Abstract
Tea contains bioactive components that provide many health benefits, but overdoses can also cause health problems related to fluorosis, among other things. The analysis of tea quality is complicated due to its diverse chemical composition, and also depends on multistep processing of raw tea leaves. In this work, chemosensitive microparticles incorporated with various metalloporphyrins that are sensitive to fluoride and chloride ions were developed. A set of seven types of microparticle suspensions was used to form an optical “smart tongue” applied for the recognition of tea infusions. Using principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) for the analysis of the spectrophotometric and spectrofluorimetric responses of the microparticles, the infusions were identified with high accuracy. Moreover, the “smart tongue” enabled the discrimination according to the fluorine content and fermentation status. These results highlight the potential of chemosensitive microparticles as versatile tests in assessing tea quality.
1. Introduction
Tea is one of the most popular beverages around the world, with a history dating back nearly 2000 years in China [1,2,3]. Numerous studies have shown that the bioactive components of tea possess multiple health functions, having antioxidation, anti-inflammation, immunoregulation, anticancer, cardiovascular protection, antidiabetes, antiobesity, and hepatic protection properties [4]. The quality of tea infusions is a crucial issue, however. Quality analysis can be problematic, as tea contains several chemicals, including tannin compounds, amino acid alkaloids, and inorganic species (e.g., fluoride and chloride ions). It should also be noted that these components can vary depending on the type of tea (black, green, red), its age, or the processes it has undergone [5].
Fluoride is an essential element for organisms, affecting calcium and phosphate metabolism in the mineralization of bones and teeth. Nevertheless, an excess of fluoride can cause fluorosis. Its content varies depending on the type of tea: it is generally highest in black teas and lowest in green teas. However, the ranges of concentration are quite wide and overlapping: black tea (0.56–6.01 mg/L) > red tea (0.33–4.96 mg/L) > green tea (0.16–6.94 mg/L) > herbal infusions (0.03–1.12 mg/L) > yerba mate (0.03–0.1 mg/L) [6,7]. It is estimated that tea leaves can accumulate fluoride at 10–15 mg per 100 g of dry weight; much less fluoride can be found in twigs and stems [8]. This amount may increase due to the fact that many growers now use phosphate fertilizers. Malinowska et al. showed the results of fluoride release for different brewing times of different teas. It was proven that as the steeping time of the tea leaves increased, so did the fluoride content of the resulting infusions [7]. The superimposition of several elements, namely increased use of plant growth products, brewing water with a high fluoride index, and, ultimately, increased tea steeping time, may contribute to an excess of daily fluoride intake (the amount assumed to ensure adequate nutrition is 4 mg/day for men, and 3 mg/day for women) [5,7,9].
The various bioactive components in teas can be determined using classical methods (HPLC, GC, ICP-OES, spectrophotometry, etc.) [10]. HPLC and GC have been widely used not only to analyze the chemical composition of teas, but also to characterize their aroma profiles. For example, Fernández et al. used HPLC to determine the amount of catechins and caffeine in different teas, allowing for effective differentiation between green, black, and instant teas [11]. HPLC was also applied to determine the amount of catechins in fermented Miang tea using UV detection at 280 nm [12]. For the aroma profile analysis, GC-MS was applied to identify volatile compounds in different types of teas and their different processing stages, and it was able to distinguish between tea varieties based on their aroma and geographical origin [13,14]. Recent publications propose alternative approaches based on sensor arrays and machine learning electronic noses and electronic tongues [15,16,17,18]. By mimicking the human sense of taste, these systems enable qualitative and quantitative analysis of complex, real samples, with special emphasis given to their quality, organoleptic properties, or components related to nutritional value [19,20,21]. The electronic tongue, based on non-specific, cross-selective sensors, was introduced as an alternative to classical methods, such as high-performance liquid chromatography [22], gas chromatography [23], or capillary electrophoresis [24,25]. Compared to the abovementioned methods, electronic tongue technology is much faster, easier to use, and does not generate high costs [19]. Many electronic tongues based on voltammetric, potentiometric sensors have been proposed and have been used for various applications, such as assessing the quality of beer [26], wine [27], or milk [28,29]. In the literature, one can also find many works on the successful application of the e-tongue to distinguish between different types of teas [19,30], or to assess their origin or their level of quality [31,32]. Nevertheless, to date, various e-tongue systems proposed for tea analysis have been based on classical sensors, and to the best of our knowledge, no e-tongue with a sensor array fabricated with microparticles, which can enable the classification of different types of tea infusions, has yet been proposed.
Modern data analysis increasingly uses machine learning methods to identify patterns, reduce dimensionality, and classify data. Among the popular techniques used in multivariate analysis, principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) stand out. PCA simplifies complex data by transforming related variables into smaller independent components that reflect key information. PCA is widely used, among other things, in the analysis of biological data, and its main advantage is the ability to visualize data in a space with fewer dimensions [33]. PLS-DA is a supervised classification method that combines a reduction in dimensionality with information about the class of data. Unlike PCA, PLS-DA considers class labels, allowing for better data group differentiation. It is especially popular in chemometric and biological data analysis, where the number of variables exceeds the number of samples [34].
The optical microparticle sensors that we propose have their origin in classical ion-selective electrodes. However, modifying their composition, i.e., replacing the polymer matrix with a surfactant, and applying optical transduction of the signal, enable in-situ testing and analysis of small volume samples [35]. In our previous work, we proposed various types of cross-sensitive micro/nanoparticles for “smart tongue” sensing, which included a chromoionophore and/or an ionophore, an ion exchanger, a plasticizer, and a surfactant [36,37]. In this work we focus on the fabrication of microparticles incorporated with metalloporphyrins, which play the dual role of both optical transducer and ionophore.
Metalloporphyrins as ionophores have become very popular over the past decade, including their use as components of liquid membrane electrodes [38]. It has been found that the selectivity patterns obtained with such sensors depend on the central metal in the porphyrin structure. In addition, the selectivity can be adjusted by adding suitable additives to the membrane phase, such as ion exchangers. Górski et al. presented anion-selective polymeric membranes using metalloporphyrins as selective ionophores for various anions [39]. Similar to M. E. Meyerhoff et al. [38,40], they demonstrated that gallium (III) and indium (III) porphyrin-based electrodes show a preference for fluoride and chloride ions, respectively. Therefore, this paper proposes microparticles based on various octaethyl- and tetraphenyl-metalloporphyrins, the responses of which were tested for fluoride and chloride ions. Further, their “smart tongue” sensing capabilities were tested for the recognition of different types of tea.
2. Experimental
2.1. Materials and Tested Tea Samples
MES, Pluronic F-127, Triton X-100, Triton N-101, 2,3,7,8,12,13,17,18-octaethyl-21H,23H-porphine manganese(III) chloride–Mn(OEP)Cl, and 5,10,15,20-tetraphenyl-21H,23H-porphine manganese(III) chloride–Mn(TPP)Cl were supplied by Sigma-Merck (Poznań, Poland). Gallium(III) 2,3,7,8,12,13,17,18-(octaethyl)porphyrin chloride–Ga(OEP)Cl, indium(III) 2,3,7,8,12,13,17,18-(octaethyl)porphyrin chloride–In(OEP)Cl, gallium(III) 5,10,15,20-(tetraphenyl)porphyrin chloride–Ga(TPP)Cl, and indium(III) 5,10,15,20-(tetraphenyl)porphyrin chloride–In(TPP)Cl were supplied by PorphyChem (Dijon, France), and zirconium(IV)meso-tetraphenylporphine dichloride–Zr(TPP)Cl2 was supplied by Frontier Specialty Chemicals (Logan, UT, USA). Plasticizers: 2-nitrophenyl octyl ether (o-NPOE), bis(2-Ethylhexyl) sebacate (DOS), bis(2-Ethylhexyl) phthalate (DOP); and lipophilic salts: potassium tetrakis3,5-bis(trifluoromethyl)phenyl borate (KTFPB) and potassium tetrakis(4-chlorophenyl)borate (KTpClPB) were obtained from Fluka (Selectophore). Milli-Q water was used for the preparation of all aqueous solutions, including the MES buffer pH 5.5. Tetrahydrofuran (Fluka) was used as a solvent for the microparticle components. The teas and herbals (control samples) were purchased from a Polish online supplier. A description of their composition and brewing procedures is included in Table 1. All chemicals were used as received.

Table 1.
Specification of the tested tea infusions and control samples.
2.2. Preparation and Measurements of NPs/MPs Optodes
The seven types of microparticles were prepared from the ingredients listed in Table 2. They are based on components such as plasticizers, surfactants, or ion exchangers, while suitable metalloporphyrin is applied instead of the typical optical transducer–pH-sensitive dye (chromoionophore). The role of the respective metalloporphyrins was both to provide the ion sensing and to ensure the optical response of the microparticles. Each of the microparticles was prepared analogously according to the previously applied protocol [36,37,41]. The fabrication process involves dissolving all the selected components in 1.5 mL of THF until a homogeneous solution is obtained. In order to dissolve the mixture thoroughly, each vial was placed in an ultrasonic bath (Sonic-0.5, 80 W, 40 kHz, POLSONIC Palczyński Sp. J., Warsaw, Poland). The next step was to pipette a 0.5 mL to 4.5 mL portion of deionized water onto the Vortex. The final step involves passing air through the resulting solution with a peristaltic pump. This process takes an hour, after which a clear suspension of microparticles is obtained, which is ready for further testing. The microparticles produced in this way are characterized by high fabrication repeatability [37,41] and high stability over time, allowing reproducible results in terms of their chemosensory response ([37] and Figure S1 in the Supporting Information).

Table 2.
Components used for the fabrication of the developed microparticles (MPs) incorporated with metalloporphyrins (MnOEPCl, MnTPPCl, ZrTPPCl2, GaTPPCl, GaOEPCl, InTPPCl, InOEPCl), together with their basic spectrophotometric (λmax) and spectrofluorimetric (λex/λem) properties.
All measurements were performed for fresh batches of MPs in at least four replicates. Measurements were performed with a Synergy MX Multi-Mode microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) using Greiner CELLSTAR® 96-well polystyrene microplates (Greiner Bio-One GmbH, Kremsmünster, Austria). Suspensions of GaTPPCl-MPs, GaOEPCl-MPs, InTPPCl-MPs, InOEPCl-MPs, ZrTPPCl2-MPs, MnOEPCl-MPs, and MnTPPCl-MPs were pipetted in volumes of 5 μL, 5 μL, 25 μL, 50 μL, 10 μL, 50 μL and 25 μL, respectively. The wells were successively increased to 100 μL with deionized water. The next step involved the introduction of 100 μL of a buffered solution of chloride/fluoride ions in the concentration range 10−6–10−1 M into the individual wells. In addition, spectra were also recorded for MPs with the addition of the pure buffer. Eight independent replicates were performed for the discriminant analysis of the teas using the prepared particles. The process of preparing the teas involved brewing them according to the manufacturer’s guidelines. The first step involved weighing 2 g of each tea and pouring it into 200 mL of boiling water. Deionized water was used in our study to eliminate the influence of foreign ions. The water temperature was controlled using a laboratory thermometer. The temperature and brewing time for each tea, yerba mate, and CBD hemp tea are presented in Table 1. Each infusion was filtered (Munktell, Grade: 388, Dia.: 125 mm, GSM: 84 g/m2) to ensure solution clarity. After diluting ten times, the resulting solutions (with estimated fluoride level from 7.0 μg/L to 0.33 mg/L, i.e., from 3.7 × 10−7 M to 1.7 × 10−5 M, data for undiluted infusions in Table 1) were spotted into microwells in volumes of 100 μL and topped up sequentially with an appropriate volume of respective microparticles suspensions and deionized water (according to the same procedure as applied during the calibration step).
2.3. Data Analysis
All results were obtained in the form of UV-Vis absorption spectra or emission curves recorded at the respective excitation wavelengths (Table 2). The signals in the maxima characteristic for each type of microparticle were used to determine the calibration curves. In order to perform the discriminant analysis of the teas, the results of which are not affected by the optical properties of the samples themselves, the differences in the spectra were calculated (i.e., from each spectrum obtained for the mixture of the tea sample and the microparticles, the spectrum of the pure tea sample was subtracted). The whole-spectrum matrices were then processed using principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA). All data analyses were performed using Solo software 9.5 (Eigenvector Research Inc., Manson, WA, USA), while calibration plots were generated in MS Excel 2020 (Microsoft, Redmond, WA, USA) and Origin 2021b (OriginLab Corporation, Northampton, MA, USA).
3. Results and Discussion
The microparticles fabricated for this work comprised gallium(III) and indium(III) tetraphenylporphyrins (Table 2). Group XIII metals, acting as the central ion in the selected type of metalloporphyrin, allow ion-sensitive membranes to be obtained for potentiometric/optical sensors with a selectivity pattern that deviates significantly from the classical Hofmeister series. Ionic additives, i.e., ion exchangers, can improve the selectivity of the sensing membranes. Porphyrin derivatives, whether TPP or OEP, bind and coordinate the central ion within its interior, thanks to the nitrogen in the pyrrole rings. This allows the metal ion to interact with the anions through axial ligation. The metal ion in the central part binds electrons from the free pair of nitrogen atoms in the pyrrole rings. The electrons of the metal ion are then transferred to the porphyrin molecule, resulting in the formation of delocalized π-bonds. These delocalized bonds allow the free flow of electrons within this system [40,42].
3.1. Optical Response of Microparticles Incorporated with Gallium and Indium Tetraphenylporphyrin Chloride
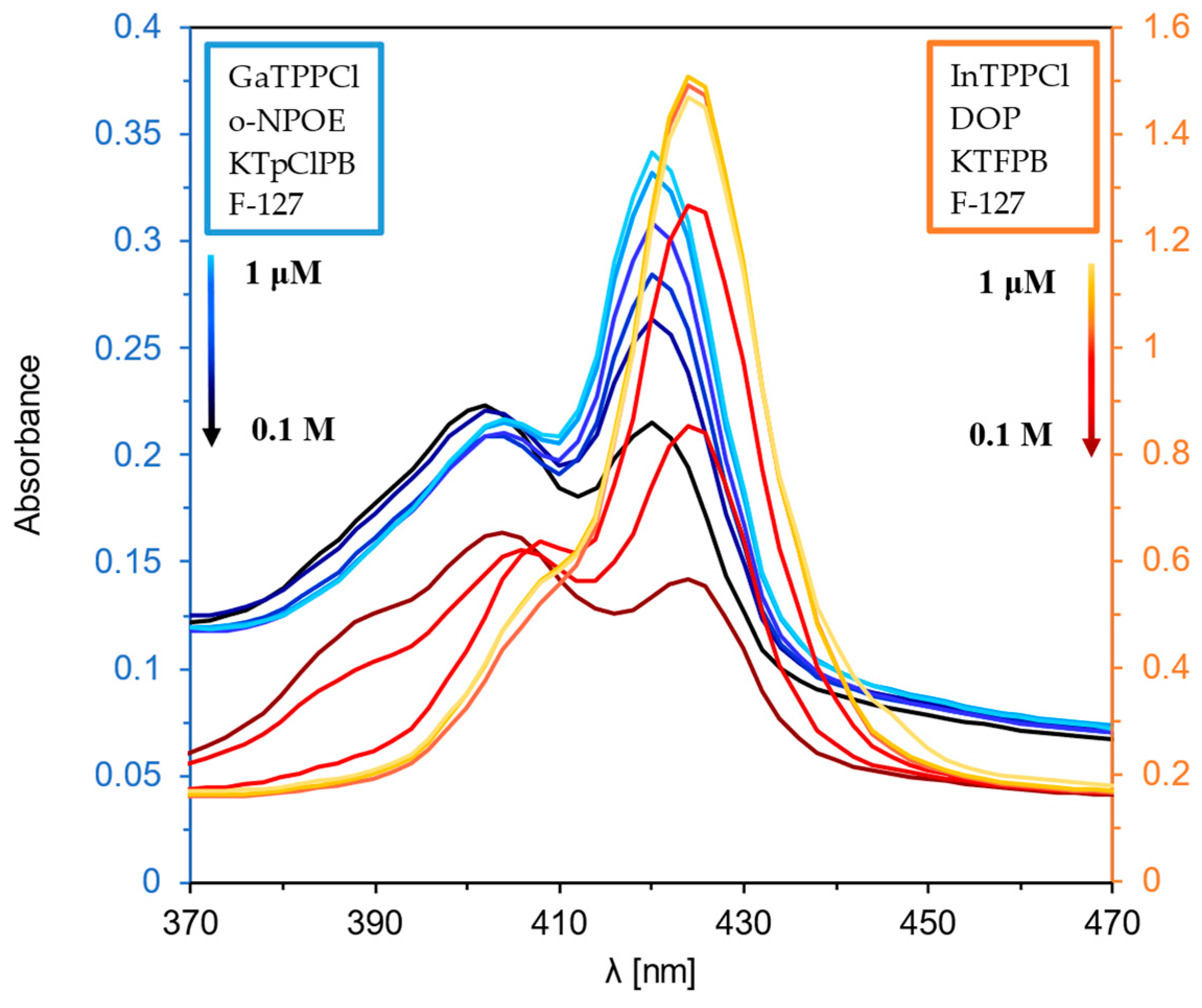
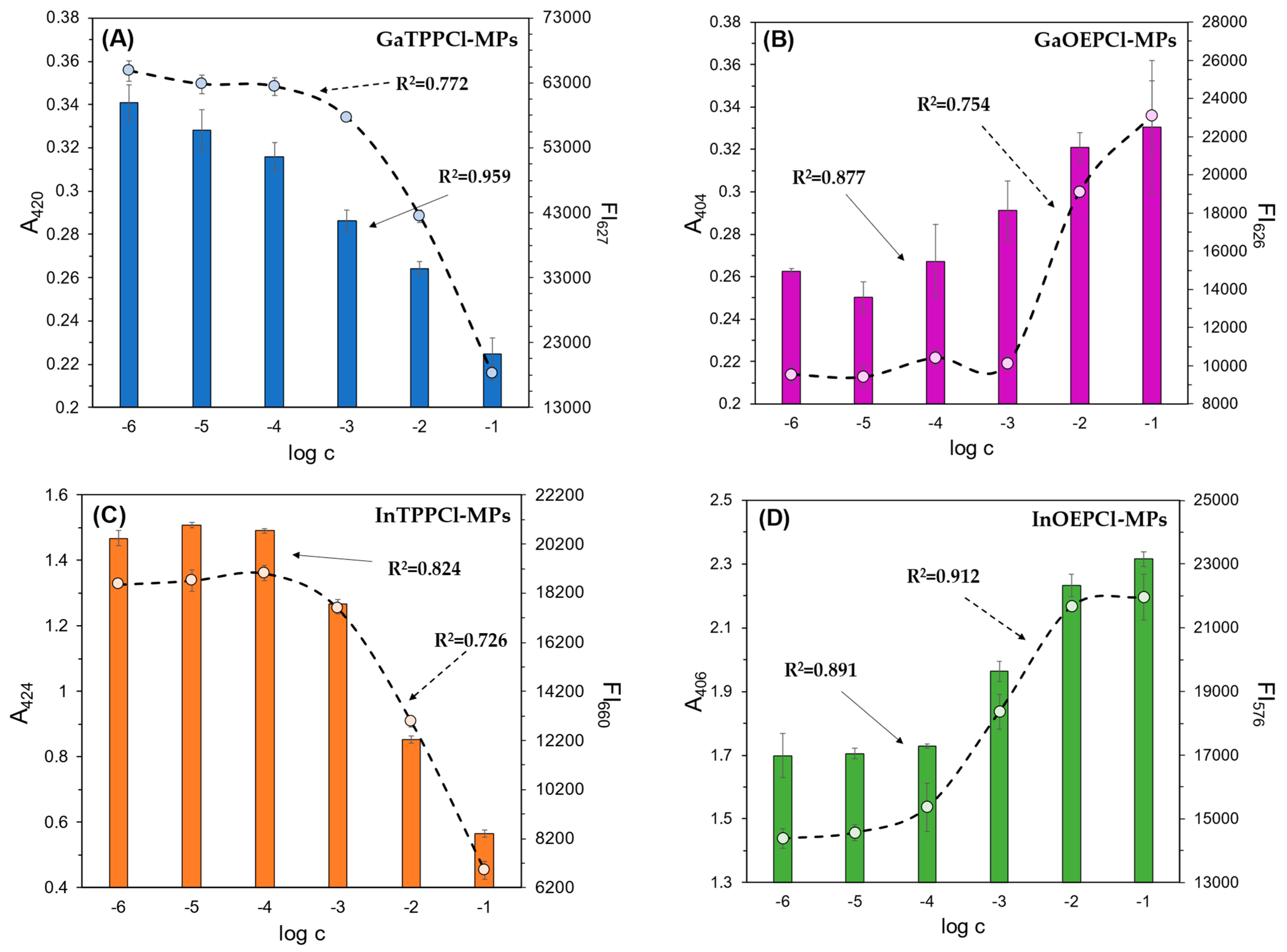
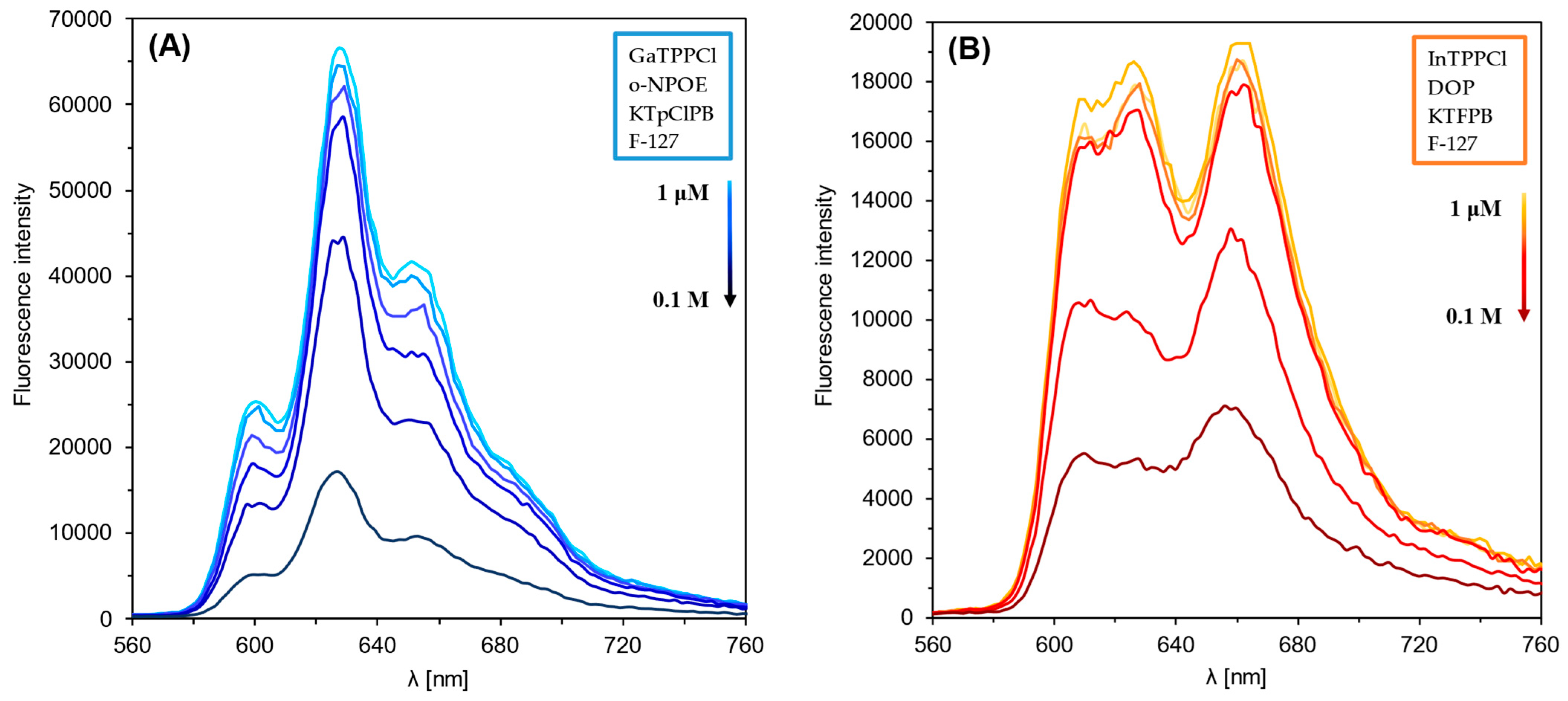
Figure 1 and Figure 2 show the optical response of GaTPPCl-MPs and InTPPCl-MPs towards fluoride anions. The selection of the type and quantity of the respective components of the MPs (plasticizer, surfactant) was carefully chosen based on optimization tests (Figure S2A,B). The spectrophotometric response (Figure 1) for both types of MPs is based on the main intense peak in the Soret band, characteristic for porphyrins. As the metal ion forms coordination bonds with the nitrogen atoms, the molecule’s symmetry increases, decreasing the number of Q-bands. In both cases, the maxima are in a similar range, i.e., 420 nm for GaTPPCl-MPs and 424 nm for InTPPCl-MPs. In the case of the metallotetraphenylporphyrins, their delocalized π-bands caused an increase in the average electron density of the porphyrin. This causes a decrease in the electron transition energy, resulting in a redshift of the Soret band. Based on absorbance in peak maximum, GaTPPCl-MPs allowed us to determine fluorides in the entire range of the analyte concentrations under study, while maintaining satisfactory sensitivity (Figure 2A). In the case of the InTPPCl-MPs, the sensing range is narrower, i.e., from 10 μM to 0.1 M (Figure 2C). The spectrofluorimetric response was also recorded for the two types of MPs. The excitation wavelength was set according to the observed absorption maximum (Table 2). Characteristic maxima in emission spectra (Figure 3A,B) were observed at 627 nm and 660 nm for GaTPPCl and InTPPCl, respectively [43]. The fluorescence intensity of the InTPPCl-MPs is much weaker compared to gallium porphyrin incorporated particles, as in this case, indium significantly attenuates the fluorescence radiation [44,45]. It should also be noted that the intensity decreases gradually with the addition of the analyte. The range of the spectrofluorimetric response coincides with the range obtained for spectrophotometric measurements (Figure 1).
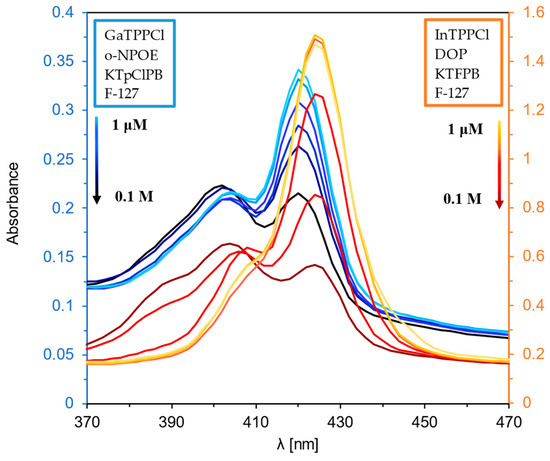
Figure 1.
UV-Vis spectra of GaTPPCl-MPs (blue lines) and InTPPCl-MPs (orange lines) in the presence of NaF analyte. Each solution of NaF was buffered (0.1 M MES buffer pH 5.5).
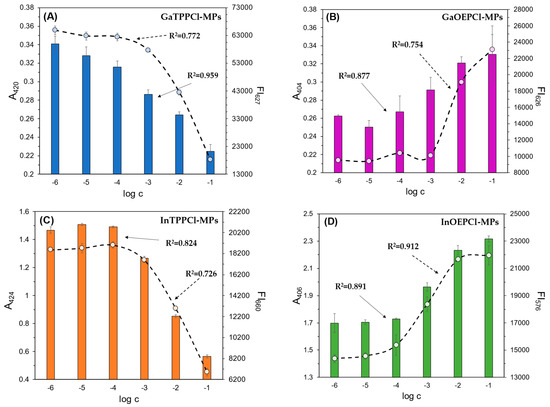
Figure 2.
Spectrophotometric (bars) and spectrofluorimetric (dashed line) responses of the fabricated microparticles: (A) GaTPPCl-MPs; (B) GaOEPCl-MPs; (C) InTPPCl-MPs; (D) InOEPCl-MPs; toward NaF (A–C) and NaCl (D) analytes. All solutions were buffered (0.1 M MES pH 5.5). The absorbance/fluorescence signals were determined in the respective absorbance/fluorescence maxima of each microparticle type (Table 1). Concentrations of anions are given on a molar scale. Calibration curve points were determined as mean ± SD; n = 4.
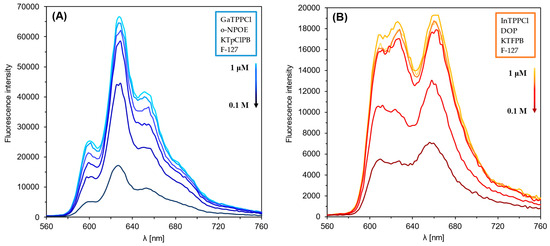
Figure 3.
Fluorescence emission spectra of GaTPPCl-MPs (A) and InTPPCl-MPs (B) in the presence of NaF analyte. Each solution of NaF was buffered (0.1 M MES buffer pH 5.5).
3.2. Optical Response of Microparticles Incorporated with Gallium and Indium Octaethylporphyrin Chloride
The optical properties of GaOEPCl-MPs and InOEPCl-MPs were also analyzed to investigate the possibility of fluoride sensing. Compared to the tetraphenylporphyrins, octaethylporphyrin is present with quadruple symmetry, significantly facilitating spectroscopic analysis. The first characteristic feature in the results obtained for the particles with octaethylporphyrin is that as the analyte concentration decreases, the absorbance value decreases, unlike that for the tetraphenylporphyrin (Figures S3A and S4A). Octaethylporphyrins are characterized by higher flexibility due to the presence of ethylene groups in the porphyrin structure. These groups are smaller and more flexible than phenyl groups present in TPP, which affects their ability to adapt to different chemical environments [45,46,47]. However, characteristic peaks for the porphyrins were recorded for both these systems in the Soret and Q bands. The metalloporphyrins are characterized by a small number of Q-bands, generally two [48]. In the case of these metalloporphyrins, their delocalized π-bonds cause a reduction in the average electron density, increasing the energy required for the electrons to pass through. This resulted in the observed shift of the Soret band towards the ultraviolet, compared to MPs based on tetraphenylporphyrins. Both microparticle types, GaOEPCl-MPs and InOEPCl-MPs, enable us to observe the optical response at a wide concentration range from 10 μM to 0.1 M (Figures S3A and S4A).
The situation is similar for the spectrofluorimetric response. The GaOEPCl-MPs allow for optical detection within the concentration range from 1 mM to 0.1 M (Figure S3B). In contrast, the InOEPCl-MPs enable optical detection of chloride anions over a broader range, from 10 μM to 0.1 M (Figure S4B).
3.3. Effect of Analyte Type on Optical Response
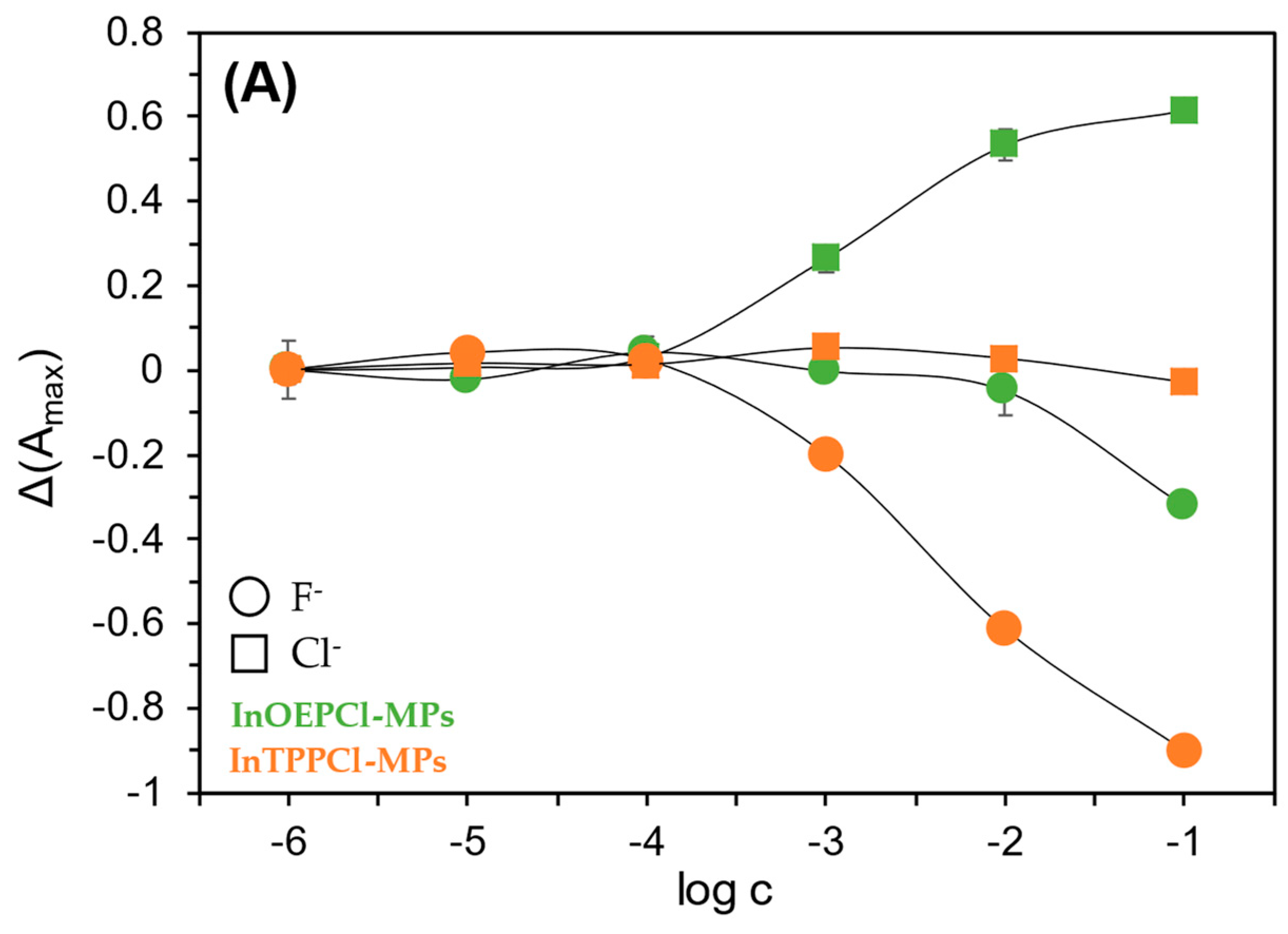
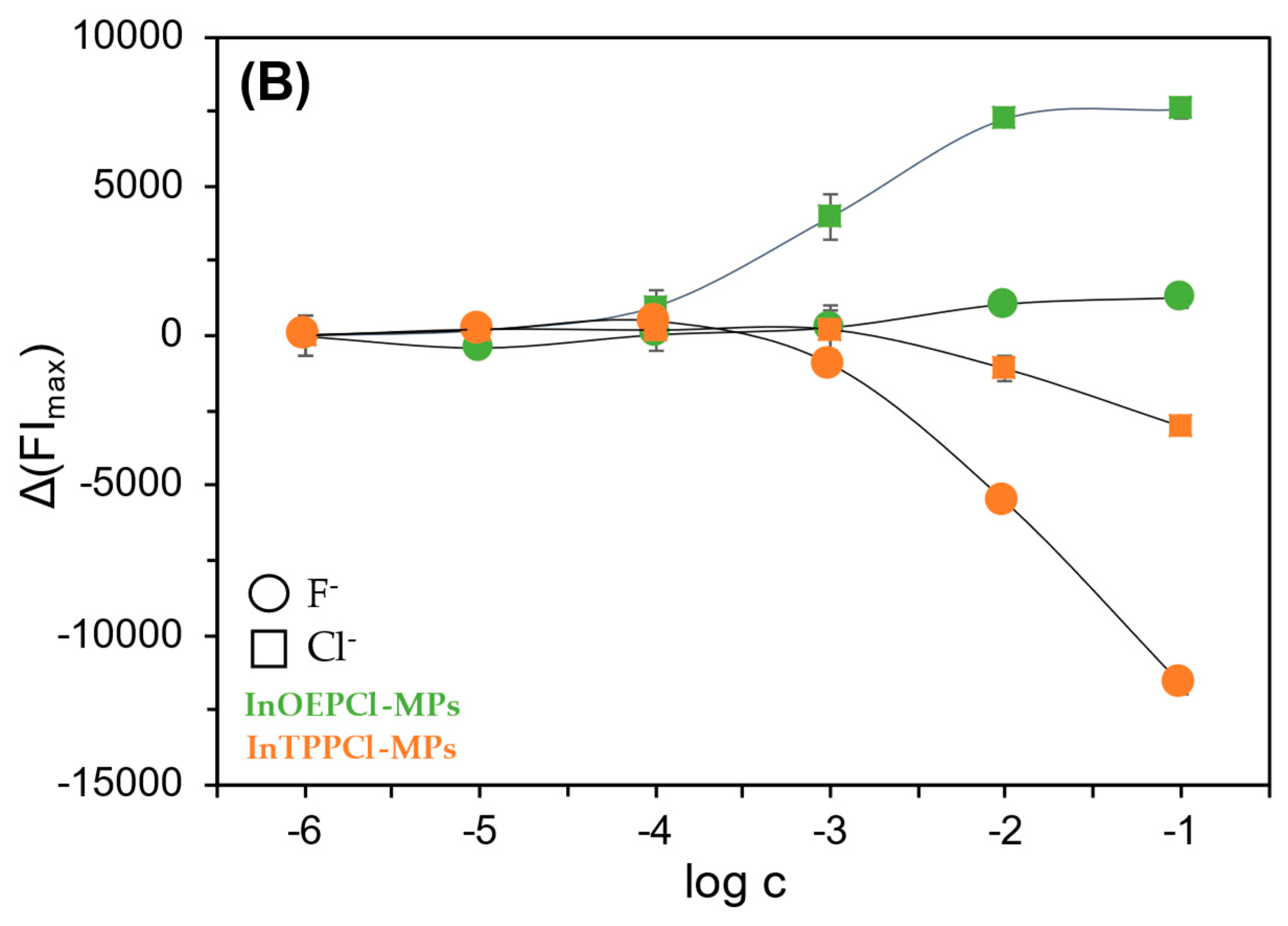
Thanks to their unique chemical, optical, and electrochemical properties, and their generally broad selectivity, metalloporphyrins are widely used in the construction of various sensors [49,50]. Metalloporphyrins act as electrode-modifying materials, improving their selectivity and sensitivity when detecting compounds such as nitric oxide, hydrogen peroxide, or glucose [49]. In optical sensors, the luminescent properties of metalloporphyrins have been used to study gases such as oxygen or carbon dioxide, and biologically active substances such as glucose or enzymes. Changes in the intensity or duration of luminescence are measured as a sensory signal, which enables their precise detection [51]. Metalloporphyrins also act as selective chemical receptors in optical sensors. Complexes formed with anions cause changes in their optical properties, enabling detection even at low concentrations [50]. In the case of anion detection, such as chlorides and fluorides, metalloporphyrins, particularly those containing indium, exhibit varying selectivity: they are more sensitive to chlorides, allowing for their determination over a wide concentration range. In contrast, their response to fluorides is significantly weaker [39]. During our study, it was observed that among all studied microparticles, those incorporated with indium metalloporphyrins, especially with octaethyl-metalloporphyrin, exhibited the least sensitivity towards fluorides.
Due to the differing selectivity towards anions, the fabricated sensors were tested with both chloride and fluoride anions, in both detection modes. Calibration curves were then prepared for both absorption and fluorescence maxima (Figure 4A,B). In the case of particles with TPP metalloporphyrin, the response towards NaF was found to be significantly better compared to NaCl. As described in Section 1, we can obtain satisfactory results over almost the entire concentration range tested with these particles. The situation is different in the case of the OEP-based MPs. In this case, the chloride anion was the analyte for which a much better optical response was obtained. Namely, by analyzing the calibration curves in Figure 4A, it can be seen that InOEPCl-MPs allow the determination of chloride anions in the range from 10 μM to 0.1 M, while fluoride anions can be determined in the 0.1 mM to 0.1 M concentration range, with incomparably lower sensitivity. Furthermore, a significant difference in the sensitivity of this system depending on the analyte selected is apparent. The spectrofluorimetric response of these microparticles yielded much better results, as chloride ions could be determined over the entire range tested, i.e., from 1 μM to 0.1 M.
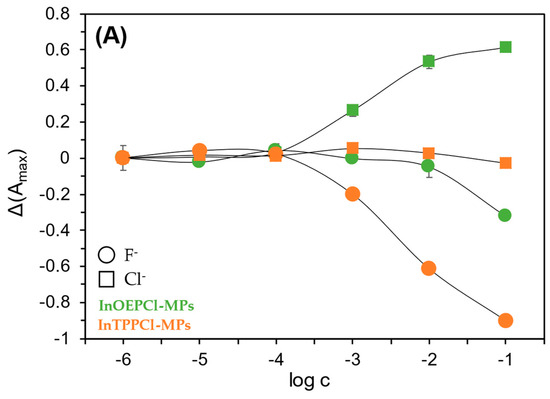
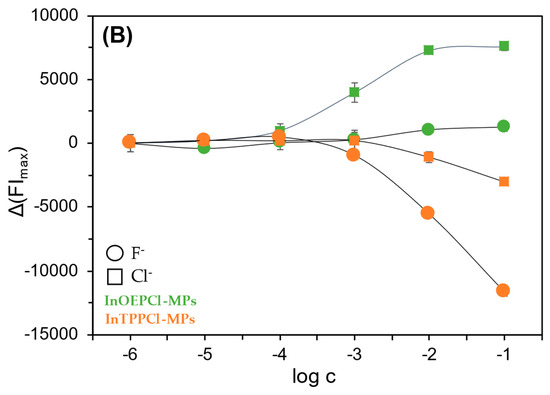
Figure 4.
Spectrophotometric (A) and spectrofluorimetric (B) responses of the fabricated InTPPCl- and InOEPCl-based microparticles toward NaCl (□) and NaF (○) analytes. The difference in absorbance is shown for maximum at 420 nm and 406 nm for InTPPCl-MPs and InOEPCl-MPs, respectively (A); the difference in fluorescence intensity is shown for maximum at λex/λem 424 nm/660 nm and λex/λem 406 nm/576 nm for InTPPCl-MPs and InOEPCl-MPs, respectively (B). All solutions were buffered (0.1 M MES pH 5.5). Concentrations of anions are given in molar scale. Calibration curve points were determined as mean ± SD; n = 4.
3.4. Comparison of Optical Responses of MPs in Spectrophotometric and Spectrofluorimetric Detection Modes
As described in the paragraphs above, we used four types of microparticles in our study-GaTPPCl-MPs, GaOEPCl-MPs, InTPPCl-MPs, and InOEPCl-MPs. All of them were tested for possible dual detection with varying NaF/NaCl analyte concentrations. Calibration curves were determined for all MP types based on the maxima observed in the respective UV-Vis and fluorescence emission spectra (Figure 2A–D). The execution of the repetition series did not contribute to obtaining large error bars, which indicates good repeatability of the measurements. Comparing Ga-based MPs with those based on In, it can be seen that smaller error bars are visible for the latter. This may be due to the starting volume of injected particles (5 μL). The determination coefficient of the linear range of the calibration curves obtained in each case exceeds 0.7, indicating a strong relationship between an increasing concentration level and the optical output (Table 3). It can be concluded that all MPs enable sensing in a wide concentration range, spanning even the micromolar level. The highest sensitivity and the broadest response range, in both detection modes, was observed for GaTTPCl-MPs.

Table 3.
Calibration parameters of the developed microparticles incorporated with metalloporphyrins in the presence of their model analytes.
3.5. Discrimination of Different Varieties of Tea Infusions Based on “Smart Tongue” Sensing
To develop a highly discriminative “smart tongue”, we used seven types of microparticles incorporated with different metalloporphyrins (Table 2). As outlined in the paragraphs above, each of these sensors exhibits varying selectivity and sensitivity to the tested NaF/NaCl analytes. To broaden the amount of discriminatory data, a zirconium metalloporphyrin-based microparticle was additionally introduced to test the real samples.
Metalloporphyrins are highly sensitive and broadly selective towards various analytes, which makes them ideal for applications in broadly sensitive sensors dedicated to electronic tongues and electronic noses. A range of sensors with different selectivity can be created by replacing only the metal coordinated to the porphyrin [52]. With the help of such sensors, quality control of alcoholic beverages, such as wines, can be successfully performed [53]. It is possible to detect compounds that can significantly affect the quality of wine [54] and control the ethanol content in beverages of different origins [55]. An integrated approach, combining e-nose and e-tongue, based on the same sensitive materials—metalloporphyrins—allows for more comprehensive information. Thanks to such integration, it is possible to improve the classification of clinical samples (urine) and food samples (milk) [56]. Thus, in our previous work [36] we used sensors based on manganese metalloporphyrins which enabled the discrimination of L-tyrosine derivatives and related dietary supplements.
In order to test the usability of the microparticles developed for smart tongue sensing, eight types of tea/herbal infusions with varying degrees of similarity were selected. Even though these infusions may differ in many components, they all have one main denominator, namely tea leaves, with a variable level of accumulated fluorine. Two types of the infusions belonged to red teas, two to green teas, and one was black tea. Additionally, yerba mate and CBD hemp tea were applied as control samples, and one infusion was a combination of black tea, green tea, and yerba (Table 1). Each infusion was prepared as described in the experimental section. Spectrophotometric and spectrofluorimetric responses were recorded for all the MPs in the presence of the eight types of infusions. For further numerical processing, whole response spectra were used to enhance the discrimination of the infusions.
In our previous works, principal components analysis (PCA) was mainly used for data analysis as an exploratory tool enabling the study of the discrimination ability of the developed nano- and microparticles [36,37]. In the present work, we additionally chose to test the recognition performance of the “smart tongue” based on the developed MPs, and thus to use the partial least squares-discriminant analysis (PLS-DA) classification method. PLS-DA is a technique that combines elements of PCA and multiple regression to predict the dependent variables (reference parameters) from a set of independent variables (measurement data) [57,58].
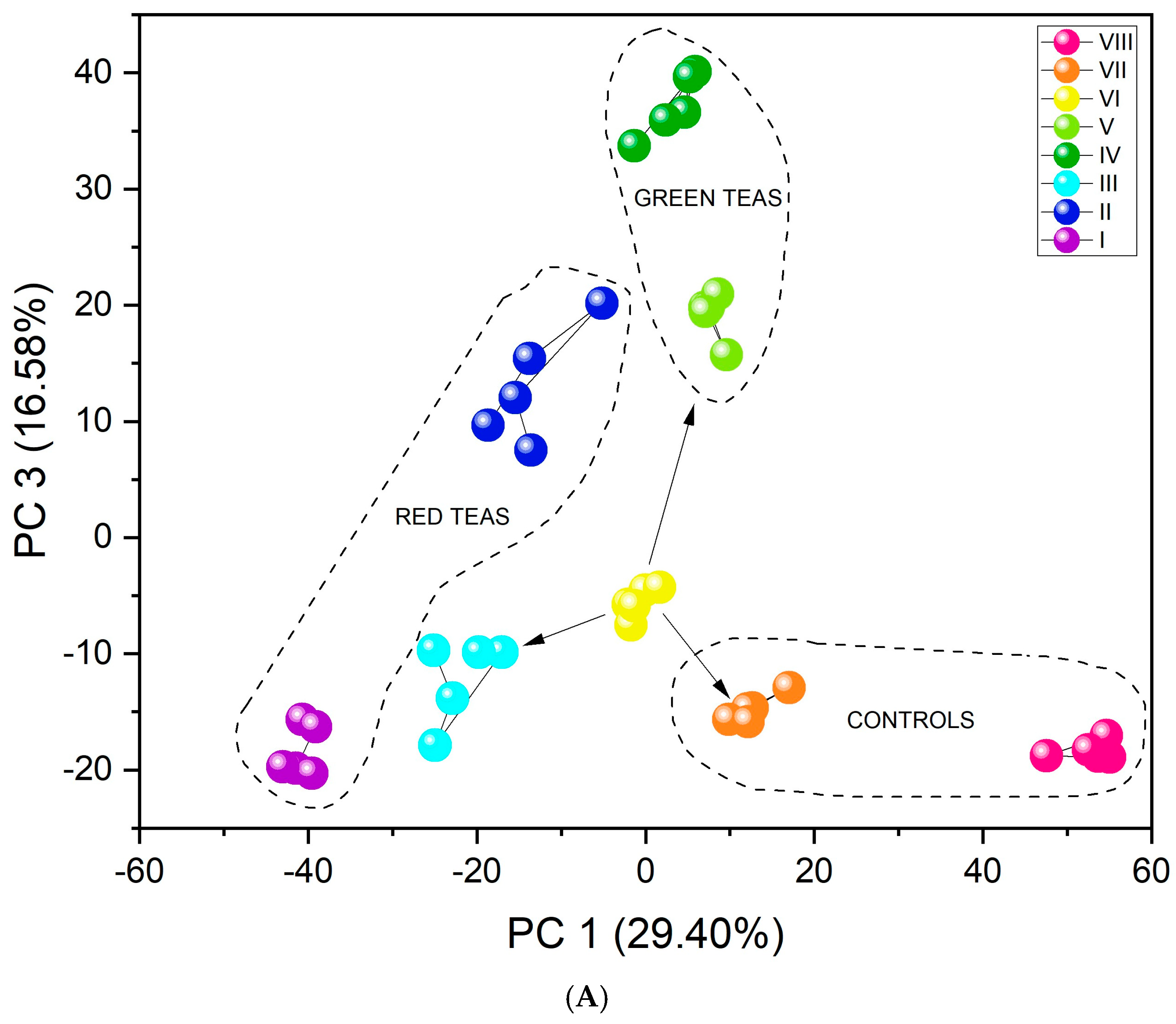
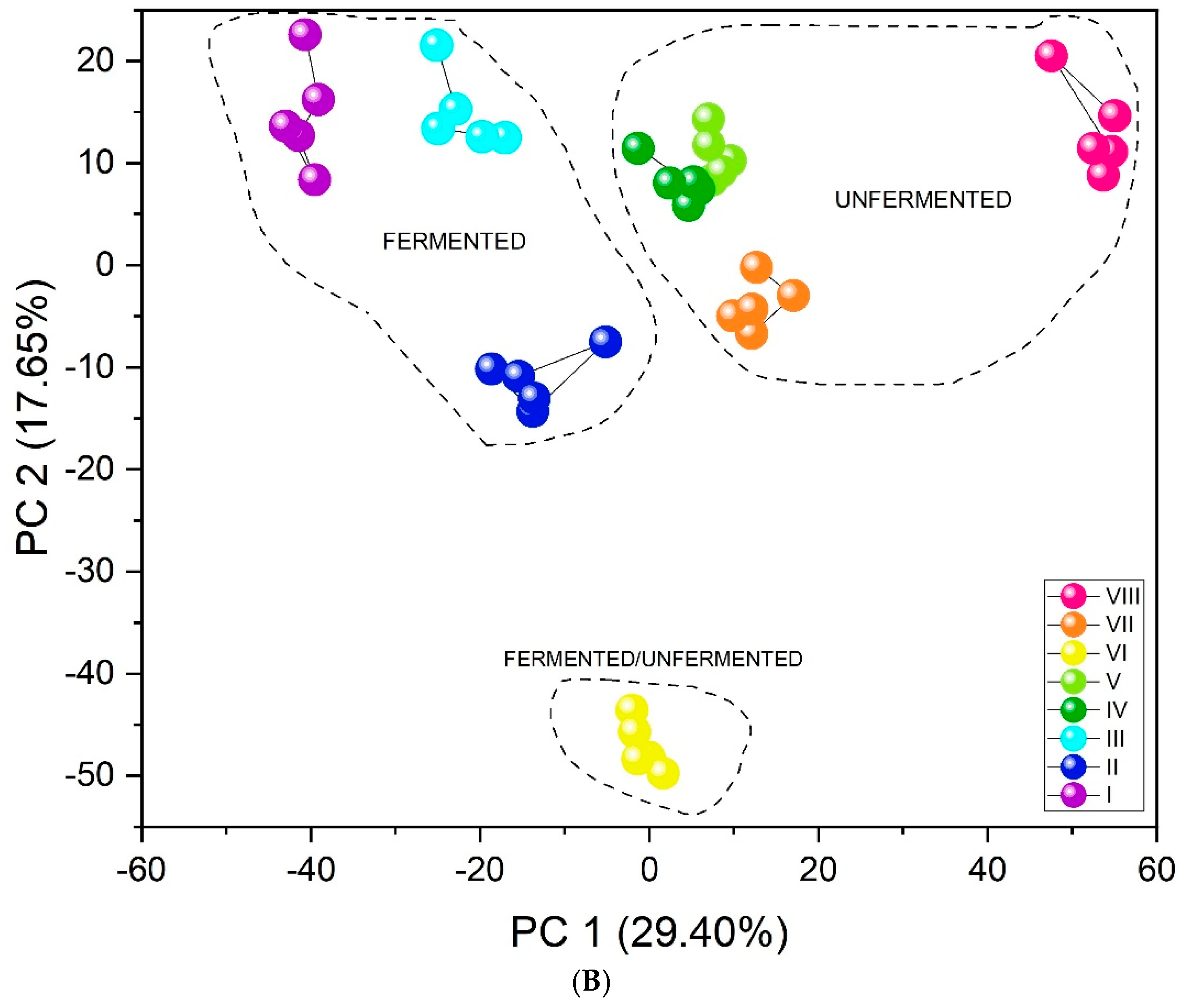
The results obtained by the unsupervised PCA analysis method (Figure 5A) show excellent differentiation. All infusion types create separate clusters. Furthermore, the three-dimensional PCA plot (Figure S5) visually confirms this separation, showing distinct clusters for all infusion types in the three-dimensional space defined by the first three principal components. The first important feature in this case is their arrangement relative to PC1. As the value of this component increases (PC1 > 0), the fluorine content in the tested infusions decreases. Black and red teas are characterized by the highest concentration of this element, followed by green teas, then yerba mate, and CBD hemp tea. An additional, equally important aspect in identifying infusions may be that CBD, significantly distant from the other clusters, contains flavonoids. The similar PC1 value for green teas and yerba mate may be related to the presence of quinic acid in their composition [59]. Tea VI includes a mix of teas: black tea, green tea, and yerba mate (Table 1). The location of its cluster relative to both PC1 and PC3 is adequate because it is located between pure green teas, pure black teas, and pure yerba mate (marked with arrows in Figure 5A). Furthermore, unsupervised analysis revealed a clear and significant differentiation between fermented and unfermented tea samples. Figure 5B shows the division of teas according to their fermentation state. The PC1 value may be associated with the fermentation process. PC1 > 0 is demonstrated by infusions that have undergone the fermentation process (red and black teas), and PC1 < 0 by unfermented infusions (green teas, yerba mate, CBD hemp tea). Since one of the teas was a mixture of fermented and unfermented teas and yerba mate, its PC1 value oscillates around 0, just between fermented and unfermented tea infusions.
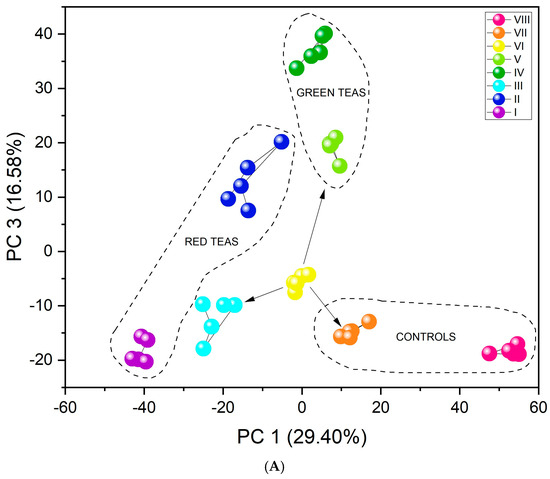
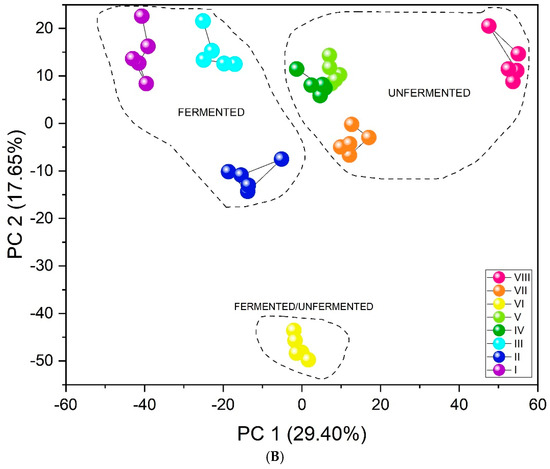
Figure 5.
PCA score plots showing the discrimination of eight types of infusions using the fabricated set of chemosensory microparticles: MnOEPCl-MPs, MnTPPCl-MPs, ZrTPPCl2-MPs, GaTPPCl-MPs, GaOEPCl-MPs, InTPPCl-MPs, and InOEPCl-MPs. Data fusion and autoscaling for preprocessing were applied: (A) PC1-PC3 score plot; (B) PC1-PC2 score plot.
PLS-DA was performed to confirm the ability of microparticles to perform identification of various kinds of tea infusions. PLS-DA models were established for UV-Vis data and fluorescence data separately; also, data fusion based on both spectrophotometric and spectrofluorimetric data was considered. A set of so-called latent variables was created for each model to indicate the correlation between the independent and dependent variables:
- Independent variables:
- 40 UV-Vis and 40 fluorescence spectra for all seven types of MPs (eight types of infusions × five replicates)
- Data vectors:
- Absorbance data: 40 samples × 1407 data points
- Fluorescence data: 40 samples × 943 data points
- Data fusion: 40 samples × 2350 data points
- Target matrix: eight sample types, 0–1 coding
- Absorbance data: 40 × 8
- Fluorescence data: 40 × 8
- Data fusion: 40 × 8
- Data split: 24 samples for train set, 16 samples for test/validation (three replicates for training. and two replicates for validation in each class)
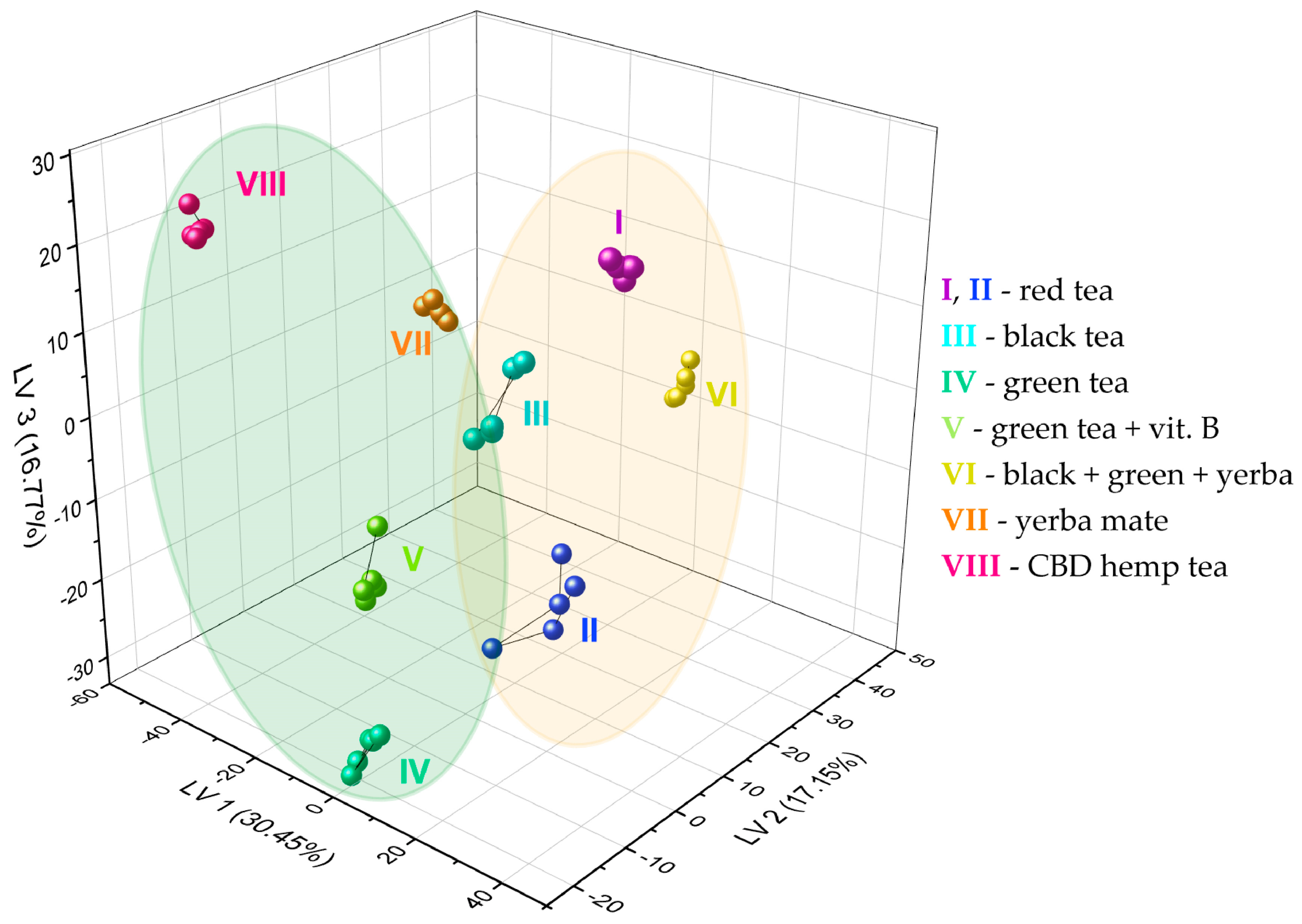
Figure 6 shows the results of the sample discrimination obtained for the data fusion case. About 64% of the original set variance was represented by the first three components, i.e., roughly 36% of the information was not used for data visualization on the 3D PLS-DA score plot. Nevertheless, this did not contribute to a deterioration of the discrimination visualized in the score plot, and even contributed to a clear grouping of all infusions into eight specified classes. Moreover, higher-level grouping can be seen—it is possible to distinguish two subsets according to the fluoride level. The actual F− concentrations (undiluted) in the prepared infusions are presented in Table 1. The subset marked with a yellow oval includes infusions with an expected higher level of fluoride ions (red teas—I, II, black tea—III, black + green + yerba—VI). In contrast, the green subgroup consists of two types of green tea (IV, V), yerba mate (VII), and CBD hemp tea (VIII), which we used as controls and are characterized by an expected lower level of fluoride ions. The fluoride level may, however, differ slightly from the estimated values. In the case of tea I, there are significantly older leaves compared to the other samples, because this is a tea that has been maturing for 15 years, and fluorides are concentrated in older leaves. In tea VI, the F− level depends on the dominant tea in this blend (there is no specific information about this). In addition, longer brewing time and higher water temperature can increase the extraction of fluoride ions.
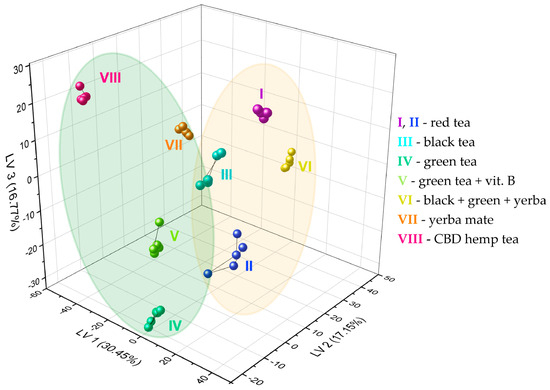
Figure 6.
PLS-DA score plot showing the discrimination of eight types of tea infusions using the fabricated set of chemosensory microparticles: MnOEPCl-MPs, MnTPPCl-MPs, ZrTPPCl2-MPs, GaTPPCl-MPs, GaOEPCl-MPs, InTPPCl-MPs, and InOEPCl-MPs. Autoscaling was used for data preprocessing (fusion of spectrophotometric and spectrofluorimetric responses). Green oval: tea infusions with lower level of fluoride ions; yellow oval: tea infusions with higher level of fluoride ions (details in the text).
The classification performance of the resulting models was evaluated using confusion matrices, created by comparing the actual values of class assignation with those predicted by the model (Table 4). Based on these matrices, four metrics were calculated to assess the quality of the models: accuracy, sensitivity, precision, and specificity. Values corresponding to true negative (TN), false negative (FN), false positive (FP), and true positive (TP) cases were used in the calculations [60,61]. Based on the confusion matrices (Table 4), for the spectrophotometric results, an accuracy (ACC) of 87.5% and 81.25% was obtained for the train and test, respectively. Other parameters, such as precision, sensitivity, and specificity, were also high, i.e., > 81%. Nevertheless, the results obtained for both sets in the case of the fluorescence data are much more favorable, as all samples could be classified correctly (100%). Finally, additional analysis based on data fusion was attempted. Again, perfect accuracy of 100% was achieved for both the train and test sets. The obtained results suggest that the “smart tongue” based on optical, chemically sensitive microparticles can be applied for tea infusion recognition. Moreover, the identification can also be based on their (dis)similar composition and production methods.

Table 4.
Confusion matrices of PLS-DA models applied to identify tea infusions using the developed microparticles: (A) results for the train set based on UV-vis data; (B) results for the test set based on UV-vis data; (C) results for the train set based on fluorescence data; (D) results for the test set based on fluorescence data. In the case of data fusion (UV-vis and fluorescence), the matrices obtained for train and test sets were the same as (C,D), respectively.
4. Conclusions
Tea is a popular beverage with many health benefits, such as anti-inflammatory, antioxidant, and anticancer effects. However, it can also pose a particular risk to humans. Fluoride is an element that accumulates most often in plant leaves, and its concentration can increase due to the increasingly common use of fertilizers. Excessive consumption of this element can lead to the risk of fluorosis in children, and over time, it can also contribute to skeletal fluorosis [7]. Moreover, adulteration of tea is an increasing problem since some adulterants can be harmful. Therefore, appropriate quality control of tea is essential from the point of view of human health, as well for as economic reasons.
This paper presents the development of a group of chemosensitive microparticles based on metalloporphyrins, which have been tested for sensitivity to chlorides and fluorides. An array of microparticles was applied as a “smart tongue” device, allowing for rapid analysis of various tea infusions. With satisfactory results, it enables the identification of tea type, distinguishing teas in terms of the level of fluoride concentration, and it can discern variously processed tea infusions (i.e., black/red/green tea). The proposed machine-learning assisted microparticle test for tea profiling can become a much simpler and cheaper alternative to classical analytical methods in tea quality analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13060203/s1, Figure S1: Time stability of spectrophotometric response of MnTPPCl-MPs towards NaCl as model analyte (0.1 M MES, pH 4.5); Figure S2: Exemplary results of optimization of MPs components; Figure S3: UV-Vis spectra and fluorescence emmision spectra of GaOEPCl-MPs in the presence of NaF analyte; Figure S4: UV-Vis spectra and fluorescence emission spectra of InOEPCl-MPs towards NaCl analyte. Figure S5: 3D PCA score plot showing the discrimination of 8 types of infusions using the fabricated set of chemosensory microparticles: MnOEPCl-MPs, MnTPPCl-MPs, ZrTPPCl2-MPs, GaTPPCl-MPs, GaOEPCl-MPs, InTPPCl-MPs, InOEPCl-MPs.
Author Contributions
Conceptualization, P.C.-S.; Methodology, A.K. and P.C.-S.; Validation, P.C.-S.; Formal analysis, A.K. and P.C.-S.; Investigation, A.K. and N.J.; Resources, P.C.-S.; Data curation, A.K., N.J. and P.C.-S.; Writing—original draft, A.K. and P.C.-S.; Writing—review & editing, P.C.-S.; Visualization, A.K. and P.C.-S.; Supervision, P.C.-S.; Project administration, P.C.-S.; Funding acquisition, P.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland, grant number UMO-2018/30/E/ST4/00481 and UMO-2024/53/B/NZ9/01654.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
This work was financially supported by the National Science Centre, Poland within the framework of project No. UMO-2018/30/E/ST4/00481 and No UMO-2024/53/B/NZ9/01654. Aleksandra Kossakowska acknowledges financial support from the IDUB project (Scholarship Plus program). Authors would like to thank Łukasz Górski for helpful discussions on metalloporphyrins.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Q.; Liu, A.; Zhao, J.; Ouyang, Q. Classification of Tea Category Using a Portable Electronic Nose Based on an Odor Imaging Sensor Array. J. Pharm. Biomed. Anal. 2013, 84, 77–83. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, X.; Deng, X. Discrimination of Varieties of Tea Using near Infrared Spectroscopy by Principal Component Analysis and BP Model. J. Food Eng. 2007, 79, 1238–1242. [Google Scholar] [CrossRef]
- Pattaravisitsate, N.; Phetrak, A.; Denpetkul, T.; Kittipongvises, S.; Kuroda, K. Effects of Brewing Conditions on Infusible Fluoride Levels in Tea and Herbal Products and Probabilistic Health Risk Assessment. Sci. Rep. 2021, 11, 14115. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Chandrajith, R.; Abeypala, U.; Dissanayake, C.B.; Tobschall, H.J. Fluoride in Ceylon Tea and Its Implications to Dental Health. Environ. Geochem. Health 2007, 29, 429–434. [Google Scholar] [CrossRef]
- Szmagara, A.; Krzyszczak, A.; Stefaniak, E.A. Determination of Fluoride Content in Teas and Herbal Products Popular in Poland. J. Environ. Health Sci. Eng. 2022, 20, 717–727. [Google Scholar] [CrossRef]
- Malinowska, E.; Inkielewicz, I.; Czarnowski, W.; Szefer, P. Assessment of Fluoride Concentration and Daily Intake by Human from Tea and Herbal Infusions. Food Chem. Toxicol. 2008, 46, 1055–1061. [Google Scholar] [CrossRef]
- Mazurek, A.; Kowalska, G.; Włodarczyk-Stasiak, M.; Wyrostek, J.; Kowalski, R. The Influence of the Preparation of Tea Infusion on the Content of Fluoride and the Assessment of Health Risk for the Consumer in Poland. Appl. Sci. 2023, 13, 5075. [Google Scholar] [CrossRef]
- Pehrsson, P.R.; Patterson, K.Y.; Perry, C.R. The Fluoride Content of Select Brewed and Microwave-Brewed Black Teas in the United States. J. Food Compos. Anal. 2011, 24, 971–975. [Google Scholar] [CrossRef]
- Dippong, T.; Cadar, O.; Kovacs, M.H.; Dan, M.; Senila, L. Chemical Analysis of Various Tea Samples Concerning Volatile Compounds, Fatty Acids, Minerals and Assessment of Their Thermal Behavior. Foods 2023, 12, 3063. [Google Scholar] [CrossRef]
- Fernández, P.L.; Martin, M.J.; González, A.G.; Pablos, F. HPLC Determination of Catechins and Caffeine in Tea. Differentiation of Green, Black and Instant Teas. Analyst 2000, 125, 421–425. [Google Scholar] [CrossRef]
- Wangkarn, S.; Grudpan, K.; Khanongnuch, C.; Pattananandecha, T.; Apichai, S.; Saenjum, C. Development of HPLC Method for Catechins and Related Compounds Determination and Standardization in Miang (Traditional Lanna Fermented Tea Leaf in Northern Thailand). Molecules 2021, 26, 6052. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, D.; Hu, J.; Tang, J.; Zhou, X.; Wang, L.; Xie, J.; Jiang, Y.; Yuan, H.; Yang, Y. Unraveling the Dynamic Changes of Volatile Compounds during the Rolling Process of Congou Black Tea via GC-E-Nose and GC–MS. Front. Sustain. Food Syst. 2024, 8, 1436542. [Google Scholar] [CrossRef]
- Niu, X.; Ao, C.; Yu, J.; Zhao, Y.; Huang, H. GC-MS Combined with Proteomic Analysis of Volatile Compounds and Formation Mechanisms in Green Teas with Different Aroma Types. Foods 2024, 13, 1848. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróblewski, W. Sensor Arrays for Liquid Sensing—Electronic Tongue Systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.; Wang, Z.; Ma, J.; Bai, X.; Wang, Z.; Lan, Y.; Yuan, W. Rapid Identification of Oolong Tea Category by Synergetic Application of E-Nose and E-Tongue Combined with a Modified GAN—TCN Composite Model. J. Food Meas. Charact. 2024, 5903, 5887–5903. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, X.; Wu, R.; Yin, L.; Hu, W.; Zhang, Z. Rapid Characterization of Black Tea Taste Quality Using Miniature NIR Spectroscopy and Electronic Tongue Sensors. Biosensors 2023, 13, 92. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Q.; Wu, C.; Hsia, K.J. (Eds.) Bioinspired Smell and Taste Sensors; Springer: Dordrecht, The Netherlands, 2015; ISBN 9789401773324. [Google Scholar]
- Chen, Q.; Zhao, J.; Vittayapadung, S. Identification of the Green Tea Grade Level Using Electronic Tongue and Pattern Recognition. Food Res. Int. 2008, 41, 500–504. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Cheli, F.; Chiofalo, V. Fusion of Electronic Nose, Electronic Tongue and Computer Vision for Animal Source Food Authentication and Quality Assessment—A Review. J. Food Eng. 2017, 210, 62–75. [Google Scholar] [CrossRef]
- Chen, J.; Lin, B.; Zheng, F.J.; Fang, X.C.; Ren, E.F.; Wu, F.F.; Verma, K.K.; Chen, G.L. Characterization of the Pure Black Tea Wine Fermentation Process by Electronic Nose and Tongue-Based Techniques with Nutritional Characteristics. ACS Omega 2023, 8, 12538–12547. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous Determination of Catechins, Caffeine and Gallic Acids in Green, Oolong, Black and Pu-Erh Teas Using HPLC with a Photodiode Array Detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Togari, N.; Kobayashi, A.; Aishima, T. Pattern Recognition Applied to Gas Chromatographic Profiles of Volatile Components in Three Tea Categories. Food Res. Int. 1995, 28, 495–502. [Google Scholar] [CrossRef]
- Horie, H.; Mukai, T.; Kohata, K. Simultaneous Determination of Qualitatively Important Components in Green Tea Infusions Using Capillary Electrophoresis. J. Chromatogr. A 1997, 758, 332–335. [Google Scholar] [CrossRef]
- Ha, D.; Sun, Q.; Su, K.; Wan, H.; Li, H.; Xu, N.; Sun, F.; Zhuang, L.; Hu, N.; Wang, P. Recent Achievements in Electronic Tongue and Bioelectronic Tongue as Taste Sensors. Sens. Actuators B Chem. 2015, 207, 1136–1146. [Google Scholar] [CrossRef]
- Kutyła-Olesiuk, A.; Zaborowski, M.; Prokaryn, P.; Ciosek, P. Monitoring of Beer Fermentation Based on Hybrid Electronic Tongue. Bioelectrochemistry 2012, 87, 104–113. [Google Scholar] [CrossRef]
- Cetó, X.; Gutiérrez, J.M.; Gutiérrez, M.; Céspedes, F.; Capdevila, J.; Mínguez, S.; Jiménez-Jorquera, C.; Del Valle, M. Determination of Total Polyphenol Index in Wines Employing a Voltammetric Electronic Tongue. Anal. Chim. Acta 2012, 732, 172–179. [Google Scholar] [CrossRef]
- Ciosek, P.; Sobański, T.; Augustyniak, E.; Wróblewski, W. ISE-Based Sensor Array System for Classification of Foodstuffs. Meas. Sci. Technol. 2006, 17, 6–11. [Google Scholar] [CrossRef]
- Winquist, F.; Holmin, S.; Krantz-Rülcker, C.; Wide, P.; Lundström, I. A Hybrid Electronic Tongue. Anal. Chim. Acta 2000, 406, 147–157. [Google Scholar] [CrossRef]
- Palit, M.; Tudu, B.; Bhattacharyya, N.; Dutta, A.; Dutta, P.K.; Jana, A.; Bandyopadhyay, R.; Chatterjee, A. Comparison of Multivariate Preprocessing Techniques as Applied to Electronic Tongue Based Pattern Classification for Black Tea. Anal. Chim. Acta 2010, 675, 8–15. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Taylor, A.; Drozdov, I.; Bodei, L.; Kidd, M. Neuroendocrine Tumor Biomarkers: Current Status and Perspectives. Neuroendocrinology 2014, 100, 265–277. [Google Scholar] [CrossRef]
- Buratti, S.; Casiraghi, A.; Minghetti, P.; Giovanelli, G. The Joint Use of Electronic Nose and Electronic Tongue for the Evaluation of the Sensorial Properties of Green and Black Tea Infusions as Related to Their Chemical Composition. Food Nutr. Sci. 2013, 4, 605–615. [Google Scholar] [CrossRef]
- Rahman, M.M.; Charoenlarpnopparut, C.; Suksompong, P. Signal Processing for Multi-Sensor E-Nose System: Acquisition and Classification. In Proceedings of the 2015 10th International Conference on Information, Communications and Signal Processing (ICICS), Singapore, 2–4 December 2015; pp. 1–5. [Google Scholar] [CrossRef]
- Ruiz-Perez, D.; Guan, H.; Madhivanan, P.; Mathee, K.; Narasimhan, G. So You Think You Can PLS-DA? BMC Bioinform. 2020, 21, 2. [Google Scholar] [CrossRef]
- Xie, X.; Bakker, E. Ion Selective Optodes: From the Bulk to the Nanoscale. Anal. Bioanal. Chem. 2015, 407, 3899–3910. [Google Scholar] [CrossRef]
- Kossakowska, A.; Kociszewska, K.; Kochman, K.; Wojciechowski, K.; Górski, Ł.; Ciosek-Skibińska, P. Toward an Electronic Tongue Based on Surfactant-Stabilized Chemosensory Microparticles with a Dual Detection Mode. ACS Appl. Mater. Interfaces 2022, 14, 50375–50385. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, A.; Wicik, M.; Matusiak, P.; Ciosek-Skibińska, P. Chemosensory Optode Array Based on Pluronic-Stabilized Microspheres for Differential Sensing. Chemosensors 2022, 10, 2. [Google Scholar] [CrossRef]
- Zhang, W.; Rozniecka, E.; Malinowska, E.; Parzuchowski, P.; Meyerhoff, M.E. Optical Chloride Sensor Based on Dimer-Monomer Equilibrium of Indium(III) Octaethylporphyrin in Polymeric Film. Anal. Chem. 2002, 74, 4548–4557. [Google Scholar] [CrossRef]
- Górski, Ł.; Malinowska, E.; Parzuchowski, P.; Zhang, W.; Meyerhoff, M.E. Recognition of Anions Using Metalloporphyrin-Based Ion-Selective Membranes: State-of-the-Art. Electroanalysis 2003, 15, 1229–1235. [Google Scholar] [CrossRef]
- Steinle, E.D.; Schaller, U.; Meyerhoff, M.E. Response Characteristics of Anion-Selective Polymer Membrane Electrodes Based on Gallium(III), Indium(III) and Thallium(III) Porphyrins. Anal. Sci. 1998, 14, 79–84. [Google Scholar] [CrossRef]
- Kossakowska, A.; Szajda, E.; Jędryka, N.; Ciosek-Skibińska, P. Development of Lasalocid A—Based Amine-Sensitive Nanoparticles for “Smart Tongue” Sensing of Dietary Supplements. Sens. Actuators B Chem. 2024, 407, 135463. [Google Scholar] [CrossRef]
- Zheng, W.; Shan, N.; Yu, L.; Wang, X. UV-Visible, Fluorescence and EPR Properties of Porphyrins and Metalloporphyrins. Dyes Pigments 2008, 77, 153–157. [Google Scholar] [CrossRef]
- Nasri, H. Porphyrins and Metalloporphyrins: An Overview. In Proceedings of the 2020 IEEE International Conference on Design and Test of Integrated Micro and Nano-Systems (DTS), Hammamet, Tunisia, 7–10 June 2020; pp. 1–6. [Google Scholar]
- Ük, N.; Aykut, S.; Jahangiri, H.; Nar, I.; Ünlü, C. Indium-Based Quantum Dots Trapped in Solid-State Matrices: A One-Pot Synthesis, Thermoresponsive Properties, and Enhanced Micropollutant Removal. New J. Chem. 2024, 48, 10074–10086. [Google Scholar] [CrossRef]
- Bahmani Jalali, H.; De Trizio, L.; Manna, L.; Di Stasio, F. Indium Arsenide Quantum Dots: An Alternative to Lead-Based Infrared Emitting Nanomaterials. Chem. Soc. Rev. 2022, 51, 9861–9881. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, M. Porphyrins and Metalloporphyrins in Electroanalytical Chemistry. In Advances in Chemistry Research; Taylor, J.C., Ed.; Nova: New York, NY, USA, 2017; Volume 36, pp. 95–152. ISBN 978-1-53610-764-7. [Google Scholar]
- Prins, P.T.; Rutkowska-Zbik, D.; Pullen, S.; Baumgartner, B. Porphyrins on Acid: Kinetics of the Photoinduced-Protonation of Tetrakis(4-Carboxyphenyl)-Porphyrin. Phys. Chem. Chem. Phys. 2024, 26, 24524–24532. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, R. The Use of Spectrophotometry UV-Vis for the Study of Porphyrins. In Macro to Nano Spectroscopy; Uddin, J., Ed.; InTech: London, UK, 2012. [Google Scholar]
- Bakker, E.; Qin, Y. Electrochemical Sensors. Anal. Chem. 2006, 78, 3965–3984. [Google Scholar] [CrossRef]
- Wang, E.; Meyerhoff, M.E. Anion Selective Optical Sensing with Metalloporphyrin-Doped Polymeric Films. Anal. Chim. Acta 1993, 283, 673–682. [Google Scholar] [CrossRef]
- Papkovsky, D.B. Luminescent Porphyrins as Probes for Optical (Bio)Sensors. Sens. Actuators B Chem. 1993, 11, 293–300. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Macagnano, A.; Mantini, A.; Goletti, C.; D’Amico, A. Characterization and Design of Porphyrins-Based Broad Selectivity Chemical Sensors for Electronic Nose Applications. Sens. Actuators B Chem. 1998, 52, 162–168. [Google Scholar] [CrossRef]
- Di, C.; Paolesse, R.; Macagnano, A.; Mantini, A.; Amico, A.D.; Ubigli, M.; Legin, A.; Lvova, L.; Rudnitskaya, A.; Vlasov, Y. Application of a Combined Artificial Olfaction and Taste System to the Quantification of Relevant Compounds in Red Wine. Sens. Actuators B Chem. 2000, 69, 342–347. [Google Scholar]
- Lvova, L.; Yaroshenko, I.; Kirsanov, D.; Di Natale, C.; Paolesse, R.; Legin, A. Electronic Tongue for Brand Uniformity Control: A Case Study of Apulian Red Wines Recognition and Defects Evaluation. Sensors 2018, 18, 2584. [Google Scholar] [CrossRef]
- Lvova, L.; Paolesse, R.; Di Natale, C.; D’Amico, A. Detection of Alcohols in Beverages: An Application of Porphyrin-Based Electronic Tongue. Sens. Actuators B Chem. 2006, 118, 439–447. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Macagnano, A.; Mantini, A.; D’Amico, A.; Legin, A.; Lvova, L.; Rudnitskaya, A.; Vlasov, Y. Electronic Nose and Electronic Tongue Integration for Improved Classification of Clinical and Food Samples. Sens. Actuators B Chem. 2000, 64, 15–21. [Google Scholar] [CrossRef]
- Krishnan, A.; Williams, L.J.; McIntosh, A.R.; Abdi, H. Partial Least Squares (PLS) Methods for Neuroimaging: A Tutorial and Review. Neuroimage 2011, 56, 455–475. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Reviews, C.; Science, F. Yerba Mate Tea (Ilex Paraguariensis): A Comprehensive Review on Chemistry, Health Implications, and Technological Considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef]
- Głowacz, K.; Wawrzyniak, U.E.; Ciosek-Skibińska, P. Comparison of Various Data Analysis Techniques Applied for the Classification of Oligopeptides and Amino Acids by Voltammetric Electronic Tongue. Sens. Actuators B Chem. 2021, 331, 129354. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Pérez-Castaño, E.; Ruiz-Samblás, C. Quality Performance Metrics in Multivariate Classification Methods for Qualitative Analysis. TrAC—Trends Anal. Chem. 2016, 80, 612–624. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).