Enhanced Room Temperature NO2 Detection by Carbon Nanofibers and Single-Walled Carbon Nanotubes: Experimental and Molecular Dynamics
Abstract
1. Introduction
2. Materials and Methods
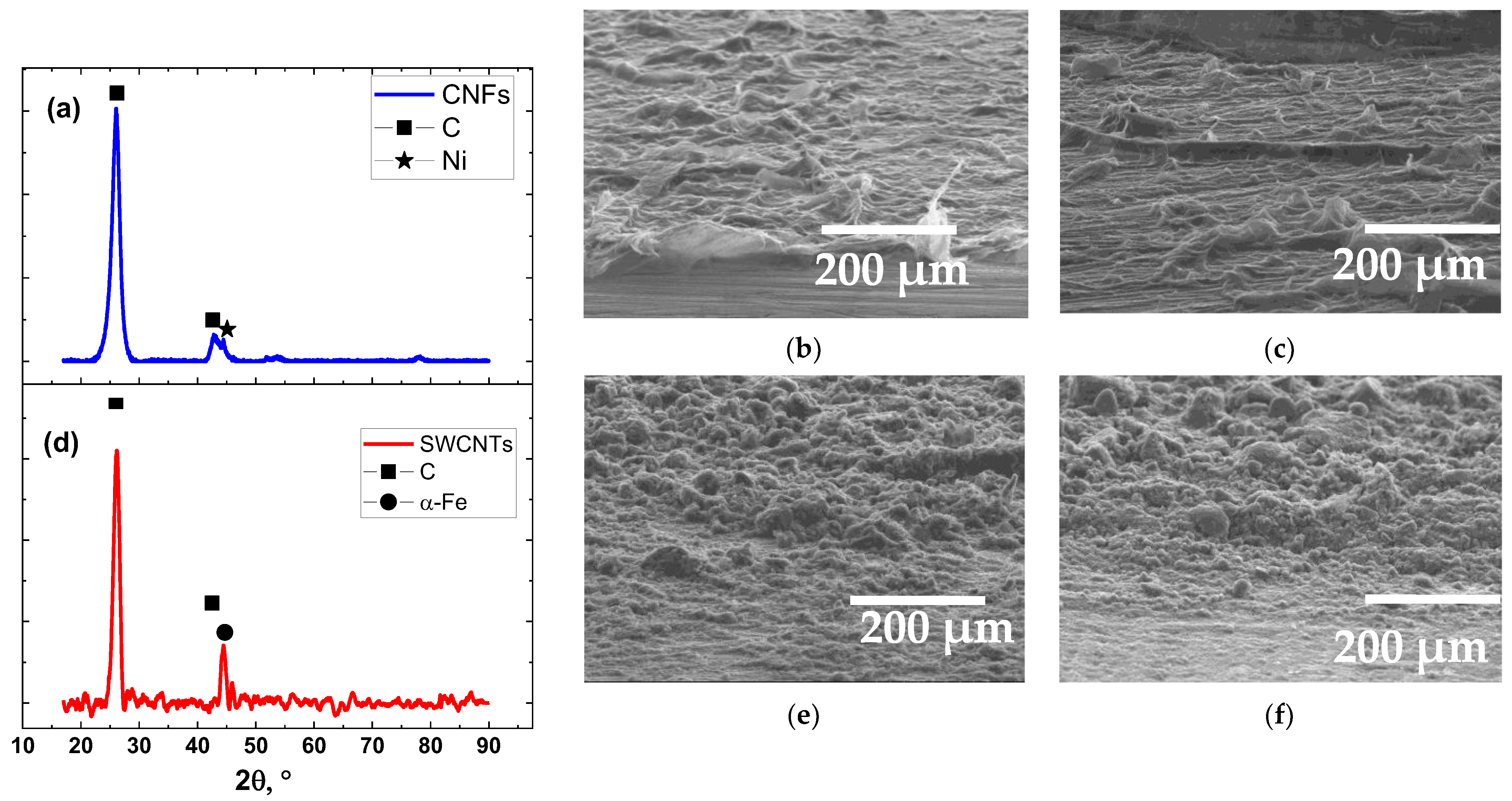
2.1. Carbon Nanomaterials
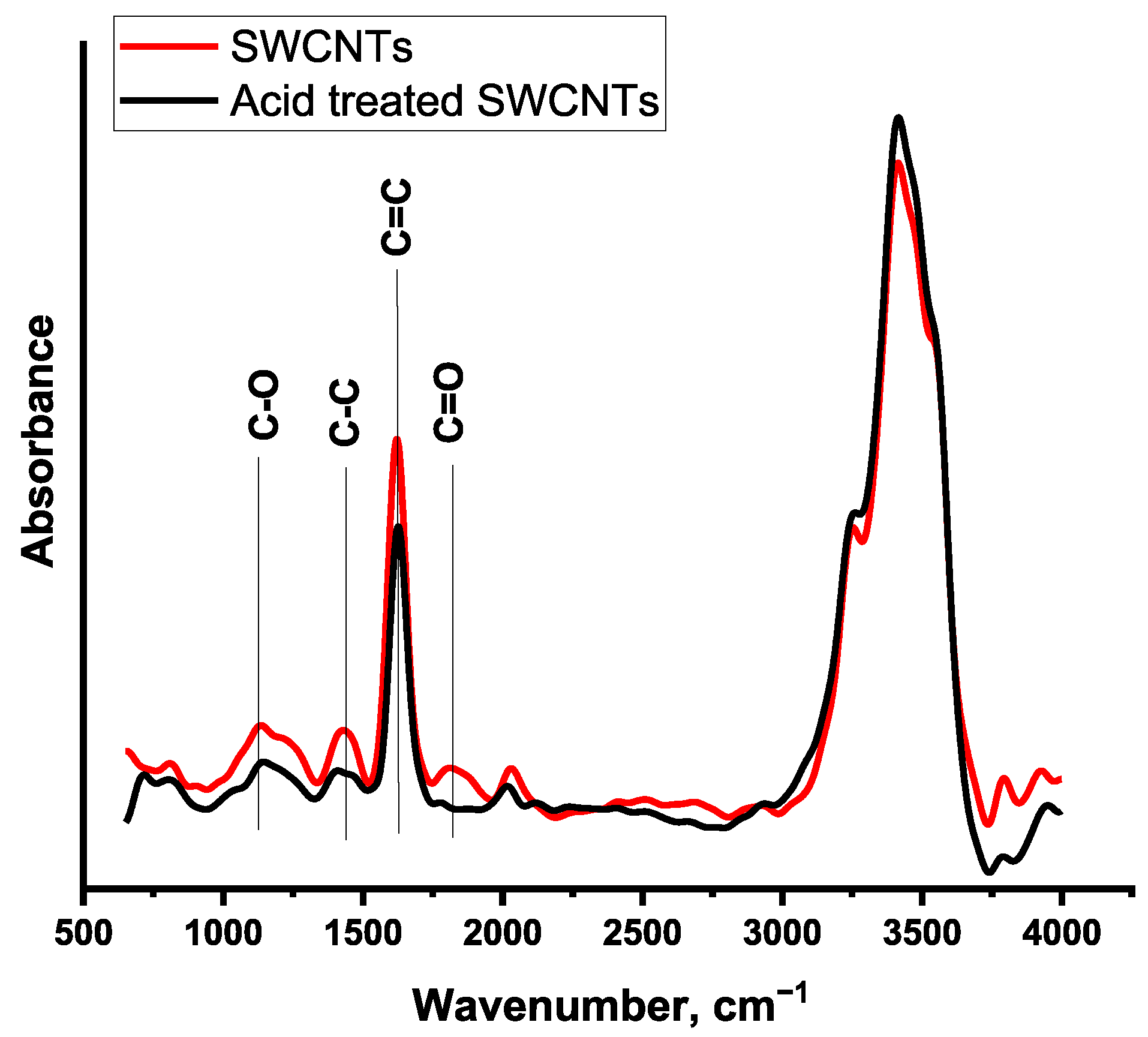
2.2. Acid Treatment of SWCNTs
2.3. Characterization of Carbon Nanomaterials
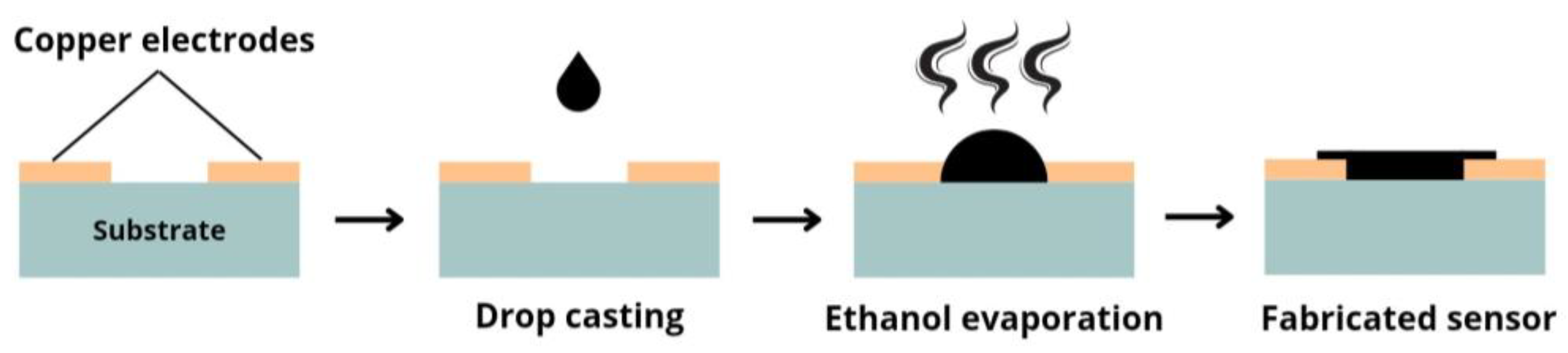
2.4. Sensor Fabrication and Gas Sensing Tests
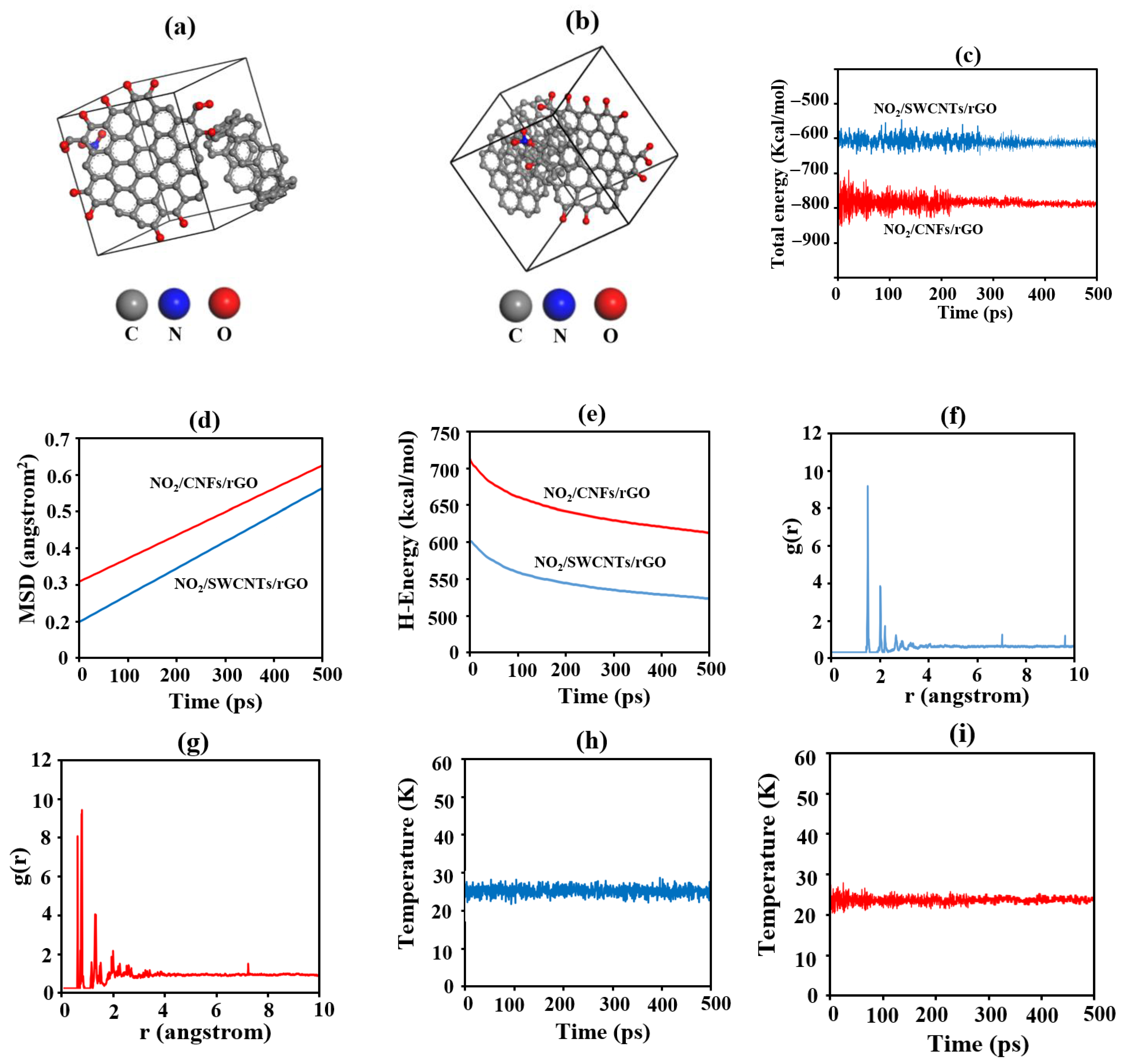
2.5. Computational Details
3. Results and Discussion
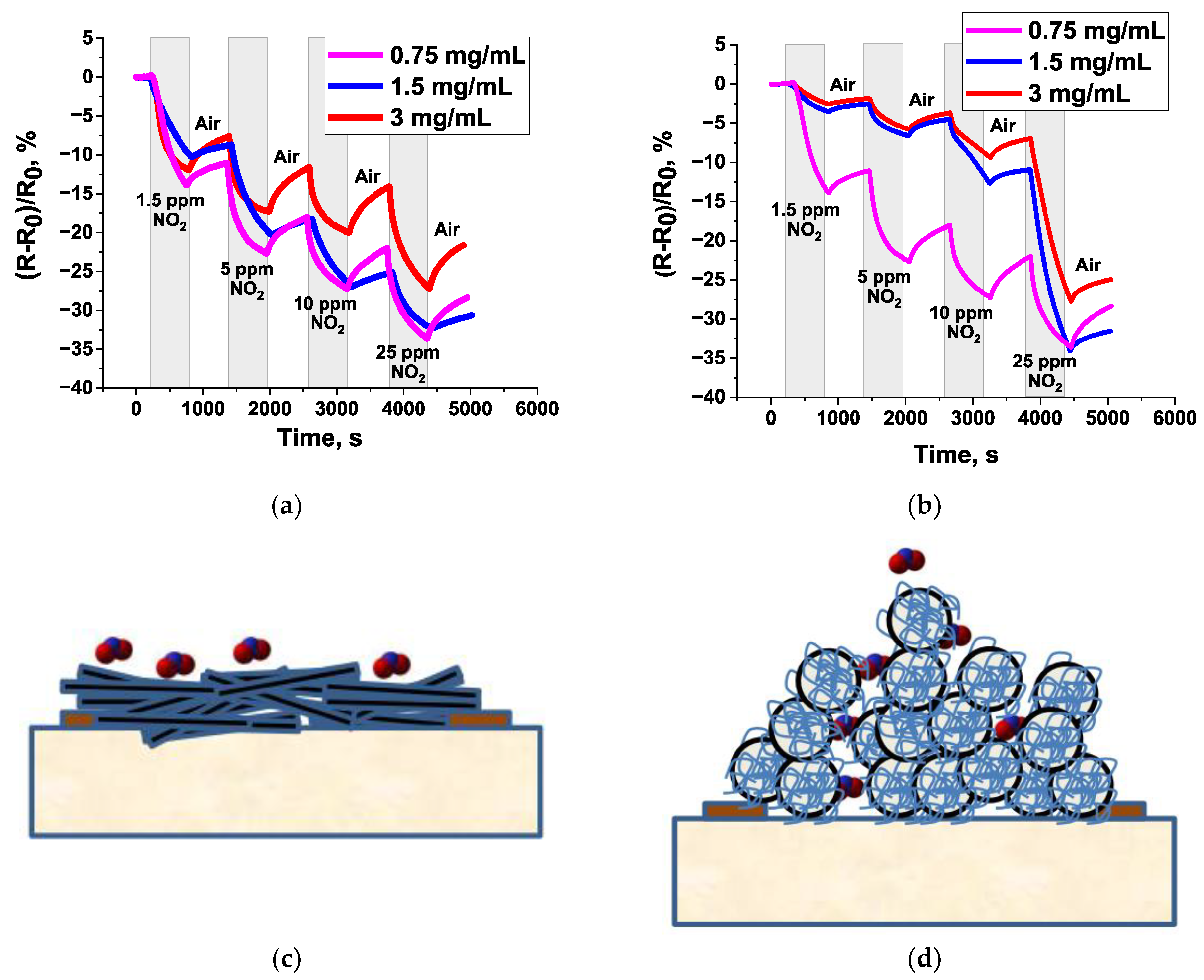
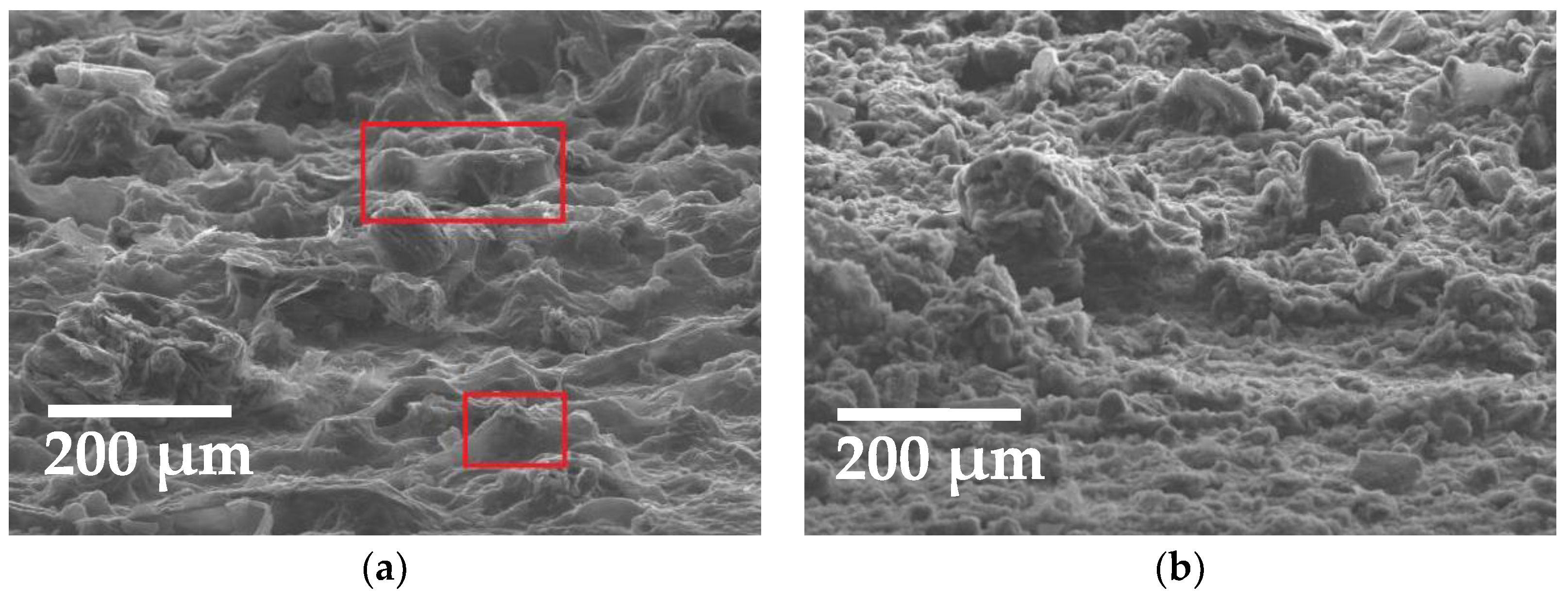
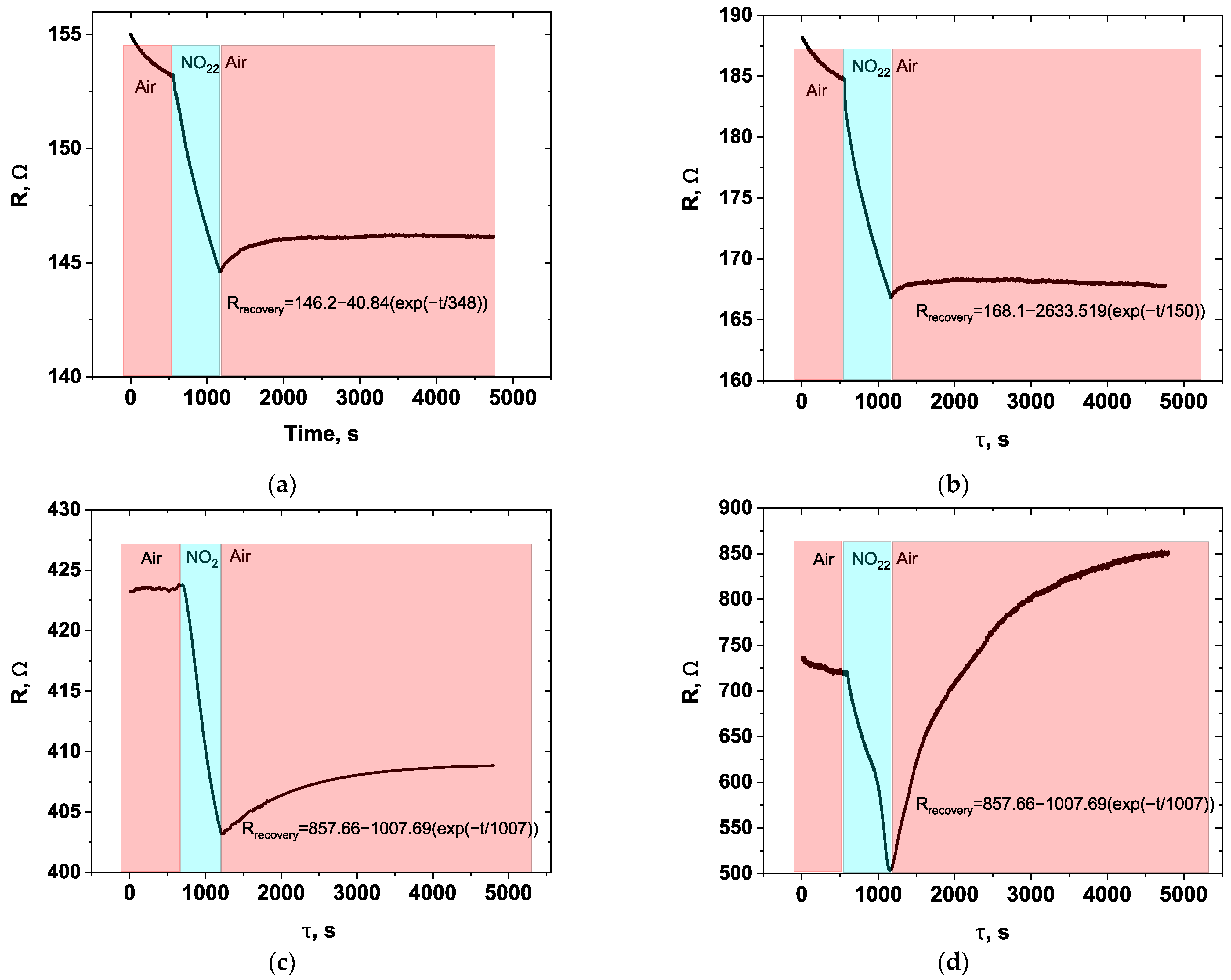
3.1. Sensors Based on SWCNTs and CNFs
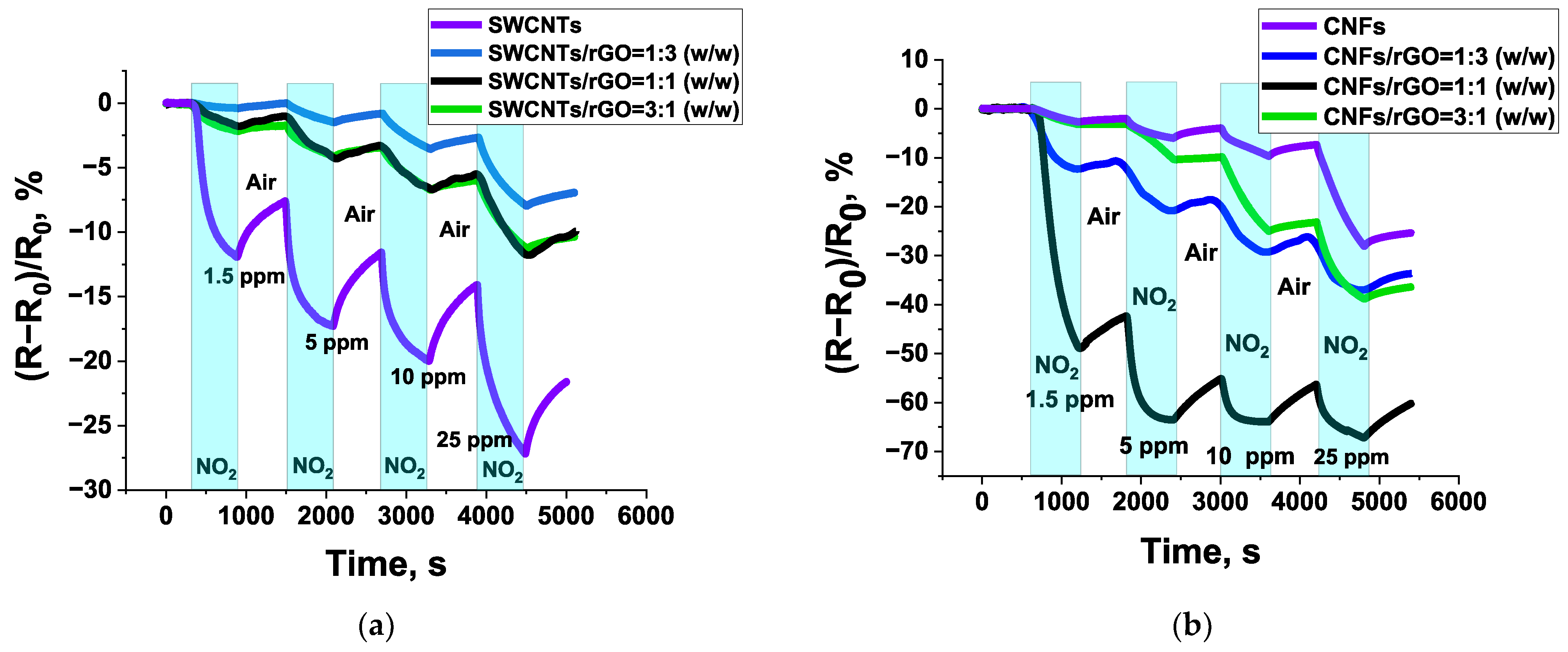
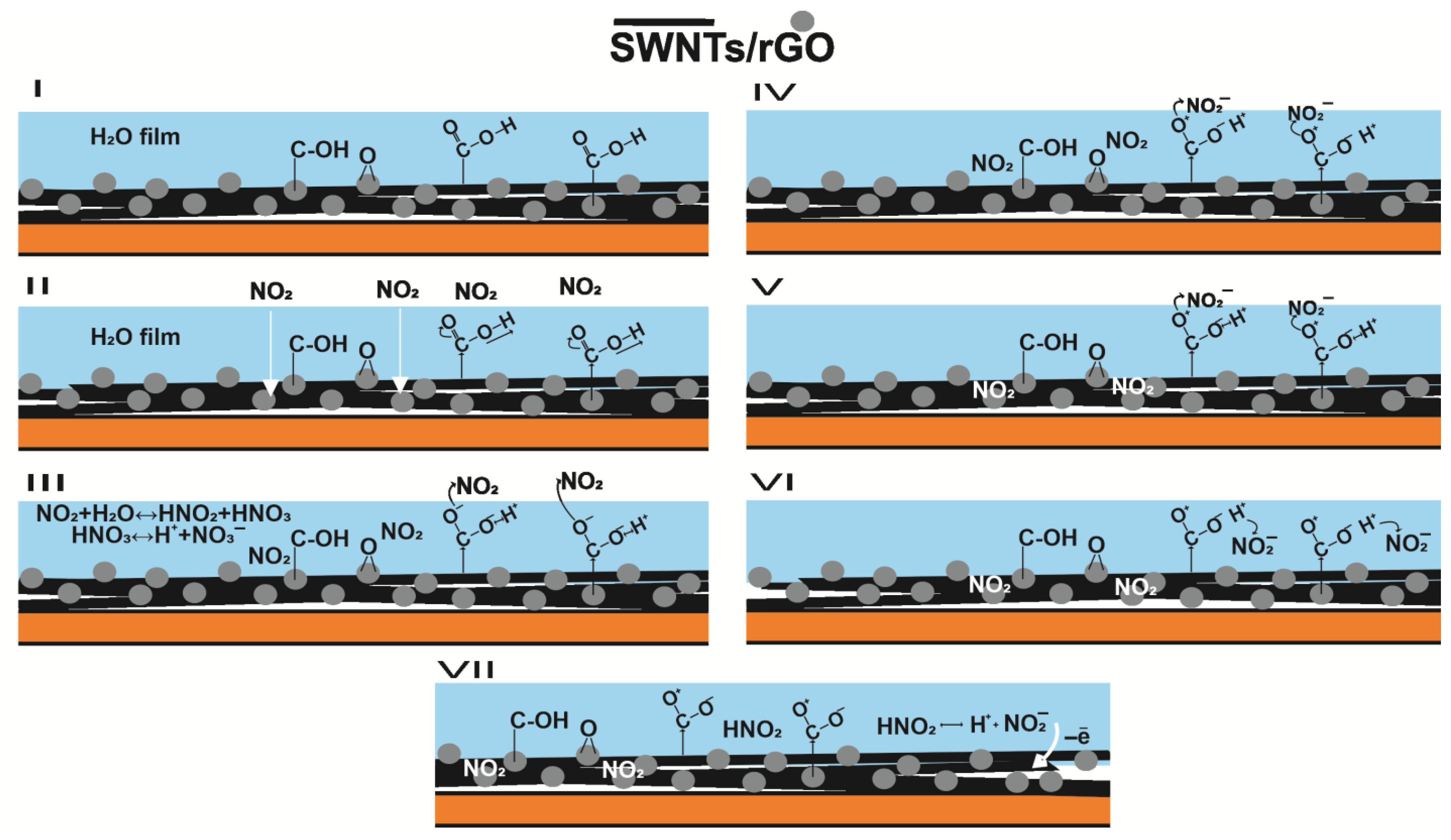
3.2. Sensors Based on Composites of SWCNTs and CNFs with rGO
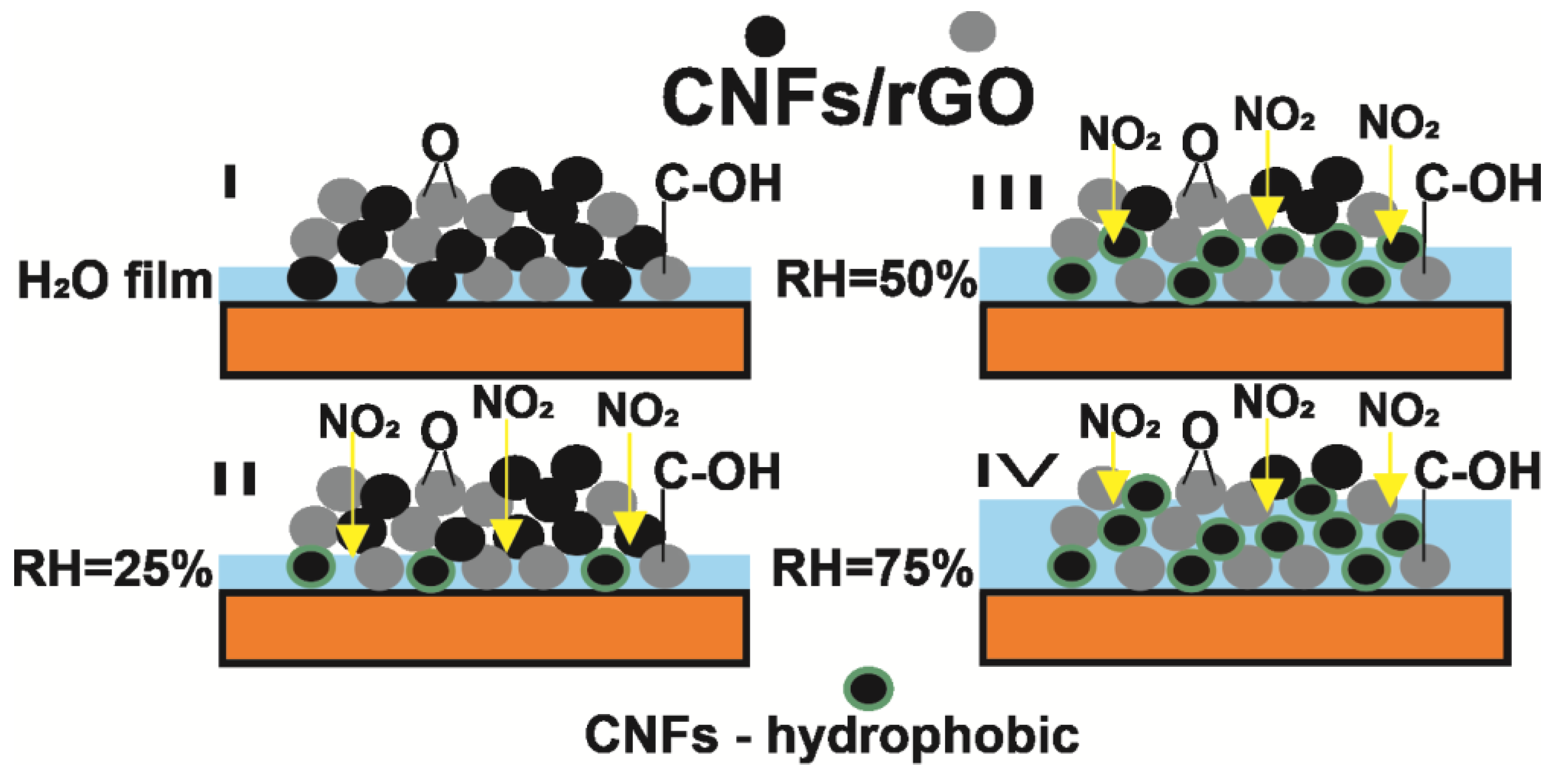
3.3. Effect of Relative Humidity on the NO2 Sensor Response in Composites
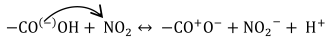
3.4. Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usmanova, R.M.; Sattarova, N.A.; Boyko, N.N. Influence of Automobiles on Environmental Pollution. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1079, 062040. [Google Scholar] [CrossRef]
- Williams, I.D.; Blyth, M. Autogeddon or autoheaven: Environmental and social effects of the automotive industry from launch to present. Sci. Total Environ. 2023, 858, 159987. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sensors Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Onyancha, R.B.; Ukhurebor, K.E.; Aigbe, U.O.; Osibote, O.A.; Kusuma, H.S.; Darmokoesoemo, H.; Balogun, V.A. A systematic review on the detection and monitoring of toxic gases using carbon nanotube-based biosensors. Sens. Bio-Sens. Res. 2021, 34, 100463. [Google Scholar] [CrossRef]
- Freddi, S.; Vergari, M.; Pagliara, S.; Sangaletti, L. A Chemiresistor Sensor Array Based on Graphene Nanostructures: From the Detection of Ammonia and Possible Interfering VOCs to Chemometric Analysis. Sensors 2023, 23, 882. [Google Scholar] [CrossRef]
- Hejazi, M.A.; Eksik, O.; Taşdelen-Yücedağ, Ç.; Ünlü, C.; Trabzon, L. Carbon-Based Nanomaterials in Gas Sensing Applications; Springer International Publishing: Cham, Switzerland, 2023; Volume 6, ISBN 0123456789. [Google Scholar]
- Shahzad, S.; Wang, H.; Li, W.; Sun, Y.; Xie, D.; Ren, T. The Effect of Thin Film Fabrication Techniques on the Performance of rGO Based NO2 Gas Sensors at Room Temperature. Chemosensors 2022, 10, 119. [Google Scholar] [CrossRef]
- Fu, L.; Yu, A.M. Carbon nanotubes based thin films: Fabrication, characterization and applications. Rev. Adv. Mater. Sci. 2014, 36, 40–61. [Google Scholar]
- Niu, H.; Zhou, H.; Lin, T. 21—Electrospun carbon nanofibers as electrode materials for supercapacitor applications. In Electrospun Polymers and Composites; Dong, Y., Baji, A., Ramakrishna, S., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Sawston, UK, 2021; pp. 5644–5656. ISBN 978-0-12-819611-3. [Google Scholar]
- Zhao, Y.; Huang, B.; Ji, Y.; Yu, Y.; Gao, X.; Zhang, Z.; Fei, H.-F. Porous Carbon Nanofiber Flexible Membranes via a Bottlebrush Copolymer Template for Enhanced High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 5644–5656. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, J.; Qu, Q.; Ma, W.; Li, F.; Du, W.; Liu, K.; Zhang, Q.; He, S.; Huang, C. Free-standing porous carbon nanofiber membranes obtained by one-step carbonization and activation for high-performance supercapacitors. Microporous Mesoporous Mater. 2022, 329, 111545. [Google Scholar] [CrossRef]
- Golovakhin, V.; Litvinova, V.I.; Manakhov, A.; Latypova, A.R.; Novgorodtseva, O.N.; Ukhina, A.V.; Ishchenko, A.V.; Al-qasim, A.S.; Maksimovskiy, E.A.; Bannov, A.G. Conductive polymer-multi-walled carbon nanotube composites for gas sensors and supercapacitors. Mater. Today Commun. 2024, 39, 109163. [Google Scholar] [CrossRef]
- Golovakhin, V.; Kim, E.Y.; Novgorodtseva, O.N.; Maksimovskiy, E.A.; Ukhina, A.V.; Ishchenko, A.V.; Bannov, A.G. Treatment of Multi-Walled Carbon Nanotubes with Dichromic Acid: Oxidation and Appearance of Intercalation. Membranes 2023, 13, 729. [Google Scholar] [CrossRef]
- Vincent, C.; Heintz, J.M.; Silvain, J.F.; Chandra, N. Cu/CNF Nanocomposite Processed By Novel Salt Decomposition Method. Open J. Compos. Mater. 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Sharafeldin, I.; Garcia-Rios, S.; Ahmed, N.; Alvarado, M.; Vilanova, X.; Allam, N.K. Metal-decorated carbon nanotubes-based sensor array for simultaneous detection of toxic gases. J. Environ. Chem. Eng. 2021, 9, 104534. [Google Scholar] [CrossRef]
- Naganaboina, V.R.; Singh, S.G. Graphene-CeO2 based flexible gas sensor: Monitoring of low ppm CO gas with high selectivity at room temperature. Appl. Surf. Sci. 2021, 563, 150272. [Google Scholar] [CrossRef]
- Mäklin, J.; Mustonen, T.; Halonen, N.; Tóth, G.; Kordás, K.; Vähäkangas, J.; Moilanen, H.; Kukovecz, Á.K.; Kónya, Z.; Haspel, H.; et al. Inkjet printed resistive and chemical-FET carbon nanotube gas sensors. Phys. Status Solidi Basic Res. 2008, 245, 2335–2338. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.H.; Lee, J.Y.; Kim, K.K.; Choa, Y.H.; Lim, J.H. Single-Walled Carbon Nanotube-Based Chemi-Capacitive Sensor for Hexane and Ammonia. Electron. Mater. Lett. 2019, 15, 712–719. [Google Scholar] [CrossRef]
- Tang, Q.B.; Guo, Y.J.; Tang, Y.L.; Long, G.D.; Wang, J.L.; Li, D.J.; Zu, X.T.; Ma, J.Y.; Wang, L.; Torun, H.; et al. Highly sensitive and selective Love mode surface acoustic wave ammonia sensor based on graphene oxides operated at room temperature. J. Mater. Sci. 2019, 54, 11925–11935. [Google Scholar] [CrossRef]
- Yu, C.; Wu, Y.; Liu, X.; Fu, F.; Gong, Y.; Rao, Y.J.; Chen, Y. Miniature fiber-optic NH3 gas sensor based on Pt nanoparticle-incorporated graphene oxide. Sensors Actuators B Chem. 2017, 244, 107–113. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Yao, Y.; Li, N.; Chen, X. High-stability quartz crystal microbalance ammonia sensor utilizing graphene oxide isolation layer. Sensors Actuators B Chem. 2014, 196, 183–188. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238. [Google Scholar] [CrossRef]
- Valentini, L.; Armentano, I.; Kenny, J.M.; Cantalini, C.; Lozzi, L.; Santucci, S. Sensors for sub-ppm NO2 gas detection based on carbon nanotube thin films. Appl. Phys. Lett. 2003, 82, 961–963. [Google Scholar] [CrossRef]
- Usman, F.; Ghazali, K.H.; Muda, R.; Dennis, J.O.; Ibnaouf, K.H.; Aldaghri, O.A.; Alsadig, A.; Johari, N.H.; Jose, R. Detection of Kidney Complications Relevant Concentrations of Ammonia Gas Using Plasmonic Biosensors: A Review. Chemosensors 2023, 11, 119. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.G.; Wróbel, P.S.; Bachmatiuk, A.; Sun, J.; Gemming, T.; Liu, Z.; Rümmeli, M.H. Carbon nanostructures as a multi-functional platform for sensing applications. Chemosensors 2018, 6, 60. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, C.; Zhu, J.; Wang, J.; Zhang, X.; Lou, X.W. Flexible films derived from electrospun carbon nanofibers incorporated with Co3O4 hollow nanoparticles as self-supported electrodes for electrochemical capacitors. Adv. Funct. Mater. 2013, 23, 3909–3915. [Google Scholar] [CrossRef]
- Randeniya, L.K.; Martin, P.J.; Bendavid, A.; McDonnell, J. Ammonia sensing characteristics of carbon-nanotube yarns decorated with nanocrystalline gold. Carbon 2011, 49, 5265–5270. [Google Scholar] [CrossRef]
- Feng, X.; Irle, S.; Witek, H.; Morokuma, K.; Vidic, R.; Borguet, E. Sensitivity of ammonia interaction with single-walled carbon nanotube bundles to the presence of defect sites and functionalities. J. Am. Chem. Soc. 2005, 127, 10533–10538. [Google Scholar] [CrossRef]
- Mangu, R.; Rajaputra, S.; Clore, P.; Qian, D.; Andrews, R.; Singh, V.P. Ammonia sensing properties of multiwalled carbon nanotubes embedded in porous alumina templates. Mater. Sci. Eng. B 2010, 174, 2–8. [Google Scholar] [CrossRef]
- Rigoni, F.; Tognolini, S.; Borghetti, P.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Enhancing the sensitivity of chemiresistor gas sensors based on pristine carbon nanotubes to detect low-ppb ammonia concentrations in the environment. Analyst 2013, 138, 7392–7399. [Google Scholar] [CrossRef]
- Bekyarova, E.; Davis, M.; Burch, T.; Itkis, M.E.; Zhao, B.; Sunshine, S.; Haddon, R.C. Chemically Functionalized Single-Walled Carbon Nanotubes as Ammonia Sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar] [CrossRef]
- Sidek, R.M.; Yusof, F.A.M.; Yasin, F.M.; Wagiran, R.; Ahmadun, F. Electrical response of multi-walled carbon nanotubes to ammonia and carbon dioxide. In Proceedings of the 2010 IEEE International Conference on Semiconductor Electronics, Malacca, Malaysia, 28–30 June 2010; pp. 263–266. [Google Scholar] [CrossRef]
- Kombarakkaran, J.; Clewett, C.F.M.; Pietraß, T. Ammonia adsorption on multi-walled carbon nanotubes. Chem. Phys. Lett. 2007, 441, 282–285. [Google Scholar] [CrossRef]
- Han, J.-W.W.; Kim, B.; Li, J.; Meyyappan, M. A carbon nanotube based ammonia sensor on cellulose paper. RSC Adv. 2014, 4, 549. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. Room temperature trace level detection of NO2 gas using SnO2 modified carbon nanotubes based sensor. J. Mater. Chem. 2012, 22, 23608. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhu, C. Various characteristic of Carbon nanotubes film methane Gas sensor. In Proceedings of the 2006 1st IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Zhuhai, China, 18–21 January 2006; IEEE: Piscataway, NJ, USA, 2016; pp. 1453–1456. [Google Scholar]
- Lanka, S. Hydrogen and Methane Gas Sensors Synthesis of Multi-Walled Carbon Nanotubes P. Samarasekara. 2009, 47, 361–369. [Google Scholar]
- Dai, H.; Xiao, P.; Lou, Q. Application of SnO2/MWCNTs nanocomposite for SF6 decomposition gas sensor. Phys. Status Solidi 2011, 208, 1714–1717. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Li, G.; Tan, F.; Liu, R.; Li, R.; Zhang, T.; Jin, H.; Li, Q. Triton assisted fabrication of uniform semiconducting single-walled carbon nanotube networks for highly sensitive gas sensors. Carbon 2014, 66, 369–376. [Google Scholar] [CrossRef]
- Mendoza, F.; Hernández, D.M.; Makarov, V.; Febus, E.; Weiner, B.R.; Morell, G. Room temperature gas sensor based on tin dioxide-carbon nanotubes composite films. Sensors Actuators B Chem. 2014, 190, 227–233. [Google Scholar] [CrossRef]
- Ong, K.G.; Grimes, C.A. A Carbon Nanotube-based Sensor for CO2 Monitoring. Sensors 2001, 1, 193–205. [Google Scholar] [CrossRef]
- Desmaris, V.; Saleem, M.A.; Shafiee, S. Examining carbon nanofibers: Properties, growth, and applications. IEEE Nanotechnol. Mag. 2015, 9, 33–38. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon nanofiber-based functional nanomaterials for sensor applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef]
- Bannov, A.G.; Lapekin, N.I.; Kurmashov, P.B.; Ukhina, A.V.; Manakhov, A. Room-Temperature NO2 Gas Sensors Based on Granulated Carbon Nanofiber Material. Chemosensors 2022, 10, 525. [Google Scholar] [CrossRef]
- Shooshtari, M. Gold-decorated vertically aligned carbon nanofibers for high-performance room-temperature ethanol sensing. Microchim. Acta 2025, 192, 517. [Google Scholar] [CrossRef]
- Bannov, A.G.; Jašek, O.; Prášek, J.; Buršík, J.; Zajíčková, L. Enhanced ammonia adsorption on directly deposited nanofibrous carbon films. J. Sensors 2018, 2018, 7497619. [Google Scholar] [CrossRef]
- Venegas, C.J.; Bollo, S.; Sierra-Rosales, P. Carbon-Based Electrochemical (Bio)sensors for the Detection of Carbendazim: A Review. Micromachines 2023, 14, 1752. [Google Scholar] [CrossRef]
- Predtechenskiy, M.R.; Khasin, A.A.; Bezrodny, A.E.; Bobrenok, O.F.; Dubov, D.Y.; Muradyan, V.E.; Saik, V.O.; Smirnov, S.N. New perspectives in SWCNT applications: Tuball SWCNTs. Part 1. Tuball by itself—All you need to know about it. Carbon Trends 2022, 8, 100175. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Kurmashov, P.B. Thermal analysis of carbon nanomaterials: Advantages and problems of interpretation. J. Therm. Anal. Calorim. 2020, 142, 349–370. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Han, D.; Zhai, L.; Gu, F.; Wang, Z. Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at low temperature. Sensors Actuators B Chem. 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Vaghela, C.; Kulkarni, M.; Haram, S.; Karve, M.; Aiyer, R. Biopolymer-Polyaniline Composite for a Wide Range Ammonia Gas Sensor. IEEE Sens. J. 2016, 16, 4318–4325. [Google Scholar] [CrossRef]
- Na, W.; Kim, J.; Kim, Y.K.; Kim, S.G.; Jang, J. Fluorination of shape-controlled porous carbon nanoweb layers for ammonia gas sensor application. Carbon 2020, 165, 185–195. [Google Scholar] [CrossRef]
- Khajavian, M.; Ismail, S.; Afshari, M. Surface functionalization of the all-triazine C3N3 framework for efficient As5+ adsorption: Experimental, density functional theory, and molecular dynamics. Sep. Purif. Technol. 2025, 373, 133611. [Google Scholar] [CrossRef]
- Al-Ostaz, A.; Pal, G.; Mantena, P.R.; Cheng, A. Molecular dynamics simulation of SWCNT–polymer nanocomposite and its constituents. J. Mater. Sci. 2008, 43, 164–173. [Google Scholar] [CrossRef]
- Mazo, M.A.; Sanguino, J.; Martín-Gullón, I.; Rubio, J. Formation of carbon nanofibers with Ni catalyst supported on a micro-mesoporous glass. Microporous Mesoporous Mater. 2021, 323, 111168. [Google Scholar] [CrossRef]
- Predtechenskiy, M.R.; Khasin, A.A.; Smirnov, S.N.; Bezrodny, A.E.; Bobrenok, O.F.; Dubov, D.Y.; Kosolapov, A.G.; Lyamysheva, E.G.; Muradyan, V.E.; Saik, V.O.; et al. New Perspectives in SWCNT Applications: Tuball SWCNTs. Part 2. New Composite Materials Through Augmentation with Tuball. Carbon Trends 2022, 8, 100176. [Google Scholar] [CrossRef]
- Brusko, V.; Khannanov, A.; Rakhmatullin, A.; Dimiev, A.M. Unraveling the infrared spectrum of graphene oxide. Carbon 2024, 229, 119507. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Chou, Y.C.; Lin, C.P.; Hsieh, T.F.; Shu, C.M. Thermal analysis of multi-walled carbon nanotubes by Kissinger’s corrected kinetic equation. Aerosol Air Qual. Res. 2010, 10, 212–218. [Google Scholar] [CrossRef]
- Sohn, Y.; Kim, D.G.; Lee, J.H.; Lee, S.; Hwang, I.S.; Lee, S.H.; Yoo, S.J.; Kim, P. Defect-controlled Fe-N-doped carbon nanofiber by ball-milling for oxygen reduction reaction. Korean J. Chem. Eng. 2020, 37, 938–945. [Google Scholar] [CrossRef]
- Drewniak, S.; Drewniak, Ł.; Pustelny, T. Mechanisms of NO2 Detection in Hybrid Structures Containing Reduced Graphene Oxide: A Review. Sensors 2022, 22, 5316. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Yamaletdinov, R.D.; Plyusnin, P.E.; Okotrub, A.V.; Bulusheva, L.G. Adsorption kinetics of NO2 gas on oxyfluorinated graphene film. Phys. Chem. Chem. Phys. 2023, 25, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lefferts, M.J.; Wang, Y.; Lister, A.M.; Forssberg, A.; Chen, R.; Wang, L.; Castell, M.R. Chemiresistive Polymer Percolation Network Gas Sensor Created with a Nanosphere Template. Adv. Mater. Interfaces 2023, 10, 2202042. [Google Scholar] [CrossRef]
- Shin, W.; Jung, G.; Hong, S.; Jeong, Y.; Park, J.; Kim, D.; Jang, D.; Kwon, D.; Bae, J.-H.; Park, B.-G.; et al. Proposition of deposition and bias conditions for optimal signal-to-noise-ratio in resistor- and FET-type gas sensors. Nanoscale 2020, 12, 19768–19775. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Cai, Y.; Pan, P.; Li, J.; Ren, Q.; Xu, J. Enhanced Humidity Sensing Response of SnO2/Silicon Nanopillar Array by UV Irradiation. Sensors 2019, 19, 2141. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Y.; Yuan, Y.; Meng, W.; Jiang, W.; Wang, X. High-response humidity sensing with graphene oxide/lignosulfonate and laser-induced graphene for respiratory health. RSC Adv. 2025, 15, 11739–11748. [Google Scholar] [CrossRef]
- Du, H.; Xie, G.; Su, Y.; Tai, H.; Du, X.; Yu, H.; Zhang, Q. A New Model and Its Application for the Dynamic Response of RGO Resistive Gas Sensor. Sensors 2019, 19, 889. [Google Scholar] [CrossRef]
- Piloto, C.; Mirri, F.; Bengio, E.A.; Notarianni, M.; Gupta, B.; Shafiei, M.; Pasquali, M.; Motta, N. Room temperature gas sensing properties of ultrathin carbon nanotube films by surfactant-free dip coating. Sensors Actuators B Chem. 2016, 227, 128–134. [Google Scholar] [CrossRef]
- Ma, X.F.; Xie, G.Z.; Su, Y.J.; Du, H.F.; Xie, T.; Jiang, Y.D. Polyvinylpyrrolidone/graphene oxide thin films coated on quartz crystal microbalance electrode for NH3 detection at room temperature. Sci. China Technol. Sci. 2016, 59, 1377–1382. [Google Scholar] [CrossRef]
- Pisarkiewicz, T.; Maziarz, W.; Małolepszy, A.; Stobiński, L.; Michoń, D.A.; Szkudlarek, A.; Pisarek, M.; Kanak, J.; Rydosz, A. Nitrogen dioxide sensing using multilayer structure of reduced graphene oxide and α-Fe2O3. Sensors 2021, 21, 1011. [Google Scholar] [CrossRef]
- Kumar, S.; Dmitrieva, V.A.; Meng, G.; Evlashin, S.A.; Sukhanova, E.V.; Kvashnin, D.G.; Popov, Z.I.; Bannov, A.G.; Fedorov, F.S.; Nasibulin, A.G. Structured Graphene Oxide/Reduced Graphene Oxide Interfaces for Improved NO2 Sensing. ACS Appl. Nano Mater. 2023, 6, 14083–14093. [Google Scholar] [CrossRef]
- Chae, M.; Lee, D.; Kim, H.D. Dynamic Response and Swift Recovery of Filament Heater-Integrated Low-Power Transparent CNT Gas Sensor. Adv. Funct. Mater. 2024, 34, 2405260. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Rumyantseva, M.; Konstantinova, E.; Marikutsa, A.; Tokarev, S.; Yaltseva, P.; Fedorova, O.; Gaskov, A. Effect of Humidity on Light-Activated NO and NO2 Gas Sensing by Hybrid Materials. Nanomaterials 2020, 10, 915. [Google Scholar] [CrossRef]
- Yan, W.; Worsley, M.A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. Effects of ambient humidity and temperature on the NO2 sensing characteristics of WS2/graphene aerogel. Appl. Surf. Sci. 2018, 450, 372–379. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Cui, X.; Cheng, P.; Wang, B.; Gao, Y.; Li, X.; Yang, T.; Zhang, T.; Lu, G. Humidity-Sensing Properties of Urchinlike CuO Nanostructures Modified by Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 3888–3895. [Google Scholar] [CrossRef]
- Peng, N.; Zhang, Q.; Chow, C.L.; Tan, O.K.; Marzari, N. Sensing Mechanisms for Carbon Nanotube Based NH3 Gas Detection. Nano Lett. 2009, 9, 1626–1630. [Google Scholar] [CrossRef]
- Kyokunzire, P.; Zaraket, J.; Teresa, M.; Fierro, V.; Celzard, A. p-/n-type conduction in activated carbon NO2 sensors with enhanced low-concentration sensitivity at room temperature. Carbon 2026, 246, 120891. [Google Scholar] [CrossRef]
- Travlou, N.A.; Rodríguez-Castellón, E.; Bandosz, T.J. Sensing of NH3 on heterogeneous nanoporous carbons in the presence of humidity. Carbon 2016, 100, 64–73. [Google Scholar] [CrossRef]
- Ogawa, T.; Kamiguchi, K.; Tamaki, T.; Imai, H.; Yamaguchi, T. Differentiating Grotthuss proton conduction mechanisms by nuclear magnetic resonance spectroscopic analysis of frozen samples. Anal. Chem. 2014, 86, 9362–9366. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Z.; Yuan, S.; Han, A.; Shen, Y.; Cheng, X.; Liang, Y.; Shen, S.; Zhang, J. Structural and transport properties of ultrathin perfluorosulfonic acid ionomer film in proton exchange membrane fuel cell catalyst layer: A review. J. Power Sources 2022, 536, 231523. [Google Scholar] [CrossRef]


| Characteristics | SWCNTs | CNFs | Method |
|---|---|---|---|
| Average diameter, nm | 1.6 ± 0.4 | 44.9 ± 5.5 | TEM |
| Surface area, m2/g | 924 | 119 | Low-temperature nitrogen adsorption |
| I(G)/I(D) | 35 | 1.0 | Raman spectroscopy |
| Samples | C, % | H, % | N, % | S, % | O, % |
|---|---|---|---|---|---|
| SWCNTs | 97.9 | ≤0.3 | ≤0.3 | - | 1.5 |
| Acid treated SWCNTs | 92.6 | 0.7 | 0.6 | - | 6.1 |
| CNFs | 97.43 | 0.5 | 0 | - | 2.07 |
| Concentration, mg/mL | τ at 1.5 ppm, s | n at 1.5 ppm, % | R0, Ω | Sensitivity, %/ppm | SNR |
|---|---|---|---|---|---|
| SWCNTs | |||||
| 0.75 | 431 ± 15 | 20 ± 1 | 29 ± 1.5 | −0.73 ± 0.23, R2 = 0.91 | 458 |
| 1.5 | 500 ± 8.5 | 14 ± 2.5 | 10 ± 7 | −0.81 ± 0.68, R2 = 0.79 | 1000 |
| 3.0 | 382 ± 24 | 36 ± 1.7 | 30 ± 3 | −0.60 ± 0.10, R2 = 0.94 | 795 |
| CNFs | |||||
| 0.75 | 410 ± 16 | 10 ± 2 | 601 ± 8 | −0.92 ± 0.03, R2 = 0.99 | 3010 |
| 1.5 | 438 ± 13 | 26 ± 1.3 | 427 ± 17 | −1.32 ± 0.06, R2 = 0.99 | 2510 |
| 3.0 | 481 ± 22 | 22 ± 4 | 191 ± 9 | −1.09 ± 0.07, R2 = 0.99 | 830 |
| Initial GO | ||
|---|---|---|
| C:O, at. | 3.1 | EDX |
| I(D)/I(G) | 0.87 | Raman spectroscopy |
| rGO | ||
| C:O, at. | 4.7 | EDX |
| I(D)/I(G) | 0.71 | Raman spectroscopy |
| rGO Concentration, % | τ at 1.5 ppm, s | n at 1.5 ppm, % | R0, Ω | Sensitivity, %/ppm | SNR |
|---|---|---|---|---|---|
| SWCNTs | |||||
| 0 | 500 ± 8.5 | 14 ± 2.5 | 10 ± 7 | −0.81 ± 0.68, R2 = 0.79 | 1000 |
| 25 | 444 ± 34 | 25 ± 3 | 12 ± 0.4 | −0.37 ± 0.04, R2 = 0.97 | 928 |
| 50 | 480 ± 7 | 45 ± 4 | 186 ± 4.5 | −0.40 ± 0.05, R2 = 0.97 | 680 |
| 75 | 450 ± 44 | 100 ± 1.5 | 5 ± 0.2 | −0.32 ± 0.01, R2 = 0.99 | 1360 |
| CNFs | |||||
| 0 | 481 ± 10 | 22 ± 3 | 191 ± 2 | −1.09 ± 0.07, R2 = 0.99 | 830 |
| 25 | 421 ± 5 | 13 ± 1.3 | 50,461 ± 148 | −0.97 ± 0.26, R2 = 0.87 | 60 |
| 50 | 427 ± 32 | 12 ± 2 | 735 ± 12 | −0.57 ± 0.39, R2 = 0.51 | 795 |
| 75 | 555 ± 8 | 2 ± 0.5 | 678 ± 6 | −1.47 ± 0.28, R2 = 0.93 | 920 |
| Sensing Material | Response to NO2, % | Concentration, ppm | Total Flow Rate in Chamber, Sccm | Temperature, °C | Reference |
|---|---|---|---|---|---|
| DWCNTs | 1.5 (n/a) | 1 | 200 | Room temperature | [72] |
| Polyvinylpyrrolidone/GO | 28.8 (n/a) | 10 | 100 | Room temperature | [73] |
| Fe2O3/rGO | 4.68 (RH = 50%) | 2.5 | n/a | 200 | [74] |
| GO/rGO | 18 (RH = 50%) | 100 | 100 | Room temperature | [75] |
| CNFs (0.75 mg/mL) | 14.8 (RH = 2%) | 1.5 | 1000 | Room temperature | This work |
| CNFs/rGO (1:1) | 49 (RH = 2%) | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozben’, A.D.; Smagulova, A.R.; Khajavian, M.; Golovakhin, V.; Shishin, A.A.; Shpakova, S.A.; Ostertak, D.I.; Ukhina, A.V.; Maksimovskiy, E.A.; Bogomolova, A.I.; et al. Enhanced Room Temperature NO2 Detection by Carbon Nanofibers and Single-Walled Carbon Nanotubes: Experimental and Molecular Dynamics. Chemosensors 2025, 13, 389. https://doi.org/10.3390/chemosensors13110389
Lozben’ AD, Smagulova AR, Khajavian M, Golovakhin V, Shishin AA, Shpakova SA, Ostertak DI, Ukhina AV, Maksimovskiy EA, Bogomolova AI, et al. Enhanced Room Temperature NO2 Detection by Carbon Nanofibers and Single-Walled Carbon Nanotubes: Experimental and Molecular Dynamics. Chemosensors. 2025; 13(11):389. https://doi.org/10.3390/chemosensors13110389
Chicago/Turabian StyleLozben’, Arina D., Arina R. Smagulova, Mohammad Khajavian, Valery Golovakhin, Artyom A. Shishin, Sofia A. Shpakova, Dmitriy I. Ostertak, Arina V. Ukhina, Eugene A. Maksimovskiy, Alexandra I. Bogomolova, and et al. 2025. "Enhanced Room Temperature NO2 Detection by Carbon Nanofibers and Single-Walled Carbon Nanotubes: Experimental and Molecular Dynamics" Chemosensors 13, no. 11: 389. https://doi.org/10.3390/chemosensors13110389
APA StyleLozben’, A. D., Smagulova, A. R., Khajavian, M., Golovakhin, V., Shishin, A. A., Shpakova, S. A., Ostertak, D. I., Ukhina, A. V., Maksimovskiy, E. A., Bogomolova, A. I., Smovzh, D. V., & Bannov, A. G. (2025). Enhanced Room Temperature NO2 Detection by Carbon Nanofibers and Single-Walled Carbon Nanotubes: Experimental and Molecular Dynamics. Chemosensors, 13(11), 389. https://doi.org/10.3390/chemosensors13110389






