Abstract
Mercury’s severe toxicity and persistence demand fast, low-cost, and sustainable detection. In this work, a Juglans regia ethanolic extract is introduced as an efficient biogenic reducing and stabilizing agent for the green synthesis of silver nanoparticles (AgNPs). This plant-mediated route enables environmentally friendly nanoparticle formation with suitable optical properties for sensing applications. To overcome the poor visual selectivity observed in the colloidal AgNPs suspension, the nanoparticles were immobilized onto filter paper to produce a solid-phase colorimetric sensor. The paper-based platform exhibited a highly selective response toward Hg2+, showing complete suppression of the yellow coloration exclusively in the presence of Hg2+, even when challenged with a 200-fold excess of potentially interfering ions. Quantitative colorimetric analysis revealed a broad linear detection range from 1 × 10−8 to 1 × 10−3 mol dm−3 and an excellent limit of detection of 1.065 × 10−8 mol dm−3, with visible color changes consistent with the calculated values. The sensor’s performance was further validated using real tap water samples, with recovery values ranging from 96% to 102%, confirming minimal matrix interference and reliable quantification. Altogether, this study demonstrates that Juglans regia-mediated AgNPs, integrated into a simple paper-based format, provide a fully green, low-cost, and portable platform for sensitive and selective on-site detection of Hg2+ in environmental waters.
1. Introduction
Mercury contamination poses a serious environmental and public health concern due to its extreme toxicity, environmental persistence, and bioaccumulation in aquatic ecosystems [1]. Once released into water bodies through industrial discharge, mining activities, or improper waste disposal, mercury can be transformed by microorganisms into methylmercury, a highly toxic and bioavailable form [2,3]. Exposure to mercury, even at trace levels, can lead to severe neurological, renal, and developmental disorders, making its detection in water sources a global priority [4,5].
Accurate detection of mercury in environmental samples is traditionally performed using sophisticated analytical techniques such as atomic absorption spectroscopy (AAS) [6], inductively coupled plasma mass spectrometry (ICP-MS) [7], or cold vapor atomic fluorescence spectroscopy (CV-AFS) [8]. While these techniques provide excellent sensitivity and selectivity, they are limited by high operational costs, the need for skilled personnel, and laboratory-bound instrumentation, making them unsuitable for routine on-site monitoring, especially in low-resource settings [9]. To address these limitations, colorimetric sensors based on silver nanoparticles (AgNPs) have received increasing attention as a simple, cost-effective, and portable alternative for mercury detection [10,11]. AgNPs exhibit localized surface plasmon resonance (LSPR), a phenomenon that results in intense color depending on the particle size, shape, and interparticle distance [12]. The addition of Hg2+ to a colloidal AgNP solution induces visible color changes due to the oxidative dissolution of Ag0 or nanoparticle aggregation, providing a straightforward, instrument-free means of detection [13,14]. This colorimetric response is highly advantageous for field applications, where quick decision-making is required.
Among the noble metal nanoparticles, AgNPs are particularly suitable for mercury detection because of the strong redox interaction between Hg2+ and Ag0. The standard electrode potential of Hg2+/Hg0 (0.85 V) is higher than that of Ag+/Ag0 (0.80 V), enabling a spontaneous redox reaction that results in the formation of an amalgam or dissolution of silver into the solution [15]. These interactions disrupt the LSPR of AgNPs and cause a noticeable color change from yellow-brown to colorless or gray, which correlates with Hg2+ concentration [16].
To enhance the practicality of AgNP-based sensing systems, immobilizing AgNPs on solid substrates, such as cellulose filter paper, has emerged as a promising strategy [17]. Cellulose-based substrates offer several advantages: they are renewable, inexpensive, biodegradable, and possess a high surface area and porous structure that promotes efficient analyte–nanoparticle interaction [18]. Furthermore, filter paper enables capillary-driven fluid transport, eliminating the need for external pumps or power sources [18]. Immobilized sensors also exhibit improved nanoparticle stability, reduced aggregation, and allow for storage and portability, making them highly suitable for real-world environmental monitoring [19].
Several studies have demonstrated that AgNPs can be successfully immobilized on paper substrates to create paper-based sensors for heavy metal detection [19,20]. These platforms typically consist of AgNPs deposited on paper strips that change color upon exposure to mercury-containing water [21]. For example, a paper-based sensor fabricated by immobilizing thiamine-functionalized AgNPs onto Whatman filter paper enabled visual colorimetric detection of Hg2+ with a limit of detection (LOD) of 5 × 10−5 mol dm−3. Although no limit of quantification (LOQ) value was reported, the sensing mechanism relied solely on a distinct color change from yellowish to white, driven by plasmonic quenching of AgNPs in the presence of Hg2+, allowing detection with the naked eye [22]. Chitosan-stabilized silver nanoparticles (AgNPs-Ch) prepared via chemical reduction have likewise been reported as selective colorimetric probes for Hg2+ in aqueous media, enabling detection down to 1.23 × 10−5 mol dm−3 through Hg2+-induced modulation of the AgNP localized surface plasmon resonance, observable both visually and spectroscopically within the linear range of 9.27 × 10−6–4.64 × 10−5 mol dm−3 [23]. Moreover, a smartphone-assisted colorimetric paper strip based on chitosan-stabilized AgNPs demonstrated an LOD of 7.6 × 10−5 mol dm−3, in which Hg2+ exposure triggered AgNP aggregation, producing a quantifiable color shift detectable by UV–Vis spectroscopy or smartphone imaging [24]. Andrographolide-stabilized AgNPs (andro-AgNPs) have also been used as a colorimetric Hg2+ probe, showing a linear response between 1.5 × 10−5–1.2 × 10−4 mol dm−3 with an LOD of 1.12 × 10−5 mol dm−3 and an LOQ of 3.72 × 10−5 mol dm−3. The sensing response arises from Hg2+-induced changes in the AgNP surface plasmon resonance [25]. However, many of these systems rely on chemically synthesized AgNPs, which involve toxic reducing agents such as sodium borohydride or hydrazine, raising concerns about environmental safety and sustainability.
To overcome this challenge, green synthesis methods employing plant extracts have been explored as an eco-friendly alternative for AgNP production [26]. Plant-based synthesis utilizes phytochemicals such as polyphenols, flavonoids, terpenoids, and alkaloids, which function both as reducing and capping agents [27]. Among various plant sources, Juglans regia (common walnut) has emerged as a particularly effective candidate due to its high levels of antioxidant compounds, especially phenolics and flavonoids, which facilitate rapid, stable reduction of Ag+ to Ag0 [28]. The use of Juglans regia extract offers a sustainable route to nanoparticle synthesis without the need for harsh chemicals or elevated temperatures, aligning with the principles of green chemistry [28,29].
In this work, we report the eco-friendly synthesis of AgNPs using Juglans regia extract and their subsequent immobilization onto cellulose filter paper to develop a portable, colorimetric sensor for Hg2+ detection in water. The sensor exhibits a clear, quantifiable color change upon interaction with Hg2+ ions, enabling rapid visual detection without specialized equipment. The integration of green synthesis, paper-based immobilization, and colorimetric sensing into a single platform represents a significant step forward in developing sustainable, accessible tools for environmental monitoring.
The novelty of this study lies in the combination of environmentally benign synthesis of AgNPs with a naturally derived reductant, the stable and homogeneous immobilization of the nanoparticles on cellulose support, and the demonstration of the sensor’s applicability for the detection of mercury in aqueous environments. Compared to previous studies, our approach enhances the eco-compatibility, practicality, and field-deployability of heavy metal sensors, opening new possibilities for decentralized water quality assessment.
2. Materials and Methods
2.1. Materials and Reagents
Whole green walnuts were used as the plant material for the synthesis of AgNPs. Absolute ethanol was employed as the extraction solvent. Salts AgNO3, Pb(NO3)2, CdSO4×8/3H2O, NiCl2×6H2O, HgCl2, AlCl3×6H2O, CaCl2, LiCl, KCl, NaCl, Na2SO4, Na2CO3, Na3PO4, MgCl2×6H2O, CoCl2×6H2O, ZnCl2, CuSO4×6H2O, MnSO4×6H2O, FeCl3, CrCl3×6H2O, which are used as sources of cations, were purchased from Sigma-Aldrich (Pestanal, Sigma Aldrich, Søborg, Denmark). All chemicals used in this work were of analytical grade and used as received without further purification. Deionized water from a water purification Milli-Q integral water system was used throughout the experiments.
2.2. Preparation of Juglans Regia Extract
In this study, 40 whole green walnuts (~200 g) were immersed in 1 L of ethanol and stored for 6 weeks in a dark place. After the extraction period, the liquid phase was separated from the solid residues by filtration.
2.3. Synthesis of AgNPs Using Juglans Regia Extract
AgNPs were synthesized using a green method based on the ethanolic extract of Juglans regia as a reducing and stabilizing agent. A total of 89 mL of a 150-fold water-diluted ethanolic extract of Juglans regia was mixed with 1 mL of 1 mol dm−3 KOH solution and 10 mL of 1 × 10−3 mol dm−3 AgNO3 solution. The reaction mixture was heated on a hot plate set to 200 °C for 15 min. After cooling, the resulting colloidal suspension was centrifuged twice at 6000 rpm to separate the nanoparticles. The successful synthesis and detailed physicochemical properties of the AgNPs were confirmed through a multi-technique characterization approach, providing insight into their optical behavior, chemical environment, size distribution, crystalline structure, and colloidal stability. The precipitated AgNPs were collected, transferred to Falcon tubes, and stored at 4 °C in a refrigerator until further use.
2.4. Physicochemical Characterization
UV–Vis spectrophotometry (Perkin Elmer Lambda 35, Traiskirchen, Austria) was used to record absorption spectra and to validate the successful synthesis of AgNPs by detecting the characteristic SPR peak. UV–Vis spectra were recorded in the range 300–700 nm.
Concentrations of Ag in colloidal suspension that exhibited an optical density of approximately 1 were quantified using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) with a Thermo Scientific iCAP 7000 Series instrument (Waltham, MA, USA).
Surface morphology and elemental composition, including elemental mapping, were examined using Field Emission Scanning Electron Microscopy coupled with Energy-Dispersive X-ray Spectroscopy (FESEM-EDX) using an FEI Scios 2 microscope (Thermo Fisher Scientific, Waltham, MA, USA). FESEM imaging and EDX spectra were acquired under high vacuum conditions at an accelerating voltage of 10 kV. FESEM-EDX analysis of AgNPs deposited on filter paper before and after treatment with Hg2+ was performed after coating with Au to increase the conductivity and at an accelerating voltage of 20 kV.
Fourier transform infrared (FTIR) spectra were recorded with a Nicolet iS20 FTIR spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Measurements were conducted over the wavenumber range of 4000 to 400 cm−1, with 64 scans per sample and a spectral resolution of 4 cm−1.
Zeta potential measurements and particle size analysis via Dynamic Light Scattering (DLS) were carried out using a Nano ZS Zetasizer system equipped with a 633 nm He-Ne laser (Malvern Instruments, Malvern, UK). These analyses provided information on the surface charge and hydrodynamic size distribution of colloidal nanoparticles.
The structural properties of the synthesized silver nanoparticles were investigated by powder X-ray diffraction (XRD) using a Rigaku Ultima IV diffractometer equipped with CuKα1,2 radiation (Rigaku Tokyo, Japan). Measurements were performed at 40 kV and 40 mA over a 2θ range of 10–80° with a step size of 0.02° and a scan speed of 5° min−1, while data acquisition was carried out via a D/Tex digital detector. Phase identification was carried out with PDXL2 software (version 2.8.3.0) using the International Centre for Diffraction Data (ICDD) database [30].
2.5. Preparation of AgNPs@FP
AgNPs were immobilized onto filter paper to form a solid-phase sensing platform (AgNPs@FP). A strip of filter paper was immersed in the colloidal AgNP solution (30 ppm) for 3 s, then dried at room temperature. This process enabled uniform adhesion of AgNPs onto the cellulose matrix, producing a yellow-colored sensing substrate.
2.6. Screening Experiments
To assess the intrinsic responsiveness of green-synthesized silver nanoparticles (AgNPs) toward different metal ions and to evaluate potential interferences in detection, a qualitative screening assay was conducted. The initial screening was performed using a colloidal solution of AgNPs containing 30 ppm silver, corresponding to an optical density of approximately 1.0 at the characteristic LSPR peak. In the screening experiment, the AgNP colloid was exposed to three concentrations (5 × 10−2, 5 × 10−4, and 5 × 10−6 mol dm−3) of six selected metal ions: Hg2+, Pb2+, Cd2+, Ni2+, Cu2+, and Al3+.
The impregnated paper was tested by dipping into aqueous solutions of selected metal ions (0.5 mL, 5 × 10−4 mol dm−3), after which the strips were again air-dried. To further probe the selectivity and rule out interference, additional experiments were performed using the same Hg2+ concentration (5 × 10−4 mol dm−3) in parallel with significantly higher concentrations (0.1 mol dm−3) of other potentially interfering ions. To investigate the impact of anions, interactions with four sodium salts were tested.
2.7. Colorimetric Quantification of Hg2+ Using AgNPs@FP
To quantitatively assess the colorimetric response of the AgNPs@FP sensor toward varying concentrations of mercury ions, uniform squares of filter paper (2.5 cm × 2.5 cm) were immersed for 3 s in the AgNPs colloidal solution and air-dried. A control sample labeled as FP was dipped in distilled water and dried under identical conditions. The test samples (AgNPs@FP) were then individually immersed in 0.5 mL of HgCl2 solutions at concentrations ranging from 1 × 10−8 to 2 × 10−3 mol dm−3. After exposure, the papers were allowed to dry for 1 h at ambient conditions.
All sensor squares were labeled and photographed with a high-resolution digital camera under uniform ambient daylight to ensure consistent illumination and minimize reflections and shadows.
The acquired image was processed using a custom pipeline developed in Python 3.11 with OpenCV. First, the image was converted from OpenCV’s native BGR color space to RGB. To enhance contrast between the sensor zones and the white background, the image was converted to grayscale, followed by adaptive mean thresholding (window size = 51, offset = 12), producing a binary image optimized for contour detection. Contours were extracted using the cv2.findContours function in external mode to isolate only the outer boundaries. To remove small noise elements, contours were filtered based on area, retaining only the 16 largest, corresponding to the expected number of test squares. Bounding rectangles for these contours were computed and sorted first by vertical (y-axis) and then by horizontal (x-axis) position to reconstruct the original spatial layout. All data processing, analysis, and plotting were performed using NumPy (version 2.3.2.) and Matplotlib (version 3.10.5.) libraries.
From each identified region of interest (ROI), the mean RGB pixel values were extracted. A custom “yellow index” was calculated using the formula:
where R, G, and B represent the mean red, green, and blue intensities, respectively. This index emphasizes yellowing resulting from red and green enhancement and blue suppression. The calculated yellow index values were plotted against the logarithm of Hg2+ concentration to evaluate the sensor’s colorimetric response.
Yellow Index=(R+G)/2 B
The standard deviation was calculated from 10 independently prepared filter papers, treated identically and immersed in the AgNPs solution for 3 s, followed by air-drying. Since the yellow index decreases with increasing analyte concentration, the LOD and LOQ signals were defined as: LOD signal = mean blank − 3 × SD and LOQ signal = mean blank − 10 × SD. The corresponding concentrations were interpolated from the calibration curve using the obtained signal values, and LOD and LOQ values were determined.
Real Sample
To verify the practical applicability and accuracy of the developed colorimetric method, a recovery study was conducted using tap water as a representative real water matrix. The water sample was used without pre-treatment to simulate realistic conditions better, thereby providing insight into the method’s performance in complex, unfiltered systems. Known amounts of Hg2+ ions were added to tap water at six different concentrations, ranging from 1 × 10−3 to 1 × 10−8 mol dm−3. Each spiked sample was analyzed in triplicate to ensure reproducibility. The concentrations of Hg2+ determined using the developed method were then compared with the spiked values, and recovery was calculated as the ratio of the measured to the theoretical concentrations.
3. Results and Discussion
3.1. Physicochemical Characterization of Synthesized AgNPs
To comprehensively understand the properties of the synthesized silver nanoparticles, a series of physicochemical characterization techniques was employed. These analyses provided complementary insights into nanoparticle formation, size, morphology, crystallinity, surface chemistry, and colloidal stability.
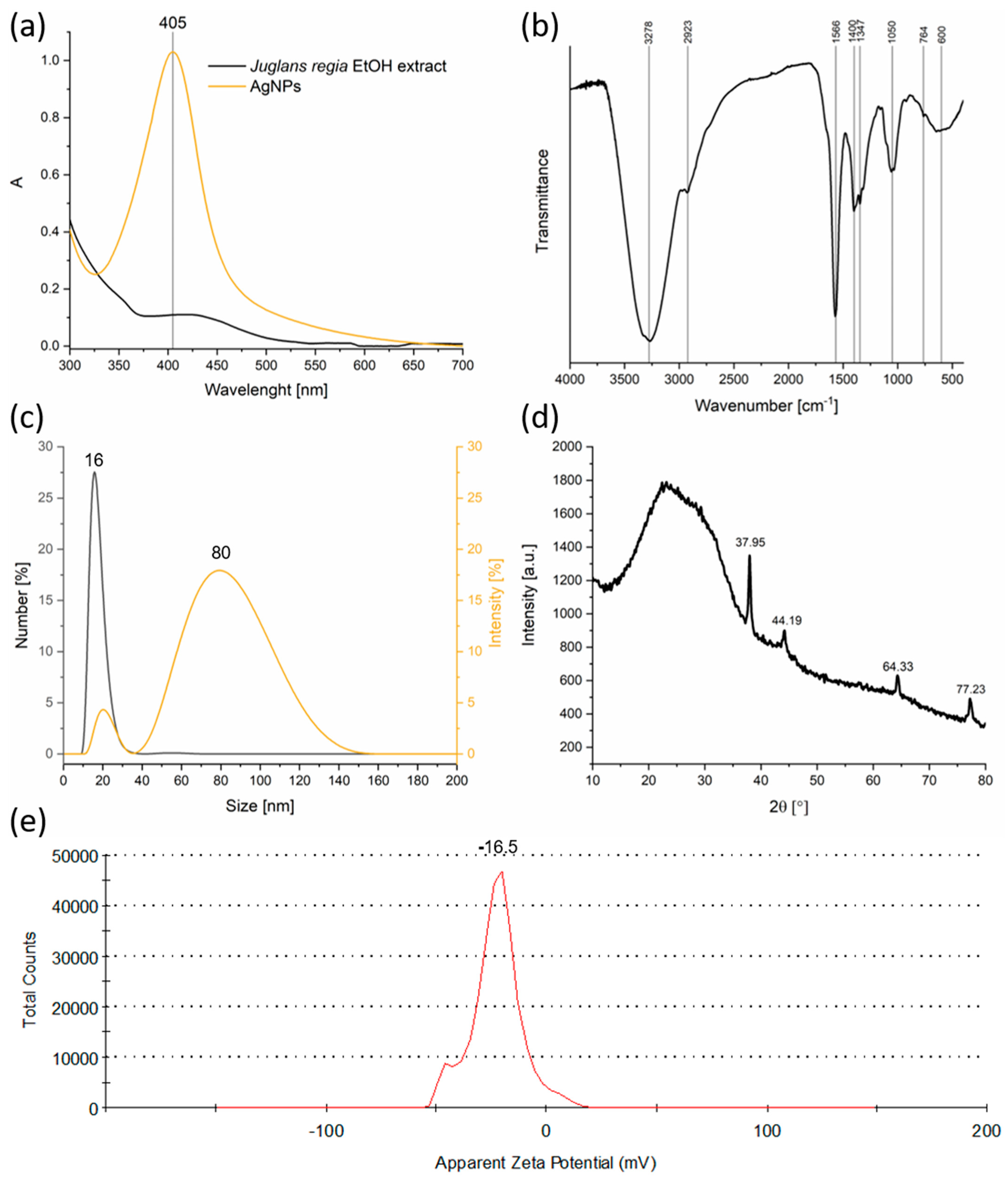
The UV–Vis spectroscopy results demonstrated a pronounced LSPR peak at 405 nm, a hallmark of spherical silver nanoparticles with nanoscale dimensions (Figure 1a). This LSPR peak confirms the reduction of Ag+ ions to metallic silver and provides a rapid, non-destructive indication of nanoparticle formation [31]. The absence of this peak in the pure extract spectrum eliminates the possibility that the observed optical density arises solely from plant metabolites, reinforcing the conclusion of nanoparticle synthesis. The relatively narrow LSPR band suggested a moderate size distribution and reasonably uniform particle shape. The concentration of silver in the colloidal solution was quantitatively evaluated by ICP. A calibration curve was established, and it was determined that a concentration of 30.525 ppm corresponded to an optical density value of 1 at the LSPR wavelength (405 nm). This calibration enabled accurate estimation of silver content in the synthesized nanoparticle suspensions, ensuring reproducibility and consistency of the green synthesis procedure. The determined concentration confirms that the synthesis yields a colloidal dispersion with sufficient silver content for subsequent applications.
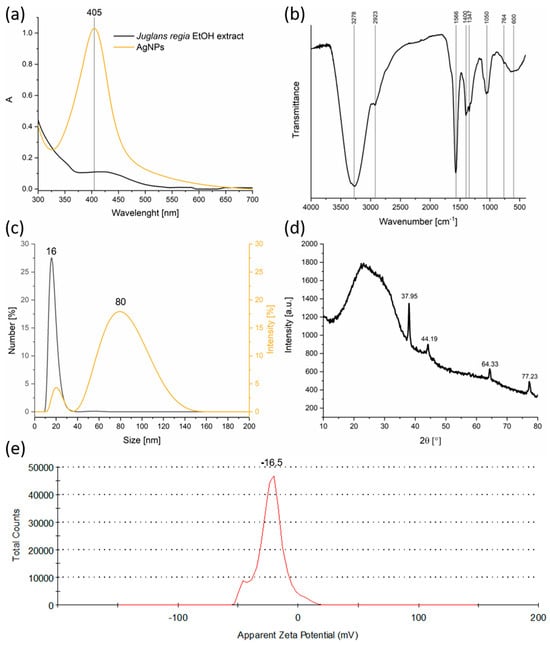
Figure 1.
Physicochemical characterization of AgNPs synthesized using Juglans regia extract: (a) UV–Vis absorption spectra; (b) FTIR spectrum; (c) DLS particle size distribution; (d) XRD pattern; (e) Zeta potential profile.
FTIR spectroscopy provided valuable information on the nature of the organic moieties involved in nanoparticle synthesis and stabilization (Figure 1b). The existence of broad O–H stretching bands (~3278 cm−1) indicates the presence of phenolic and alcoholic groups, which are well-known antioxidants capable of donating electrons to reduce metal ions [32]. Aliphatic C–H stretching vibrations (~2923 cm−1) and bands assigned to C=O and C=C stretching (1600–1400 cm−1) [33] indicate the presence of flavonoids, terpenoids, and other aromatic compounds, all of which can interact with nanoparticle surfaces. The identification of C–O–C ether vibrations (~1100 cm−1) supported the idea that complex carbohydrates or glycosides might also play a role in capping. These organic molecules stabilize the nanoparticles by adsorbing onto their surface, forming a protective layer that limits aggregation and controls growth during synthesis [34].
DLS revealed a bimodal size distribution, with a smaller mode around 16 nm and a larger one near 80 nm (Figure 1c). The smaller population size corresponds well with the expected size of individual nanoparticles observed in similar green syntheses, while the larger size peak likely results from aggregates or loosely bound clusters. This degree of polydispersity is typical of biosynthesized nanoparticles, where capping efficiency can vary with biomolecule concentration and composition. The presence of aggregates, while not ideal for certain applications that require monodisperse samples, does not preclude effective functionality in antimicrobial or catalytic applications, where some degree of clustering can even be beneficial.
XRD analysis (Figure 1d) confirmed the formation of a face-centered cubic (fcc) crystalline phase characteristic of metallic silver (Silver-3C, syn, ICDD DB Number 03-065-2871) [35]. The diffraction pattern displayed intense peaks at 2θ values of 37.95°, 44.19°, 64.33°, and 77.23°, which were indexed to the (111), (200), (220), and (311) crystallographic planes, respectively. These sharp peaks indicate the formation of well-crystallized silver nanoparticles. Additionally, a broad amorphous hump observed between 10° and 30° was attributed to organic compounds from the Juglans regia extract, evidencing the presence of biomolecular capping and stabilization layers [36].
The crystallite size estimated by the Williamson–Hall method was approximately 6.4 nm, with negligible lattice strain, which suggests the formation of highly ordered nanocrystals with minimal internal stress or defects. Moreover, the calculated lattice parameter of a = 4.0969 Å is consistent with the literature values for fcc silver, further confirming the purity and crystallinity of the synthesized nanoparticles.
Zeta potential measurements (Figure 1e) yielded a value of −16.5 mV, indicating moderate electrostatic stabilization of the colloidal suspension. While values above ±30 mV are generally considered highly stable, the observed moderately negative charge suggests a balance between repulsive forces and potential van der Waals attractions leading to some aggregation, as seen in DLS. The negative surface charge arises from the adsorption of phytochemicals bearing ionizable groups (–OH and –COO−), thereby contributing to nanoparticle dispersion in aqueous media. For applications requiring long-term stability, such as drug delivery or sensor development, further surface modification or the addition of stabilizing agents might be necessary. However, for many environmental and antimicrobial uses, this level of stability is sufficient.
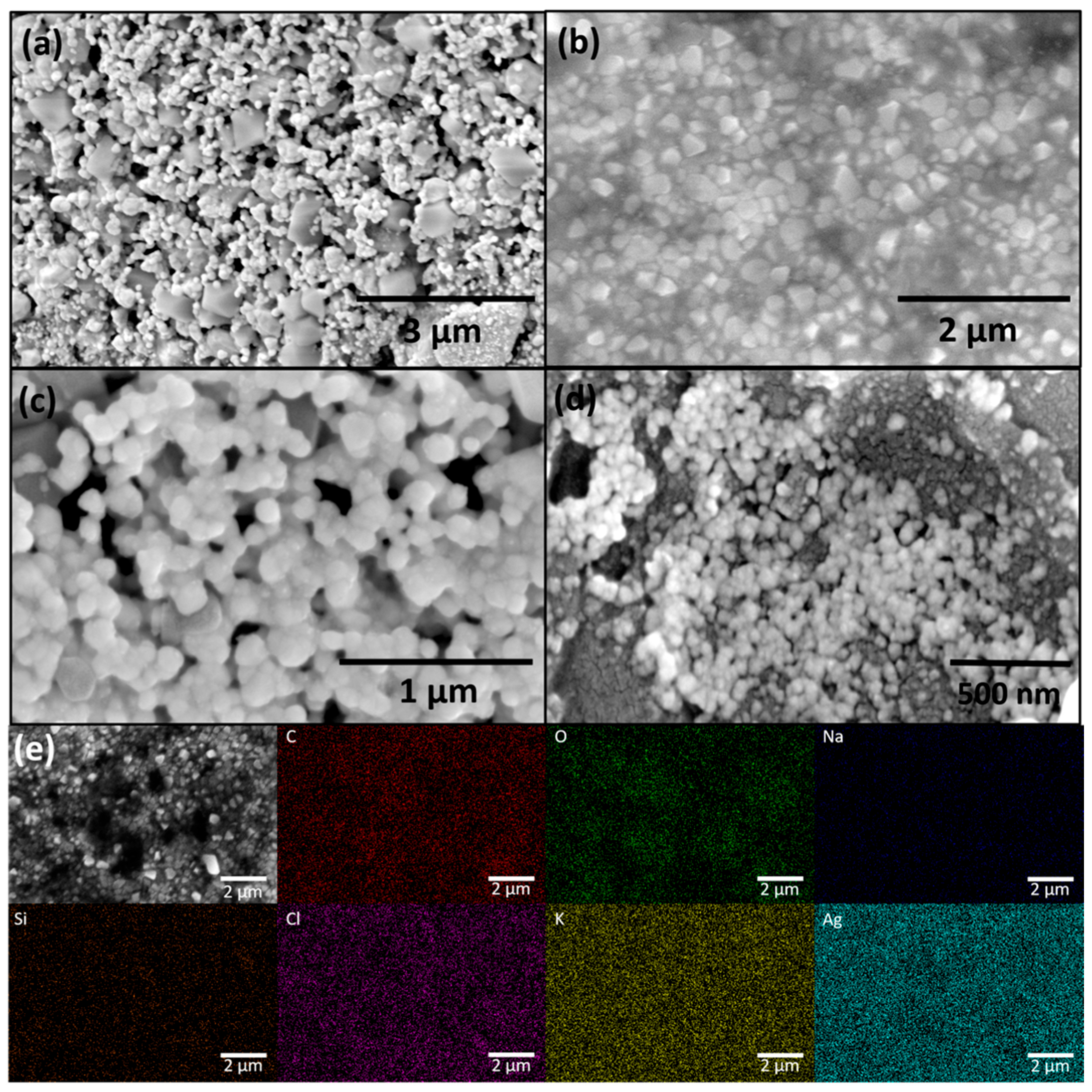
FESEM analysis (Figure 2a–d) revealed that the synthesized AgNPs are predominantly spherical in shape, with an average diameter of approximately 30 nm. A high degree of agglomeration was observed, likely due to the drying of the colloidal solution prior to SEM imaging. This agglomeration is common in drop-cast samples and does not necessarily reflect the dispersion state of nanoparticles in the original colloidal suspension.
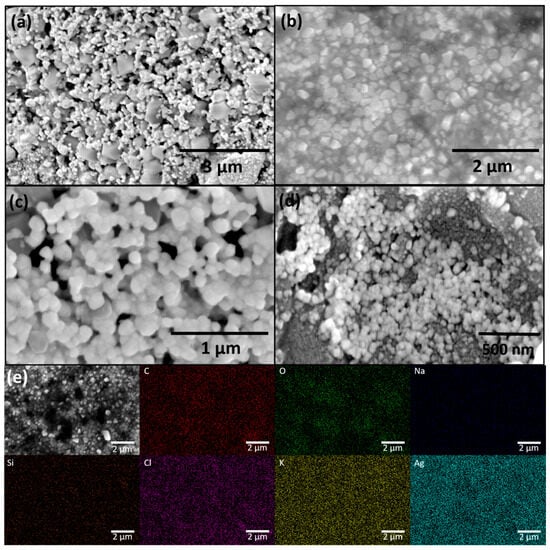
Figure 2.
FESEM micrographs of green synthesized AgNPs at different magnifications: (a) 25,000×, (b) 35,000×, (c) 80,000×, (d) 100,000×; and (e) EDX elemental mapping of AgNPs at magnification 35,000×.
EDX analysis (Figure 2e) confirmed the presence of uniformly distributed Ag as the major elemental component, verifying successful nanoparticle formation. In addition to Ag, elements such as C and O were detected, originating from the plant-based organic compounds adsorbed on the nanoparticle surface, which serve as natural capping and stabilizing agents. The presence of Na, Si, Cl, and K can be attributed to phytoconstituents in the Juglans regia extract or trace impurities commonly found in plant-derived materials.
3.2. Colorimetric Screening and Selectivity Assessment of AgNP-Based Sensors for Metal Ions Detection
To assess the intrinsic responsiveness of green-synthesized AgNPs toward different metal ions and evaluate potential interferences in detection, a qualitative screening assay was conducted.
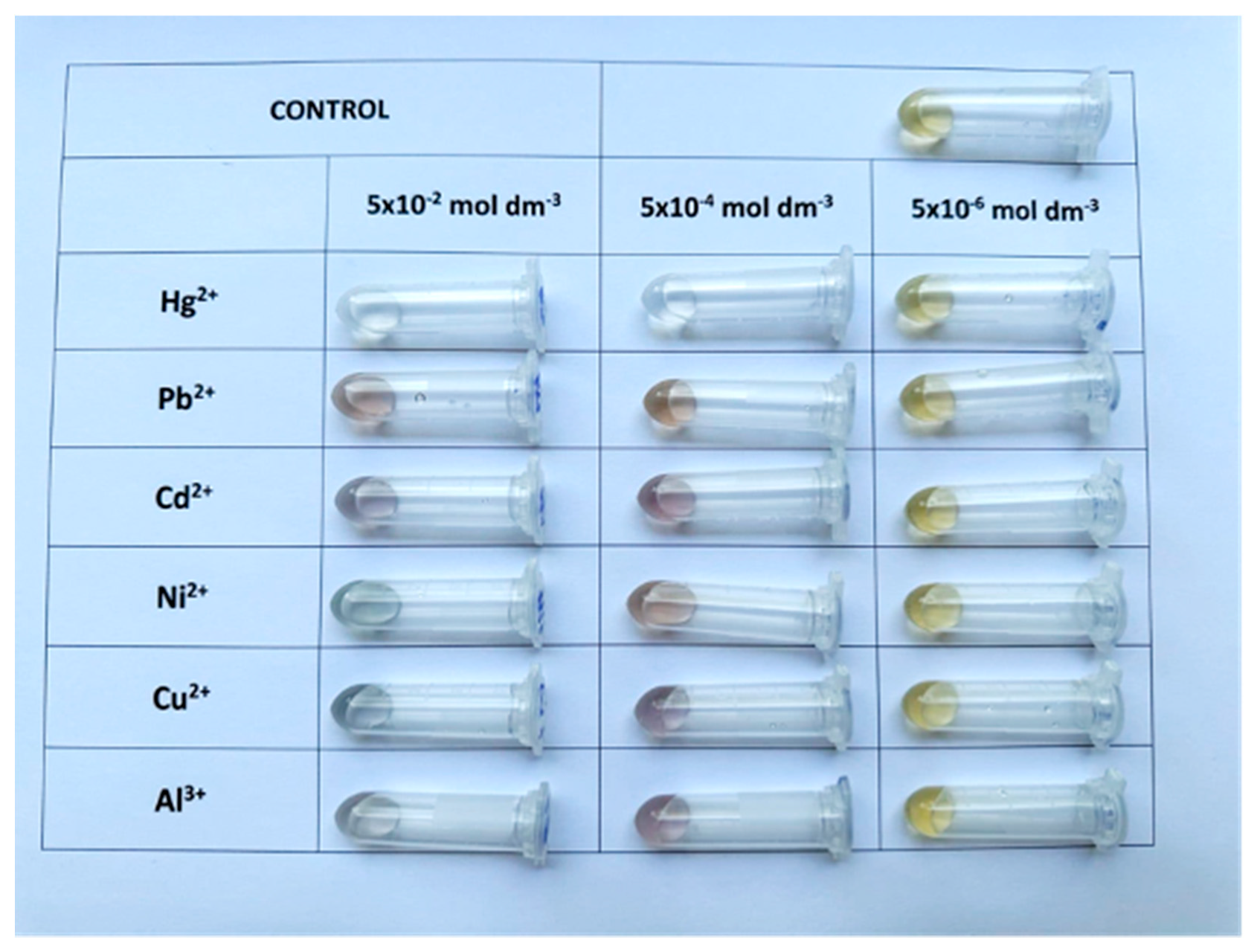
Upon visual inspection (Figure 3), it was evident that the presence of metal ions induced observable color changes in the colloidal system, which varied depending on the type and concentration of the ion. In most cases, a gradual fading of color or a shift in hue was noticed, particularly at higher concentrations. Hg2+ triggered the most significant visual transformation, leading to the complete disappearance of the yellow tone, suggesting strong interaction with AgNPs and potential redox transformation. Recorded UV–Vis spectra are provided in the Supplementary Materials (Figure S2) to support the diverse response of the synthesized AgNPs to selected metals.
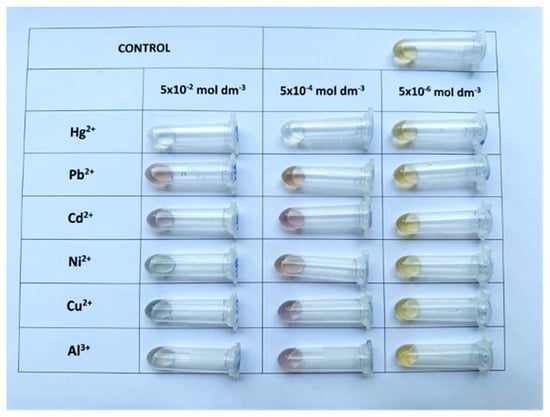
Figure 3.
Colorimetric response of AgNPs to selected metal ions at three concentrations (from right to left: 5 × 10−6, 5 × 10−4, and 5 × 10−2 mol dm−3).
However, despite clear evidence of AgNP responsiveness, this colloidal system demonstrated limited visual selectivity. At lower concentrations (e.g., 5 × 10−6 mol dm−3), the differences between samples became subtle and difficult to distinguish by the naked eye, making qualitative analysis unreliable. Furthermore, certain ions produced similar responses, complicating the interpretation of results and limiting practical applicability in real-sample screening scenarios.
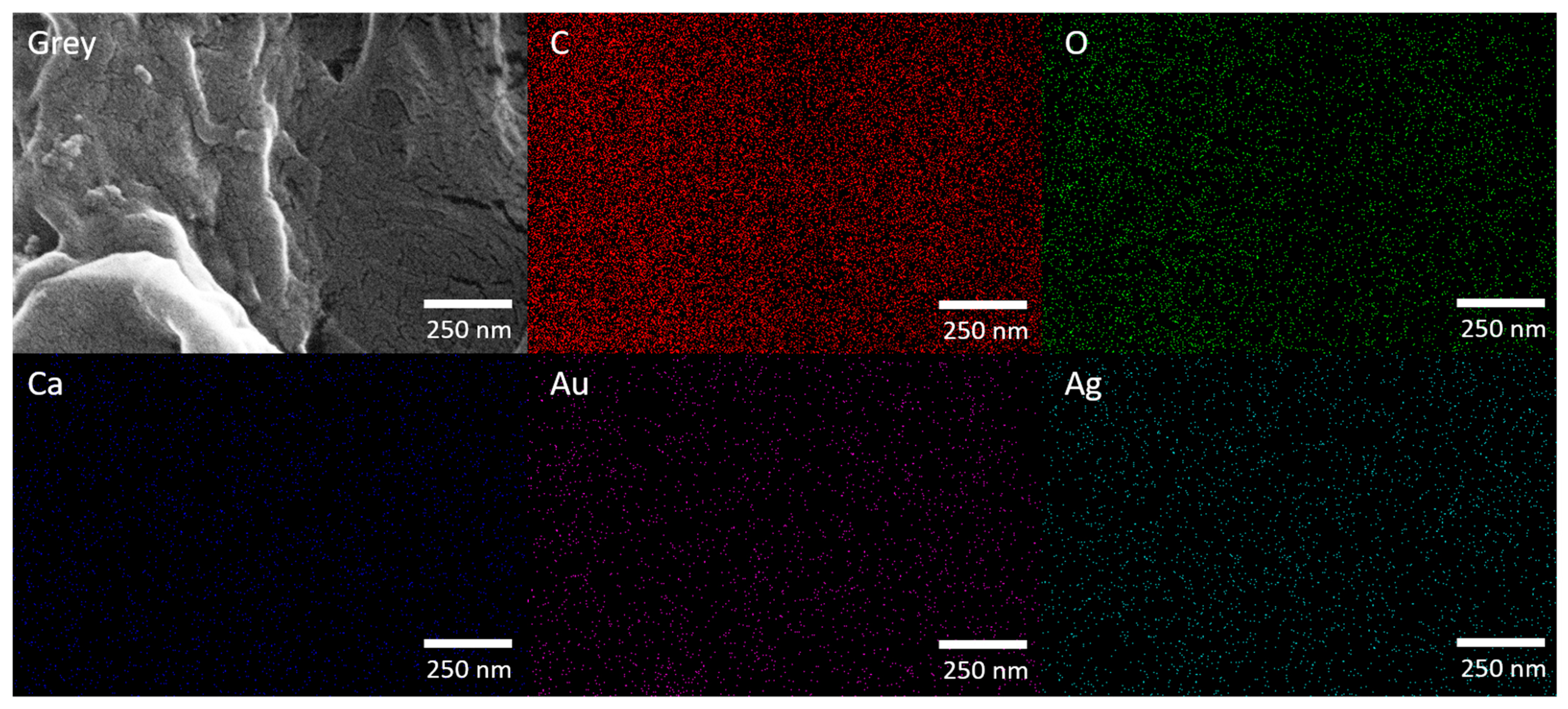
To overcome these limitations, AgNPs were immobilized onto filter paper to form a solid-phase sensing platform (AgNPs@FP). The SEM micrograph and EDS map of AgNPs@FP are shown in Figure 4.
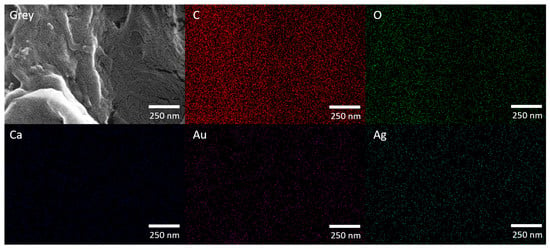
Figure 4.
FESEM micrograph (150,000 × magnification) and corresponding EDX elemental mapping of AgNPs@FP.
The FESEM micrograph reveals that the FP’s fibrous structure remains intact after AgNP modification, indicating that the synthesis process does not disrupt its morphology. Elemental mapping confirms a uniform distribution of C and O, originating from both the cellulose framework of the paper and the phytochemicals introduced through the plant extract used in the green synthesis. Ca signal was also observed, which is attributed to residual inorganic components naturally present in the paper matrix. Ag was evenly dispersed across the surface, demonstrating the effective and homogeneous incorporation of AgNPs. The Au signal results from the conductive coating applied prior to SEM analysis to enhance image quality.
The impregnated paper was then tested by dipping into aqueous solutions of selected metal ions (0.5 mL, 5 × 10−4 mol dm−3), after which the strips were again air-dried. Remarkably, a distinct and reproducible color change was observed only in the case of Hg2+, while no noticeable changes occurred with the other tested ions.
To further probe the selectivity and rule out interference, additional experiments were performed using the same Hg2+ concentration (5 × 10−4 mol dm−3) in parallel with significantly higher concentrations (0.1 mol dm−3) of other potentially interfering ions. Even under these challenging conditions, only the Hg2+-treated strips exhibited a visible color change, while the presence of other ions did not lead to any perceptible alteration in color (Figure 5). These results clearly demonstrate the high specificity of the paper-based AgNPs sensor toward mercury ions, which contrasts with the ambiguous responses of the colloidal system.
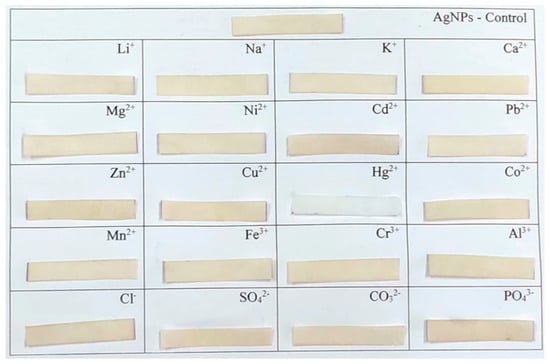
Figure 5.
Visual response of AgNPs@FP strips after exposure to various metal ions and anions (c = 0.1 mol dm−3) and Hg2+ (c = 5 × 10−4 mol dm−3).
This enhancement in selectivity can be attributed to several factors. Firstly, immobilizing AgNPs on filter paper restricts their mobility and stabilizes their dispersion, thereby reducing nonspecific aggregation. Secondly, the matrix facilitates better visualization by providing a neutral background that amplifies subtle color differences. Finally, the strong affinity between Hg2+ and silver likely promotes a redox or amalgamation reaction that alters the optical properties of AgNPs irreversibly and selectively in the solid state.
3.3. Colorimetric Quantification of Hg2+ Ions Using AgNPs@FP Sensor
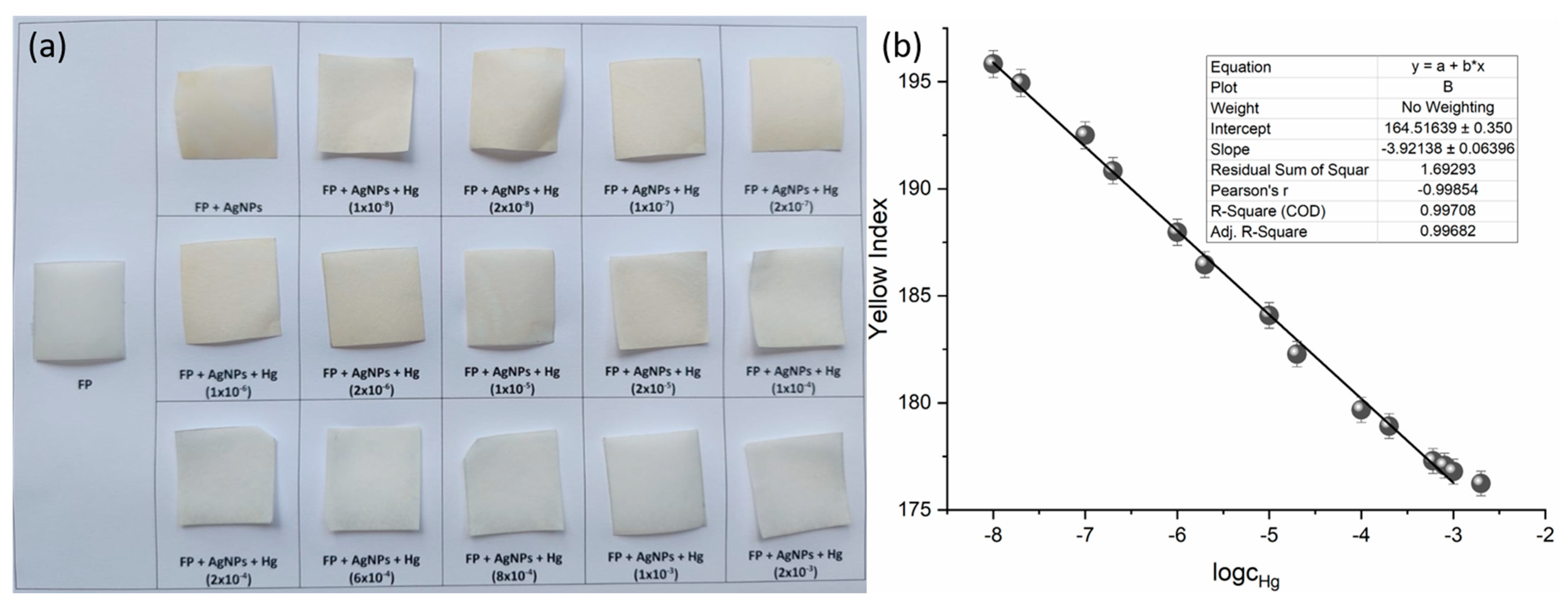
AgNPs@FP were individually immersed in 0.5 mL of HgCl2 solutions with concentrations ranging from 1 × 10−8 to 2 × 10−3 mol dm−3. The photograph of sensor squares is given in Figure 6a.
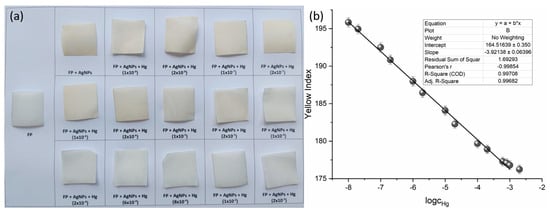
Figure 6.
(a) Visual response of AgNPs@FP synthesized using Juglans regia extract, after exposure to increasing concentrations of Hg2+ (from 1 × 10−8 to 2 × 10−3 mol dm−3). (b) Calibration curve showing the linear dependence of yellow index on the logarithm of Hg2+ concentration.
The calculated yellow index values were plotted against the logarithm of Hg2+ concentration to evaluate the sensor’s colorimetric response (Figure 6b). A clear linear correlation was observed over the concentration range of 1 × 10−8 to 1 × 10−3 mol dm−3, defining the sensor’s dynamic range. The data point at 2 × 10−3 mol dm−3 was excluded from the analysis because it falls outside the established linearity range.
The mean yellow index of the blank was determined to be 197.273 ± 0.475, with the standard deviation calculated from 10 independently prepared filter papers, treated identically and immersed for 3 s in the AgNPs solution, followed by air-drying.
The corresponding concentrations were interpolated from the calibration curve (Figure 6b) using the obtained signal values, and the LOD and LOQ were determined to be 1.065 × 10−8 mol dm−3 and 3.263 × 10−8 mol dm−3, respectively.
These results demonstrate that the sensing platform enables reliable detection of Hg2+ at low nanomolar levels, with a visible color change detectable down to 1 × 10−8 mol dm−3, in agreement with the calculated LOD.
The FESEM image reveals a well-preserved fibrous network of the filter paper, indicating that the surface architecture remains intact after AgNP deposition and Hg2+ detection (Figure S2). C and O are uniformly distributed and originate from both the cellulose structure of the filter paper and from phytochemicals present in the plant extract used for the green synthesis. Ag is homogeneously dispersed, confirming the successful modification of the paper with silver nanoparticles. Hg signal appears in the same regions as Ag, suggesting a strong interaction between AgNPs and Hg2+ ions, likely through amalgamation or surface complexation. Cl and Na may derive from residual salts used in the synthesis or treatment steps. Au signal may stem from the gold coating typically applied before SEM imaging to improve conductivity. The co-localization of Ag and Hg in the map supports the sensor’s capability to capture Hg2+ ions effectively, providing visual confirmation of the detection mechanism.
3.4. Detection of Hg2+ in Tap Water Samples Using AgNPs@FP
As shown in Table 1, the recovery values ranged from 96% to 102%, confirming the method’s strong accuracy across a broad concentration range. Importantly, even at the lowest tested concentration (1.0 × 10−8 mol dm−3), the recovery remained within acceptable limits (96 ± 5), indicating reliable quantification of trace mercury ions in untreated water. These results highlight the robustness of the sensing system, which maintained a consistent response even in the presence of potential interferents naturally present in tap water. The minimal deviation in recoveries suggests that matrix effects were negligible, likely due to the selectivity of the sensing mechanism toward Hg2+ ions. Furthermore, the ease of sample preparation, requiring no filtration or chemical treatment, supports the suitability of the method for rapid and routine monitoring of mercury contamination in environmental waters.

Table 1.
Detection of Hg2+ in spiked tap water samples using AgNPs@FP sensor.
3.5. Comparison with Literature-Reported AgNPs-Based Paper Sensors for Hg2+ Detection
Table 2 summarizes the synthesis approaches of AgNPs applied on filter paper for the colorimetric detection of Hg2+ ions. Different green and chemical routes were reported, ranging from plant-mediated syntheses using extracts of C. cneorum, Ocimum sanctum, Achillea wilhelmsii, and Juglans regia, to conventional wet-chemical methods employing AgNO3 with NaBH4 and stabilizers such as PVP or citrate. The reported linearity ranges vary significantly, spanning from 10−3 to 10−8 mol dm−3, with the lowest LOD reaching the nanomolar level (5 × 10−9 mol dm−3). Notably, plant extracts generally provided simple and sustainable synthetic routes, although with slightly higher LOD values compared to chemical reduction methods. The present work, based on Juglans regia extract, demonstrates competitive sensitivity (LOD = 1.065 × 10−8 mol dm−3) while employing an environmentally friendly and low-cost approach.

Table 2.
Reported AgNP-based paper sensors for Hg2+ detection compared with the present study.
4. Conclusions
The present work highlights the potential of plant-mediated green synthesis to produce silver nanoparticles with well-defined crystallinity, controlled size, and functional surface chemistry, using Juglans regia extract as a sustainable reducing and capping agent. By immobilizing these nanoparticles onto filter paper, a highly selective and sensitive colorimetric platform for Hg2+ detection was developed, capable of detecting trace concentrations down to nanomolar levels (LOD = 1.065 × 10−8 mol dm−3) over a wide linear range without complex instrumentation. This study stands out for its combination of high sensitivity, a broad linear range, and entirely green, plant-mediated synthesis, thereby avoiding hazardous chemicals. The integration onto filter paper produces a portable, low-cost, and user-friendly sensor suitable for real-world applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors13120433/s1. Figure S1: UV/Vis spectra of AgNPs after exposure to different concentration of: (a) Hg2+; (b) Pb2+; (c) Cd2+; (d) Ni2+; (e) Cu2+; (f) Al3+ ions. Figure S2: FESEM micrograph (150,000 × magnification) and corresponding EDX elemental mapping of AgNPs@FP after exposure to Hg2+ ions (5 × 10−4 mol dm−3).
Author Contributions
Conceptualization, N.R. and V.M.; methodology, V.M., S.G., V.R. and N.P.; validation, V.M., S.G. and T.L.-P.; formal analysis, N.R., S.K., V.R., T.T. and N.P.; investigation, N.R., S.K., V.R. and T.T.; resources, S.G., V.R., N.P. and T.L.-P.; data curation, N.R., S.K., S.G., T.T. and N.P.; writing—original draft preparation, N.R. and S.K.; writing—review and editing, V.M., V.R., N.P. and T.L.-P.; visualization, V.M.; supervision, V.M.; funding acquisition, V.M., S.G. and T.L.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Serbian Ministry of Science, Technological Development, and Innovation (grant number: 451-03-136/2025-03/200017 and grant number: 451-03-136/2025-03/200162).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Ongulu, J.; Otunuya, C.F.; Opiti, A.R.; Ajakaye, R.S.; et al. Environmental persistence, bioaccumulation, and ecotoxicology of heavy metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Basu, N.; Bastiansz, A.; Dórea, J.G.; Fujimura, M.; Horvat, M.; Shroff, E.; Weihe, P.; Zastenskaya, I. Our evolved understanding of the human health risks of mercury. Ambio 2023, 52, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Ali, W.; Zinck, P.; Souissi, S.; Lee, J.-S. Toxicity of methylmercury in aquatic organisms and interaction with environmental factors and coexisting pollutants: A review. Sci. Total Environ. 2024, 943, 173574. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.B.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Leskovac, A.; Petrović, S.; Mitić, M.; Lazarević-Pašti, T.; Novković, M.; Potkonjak, N. Metals on the Menu—Analyzing the Presence, Importance, and Consequences. Foods 2024, 13, 1890. [Google Scholar] [CrossRef]
- Shuvaeva, O.V.; Gustaytis, M.A.; Anoshin, G.N. Mercury speciation in environmental solid samples using thermal release technique with atomic absorption detection. Anal. Chim. Acta 2008, 621, 148–154. [Google Scholar] [CrossRef]
- Louie, H.; Wong, C.; Huang, Y.J.; Fredrickson, S. A study of techniques for the preservation of mercury and other trace elements in water for analysis by inductively coupled plasma mass spectrometry (ICP-MS). Anal. Methods 2012, 4, 522–529. [Google Scholar] [CrossRef]
- da Silva, D.G.; Portugal, L.A.; Serra, A.M.; Ferreira, S.L.C.; Cerdà, V. Determination of mercury in rice by MSFIA and cold vapour atomic fluorescence spectrometry. Food Chem. 2013, 137, 159–163. [Google Scholar] [CrossRef]
- Ali, S.; Mansha, M.; Baig, N.; Khan, S.A. Recent Trends and Future Perspectives of Emergent Analytical Techniques for Mercury Sensing in Aquatic Environments. Chem. Rec. 2022, 22, e202100327. [Google Scholar] [CrossRef]
- Pomal, N.C.; Bhatt, K.D.; Modi, K.M.; Desai, A.L.; Patel, N.P.; Kongor, A.; Kolivoška, V. Functionalized Silver Nanoparticles as Colorimetric and Fluorimetric Sensor for Environmentally Toxic Mercury Ions: An Overview. J. Fluoresc. 2021, 31, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Chahal, V.; Issar, U.; Nagpal, M.; Vashistha, V.K.; Mittal, A. Nanomaterials as fluorescent sensor and colorimetric sensor for toxic Hg(II) ion: A review. Ionics 2024, 30, 6811–6833. [Google Scholar] [CrossRef]
- Serna-Gallén, P.; Mužina, K. Metallic nanoparticles at the forefront of research: Novel trends in catalysis and plasmonics. Nano Mater. Sci. 2024; in press. [Google Scholar] [CrossRef]
- Sanjeevappa, H.K.; Nilogal, P.; Rayaraddy, R.; Martis, L.J.; Osman, S.M.; Badiadka, N.; Yallappa, S. Biosynthesized unmodified silver nanoparticles: A colorimetric optical sensor for detection of Hg2+ ions in aqueous solution. Results Chem. 2022, 4, 100507. [Google Scholar] [CrossRef]
- Ghosh, S.; Mondal, A. Aggregation chemistry of green silver nanoparticles for sensing of Hg2+ and Cd2+ ions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125335. [Google Scholar] [CrossRef]
- Bothra, S.; Solanki, J.N.; Sahoo, S.K. Functionalized silver nanoparticles as chemosensor for pH, Hg2+ and Fe3+ in aqueous medium. Sens. Actuators B Chem. 2013, 188, 937–943. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; Taha, G.M.; Hagaggi, N.S.A.; Moghazy, M.A. Green synthesis of silver nanoparticles and its environmental sensor ability to some heavy metals. BMC Chem. 2024, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Abouzeid, R.E.; Owda, M.E.; Cruz-Maya, I.; Guarino, V. Cellulose–Silver Composites Materials: Preparation and Applications. Biomolecules 2021, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.H.; Li, D.; Simon, G.P.; Garnier, G. Paper surfaces functionalized by nanoparticles. Adv. Colloid Interface Sci. 2011, 163, 23–38. [Google Scholar] [CrossRef]
- Mohamed, A.; Li, X.; Li, C.; Li, X.; Yuan, C.; Barakat, H. Smartphone-Based Colorimetric Detection of Chromium (VI) by Maleic Acid-Functionalized Gold Nanoparticles. Appl. Sci. 2021, 11, 10894. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.-J.; Du, A.; Kou, Y.; Xu, X.; Hu, D.; Lu, Z. Advances in paper-based analytical devices relying on optical detection. Adv. Compos. Hybrid Mater. 2025, 8, 260. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Padhye, R.; Wang, X.; Houshyar, S. Advances in nanoparticle-enhanced paper sensor for detecting toxic metals in water. Talanta 2025, 293, 128146. [Google Scholar] [CrossRef]
- Budlayan, M.L.; Dalagan, J.; Lagare-Oracion, J.P.; Patricio, J.; Arco, S.; Latayada, F.; Vales, T.; Baje, B.; Alguno, A.; Capangpangan, R. Detecting mercury ions in water using a low-cost colorimetric sensor derived from immobilized silver nanoparticles on a paper substrate. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100736. [Google Scholar] [CrossRef]
- Avissa, M.; Alauhdin, M. Selective Colorimetric Detection of Mercury(II) using Silver Nanoparticles-Chitosan. Molekul 2022, 17, 107–115. [Google Scholar] [CrossRef]
- Adlim, M.; Surbakti, M.S.; Omar, A.F.; Rahmayani, R.F.I.; Hasmar, A.H.; Ozmen, I.; Yavuz, M. Detecting dissolved mercury(ii) ions using chitosan-AgNP strips integrated with smartphones. RSC Adv. 2024, 14, 27504–27513. [Google Scholar] [CrossRef] [PubMed]
- Talodthaisong, C.; Sangiamkittikul, P.; Chongwichai, P.; Saenchoopa, A.; Thammawithan, S.; Patramanon, R.; Kosolwattana, S.; Kulchat, S. Highly Selective Colorimetric Sensor of Mercury(II) Ions by Andrographolide-Stabilized Silver Nanoparticles in Water and Antibacterial Evaluation. ACS Omega 2023, 8, 41134–41144. [Google Scholar] [CrossRef] [PubMed]
- Revathi, S.; Sutikno, S.; Hasan, A.F.; Altemimi, A.B.; Alkaisy, Q.H.; Phillips, A.J.; Hesarinejad, M.A.; Abedelmaksoud, T.G. Green synthesis and characterization of silver nanoparticles (AgNP) using Acacia nilotica plant extract and their anti-bacterial activity. Food Chem. Adv. 2024, 4, 100680. [Google Scholar] [CrossRef]
- Khan, H.; Piccolella, S.; Pacifico, S. Harnessing plant extracts for green nanoparticle synthesis: Toward a sustainable future. Mater. Today Sustain. 2025, 31, 101195. [Google Scholar] [CrossRef]
- Eshghi, M.; Vaghari, H.; Najian, Y.; Najian, M.J.; Jafarizadeh-Malmiri, H.; Berenjian, A. Microwave-Assisted Green Synthesis of Silver Nanoparticles Using Juglans regia Leaf Extract and Evaluation of Their Physico-Chemical and Antibacterial Properties. Antibiotics 2018, 7, 68. [Google Scholar] [CrossRef]
- Ebrahim Mohammadzadeh, S.; Faghiri, F.; Ghorbani, F. Green synthesis of phenolic capping Ag NPs by green walnut husk extract and its application for colorimetric detection of Cd2+ and Ni2+ ions in environmental samples. Microchem. J. 2022, 179, 107475. [Google Scholar] [CrossRef]
- International Crystallographical Database (ICDD), N.S. 12 Campus Blvd, PA 19073, USA. 2012. Available online: https://www.icdd.com/ (accessed on 10 June 2025).
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Adil, M.; Alam, S.; Amin, U.; Ullah, I.; Muhammad, M.; Ullah, M.; Rehman, A.; Khan, T. Efficient green silver nanoparticles-antibiotic combinations against antibiotic-resistant bacteria. AMB Express 2023, 13, 115. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F. Phytofabrication of Silver Nanoparticles (AgNPs) with Pharmaceutical Capabilities Using Otostegia persica (Burm.) Boiss. Leaf Extract. Nanomaterials 2021, 11, 1045. [Google Scholar] [CrossRef]
- Powder Diffraction File P-D, Announcement of New Data-Base Release 2012; International Centrefor Diffraction Data (ICDD): Newtown Square, PA, USA, 2012.
- Zhen, D.; Deng, Q.; Yu, Y.; Chen, S.; Li, L.; Cai, Q.; Liu, Y. A covalent organic polymer fluorescent probe for highly selective and sensitive UO22+ detection in water and food samples. Biosens. Bioelectron. 2025, 290, 117950. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Akhtar, K.; Seo, J.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Phytosynthesis of silver nanoparticles; naked eye cellulose filter paper dual mechanism sensor for mercury ions and ammonia in aqueous solution. J. Mater. Sci. Mater. Electron. 2019, 30, 7367–7383. [Google Scholar] [CrossRef]
- Monisha; Shrivas, K.; Kant, T.; Patel, S.; Devi, R.; Dahariya, N.S.; Pervez, S.; Deb, M.K.; Rai, M.K.; Rai, J. Inkjet-printed paper-based colorimetric sensor coupled with smartphone for determination of mercury (Hg2+). J. Hazard. Mater. 2021, 414, 125440. [Google Scholar] [CrossRef]
- Apilux, A.; Siangproh, W.; Praphairaksit, N.; Chailapakul, O. Simple and rapid colorimetric detection of Hg(II) by a paper-based device using silver nanoplates. Talanta 2012, 97, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, C.; Mishra, R.; Sahu, A.K.; Sahoo, L.N.; Sahoo, N.K.; Tripathy, S.K.; Sahoo, S. Green synthesis of glucose-capped stable silver nanoparticles: A cost-effective sensor for the selective detection of Hg2+ ions in aqueous solutions. Sens. Diagn. 2023, 2, 647–656. [Google Scholar] [CrossRef]
- Meelapsom, R.; Jarujamrus, P.; Amatatongchai, M.; Chairam, S.; Kulsing, C.; Shen, W. Chromatic analysis by monitoring unmodified silver nanoparticles reduction on double layer microfluidic paper-based analytical devices for selective and sensitive determination of mercury(II). Talanta 2016, 155, 193–201. [Google Scholar] [CrossRef]
- Mavaei, M.; Chahardoli, A.; Fattahi, A.; Khoshroo, A. A Simple Method for Developing a Hand-Drawn Paper-Based Sensor for Mercury; Using Green Synthesized Silver Nanoparticles and Smartphone as a Hand-Held-Device for Colorimetric Assay. Glob. Chall. 2021, 5, 2000099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).