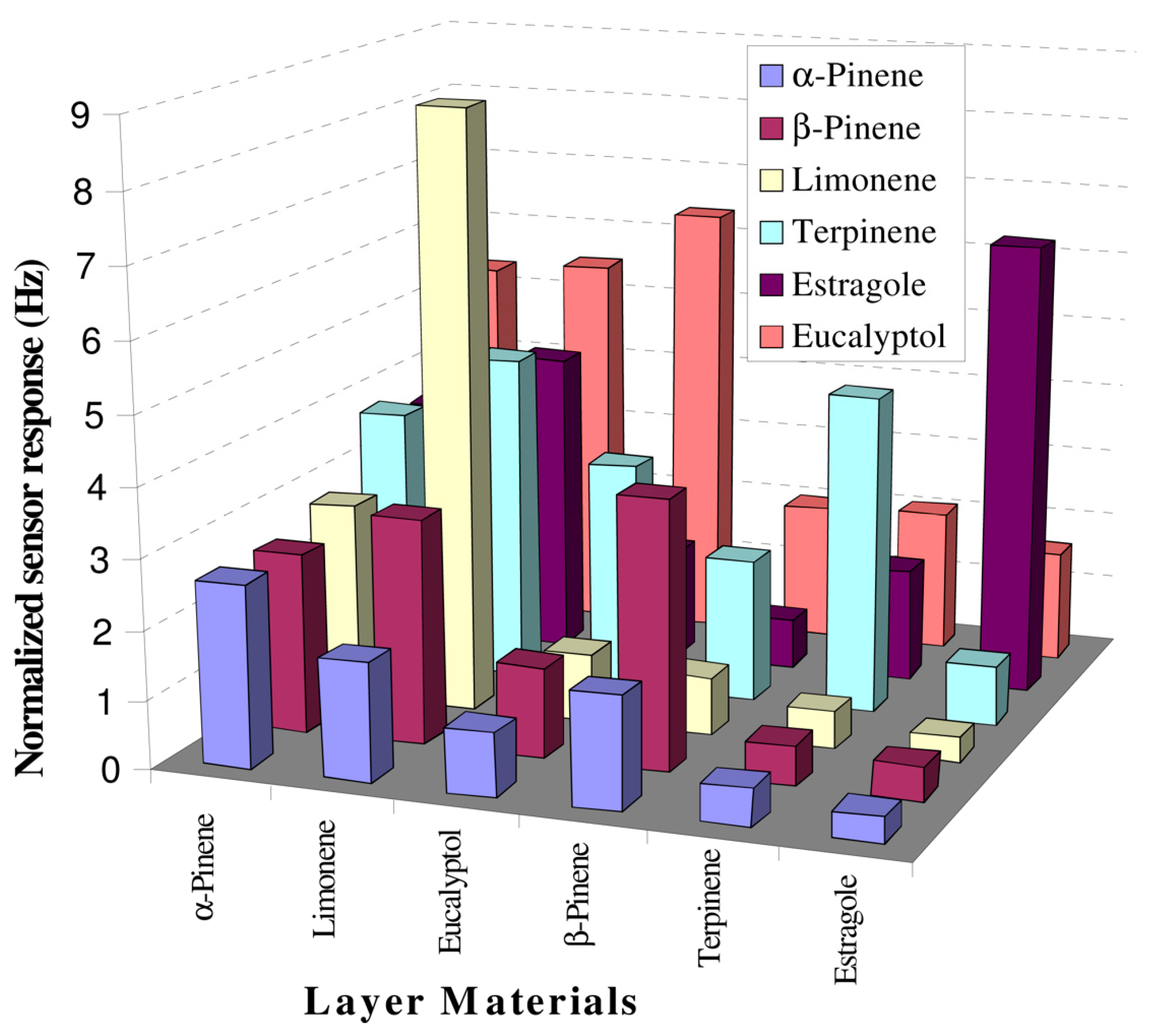
A sample sensor built up with piezoelectric 10 MHz multichannel quartz crystal microbalance (MQCM) coated with six MIP based on polystyrene was investigated for its detection of terpenes from fresh and dried Lamiaceae family species, like rosemary, basil and sage. The sensitivity was proven to be <20 ppm within a linear concentration range of 20–250 ppm, with sensor data being validated by GC-MS. Such an array in association with data analysis tools can be utilized for characterizing complex mixtures (Figure 8) [137].
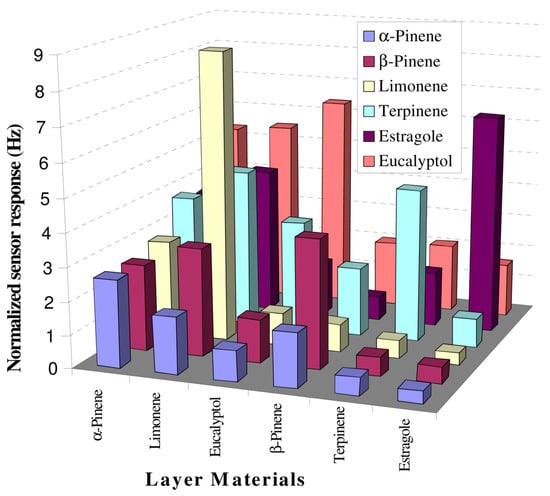
Figure 8.
Selectivity profile of polymer layers at 50 ppm concentration of given analyte, (reprinted with permission from Ref. [137]).
Fruit maturity in vapours emanating from fruit, and consequently containing limonene, was proven with a MIP sensor membrane based on methacrylic acid on a PET basis, where signal transduction was performed by an interdigitated electrode structure. What is more, the sensor could be reused after removing the adsorbed D-limonene molecules by immersion in a specific solution (not named by the authors) [138].
The summary of all aforementioned sensors, along with the sensing principles and representative references investigating those sensors can be found in Table 2.

Table 2.
Summary of different sensing principles for limonene with representative original research or review articles.
4. Advantages and Disadvantages of Specific Limonene Sensors for Breath Analysis
The potential and challenges associated with using limonene sensors for breath analysis are highlighted by an examination of the advantages and disadvantages.
4.1. Advantages
- High sensitivity and specificity: Analysis techniques such as GC-MS, PTR-MS, and SIFT-MS offer high sensitivity and specificity, capable of detecting trace amounts of limonene, which is crucial for accurate breath analysis. GC-MS is generally considered to provide the highest sensitivity and specificity for detecting limonene, especially in complex mixtures. However, SAW, QCM, and IMS also offer high sensitivity and may be preferred in applications requiring real-time monitoring or specific environmental conditions.
- Non-invasiveness: Breath analysis is a non-invasive method, making it more comfortable and safer for patients compared to blood tests or biopsies. This can improve patient compliance and make frequent monitoring feasible.
- Rapid and real-time analysis: PTR-MS and e-Nose facilitate rapid and real-time analysis, enabling immediate results that are beneficial in clinical diagnostics and monitoring applications.
- Portability: Sensors such as MOS and e-Nose can be made portable, enabling point-of-care diagnostics and on-site analysis, a particularly advantageous feature in remote or field settings.
- Cost-effectiveness: Sensors like MOS and ESNs can be relatively inexpensive to produce and operate, making them accessible for widespread use.
- Potential for integration with wearable devices: The lightweight and flexible nature of ESNs renders them suitable for integration with wearable devices for continuous monitoring.
4.2. Disadvantages
- 7.
- Calibration and standardization: Many sensors require frequent calibration to maintain accuracy. The lack of standardized calibration protocols can lead to variability in results between different devices and studies.
- 8.
- Interference from other compounds: breath contains a complex mixture of VOCs, and certain sensors may suffer from cross-sensitivity or interference from other compounds, which can affect the accuracy of limonene detection.
- 9.
- Environmental conditions: sensors like MOS can be affected by environmental conditions such as humidity and temperature, which can impact their performance and reliability.
- 10.
- Complexity and cost of high-sensitivity techniques: Techniques like GC-MS and PTR-MS, while highly sensitive, are complex and expensive, requiring sophisticated equipment and skilled operators. This limits their use to specialized laboratories and reduces accessibility for routine screening.
- 11.
- Durability and longevity: Some sensors, particularly those using nanomaterials, may face issues with durability and longevity. Their performance can degrade over time, necessitating frequent replacement or maintenance.
- 12.
- Sample collection and handling: Proper sample collection and handling are critical for accurate results, especially for techniques requiring breath samples to be stored and transported (e.g., GC-MS analysis using Tedlar bags). Improper handling can lead to contamination or loss of analytes.
5. Limonene Sensors in Comparison
5.1. Sensitivity and Detection Limits
Several studies have demonstrated the capability of different sensor technologies to detect limonene at sub-ppm levels in air. For instance, a chemoresistive sensor based on WO3 was found to be highly sensitive to limonene, with a response of 2.5 at 100 ppb and effective detection at lower operating temperatures (200 °C) [114]. Additionally, a compact detector using Si/WO3 nanoparticles demonstrated selective detection of limonene down to 20 ppb in breath samples [73].
5.2. Selectivity and Specificity
The selectivity of sensors is crucial for accurate limonene detection in the presence of other volatile organic compounds (VOCs). The WO3-based chemoresistive sensor exhibited remarkable cross-selectivity, making it suitable for real-life applications [114]. Similarly, a gFET functionalized with fly olfactory receptor mimetic peptides achieved highly sensitive and selective detection of limonene, with a detection range of 8–1000 pM [134]. Enzyme-based sensors also showed promise, with AChE selectively interacting with limonene, allowing for detection at 5.7 μM [133]. The selectivity of sensors is crucial for accurate limonene detection in the presence of other volatile organic compounds (VOCs). The WO3-based chemoresistive sensor exhibited remarkable cross-selectivity, making it suitable for real-life applications [114]. Similarly, a gFET functionalized with fly olfactory receptor mimetic peptides achieved highly sensitive and selective detection of limonene, with a detection range of 8–1000 pM [134]. Enzyme-based sensors also showed promise, with AChE selectively interacting with limonene, allowing for detection at 5.7 μM [133].
5.3. Practical Applications
The practical applications of these sensors are diverse. For instance, the sulfur-doped graphene-based sensors were successfully used to determine limonene concentrations in beverages like triple sec liqueur and limoncello, achieving high recoveries and low relative standard deviation (RSD) values [141]. The compact breath detector for limonene demonstrated potential for non-invasive liver disease screening by monitoring limonene levels in exhaled breath [73]. Additionally, human olfactory studies revealed that individuals could detect limonene at concentrations as low as 8 ppb, suggesting the potential for human-based detection methods in controlled environments [162].
5.4. Summary
Breath analysis can be achieved with the help of many methods, as highlighted by this review. However, the development of sensors and detectors of limonene for quick and accurate diagnosis of patients is in a rather early and rudimentary stage. There is a limited number of studies where the sensors were tested on patients, and even then, the pool of subjects is relatively small. Nonetheless, the findings of these studies are encouraging and provide a foundation for further research.
The table below summarizes various methods for detecting limonene, highlighting their sensitivity and detection limits (Table 3). It has been demonstrated that both enzyme inhibition and chemoresistive sensors exhibit high sensitivity and selectivity, with detection limits in the micromolar and sub-ppm ranges, respectively. It has been shown that sulfur-doped graphene sensors and olfactory detection methods also demonstrate high sensitivity, with the latter being particularly effective in controlled environments. Plastic fibre optic sensors and molecularly imprinted polymer-based QCM sensors offer cost-effective and highly sensitive alternatives, suitable for various applications including real-time monitoring and food analysis.

Table 3.
Comparison of sensitivity and detection limits of limonene analysis in air by different detection methods.
6. Conclusions, Challenges and Future Directions
Breath analysis for the detection of limonene is a promising non-invasive method for the diagnosis of liver diseases, such as cirrhosis, due to limonene’s presence in the breath of affected individuals. Developing novel limonene sensors to screen exhaled breath volatiles as biomarkers for liver dysfunction has several potential benefits, as early detection could lead to improved diagnosis, survival rates, and significant healthcare savings. Future research in this area should focus on improving the sensitivity and selectivity of the sensors. Additionally, integrating these sensors into portable and user-friendly devices will be crucial for widespread adoption in healthcare. Recent research has made significant strides in developing sensitive and selective sensors for limonene detection. Technologies such as WO3-based chemoresistive sensors, sulfur-doped graphene-based electrochemical sensors, and enzyme-based sensors have shown promising results at sub-ppm levels. Sensors, such as those using thiol-capped gold nanoparticles and Cr2O3-doped graphene, show further promise in detecting limonene with high sensitivity and selectivity. Further research should aim to improve these properties to ensure accurate detection in complex breath samples. Another area where focused research is still needed, is the miniaturization and portability of sensors. Developing compact, low-cost, and portable sensors is crucial for practical applications. Technologies like chemoresistive sensors offer potential for miniaturization and integration into wearable devices. Sensor systems should be designed for use by anyone, anywhere, without the need for technical expertise or medical personnel, reducing screening costs and increasing accessibility. Future sensors should be designed for integration with Internet of Things (IoT) systems to enable real-time monitoring and data analysis. This could facilitate continuous health monitoring and early detection of disease. Low-cost systems will be important in enabling mass screening, especially in low-income countries, where the incidence of liver diseases is even higher than in developed countries. Potential early mass screening with low-cost sensors could make a difference in providing better treatment and its outcome. Systems such as the colourimetric sensor have the potential to be considered as candidates for such sensor types. Yet, it is imperative to acknowledge that the development of these systems will require extensive research and development in the future in order to ensure their suitability for the aforementioned applications. Such limonene sensors will offer rapid analysis and easy interpretation of results, providing users with actionable healthcare information and guidance on whether further diagnostic tests are necessary. Future widespread low-cost screening will ensure a meaningful societal impact by addressing hitherto unmet healthcare needs. Research should explore further dynamic breath testing methods, which have shown improved diagnostic performance for cirrhosis by monitoring limonene kinetics in real time. This approach could improve the accuracy and reliability of breath analysis. Addressing cross-selectivity issues and managing interference from other volatile organic compounds in breath is essential. Strategies such as the use of specific olfactory receptor mimetic peptides and advanced material composites can help achieve this. Future research in breath analysis for limonene detection should therefore focus on improving sensor sensitivity, selectivity, and integration into portable, real-time monitoring systems. These advances will enhance the diagnostic capabilities for liver diseases, making non-invasive breath analysis a viable tool in clinical settings.
Author Contributions
Writing—original draft preparation, E.K., C.K., W.L.; writing—review and editing, E.K., C.K., W.L.; supervision, E.K., C.K.; project administration, C.K.; funding acquisition, C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Austrian Research Promotion Agency (FFG) within the COMET Project “PI-SENS” (Project No. 915477) as well as by the Federal Provinces of Lower Austria and Tirol.
Conflicts of Interest
None of the authors have any conflicts of interest and are not involved in any commercial activities related to the topic of the review paper.
Abbreviations
The following abbreviations are used in this manuscript:
| AChE | Acetylcholinesterase |
| AIH | Autoimmune hepatitis |
| ALD | Alcohol-related liver disease |
| APCI | Atmospheric pressure chemical ionization |
| AUROC | Area under the receiver operating characteristic |
| CNT | Carbon nanotube |
| CRDS | Cavity ring-down spectroscopy |
| CS | Colorimetric sensor |
| CT | Computer tomography |
| DB-SPME | Direct-breath solid-phase microextraction |
| DILI | Drug-induced liver injury |
| EC | Electrochemical sensor |
| FAIMS | Field asymmetric ion mobility spectrometry |
| FET | Field-effect transistor |
| FGC-SAW | Fast gas chromatography surface acoustic wave |
| GC | Gas chromatography |
| GC-IMS | Gas chromatography ion mobility spectrometry |
| GC-MS | Gas chromatography-mass spectrometry |
| gFET | Graphene field-effect transistor |
| HCC | Hepatocellular carcinoma |
| HE | Hepatic encephalopathy |
| IMS | Ion mobility spectrometry |
| IMS-MS | Ion mobility spectrometry-mass spectrometry |
| IoT | Internet of things |
| IUPAC | International Union of Pure and Applied Chemistry |
| LAS | Laser absorption spectroscopy |
| LFT | Liver function test |
| LoD | Limit of detection |
| MEMS | Micro-electro-mechanical systems |
| MIP | Molecularly imprinted polymer |
| MIR | Mid-infrared |
| MOF | Motel organic frameworks |
| MOS | Metal-oxide semiconductor |
| MRI | Magnetic resonance imaging |
| MS | Mass spectrometry |
| MQCM | Multichannel quartz crystal microbalance |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NIR-PAS | Near-infrared photoacoustic spectroscopy |
| PAA | Polyacrylic acid |
| PAS | Photoacoustic spectroscopy |
| PBC | Primary biliary cholangitis |
| PDMS | Polydimethylsiloxane |
| PLC | Primary liver cancer |
| ppb | Parts per billion |
| ppt | Parts per trillion |
| PTR-MS | Proton transfer reaction-mass spectrometry |
| PVP | Polyvinylpyrrolidone |
| RSD | Relative standard deviation |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SAW | Surface acoustic wave |
| SELEX | Systemic evolution of ligands by exponential enrichment |
| SIFT | Selected ion flow tube |
| SIFT-MS | Selected ion flow tube-mass spectrometry |
| SOF | Silica optical fibre |
| SPME | Solid-phase microextraction |
| TDLAS | Tuneable diode laser absorption spectroscopy |
| QCM | Quartz crystal microbalance |
| VOC | Volatile organic compound |
References
- Solomons, T.W.G.; Fryhle, C.B.; Snyder, S.A. Organic Chemistry, 12th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Erasto, P.; Viljoen, A.M. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Limonene Priority Existing Chemical Assessment Report; NICNAS: Sydney, Australia, 2002; p. 149.
- Malko, M.; Wróblewska, A. The Importance of R-(+)-Limonene as the Raw Material for Organic Syntheses and for Organic Industry. CHEMIK Nauka-Technika-Rynek 2016, 70, 198–202. [Google Scholar]
- Rowe, D. Practical Analysis of Flavor and Fragrance Materials; Goodner, K., Rouseff, R., Eds.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of Toxicological Assessment of D-Limonene, a Food and Cosmetics Additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef]
- Matura, M.; Sköld, M.; Börje, A.; Andersen, K.E.; Bruze, M.; Frosch, P.; Goossens, A.; Johansen, J.D.; Svedman, C.; White, I.R.; et al. Not Only Oxidized R-(+)- but Also S-(−)-Limonene Is a Common Cause of Contact Allergy in Dermatitis Patients in Europe. Contact Dermat. 2006, 55, 274–279. [Google Scholar] [CrossRef]
- Sunil, V.R.; Laumbach, R.J.; Patel, K.J.; Turpin, B.J.; Lim, H.-J.; Kipen, H.M.; Laskin, J.D.; Laskin, D.L. Pulmonary Effects of Inhaled Limonene Ozone Reaction Products in Elderly Rats. Toxicol. Appl. Pharmacol. 2007, 222, 211–220. [Google Scholar] [CrossRef]
- Anderson, S.E.; Khurshid, S.S.; Meade, B.J.; Lukomska, E.; Wells, J.R. Toxicological Analysis of Limonene Reaction Products Using an in Vitro Exposure System. Toxicol. In Vitro 2013, 27, 721–730. [Google Scholar] [CrossRef]
- Dales, R.E.; Cakmak, S. Is Residential Ambient Air Limonene Associated with Asthma? Findings from the Canadian Health Measures Survey. Environ. Pollut. 2019, 244, 966–970. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase I and Pharmacokinetic Study of D-Limonene in Patients with Advanced Cancer. Cancer Chemother. Pharmacol. 1998, 42, 111–117. [Google Scholar] [CrossRef]
- Igimi, H.; Hisatsugu, T.; Nishimura, M. The Use Ofd-Limonene Preparation as a Dissolving Agent of Gallstones. Am. J. Dig. Dis. 1976, 21, 926–939. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and Clinical Applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Crowell, P.L.; Gould, M.N. Chemoprevention and Therapy of Cancer by d-Limonene. Crit. Rev. Oncog. 1994, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Santana, H.S.R.; de Carvalho, F.O.; Santos, D.M.D.; da Silva, E.A.P.; Silva, É.R.; Shanmugam, S.; Heimfarth, L.; Nunes, P.S.; de Oliveira e Silva, A.M.; de Souza Araújo, A.A.; et al. Inhaled D-Limonene Minimizes Acute Lung Injury and Reduces Oxidative Stress Induced by Smoke in Rats. Phytomed. Plus 2022, 2, 100308. [Google Scholar] [CrossRef]
- Zhu, T.; Li, Y.; Feng, T.; Yang, Y.; Zhang, K.; Gao, J.; Quan, X.; Qian, Y.; Yu, H.; Qian, B. d-Limonene Inhibits the Occurrence and Progression of Luad through Suppressing Lipid Droplet Accumulation Induced by Pm2.5 Exposure in Vivo and in Vitro. Respir. Res. 2022, 23, 338. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Sarbach, C.; Vergès, S.; Coiffard, F.; Constancias, C.; Champigneulle, B.; Doutreleau, S.; Andreucci, P.; Duraffourg, L.; Postaire, E. Evidence of Limonene in Breath Samples in Men from the World’s Highest City. Med. Res. Arch. 2022, 10, 1–18. [Google Scholar] [CrossRef]
- Miyazawa, M.; Shindo, M.; Shimada, T. Metabolism of (+)- and (−)-Limonenes to Respective Carveols and Perillyl Alcohols by Cyp2c9 and Cyp2c19 in Human Liver Microsomes. Drug Metab. Dispos. 2002, 30, 602–607. [Google Scholar] [CrossRef]
- Xie, Z.; Sutaria, S.R.; Chen, J.Y.; Gao, H.; Conklin, D.J.; Keith, R.J.; Srivastava, S.; Lorkiewicz, P.; Bhatnagar, A. Evaluation of Urinary Limonene Metabolites as Biomarkers of Exposure to Greenness. Environ. Res. 2024, 245, 117991. [Google Scholar] [CrossRef]
- Schmidt, L.; Göen, T. R-Limonene Metabolism in Humans and Metabolite Kinetics after Oral Administration. Arch. Toxicol. 2017, 91, 1175–1185. [Google Scholar] [CrossRef]
- Stavropoulos, G.; van Munster, K.; Ferrandino, G.; Sauca, M.; Ponsioen, C.; van Schooten, F.-J.; Smolinska, A. Liver Impairment—The Potential Application of Volatile Organic Compounds in Hepatology. Metabolites 2021, 11, 618. [Google Scholar] [CrossRef]
- Sinha, R.; Lockman, K.A.; Homer, N.Z.M.; Bower, E.; Brinkman, P.; Knobel, H.H.; Fallowfield, J.A.; Jaap, A.J.; Hayes, P.C.; Plevris, J.N. Volatomic Analysis Identifies Compounds That Can Stratify Non-Alcoholic Fatty Liver Disease. JHEP Rep. 2020, 2, 100137. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.I.; Preti, G.; Deems, R.O.; Friedman, L.S.; Munoz, S.J.; Maddrey, W.C. Limonene in Expired Lung Air of Patients with Liver Disease. Dig. Dis. Sci. 1994, 39, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, G.; Orf, I.; Smith, R.; Calcagno, M.; Thind, A.K.; Debiram-Beecham, I.; Williams, M.; Gandelman, O.; de Saedeleer, A.; Kibble, G.; et al. Breath Biopsy Assessment of Liver Disease Using an Exogenous Volatile Organic Compound—Toward Improved Detection of Liver Impairment. Clin. Transl. Gastroenterol. 2020, 11, e00239. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.E.; del Río, R.F.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Limonene in Exhaled Breath Is Elevated in Hepatic Encephalopathy. J. Breath Res. 2016, 10, 046010. [Google Scholar] [CrossRef]
- Fernández del Río, R.; O’Hara, M.E.; Holt, A.; Pemberton, P.; Shah, T.; Whitehouse, T.; Mayhew, C.A. Volatile Biomarkers in Breath Associated with Liver Cirrhosis—Comparisons of Pre- and Post-Liver Transplant Breath Samples. EBioMedicine 2015, 2, 1243–1250. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile Biomarkers in the Breath of Women with Breast Cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of Head-and-Neck Cancer from Exhaled Breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef]
- Fedeli, U.; Amidei, C.B.; Casotto, V.; Grande, E.; Saia, M.; Zanetto, A.; Russo, F.P. Mortality from Chronic Liver Disease: Recent Trends and Impact of the Covid-19 Pandemic. World J. Gastroenterol. 2023, 29, 4166–4173. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global Epidemiology of Cirrhosis—Aetiology, Trends and Predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-Alcoholic Fatty Liver Disease—A Global Public Health Perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Non-Alcoholic Fatty Liver Disease: An Overview of Risk Factors, Pathophysiological Mechanisms, Diagnostic Procedures, and Therapeutic Interventions. Life Sci. 2021, 271, 119220. [Google Scholar] [CrossRef] [PubMed]
- Juanola, O.; Martínez-López, S.; Francés, R.; Gómez-Hurtado, I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 5227. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global Epidemiology of Alcohol-Associated Cirrhosis and Hcc: Trends, Projections and Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. [Google Scholar] [CrossRef]
- Alcohol and the Human Body. National Institute on Alcohol Abuse and Alcoholism. Available online: https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics-z/alcohol-facts-and-statistics/alcohol-and-human-body (accessed on 12 March 2025).
- Gazda, J.; Drazilova, S.; Janicko, M.; Jarcuska, P. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 9151525. [Google Scholar] [CrossRef]
- Olynyk, J.K.; Ramm, G.A. Hemochromatosis. N. Engl. J. Med. 2022, 387, 2159–2170. [Google Scholar] [CrossRef]
- Gao, J.; Brackley, S.; Mann, J.P. The Global Prevalence of Wilson Disease from Next-Generation Sequencing Data. Genet. Med. 2019, 21, 1155–1163. [Google Scholar] [CrossRef]
- Fregonese, L.; Stolk, J. Hereditary Alpha-1-Antitrypsin Deficiency and Its Clinical Consequences. Orphanet J. Rare Dis. 2008, 3, 16. [Google Scholar] [CrossRef]
- Li, X.; Tang, J.; Mao, Y. Incidence and Risk Factors of Drug-Induced Liver Injury. Liver Int. 2022, 42, 1999–2014. [Google Scholar] [CrossRef]
- Jagdish, R.K.; Maras, J.S.; Sarin, S.K. Albumin in Advanced Liver Diseases: The Good and Bad of a Drug! Hepatology 2021, 74, 2848–2862. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.F. The Misunderstood Coagulopathy of Liver Disease: A Review for the Acute Setting. West. J. Emerg. Med. 2018, 19, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Wright, G.; Gatt, A.; Riddell, A.; Vemala, V.; Mallett, S.; Chowdary, P.; Davenport, A.; Jalan, R.; Burroughs, A. Evaluation of Coagulation Abnormalities in Acute Liver Failure. J. Hepatol. 2012, 57, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Loaeza-del-Castillo, A.; Paz-Pineda, F.; Oviedo-Cárdenas, E.; Sánchez-Ávila, F.; Vargas-Vorácková, F. AST to Platelet Ratio Index (APRI) for the Noninvasive Evaluation of Liver Fibrosis: Original Article. Ann. Hepatol. 2008, 7, 350–357. [Google Scholar] [CrossRef]
- Aslam, F.; Loveday, T.; Junior, P.L.S.U.; Borad, M.J. Utility of Ast to Platelet Ratio Index (APRI) Score in Predicting Post-Surgical Outcomes in Patients with Cholangiocarcinoma. J. Clin. Oncol. 2023, 41, 502. [Google Scholar] [CrossRef]
- Kim, W.R.; Berg, T.; Asselah, T.; Flisiak, R.; Fung, S.; Gordon, S.C.; Janssen, H.L.A.; Lampertico, P.; Lau, D.; Bornstein, J.D.; et al. Evaluation of Apri and FIB-4 Scoring Systems for Non-Invasive Assessment of Hepatic Fibrosis in Chronic Hepatitis B Patients. J. Hepatol. 2016, 64, 773–780. [Google Scholar] [CrossRef]
- Sonneveld, M.J.; Brouwer, W.P.; Chan, H.L.Y.; Piratvisuth, T.; Jia, J.-D.; Zeuzem, S.; Liaw, Y.-F.; Hansen, B.E.; Choi, H.; Wat, C.; et al. Optimisation of the Use of APRI and FIB-4 to Rule out Cirrhosis in Patients with Chronic Hepatitis B: Results from the Sonic-B Study. Lancet Gastroenterol. Hepatol. 2019, 4, 538–544. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Wei, L.; Tang, Q.; Hu, P. Optimized Cutoffs of Gamma-Glutamyl Transpeptidase-to-Platelet Ratio, Aspartate Aminotransferase-to-Platelet Ratio Index, and Fibrosis-4 Scoring Systems for Exclusion of Cirrhosis in Patients with Chronic Hepatitis B. Hepatol. Commun. 2022, 6, 1664–1672. [Google Scholar] [CrossRef]
- Van Beers, B.E.; Daire, J.-L.; Garteiser, P. New Imaging Techniques for Liver Diseases. J. Hepatol. 2015, 62, 690–700. [Google Scholar] [CrossRef]
- Van Beers, B.E.; Garteiser, P.; Leporq, B.; Rautou, P.-E.; Valla, D. Quantitative Imaging in Diffuse Liver Diseases. Semin. Liver Dis. 2017, 37, 243–258. [Google Scholar]
- Mathew, R.P.; Venkatesh, S.K. Imaging of Hepatic Fibrosis. Curr. Gastroenterol. Rep. 2018, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Babu, A.; Wells, M.L.; Teytelboym, O.M.; Mackey, J.E.; Miller, F.H.; Yeh, B.M.; Ehman, R.L.; Venkatesh, S.K. Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions. RadioGraphics 2016, 36, 1987–2006. [Google Scholar] [CrossRef] [PubMed]
- Haj-Mirzaian, A.; Kadivar, A.; Kamel, I.R.; Zaheer, A. Updates on Imaging of Liver Tumors. Curr. Oncol. Rep. 2020, 22, 46. [Google Scholar] [CrossRef]
- Park, C.C.; Nguyen, P.; Hernandez, C.; Bettencourt, R.; Ramirez, K.; Fortney, L.; Hooker, J.; Sy, E.; Savides, M.T.; Alquiraish, M.H.; et al. Magnetic Resonance Elastography Vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 598–607.e2. [Google Scholar] [CrossRef]
- European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis. J. Hepatol. 2015, 63, 237–264. [Google Scholar] [CrossRef]
- Germani, G.; Hytiroglou, P.; Fotiadu, A.; Burroughs, A.K.; Dhillon, A.P. Assessment of Fibrosis and Cirrhosis in Liver Biopsies: An Update. Semin. Liver Dis. 2011, 31, 082–090. [Google Scholar] [CrossRef]
- Crossan, C.; Tsochatzis, E.A.; Longworth, L.; Gurusamy, K.; Davidson, B.; Rodríguez-Perálvarez, M.; Mantzoukis, K.; O’Brien, J.; Thalassinos, E.; Papastergiou, V.; et al. Cost-Effectiveness of Non-Invasive Methods for Assessment and Monitoring of Liver Fibrosis and Cirrhosis in Patients with Chronic Liver Disease: Systematic Review and Economic Evaluation. Health Technol. Assess. 2015, 19, 9. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.Y. Non-Invasive Diagnosis of Hepatitis B Virus-Related Cirrhosis. World J. Gastroenterol. 2014, 20, 445–459. [Google Scholar] [CrossRef]
- Muir, A.J. Understanding the Complexities of Cirrhosis. Clin. Ther. 2015, 37, 1822–1836. [Google Scholar] [CrossRef]
- Berzigotti, A. Advances and Challenges in Cirrhosis and Portal Hypertension. BMC Med. 2017, 15, 200. [Google Scholar] [CrossRef]
- Gupta, S.; Walker, S. Testing for Cirrhosis. Aust. Prescr. 2021, 44, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Aldulaimi, S.; Mendez, A.M. Splenomegaly: Diagnosis and Management in Adults. Am. Fam. Physician 2021, 104, 271–276. [Google Scholar] [PubMed]
- Borrell-Carrió, F.; Poveda, B.F.; Seco, E.M.; Castillejo, J.A.P.; González, M.P.; Rodríguez, E.P. Family Physicians’ Ability to Detect a Physical Sign (Hepatomegaly) from an Unannounced Standardized Patient (Incognito Sp). Eur. J. Gen. Pract. 2011, 17, 95–102. [Google Scholar] [CrossRef]
- Yilma, M.; Xu, R.H.; Saxena, V.; Muzzin, M.; Tucker, L.-Y.; Lee, J.; Mehta, N.; Mukhtar, N. Survival Outcomes among Patients with Hepatocellular Carcinoma in a Large Integrated Us Health System. JAMA Netw. Open 2024, 7, e2435066. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, B.; Xu, Y.; Wang, W.; Shen, Y.; Zhang, A.; Xu, Z. Prospective Study of Early Detection for Primary Liver Cancer. J. Cancer Res. Clin. Oncol. 1997, 123, 357–360. [Google Scholar] [CrossRef]
- Burra, P.; Becchetti, C.; Germani, G. Nafld and Liver Transplantation: Disease Burden, Current Management and Future Challenges. JHEP Rep. 2020, 2, 100192. [Google Scholar] [CrossRef]
- Haque, M. Liver Transplantation Costs in the Era of a Health Care Crisis: Are We Looking Beyond and Thinking About the Economics? Liver Transplant. 2010, 16, 534. [Google Scholar] [CrossRef]
- Ferrandino, G.; Ricciardi, F.; Murgia, A.; Banda, I.; Manhota, M.; Ahmed, Y.; Sweeney, K.; Nicholson-Scott, L.; McConville, L.; Gandelman, O.; et al. Exogenous Volatile Organic Compound (Evoc®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension. Biomedicines 2023, 11, 2957. [Google Scholar] [CrossRef]
- Weber, I.C.; Oosthuizen, D.N.; Mohammad, R.W.; Mayhew, C.A.; Pratsinis, S.E.; Güntner, A.T. Dynamic Breath Limonene Sensing at High Selectivity. ACS Sens. 2023, 8, 2618–2626. [Google Scholar] [CrossRef]
- Patnaik, R.K.; Lin, Y.-C.; Agarwal, A.; Ho, M.-C.; Yeh, J.A. A Pilot Study for the Prediction of Liver Function Related Scores Using Breath Biomarkers and Machine Learning. Sci. Rep. 2022, 12, 2032. [Google Scholar] [CrossRef]
- Hu, B. Mass Spectrometric Analysis of Exhaled Breath: Recent Advances and Future Perspectives. TrAC Trends Anal. Chem. 2023, 168, 117320. [Google Scholar] [CrossRef]
- Yuan, Z.-C.; Zhang, Y.; Cai, S.-H.; Chen, W.; Hu, B. Solid Phase Microextraction for Human Breath Analysis of Environmental and Occupational Exposures: A Review. Adv. Sample Prep. 2022, 3, 100023. [Google Scholar] [CrossRef]
- Ghawanmeh, A.A.; Tanash, S.A.; Al-Rawashdeh, N.A.F.; Albiss, B. The Recent Progress on Nanomaterial-Based Chemosensors for Diagnosis of Human Exhaled Breath: A Review. J. Mater. Sci. 2024, 59, 8573–8605. [Google Scholar] [CrossRef]
- Hu, W.; Wu, W.; Jian, Y.; Haick, H.; Zhang, G.; Qian, Y.; Yuan, M.; Yao, M. Volatolomics in Healthcare and Its Advanced Detection Technology. Nano Res. 2022, 15, 8185–8213. [Google Scholar] [CrossRef]
- Henderson, B.; Khodabakhsh, A.; Metsälä, M.; Ventrillard, I.; Schmidt, F.M.; Romanini, D.; Ritchie, G.A.D.; Hekkert, S.T.L.; Briot, R.; Risby, T.; et al. Laser Spectroscopy for Breath Analysis: Towards Clinical Implementation. Appl. Phys. B 2018, 124, 161. [Google Scholar] [CrossRef]
- McCurdy, M.R.; Bakhirkin, Y.; Wysocki, G.; Lewicki, R.; Tittel, F.K. Recent Advances of Laser-Spectroscopy-Based Techniques for Applications in Breath Analysis. J. Breath Res. 2007, 1, 014001. [Google Scholar] [CrossRef]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef]
- Di Gilio, A.; Palmisani, J.; Ventrella, G.; Facchini, L.; Catino, A.; Varesano, N.; Pizzutilo, P.; Galetta, D.; Borelli, M.; Barbieri, P.; et al. Breath Analysis: Comparison among Methodological Approaches for Breath Sampling. Molecules 2020, 25, 5823. [Google Scholar] [CrossRef]
- Berna, A.Z.; Schaber, C.L.; Bollinger, L.B.; Mwale, M.; Mlotha-Mitole, R.; Trehan, I.; John, A.R.O. Comparison of Breath Sampling Methods: A Post Hoc Analysis from Observational Cohort Studies. Analyst 2019, 144, 2026–2033. [Google Scholar] [CrossRef]
- Schulz, E.; Woollam, M.; Grocki, P.; Davis, M.D.; Agarwal, M. Methods to Detect Volatile Organic Compounds for Breath Biopsy Using Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry. Molecules 2023, 28, 4533. [Google Scholar] [CrossRef]
- van Oort, P.M.P.; White, I.R.; Ahmed, W.; Johnson, C.; Bannard-Smith, J.; Felton, T.; Bos, L.D.; Goodacre, R.; Dark, P.; Fowler, S.J. Detection and Quantification of Exhaled Volatile Organic Compounds in Mechanically Ventilated Patients—Comparison of Two Sampling Methods. Analyst 2021, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Biehl, W.; Schmetzer, H.; Koczulla, R.; Hattesohl, A.; Jörres, R.; Duell, T.; Althöhn, U. P01.03|Voc Pattern Recognition of Lung Cancer: A Comparative Evaluation of Different Dog- and Enose-Based Strategies Using Different Sampling Materials. J. Immunother. Cancer 2020, 8 (Suppl. S2), A9. [Google Scholar]
- Dadamio, J.; Van den Velde, S.; Laleman, W.; Van Hee, P.; Coucke, W.; Nevens, F.; Quirynen, M. Breath Biomarkers of Liver Cirrhosis. J. Chromatogr. B 2012, 905, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, G.; Huang, H.; Liu, T.; Wang, J.; Sun, J.; Wang, H. A Semi-Packed Micro Gc Column for Separation of the Nafld Exhaled Breath VOCs. Surf. Coat. Technol. 2019, 363, 322–329. [Google Scholar] [CrossRef]
- Materić, D.; Bruhn, D.; Turner, C.; Morgan, G.; Mason, N.; Gauci, V. Methods in Plant Foliar Volatile Organic Compounds Research. Appl. Plant Sci. 2015, 3, 1500044. [Google Scholar] [CrossRef]
- Zhan, X.; Duan, J.; Duan, Y. Recent Developments of Proton-Transfer Reaction Mass Spectrometry (PTR-MS) and Its Applications in Medical Research. Mass Spectrom. Rev. 2013, 32, 143–165. [Google Scholar] [CrossRef]
- Brown, P.A. Investigations of Proton Transfer Reaction Mass Spectrometry for Applications in Organophosphate Detection and Breath Analysis. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2012. [Google Scholar]
- Španěl, P.; Smith, D. Selected Ion Flow Tube: A Technique for Quantitative Trace Gas Analysis of Air and Breath. Med. Biol. Eng. Comput. 1996, 34, 409–419. [Google Scholar] [CrossRef]
- Lehnert, A.S.; Behrendt, T.; Ruecker, A.; Pohnert, G.; Trumbore, S.E. SIFT-MS Optimization for Atmospheric Trace Gas Measurements at Varying Humidity. Atmos. Meas. Tech. 2020, 13, 3507–3520. [Google Scholar] [CrossRef]
- Belluomo, I.; Boshier, P.R.; Myridakis, A.; Vadhwana, B.; Markar, S.R.; Spanel, P.; Hanna, G.B. Selected Ion Flow Tube Mass Spectrometry for Targeted Analysis of Volatile Organic Compounds in Human Breath. Nat. Protoc. 2021, 16, 3419–3438. [Google Scholar] [CrossRef]
- Markar, S.R.; Wiggins, T.; Antonowicz, S.; Chin, S.-T.; Romano, A.; Nikolic, K.; Evans, B.; Cunningham, D.; Mughal, M.; Lagergren, J.; et al. Assessment of a Noninvasive Exhaled Breath Test for the Diagnosis of Oesophagogastric Cancer. JAMA Oncol. 2018, 4, 970–976. [Google Scholar] [CrossRef]
- Langford, V.S.; Billiau, C.; McEwan, M.J. Evaluation of the Efficacy of Sift-Ms for Speciation of Wastewater Treatment Plant Odors in Parallel with Human Sensory Analysis. Environments 2020, 7, 90. [Google Scholar] [CrossRef]
- Lacko, M.; Wang, N.; Sovová, K.; Pásztor, P.; Španěl, P. Addition of Fast gas Chromatography to Selected Ion Flow Tube Mass Spectrometry for Analysis of Individual Monoterpenes in Mixtures. Atmos. Meas. Tech. 2019, 12, 4965–4982. [Google Scholar] [CrossRef]
- Wang, T.; Španěl, P.; Smith, D. Selected Ion Flow Tube, Sift, Studies of the Reactions of H3O+, NO+ and O2+ with Eleven C10h16 Monoterpenes. Int. J. Mass Spectrom. 2003, 228, 117–126. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.; Zhou, Y.; Wang, J.; You, R. Research Progress of Electronic Nose Technology in Exhaled Breath Disease Analysis. Microsyst. Nanoeng. 2023, 9, 129. [Google Scholar] [CrossRef]
- Nazir, N.U.; Abbas, S.R.; Nasir, H.; Hussain, I. Electrochemical Sensing of Limonene Using Thiol Capped Gold Nanoparticles and Its Detection in the Real Breath Sample of a Cirrhotic Patient. J. Electroanal. Chem. 2022, 905, 115977. [Google Scholar] [CrossRef]
- Voss, A.; Schroeder, R.; Schulz, S.; Haueisen, J.; Vogler, S.; Horn, P.; Stallmach, A.; Reuken, P. Detection of Liver Dysfunction Using a Wearable Electronic Nose System Based on Semiconductor Metal Oxide Sensors. Biosensors 2022, 12, 70. [Google Scholar] [CrossRef]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of Volatile Organic Compounds by Self-Assembled Monolayer Coated Sensor Array with Concentration-Independent Fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef]
- Yang, Y.; Nagano, K.; Chaoluomeng; Iwasa, T.; Fukuda, H. Analysis of Surface Acoustic Wave Propagation Velocity in Biological Function-Oriented Odor Sensor. J. Sens. 2018, 2018, 8129484. [Google Scholar] [CrossRef]
- Grate, J.W.; Abraham, M.H. Solubility Interactions and the Design of Chemically Selective Sorbent Coatings for Chemical Sensors and Arrays. Sens. Actuators B Chem. 1991, 3, 85–111. [Google Scholar] [CrossRef]
- Du, X.; Rouseff, R. Comparison of Fast Gas Chromatography−Surface Acoustic Wave Sensor (FGC-SAW) and Capillary Gc-Ms for Determining Strawberry and Orange Juice Volatiles. In Recent Advances in the Analysis of Food and Flavors; American Chemical Society: Washington, DC, USA, 2012; pp. 177–189. [Google Scholar]
- Vaughan, S.R.; Speller, N.C.; Chhotaray, P.; McCarter, K.S.; Siraj, N.; Pérez, R.L.; Li, Y.; Warner, I.M. Class Specific Discrimination of Volatile Organic Compounds Using a Quartz Crystal Microbalance Based Multisensor Array. Talanta 2018, 188, 423–428. [Google Scholar] [CrossRef]
- Cao, Y.; Fu, M.; Fan, S.; Gao, C.; Ma, Z.; Hou, D. Hydrophobic MOF/PDMs-Based QCM Sensors for VOCs Identification and Quantitative Detection in High-Humidity Environments. ACS Appl. Mater. Interfaces 2024, 16, 7721–7731. [Google Scholar] [CrossRef] [PubMed]
- Penza, M.; Cassano, G.; Aversa, P.; Cusano, A.; Cutolo, A.; Giordano, M.; Nicolais, L. Carbon Nanotube Acoustic and Optical Sensors for Volatile Organic Compound Detection. Nanotechnology 2005, 16, 2536. [Google Scholar] [CrossRef]
- Torad, N.L.; Kim, J.; Kim, M.; Lim, H.; Na, J.; Alshehri, S.M.; Ahamad, T.; Yamauchi, Y.; Eguchi, M.; Ding, B.; et al. Nanoarchitectured Porous Carbons Derived from Zifs toward Highly Sensitive and Selective QCM Sensor for Hazardous Aromatic Vapors. J. Hazard. Mater. 2021, 405, 124248. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Sang, M.; Wang, M.; Han, L.; Gong, Z.; Tang, X.; Long, X.; Xiong, H.; Peng, H. Rapid Detection of D-Limonene Emanating from Citrus Infestation by Bactrocera dorsalis (Hendel) Using a Developed Gas-Sensing System Based on QCM Sensors Coated with Ethyl Cellulose. Sens. Actuators B Chem. 2021, 328, 129048. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C. Volatile Organic Compounds Gas Sensor Based on Quartz Crystal Microbalance for Fruit Freshness Detection: A Review. Food Chem. 2021, 334, 127615. [Google Scholar] [CrossRef]
- Völkle, J.; Kumpf, K.; Feldner, A.; Lieberzeit, P.; Fruhmann, P. Development of Conductive Molecularly Imprinted Polymers (cMIPs) for Limonene to Improve and Interconnect QCM and Chemiresistor Sensing. Sens. Actuators B Chem. 2022, 356, 131293. [Google Scholar] [CrossRef]
- Sasaki, K.; Furusawa, H.; Nagamine, K.; Tokito, S. Detection of Odorant Molecules in the Gaseous Phase Using A-, Β-, and Γ-Cyclodextrin Films on a Quartz Crystal Microbalance. Technologies 2018, 6, 63. [Google Scholar] [CrossRef]
- Rossi, A.; Spagnoli, E.; Tralli, F.; Marzocchi, M.; Guidi, V.; Fabbri, B. New Approach for the Detection of Sub-Ppm Limonene: An Investigation through Chemoresistive Metal-Oxide Semiconductors. Sensors 2023, 23, 6291. [Google Scholar] [CrossRef]
- Xia, J.; Zhu, F.; Bounds, J.; Aluauee, E.; Kolomenskii, A.; Dong, Q.; He, J.; Meadows, C.; Zhang, S.; Schuessler, H. Spectroscopic Trace Gas Detection in Air-Based Gas Mixtures: Some Methods and Applications for Breath Analysis and Environmental Monitoring. J. Appl. Phys. 2022, 131, 22. [Google Scholar] [CrossRef]
- Dumitras, D.C.; Petrus, M.; Bratu, A.-M.; Popa, C. Applications of near Infrared Photoacoustic Spectroscopy for Analysis of Human Respiration: A Review. Molecules 2020, 25, 1728. [Google Scholar] [CrossRef]
- Selvaraj, R.; Vasa, N.J.; Nagendra, S.M.S.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy-Based Sensing Techniques for Exhaled Breath Diagnostics. Molecules 2020, 25, 2227. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Sun, F.; Bian, Y.; Ni, J.; Wang, Q.J.; Yu, X. Mid-Infrared Absorption Spectroscopy with Enhanced Detection Performance for Biomedical Applications. Appl. Spectrosc. Rev. 2023, 58, 834–868. [Google Scholar] [CrossRef]
- Covington, J.A.; der Schee, M.P.V.; Edge, A.S.L.; Boyle, B.; Savage, R.S.; Arasaradnam, R.P. The Application of Faims Gas Analysis in Medical Diagnostics. Analyst 2015, 140, 6775–6781. [Google Scholar] [CrossRef] [PubMed]
- Eiceman, G.A.; Karpas, Z.; Hill, H.H., Jr. Ion Mobility Spectrometry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Janzen, M.C.; Ponder, J.B.; Bailey, D.P.; Ingison, C.K.; Suslick, K.S. Colorimetric Sensor Arrays for Volatile Organic Compounds. Anal. Chem. 2006, 78, 3591–3600. [Google Scholar] [CrossRef]
- Gurung, A.; Gurung, R.; Kumari, P.; Bhutia, T.; Poudyal, P.; Pradhan, G. Fabrication of Porphyrin Based Colorimetric Sensor for the Detection of Liver Cirrhosis Biomarker. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Gan, Z.; Wang, J. A Mof-Stabilized Enzyme-Based Colorimetric Biosensor Integrated with Ph Test Strips for Portable Detection of D-Limonene in Agricultural Applications. Chem. Eng. J. 2025, 510, 161719. [Google Scholar] [CrossRef]
- Ma, H.; Yang, Y.; Wang, J.; Li, C.; Fan, Z.; Wang, J. Recent Advances in Luminescent Metal-Organic Frameworks for Detection of Gas and Volatile Organic Molecules. IEEE Trans. Instrum. Meas. 2023, 72, 1500712. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun Nanomaterials for Ultrasensitive Sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Aliheidari, N.; Aliahmad, N.; Agarwal, M.; Dalir, H. Electrospun Nanofibers for Label-Free Sensor Applications. Sensors 2019, 19, 3587. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, Y.; Cui, T.; Wu, F.; Ji, S.; Yang, Y.; Tian, H.; Ren, T. Electrospun Nanofibers for Integrated Sensing, Storage, and Computing Applications. Appl. Sci. 2022, 12, 4370. [Google Scholar] [CrossRef]
- Chen, Z.; Guan, M.; Bian, Y.; Yin, X. Multifunctional Electrospun Nanofibers for Biosensing and Biomedical Engineering Applications. Biosensors 2024, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Halicka, K.; Cabaj, J. Electrospun Nanofibers for Sensing and Biosensing Applications—A Review. Int. J. Mol. Sci. 2021, 22, 6357. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Macagnano, A.; Molinari, F.N.; Mancini, T.; Lupi, S.; De Cesare, F. Uv Light Stereoselective Limonene Sensor Using Electrospun Pvp Composite Nanofibers. Proceedings 2024, 97, 131. [Google Scholar] [CrossRef]
- Gaggiotti, S.; Della Pelle, F.; Mascini, M.; Cichelli, A.; Compagnone, D. Peptides, DNA and Mips in Gas Sensing. From the Realization of the Sensors to Sample Analysis. Sensors 2020, 20, 4433. [Google Scholar] [CrossRef]
- Saito, T.; Nishida, Y.; Tabata, M.; Isobayashi, A.; Tomizawa, H.; Miyahara, Y.; Sugizaki, Y. Molecular Interactions between an Enzyme and Its Inhibitor for Selective Detection of Limonene. Anal. Chem. 2022, 94, 7692–7702. [Google Scholar] [CrossRef]
- Rungreungthanapol, T.; Homma, C.; Akagi, K.I.; Tanaka, M.; Kikuchi, J.; Tomizawa, H.; Sugizaki, Y.; Isobayashi, A.; Hayamizu, Y.; Okochi, M. Volatile Organic Compound Detection by Graphene Field-Effect Transistors Functionalized with Fly Olfactory Receptor Mimetic Peptides. Anal. Chem. 2023, 95, 4556–4563. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hitomi, T.; Homma, C.; Rungreungthanapol, T.; Tanaka, M.; Yamada, K.; Hamasaki, H.; Sugizaki, Y.; Isobayashi, A.; Tomizawa, H.; et al. Enantioselective Detection of Gaseous Odorants with Peptide-Graphene Sensors Operating in Humid Environments. ACS Appl. Mater. Interfaces 2024, 16, 18564–18573. [Google Scholar] [CrossRef]
- Cuypers, W.; Lieberzeit, P.A. Combining Two Selection Principles: Sensor Arrays Based on Both Biomimetic Recognition and Chemometrics. Front. Chem. 2018, 6, 268. [Google Scholar] [CrossRef]
- Iqbal, N.; Mustafa, G.; Rehman, A.; Biedermann, A.; Najafi, B.; Lieberzeit, P.A.; Dickert, F.L. Qcm-Arrays for Sensing Terpenes in Fresh and Dried Herbs Via Bio-Mimetic Mip Layers. Sensors 2010, 10, 6361–6376. [Google Scholar] [CrossRef]
- Hawari, H.F.; Samsudin, N.M.; Ahmad, M.N.; Shakaff, A.Y.M.; Ghani, S.A.; Wahab, Y.; Hashim, U. Recognition of Limonene Volatile Using Interdigitated Electrode Molecular Imprinted Polymer Sensor. In Proceedings of the 2012 Third International Conference on Intelligent Systems Modelling and Simulation, Kota Kinabalu, Malaysia, 8–10 February 2012; pp. 723–726. [Google Scholar]
- Wilson, A.D. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef] [PubMed]
- Niculae, A.-R.; Staden, R.-I.S.-V.; van Staden, J.F.; State, R.G. Sulfur-Doped Graphene-Based Electrochemical Sensors for Fast and Sensitive Determination of (R)-(+)-Limonene from Beverages. Sensors 2022, 22, 5851. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.G.; Stradiotto, N.R. Electrochemical Sensor Based on Nanostructured Surface of Reduced Graphene Oxide and Gold Nanoparticles Containing Electropolymerized Poly-Pyrrole for Selective Determination of Limonene Enantiomers in Orange and Pine Essential Oils. Microchem. J. 2025, 209, 112781. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Wex, K.; Sabel, B.; Schubert, J.K.; Miekisch, W. Electrochemical Sensor System for Breath Analysis of Aldehydes, Co and No. J. Breath Res. 2015, 9, 016008. [Google Scholar] [CrossRef]
- Maiti, K.S.; Lewton, M.; Fill, E.; Apolonski, A. Sensitive Spectroscopic Breath Analysis by Water Condensation. J. Breath Res. 2018, 12, 046003. [Google Scholar] [CrossRef]
- Namjou, K.; Roller, C.B.; McMillen, G. Breath-Analysis Using Mid-Infrared Tunable Laser Spectroscopy. In Proceedings of the IEEE Sensors, Atlanta, GA, USA, 28–31 October 2007. [Google Scholar]
- Naz, F.; Groom, A.G.; Mohiuddin, M.D.; Sengupta, A.; Daigle-Maloney, T.; Burnell, M.J.; Michael, J.C.R.; Graham, S.; Beydaghyan, G.; Scheme, E.; et al. Using Infrared Spectroscopy to Analyze Breath of Patients Diagnosed with Breast Cancer. J. Clin. Oncol. 2022, 40, e13579. [Google Scholar] [CrossRef]
- Liu, S.; Dong, X.; Cao, H.; Lv, J.; Zhao, L.; Xia, Y.; Wang, Y.; Lv, Z. Mid-Infrared Photothermal Spectroscopy for Breath Nitric Oxide Testing with an Anti-Resonant Fiber. Opt. Laser Technol. 2022, 152, 108158. [Google Scholar] [CrossRef]
- Zhou, J.; Al Husseini, D.; Li, J.; Lin, Z.; Sukhishvili, S.; Coté, G.L.; Gutierrez-Osuna, R.; Lin, P.T. Detection of Volatile Organic Compounds Using Mid-Infrared Silicon Nitride Waveguide Sensors. Sci. Rep. 2022, 12, 5572. [Google Scholar] [CrossRef]
- Flores Rangel, G.; de León Martinez, L.D.; Mizaikoff, B. Helicobacter Pylori Breath Test Via Mid-Infrared Sensor Technology. ACS Sens. 2025, 10, 1005–1010. [Google Scholar] [CrossRef]
- D’Arco, A.; Mancini, T.; Paolozzi, M.C.; Macis, S.; Mosesso, L.; Marcelli, A.; Petrarca, M.; Radica, F.; Tranfo, G.; Lupi, S.; et al. High Sensitivity Monitoring of VOCs in Air through FTIR Spectroscopy Using a Multipass Gas Cell Setup. Sensors 2022, 22, 5624. [Google Scholar] [CrossRef] [PubMed]
- Yost, M.G.; Rose, M.A.; Morgan, M.S. An Evaluation of Fourier Transform Infrared (FTIR) Spectroscopy for Detecting Organic Solvents in Expired Breath. Appl. Occup. Environ. Hyg. 2003, 18, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hauer, J.; Maiti, K.S. Development of Non-Invasive Diagnosis Based on FTIR Spectroscopy. Vib. Spectrosc. 2024, 134, 103724. [Google Scholar] [CrossRef]
- Zhong, X.; Li, D.; Du, W.; Yan, M.; Wang, Y.; Huo, D.; Hou, C. Rapid Recognition of Volatile Organic Compounds with Colorimetric Sensor Arrays for Lung Cancer Screening. Anal. Bioanal. Chem. 2018, 410, 3671–3681. [Google Scholar] [CrossRef]
- Chevrot, A.; Venckute, J.; Cuesta, S.; Golparvar, A.; Kapic, A.; Barbruni, G.L.; Carrara, S. Portable Breathalyzer for Exhaled Volatile Organic Compounds Monitoring in Lung Diseases. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022. [Google Scholar]
- Bunkowski, A.; Maddula, S.; Davies, A.N.; Westhoff, M.; Litterst, P.; Bödeker, B.; Baumbach, J.I. One-Year Time Series of Investigations of Analytes within Human Breath Using Ion Mobility Spectrometry. Int. J. Ion Mobil. Spectrom. 2010, 13, 141–148. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gómez-Ríos, G.A.; Silva, É.A.S.; Pawliszyn, J. Coupling Needle Trap Devices with Gas Chromatography–Ion Mobility Spectrometry Detection as a Simple Approach for on-Site Quantitative Analysis. J. Chromatogr. A 2013, 1300, 193–198. [Google Scholar] [CrossRef]
- Molinari, F.N.; Marelli, M.; Berretti, E.; Serrecchia, S.; Coppola, R.E.; De Cesare, F.; Macagnano, A. Cutting-Edge Sensor Design: Mip Nanoparticle-Functionalized Nanofibers for Gas-Phase Detection of Limonene in Predictive Agriculture. Polymers 2025, 17, 326. [Google Scholar] [CrossRef]
- González-Vila, Á.; Debliquy, M.; Lahem, D.; Zhang, C.; Mégret, P.; Caucheteur, C. Molecularly Imprinted Electropolymerization on a Metal-Coated Optical Fiber for Gas Sensing Applications. Sens. Actuators B Chem. 2017, 244, 1145–1151. [Google Scholar] [CrossRef]
- Okabayashi, T.; Toda, T.; Yamamoto, I.; Utsunomiya, K.; Yamashita, N.; Nakagawa, M. Temperature-Programmed Chemiluminescence Measurements for Discrimination and Determination of Fragrance. Sens. Actuators B Chem. 2001, 74, 152–156. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, X.; Liu, Y.; Chang, X. Development of a Simple Cataluminescence Sensor System for Detecting and Discriminating Volatile Organic Compounds at Different Concentrations. Anal. Chem. 2013, 85, 3802–3806. [Google Scholar] [CrossRef]
- Lu, Y.; Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Pang, W.; Zhang, H.; Zhang, D.; Duan, X. Detection of Volatile Organic Compounds Using Microfabricated Resonator Array Functionalized with Supramolecular Monolayers. ACS Appl. Mater. Interfaces 2015, 7, 17893–17903. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.S.; Schmidt, R.; Wolkoff, P. Olfactory Detection of Ozone and D-Limonene: Reactants in Indoor Spaces. Indoor Air 2007, 17, 337–347. [Google Scholar] [CrossRef]
- Ghatak, B.; Ali, S.B.; Naskar, H.; Tudu, B.; Pramanik, P.; Mukherji, S.; Bandyopadhyay, R. Selective and Sensitive Detection of Limonene in Mango Using Molecularly Imprinted Polymer Based Quartz Crystal Microbalance Sensor. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).