Abstract
New faster and more sensitive detection methods are required for food microbiological contamination quality control throughout the entire handling process. The adenosine triphosphate (ATP) bioluminescence detection method based on the firefly luciferin–luciferase system has been a widely used approach for decades due to its practicality, efficiency, and rapidity. The luciferase of the Amydetes vivianii firefly, cloned and produced in our laboratory, displays a high bioluminescence and stability, showing desirable properties for ATP assays. Using this enzyme, in this study we developed and validated a swab-based ATP detection method for meat, chicken, and milk, allowing us to distinguish between contaminated and non-contaminated forms of these foods. This method demonstrated robust interday and intraday precision, an accuracy of 70–71% across different matrices, and a limit of detection of 1.0 × 104 cps for the dilutions and 2.7 × 103 cps for the swabs, fulfilling all validation criteria and ensuring reliability for routine applications. Except for milk, which has very low endogenous ATP levels due to pasteurization, thus requiring sample pre-processing, the method allowed us to luminometrically detect ATP on the surface of meat and chicken in less than an hour using an assay solution. The method showed higher sensitivity compared to an available commercial kit due to its intense signal, with a remarkable ~22-fold increase in luminescence intensity when comparing the highest ATP concentration of Amydetes luciferase with a commercially available luciferase, allowing for detecting microbiological contamination at lower levels or using less sensitive luminometers.
1. Introduction
Bioluminescence, the emission of light by living organisms, is commonly used as a communication mechanism among organisms [1,2], and humans have employed it for bioindication purposes. The firefly luciferin–luciferase system has been extensively used for ATP detection in microbiological contamination assays [3,4]. This system catalyzes light emission through the oxidation of a benzothiazolic luciferin in the presence of ATP and magnesium [4].
Since ATP is a viable cell indicator, the firefly luciferin–luciferase enzyme system has been extensively used during recent decades as an analytical reagent in a wide array of kits, where the luminescence intensity is proportional to the ATP levels [3]. It finds applications in cell growth measurements, cell viability assays, and microbiological contamination detection. This principle has proven useful in pre-screening quality control assays, preservative efficacy, and in disinfectant or sanitizing validation studies by directly and indirectly measuring ATP in microbiological contamination [5]. The increasing use of bioluminescence assays for ATP analysis reflects its faster, more reliable, efficient, and sensitive assessment of microbial contamination suitable for a high number of microbiological samples without the need for long incubation times with minimal intervention in the assays [6].
In the food industry, detecting the presence of pathogens or potential food deterioration due to inadequate processing, transport, and storage conditions is crucial for quality control and food safety assessments. Current analytical methods target microorganisms such as fecal coliforms, enterococci, total enterobacteria, and E. coli, as indicators of fecal contamination and food toxin production.
The national and international standards specified in International Organization for Standardization (ISO) 4831 ensure food quality by setting total coliforms limits and recommending specific methods including aerobic plate count (APC) and the most probable number enumeration (MPN) for meat, meat products, and milk. Alternative methods can be used if validated as equivalent to the described methodologies in accordance with ISO 16140 or other similar protocols, where negative results are definitive and positive results are presumptive and require confirmation using standard methods. Although these methods are simple, sensitive, and cost-effective, often considered the “gold standards”, they usually require long incubation periods of 18 to 24 h for visible colonies to grow, leading to questionable results that need confirmation [7]. Additionally, real-time results throughout the processing line require considerable effort, starting from the beginning of production, to minimize problems and perform microbial control in critical steps.
Therefore, due to the need for faster, more sensitive, accurate, and robust assays for microorganisms’ quantification, new methods are constantly being published in the literature in an effort to improve current methods. Considering such demands, bioluminescence-based ATP assays using brighter and more stable luciferases are highly desirable.
Here, we used a novel efficient firefly luciferase (Amydetes vivianii) that displays higher bioluminescence activity and stability and desirable properties for ATP assays to develop and validate a swab-based method to detect ATP released by bacteria and the application of this method for the quality control of foods such as meat, chicken, and milk. This method allows us to detect higher levels of microorganism contamination, up to 108 CFUs/mL, using less sensitive light-detecting devices.
2. Materials and Methods
2.1. Overview of the Development of an ATP Quantification Method for Detecting Microbiological Contamination in Cultures and in Swabs
Aiming to validate the proposed methods for microbiological quality control assays, the ATP bioluminescence technique was first validated using the standard ATP. The same method was then validated using ATP extracted from E. coli Xl1-Blue cultures prepared on the day of each experiment and diluted in phosphate buffer. The same method was again validated with ATP extracted from E. coli Xl1-Blue cultures diluted in phosphate buffer and adsorbed on a swab in order to verify if the swab could interfere with the luminescence detection of the equipment.
2.2. Bioluminescence Measurements
The luminescence measurements were performed using an AB2200 luminometer (ATTO, Tokyo, Japan) by mixing 75 μL of the assay solution in a luminometer tube with 5 μL of Amydetes vivianii luciferase (~0.1 mg/mL) and 5 μL of 10 mM luciferin. The assay solution was prepared on the same day with 45 μL Tris-HCl 0.10 M, 5 μL MgSO4 80 mM, 10 μL of 1 mg/mL bovine serum albumin (BSA), 5 μL of 5 mM dithiothreitol (DTT), and 15 μL glycerol. The luminometer analysis results were given in relative luminescence intensity–counts per second (cps). For each analysis, five replicates were used, and the averages, standard deviations, and precision were calculated, according to parameters described in the following sections. Microsoft Office Excel was used to create graphs and tables.
- Standard ATP quantitation. Curves were created for the standardization of commercial ATP (Sigma-CAS 34369-07-8) at the following concentrations: 5.0 × 10−3, 5.0 × 10−4, 5.0 × 10−5, 5.0 × 10−6 and 5.0 × 10−7 mM diluted in 25 mM phosphate buffer pH 7.0. Then, the analyses were carried out in a luminometer tube using 75 μL of the analysis solution, 5 μL of A. vivianii luciferase (~0.1 mg/mL), and 5 μL of 10 mM luciferin.
- ATP extraction and quantification from diluted culture cells. Dilution cell culture curves were created in order to standardize the number of microorganisms that would be used throughout the assay. For this purpose, E. coli Xl1-Blue cells (Agilent) were cultivated in liquid Luria–Bertani (LB) broth containing tetracycline at 37 °C until they reached an optical density (OD600) of 1.0. From the culture, the dilutions were prepared in phosphate buffer, pH 7.0: 1000, 100, 10, 1, 0.1 and 0.01 μL/mL. The dilutions were then diluted again in equal parts of extraction buffer so that all of the ATP inside the cells could be quantified. The extraction buffer consisted of 0.10 M Tris-HCl pH 8.0, 1% trichloroacetic acid (TCA), and 2% Triton X-100. After 10 min, 10 μL of the dilutions in extraction buffer was analyzed in a luminometer with 75 μL of the analysis solution, 5 μL of A. vivianii luciferase, and 5 μL of 10 mM luciferin after 4 min of contact between the dilutions and the analysis solution.
- ATP extraction and quantification from diluted culture cells in a swab. The last standardization involved testing the ability of a swab to capture microorganisms from different matrices, first to validate the method, and later in food. Therefore, considering the 120 μL capacity of each swab, which aligns with the capacity reported for cotton swabs in the literature [8], 60 μL of extraction buffer was added to the swab followed by 60 μL of previously prepared culture dilutions in phosphate buffer, pH 7.0. The dilutions were seeded in a previously sterilized plate to confirm the swab’s ability to adsorb all seeded content. The swabs were allowed to rest for 40 min before being inserted into a luminometer tube 4 min after being in contact with the analysis solution.
2.3. Bioluminescent Method Validation Procedures for Detecting ATP Extracted from E. coli Cultures
The proposed method was validated using the microbiology validation protocols described in European Pharmacopoeia (Ph. Eur.) Chapter 5.1.6 (Alternative methods for control of microbiological quality) and United States Pharmacopeia (USP) Chapter 1223 [9,10]. The bioanalytical assay validation procedures were carried out in accordance with the described regulatory guidelines, and included the following measures and requirements:
- Linearity. The linearity of the calibration curve was validated over three days at five different concentrations of ATP using the three proposed methods: standard ATP, ATP extracted from the culture alone, and the culture in a swab. The average, standard deviations, equation of the line, and coefficient of determination (R2) were calculated and compared on linear curves. For the correlation coefficient, the acceptance criterion was 0.99. Acceptance criteria for reproducibility and precision were set to 35%, based on the average of each point on the calibration curve, calculated using the following formula: (standard deviation/mean) × 100.
- Limit of detection. The following formulas were used to calculate the limit of detection (LOD) with the average of the values of the analysis solution without the standard ATP (blank, LOB), the standard deviation (SD) of the blank, and the standard deviation of the sample with the lowest concentration of the linearity curve, according to the following formulas:
- LOB = averageblank + 1.645(SDblank)
- LOD = LOB + 1.645(SDlow concentration sample)
- Limit of quantification. This parameter was derived from a curve containing successive dilutions of standard ATP, with the lowest safely quantifiable value serving as both the quantification limit and the lowest point of the linearity curve.
- Interday and intraday precision. The method was reproduced over the same day (intraday) and over three different days for this parameter (interday) with the cultures in the dilutions and swabs, at the highest, medium, and lowest concentrations. The acceptance criterion for the final precision was up to 35%.
- Accuracy. This criterion was evaluated by comparing data obtained by a standard analyte (ATP) to data obtained from ATP extracted from a standardized culture (using or not using the swab) to determine how close they are. The comparison was analyzed using Student’s t-test, with a recovery criterion of 70–100% allowed.
- Matrix effect. The interference of the response on the sample matrix was evaluated at the lower and upper concentrations of the ATP linearity curve. For dilution, the culture was used without extracting the ATP from the cells but adding standard ATP to verify if the result would be close to the ATP standard curve. For the swab, 110 μL of sterile water was used as a diluent in the swab with 10 μL of standard ATP. The accuracy criterion was established by Student’s t-test between the standard ATP and the ATP evaluated under the described conditions, which had to be between 70 and 100%.
- Stability. This criterion was evaluated over time at the highest, medium, and lowest concentrations of the linearity curve. The stock analysis solution stability was tested with and without the presence of luciferin, under storage conditions of −20 °C (freezer) and at 5 °C (refrigerator) after 15 days and 1, 3, and 6 months of storage, comparing with an analysis performed at time zero (fresh solution) using Student’s t-test. The maximum deviation of 30% of the fresh stock was used as the acceptance criterion.
2.4. Correlation Between CFUs and Bioluminescence
Following method validation, it was necessary to correlate the luminescence intensity results with real values used as a standard evaluation, expressed in colony-forming units (CFUs), which is used as the standard unit in current regulations to define the limits of acceptable microorganisms in food. To estimate the number of CFUs, tests were performed in triplicate and at three different optical densities (OD600) in five dilutions of E. coli Xl1-Blue culture in phosphate buffer, pH 8.0 (1000, 100, 10, 1 and 0.1 μL/mL). After adjusting the dilution factor and background definition, a graph was created using the averages of the three optical densities, obtained in CFUs/mL and cps. In subsequent measurements, the equation of the line generated by the graph of the correlation between colony count (CFUs/mL) and luminescence intensity (cps) was used to estimate the number of CFUs/mL based on the luminescence intensity results.
The same correlation was obtained with a commercial swab, which is very similar to our swab consisting of a plastic handle with a cotton tip [11,12], aiming to compare the final results in foods and to compare the benefits and drawbacks of using a standard commercial luciferase and our proprietary luciferase. The maximum limit considered as intermediate quality was 1 × 106 CFUs/g. This is a parameter of mesophilic aerobes/gram used to verify food quality in accordance with current regulations (Brazil, 2022). The Normative Instruction that provides for the conservation, transport, and reception of raw milk in establishments requires a maximum limit for the standard count in plates of up to 9 × 105 CFUs/mL before its processing into pasteurized milk.
2.5. Food Analysis
Following the development of the method using a transformed E. coli cell culture, the same method was applied in real samples to ensure that the established parameters were maintained. Meats such as raw beef and raw chicken, as well as dairy products such as type A whole milk (high quality), were chosen for this purpose.
- Food matrix effect. The interference of the food matrix in ATP quantification was evaluated by adding a known concentration of ATP standard solution on the food surface or solution—120 μL in solid food and the ATP dilution in milk. Instead of the ATP extraction from the microorganisms present in the food, the lower and upper concentrations of the linearity curve (5.00 × 10−3 and 5.00 × 10−7 mM) were used for this purpose. Student’s t-test was used to compare the standard ATP to the ATP evaluated under the described conditions, which should be between 70 and 100%.
- Application of the method to food. The food samples were purchased on the same day as the experiment to ensure freshness and consistency, stored at room temperature, and handled aseptically to avoid external contamination prior to testing. For solid food, commercial and standard swabs were rubbed onto a 1 cm2-sized quadrant of the surface. For liquid food, the swab was dipped into a 1 mL sample for the analysis. The test was conducted on three different days. To ensure compliance with current legislation, the averages, standard deviations, and precision were calculated for each day and for the three days using the respective equations of the straight lines obtained in the correlation graphs between luminescence intensity and CFUs/mL described in the section entitled “Correlation between CFUs and bioluminescence”.
- Forced degradation study. To evaluate whether the method could be applied in cases of contaminated food or those with microbial levels exceeding the legal limits specified by law, the samples underwent controlled processes to slightly accelerate their natural degradation. This approach aimed to increase contamination levels enough to determine whether it was possible to distinguish between contaminated and uncontaminated food. The food samples were incubated at 36 °C for 12 h before being analyzed and the calculation of averages, standard deviations, and precision was performed using A. vivianii luciferase. The equation of the line obtained in the correlation graph between luminescence intensity and CFUs/mL described in the section entitled “Linearity” was used to estimate the average CFUs for each cps.
3. Results
3.1. Development and Validation of a Microbiological Contamination Detection Method
We developed a method for the fast detection of ATP from microorganisms present in food using proprietary Amydetes vivianii firefly luciferase. This method aims to compare the ATP content from microorganisms in diluted and controlled E. coli Xl1-Blue culture samples with the same cultures adsorbed in swabs and with standard ATP. A schematic figure provides further understanding of our swab-based methodology in Figure 1.
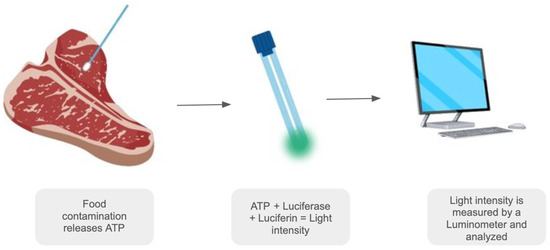
Figure 1.
Schematic figure representing the process of the swab-based bioluminescence detection method. Image created with Canva.com under Canva’s free license.
3.2. Linearity
The average, standard deviation, and precision are shown in Tables S1 and S2. The line graphs generated for five dilutes with extracted ATP obtained over the three study days are shown in Table S3, and the linearity criteria were met for both modes, with luminescence intensity being directly proportional to the ATP concentration. The average coefficient of determination (R2) obtained over the three study days was 0.9975 for dilutions and 0.9960 for swabs (Table S4 and Figure S1, respectively). The precision, ranging from 7.6% to 26.8%, was within the acceptable range of up to 35% (Tables S1 and S2).
Additionally, the R2 values greater than 0.99 demonstrate a robust linear relationship between concentration measurements and luminescence intensity. Table S3 shows the values for standard ATP results for comparative purposes and to calculate the precision in the subsequent measurements.
A graph (Figure 2) was created using the data obtained from the standard ATP, diluted cultures, and swabs to provide a visual comparison between these methods. This graph illustrates the linearity of the calibration curves for the dilutions, swabs, and standard ATP, all of which show a strong linear correlation, as indicated by their high R2 values and supported by consistent statistical analysis. The software GraphPad Prism (Version 8.0) was used to create the graph and perform statistical analysis.
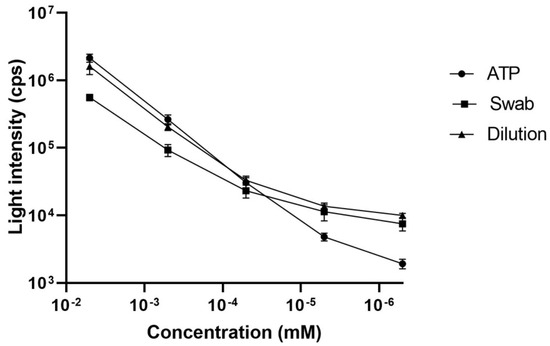
Figure 2.
Linearity curves constructed for standard ATP (ATP), ATP extracted from the diluted cultures (Dilution), and from the cultures in the swabs (Swab) at five different concentrations along with their respective luminescence intensities.
3.3. Limits of Detection and Quantification
The lowest amount of ATP that could be detected in bacterial cultures using this method was determined using the values of the solution without ATP (control), being 58.2 cps for the dilutions and 45.2 cps for the swabs (Table S5). Based on these values and the standard deviation of the sample with the lowest ATP concentration from the linearity curve, the limit of detection (LOD) was calculated and used as a background value in the next parameters’ measurements using our luminometric detection system. The limit of detection was defined as the average value of the lowest ATP concentration measured along the linearity curve (5.00 × 10−1 nM), which was 1.0 × 104 cps for the dilutions and 2.7 × 103 cps for the swabs, according to the “Linearity” parameter.
3.4. Interday and Intraday Precision
All precision acceptance criteria of three different concentrations (high, medium and low) when comparing with the standard ATP value were met for the cultures in dilutions and swabs (Tables S6–S9). In the interday assessment, the requirement was between 15.4% and 27.4%, and in the intraday assessment, it was between 4.9% and 16.5%, both within the accepted limit of 35%. The precision values indicate that the method is repeatable on the same day (intraday) or on different days (interday).
3.5. Accuracy
According to the criteria established to verify the accuracy of the extracted ATP concentrations in dilutions and swab, when compared with a standard curve of ATP established by means of Student’s t-test, all acceptance criteria were met: the dilutions and the swabs reached 72% and 71% accuracy, respectively, both within the allowed recovery criteria (between 70 and 100%).
3.6. Matrix Effect
The results for the accuracy of the matrix effect evaluation (Table S10) were 71% and 70% for the dilutions and the swabs, respectively, within the established criteria (70–100%), demonstrating that the method was not affected by endogenous components of the matrix or of the swab. Considering that the accuracy of the method calculated for the culture in dilution mode and in the swabs was 72% and 71%, respectively, the accuracy calculated by the matrix effect is very close to the values established in the culture.
3.7. Stability of the Analysis Solution
The results of the evaluation of the analysis solution stability are shown in Table S11. According to the results, the solution containing luciferin stored at 5 °C for 3 months lost activity comparing to the solution at the initial time, indicating that this solution was degraded under such conditions. Table S12 shows the Student’s t-test analysis comparing between time zero and the respective condition and time period. The acceptable accuracy value is greater than 70%, so only solutions kept at −20 °C were preserved over time.
3.8. Correlation Between CFUs and Bioluminescence
The average for the triplicate analysis of each optical density (OD600 = 1.08, 1.25 and 1.17) is shown in Tables S13 and S14 for results in CFUs/mL and luminescence intensity (cps), respectively. A graph was created (Figure 3) using the average of the three optical densities used, with the results in CFUs/mL and in cps after adjusting the dilution factor and including the background as a point of intersection in the axis. The generated equation (y = 5.1952x + 2726) was used in subsequent food analysis in order to determine the CFUs/mL result based on the corresponding luminescence intensity (cps), therefore using this established correlation.
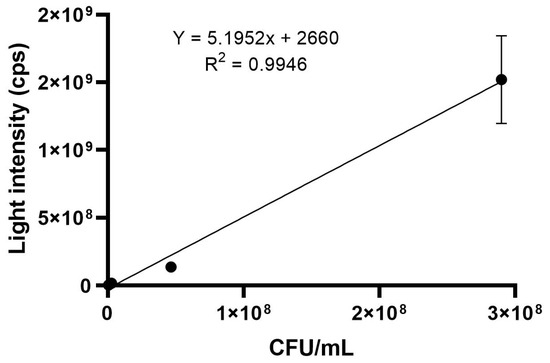
Figure 3.
Correlation graph between luminescence intensity (cps) and bacterial count (CFUs/mL) for the average of three different optical densities (OD600 = 1.08; 1.25 and 1.17).
The same assay was performed with a commercial swab, allowing us to compare the results of food analysis. The luminescence intensity (cps) average of the optical densities (OD600 = 1.08, 1.25 and 1.17) is shown in Table S15. The same adjustments were performed, and a graph (Figure 4) was also created with the averages of optical density, obtained in CFUs/mL and in cps (equation of line: y = 0.4231x + 2726) for further correlation analysis.
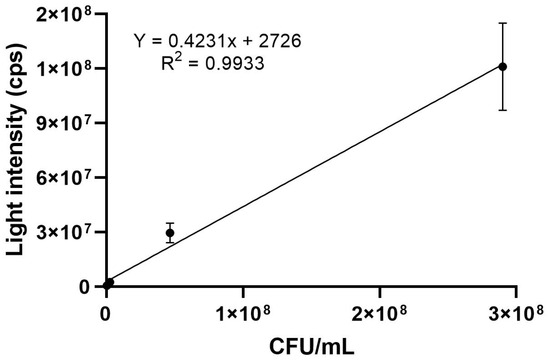
Figure 4.
Correlation graph between luminescence intensity (cps) and bacterial count (CFUs/mL) for the average of three different optical densities (OD600 = 1.08; 1.25 and 1.17) using a commercial swab (3M).
3.9. Food Analysis
The same method used for cell cultures was also used in foods to verify its repeatability and final reproducibility. For this purpose, meat, chicken, and milk were tested with Amydetes vivianii firefly luciferase stock solution and a commercial swab. The results highlight the superior sensitivity of the Amydetes luciferase over a commercially available luciferase, with a ~22-fold increase in luminescence intensity when comparing the highest ATP concentration (5.00 µM), which may allow detection using less sensitive equipment, such as CCD-based and photodiode-based field detectors. These results will be discussed in the following sections.
3.10. Food Matrix Effect
The accuracy was 73% for meat, 80% for chicken, and 70% for milk, all within the established criteria (70–100%), demonstrating that the method was not affected by components of the matrix of the food or by the swab (Table S16). Considering that the matrix effect of the method calculated for the culture in dilution mode and in the swab were 71% and 70%, respectively, the matrix effect in the food is very close to the values found previously.
3.11. Method Application in Food
The results obtained using Amydetes firefly luciferase and the commercial swab are shown in Table 1. The results in CFUs/g and CFUs/mL were obtained by solving the line equations of the correlation graphs between cps and CFUs/mL in Figure 3 and Figure 4, allowing us to compare the results to the limits set by regulatory agencies.

Table 1.
Results of the quantification of ATP in triplicate in cps and in CFUs/g and CFUs/mL of three different food samples on three different days using the Amydetes vivianii luciferase assay and a commercial swab (3M).
Therefore, based on the limits established by Brazilian regulatory agencies (IN 60/2019), meat and chicken have acceptable limits of 3.00 × 106 CFUs/g and milk has acceptable limits of 3.00 × 105 CFUs/mL. The results using homemade Amydetes vivianii remained within the established limits, whereas the commercial swab results were outside the allowed limits. The difference between the results is most likely due to the higher efficiency and signal of the A. vivianii luciferase, which reach higher detection limits in the correlation curve of the graph in Figure 3, allowing the results to follow the trend line. In our conditions, the commercial swab had a lower acceptance limit, as shown in Figure 4 and in Figure S2, when comparing with the A. vivianii luciferase in the linearity curves using or not using the swab. The slope coefficient in the equation y = zx + 2726 reflects the sensitivity of a luciferase: the higher the slope coefficient, the more sensitive the luciferase, resulting in higher-intensity cps readings for lower bacterial concentrations (CFUs/mL). This high sensitivity was also tested at saturation with ATP, where the luciferase of A. vivianii displayed higher brightness in the luminometer, 1.16 × 108, and 9.65 × 106 for the commercial swab. With a higher slope coefficient, for the same intensity (cps) reading, the bacterial concentration is lower. This suggests that a luciferase with a higher slope coefficient can detect bacteria at lower CFUs/g concentrations, making it more sensitive. Therefore, if a luciferase has a higher slope coefficient, it generates a more intense light response for the same bacterial concentration, or the same light response for a lower bacterial concentration. This means that fewer bacteria are needed to produce a detectable amount of light, indicating a more efficient or sensitive luciferase. The opposite happens with less sensitive luciferases, requiring higher bacterial concentrations to generate a significant cps reading. Thus, a more sensitive luciferase may have a broader range, allowing it to measure a wider range of bacterial concentrations before saturating.
3.12. Forced Degradation Study
The results allowed for the quantification and correlation of ATP according to the regulatory CFUs/g limits, indicating whether the food was contaminated or not (Table 2). The calculation was performed based on the equation of the correlation line between cps and CFUs/mL of Figure 3 using 1 g of each food. Therefore, according to the results and the possibility to detect contamination, A. vivianii firefly luciferase is suitable for use in ATP quantification in foods because it has higher efficiency, giving more intense signals in cps than the commercial swab luciferase, allowing us to detect contaminations above the allowed limits.

Table 2.
Results of the quantification of ATP in luminescence intensity (cps) and the corresponding value in CFUs/g and CFUs/mL of forced degradation in three different foods.
3.13. Contextualization on the Use of Amydetes Luciferase for Bioluminescence Assays in Food Quality Control and Differentials in Its Use
From the presented results, it is possible to differentiate suitable and unsuitable food samples for consumption due to a high level of microbiological contamination. It is also possible to enable studies using luciferase from the Amydetes vivianii firefly in the quantification of ATP from microorganisms in food surfaces. These studies do not exclude the need for other tests to validate the method to determine the possibility of consuming food of animal origin but provide a fast, sensitive screening method.
The use of the Amydetes vivianii firefly luciferase is this technique’s main advantage, as it allows the detection of ATP with a wider range of luminescence intensities, with more intense luminescence intensities than some luciferases in commercially available kits. For this reason, elevated levels of ATP above the limits of acceptable microorganisms in food can be detected. When the same assay is performed using a commercially available luciferase kit, the lower limit and, as a result, the lower range of luminescence intensities do not allow the extrapolation of ATP detection levels, making it impossible to distinguish between contaminated food and viable food for consumption, according to the limits of government agencies. Furthermore, this method, due to the high efficiency and brightness of this new luciferase, allows, in principle, the use of slightly less sensitive detecting equipment.
Many studies have shown the low quality of commercialized meat [13,14]. Therefore, due to the need for faster and easier quality control methods, rapid screening methods for estimating microbial counts in foods are being published. Among the rapid methods in microbiology, there are techniques that use microscopic counting, immunoautomation, RT-qPCR, and biochemical techniques such as impedometric detection or bioluminescence [5]. In addition to these, RMMs (rapid microbiological methods) are growth-based technologies measuring biochemical or physiological parameters that reflect the growth of microorganisms, such as those based on ATP. RMMs can be useful when applied to quality control tests, preservative effectiveness, disinfectant or sanitizer validation studies, antibiotic potency determination tests, and the validation of microbiological cleaning efficiency for machines, equipment, or specific areas, promoting faster and more reliable and efficient results in high-volume microbiological tests [15,16,17].
The bioluminescence technique is one of the faster techniques, with fewer preparation steps, but it lacks specificity because it does not distinguish between the type and species of contaminant in microbial sources of ATP, which can be microbial or somatic. In many cases, this can be solved by pre-treating the sample depending on the characteristics of the analyzed microorganism suspension [18]. These steps lengthen the assay and reduce its sensitivity [19].
Sample pre-processing is not critical to the process, and many authors have overlooked it [20]. Oto et al., 2013, investigated the ATP content on the surface of pork meat in real time for sanitation monitoring, demonstrating a good coefficient of determination with plate counts [21]. Oshita et al., 2011, used pork loin samples in another study, in which the amount of ATP and the plaque count were found to have a linear correlation with a coefficient of determination of 0.95 [22].
With somatic and microbial cell counts, ATP bioluminescent methods have already been used to monitor raw cow milk quality [23]. Cunha et al., 2014, compared the results of 102 samples of whole milk using traditional culture methods with ATP–bioluminescence results obtained using the 3M equipment “TM Microbial Luminescence System II” (MLS-3M Microbiology Products, St. Paul, MN, USA), and significant correlations between the counts of aerobic mesophilic microorganisms were obtained [24]. Studies show that bioluminescence methods can detect up to 104 CFUs/mL of bacteria in milk [25].
At low ATP concentrations, the presence of non-microbial or extracellular ATP generates a background signal and an overestimation of contamination. To accurately determine bacterial levels, the ATP noise should first be removed from the sample by pre-treating it. Hence, ATP bioluminescence measurements could be used to count the total number of microorganisms present in a sample if the concentration is high enough (>104 CFUs/g) [26,27,28]. Given that our research emphasized higher levels of microorganism detection (up to 106 CFUs/mL), we believe that sample pre-processing is unnecessary, except for milk, which has lower ATP levels, and therefore this method of validation, at the present stage, is not suitable for milk contamination detection. To adapt the ATP bioluminescence detection system for milk samples, modifications such as sample pre-processing to concentrate ATP, optimizing assay sensitivity, and mitigating matrix effects from milk components would be necessary, enabling the use of various luciferases.
4. Conclusions
The bioluminescence-based ATP assay method for food microbiological contamination using the brighter Amydetes vivianii firefly luciferase allowed for the comparison of standard ATP, ATP extracted from diluted cultures of E. coli Xl1-Blue, and cultures diluted in swabs following established parameters. The method employing the Amydetes vivianii firefly luciferase was applied to food and compared with a commercially available swab test for fast microbiological evaluation via ATP detection in meat and chicken. Our developed luciferase showed a remarkable ~22-fold increase in luminescence intensity when comparing the highest ATP concentration and a ~12-fold increase in the most concentrated optical dilution in relation to the commercially available test. However, this type of analysis is not suitable for milk yet, which has lower ATP levels, because sample pre-processing is required. Furthermore, the results complied with the limits set by ANVISA and MAPA (Brazilian Regulatory Agencies-Agência Nacional de Vigilância Sanitária and Ministério da Agricultura e Pecuária, respectively), in which the acceptable limits for meat and chicken are 3.00 × 106 CFUs/g, while milk has an acceptable limit of 3.00 × 105 CFUs/mL.
The use of the A. vivianii firefly luciferase proved to be suitable to differentiate between contaminated and non-contaminated meat and poultry. The higher luminescence signal allows microbiological detection in a wide range of scales with sensitivity, using less sensitive and therefore cheaper detecting equipment. The proposed method yielded results in less than an hour, significantly faster than typical quality control assays. Although further analyses are required before commercialization, the results indicate that the use of this luciferase is an improvement for rapid bioluminescence-based quality control screening assays in the food industry, with positive results being confirmed by conventional quality control methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13020027/s1, Table S1. Luminescence intensity values (cps) for each ATP concentration tested over three days for E. coli Xl1-Blue diluted cultures; Table S2. Luminescence intensity values (cps) for each concentration tested over three days for E. coli Xl1-Blue diluted cultures using swabs; Table S3. Luminescence intensity values (cps) for each ATP concentration tested over three days; Table S4. Values from the equation of the lines over three days of linearity evaluation; Figure S1. Linearity graphs of the ATP extracted (A) from the diluted culture, and (B) from the culture in swab of five concentrations and their respective results in cps; Table S5. Luminescence intensity values (cps) for solutions without ATP; Table S6. Luminescence intensity results (cps) in the interday evaluation of the E. coli Xl1-Blue diluted culture; Table S7. Luminescence intensity results (cps) in the interday evaluation of the E. coli Xl1-Blue diluted culture in swab; Table S8. Luminescence intensity results (cps) in the intraday evaluation of the E. coli Xl1-Blue diluted culture; Table S9. Luminescence intensity results (cps) in the intraday evaluation of the E. coli Xl1-Blue diluted culture in swab; Table S10. Luminescence intensity results (cps) of the matrix effect evaluation in two standard ATP concentrations (5.00 × 10−3 and 5.00 × 10−7 mM); Table S11. Luminescence intensity of four conditions under which the solution was evaluated in for stability over four different time periods; Table S12. Analysis of the assay solution stability at different storage conditions and time periods by comparison using test-t of Student’s with time zero; Table S13. Average results of triplicate analysis in CFU/mL at three different optical densities of five different dilutions; Table S14. Average results of triplicate analysis in luminescence intensity (cps) using A. vivianii assay at three different optical densities of five different dilutions; Table S15. Average results in luminescence intensity (cps) using a commercially available swab (3M) at three different optical densities of five different dilutions; Table S16. Luminescence intensities (cps) of food matrix effect evaluation at two standard ATP concentrations (5.00 × 10−3 and 5.00 × 10−7 mM) and Figure S2. Linearity curves constructed for standard ATP (ATP), ATP extracted from the cultures in the swab (Swab) and the commercial swab (3M) at five different concentrations along with their respective luminescence intensities.
Author Contributions
Conceptualization, V.R.V., A.F.S. and D.R.d.S.; Methodology, A.F.S. and D.R.d.S.; Validation, A.F.S.; Formal Analysis, A.F.S. and V.R.V.; Investigation, A.F.S. and V.R.V.; Resources, V.R.V.; Data Curation, A.F.S. and V.R.V.; Writing—Original Draft Preparation, Review and Editing, A.F.S. and V.R.V.; Supervision, V.R.V.; Project Administration, V.R.V.; Funding Acquisition, V.R.V. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to FAPESP for the grants n. 2010/05426-8 and 2022/04800-1 provided to VRV, the fellowship n. 2021/03901-5 provided to AFS, the fellowship n. 2018/17855-2 provided to DRdS, and the Multiusuary Equipment EMU 2017/22262-8 and CNPq 405060/2021-1 provided to VRV.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript, and additional information can be obtained upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest such as affiliations with or involvement in any organization or entity with any financial interest.
References
- Brodl, E.; Winkler, A.; Macheroux, P. Molecular mechanisms of bacterial bioluminescence. Comput. Struct. Biotechnol. J. 2018, 16, 551. [Google Scholar] [CrossRef] [PubMed]
- Vacher, M.; Fdez Galván, I.; Ding, B.W.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Lindh, R. Chemi-and bioluminescence of cyclic peroxides. Chem. Rev. 2018, 118, 6927. [Google Scholar] [CrossRef]
- Campbell, A.K. Chemiluminescence: Principles and Applications in Biology and Medicine, 1st ed.; VCH/Ellis Horwood: Hertfordshire, UK, 1988; ISBN 978-0895735010. [Google Scholar]
- Viviani, V.R.; Ohmiya, Y. Photoproteins in Bioanalysis; Wiley: Hoboken, NJ, USA, 2006; p. 49. [Google Scholar]
- Roesti, D.; Wilkens, E. Implementation of a rapid methods portfolio at a pharmaceutical manufacturing site. Rapid Micro Methods 2015, 20, 52. [Google Scholar]
- Henriques, J.; Cardoso, C.; Vitorino, C.; Rapid microbiological methods. They are rapid! Are they fast? Res. Trends Microbiol. 2019, 1–8. Available online: https://meddocsonline.org/ebooks/ebook-microbiology/rapid-microbiological-methods-they-are-rapid-are-they-fast.pdf (accessed on 7 January 2025).
- Forsythe, S.J. Microbiologia da Segurança dos Alimentos, 2nd ed.; Artmed Editora: Porto Alegre, Brazil, 2013; ISBN 978-8536327051. [Google Scholar]
- Ellis, O.; Godwin, H.; David, M.; Morse, D.J.; Humphries, R.; Uslan, D.Z. How to better monitor and clean irregular surfaces in operating rooms: Insights gained by using both ATP luminescence and RODAC assays. Am. J. Infect. Control 2018, 46, 906–912. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia. Alternative methods for control of microbiological quality. In Phareuropa, 6th, ed.; European Pharmacopoeia: Strasbourg, France, 2007. [Google Scholar]
- United States Pharmacopeia. USP 40, Validation of Alternative Microbiological Methods. In The National Formulary; NF 35; The United States Pharmacopeial Convention: Rockville, MD, USA, 2017. [Google Scholar]
- Rigotti, M.A.; Ferreira, A.M.; Nogueira, M.C.L.; de Almeida, M.T.G.; Guerra, O.G.; de Andrade, D. Evaluation of three surface friction techniques for the removal of organic matter. Texto Contexto-Enferm. 2015, 24, 1061–1070. [Google Scholar] [CrossRef][Green Version]
- Ali, S.; Moore, G.; Wilson, A.P.R. Effect of surface coating and finish upon the cleanability of bed rails and the spread of Staphylococcus aureus. J. Hosp. Infect. 2012, 80, 192–198. [Google Scholar] [CrossRef]
- Vidal Junior, P.O.; Menezes, A.C.R.; de Souza, L.M.P.; Guimarães, A.G.; de Cassia Vieira Cardoso, R. Trade and safety issues of raw beef from the countryside of Bahia state, Brazil. J. Public Health Res. 2020, 9, 1752. [Google Scholar] [CrossRef]
- Camargo, A.C.; Cossi, M.V.C.; Silva, W.P.D.; Bersot, L.D.S.; Landgraf, M.; Baranyi, J.; Luís Augusto, N. Microbiological testing for the proper assessment of the hygiene status of beef carcasses. Microorganisms 2019, 7, 86. [Google Scholar] [CrossRef]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G. Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106. [Google Scholar] [CrossRef]
- Poghossian, A.; Geissler, H.; Schöning, M.J. Rapid methods and sensors for milk quality monitoring and spoilage detection. Biosens. Bioelectron. 2019, 140, 111272. [Google Scholar] [CrossRef]
- Ziyaina, M.; Rasco, B.; Sablani, S.S. Rapid methods of microbial detection in dairy products. Food Control. 2020, 110, 107008. [Google Scholar] [CrossRef]
- Lomakina, G.Y.; Modestova, Y.A.; Ugarova, N.N. Bioluminescence assay for cell viability. Bbiochem 2015, 80, 701. [Google Scholar] [CrossRef]
- Bottari, B.; Santarelli, M.; Neviani, E. Determination of microbial load for different beverages and foodstuff by assessment of intracellular ATP. Trends Food Sci. Technol. 2015, 44, 36. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.; Tian, Q.; Yue, W.; Zeng, J.; Chen, G.; Cai, X. Disposable bioluminescence-based biosensor for detection of bacterial count in food. Anal. Biochem. 2009, 394, 1. [Google Scholar] [CrossRef] [PubMed]
- Ot, N.; Oshita, S.; Makino, Y.; Kawagoe, Y.; Sugiyama, J.; Yoshimura, M. Non-destructive evaluation of ATP content and plate count on pork meat surface by fluorescence spectroscopy. Meat Sci. 2013, 93, 579. [Google Scholar] [CrossRef] [PubMed]
- Oshita, S.; Al-Haq, M.I.; Kawagishi, S.; Makino, Y.; Kawagoe, Y.; Ye, X.; Shinozaki, S.; Hiruma, N. Monitoring of ATP and viable cells on meat surface by UV–Vis reflectance spectrum analysis. J. Food Eng. 2011, 107, 262. [Google Scholar] [CrossRef]
- Frundzhyan, V.G.; Parkhomenko, I.M.; Brovko, L.Y.; Ugarova, N.N. Improved bioluminescent assay of somatic cell counts in raw milk. J. Dairy Res. 2008, 75, 279. [Google Scholar] [CrossRef]
- Cunha, A.F.; Lage, A.D.; Pereira e Araújo, M.M.; Abreu, C.F.; Tassinari, A.R.; Ferraz, M.A.; Davenport, K.; Cerqueira, M.M.O.P. ATP-Bioluminescence as a method to evaluated microbiological quality of UHT milk. Arq. Bras. Med. Vet. Zootec. 2014, 66, 1909. [Google Scholar] [CrossRef][Green Version]
- Dostalek, P.; Brányik, T. Czech J. Prospects for rapid bioluminescent detection methods in the food industry–a review. Food Sci. 2005, 23, 85. [Google Scholar]
- Lopez-Campos, G.; Martinez-Suarez, J.V.; Aguado-Urda, M.; Lopez-Alonso, V. Detection, Identification, and Analysis of Foodborne Pathogens. In SpringerBriefs in Food, Health, and Nutrition; Springer: New York, NY, USA, 2012; pp. 13–32. [Google Scholar]
- Samkutty, P.J.; Gough, R.H.; Adkinson, R.W.; Mcgrew, P. Rapid assessment of the bacteriological quality of raw milk using ATP bioluminescence. J. Food Prot. 2011, 64, 208. [Google Scholar] [CrossRef]
- Jasson, V.; Jacxsens, L.; Luning, P.; Rajkovic, A.; Uyttendaele, M. Alternative microbial methods: An overview and selection criteria. Food Microbiol. 2010, 27, 710. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).